Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most frequent B-cell type of non-Hodgkin’s lymphoma. Recent genomic studies have highlighted the importance of genetic alterations in apoptotic pathways that help malignant DLBCL cells to evade apoptosis. Apoptosis evasion by DLBCL cells is known to mediate resistance to chemotherapy. Advances in the field of regulated cell death (RCD) research have identified novel therapeutic avenues in cancer. In particular, non-apoptotic RCDs can be targeted to overcome resistance to apoptosis in cancer and ensure cell death. In this review, we have highlighted the contribution of multiple RCDs, including apoptosis, necroptosis, ferroptosis, pyroptosis, PANoptosis, NETotic cell death, autophagy-dependent cell death, cuproptosis, methuosis, or mitotic death, to normal development of B lymphocytes and DLBCL pathogenesis. We have summarized molecular mechanisms governing distinct RCDs in DLBCL, differences in cell death pathways in activated B-cell (ABC) and germinal center B-cell (GCB) DLBCL subtypes, prognostic values of RCD-related genes, and discussed the implication of RCD pathways for DLBCL treatment. Notably, the impact of RCDs goes far beyond just killing tumor cells. RCD modalities are important for orchestrating the immune response and modulating the tumor microenvironment. The current review also aims to reveal the effect of different RCDs on the tumor microenvironment in DLBCL. Most RCDs play a dual role in DLBCL, demonstrating both tumor-inducing and tumor-suppressing effects, which suggests that their targeting should be exploited with caution. Our analysis suggests that pharmacological ferroptosis induction may be the most promising RCD-targeting strategy in DLBCL.
1. Introduction
Diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) represents the most frequent subtype (up to 40% of cases) of non-Hodgkin lymphomas (NHLs). Generally, NHLs are a group of heterogenous malignant hematological tumors derived either from B lymphocytes (approximately 90% of cases) or T and NK cells (about 10%) [1]. DLBCL is an aggressive, morphologically and genetically diverse NHL type [2,3], with about 40% of patients relapsing after the current standard of care frontline chemoimmunotherapy [4]. In addition to DLBCL NOS, the recently updated WHO-HAEM5 (the 5th edition of the World Health Organization Classification of Haematolymphoid Tumours) identifies and defines the entire family of large B-cell lymphomas (LBCLs), which includes T-cell/histiocyte-rich LBCL; DLBCL/high grade B-cell lymphoma with MYC and BCL2 rearrangements; ALK-positive LBCL; LBCL with IRF4 rearrangement; high-grade B-cell lymphoma with 11q aberrations; lymphomatoid granulomatosis; EBV-positive DLBCL; DLBCL associated with chronic inflammation; fibrin-associated LBCL; fluid overload-associated LBCL; plasmablastic lymphoma; primary LBCL of immune-privileged sites; primary cutaneous DLBCL, leg type; intravascular LBCL; primary mediastinal LBCL; mediastinal grey zone lymphoma; and high-grade B-cell lymphoma, NOS [5,6]. Based on the cell of origin classification defined by gene expression profiling, DLBCL could be subdivided into activated B-cell like (ABC), germinal center B-cell like (GCB), and unclassified subtypes [7,8]. Recently published genomic studies have further defined multiple parallel DLBCL genetic subtypes and revealed a wide spectrum of mutations that affect survival/cell death signaling in DLBCL [9,10,11,12]. Of note, B-cell receptor (BCR) signaling plays a key role in the regulation of survival for both ABC and GCB DLBCL subtypes, relying on the so-called autoantigen-activated or antigen-independent cell autonomous “chronic active” and antigen-independent “tonic” signaling, respectively [13,14,15,16]. Thus, like in the case of other malignancies [17], evasion of cell death as a consequence of altered survival and cell death signaling is a common hallmark of DLBCL. This deregulation is in the focus of continuous research due to the associated therapeutic potential.
Specifically, the availability of advanced technologies has allowed researchers to describe various molecular mechanisms of cell death and to show that they are quite distinctive. This resulted in an unprecedented growth of identified distinct regulated cell death (RCD) modalities characterized by specific cellular signaling and immunogenic consequences [18]. It is important to note that in contrast to accidental cell death, RCDs are associated with strictly regulated and specific signaling events and defined molecular machinery. On the other hand, interconnection between alternative cell death pathways is frequent [19]. Detailed knowledge of cell death pathways in cancer opens a wide range of options to target malignant cells directly and to modulate the tumor microenvironment in order to fine-tune the anti-tumor immune response [20]. An increasing number of studies indicate that alkaliptosis [21], anoikis [22], apoptosis [23], autophagy-related cell death [24], autosis [25], cuproptosis [26], disulfidptosis [27], entosis [28], ferroptosis [29], lysosome-dependent cell death [30], methuosis [31], mitochondrial permeability transition-driven necrosis [32], mitotic death [33], necroptosis [34], NETosis [35], oxeiptosis [36], PANoptosis [37], parthanatos [38], and pyroptosis [39] contribute to tumorigenesis and might be therapeutically modulated. However, in recent years, it has become clear that the role of a particular RCD in cancer is not straightforward. On the contrary, it is quite complex and often Janus-faced, especially in the case of immunogenic cell death (ICD) [40]. ICD, which is a stress response-associated RCD accompanied by antigen presentation, release of damage-associated molecular patterns (DAMPs) and recruitment of antigen-presenting cells, evokes adaptive immunity that can be targeted for cancer immunotherapy [41]. Notably, since RCD pathways are frequently interconnected by extensive crosstalk and backup mechanisms, it might be possible to therapeutically target several lethal subroutines simultaneously to improve the overall treatment effectiveness [42].
In this review article, we summarize the complex contribution of multiple different lethal subroutines to DLBCL development (Figure 1), with an emphasis on the immunogenic effects of RCDs (of malignant cells as well as of tumor microenvironment cells) and signaling pathways crucial for regulation of survival/cell death in DLBCL. Complex understanding of these processes will help uncover novel therapeutic avenues aimed to control RCD-based tumor destruction and adjust the adaptive immune response.
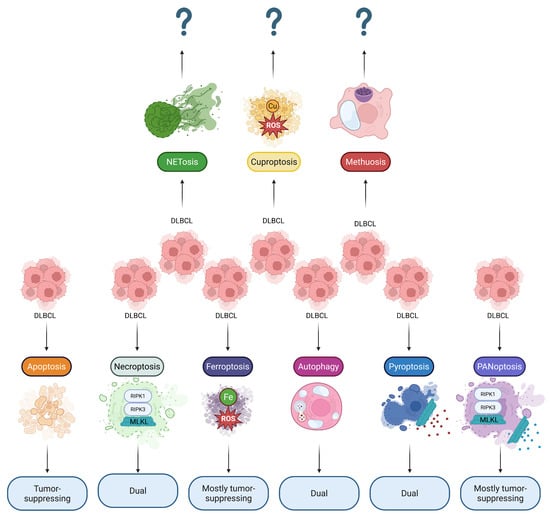
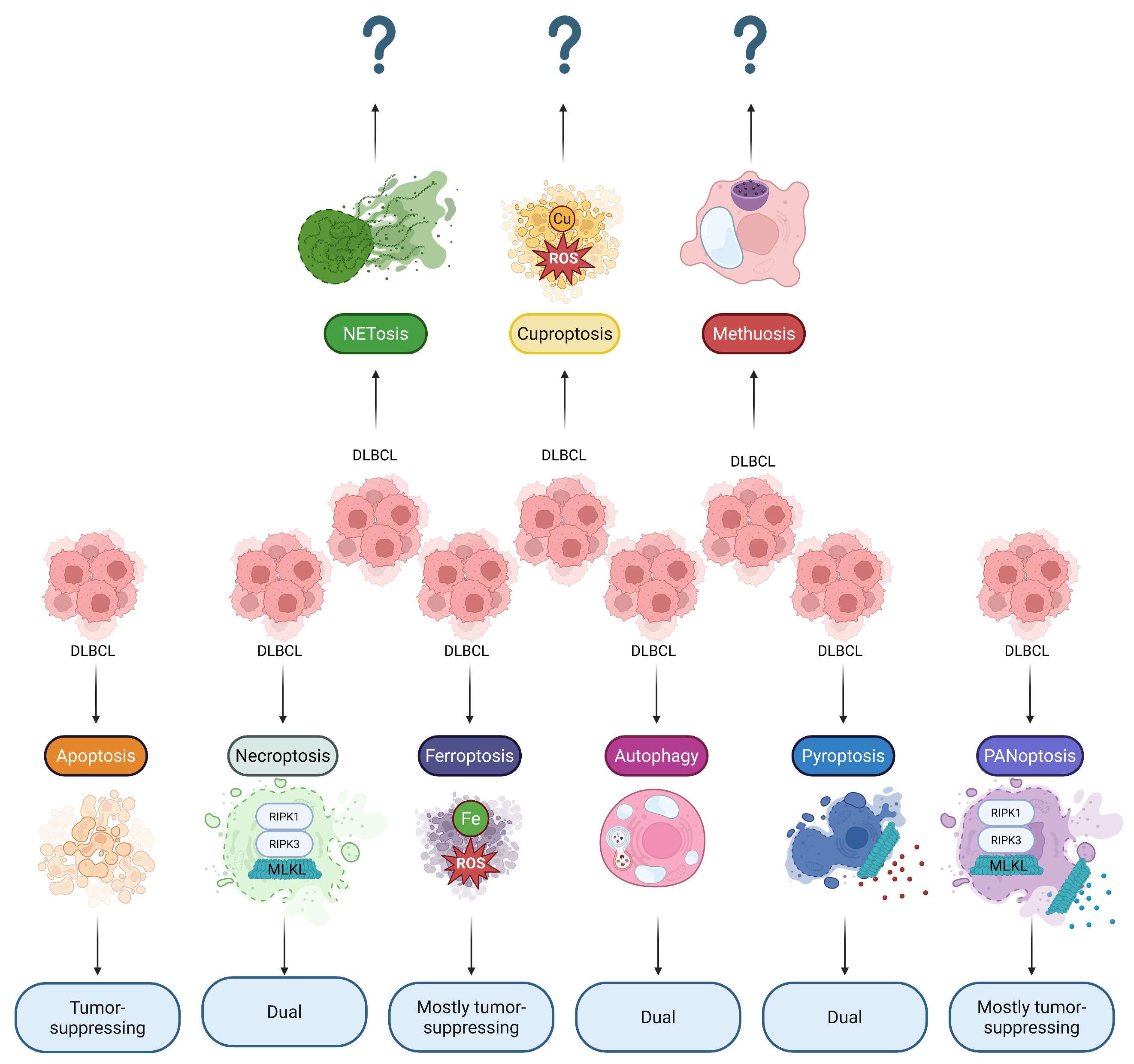
Figure 1.
Reported involvement of RCDs in DLBCL. Diverse RCD modalities can simultaneously ensure cell death of DLBCL malignant cells and modify the tumor microenvironment through immunogenic cell death pathways. Thus, most RCDs play a dual role in DLBCL, suggesting that their pharmacological targeting should be treated with caution. Note: ? sign indicates that the effects are not yet reported; DLBCL, Diffuse large B-cell lymphoma; MLKL, Mixed lineage kinase domain-like protein; RIPK1, Receptor-interacting protein kinase 1; RIPK3, Receptor-interacting protein kinase 3; ROS, Reactive oxygen species.
2. DLBCL Cells Evade Apoptosis
The concept of apoptosis as a morphologically distinct cell death type was introduced by Kerr and colleagues in 1972 [43]. Due to a lack of options to study associated molecular signaling, apoptosis was initially described as a cell death associated with typical morphological alterations such as cell shrinkage, chromatin condensation-mediated pyknosis, plasma membrane blebbing, and karyorrhexis, culminating in the formation of apoptotic bodies [44]. According to the Nomenclature Committee on Cell Death, apoptosis is executed by cysteine-aspartic proteases called caspases, especially caspase-3, involved in degradation of intracellular proteins [45]. Depending on the patterns involved in caspase-3 activation, apoptosis can be mediated by two distinct signaling pathways: intrinsic and extrinsic [46]. The intrinsic stress-associated pathway is triggered by internal stimuli and is associated with Bcl-2 family proteins that regulate mitochondrial outer membrane permeabilization (MOMP), a key event of the intrinsic apoptotic pathway [47]. Upon activation, pro-apoptotic Bcl-2 proteins like BAX and BAK form pores in the mitochondrial membrane, resulting in MOMP and consequent release of cytochrome c. Cytochrome c then binds to apoptosome-containing adaptor Apaf-1 to recruit caspases such as caspase-9, caspase-3, and caspase-7 [48]. BAX and BAK are positively regulated by BH3-only proteins (BID, BIM, PUMA) and inhibited by anti-apoptotic members of the Bcl-2 protein family (Bcl-2, Bcl-XL, MCL-1). Furthermore, BH3-only “sensitizers” (BAD, NOXA, HRK, BIK, BMF) can inhibit anti-apoptotic proteins to ensure apoptosis [49]. In its turn, extrinsic apoptosis is mediated by cell death receptors, including tumor necrosis factor receptors (TNFRs), Fas, or TRAIL (TNF-related apoptosis-inducing ligand) receptors. Receptor activation leads to recruitment of either death-inducing signaling complex (DISC) or TNF-associated death domain (TRADD) as an adaptor protein, which consequently activates caspase-8 and leads to downstream cleavage of pro-caspase-3 [50].
From a physiological point of view, apoptosis primarily sustains the integrity of various body tissues through elimination of damaged, compromised or unwanted cells during the entire ontogenesis, supporting embryogenesis and tissue homeostasis. Moreover, apoptosis has a constructive function as well. It enables tissue remodeling via emitting migration, pro-survival, pro-proliferation and pro-differentiation signals to adjacent live cells [51]. Apoptosis is known to play a crucial role in B-cell development and homeostasis [52], being the most common mode of lymphocytic RCD and a critical factor for normal lymphopoiesis [53]. B lymphocytes, and their precursors, express different levels of pro-apoptotic proteins, which makes them differentially sensitive to apoptosis [54,55]. Apoptosis is triggered to eliminate self-reactive B cells, mediating tolerance [56]. It also constrains competition-induced atrophy in follicular B cells [57]. Importantly, the presence of tonic BCR signaling is required to prevent apoptosis of mature B lymphocytes, which emphasizes the importance of apoptosis in the maintenance of the population of active B cells [58]. Furthermore, apoptosis acts as a quality control for BCR functionality (Figure 2) [59]. To summarize, apoptosis controls the number of B cells in the periphery, mediates B-cell tolerance, participates in B lymphopoiesis, and ensures proper B-cell activation and elimination of “incorrectly” activated B lymphocytes [60].
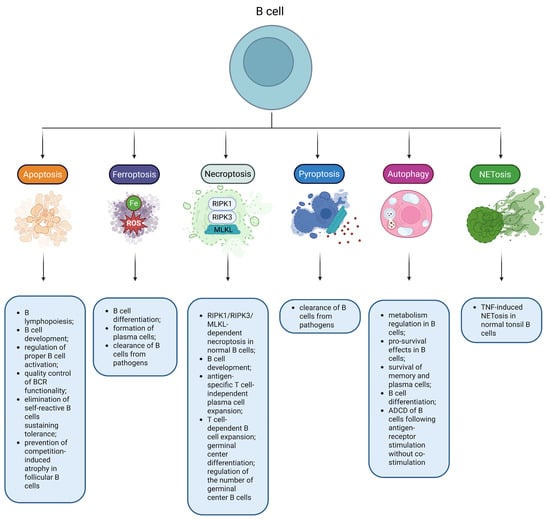
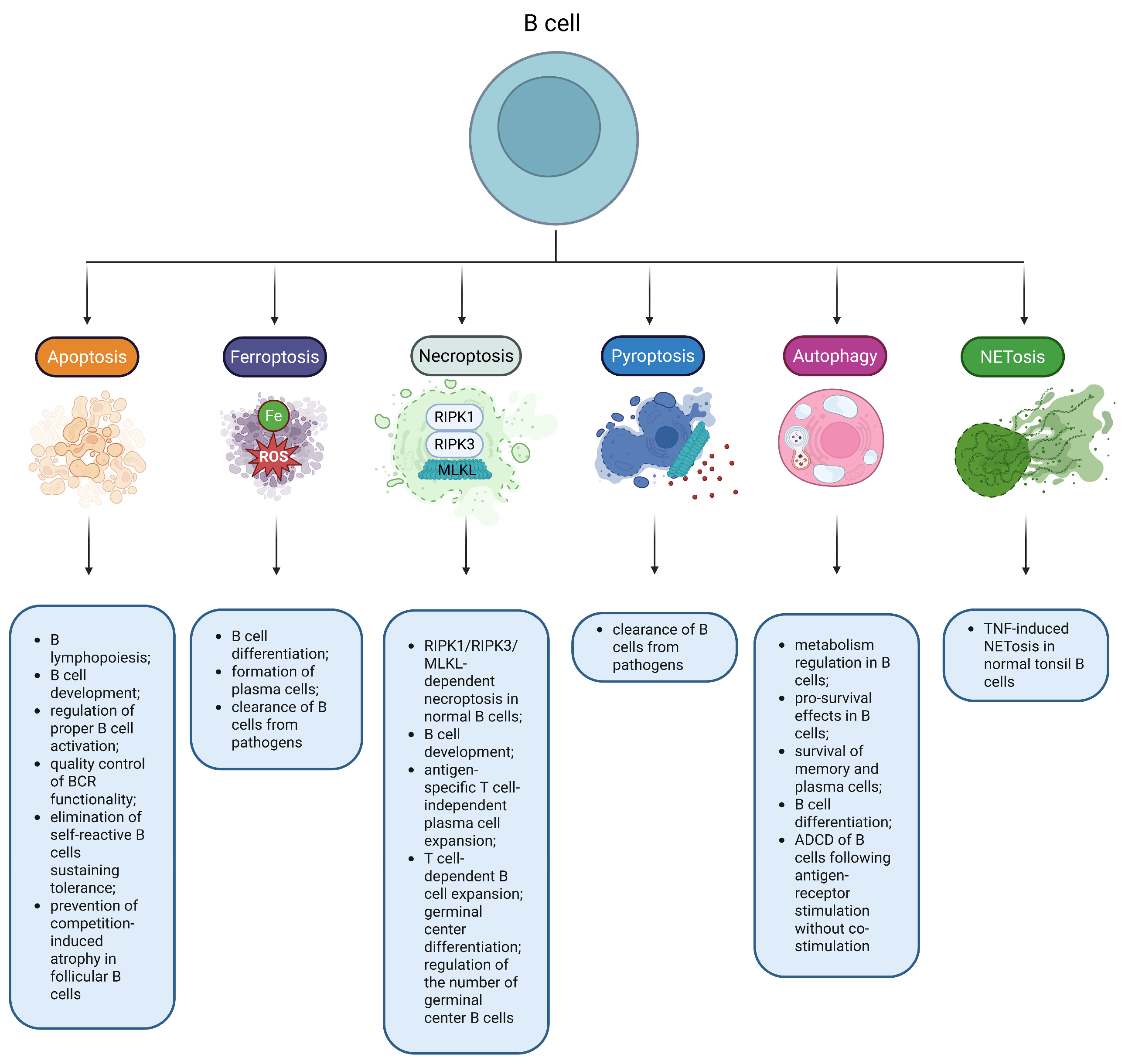
Figure 2.
