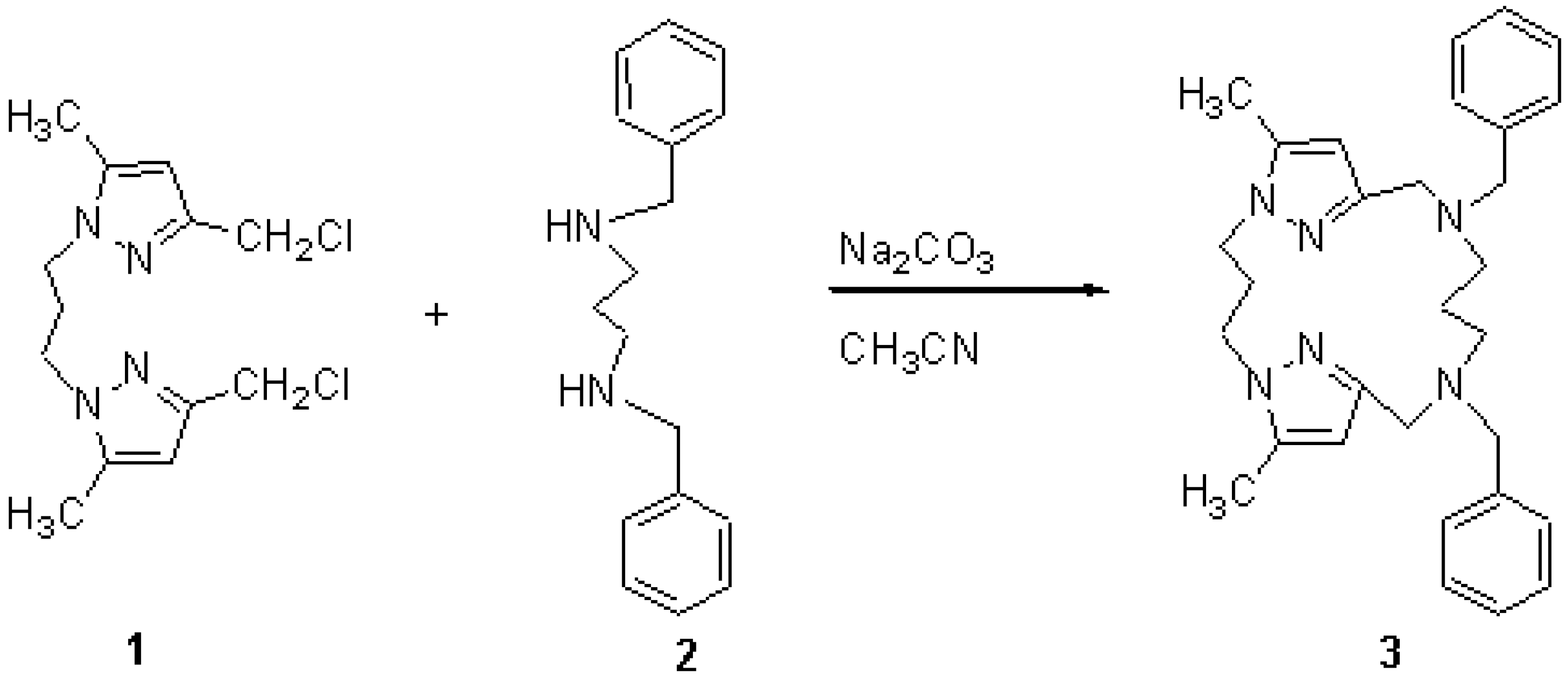
A suspension of sodium carbonate (12 g, 120 mmol) in acetonitrile (250 mL) was refluxed under magnetic stirring, then a solution of bis-(3-chloromethyl-5-methylpyrazolyl)propane (1) (2.03 g, 7 mmol) [1,2,3] and diamine 2 (1.86 g , 7 mmol) in acetonitrile (50 mL) was added dropwise. The solution was refluxed under stirring for two hours, filtered and the solvent was removed in vacuum, the residue was purified on alumina column with (CH2Cl2/MeOH : 97/3) as eluent to give the macrocycle 3 as an oily substance.
Yield: (2 g, 70 %).
(FAB)+ [M+H]+ = 485.
1H NMR (250 MHz, CDCl3) d ppm: 7.40 (m, 10H, Ph) ; 5.85 (s, 2H, HPz) ; 3.95 (t, 4H, CH2-Pz) ;3.60 (s, 4H, N-CH2-Ph) ; 3.50 (s, 4H, Pz-CH2-N) ; 2.45 (t, 4H, N-CH2-CH2) ; 2.40 (m, 2H, N-CH2-CH2) ; 2.20 (s, 6H, CH3-Pz) ;1.30 (m, 2H, CH2-CH2-CH2).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Tarrago, G.; El Kadiri, S.; Marzin, C.; Coquelet, C. New J. Chem. 1991, 15, 677.
- Bienvenue, E.; Choua, S.; Lobo-Recio, M-Has.; Marzin, C.; Pacheco, P.; Seta, P.; Tarrago, G. Inorg. Biochem. 1995, 57, 157.
- Bol, J. E.; Driessen, W. L.; Reedjik, J. J. Chem. Soc., Chem. Commun 1995, 13, 65.
© 2003 MDPI. All rights reserved.