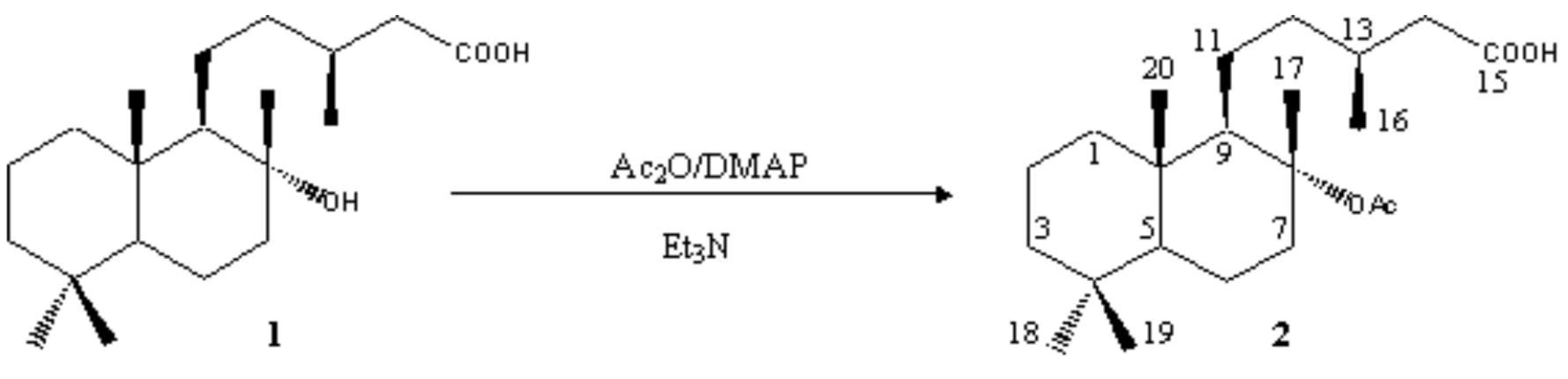
Acetic anhydride (0.09 mL, 0.98 mmol) and N,N-dimethylaminopyridine (2 mg, 0.02 mmol) were added to a stirred solution of the alcohol 1 (75 mg, 0.23 mmol) in freshly distilled triethylamine (1 mL) [1]. The reaction was mantained between 35-40 °C for 72 h and, then, water was added (10 mL) and the mixture extracted with Et2O (3×25 mL). The combined organic layers were washed with 2N HCl (25 mL) and brine (3×25 mL). The organic phase was dried over anhydrous Na2SO4 and the solvent evaporated under reduced pressure to yield a residue (77 mg) which was purified by flash chromatography on silica gel, using a 3:2 hexane/EtOAc mixture as eluent, to give the pure title compound 2 (55 mg, 0.15 mmol, 65%).
Mp: 131.0-132.8 °C (white crystals, from hexane).
[a]D = -53.1° (c 0.83 cg·mL−1, CHCl3).
IR (neat, n, cm−1): 3400-2300, 1700 (COOH), 1732, 1288, 1188 (OAc).
1H NMR (300 MHz, CDCl3, d, ppm): 0.78 (3H, s, Meb-4), 0.83 (3H, s, Me-10), 0.86 (3H, s, Mea-4), 0.98 (3H, d, J=6.6 Hz, Me-13), 1.45 (3H, s, Me-8), 1.92 (3H, s, OAc), 0.94-1.98 (16H, m, H-1,2,3,5,6,7a,9,11,12,13), 2.16 (1H, dd, J=15.0 Hz, 7.6 Hz, H-14), 2.33 (1H, dd, J=15.0 Hz, 6.6 Hz, H'-14), 2.63 (1H, dt, J=12.7 Hz, 3.5 Hz, Hb-7).
13C NMR (75 MHz, CDCl3, d, ppm): 39.50 (C-1), 18.28 (C-2), 41.87 (C-3), 33.06 (C-4), 55.58 (C-5), 19.96 (C-6), 38.72 (C-7), 88.01 (C-8), 59.01 (C-9), 39.33 (C-10), 23.11 (C-11), 39.79 (C-12), 30.87 (C-13), 41.55 (C-14), 179.41 (C-15), 19.76 (C-16), 20.34 (C-17), 33.29 (C-18), 21.38 (C-19), 15.67 (C-20), 170.34 (OAc), 22.81 (OAc).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
We wish to thank the Junta de Andalucía for financial support and the Ministerio de Educación, Cultura y Deporte for a Fellowship to J. M. Castro.
References and Notes
- Urones, J. G.; Basabe, P.; Marcos, I. S.; González, J. L.; Jiménez, V.; Sexmero, M. J.; Lithgow, A. M. Ambergris Compounds from Labdanolic Acid. Tetrahedron 1992, 48, 9991–9998. [Google Scholar] [CrossRef]
- Sample availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.