RCDs play an important role in B-cell biology. Multiple RCD modes have been reported to regulate physiological functions in normal B cells. Note: ADCD, Autophagy-dependent cell death; BCR, B-cell receptor; MLKL, Mixed lineage kinase domain-like protein; RIPK1, Receptor-interacting protein kinase 1; RIPK3, Receptor-interacting protein kinase 3; ROS, Reactive oxygen species; TNF, Tumor necrosis factor.
Since apoptosis evasion is considered as one of the hallmarks of tumorigenesis [61], its induction has been considered as a therapeutic strategy of anti-cancer therapy for decades [62]. However, these approaches are complicated by tumor heterogeneity, as multiple different pathways can be affected and can mediate apoptosis resistance. In particular, BAX mutations, caspase-8 mutations, p53 dysregulation, Bcl-2 upregulation, dysregulation of the pro-survival PI3K (phosphoinositide 3-kinase)/AKT (protein kinase B) pathway and others are common apoptosis resistance-mediating mechanisms [61,63,64,65]. Therefore, resistance to apoptosis in cancer could be quite complex and may be associated with downregulation of pro-apoptotic proteins and/or upregulation of anti-apoptotic factors. Apoptosis deregulation in cancer also mediates drug resistance, further challenging successful cancer treatment [66,67]. Nevertheless, Food and Drug Administration-approved apoptosis inducers have already proven their efficiency. The already approved drugs and currently investigated therapeutic agents target the intrinsic (Bcl-2 inhibitors, BH3 mimetics, Bcl-XL inhibitors, MCL-1 inhibitors, IAP inhibitors) and extrinsic (death receptor agonists) pathways [62]. However, it is important to note that high-grade aggressive tumors with unfavorable prognosis are paradoxically associated with high rates of apoptosis, promoting cancer evolution and progression also by affecting the tumor microenvironment [68].
DLBCL, like other cancers, is characterized by multiple genetic events associated with apoptosis inhibition to promote cell survival and tumor proliferation. DLBCL-associated genetic and other alterations that negatively affect apoptosis include Bcl-2 overexpression, MCL-1 overexpression, Fas downregulation, TRAIL-R1/TRAIL-R2 downregulation, reduced expression of caspase-10, downregulation of the p53 pathway, upregulation of NF-kB and PI3K/AKT pathway signaling, PTEN mutations, CD79A/CD79B overexpression, upregulation of the components of the CARD11-BCL10-MALT1 complex, or MYD88 upregulation [69,70]. Thus, pro-survival and anti-apoptotic genetic alterations increase BCR, PI3K/AKT, and NF-kB signaling, as well as inhibit the p53 pathway and both (intrinsic and extrinsic) apoptotic pathways. For instance, apolipoprotein C1 upregulation prevents apoptosis in DLBCL by maintaining PI3K/AKT/mTOR signaling [71]. Additionally, DLBCL cells overexpress C-terminal binding protein 2 (CtBP2) to inhibit apoptosis by activating the Wnt/β-catenin signaling pathway in an EGR1 (early growth response-1)-mediated fashion [72]. Likewise, DLBCL-associated overexpression of NCAPD3 (non-SMC condensin II complex subunit D3), a condensin II subunit involved in chromatin condensation, reduces the sensitivity of cells to apoptosis by activating SIRT1 transcription [73]. It is important to note that regulatory RNA molecules are also involved in apoptosis inhibition as a pro-survival strategy of DLBCL cells. For instance, c-Myc-dependent MiR-7-5p overexpression blocks apoptosis through the autophagy/beclin 1 regulator 1 (AMBRA1) signaling pathway [74]. Furthermore, MiR-93 overexpression in DLBCL leads to a more aggressive phenotype and inhibition of apoptosis via SMAD5 downregulation [75]. Thus, DLBCL cells have a wide array of adaptations that help them to evade apoptosis. DLBCL elicits a striking heterogeneity in terms of the anti-apoptotic adaptive mechanisms.
Genomic studies have already described significant differences in the genomic landscape between ABC and GCB DLBCL subtypes [76]. For instance, ABC DLBCL is associated with mutations in genes encoding components and regulators of the NF-kB pathway [77,78]. In GCB DLBCL, apoptosis evasion can be associated with BCL6 overexpression and mutations [79]. BCL-2 translocations associated with Bcl-2 upregulation are also frequently observed in GCB, but not in ABC DLBCL [80]. Moreover, FAS alterations leading to downregulation of this cell death receptor are more commonly identified in the GCB subtype compared to the ABC DLBCL [81].
Importantly, genomic studies have identified genetic DLBCL subgroups characterized by specific gene expression patterns. The MCD genomic subtype (frequently ABC DLBCL) is associated with high frequency of MYD88L265P and CD79B mutations, ensuring enhanced anti-apoptotic NF-kB signaling [9,82]. Additionally, another ABC DLBCL-associated BN2 genetic subtype is characterized by high frequency of BCL6 fusions [9,82]. TP53 mutations resulting in p53 downregulation are the most frequent event of the A53 subtype (also dominated by ABC DLBCL cases) [9,82]. Frequent BCL-2 and FAS mutations are observed in the EZB genetic subtype (mainly GCB DLBCL cases) [82]. Additionally, EZB DLBCL is associated with PTEN deletions/mutations, leading to constant anti-apoptotic PI3K/AKT signaling [9,12]. Thus, all individual DLBCL subtypes have frequent mutations in genes encoding components of the apoptotic machinery, helping them to escape apoptosis and mediating survival of malignant cells. However, specific altered pathways are different between ABC and GCB DLBCL, as well as between various genetic DLBCL subgroups. In this regard, the genetic subclassification specifically highlights the various ways of apoptosis evasion in DLBCL and may enable precision medicine-based apoptosis targeting.
It has been hypothesized that a high frequency of genetic alterations that allow DLBCL cells to evade apoptosis might be one of the reasons for primary or secondary resistance to standard treatment in DLBCL (30–40% of cases) [83]. This suggests that on top of apoptosis reactivation, treatment effectiveness might be improved by targeting RCDs alternative to apoptosis. In this review article, we have summarized the currently available knowledge about dysregulation of individual RCD modalities in DLBCL to identify the most promising cell death pathways that can be targeted to overcome apoptosis resistance in DLBCL.
3. Necroptosis Contributes to DLBCL Pathogenesis
Necroptosis is a lethal subroutine referred to as programmed necrosis, which requires activation of receptor-interacting protein kinase 1 (RIPK1), receptor-interacting protein kinase 3 (RIPK3), and mixed lineage kinase domain-like protein (MLKL) [84]. These three crucial components of necroptosis signaling can be activated in response to multiple stimuli, including ligand-mediated activation of Fas, TNFR1, Toll-like receptors (TLRs), TRAIL-R1, etc. [85]. The most important regulator of necroptosis is caspase-8. Its activation prevents switching from necroptosis to apoptosis. Caspase-8 status thus determines whether a cell dies by apoptosis or necroptosis [86]. This decision is quite important also for its immunogenic consequences. In contrast to apoptosis, necroptosis is strongly pro-inflammatory. It has a lytic character and promotes inflammation in a DAMP-mediated manner. As a result, necroptosis has been implicated in multiple inflammatory diseases [87]. Apoptosis is the most physiological RCD [88], and necroptosis acts as its backup [84]. Moreover, necroptosis is believed to play a key role in clearance of infected cells, simultaneously driving inflammation as a strategy to eliminate pathogens [89]. Thus, modulation of inflammation in response to infection is one of the key functions of necroptosis. In addition, contribution of necroptosis to embryonic development has been reported [90]. Normal B cells can undergo RIPK1/RIPK3/MLKL-dependent necroptosis [91]. RIPK1 and caspase-8 are crucial for normal B-cell development, antigen-specific T cell-independent plasma cell expansion, T cell-dependent B cell expansion, and germinal center differentiation (Figure 2) [92]. Interestingly, caspase-9 was documented to protect germinal center B cells against necroptosis to maintain their pool [91].
In oncology, necroptosis has attracted a great deal of attention due to its ability to simultaneously kill apoptosis-resistant cancer cells and boost the anti-tumor immune response (as a pro-inflammatory RCD) [34]. Tumor cell demise by necroptosis is associated with release of DAMPs and tumor antigens, recruitment of macrophages and naïve dendritic cells and their activation, as well as with cross-priming and activation of cytotoxic T cells. On the other hand, other aspects of this pro-inflammatory tumor microenvironment might support tumorigenesis and tumor progression. It recruits tumor-associated macrophages, promotes angiogenesis and metastasis, and increases generation of reactive oxygen species (ROS)/reactive nitrogen species (RNS). ROS/RNS may in turn promote mutagenesis and fuel genomic instability and tumor evolution [93].
Multiple studies have shown that necroptosis contributes to DLBCL pathogenesis. Importantly, like in most cancers, necroptosis in DLBCL acts as a double-edged sword. However, a growing body of evidence suggests that necroptosis in DLBCL seems to be mostly tumor-suppressing [94,95]. In addition, RIPK1, a crucial necroptosis-associated kinase, elicits an anti-tumor effect in DLBCL [96]. As a part of the adaptive mechanisms of cancer cell survival, DLBCL cells can impede mitophagy-dependent necroptosis by modifying choline metabolism in a Myc-driven, choline-phosphate cytidylyltransferase A (encoded by PCYT1A)-dependent manner [97]. Another mechanism that supports survival and prevents necroptosis of DLBCL cells is RIPK1 downregulation. This corroborates a reported increase in the proliferation of DLBCL cells in response to RIPK1 inhibition [96].
The search for pharmacological agents that can target necroptosis and its machinery in DLBCL is ongoing. Thymoquinone, a pleiotropic plant-derived compound, is capable of triggering necroptosis of GCB DLBCL cell lines, which is dependent on Ca2+ signaling and linked to a release of high mobility group box 1 protein (HMGB1), a necroptosis-associated DAMP [98]. Furthermore, diclofenac, a non-steroidal anti-inflammatory drug, has been speculated to induce p53-indepdendent necroptosis along with apoptosis in ABC DLBCL and GCB DLBCL cell lines [99].
Additionally, necroptosis and necroptosis-associated tumor cell features might be useful biomarkers. Omics-based studies have allowed researchers to identify novel prognostic markers in cancer on a large scale [100]. Since necroptosis contributes to DLBCL pathogenesis, analysis of necroptosis-related genes might be used to improve treatment outcome prediction. Zhang et al. constructed a necroptosis-related gene-based molecular signature (analyzing expression of 25 necroptosis-related genes), which allowed them to predict the disease outcome by assigning patients into two clusters. Cluster A was associated mostly with downregulation of necroptosis-related genes and was linked to poor prognosis. Cluster B was characterized by upregulation of necroptosis-related genes and was associated with a favorable prognosis [95]. Pan et al. analyzed the expression of 155 necroptosis-related genes and identified 3 clusters of patients with different prognosis. Protective necroptosis-related genes were mainly overexpressed in cluster 1 patients with the best prognosis, whereas detrimental necroptosis-related genes were upregulated in cluster 3 of patients with the worst prognosis. Additionally, the authors established a 6-genes-based (FSTL4, ACTB, SNRPD2, WHSC1L1, PAICS, and CLTC) predictive model for DLBCL patients [94].
Available evidence confirms that necroptosis modulates the DLBCL tumor microenvironment. For instance, different gene expression clusters (based on analysis of necroptosis-related genes) were associated with considerable variations in cellular components of the tumor microenvironment. Tumors within a high necroptosis degree and good prognosis were enriched with memory resting CD4+ T cells, memory activated CD4+ T cells, and M1 macrophages. In contrast, tumors within a cluster with low rate of necroptosis (and poor prognosis) were abundant in M2 macrophages [94]. This observed correlation of prognosis, infiltration with M1 or M2 macrophages, and gene expression clusters based on necroptosis-related genes was subsequently supported by Zhang et al. [95]. Notably, expression patterns of necroptosis-related genes determine the sensitivity of DLBCL cells to certain anti-lymphoma drugs, including NVP.BEZ235 (a PI3K inhibitor), BX795 (an inhibitor of IkB kinases), tipifarnib (a farnesyltransferase inhibitor), sorafenib (a multi-targeted kinase inhibitor), MS.275 (a histone deacetylase inhibitor), and methotrexate (a dihydrofolate reductase inhibitor) [95].
There are only a limited number of studies so far documenting sensitivity of DLBCL to necroptosis, contribution of necroptosis to DLBCL development and metastasis, as well as the prognostic significance of this RCD. However, it is clear that DLBCL cells develop mechanisms to prevent necroptosis and that necroptosis induction might be an effective therapeutic avenue for DLBCL treatment. On the other hand, necroptosis induction should be approached with caution due to the context-dependent effect on the tumor microenvironment. It remains to be determined if detected prognostic associations of necroptosis find a clinical utilization in clinical decision-making and prediction of responses to conventional drugs.
4. Ferroptosis Induction Is a Promising Anti-DLBCL Therapeutic Strategy
Ferroptosis is referred to as an iron-driven and ROS-dependent RCD distinct from apoptosis [101]. Ferroptosis requires iron to generate ROS in a Fenton reaction-mediated manner, available polyunsaturated fatty acids (which can undergo peroxidation to produce lipid peroxides), and depletion of the antioxidant system that does not allow further coping with iron-induced oxidative stress [102]. Formation of phospholipid peroxides, which act as drivers of ferroptosis, is facilitated by acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) [103]. System Xc−, which acts as a cystine/glutamate antiporter with its key component encoded by SLC7A11, plays an important role in ferroptosis by providing the influx of cystine required for the formation of reduced glutathione (GSH). GSH depletion diminishes the antioxidant capacity of cells and promotes ferroptosis [104]. Along with GSH bioavailability, prevention of ferroptosis is critically dependent on glutathione peroxidase 4 (GPX4), suggesting that the system Xc−/GSH/GPX4 axis is crucial to hold ferroptosis under proper control [105]. Ferroptosis has been implicated in multiple physiological processes such as development or aging, as well as in many pathologies, including deregulation of the immune response, inflammation, and tumorigenesis [103]. Notably, it has been suggested that ferroptosis might participate in B-cell differentiation and formation of plasma cells [106]. Moreover, Epstein–Barr virus increases the susceptibility of B cells to ferroptosis (Figure 2) [107].
Ferroptosis has attracted the attention of the cancer research community due to its involvement in tumorigenesis. Tumor cells have developed several mechanisms to avoid this RCD, including restriction of polyunsaturated fatty acid-phospholipid synthesis to diminish generation of ferroptosis-driving phospholipid peroxides, reduction of iron bioavailability to decrease the amount of pro-ferroptotic ROS, upregulation of negative ferroptosis regulators like GPX4 to boost the antioxidant capacity, and activation of the anti-ferroptotic PI3K/AKT/mTOR signaling pathway [29,108]. Abundant evidence suggests that ferroptosis dysregulation is common for tumors, as its tumor-suppressing role has been documented in many cancer types [109]. In addition, multiple cancer types have been reported to be vulnerable to ferroptosis induction [110]. As a result, multiple approaches based on ferroptosis induction were developed to potentially overcome tumor drug resistance and to improve the effectiveness of immunotherapy [111]. On the other hand, mechanisms that underlie ferroptosis vulnerability in each cancer type are cancer type-dependent and require detailed evaluation for each particular tumor type [112]. Additionally, there is extensive and complex crosstalk between ferroptosis, the tumor microenvironment, and anti-tumor immunity. Induction of ferroptosis of certain tumor microenvironment immune cells that mediate anti-tumor immunity might compromise the anti-cancer immune response [113]. This suggests that ferroptosis induction in cancer treatment could be Janus-faced.
Despite some challenges in targeting ferroptosis pharmacologically, including unavailability of a generally accepted animal model to assess the effectiveness of ferroptosis inducers in vivo, uncertainty in the selection of proper therapeutic windows, the lack of highly effective ferroptosis inducers, adverse effects related to anti-tumor immunity, and a limited number of genomic studies to identify the most sensitive tumor subtypes that would allow a personalized treatment approach [114], the field has significantly advanced, and there is a growing number of studies focusing on the role of ferroptosis in DLBCL (including its inhibition). Ferroptosis modulation as a promising strategy to treat lymphoma has been summarized in recently published reviews [108,115].
In DLBCL lines, multiple mechanisms of ferroptosis evasion have been described; for instance, progesterone and adiponectin receptor 3 (PAQR3) downregulation. Under normal circumstances, PARQT3 modulates the expression of low-density lipoprotein receptor, leading to associated inhibition of the anti-ferroptotic PI3K/AKT pathway [116]. Generally, activation of the PI3K/AKT/mTOR signaling pathway plays a crucial role in maintaining survival of malignant DLBCL cells [117,118]. PI3K/AKT/mTOR signaling-dependent GPX4 upregulation protects cells from ferroptosis [119]. As in other cancer types, DLBCL tumors frequently harbor p53 mutations (in approximately 20% of patients) [120]. Notably, p53-mediated regulation of ferroptosis in lymphoma has been reported as highly context-dependent, being either pro- or anti-ferroptotic. Therefore, TP53 mutations may either reduce or increase the susceptibility of tumor cells to ferroptosis [121]. Another frequent DLBCL alteration, Myc overexpression [122], can contribute to ferroptosis evasion through upregulation of the above-mentioned SLC7A11 [123]. Another anti-ferroptotic mechanism utilized by DLBCL cells is peroxiredoxin 1 (PRDX1) upregulation, since PRDX1 protects cells from ferroptosis by activating the MAPK/ERK pathway [124]. A wide array of adaptive pathways exploited by DLBCL cells to avoid ferroptosis indicates that it has a tumor-suppressing role in DLBCL [125]. This role might be compensatory to their other malignant characteristics, which would make them otherwise too sensitive to ferroptosis: high iron requirements to support their growth and proliferation; overexpression of fatty acid synthase as a part of metabolic rewiring, providing extra polyunsaturated fatty acids; and elevated baseline ROS levels [108]. Moreover, Myc overexpression, which can be protective in ferroptosis as outlined above, can promote cysteine depletion and iron accumulation, triggering ferroptosis [121]. Thus, in addition to adaptive mechanisms aimed at providing survival and reducing the degree of ferroptosis, DLBCL cells have metabolic vulnerabilities that might be exploitable to trigger their death by ferroptosis. These mechanisms are summarized in Figure 3.
