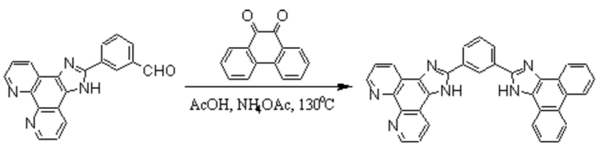
2-(3-Formylphenyl)imidazo[4,5-f][1,10]phenanthroline was prepared by a previously published method []. A mixture of 2-(3-formylphenyl)imidazo[4,5-f][1,10]phenanthroline (0.15 g, 0.46 mmol), 9,10-phenanthrenequinone (0.104 g, 0.50 mmol), ammonium acetate (0.713 g, 9.3 mmol) and glacial acetic acid (20 cm3) was refluxed for about 2 h. The cooled solution was filtered, diluted with water (ca, 60 cm3) and neutralized with concentrated aqueous ammonia. The crude product was collected and purified by column chromatography on alumina with ethanol-toluene (1:4 v/v) as eluent to give the title compound as pale yellow powders. Yield 0.183 g, 77.5%.
1H NMR (500 MHz, d6-DMSO): 13.99 (s, 1H), 13.71 (s, 1H), 9.25 (s, 1H), 9.07 (dd, 2H, J = 2), 9.05 (d, 2H, J = 8.5), 8.88 (d, 2H, J = 8.5), 8.69 (d, 2H, J = 7.5), 8.45 (d, 1H, J = 8.0), 8.40 (d, 1H, J = 8.5), 7.88-7.84 (m, 3H), 7.78 (t, 2H. J = 7.5), 7.67 (t, 2H, J = 7.5).
13C NMR (125 MHz, d6-DMSO): 150.2, 148.7, 147.9, 143.8, 137.1, 135.8, 131.3, 130.8, 129.7, 127.9, 127.8, 127.6, 127.2, 126.9, 126.6, 125.5, 125.2, 124.2, 123.7, 123.1, 122.4, 122.1, 119.4.
IR (KBr, cm-1): 3429.3, 1645, 1610, 1556, 1454, 1413, 1356, 1298, 1236, 1191, 1076, 954, 808, 756, 738, 710, 695, 672
UV-Vis (l, nm, in ethanol): 260, 324, 361.
FAB-MS ([M+1]+): 513.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
Support for this research provided by the National Natural Science Foundation of China, the National Science Foundation of Guangdong Province and Research Fund of Royal Society of Chemistry U.K. is gratefully acknowledged.
References
- Chao, H.; Ye, B.-H.; Li, H.; Li, R.-H.; Zhou, J.-Y.; Ji, L.-N. Polyhedron 2000, 19, 1975.
© 2003 MDPI. All rights reserved.