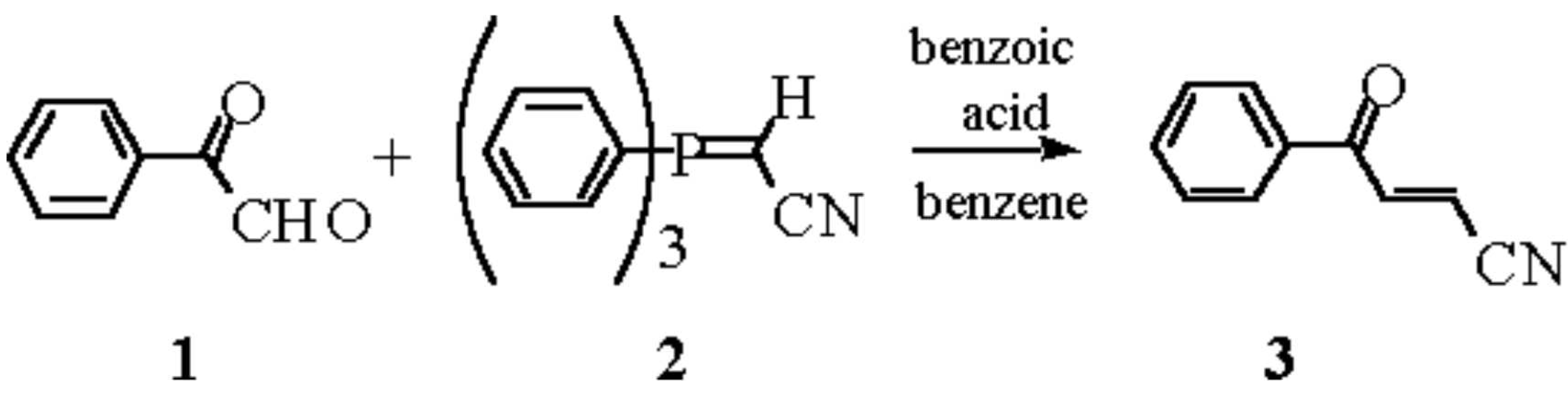
3-Benzoylacrylonitrile [(E)-4-Oxo-4-phenyl-2-butenenitrile]
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Hosokawa, T.; Aoki, S.; Murahashi, S. Synthesis 1992, 558. [CrossRef]
- Nudelman, A.; Keinan, E. Synthesis 1982, 687. [CrossRef]
- Severin, T.; Poehlmann, H. Chem. Ber. 1978, 111, 1564.
- Nesmeyanov, A. N.; Rybinskaya, M. I. Dokl. Akad. Nauk SSSR 1957, 115, 315, Chem. Abstr. 1958, 52, 7158g.
- Raulet, C. Compt. Rend. Sci. Acad. Sci., Ser. C 1973, 276, 903.
- Raulet C. Bull. Soc. Chim. Fr. 1974, 531.
- Akiyama, Y.; Abe, J.; Takano, T.; Kawasaki, T.; Sakamoto, M. Chem. Pharm. Bull. 1984, 32, 2821. [CrossRef]
- Colonna, M.; Marchetti, L. Gazz. Chim. Ital. 1966, 96, 1175.
- Ger. Offenl. DE 2.116.293 (Inv.: Raabe, T.; Stachel, A.; Schottholt, J.; Nitz, R. E.; Casella Farbwerke Mainkur A.-G.; 19.10.1972 [Appl. 2.4.1971]) Chem. Abstr. 1973, 78, 16219.
- Trippett, S.; Walker, D.M. J. Chem. Soc. 1959, 3874.
- Sample Availability: Available from the authors
© 2003 MDPI. All rights reserved.
Share and Cite
Thiemann, T.; Watanabe, M. 3-Benzoylacrylonitrile [(E)-4-Oxo-4-phenyl-2-butenenitrile]. Molbank 2003, 2003, M334. https://doi.org/10.3390/M334
Thiemann T, Watanabe M. 3-Benzoylacrylonitrile [(E)-4-Oxo-4-phenyl-2-butenenitrile]. Molbank. 2003; 2003(3):M334. https://doi.org/10.3390/M334
Chicago/Turabian StyleThiemann, Thies, and Masataka Watanabe. 2003. "3-Benzoylacrylonitrile [(E)-4-Oxo-4-phenyl-2-butenenitrile]" Molbank 2003, no. 3: M334. https://doi.org/10.3390/M334
APA StyleThiemann, T., & Watanabe, M. (2003). 3-Benzoylacrylonitrile [(E)-4-Oxo-4-phenyl-2-butenenitrile]. Molbank, 2003(3), M334. https://doi.org/10.3390/M334