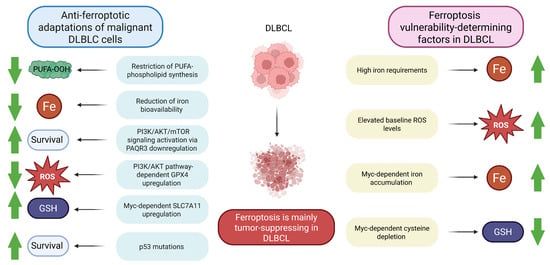
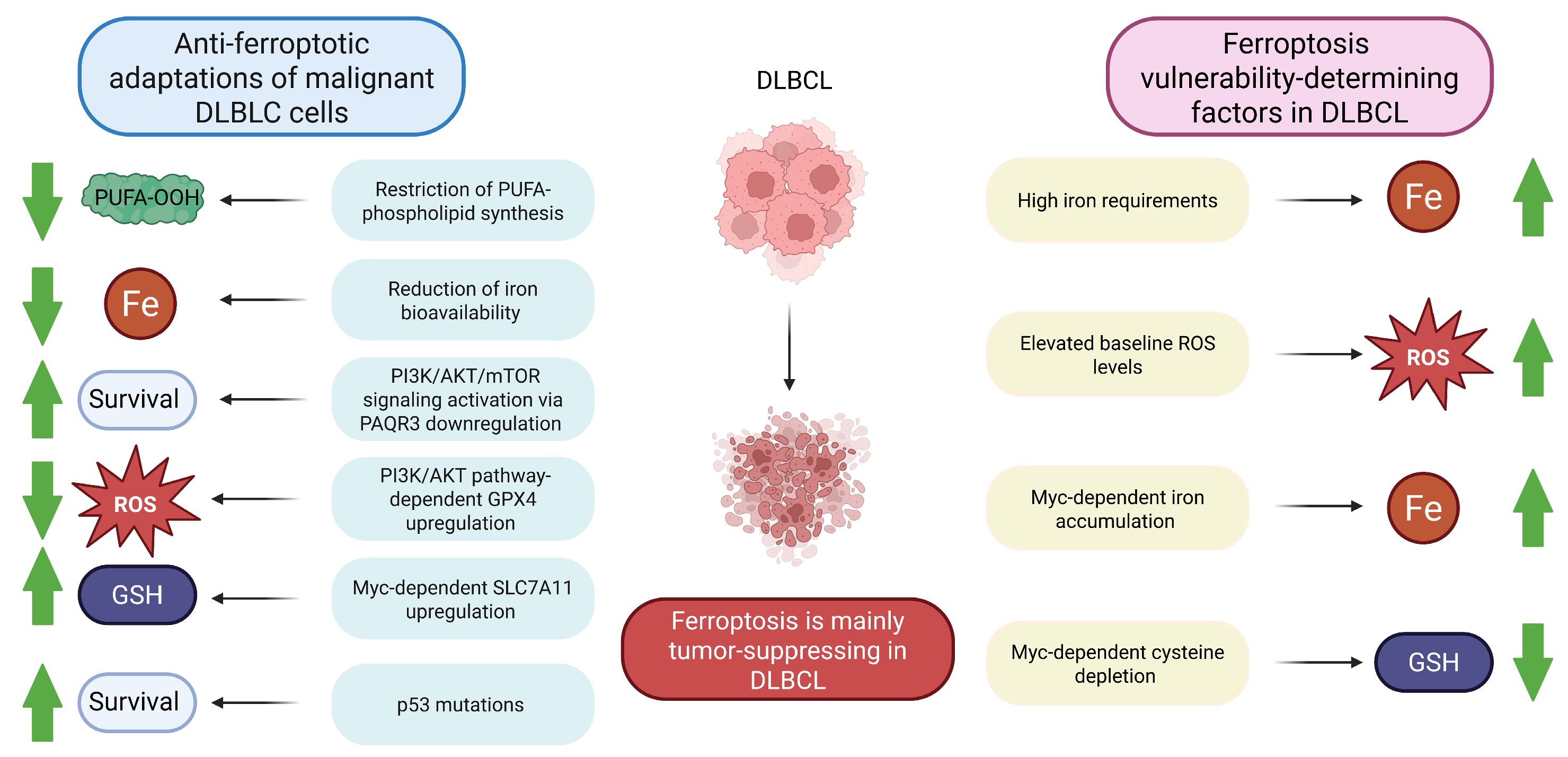
Figure 3.
Ferroptosis susceptibility in DLBCL. DLBCL malignant cells have multiple mechanisms to evade tumor-suppressing ferroptosis. On the other hand, a number of factors make DLBCL cells vulnerable to ferroptosis. Taken together, ferroptosis induction is a promising therapeutic strategy in DLBCL. Note: AKT, Protein kinase B; DLBCL, Diffuse large B-cell lymphoma; GPX4, Glutathione peroxidase 4; GSH, Reduced glutathione; PAQR3, Progesterone and adiponectin receptor 3; PI3K, Phosphoinositide 3-kinase; PUFA, Polyunsaturated fatty acid; PUFA-OOH, Polyunsaturated fatty acid-containing phospholipid hydroperoxide; ROS, Reactive oxygen species; SLC7A11, Solute carrier family 7 member 11; green arrows demonstrate whether the reported effects are enhanced or diminished.
It has been clearly shown that ferroptosis inducers inhibit tumor growth in vivo [126], which has been reported for DLBCL as well. Interestingly, GCB DLBCL is more vulnerable to ferroptosis than the ABC subtype [127]. The mechanisms that explain this difference are yet-to-be-discovered. However, Schmitt et al. showed that BRD4 (Bromodomain containing 4) protein, an embryogenesis-regulating transcription factor, was able to upregulate ferroptosis suppressor protein 1 (FSP1) in GCB DLBCL [128]. In addition, the high sensitivity of GCB DLBCL to ferroptosis inducers could be potentially explained by generally lower levels of ferroptosis-protecting GSH and GPX4, as well as higher levels of pro-ferroptotic 5-lipooxygenease (5-LOX) [129]. Thus, pharmacological inhibition of ferroptosis by bromodomain and extraterminal motif (BET) inhibitors, along with simultaneous application of ferroptosis inducers, can highly effectively trigger ferroptosis in DLBCL [127]. Moreover, tailless complex polypeptide 1 (TCP1) promoted ACSL4/LPCAT3 pathway-dependent ferroptosis in GCB DLBCL cells but not in the ABC subtype [130]. At the same time, dimethyl fumarate was found to induce ferroptosis primarily in the GCB DLBCL as well, whereas in ABC DLBCL, its cytotoxicity was primarily attributable to inhibition of NF-κB/STAT3 signaling [129]. There are also other factors that might determine the different susceptibilities of ABC and GCB DLBCL cells to ferroptosis. For example, expression levels of GCLC, LPCAT3, SLC1A5, and GOT1 are higher in ABC DLBCL in comparison to the GCB subtype [131].
Of note, it seems that expression levels of several components of ferroptotic machinery have prognostic significance in DLBCL. For instance, GPX4 expression negatively correlated with overall survival [132]. This finding corroborates data obtained in a study by Long et al. in which co-expression of GPX4 and STAT1 was also associated with poor patient survival [133]. Additionally, poor DLBCL prognosis was associated also with 4-hydroxy-2-nonenal (4-HNE) abundance and high FST1 expression [134].
Further ferroptosis-based risk stratification of DLBCL patients came from transcriptomic studies. Wu et al. reported that the expression levels of 27 ferroptosis-related genes could be used to predict overall survival in DLBCL patients and further elaborated a model based on the expression of 4 specific genes (CAPG, HAMP, NOX4, and SLC1A5) to assign patients to either a low-risk or a high-risk group [135]. Another ferroptosis-related gene-based prognostic signature for DLBCL was developed by Wang et al. [131]. Expression levels of 19 ferroptosis-related genes were found to be predictive in this study. The model suggested by these authors was based on the expression levels of six ferroptosis-related genes (GCLC, LPCAT3, NFE2L2, ABCC1, SLC1A5, and GOT1), allowing them to identify two different groups of patients with different overall survival. Alternatively, a prognostic model based on ZEB1, PSAT1, NGB, NFE2L2, LAMP2, HIF1A, FH, and CXCL2 expression was also suggested as another means to identify low-risk and high-risk DLBCL patients [136]. In another study, Li et al. suggested a five genes-based model (GOP1, GPX2, SLC7A5, ATF4, and CXCL2) for DLBCL prognosis prediction [137]. As could be seen in the above-outlined studies, the SLC7A5 gene was identified in most prognostic models. It encodes a component of an amino acid exchanger involved in the uptake of several essential amino acids, a crucial function for highly demanding cancer cell metabolism and proliferation [138]. As for its ferroptosis-related functions, SLC7A5 promotes ferroptosis through downregulation of glutathione reductase in a nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent way [139]. Identification of novel ferroptosis-related prognostic markers in DLBCL supports a key role of ferroptosis in this malignancy and provides possible tools for patient risk stratification and treatment response prediction. Ferroptosis-related gene expression analysis studies in DLBCL should be expanded and refined to further describe the role of ferroptosis-related genes as possible driver genes in DLBCL to broaden the available prognostic toolkit and to shed more light on the molecular differences between ABC and GCB DLBCL subtypes.
Importantly, there is also evidence that ferroptosis induction can be a novel therapeutical avenue for refractory DLBCL [140]. To improve the treatment efficiency/toxicity balance in relapse/refractory (R/R) DLBCL, a combination of lenalidomide (a cereblon E3 ligase modulator) with etoposide (a topoisomerase inhibitor) was suggested. This combination triggered ferroptosis of malignant cells via iron-dependent ROS-mediated mechanisms [141]. Another direction of ferroptosis induction research for DLBCL therapy is the improvement of radiotherapy efficiency, since fludarabine, a STAT1 inhibitor, increases radiosensitivity of DLBCL cells via ferroptosis modulation [133]. Additionally, Manara et al. demonstrated that cap-dependent translation inhibition by rocaglate increased susceptibility of DLBCL cells to ferroptosis as well [142]. Xiong et al. showed that doxorubicin toxicity in DLBCL was aggravated by artesunate, an anti-malarial agent, through ferroptosis induction via metallothionein 1G-dependent, ROS- and iron-mediated pathways [143]. Ferroptosis studies have also revealed that ferroptosis induction might be combined with immunotherapy-based strategies in DLBCL [108], since ferroptosis of malignant cells may have a vast effect on tumor microenvironment cells [121].
Nanotechnology is one of the alternative and promising approaches to induce ferroptosis in DLBCL. For instance, iron supplementation using iron oxide nanoparticles can trigger this iron-driven RCD [144]. Moreover, the effectiveness of a nanocarrier based on Prussian blue nanoparticles coated with manganese ions and encapsulated with poly(ethyleneglycol) as a ferroptosis-inducing agent has been shown to be effective in DLBCL cell lines [140]. Importantly, nanomaterials designed to induce ferroptosis in DLBCL act primarily through induction of ROS production in a Fenton chemistry-mediated way. However, nanomaterials can also exploit the ability of cholesterol depletion to induce ferroptosis. Cholesterol-depleting functional lipoprotein-like nanoparticles trigger ferroptosis in DLBCL cell lines and animal-based models via GPX4 downregulation and increased formation of lipid peroxides [145]. Moreover, PEG-PLGA nanoparticles have been investigated as a drug delivery system for ferroptosis inducers, demonstrating good biocompatibility and the ability to improve the efficiency of other ferroptosis-promoting agents [126]. It is important to note that different DLBCL cell lines have different levels of sensitivity to ferroptosis inducers [126], which highlights the need for careful selection of cell lines when performing experiments aiming to study features of ferroptosis in DLBCL.
Taken together, compelling evidence suggests that ferroptosis plays an important role in DLBCL tumorigenesis. DLBCL malignant cells have developed mechanisms to evade this RCD, but other adaptive mechanisms required for maintaining survival, growth and proliferation of DLBCL cells make them vulnerable to ferroptosis. Multiple studies indicate a possible prognostic application of ferroptosis markers. Evidence of the heterogeneity of DLBCL in relation to the ferroptosis pathway might pave the way for ferroptosis-based precision medicine in anti-DLBCL therapy. In general, it has been suggested that ferroptosis targeting in DLBCL might include inhibition of the system Xc (to decrease the antioxidant capacity), modulation of the BRD4/FSP1 axis, elevation of labile iron availability, inhibition of GPX4, p53 activation in combination with ferroptosis inducers, combination of ferroptosis inducers and cap-dependent translation inhibitors, combination of ferroptosis inducing-agents with immune checkpoint inhibitors, or application of ferroptosis-promoting nanomaterials.
5. Pyroptosis Plays a Dual Role in DLBCL
Pyroptosis is a caspase-1- or caspase-11/4/5-dependent cell death mode associated with inflammasome formation. It is mediated by the family of gasdermin (GSDM) proteins and subsequent GSDM-mediated release of pro-inflammatory IL-1β and IL-18 [146]. Canonically, the action of DAMPs and pathogen-associated molecular patterns (PAMPs) results in inflammasome assembly and further caspase-1 activation that mediates cleavage and hence maturation of pro-IL-1β and pro-IL-18. It also mediates cleavage of gasdermin D (GSDMD), which then forms pores to secrete the above-mentioned cytokines. Alternatively, pyroptosis may be activated by the non-canonical pathway in a caspase-11/4/5-mediated manner [147]. Pyroptosis has been initially described in myeloid cells. However, this RCD has been demonstrated in multiple other cell types, including endothelial cells [148], enterocytes [149], keratinocytes [150], etc. In normal B cells, the NLRP3/ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain)/caspase-1 pathway is regulated by B cell-activating factor (BAFF) and BCR signaling to maintain cell survival [151]. Importantly, pyroptosis confers a strong inflammatory response associated with liberation of cytokines, activation of immune cells, and phagocytosis [152]. Thus, physiologically, pyroptosis primarily acts to initiate an immune response and eliminate pathogens [153]. Importantly, B cells can undergo pyroptosis triggered by iron in a Tom20/Bax/caspase/GSDME pathway-mediated manner (Figure 2) [154]. Importantly, the linear ubiquitin chain assembly complex (LUBAC), a ubiquitin ligase, protects germinal center B cells from pyroptosis and hence ensures antibody responses [155]. Additionally, B lymphocytes were found to be protected from cell death through inhibiting TLR4 signaling via pyroptotic cell-derived ASC-containing vesicles [156]. However, the precise physiological role of pyroptosis in B lymphocytes remains elusive.
A growing number of studies have tried to determine the role of pyroptosis in carcinogenesis. Pyroptosis contribution to cancer is not yet fully clarified, and this RCD seems to play a Janus-faced role in carcinogenesis due to its ability to modulate innate immunity. On the one hand, pyroptosis triggers the immune response, promoting tumor destruction. On the other hand, pyroptosis facilitates the formation of the inflammatory tumor microenvironment, which frequently promotes tumor growth and metastasis [157,158]. However, this discrepancy does not decrease the interest in pyroptosis as a target for therapeutic interventions in cancer, specifically within the concept of immunotherapy.
Specifically in DLBCL, accumulating evidence indicates that pyroptosis might be involved in shaping the cellular structure of the tumor microenvironment [159]. Pyroptosis of DLBCL cells has been clearly shown to depend on sterile alpha motif and HD domain-containing protein 1 (SAMHD1) and stimulator of interferon genes (STING), both upregulated in DLBCL. Notably, STING-mediated pyroptosis of DLBCL cell lines primarily relied on the BAK/BAX/caspase-3/GSDME pathway and not on the ASC/NLRP3/caspase-1/GSDMD axis [160]. Moreover, overexpression of pyroptosis-associated IL-1β and IL-18 was detected as well, while expression patterns of proteins from the GSDM family were not altered in DLBCL [161]. The NLRP3 inflammasome, which is tightly linked with pyroptosis [162], regulates PD-L1 expression and contributes to IL-18-mediated inflammatory tumor microenvironment modulation. Moreover, NLRP3 blockade in an animal-based model resulted in reduction of tumor growth and immunosuppression mediated by PD-L1 downregulation [163]. Importantly, experimental evidence indicates that the anti-DLBCL effects of bendamustine–rituximab are attributable to pyroptosis induction via the activation of the cGAS (cyclic GMP-AMP synthase)/STING pathway with subsequent release of pyroptosis-associated pro-inflammatory cytokines [164].
Expression of pyroptosis-related genes was reported to be either beneficial (HTRA1, RBBP7, NFE2L2, and SCAF11) or detrimental (ABL1, PAK2, CPTP and ADORA3) for DLBCL development [159]. SCAF11 upregulation was also shown in another study, while CASP8, CASP9, NLRP1, NLRP6, and TIRAP were downregulated (analysis of pyroptosis markers with no confirmation of pyroptosis occurrence) [165].
A consensus clustering-based study analyzing the expression levels of 52 pyroptosis-related genes revealed that most pyroptosis-related genes were abnormally expressed in DLBCL, which might be of prognostic significance. Of note, in the outlined study, pyroptosis played a critical role in shaping the tumor microenvironment. Furthermore, three pyroptosis-related clusters were identified, which could be applied to predict clinical outcome and sensitivity to chemotherapy in DLBCL [161]. Additionally, another risk model based on 8 pyroptosis-related genes (from a study analyzing 24 such genes) was also proposed to predict the clinical outcome and selection of the appropriate chemotherapy [159]. Likewise, Lv et al. demonstrated that a model based on the expression of 19 pyroptosis-related genes predicted overall survival of DLBCL patients and allowed for their stratification into low-risk and high-risk groups [165].
In general, pyroptosis in DLBCL might simultaneously enhance innate immunity and remodel the immune landscape to create the inflammatory tumor microenvironment, providing evidence for its dual role for DLBCL progression. At the same time, accumulating evidence suggests that investigation of pyroptosis in DLBCL has immense prognostic and therapeutic perspectives.
6. PANoptosis: A Bridge Between Anti-Tumor Immunity and Inflammation in DLBCL
The crosstalk between different cellular lethal subroutines is well-documented. Particularly, pyroptosis, apoptosis and necroptosis pathways are tightly interconnected, which resulted in the description of a unique and separated RCD termed “PANoptosis”. PANoptosis shares features of all the above-mentioned cell death pathways and is regulated by structurally variable PANoptosome complexes composed of sensor proteins, adaptor proteins, and effector proteins with catalytic activity [166,167]. PANoptosis is a pro-inflammatory RCD and is considered separately in a more holistic way than just the interplay and coordination between three individual RCDs. Unfortunately, there are no data available in the literature that would evaluate PANoptosis or describe whether PANoptosis even occurs in normal B cells, but this RCD is documented for hematological malignancies [168].
Notably, PANoptosis can ensure death in tumor cells resistant to apoptosis, necroptosis, and pyroptosis [169]. Furthermore, PANoptosis can drive the innate immune response in cancer, modulating the tumor microenvironment [170]. Thus, similarly to other immunogenic RCD modalities, its therapeutic activation in cancer can reinforce the destruction of tumor cells and enhance anti-tumor immunity at the same time.
There is scarce evidence supporting the role of PANoptosis in DLBCL biology due to a lack of relevant studies. In particular, SAMHD1 deficiency has been demonstrated to trigger PANoptosis in DLBCL through the cGAS/STING pathway in a caspase-8/RIPK3/ASC-dependent manner, reducing tumor cell proliferation and tumor growth [171]. Furthermore, this study linked STING-mediated PANoptosis and the effectiveness of immunotherapy based on PD-L1 inhibitors, supporting the idea that PANoptosis modulation might be exploited to improve immunotherapy outcomes. Additionally, IRF3 (interferon regulatory factor 3)/IFN-β (interferon-β) signaling-mediated FDX1 (ferredoxin 1)-dependent PANoptosis induction was found to be partly responsible for anti-DLBCL effects of elesclomol, an anti-cancer copper ionophore [172].
It has also been recently demonstrated that expression levels of PANoptosis-related genes hold promise as diagnostic and prognostic tools in DLBCL. Xu et al. introduced a PANoptosis-related gene prognostic index (PANGPI) score in DLBCL, which might be of prognostic and treatment monitoring significance. It was based on the evaluation of ZBP1, RIPK1, MLKL, TLR2, TLR3, TLR4, RAVER1, CASP1, MEFV, and TUFM expression levels [173].
These preliminary data indicate that PANoptosis induction is beneficial, since tumor cells tend to evade PANoptosis by overexpressing SAMHD1, inhibiting STING-dependent PANoptosis. Understanding of the factors that contribute to PANoptosis in DLBCL might provide a foundation for its therapeutic modulation. It might be specifically beneficial for its innate immunity-regulatory features. Additionally, we believe that further analysis of PANoptosis-related genes might reveal novel prognostic markers in DLBCL.
7. NETotic Cell Death Is an Emerging Contributor to DLBCL
NETotic cell death (or NETosis) is a type of RCD observed primarily in neutrophils. It is associated with the formation of neutrophil extracellular traps (NETs) that contain chromatin, histones, and DNA (mitochondrial and nuclear) with the primary function of trapping microorganisms [174,175]. NET formation relies on Raf/MEK/ERK and PKC signaling, which culminates in NADPH oxidase activation and NADPH oxidase-mediated ROS generation [45,176,177]. Excessive NADPH oxidase derived ROS trigger NETotic cell death via considerable DNA damage, consecutive activation of compensatory DNA repair mechanisms and resulting chromatin decondensation and NET extrusion [178]. Notably, normal tonsillar B lymphocytes have been shown to die through NETosis, which is promoted by TNF-α at high cell density. This effect is not characteristic of peripheral blood B cells (Figure 2) [179]. Additionally, a growing body of evidence demonstrates that NETs promote activation of B cells in autoimmune disorders [180,181].
Tumor cells have been widely described as inducers of NETosis, and NETs have been reported to promote circulatory impairment in cancer, trigger metastasis and proliferation, mediate PD-L1-associated immunosuppression, activate angiogenesis, or mechanistically prevent interaction of tumor cells with cytotoxic immune cells [182,183]. Accumulating evidence on the involvement of NETs and NETosis in carcinogenesis indicates that its inhibition can be a tempting therapeutic strategy. Multiple anti-NET therapeutics are currently under investigation [184].
In general, little is reported on the contribution of NETotic cell death and NETs to DLBCL pathogenesis. DLBCL cell line-based experiments showed that NETs induce proliferation and increase the migratory capacity of DLBCL cells through TLR9-mediated signaling. Importantly, NETotic cell death in DLBCL was induced by malignant cell-derived IL-8 and its binding to CXCR2, which activated multiple downstream effectors (including Src, ERK, and p38 MAPK) [185]. This pioneering study also identified NETosis as a promising target in DLBCL treatment. Moreover, additional studies suggested that NETs might be of prognostic significance in DLBCL. Enhanced circulating levels of NETs were associated with an unfavorable prognosis in DLBCL patients [185]. Additionally, Shi et al. investigated the prognostic role of NETosis-related genes in DLBCL and showed that expression levels of eight NETosis-related genes (PARVB, LYZ, PPARGC1A, HIF1A, SPP1, CDH1, S100A9, and CXCL2), and the associated risk model, could predict patient survival [186].
A recently published review has highlighted the possibility to target NET formation in hematological malignancies [187]. On the other hand, our summary suggests that further studies of NETs and NETotic cell death will help identify novel prognostic biomarkers and therapeutic options.
8. Autophagy and Autophagy-Dependent Cell Death Regulate Tumor Progression in DLBCL
Autophagy is an evolutionarily conserved catabolic cellular mechanism aimed at maintaining homeostasis, primarily under nutritional or metabolic stress. It ensures degradation of organelles and macromolecules to provide recycling of cellular components [188]. Due to its protective effect, autophagy has been considered a pro-survival process [189]. However, it is now clear that the autophagy machinery can mediate an RCD called autophagy-dependent cell death (ADCD) [45]. Notably, this cell death modality can be accompanied by the formation of autophagosomes, since it can be triggered to avoid apoptosis to ensure cell survival. However, if these survival mechanisms fail and cells die by apoptosis, accumulation of autophagosomes can be observed [190]. Alternatively, autophagy might be required to induce apoptosis, ferroptosis, or necroptosis [191]. Thus, it is suggested to specifically define ADCD as an RCD only when cell death accompanied by autophagy occurs without signs of other RCDs, and when this process could be blocked by autophagy inhibitors [189]. In autophagy, the activated ATG1/ULK1 complex phosphorylates and hence recruits downstream autophagy proteins, including ATG13 and ATG14 [192]. Thereafter, the autophagy-specific class III phosphatidylinositol 3-kinase complex I (PtdIns3K-C1) composed of PIK3C3/VPS34, PIK3R4/VPS15, BECN1/Beclin 1, and ATG14 produces phosphatidylinositol 3-phosphate, which in turn mediates vesicle nucleation [193]. A further step requires cooperation between the ATG12/ATG5/ATG16L1 and LC3/GABARAP systems to ensure vesicle expansion [194]. Then, SNARE proteins mediate autophagosome–lysosome fusion [195].
Physiologically, autophagy acts as a quality control process to regulate survival/cell death pathways [196]. It has been shown that autophagy is not crucial for correct B-cell development [197]. On the other hand, it has been shown that it is required for B-cell differentiation. Moreover, autophagy is involved in maintaining homeostasis and survival of plasma and memory cells [198] and is necessary for TLR-mediated activation of B lymphocytes [199]. Like in other cells, autophagy mediates self-renewal and regulates metabolism of B cells [200]. Specifically in B lymphocytes, autophagy is an integral part of BCR internalization in an ATG5-dependent fashion and contributes to the process of MHC class II-restricted antigen presentation [201]. Moreover, autophagy contributes to chromatin accessibility regulation in germinal center B cells [202]. However, little is known about ADCD in B lymphocytes and the contribution of autophagy to their cell death. Importantly, autophagy is linked to cell death of B cells following antigen receptor stimulation in the absence of co-stimulation [203]. BCR signaling promotes autophagy and apoptosis in primary B cells, and CD40 co-stimulation switches the balance towards autophagy, restricting cell death [204]. Therefore, as expected, autophagy is considered to be a pro-survival pathway in B lymphocytes (Figure 2) [199].
Generally, there is a consensus that autophagy is protective in normal cells and during early tumorigenesis. However, autophagy-related pathways might contribute to tumor progression and metastasis, providing adaptation of malignant cells to harsh stressors. This indicates a dichotomous and context-dependent involvement of ADCD in cancer [24]. In addition, a growing body of evidence supports the hypothesis that autophagy shapes the tumor microenvironment. Autophagy of immune cells could critically affect immunosurveillance by adjusting the function of tumor-infiltrating cells [205] and mediating the epithelial-to-mesenchymal transition [206]. Importantly, autophagy in malignant cells affects various cell death pathways and mediates the eventual switch between them [207]. Thus, given the well-established tumor-promoting effect, autophagy inhibitors have been evaluated in multiple clinical trials (including ULK1, VPS34 and ATG4B inhibitors) [208].
It is now clear that autophagy is an important pathogenic factor and drug target in hematolymphoid malignancies as well [209]. Autophagy activation has been shown to promote DLBCL progression [210,211,212,213]. Higher LC3B expression, which is one of the key autophagy-associated proteins and an autophagy marker, was associated with more aggressive DLBCL behavior [214]. ATG4D, HIF1A, LAMP2, RPTOR, ULK1, and MAP1LC3B have been shown as key regulators of autophagy, mediating the crosstalk between autophagy and apoptosis in DLBCL [215]. The accelerated LC3B-II-dependent autophagic flux has been detected in both main DLBCL subtypes (ABC and GCB), suggesting that DLBCL relies on autophagy [214]. And p62, an autophagy adaptor protein, was upregulated in DLBCL [216]. Notably, autophagy inhibition in DLBCL triggered apoptosis [217]. On the other hand, apoptosis could be also triggered by autophagy induction, specifically in Myc-expressing DLBCL cells following inhibition of positive cofactor 4 (PC4, an upstream regulator of c-Myc) [218]. Furthermore, simultaneous induction of apoptosis and autophagy inhibited the growth of DLBCL cells [219,220]. Unexpectedly, Bcl-2-associated BECN1 overexpression was associated with a positive outcome in DLBCL [221,222,223]. Interestingly, a recent study showed that BECN1 expression negatively correlated with Bcl-2 expression in DLBCL, which might suggest dysregulated crosstalk between autophagy and apoptosis [224]. Thus, the available literature points to a controversial role of autophagy in DLBCL. Similarly, the crosstalk between autophagy and apoptosis is far from being straightforward.
Our understanding of autophagy and the interplay between autophagic and apoptotic pathways in DLBCL is still very limited. There is evidence that autophagy is deregulated in DLBCL and that there is an extensive interplay between autophagy and apoptosis. It seems that DLBCL cells upregulate autophagy-related proteins to avoid apoptosis. For instance, ATG5 upregulation promoted autophagy and facilitated the progression in DLBCL. This process was mediated by transcription factor PAX5-dependent ARRDC1-AS1 activation [212]. Likewise, AMBRA1 (a positive regulator of autophagy) was overexpressed in DLBCL, and AMBRA1 autophagy pathway inhibition resulted in apoptosis activation. This suggests that autophagy is protective in DLBCL and prevents DLBCL cells from apoptosis [74]. Moreover, JNK signaling-dependent autophagy upregulation by CUL4B, a scaffold protein required for the CUL4B-RING E3 ubiquitin ligase complex, was required to promote growth and progression of DLBCL [211]. Little is known about the regulation of autophagy in DLBCL. For instance, LINC00963, a sponge of miRNA, induced autophagy in DLBCL by downregulating miR-320a. Although the majority of studies support the pro-survival role of autophagy in DLBCL, autophagy-inducing LINC00963 overexpression was found to suppress DLBCL tumor growth in vivo [225]. Taken together, these studies provide insights that the autophagic flux in DLBCL is subject to multiple regulators and is largely context-dependent.
Notably, subtype-specific differences in autophagy have been documented in DLBCL. Upregulation of the ULK1 complex has been reported specifically in GCB DLBCL [214]. Moreover, chronic selective autophagy has been suggested to mediate response to BTK inhibitors in the MCD DLBCL genetic subtype. This seems to be associated with the ability to target ubiquitinated MYD88L265P for further proteolysis [226].
There is growing evidence that autophagy might shape the tumor microenvironment in DLBCL. A link between higher immune cell infiltration and lower autophagy degree was identified in low-risk DLBCL patients [213]. As for the cellular composition of the immune infiltrate, expression levels of autophagy-related genes showed that their low expression was associated with high risk, a higher proportion of Treg and native B lymphocytes, and with a lower proportion of CD8+ T cells, CD4+ memory activated T cells, gamma delta T cells, macrophages M1 and resting mast cells, all compared to low-risk patients with a higher expression of autophagy-related genes [215]. This suggests that expression of autophagy-related genes might allow for risk stratification and treatment response prediction in DLBCL, similar to other RCDs. For example, Zhou et al. studied the expression of 309 autophagy-related genes and identified 5 genes (TP53INP2, PRKCQ, TUSC1, PRKAB1, and HIF1A) that formed a specific gene signature, allowing for survival and treatment response prediction. Of note, analysis of signaling pathways revealed that the NF-κB pathway was activated in high-risk patients. In low-risk patients, the PI3K/AKT pathway (which might inhibit autophagy) was upregulated [213]. Moreover, a different pattern of autophagy-related gene expression in DLBCL was demonstrated by Xiong et al., who constructed a predictive model based on eight autophagy-related genes (ADD3, IGFBP3, TPM1, LYZ, AFDN, DNAJC10, GLIS3, and CCDC102A) [215].
The well-documented involvement of autophagy in the pathogenesis of DLBCL indicates its possible therapeutic utilization. It is important to note that combined BTK and ULK1 inhibition reduces the viability of DLBCL cells [214]. Expression of certain autophagy-related genes like LYZ and ADD3 was linked with drug resistance to the majority of chemotherapeutic drugs [215]. However, an alternative anti-DLBCL strategy comprising the induction of autophagy-dependent apoptosis through AMPK-dependent ULK1 activation was suggested and proven to be effective as well [227]. Additionally, chronic selective autophagy induction by BTK inhibitors promoted MYD88L265P degradation in MCD DLBCL [226]. These studies are in line with other reports suggesting that accelerated autophagy in DLBCL sensitizes malignant cells to anti-cancer treatment [228]. For example, BECN1 overexpression increased sensitivity of DLBCL cells to doxorubicin [224]. On the other hand, bortezomib, a proteosome inhibitor, required autophagy to degrade IκBα. This activated the compensatory pro-survival NF-κB pathway. Inhibition of autophagy could make cells much more sensitive to bortezomib, especially in NF-κB signaling-dependent ABC DLBCL [229]. Furthermore, miR-7-5p targeting was suggested as one of the anti-DLBCL strategies due to the ability of this miRNA to regulate autophagy and apoptosis in an AMBRA1-dependent way [74]. Tenovin-6, an inhibitor of sirtuins, was found to reduce proliferation of ABC DLBCL cells by inhibiting autophagy as well [210].
In summary, autophagy plays a pivotal role in DLBCL, regulating the survival of malignant cells and tumor progression, shaping the tumor microenvironment, and mediating the response to multiple chemotherapeutical agents. Similarly to other RCDs, the possibly opposing effects of autophagy, widely reported in the literature, suggest that its therapeutic targeting should be treated with caution. Notably, both induction and inhibition of autophagy have been suggested as therapeutic options in DLBCL [214,230]. We believe that identification of subtype-specific autophagy-related features might result in the development of more targeted approaches to modulate autophagy in an informed way and according to particular alterations in individual tumors.
9. Cuproptosis: An Underexplored RCD in DLBCL
Cuproptosis is a copper-driven RCD which relies on lipoylated components of the mitochondrial energy metabolism-related tricarboxylic acid cycle [231]. Their aggregation reduces the number of iron–sulfur clusters, promoting proteotoxic stress [232]. The physiological aspects of cuproptosis remain to be determined, as well as its eventual occurrence in normal B cells. However, cuproptosis has been widely investigated in cancer and was shown to reduce tumor growth and prevent metastasis [233].
Little is known about the role of the cuproptosis machinery in DLBCL. For instance, cuproptosis-related lipoic acid synthetase (LIAS) is upregulated in DLBCL [234]. At the same time, antioxidant 1 copper chaperone (Atox1), a cuproptosis-related transcription factor, was shown to promote DLBCL proliferation by activating the MAPK signaling pathway [235]. Notably, MALAT1, a cuproptosis-associated lncRNA, was also demonstrated to promote DLBCL proliferation [236]. These findings underscore the importance of cuproptosis-associated proteins and regulatory RNAs in DLBCL biology. However, the eventual contribution of cuproptosis (as a distinct RCD) to DLBCL tumorigenesis and its progression remains to be determined.
In several studies, multiple cuproptosis-related genes like FDX1, LIAS, LIPT1, LIPT2, DLD, GCSH, DBT, DLST, DLAT, PDHA1, PDHB, SLC31A1, MTF1, NFE2L2, GLS, NLRP3, CDKN2A, ATP7A, and ATP7B, primarily encoding components of the lipoic acid pathway, iron–sulfur cluster biosynthesis, or tricarboxylic acid cycle, as well as fatty acid and pyruvate metabolism, have been described [237,238]. Of note, a consensus clustering-based approach identified two distinct subtypes of DLBCL (subtypes A and B) depending on the expression profiles of 12 cuproptosis-related genes (FDX1, LIAS, LIPT1, DLD, DLAT, PDHA1, PDHB, MTF1, GLS, CDKN2A, SLC31A1, and ATP7B). This allowed for the development of a specific prognostic model [239]. Subtype A, characterized by a higher expression of negative cuproptosis regulators, had a worse survival compared to Subtype B [239]. These two subtypes of DLBCL differed in their response to treatment and the features of their tumor microenvironment [239]. Furthermore, the CDKN2A gene was found to be the most frequently mutated cuproptosis-related gene in DLBCL [239]. Its protein product, a cyclin-dependent kinase inhibitor 2A, was reported to contribute to tumorigenesis in DLBCL and was suggested to be pharmacologically targetable [240]. Additionally, Li et al. suggested a cuproptosis-associated prognostic model for DLBCL, which took into consideration the expression of five cuproptosis-related genes (CDKN2A, DLAT, DLD, LIPT1, and MTF1). It also allowed for the identification of low- and high-risk DLBCL patients [240]. Recently, Wang et al. developed a prognostic model for DLBCL utilizing four cuproptosis- and ICD-associated lncRNAs, namely ANKRD10-IT1, HOXB-AS1, LINC00520, and LINC01165, capable of assigning patients into low- or high-risk groups. Notably, high-risk patients had higher levels of tumor-infiltrating Tregs and neutrophils, which, according to the authors, indicated tumor growth-promoting immunosuppression. Additionally, high-risk individuals with DLBCL were characterized by a higher resistance to conventional chemotherapeutic drugs [241]. Likewise, Bai et al. suggested a risk model for DLBCL patients based on the expression status of seven lncRNAs, namely LINC00294, RNF139-AS1, LINC00654, WWC2-AS2, LINC00661, LINC01165, and LINC01398 [242].
Growing interest in cuproptosis as a cancer-associated RCD with tumor biology consequences has resulted in the appearance of the first studies documenting its impact in DLBCL. It has been confirmed that the expression of cuproptosis-related genes can be assessed to risk stratify DLBCL patients, to predict certain features of the tumor microenvironment, and to evaluate drug resistance. This all can potentially support precision medicine-based DLBCL treatment in the future. More studies are certainly needed to validate these findings, but cuproptosis inducers are already being developed as promising agents for cancer treatment [26]. Nevertheless, it is not clear whether cuproptosis is protective in DLBCL or not.
10. Methuosis Is a Potential Target in DLBCL
Methuosis is a caspase-independent RCD mode characterized by cytosolic accumulation of micropinosome-derived liquid-filled vacuoles [31]. Methuosis signaling is still poorly understood. However, it has been reported that Ras/Rac, AKT/mTOR and AMP-activated protein kinase (AMPK) pathways are involved in this RCD [243]. To our knowledge, there is no evidence that methuosis can occur in normal B lymphocytes and contribute to their physiology. However, PI3K/AKT signaling-dependent methuosis was demonstrated in B-lymphocytic acute lymphoblastic leukemia cells [244]. Accumulating evidence suggests that induction of methuosis is a tempting strategy in cancer treatment [31,245].
Methuosis induction by HZX-02-059, an azaindole derivative and inhibitor of phosphatidylinositol-3-phosphate 5-kinase (PIKfyve) and tubulin, was studied in double-hit lymphoma [246]. Double-hit lymphoma is a highly aggressive but relatively rare subtype of DLBCL, which is associated with rearrangements of MYC and BCL2 and/or BCL6 genes [247]. The above-mentioned dual inhibitor reduced the proliferation of human double-hit lymphoma cells and induced their methuosis through the PIKfyve/transcription factor EB (TFEB) axis and tubulin inhibition (accompanied by downregulation of the mTOR/Myc pathway) [246]. Thus, induction of lymphoma cell vacuolization and death via methuosis might be another emerging strategy to treat DLBCL. Of note, mutations of certain non-methuosis signaling pathways can make cancer cells more susceptible to methuosis [243], which suggests possible application of methuosis activation in a tumor characteristics-driven and informed personalized treatment approach.
11. Mitotic Death Is Involved in DLBCL Development
Mitotic death is referred to as an RCD induced by mitotic catastrophe [45,248]. However, mitotic catastrophe, most frequently triggered by DNA damage, can also result in cellular senescence, intrinsic apoptosis, autophagy, and necrotic death. Mitotic catastrophe is an attractive target for cancer treatment. It could regulate selection of a specific cell death pathway. Importantly, p53 status heavily influences mitotic catastrophe-associated cell fate decision [249].
In general, mitotic death in DLBCL is poorly studied. Bortezomib, a proteasomal inhibitor, was reported to trigger mitotic catastrophe in rituximab-sensitive, but not rituximab-resistant, DLBCL cell lines. The suggested mechanism involved the promotion of p21-dependent accumulation of G2/M regulatory proteins. This, in turn, resulted in cellular senescence of rituximab-resistant lines and apoptosis of rituximab-sensitive cells [250]. Cellular senescence, characterized by irreversible cell cycle arrest, plays a dual role in cancer. It acts as a tumor-suppressing or tumor-promoting factor, contributing to oncogenesis through the senescence-associated secretory phenotype [251]. Additionally, the combined action of volasertib, a polo-like kinase 1 inhibitor, and belinostat, a histone deacetylase inhibitor, promoted mitotic catastrophe and mitotic death in DLBCL cell lines [252]. Likewise, CFI-400945, a polo-like kinase 4 inhibitor, impaired cytokinesis in a Hyppo signaling-mediated way in DLBCL cells, which made them vulnerable to mitotic catastrophe [253]. Additionally, it has been shown that in OxPhos DLBCL (characterized by upregulation of mitochondrial electron transport chain component-encoding genes) [254], mitosis-associated chromosome segregation integrity is maintained by the SIRT1/heat shock protein 90 α (HSP90α) axis, eliciting tumor-surviving effects. Combined inhibition of SIRT1 and HSP90α led to mitotic catastrophe [255]. Interestingly, Wee1 (a mitosis entry-regulating nuclear kinase) inhibition promoted mitotic catastrophe and subsequent apoptosis of G2 phase-arrested DLBCL cells but not G1/S phase-arrested cells. This highlights diverse sensitivity to Wee1 inhibitors following chemotherapy, which is determined by different effects on cell cycle progression and its arrest [256]. Consistently, a newly synthesized T22-auristatine nanoconjugate induced G2/M cell cycle arrest, mitotic catastrophe, and apoptosis in CXCR4-positive DLBCL cell lines [257].
Thus, mitotic catastrophe in DLBCL might be an important factor that needs to be considered in association with anti-DLBCL therapy effectiveness, drug resistance, and design of treatment combinations, all due to the possibility that several alternative mitotic catastrophe-associated events can occur.
12. Other Regulated Cell Death Modalities: What Is Not Explored?
To our knowledge, there are no studies focusing on RCDs in DLBCL other than discussed above. These include oxeiptosis, disulfidptosis, parthanatos, entosis, alkaliptosis, anoikis, and autosis. On the other hand, Wang et al. reported that disulfidptosis-related genes (CAPZB, DSTN, GYS1, IQGAP1, MYH9, NDUFA11, NDUFS1, and OXSM) were of prognostic significance in DLBCL [258]. Likewise, He et al. investigated the expression of a wider spectrum of RCD-related genes, including 3 parthanatos-related genes, 11 entotic cell death-related genes, 6 alkaliptosis-related genes, 2 oxeiptosis-related genes, and 257 anoikis-related genes, aiming to identify novel RCD-related genes with prognostic significance [259]. Guan et al. revealed that anoikis-related genes could be used to build a specific signature capable of risk stratifying DLBCL patients and predicting the response to treatment [260]. In addition, there are some data showing that crucial components of the signaling pathways involved in the above-mentioned RCDs are altered in DLBCL, which might make further investigation of these RCDs in DLBCL appealing. For instance, poly (ADP-ribose) polymerase 1 (PARP1) was reported to be upregulated in DLBCL, and its inhibition was suggested as a tempting treatment strategy [261]. In its turn, PARP hyperactivation was considered a hallmark of parthanatos, a PARP-dependent RCD [262]. Furthermore, alkaliptosis, which is a pH-dependent cell death modality mediated by intracellular alkalization, critically relied on the NF-κB signaling known to be activated in DLBCL [14,263]. Oxeiptosis was reported as a distinct ROS-dependent and caspase-independent non-inflammatory form of RCD. It required the participation of kelch-like ECH-associated protein 1 (KEAP1) as a ROS sensor and physiologically restricted pro-inflammatory ROS-dependent deaths like pyroptosis [264]. KEAP1 activation by ROS resulted in the release of Nrf2, a key transcription factor that upregulated cytoprotective antioxidant enzymes [265]. Both components of the oxeiptosis-related KEAP1/Nrf2 pathway have been demonstrated to be upregulated in DLBCL [266]. These findings underscore the importance of the exploration of alkaliptosis, oxeiptosis, and parthanatos in DLBCL.
13. Conclusions and Perspectives
Tumor heterogeneity, tumor evolution, and resistance to apoptosis are major challenges in cancer treatment [34]. Recent advances in the field of RCD have broadened our knowledge of possible novel targets for anti-cancer therapy. Distinct lethal subroutines have been widely investigated to identify such novel molecular targets. Moreover, given the significant crosstalk between RCDs and their multifaceted effects, it might seem beneficial to target simultaneously two or more RCDs [42,267]. In this review, we have summarized the current knowledge of the role of lethal subroutines in DLBCL. It has long been clear that DLBCL is capable of evading apoptosis [14,70]. However, despite the increasing number of studies focusing on non-apoptotic RCDs in DLBCL, the contribution of non-apoptotic RCDs to this lymphoma development is less well-studied. Herein, we provide evidence that DLBCL has developed mechanisms to escape from necroptosis and ferroptosis. In addition to apoptosis, necroptosis, and ferroptosis, tumor DLBCL cells can die by pyroptosis, PANoptosis, ADCD, and methuosis. Furthermore, NETosis occurring in non-malignant cells from the tumor microenvironment can promote growth and metastasis in DLBCL. In general, significance, effects, and molecular mechanisms, as well as the crosstalk between the non-apoptotic RCDs in DLBCL, remain elusive. However, it is clear even now that most non-apoptotic RCDs are Janus-faced in DLBCL. This is primarily associated with the immunogenic effects of RCDs; death of malignant cells can result in the release of multiple signaling molecules, which have a complex and variable effect on tumor growth and progression, the cellular composition of the tumor microenvironment, and the interplay between individual tumor microenvironment cell types. Accumulating evidence, summarized in this review, indicates that necroptosis, pyroptosis, and autophagy have immunogenic effects and can modulate the tumor microenvironment in DLBCL. Moreover, other non-apoptotic RCDs like PANoptosis and ferroptosis can potentially affect the tumor microenvironment in DLBCL as well. Cuproptosis has been shown to affect the tumor microenvironment in cancer in general [268], but its role in DLBCL is yet to be clarified. On the other hand, genomic studies have emphasized the possible prognostic significance of cuproptosis. Further studies should aim to determine and describe the details of the interplay between RCDs and the tumor microenvironment. This is specifically important and exploitable for the development of novel anti-DLBCL treatment strategies.
As RCDs play different roles in various cancers, it is necessary to evaluate them specifically in each malignancy, including DLBCL. Relatively abundant information is available about ferroptosis and autophagy. Additionally, there is ample evidence that metabolic reprogramming makes DLBCL cells more vulnerable to ferroptosis. Therefore, ferroptosis induction seems to be currently the most promising therapeutic strategy among non-apoptotic RCD-targeted approaches. Its effectiveness has been demonstrated in cell culture-based studies as well as in vivo. Moreover, some evidence suggests that the sensitivity of ABC DLBCL to ferroptosis induction can differ from that in GCB DLBCL. Unfortunately, little is known about the subtype-related differences in non-apoptotic RCDs in DLBCL. These studies might reveal differences in the sensitivity to inhibition of a particular RCD, which might be associated with a particular DLCBL subtype. Similarly to other personalized treatment approaches, it might help to identify patients who are most suited to modulation of a particular RCD. Moreover, differences in cell death pathways in DLBCL might determine resistance to certain treatment options. It has been reported that enhanced autophagy makes DLCBL cells more sensitive to conventional drugs [228]. Unfortunately, the link between RCDs and sensitivity to drugs has been poorly studied so far. However, if successfully described, it might provide indispensable information to overcome or reduce resistance to chemotherapy in DLBCL.
Our review suggests that malignant cells can be eliminated in DLBCL by multiple RCD modalities, including apoptosis, necroptosis, ferroptosis, ADCD, pyroptosis, PANoptosis, or methuosis. Additionally, the diversity of RCDs shapes the tumor microenvironment in an extremely sophisticated way, which needs to be further investigated. Studies focusing on expression patterns across RCD-related genes have shown a potential benefit to determine the sensitivity of DLBCL tumors to different RCDs and conventional drugs.
Author Contributions
All authors have equally contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by an EHA Ukraine Bridge Funding awarded by the European Hematology Association to AT, the Ministry of Health, Czech Republic (DRO-VFN00064165) to OH, and the National Institute for Cancer Research (EXCELES-LX22NPO5102) to OH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author, Ondrej Havranek upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4-HNE | 4-Hydroxy-2-nonenal |
| 5-LOX | 5-Lipooxygenease |
| ABC | Activated B cell |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| ADCD | Autophagy-dependent cell death |
| AKT | Protein kinase B |
| AMBRA1 | Autophagy/beclin 1 regulator 1 |
| ASC | Apoptosis-associated speck-like protein containing a caspase recruitment domain |
| Atox1 | Antioxidant 1 copper chaperone |
| BAFF | B cell-activating factor |
| BCR | B-cell receptor |
| BET | Bromodomain and extraterminal motif |
| BRD4 | Bromodomain containing 4 |
| cGAS | Cyclic GMP-AMP synthase |
| CtBP2 | C-terminal binding protein 2 |
| DAMP | Damage-associated molecular pattern |
| DISC | Death-inducing signaling complex |
| DLBCL | Diffuse large B cell lymphoma |
| DLBCL, NOS | Diffuse large B cell lymphoma, not other specified |
| EGR1 | Early growth response-1 |
| FDX1 | Ferredoxin 1 |
| FSP1 | Ferroptosis suppressor protein 1 |
| GCB | Germinal center B-cell |
| GPX4 | Glutathione peroxidase 4 |
| GSDM | Gasdermin |
| GSH | Reduced glutathione |
| HMGB1 | High mobility group box 1 protein |
| ICD | Immunogenic cell death |
| IFN | Interferon |
| IRF3 | Interferon regulatory factor 3 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| LBCL | Large B-cell lymphoma |
| LIAS | Lipoic acid synthetase |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 |
| LUBAC | Linear ubiquitin chain assembly complex |
| MLKL | Mixed lineage kinase domain-like protein |
| MOMP | Mitochondrial outer membrane permeabilization |
| NCAPD3 | Non-SMC condensing II complex subunit D3 |
| NET | Neutrophil extracellular trap |
| NHL | Non-Hodgkin lymphoma |
| NLRP3 | NLR family pyrin domain containing 3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PAMP | Pathogen-associated molecular pattern |
| PAQR3 | Progesterone and adiponectin receptor 3 |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| PI3K | Phosphoinositide 3-kinase |
| PIKfyve | Phosphatidylinositol-3-phosphate 5-kinase |
| PRDX1 | Peroxiredoxin 1 |
| PUFA | Polyunsaturated fatty acid |
| PUFA-OOH | Polyunsaturated fatty acid-containing phospholipid hydroperoxide |
| R/R | Relapse/refractory |
| RCD | Regulated cell death |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SAMHD1 | Sterile alpha motif and HD domain-containing protein 1 |
| SLC7A11 | Solute carrier family 7 member 11 |
| STING | Stimulator of interferon genes |
| TCP1 | Tailless complex polypeptide 1 |
| TFEB | Transcription factor EB |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TNFR | Tumor necrosis factor receptor |
| TRADD | TNF-associated death domain |
| TRAIL | TNF-related apoptosis-inducing ligand |
References
- Alfaifi, A.; Refai, M.Y.; Alsaadi, M.; Bahashwan, S.; Malhan, H.; Al-Kahiry, W.; Dammag, E.; Ageel, A.; Mahzary, A.; Albiheyri, R.; et al. Metabolomics: A New Era in the Diagnosis or Prognosis of B-Cell Non-Hodgkin’s Lymphoma. Diagnostics 2023, 13, 861. [Google Scholar] [CrossRef] [PubMed]
- Susanibar-Adaniya, S.; Barta, S.K. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am. J. Hematol. 2021, 96, 617–629. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Barta, S.K. 2024 Update: Advances in the risk stratification and management of large B-cell lymphoma. Am. J. Hematol. 2023, 98, 1791–1805. [Google Scholar] [CrossRef]
- García-Sancho, A.M.; Cabero, A.; Gutiérrez, N.C. Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma: New Approved Options. J. Clin. Med. 2024, 13, 70. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748, Correction in Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef]
- Hamova, I.; Maco, M.; Tkachenko, A.; Kupcova, K.; Velasova, A.; Trneny, M.; Mocikova, H.; Havranek, O. Circulating tumor DNA as a powerful tool in diagnostics and treatment outcome prediction—Focus on large B-cell lymphomas and follicular lymphomas. Expert Rev. Mol. Diagn 2025, 25, 275–295. [Google Scholar] [CrossRef]
- Shahrokh Esfahani, M.; Alig, S.; Mehrmohamadi, M.; Hamilton, E.G.; King, D.A.; Schultz, A.; Steen, C.B.; Macaulay, C.; Sworder, B.; Kurtz, D.M.; et al. Noninvasive Cell-of-Origin Classification of Diffuse Large B-Cell Lymphoma Using Inferred Gene Expression from Cell-Free DNA Sequencing. Blood 2021, 138, 37. [Google Scholar] [CrossRef]
- Shimkus, G.; Nonaka, T. Molecular classification and therapeutics in diffuse large B-cell lymphoma. Front. Mol. Biosci. 2023, 10, 1124360. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690, Correction in Nat. Med. 2018, 24, 1290–1291. Erratum in Nat. Med. 2018, 24, 1292. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.; Fernández-Miranda, I.; Pérez-Callejo, D.; Quero, C.; Rodríguez, M.; Martín-Acosta, P.; Gómez, S.; González-Rincón, J.; Santos, A.; Tarin, C.; et al. Proposal and validation of a method to classify genetic subtypes of diffuse large B cell lymphoma. Sci. Rep. 2021, 11, 1886. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Schmitz, R. Molecular Subgroups of Diffuse Large B Cell Lymphoma: Biology and Implications for Clinical Practice. Curr. Oncol. Rep. 2022, 24, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Havranek, O.; Xu, J.; Köhrer, S.; Wang, Z.; Becker, L.; Comer, J.M.; Henderson, J.; Ma, W.; Man Chun Ma, J.; Westin, J.R.; et al. Tonic B-cell receptor signaling in diffuse large B-cell lymphoma. Blood 2017, 130, 995–1006. [Google Scholar] [CrossRef]
- Tkachenko, A.; Kupcova, K.; Havranek, O. B-Cell Receptor Signaling and Beyond: The Role of Igα (CD79a)/Igβ (CD79b) in Normal and Malignant B Cells. Int. J. Mol. Sci. 2023, 25, 10. [Google Scholar] [CrossRef]
- Davis, R.E.; Ngo, V.N.; Lenz, G.; Tolar, P.; Young, R.M.; Romesser, P.B.; Kohlhammer, H.; Lamy, L.; Zhao, H.; Yang, Y.; et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010, 463, 88–92. [Google Scholar] [CrossRef]
- Eken, J.A.; Koning, M.T.; Kupcova, K.; Sepúlveda Yáñez, J.H.; de Groen, R.A.L.; Quinten, E.; Janssen, J.; van Bergen, C.A.M.; Vermaat, J.S.P.; Cleven, A.; et al. Antigen-independent, autonomous B cell receptor signaling drives activated B cell DLBCL. J. Exp. Med. 2024, 221, e20230941. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L. Deregulation of Cell Death in Cancer: Recent Highlights. Cancers 2020, 12, 3517. [Google Scholar] [CrossRef]
- You, L.; Xin, Z.; Zhou, X.; Na, F.; Zhou, J.; Ying, B. Diverse regulated cell death modes predict the immune microenvironment and drug sensitivity in lung adenocarcinoma. J. Cell. Physiol. 2023, 238, 2570–2585. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Huang, D.; Shi, Y.; Liang, Z.; Bu, H. Regulated cell death in cancer: From pathogenesis to treatment. Chin. Med. J. 2023, 136, 653–665. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kang, R.; Tang, D. Alkaliptosis: A new weapon for cancer therapy. Cancer Gene Ther. 2020, 27, 267–269. [Google Scholar] [CrossRef]
- Adeshakin, F.O.; Adeshakin, A.O.; Afolabi, L.O.; Yan, D.; Zhang, G.; Wan, X. Mechanisms for Modulating Anoikis Resistance in Cancer and the Relevance of Metabolic Reprogramming. Front. Oncol. 2021, 11, 626577. [Google Scholar] [CrossRef]
- Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I.; Anam, M.; Maharjan, S.; Amjad, Z.; Khan, S. A Systematic Review of Apoptosis in Correlation with Cancer: Should Apoptosis Be the Ultimate Target for Cancer Treatment? Cureus 2022, 14, e28496. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Bai, L.; Wu, Q.; Zhang, X.; Zhao, Y. Autosis as a selective type of cell death. Front. Cell Dev. Biol. 2023, 11, 1164681, Correction in Front. Cell Dev. Biol. 2023, 11, 1211196. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, C.; Ding, Y.; Duan, S. Disulfidptosis: A new target for metabolic cancer therapy. J. Exp. Clin. Cancer Res. 2023, 42, 103. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczuk-Bialy, I.; Dziuba, I.; Sarnecka, A.; Platos, E.; Kowalczyk, M.; Pels, K.K.; Wilczynski, G.M.; Wojcik, C.; Bialy, L.P. Entosis: From Cell Biology to Clinical Cancer Pathology. Cancers 2020, 12, 2481. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Berg, A.L.; Rowson-Hodel, A.; Wheeler, M.R.; Hu, M.; Free, S.R.; Carraway, K.L., III. Engaging the Lysosome and Lysosome-Dependent Cell Death in Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane City, Australia, 2022. [Google Scholar]
- Maltese, W.A.; Overmeyer, J.H. Methuosis: Nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am. J. Pathol. 2014, 184, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhong, B.; Hou, Y.; Wang, M.; Guo, B.; Lin, L.; Zhou, Y.; Chen, X. Fighting cancer by triggering non-canonical mitochondrial permeability transition-driven necrosis through reactive oxygen species induction. Free Radic. Biol. Med. 2023, 202, 35–45. [Google Scholar] [CrossRef]
- Bai, Z.; Zhou, Y.; Peng, Y.; Ye, X.; Ma, L. Perspectives and mechanisms for targeting mitotic catastrophe in cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188965. [Google Scholar] [CrossRef]
- Meier, P.; Legrand, A.J.; Adam, D.; Silke, J. Immunogenic cell death in cancer: Targeting necroptosis to induce antitumour immunity. Nat. Rev. Cancer 2024, 24, 299–315. [Google Scholar] [CrossRef]
- Ronchetti, L.; Boubaker, N.S.; Barba, M.; Vici, P.; Gurtner, A.; Piaggio, G. Neutrophil extracellular traps in cancer: Not only catching microbes. J. Exp. Clin. Cancer Res. 2021, 40, 231. [Google Scholar] [CrossRef]
- Pallichankandy, S.; Thayyullathil, F.; Cheratta, A.R.; Subburayan, K.; Alakkal, A.; Sultana, M.; Drou, N.; Arshad, M.; Tariq, S.; Galadari, S. Targeting oxeiptosis-mediated tumor suppression: A novel approach to treat colorectal cancers by sanguinarine. Cell Death Discov. 2023, 9, 94. [Google Scholar] [CrossRef]
- Xiong, Y. The emerging role of PANoptosis in cancer treatment. Biomed. Pharmacother. 2023, 168, 115696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Tao, S.; Yao, Y.; Wang, Y.; Wei, Q.; Shao, A.; Deng, Y. Parthanatos and its associated components: Promising therapeutic targets for cancer. Pharmacol. Res. 2021, 163, 105299. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Zhang, Z. Cancer-associated pyroptosis: A new license to kill tumor. Front. Immunol. 2023, 14, 1082165. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A. The Field of Regulated Cell Death Biology Requires Umbrella Reviews. Indian J. Clin. Biochem. 2023, 39, 444–445. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Hett, E.; Kroemer, G.; Marincola, F.M. Immunogenic cell death in cancer: Concept and therapeutic implications. J. Transl. Med. 2023, 21, 162. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Suhaili, S.H.; Karimian, H.; Stellato, M.; Lee, T.H.; Aguilar, M.I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017, 9, 443–457. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, D.; Chen, W.; Yan, Z.; Yan, C.; He, T.; Liang, Q.; Shi, Y. Molecular determinants of caspase-9 activation by the Apaf-1 apoptosome. Proc. Natl. Acad. Sci. USA 2014, 111, 16254–16261. [Google Scholar] [CrossRef]
- Westaby, D.; Jimenez-Vacas, J.M.; Padilha, A.; Varkaris, A.; Balk, S.P.; de Bono, J.S.; Sharp, A. Targeting the Intrinsic Apoptosis Pathway: A Window of Opportunity for Prostate Cancer. Cancers 2021, 14, 51. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef]
- Krasovec, G.; Horkan, H.R.; Quéinnec, É.; Chambon, J.-P. The constructive function of apoptosis: More than a dead-end job. Front. Cell Dev. Biol. 2022, 10, 1033645. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Thompson, C.B. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 2002, 109, S97–S107. [Google Scholar] [CrossRef]
- Lu, L.; Osmond, D.G. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol. Rev. 2000, 175, 158–174. [Google Scholar] [CrossRef]
- Osmond, D.G.; Lu, L. Developmental regulation of apoptosis and BCL-2/BAX expression during B lymphopoiesis in normal and RAG-2-/- mouse bone marrow. FASEB J. 1998, 12, A1080. [Google Scholar]
- Lu, L.; Chaudhury, P.; Osmond, D.G. Regulation of cell survival during B lymphopoiesis: Apoptosis and Bcl-2/Bax content of precursor B cells in bone marrow of mice with altered expression of IL-7 and recombinase-activating gene-2. J. Immunol. 1999, 162, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.J.; Newen, A.M.; McNees, C.; Sharma, S.; Pfannenstiel, D.; Moyer, T.; Stephany, D.; Douagi, I.; Wang, Q.; Mayer, C.T. Peripheral apoptosis and limited clonal deletion during physiologic murine B lymphocyte development. Nat. Commun. 2024, 15, 4691. [Google Scholar] [CrossRef] [PubMed]
- Chappaz, S.; McArthur, K.; Kealy, L.; Law, C.W.; Tailler, M.; Lane, R.M.; Lieschke, A.; Ritchie, M.E.; Good-Jacobson, K.L.; Strasser, A.; et al. Homeostatic apoptosis prevents competition-induced atrophy in follicular B cells. Cell Rep. 2021, 36, 109430. [Google Scholar] [CrossRef]
- Wright, J.A.; Bazile, C.; Clark, E.S.; Carlesso, G.; Boucher, J.; Kleiman, E.; Mahmoud, T.; Cheng, L.I.; López-Rodríguez, D.M.; Satterthwaite, A.B.; et al. Impaired B Cell Apoptosis Results in Autoimmunity That Is Alleviated by Ablation of Btk. Front. Immunol. 2021, 12, 705307. [Google Scholar] [CrossRef]
- Defrance, T.; Casamayor-Pallejà, M.; Krammer, P.H. The life and death of a B cell. Adv. Cancer Res. 2002, 86, 195–225. [Google Scholar] [CrossRef] [PubMed]
- Defrance, T. Mature B cells: Apoptosis checkpoints. Transplantation 2005, 79, S4–S7. [Google Scholar] [CrossRef]
- O’Leary, B.; Skinner, H.; Schoenfeld, J.D.; Licitra, L.; Le Tourneau, C.; Esdar, C.; Schroeder, A.; Salmio, S.; Psyrri, A. Evasion of apoptosis and treatment resistance in squamous cell carcinoma of the head and neck. Cancer Treat. Rev. 2024, 129, 102773. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Sharma, A.; Boise, L.H.; Shanmugam, M. Cancer Metabolism and the Evasion of Apoptotic Cell Death. Cancers 2019, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A Link between Cancer Genetics and Chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Yapryntseva, M.A.; Pervushin, N.V.; Tsvetcov, R.I.; Zhivotovsky, B.; Kopeina, G.S. Cancer Drug Resistance: Targeting Proliferation or Programmed Cell Death. Cells 2024, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Young, K.H. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging 2015, 7, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Leveille, E.; Johnson, N.A. Genetic Events Inhibiting Apoptosis in Diffuse Large B Cell Lymphoma. Cancers 2021, 13, 2167. [Google Scholar] [CrossRef]
- Gao, J.; Lu, X.; Wang, G.; Huang, T.; Tuo, Z.; Meng, W. APOC1 knockdown induces apoptosis and decreases angiogenesis in diffuse large B-cell lymphoma cells through blocking the PI3K/AKT/mTOR pathway. Biomol. Biomed. 2025, 25, 1205–1217. [Google Scholar] [CrossRef]
- Dong, J.; Li, L.; Zhang, X.; Yin, X.; Chen, Z. CtBP2 Regulates Wnt Signal Through EGR1 to Influence the Proliferation and Apoptosis of DLBCL Cells. Mol. Carcinog. 2025, 64, 959–969. [Google Scholar] [CrossRef]
- Lu, T.; Yang, J.; Cai, Y.; Ding, M.; Yu, Z.; Fang, X.; Zhou, X.; Wang, X. NCAPD3 promotes diffuse large B-cell lymphoma progression through modulating SIRT1 expression in an H3K9 monomethylation-dependent manner. J. Adv. Res. 2025, 68, 163–178. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, K.; Tao, J.; Zheng, C.; Zhai, L. MYC-dependent MiR-7-5p regulated apoptosis and autophagy in diffuse large B cell lymphoma by targeting AMBRA1. Mol. Cell Biochem. 2025, 480, 191–202. [Google Scholar] [CrossRef]
- Fang, X.; Huang, Q.; Dong, L.J.; Liu, Q.H.; Miao, X.N.; Xiong, J.; Jiang, X.D.; Yu, Q. MiR-93 promotes the progression and chemoresistance of diffuse large B-cell lymphoma cells via targeting SMAD5. Arch. Physiol. Biochem. 2025, 131, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Cutmore, N.H.; Krupka, J.A.; Hodson, D.J. Genetic Profiling in Diffuse Large B-Cell Lymphoma: The Promise and the Challenge. Mod. Pathol. 2023, 36, 100007. [Google Scholar] [CrossRef]
- Lenz, G. Novel NF-κB regulator in ABC DLBCL. Blood 2016, 127, 2785–2786. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.; Klein, U. Aberrant Activation of NF-κB Signalling in Aggressive Lymphoid Malignancies. Cells 2018, 7, 189. [Google Scholar] [CrossRef]
- Iqbal, J.; Greiner, T.C.; Patel, K.; Dave, B.J.; Smith, L.; Ji, J.; Wright, G.; Sanger, W.G.; Pickering, D.L.; Jain, S.; et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia 2007, 21, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Klanova, M.; Klener, P. BCL-2 Proteins in Pathogenesis and Therapy of B-Cell Non-Hodgkin Lymphomas. Cancers 2020, 12, 938. [Google Scholar] [CrossRef]
- Razzaghi, R.; Agarwal, S.; Kotlov, N.; Plotnikova, O.; Nomie, K.; Huang, D.W.; Wright, G.W.; Smith, G.A.; Li, M.; Takata, K.; et al. Compromised counterselection by FAS creates an aggressive subtype of germinal center lymphoma. J. Exp. Med. 2021, 218, e20201173. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar] [CrossRef]
- Nowakowski, G.; Maurer, M.J.; Cerhan, J.R.; Dey, D.; Sehn, L.H. Utilization of real-world data in assessing treatment effectiveness for diffuse large B-cell lymphoma. Am. J. Hematol. 2023, 98, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Chen, Z.; Xu, Y. The double-edged functions of necroptosis. Cell Death Dis. 2023, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.R.; Davies, K.A.; Zhang, Y.; Brzozowski, M.; Czabotar, P.E.; Murphy, J.M.; Lessene, G. From (Tool)Bench to Bedside: The Potential of Necroptosis Inhibitors. J. Med. Chem. 2023, 66, 2361–2385. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Yang, M.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. A Glimpse of necroptosis and diseases. Biomed. Pharmacother. 2022, 156, 113925. [Google Scholar] [CrossRef]
- Morgan, M.J.; Kim, Y.-S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med. 2022, 54, 1695–1704. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, G.; Vandenabeele, P.; Krysko, D.V. Necroptosis: A Novel Cell Death Modality and Its Potential Relevance for Critical Care Medicine. Am. J. Respir. Crit. Care Med. 2016, 194, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef]
- Zhang, J.; Kodali, S.; Chen, M.; Wang, J. Maintenance of Germinal Center B Cells by Caspase-9 through Promotion of Apoptosis and Inhibition of Necroptosis. J. Immunol. 2020, 205, 113–120. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Hägglöf, T.; Hadley, J.T.; McLennan, A.; Mattke, A.; Dudley, E.A.; Kumagai, A.; Dong, L.Q.; Leadbetter, E.A. Receptor Interacting Protein Kinase Pathways Regulate Innate B Cell Developmental Checkpoints But Not Effector Function in Mice. Front. Immunol. 2021, 12, 758407. [Google Scholar] [CrossRef]
- Sprooten, J.; De Wijngaert, P.; Vanmeerbeek, I.; Martin, S.; Vangheluwe, P.; Schlenner, S.; Krysko, D.V.; Parys, J.B.; Bultynck, G.; Vandenabeele, P.; et al. Necroptosis in Immuno-Oncology and Cancer Immunotherapy. Cells 2020, 9, 1823. [Google Scholar] [CrossRef]
- Pan, Y.B.; Wang, W.; Cai, H.K.; Zhang, J.; Teng, Y.; Xue, J.; Zhu, M.; Luo, W.D. Integrative analysis of a necroptosis-related gene signature of clinical value and heterogeneity in diffuse large B cell lymphoma. Front. Genet. 2022, 13, 911443. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Z.; Guan, J.; Zheng, C. Identification and Assessment of Necroptosis-Related Genes in Clinical Prognosis and Immune Cells in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2022, 12, 904614. [Google Scholar] [CrossRef]
- Wu, B.; Li, J.; Wang, H.; Liu, J.; Li, J.; Sun, F.; Feng, D.C. RIPK1 is aberrantly expressed in multiple B-cell cancers and implicated in the underlying pathogenesis. Discov. Oncol. 2023, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, L.; Fei, X.C.; Jiang, X.F.; Zheng, Z.; Zhao, Y.; Wang, C.F.; Li, B.; Chen, S.J.; Janin, A.; et al. MYC is a positive regulator of choline metabolism and impedes mitophagy-dependent necroptosis in diffuse large B-cell lymphoma. Blood Cancer J. 2017, 7, e582. [Google Scholar] [CrossRef]
- Berehab, M.; Rouas, R.; Akl, H.; Duvillier, H.; Journe, F.; Fayyad-Kazan, H.; Ghanem, G.; Bron, D.; Lewalle, P.; Merimi, M. Apoptotic and Non-Apoptotic Modalities of Thymoquinone-Induced Lymphoma Cell Death: Highlight of the Role of Cytosolic Calcium and Necroptosis. Cancers 2021, 13, 3579. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Varney, M.; Dave, B.J.; Singh, R.K. Diclofenac Induces Apoptosis and Suppresses Diffuse Large B-Cell Lymphoma Proliferation Independent of P53 Status. Blood 2014, 124, 5485. [Google Scholar] [CrossRef]
- Demir Karaman, E.; Işık, Z. Multi-Omics Data Analysis Identifies Prognostic Biomarkers across Cancers. Med. Sci. 2023, 11, 44. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Coradduzza, D.; Congiargiu, A.; Chen, Z.; Zinellu, A.; Carru, C.; Medici, S. Ferroptosis and Senescence: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3658. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Chen, Q.; Xiang, M.; Gao, Z.; Lvu, F.; Sun, Z.; Wang, Y.; Shi, X.; Xu, J.; Wang, J.; Liang, J. The role of B-cell ferroptosis in the pathogenesis of systemic lupus erythematosus. Clin. Immunol. 2023, 256, 109778. [Google Scholar] [CrossRef]
- Burton, E.M.; Voyer, J.; Gewurz, B.E. Epstein-Barr virus latency programs dynamically sensitize B-cells to ferroptosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2118300119. [Google Scholar] [CrossRef]
- Bian, W.; Li, H.; Chen, Y.; Yu, Y.; Lei, G.; Yang, X.; Li, S.; Chen, X.; Li, H.; Yang, J.; et al. Ferroptosis mechanisms and its novel potential therapeutic targets for DLBCL. Biomed. Pharmacother. 2024, 173, 116386. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, W.; Abdul Razak, S.R.; Han, T.; Ahmad, N.H.; Li, X. Ferroptosis as a potential target for cancer therapy. Cell Death Dis. 2023, 14, 460. [Google Scholar] [CrossRef]
- Rodriguez, R.; Schreiber, S.L.; Conrad, M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol. Cell 2022, 82, 728–740. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Dang, Q.; Sun, Z.; Wang, Y.; Wang, L.; Liu, Z.; Han, X. Ferroptosis: A double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022, 13, 925. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Guo, J.; Ma, J. The crosstalk between ferroptosis and anti-tumor immunity in the tumor microenvironment: Molecular mechanisms and therapeutic controversy. Cancer Commun. 2023, 43, 1071–1096. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, T.; Qin, Q.; Huang, X.; Wang, Y. Ferroptosis in lymphoma: Emerging mechanisms and a novel therapeutic approach. Front. Genet. 2022, 13, 1039951. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, W.; Yu, N.; Zhong, X. PAQR3 facilitates the ferroptosis of diffuse large B-cell lymphoma via the regulation of LDLR-mediated PI3K/AKT pathway. Hematol. Oncol. 2024, 42, e3219. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Xia, Z.G.; Wang, A.H.; Wang, W.F.; Liu, Z.Y.; Chen, L.Y.; Li, J.M. Activation of the PI3K/AKT/mTOR pathway in diffuse large B cell lymphoma: Clinical significance and inhibitory effect of rituximab. Ann. Hematol. 2013, 92, 1351–1358. [Google Scholar] [CrossRef]
- Xu, W.; Berning, P.; Lenz, G. Targeting B-cell receptor and PI3K signaling in diffuse large B-cell lymphoma. Blood 2021, 138, 1110–1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 2021, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Jeffrey Medeiros, L.; Bueso-Ramos, C.E.; Tang, G.; Wang, S.; Oki, Y.; Desai, P.; Khoury, J.D.; Miranda, R.N.; Tang, Z.; et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod. Pathol. 2017, 30, 194–203. [Google Scholar] [CrossRef]
- Yu, T.; Xu-Monette, Z.Y.; Yu, L.; Li, Y.; Young, K.H. Mechanisms of ferroptosis and targeted therapeutic approaches in lymphoma. Cell Death Dis. 2023, 14, 771, Correction in Cell Death Dis. 2024, 15, 19. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X. The Spectrum of MYC Alterations in Diffuse Large B-Cell Lymphoma. Acta Haematol. 2020, 143, 520–528. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, S.; Xu, M.; Ma, B.; Liu, R.; Xu, Y.; Zhang, Y. TFAP2C-Mediated lncRNA PCAT1 Inhibits Ferroptosis in Docetaxel-Resistant Prostate Cancer Through c-Myc/miR-25-3p/SLC7A11 Signaling. Front. Oncol. 2022, 12, 862015. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Xie, S.; Wang, M.; Shen, J. PRDX1 knockdown promotes erastin-induced ferroptosis and impedes diffuse large B-cell lymphoma development by inhibiting the MAPK/ERK pathway. BMC Cancer 2025, 25, 806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Z.; Dong, X.; Yao, Y.; Chen, Q.; Shi, Y.; Deng, Y.; Zhang, Q.; Yu, L.; Wang, C. Regulation and therapy: The role of ferroptosis in DLBCL. Front. Pharmacol. 2025, 15, 1458412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Daniels, J.D.; Zandkarimi, F.; Liu, H.; Brown, L.M.; Uchida, K.; O’Connor, O.A.; Stockwell, B.R. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem. Biol. 2019, 26, 623–633.e9. [Google Scholar] [CrossRef]
- Zhou, N.; Busino, L. Targeting epigenetics and ferroptosis in DLBCL. Blood 2023, 142, 1108–1109. [Google Scholar] [CrossRef]
- Schmitt, A.; Grimm, M.; Kreienkamp, N.; Junge, H.; Labisch, J.; Schuhknecht, L.; Schönfeld, C.; Görsch, E.; Tibello, A.; Menck, K.; et al. BRD4 inhibition sensitizes diffuse large B-cell lymphoma cells to ferroptosis. Blood 2023, 142, 1143–1155. [Google Scholar] [CrossRef]
- Schmitt, A.; Xu, W.; Bucher, P.; Grimm, M.; Konantz, M.; Horn, H.; Zapukhlyak, M.; Berning, P.; Brändle, M.; Jarboui, M.-A.; et al. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 2021, 138, 871–884. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Huang, G.; Xiao, X.; Xu, S.; Weng, P.; Wang, Y.; Tian, H.; Huang, H.; Chen, Y. TCP1 expression alters the ferroptosis sensitivity of diffuse large B-cell lymphoma subtypes by stabilising ACSL4 and influences patient prognosis. Cell Death Dis. 2024, 15, 611. [Google Scholar] [CrossRef]
- Wang, J.; Peng, H.; Zhang, G.; Yan, W. Identification of a novel model based on ferroptosis-related genes for predicting the prognosis of diffuse large B-cell lymphomas. Hematology 2023, 28, 2198862. [Google Scholar] [CrossRef]
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 2018, 98, 609–619. [Google Scholar] [CrossRef]
- Long, Q.; Tao, H.; Wang, P.; Wu, B.; Zhu, Q.; Chen, H.; Lao, G.; Yang, Y.; Liu, G.; Liu, S.; et al. Fludarabine Enhances Radiosensitivity by Promoting Ferroptosis in B-Cell Lymphoma. Radiat. Res. 2024, 201, 224–239. [Google Scholar] [CrossRef]
- Kawade, G.; Kurata, M.; Matsuki, Y.; Fukuda, S.; Onishi, I.; Kinowaki, Y.; Watabe, S.; Ishibashi, S.; Ikeda, M.; Yamamoto, M.; et al. Mediation of Ferroptosis Suppressor Protein 1 Expression via 4-Hydroxy-2-Nonenal Accumulation Contributes to Acquisition of Resistance to Apoptosis and Ferroptosis in Diffuse Large B-Cell Lymphoma. Lab. Investig. 2024, 104, 102027. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Fu, L.; Wu, R.; Gu, Z.; Yin, C.; He, K. Identification and Development of a 4-Gene Ferroptosis Signature Predicting Overall Survival for Diffuse Large B-Cell Lymphoma. Technol. Cancer Res. Treat. 2023, 22, 15330338221147772. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, Y.; Pan, T.; Zeng, R.; Li, Y.; Chen, S.; Li, Y.; Xiao, L.; Zhou, H. Ferroptosis-Related Gene Signature: A New Method for Personalized Risk Assessment in Patients with Diffuse Large B-Cell Lymphoma. Pharmgenomics Pers. Med. 2021, 14, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yao, R.; Yu, N.; Zhang, W. Identification of a prognostic signature based on five ferroptosis-related genes for diffuse large B-cell lymphoma. Cancer Biomark. 2024, 40, 125–139. [Google Scholar] [CrossRef]
- Nachef, M.; Ali, A.K.; Almutairi, S.M.; Lee, S.H. Targeting SLC1A5 and SLC3A2/SLC7A5 as a Potential Strategy to Strengthen Anti-Tumor Immunity in the Tumor Microenvironment. Front. Immunol. 2021, 12, 624324. [Google Scholar] [CrossRef]
- Xu, F.-L.; Wu, X.-H.; Chen, C.; Wang, K.; Huang, L.-Y.; Xia, J.; Liu, Y.; Shan, X.-F.; Tang, N. SLC27A5 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by downregulating glutathione reductase. Cell Death Dis. 2023, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, R.; Li, K.; Gao, P.; Gong, Y.; Yang, W.; Cai, K. Design of manganese ion coated Prussian blue nanocarrier for the therapy of refractory diffuse large B-cell lymphoma based on a comprehensive analysis of ferroptosis regulators from clinical cases. Nano Res. 2023, 16, 5265–5278. [Google Scholar] [CrossRef]
- Li, J.; Guo, W.; Zhao, Y.; Wang, X.; Wan, X.; Bai, O. Lenalidomide in Combination with Etoposide Induced Ferroptotic Cell Death in DLBCL. Blood 2023, 142, 5788. [Google Scholar] [CrossRef]
- Manara, P.; Barroso, A.; Alaoui, A.Y.; Cunningham, T.A.; Ho, D.H.; Hoffman, K.; Schatz, J.H. Enhanced Ferroptosis Induction Susceptibility in Diffuse Large B-Cell Lymphoma through Rocaglate-Mediated Translation Inhibition. Blood 2023, 142, 2814. [Google Scholar] [CrossRef]
- Xiong, D.; Geng, C.; Zeng, L.; Yao, H.; Tan, J.; Zhang, L.; Liu, X.; Liu, L. Artesunate induces ferroptosis by regulating MT1G and has an additive effect with doxorubicin in diffuse large B-cell lymphoma cells. Heliyon 2024, 10, e28584. [Google Scholar] [CrossRef]
- Huang, Q.T.; Hu, Q.Q.; Wen, Z.F.; Li, Y.L. Iron oxide nanoparticles inhibit tumor growth by ferroptosis in diffuse large B-cell lymphoma. Am. J. Cancer Res. 2023, 13, 498–508. [Google Scholar]
- Rink, J.; Lin, A.Y.; Yang, S.; Behdad, A.; Karmali, R.; Thaxton, C.S.; Gordon, L.I. Bio-Inspired Functional Lipoprotein-like Nanoparticles (Flip-NPs) Cause Cholesterol Starvation and Ferroptosis in B-Cell Lymphomas: Studies in Cell Lines, Xenograft Models and Primary Patient Samples. Blood 2019, 134, 2857. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Y.; Tan, X.; Merkher, Y.; Leonov, S.; Zhu, L.; Deng, Y.; Zhang, H.; Zhu, D.; Tan, Y.; et al. Emerging mechanisms of pyroptosis and its therapeutic strategy in cancer. Cell Death Discov. 2022, 8, 338. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cai, W.; Huang, J.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. Pyroptosis in development, inflammation and disease. Front. Immunol. 2022, 13, 991044. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Chen, Y.; Chen, S.; Zhang, Y.; Chen, H.; Yang, Y.; Gu, H.; Chen, Q.; Li, M.; Chen, X. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023, 14, 595. [Google Scholar] [CrossRef]
- Lim, K.-H.; Chen, L.-C.; Hsu, K.; Chang, C.-C.; Chang, C.-Y.; Kao, C.-W.; Chang, Y.-F.; Chang, M.-C.; Chen, C.G. BAFF-driven NLRP3 inflammasome activation in B cells. Cell Death Dis. 2020, 11, 820. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Jin, X.; Ma, Y.; Liu, D.; Huang, Y. Role of pyroptosis in the pathogenesis and treatment of diseases. MedComm 2023, 4, e249. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Chen, S.; Shi, L.; Liu, M.; Tu, P.; Sun, J.; Zhao, R.; Zhang, Y.; Wang, J.; et al. Iron induces B cell pyroptosis through Tom20–Bax–caspase–gasdermin E signaling to promote inflammation post-spinal cord injury. J. Neuroinflamm. 2023, 20, 171. [Google Scholar] [CrossRef]
- Fernandez, M.; Vankamamidi, S.E.; Wu, S.; Yan, H.; Xu, Z. Regulation of germinal center B cell apoptosis and pyroptosis by linear ubiquitin chain assembly complex (LUBAC) in antibody and autoantibody responses. J. Immunol. 2024, 212, 1260–5678. [Google Scholar] [CrossRef]
- Huang, W.; Wang, B.; Ou, Q.; Zhang, X.; He, Y.; Mao, X.; Wei, X.; Kou, X. ASC-expressing pyroptotic extracellular vesicles alleviate sepsis by protecting B cells. Mol. Ther. 2024, 32, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, J.; Zhang, C. What role does pyroptosis play in cancer? Mol. Metab. 2022, 65, 101587. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Song, W.J.; Ma, G.K.; Wang, H.; Yang, L. Pyroptosis and its role in cancer. World J. Clin. Cases 2023, 11, 2386–2395. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, W.; Ma, J.; Xu, Z. A Novel and Validated 8-Pyroptosis-Related Genes Based Risk Prediction Model for Diffuse Large B Cell Lymphoma. Biomolecules 2022, 12, 1835. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, X.; Hu, S.; Lu, T.; Chen, X.; Ding, M.; Wang, X. SAMHD1 Performs Tumor-Promoting Effects in Diffuse Large B-Cell Lymphoma By Inhibiting DNA Damage and Sting-Mediated Cell Pyroptosis. Blood 2021, 138, 869. [Google Scholar] [CrossRef]
- Wang, W.; Xu, S.W.; Teng, Y.; Zhu, M.; Guo, Q.Y.; Wang, Y.W.; Mao, X.L.; Li, S.W.; Luo, W.D. The Dark Side of Pyroptosis of Diffuse Large B-Cell Lymphoma in B-Cell Non-Hodgkin Lymphoma: Mediating the Specific Inflammatory Microenvironment. Front. Cell Dev. Biol. 2021, 9, 779123. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat. Rev. Cardiol. 2024, 21, 219–237. [Google Scholar] [CrossRef]
- Lu, F.; Zhao, Y.; Pang, Y.; Ji, M.; Sun, Y.; Wang, H.; Zou, J.; Wang, Y.; Li, G.; Sun, T.; et al. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett. 2021, 497, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhao, W.; Lin, W.; Xiao, Y.; Ren, J.; Zhou, Y.; Meng, W.; Bi, E.; Jiang, L. Bendamustine-rituximab elicits dual tumoricidal and immunomodulatory responses via cGAS-STING activation in diffuse large B-cell lymphoma. J. Immunother. Cancer 2024, 12, e009212. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Y.; Cai, Y.; Yu, Z.; Ge, X.; Fang, X.; Wang, C.; Kong, R.; Wang, X.; Zhou, X. IDENTIFYING A NOVEL DEFINED PYROPTOSIS-ASSOCIATED SIGNATURE CONTRIBUTES TO PREDICTING PROGNOSIS AND TUMOR MICROENVIRONMENT OF DIFFUSE LARGE B-CELL LYMPHOMA. Hematol. Oncol. 2023, 41, 637. [Google Scholar] [CrossRef]
- Shi, C.; Cao, P.; Wang, Y.; Zhang, Q.; Zhang, D.; Wang, Y.; Wang, L.; Gong, Z. PANoptosis: A Cell Death Characterized by Pyroptosis, Apoptosis, and Necroptosis. J. Inflamm. Res. 2023, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Y.; Zhang, L.; Guo, L.; Wang, X.; Pan, Z.; Jiang, X.; Wu, F.; He, G. Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases. Cell Death Dis. 2023, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X. Targeting regulated cell death (RCD) in hematological malignancies: Recent advances and therapeutic potential. Biomed. Pharmacother. 2024, 175, 116667. [Google Scholar] [CrossRef]
- Cai, H.; Lv, M.; Wang, T. PANoptosis in cancer, the triangle of cell death. Cancer Med. 2023, 12, 22206–22223. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Qian, F.; Cai, P.; Ocansey, S.; Amoah, S.; Qian, Y.; Mao, F. Current evidence and therapeutic implication of PANoptosis in cancer. Theranostics 2024, 14, 640–661. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, X.; Lu, T.; Fang, X.; Ding, M.; Yu, Z.; Hu, S.; Liu, J.; Zhou, X.; Wang, X. Activation of STING by SAMHD1 Deficiency Promotes PANoptosis and Enhances Efficacy of PD-L1 Blockade in Diffuse Large B-cell Lymphoma. Int. J. Biol. Sci. 2023, 19, 4627–4643. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Y.; Zeng, J.; Liu, D.; Feng, X.; Cheng, Y.; Lu, D.; Sun, Y.; Zhu, Q.; Zhang, X.; et al. FDX1 promotes elesclomol-induced PANoptosis in diffuse large B-cell lymphoma via activating IRF3/IFN-β signaling. Oncogene 2025, 44, 2303–2314. [Google Scholar] [CrossRef]
- Xu, M.; Ruan, M.; Zhu, W.; Xu, J.; Lin, L.; Li, W.; Zhu, W. Integrative analysis of a novel immunogenic PANoptosis-related gene signature in diffuse large B-cell lymphoma for prognostication and therapeutic decision-making. Sci. Rep. 2024, 14, 30370. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef]
- Marković, D.C.; Maslovarić, I.S.; Kovačić, M.; Vignjević Petrinović, S.; Ilić, V.L. Putative Role of Neutrophil Extracellular Trap Formation in Chronic Myeloproliferative Neoplasms. Int. J. Mol. Sci. 2023, 24, 4497. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Z.; Díaz-Godínez, C.; Mora, N.; Alemán, O.R.; Uribe-Querol, E.; Carrero, J.C.; Rosales, C. Entamoeba histolytica Induce Signaling via Raf/MEK/ERK for Neutrophil Extracellular Trap (NET) Formation. Front. Cell Infect. Microbiol. 2018, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Azzouz, D.; Khan, M.A.; Palaniyar, N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.; Kalinina, O.; Knight, K.L. Death of tonsillar B cells by NETosis. Cell Death Discov. 2023, 9, 108. [Google Scholar] [CrossRef]
- Bertelli, R.; Schena, F.; Antonini, F.; Reverberi, D.; Signa, S.; Pedemonte, N.; Consolaro, A.; Gattorno, M.; Negrini, S.; Pupo, F.; et al. Neutrophil Extracellular Traps in Systemic Lupus Erythematosus Stimulate IgG2 Production From B Lymphocytes. Front. Med. 2021, 8, 635436. [Google Scholar] [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Volkov, D.V.; Tetz, G.V.; Rubtsov, Y.P.; Stepanov, A.V.; Gabibov, A.G. Neutrophil Extracellular Traps (NETs): Opportunities for Targeted Therapy. Acta Naturae 2021, 13, 15–23. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, H.; Tan, S.; Dong, Q.; Fan, X.; Wang, Y.; Zhang, H.; He, J. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp. Hematol. Oncol. 2022, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Jaboury, S.; Wang, K.; O’Sullivan, K.M.; Ooi, J.D.; Ho, G.Y. NETosis as an oncologic therapeutic target: A mini review. Front. Immunol. 2023, 14, 1170603. [Google Scholar] [CrossRef]
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Pan, Y.; Xiang, G.; Wang, M.; Huang, Y.; He, L.; Wang, J.; Fang, Q.; Li, L.; Liu, Z. A novel NET-related gene signature for predicting DLBCL prognosis. J. Transl. Med. 2023, 21, 630. [Google Scholar] [CrossRef]
- Aydemir, D. The impact of NETosis on hematological malignancies as a promising therapeutic target. Front. Hematol. 2024, 3, 1377806. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.-W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Yu, G.; Klionsky, D.J. Life and Death Decisions-The Many Faces of Autophagy in Cell Survival and Cell Death. Biomolecules 2022, 12, 866. [Google Scholar] [CrossRef]
- Turco, E.; Fracchiolla, D.; Martens, S. Recruitment and Activation of the ULK1/Atg1 Kinase Complex in Selective Autophagy. J. Mol. Biol. 2020, 432, 123–134. [Google Scholar] [CrossRef]
- Birgisdottir, Å.B.; Mouilleron, S.; Bhujabal, Z.; Wirth, M.; Sjøttem, E.; Evjen, G.; Zhang, W.; Lee, R.; O’Reilly, N.; Tooze, S.A.; et al. Members of the autophagy class III phosphatidylinositol 3-kinase complex I interact with GABARAP and GABARAPL1 via LIR motifs. Autophagy 2019, 15, 1333–1355. [Google Scholar] [CrossRef]
- Iriondo, M.N.; Etxaniz, A.; Varela, Y.R.; Ballesteros, U.; Lázaro, M.; Valle, M.; Fracchiolla, D.; Martens, S.; Montes, L.R.; Goñi, F.M.; et al. Effect of ATG12-ATG5-ATG16L1 autophagy E3-like complex on the ability of LC3/GABARAP proteins to induce vesicle tethering and fusion. Cell Mol. Life Sci. 2023, 80, 56. [Google Scholar] [CrossRef]
- Tian, X.; Teng, J.; Chen, J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy 2021, 17, 2680–2688. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Zhao, T. The physiological roles of autophagy in the mammalian life cycle. Biol. Rev. Camb. Philos. Soc. 2019, 94, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Murera, D.; Arbogast, F.; Fauny, J.D.; Muller, S.; Gros, F. Autophagy is dispensable for B-cell development but essential for humoral autoimmune responses. Cell Death Differ. 2016, 23, 853–864. [Google Scholar] [CrossRef]
- Martinez-Martin, N.; Maldonado, P.; Gasparrini, F.; Frederico, B.; Aggarwal, S.; Gaya, M.; Tsui, C.; Burbage, M.; Keppler, S.J.; Montaner, B.; et al. A switch from canonical to noncanonical autophagy shapes B cell responses. Science 2017, 355, 641–647. [Google Scholar] [CrossRef]
- Raza, I.G.A.; Clarke, A.J. B Cell Metabolism and Autophagy in Autoimmunity. Front. Immunol. 2021, 12, 681105. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J.; Riffelmacher, T.; Braas, D.; Cornall, R.J.; Simon, A.K. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 2018, 215, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, L.; Van Kaer, L. Role of canonical and noncanonical autophagy pathways in shaping the life journey of B cells. Front. Immunol. 2024, 15, 1426204. [Google Scholar] [CrossRef]
- Sallan, M.C.; Filipsky, F.; Shi, C.H.; Pontarini, E.; Terranova-Barberio, M.; Beattie, G.; Clear, A.; Bombardieri, M.; Yip, K.Y.; Calado, D.P.; et al. Autophagy is an upstream mediator of chromatin dynamics in normal and autoimmune germinal center B cells. J. Clin. Investig. 2025, 135, e178920. [Google Scholar] [CrossRef]
- McLeod, I.X.; He, Y. Roles of autophagy in lymphocytes: Reflections and directions. Cell Mol. Immunol. 2010, 7, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ichinose, S.; Hayashizaki, K.; Tsubata, T. Induction of autophagy by B cell antigen receptor stimulation and its inhibition by costimulation. Biochem. Biophys. Res. Commun. 2008, 374, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Chen, J.; Sun, L. Crosstalk between autophagy and immune cell infiltration in the tumor microenvironment. Front. Med. 2023, 10, 1125692. [Google Scholar] [CrossRef]
- Bustos, S.O.; Antunes, F.; Rangel, M.C.; Chammas, R. Emerging Autophagy Functions Shape the Tumor Microenvironment and Play a Role in Cancer Progression—Implications for Cancer Therapy. Front. Oncol. 2020, 10, 606436. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations. J. Cell Biol. 2020, 219, e201909033. [Google Scholar] [CrossRef]
- Chen, J.-L.; Wu, X.; Yin, D.; Jia, X.-H.; Chen, X.; Gu, Z.-Y.; Zhu, X.-M. Autophagy inhibitors for cancer therapy: Small molecules and nanomedicines. Pharmacol. Ther. 2023, 249, 108485. [Google Scholar] [CrossRef]
- Pierdominici, M.; Barbati, C.; Vomero, M.; Locatelli, S.L.; Carlo-Stella, C.; Ortona, E.; Malorni, W. Autophagy as a pathogenic mechanism and drug target in lymphoproliferative disorders. FASEB J. 2014, 28, 524–535. [Google Scholar] [CrossRef]
- Yuan, H.; He, M.; Cheng, F.; Bai, R.; da Silva, S.R.; Aguiar, R.C.; Gao, S.J. Tenovin-6 inhibits proliferation and survival of diffuse large B-cell lymphoma cells by blocking autophagy. Oncotarget 2017, 8, 14912–14924. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Zhang, Y.; Yang, J.; Xu, Y.; Zhao, Y.; Wang, X. CUL4B regulates autophagy via JNK signaling in diffuse large B-cell lymphoma. Cell Cycle 2019, 18, 379–394. [Google Scholar] [CrossRef]
- Xu, H.; Yu, X.; Yang, Z.; Song, Q.; Cheng, S.; He, Z.; Dai, L. PAX5-activated lncRNA ARRDC1-AS1 accelerates the autophagy and progression of DLBCL through sponging miR-2355-5p to regulate ATG5. Life Sci. 2021, 286, 119932. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.Z.; Liu, D.; Lin, C.R.; Liang, D.; Huang, R.; Wang, L. An Autophagy-Related Gene Signature can Better Predict Prognosis and Resistance in Diffuse Large B-Cell Lymphoma. Front. Genet. 2022, 13, 862179. [Google Scholar] [CrossRef]
- Mandhair, H.K.; Radpour, R.; Westerhuis, M.; Banz, Y.; Humbert, M.; Arambasic, M.; Dengjel, J.; Davies, A.; Tschan, M.P.; Novak, U. Analysis of autophagy in DLBCL reveals subtype-specific differences and the preferential targeting of ULK1 inhibition in GCB-DLBCL provides a rationale as a new therapeutic approach. Leukemia 2024, 38, 424–429. [Google Scholar] [CrossRef]
- Xiong, D.; Wei, X.; Huang, W.; Zheng, J.; Feng, R. Prediction significance of autophagy-related genes in survival probability and drug resistance in diffuse large B-cell lymphoma. Aging 2024, 16, 1049–1076. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Ma, M.-X.; Xing, L.-N.; Zhang, J.-N.; Guo, X.-N.; Qiao, S.-K. Downregulation of autophagy is associated with poor clinical outcome after immunochemotherapy in patients with diffuse large B-cell lymphoma. Exp. Hematol. 2024, 139, 104638. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Wu, P.; Gong, S.; Gao, J.; Tao, H.; Shen, Q.; Wang, S.; Zhou, Z.; Jia, Y. Artesunate induces apoptosis, autophagy and ferroptosis in diffuse large B cell lymphoma cells by impairing STAT3 signaling. Cell. Signal. 2021, 88, 110167. [Google Scholar] [CrossRef]
- Ma, L.; Gong, Q.; Chen, Y.; Luo, P.; Chen, J.; Shi, C. Targeting positive cofactor 4 induces autophagic cell death in MYC-expressing diffuse large B-cell lymphoma. Exp. Hematol. 2023, 119–120, 42–57.e4. [Google Scholar] [CrossRef]
- Liu, H.; Wei, J.; Sang, N.; Zhong, X.; Zhou, X.; Yang, X.; Zhang, J.; Zuo, Z.; Zhou, Y.; Yang, S.; et al. The novel LSD1 inhibitor ZY0511 suppresses diffuse large B-cell lymphoma proliferation by inducing apoptosis and autophagy. Med. Oncol. 2021, 38, 124. [Google Scholar] [CrossRef]
- Xu, L.; Gao, X.; Yang, P.; Sang, W.; Jiao, J.; Niu, M.; Liu, M.; Qin, Y.; Yan, D.; Song, X.; et al. EHMT2 inhibitor BIX-01294 induces endoplasmic reticulum stress mediated apoptosis and autophagy in diffuse large B-cell lymphoma cells. J. Cancer 2021, 12, 1011–1022. [Google Scholar] [CrossRef]
- Nicotra, G.; Mercalli, F.; Peracchio, C.; Castino, R.; Follo, C.; Valente, G.; Isidoro, C. Autophagy-active beclin-1 correlates with favourable clinical outcome in non-Hodgkin lymphomas. Mod. Pathol. 2010, 23, 937–950. [Google Scholar] [CrossRef]
- Huang, J.J.; Zhu, Y.J.; Lin, T.Y.; Jiang, W.Q.; Huang, H.Q.; Li, Z.M. Beclin 1 expression predicts favorable clinical outcome in patients with diffuse large B-cell lymphoma treated with R-CHOP. Hum. Pathol. 2011, 42, 1459–1466. [Google Scholar] [CrossRef]
- Djavaheri-Mergny, M.; Giuriato, S.; Tschan, M.P.; Humbert, M. Therapeutic Modulation of Autophagy in Leukaemia and Lymphoma. Cells 2019, 8, 103. [Google Scholar] [CrossRef]
- Salwa, A.; Ferraresi, A.; Secomandi, E.; Vallino, L.; Moia, R.; Patriarca, A.; Garavaglia, B.; Gaidano, G.; Isidoro, C. High BECN1 Expression Negatively Correlates with BCL2 Expression and Predicts Better Prognosis in Diffuse Large B-Cell Lymphoma: Role of Autophagy. Cells 2023, 12, 1924. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.; Yang, Y.; Zhao, D.; Wen, Y.; Lv, C.; Qiu, H.; Wang, C. The regulation of miR-320a/XBP1 axis through LINC00963 for endoplasmic reticulum stress and autophagy in diffuse large B-cell lymphoma. Cancer Cell Int. 2021, 21, 305. [Google Scholar] [CrossRef]
- Phelan, J.D.; Scheich, S.; Choi, J.; Wright, G.W.; Häupl, B.; Young, R.M.; Rieke, S.A.; Pape, M.; Ji, Y.; Urlaub, H.; et al. Response to Bruton’s tyrosine kinase inhibitors in aggressive lymphomas linked to chronic selective autophagy. Cancer Cell 2024, 42, 238–252.e9. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, T.; Song, Z.; Peng, L.; Gao, M.; Hermine, O.; Rousseaux, S.; Khochbin, S.; Mi, J.Q.; Wang, J. Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044. Cancer Med. 2018, 7, 380–396. [Google Scholar] [CrossRef]
- García Ruiz, O.; Sánchez-Maldonado, J.M.; López-Nevot, M.Á.; García, P.; Macauda, A.; Hernández-Mohedo, F.; González-Sierra, P.A.; Martínez-Bueno, M.; Pérez, E.; Reyes-Zurita, F.J.; et al. Autophagy in Hematological Malignancies. Cancers 2022, 14, 5072. [Google Scholar] [CrossRef]
- Jia, L.; Gopinathan, G.; Sukumar, J.T.; Gribben, J.G. Blocking Autophagy Prevents Bortezomib-Induced NF-κB Activation by Reducing I-κBα Degradation in Lymphoma Cells. PLoS ONE 2012, 7, e32584. [Google Scholar] [CrossRef]
- Ortona, E.; Locatelli, S.L.; Pagano, M.T.; Ascione, B.; Careddu, G.; Dupuis, M.L.; Marconi, M.; Carlo-Stella, C.; Malorni, W.; Matarrese, P.; et al. The Natural Estrogen Receptor Beta Agonist Silibinin as a Promising Therapeutic Tool in Diffuse Large B-cell Lymphoma. Anticancer. Res. 2022, 42, 767–779. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261, Correction in Science 2022, 376, eabq4855. [Google Scholar] [CrossRef]
- Springer, C.; Humayun, D.; Skouta, R. Cuproptosis: Unraveling the Mechanisms of Copper-Induced Cell Death and Its Implication in Cancer Therapy. Cancers 2024, 16, 647. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Z.; Wang, J.; Zhao, H. Cuproptosis: Unveiling a new frontier in cancer biology and therapeutics. Cell Commun. Signal. 2024, 22, 249. [Google Scholar] [CrossRef]
- Cong, Y.; Li, N.; Zhang, Z.; Shang, Y.; Zhao, H. Cuproptosis: Molecular mechanisms, cancer prognosis, and therapeutic applications. J. Transl. Med. 2025, 23, 104. [Google Scholar] [CrossRef]
- Xie, J.; Shao, Z.; Li, C.; Zeng, C.; Xu, B. Cuproptosis-related gene ATOX1 promotes MAPK signaling and diffuse large B-cell lymphoma proliferation via modulating copper transport. Biomol. Biomed. 2024, 25, 16–28. [Google Scholar] [CrossRef]
- Ouyang, W.; Lai, Z.; Huang, H.; Ling, L. Machine learning-based identification of cuproptosis-related lncRNA biomarkers in diffuse large B-cell lymphoma. Cell Biol. Toxicol. 2025, 41, 72. [Google Scholar] [CrossRef]
- Lei, L.; Tan, L.; Sui, L. A novel cuproptosis-related gene signature for predicting prognosis in cervical cancer. Front. Genet. 2022, 13, 957744. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y. Cuproptosis-related gene subtypes predict prognosis in patients with head and neck squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2023, 52, 58. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Zheng, Z.; Lin, Z.; Wang, Q.; Zheng, D.; Chen, Z.; Ma, Y. Development and validation of a cuproptosis-associated prognostic model for diffuse large B-cell lymphoma. Front. Oncol. 2022, 12, 1020566. [Google Scholar] [CrossRef]
- Fu, L.; Jiali, L.; Rao, J.; Lin, S.; Dong, S.; Xiang, X.; Gao, L.; Zhang, X. P1191: A NOVEL PROGNOSTIC MODEL OF DLBCL PATIENTS BASED ON CUPROPTOSIS RELATED GENES. HemaSphere 2023, 7, e4495877. [Google Scholar] [CrossRef]
- Wang, N.; Shi, S.; Li, M.; Yu, X.; Ma, G. Development and validation of a combined cuproptosis and immunogenic cell death prognostic model for diffuse large B-cell lymphoma. Aging 2024, 16, 1218–1236. [Google Scholar] [CrossRef]
- Bai, X.; Lu, F.; Li, S.; Zhao, Z.; Wang, N.; Zhao, Y.; Ma, G.; Zhang, F.; Su, X.; Wang, D.; et al. Cuproptosis-related lncRNA signature as a prognostic tool and therapeutic target in diffuse large B cell lymphoma. Sci. Rep. 2024, 14, 12926. [Google Scholar] [CrossRef]
- Bielsa, N.; Casasampere, M.; Abad, J.L.; Enrich, C.; Delgado, A.; Fabriàs, G.; Lizcano, J.M.; Casas, J. Methuosis Contributes to Jaspine-B-Induced Cell Death. Int. J. Mol. Sci. 2022, 23, 7257. [Google Scholar] [CrossRef]
- Lu, Z.; Feng, L.; Chen, Q.; Xu, B. An Azaindole-Based Small Molecule Hzx-02-059 Induces Methuosis in B-Cell Acute Lymphoblastic Leukemia through the PI3K/AKT Axis. Blood 2020, 136, 9. [Google Scholar] [CrossRef]
- He, J.; Wang, L.; Mi, D.; Guan, T.; Liu, W.; He, P.; Gu, H.; Li, Y.; Peng, Y.; Jia, A.-Q.; et al. Discovery of Pyrimidinediamine Derivatives as Potent Methuosis Inducers for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2023, 66, 7421–7437. [Google Scholar] [CrossRef]
- Feng, L.; Chen, K.; Huang, W.; Jiang, Y.; Sun, X.; Zhou, Y.; Li, L.; Li, Y.; Deng, X.; Xu, B. Pharmacological targeting PIKfyve and tubulin as an effective treatment strategy for double-hit lymphoma. Cell Death Discov. 2022, 8, 39. [Google Scholar] [CrossRef]
- Riedell, P.A.; Smith, S.M. Double hit and double expressors in lymphoma: Definition and treatment. Cancer 2018, 124, 4622–4632. [Google Scholar] [CrossRef]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Petrichuk, S.V.; Kopeina, G.S.; Zhivotovsky, B. A link between mitotic defects and mitotic catastrophe: Detection and cell fate. Biol. Direct 2021, 16, 25. [Google Scholar] [CrossRef]
- Gu, J.J.; Kaufman, G.P.; Mavis, C.; Czuczman, M.S.; Hernandez-Ilizaliturri, F.J. Mitotic catastrophe and cell cycle arrest are alternative cell death pathways executed by bortezomib in rituximab resistant B-cell lymphoma cells. Oncotarget 2017, 8, 12741–12753. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.; Hong, D.; Zeng, M.; Zhang, X. The Paradoxical Role of Cellular Senescence in Cancer. Front. Cell Dev. Biol. 2021, 9, 722205, Correction in Front. Cell Dev. Biol. 2021, 9, 759761. [Google Scholar] [CrossRef]
- Nguyen, T.; Parker, R.; Hawkins, E.; Holkova, B.; Yazbeck, V.; Kolluri, A.; Kmieciak, M.; Rahmani, M.; Grant, S. Synergistic interactions between PLK1 and HDAC inhibitors in non-Hodgkin’s lymphoma cells occur in vitro and in vivo and proceed through multiple mechanisms. Oncotarget 2017, 8, 31478–31493, Correction in Oncotarget 2019, 10, 5383–5384. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Liu, J.; Ren, S.; Cai, Y.; Hu, S.; Zhou, X.; Wang, X. Cfi-400945 Induces Cytokinesis Failure By Activating Hippo Signaling Pathway in Diffuse Large B-Cell Lymphoma. Blood 2020, 136, 19–20. [Google Scholar] [CrossRef]
- Norberg, E.; Lako, A.; Chen, P.H.; Stanley, I.A.; Zhou, F.; Ficarro, S.B.; Chapuy, B.; Chen, L.; Rodig, S.; Shin, D.; et al. Differential contribution of the mitochondrial translation pathway to the survival of diffuse large B-cell lymphoma subsets. Cell Death Differ. 2017, 24, 251–262. [Google Scholar] [CrossRef]
- Białopiotrowicz-Data, E.; Noyszewska-Kania, M.; Jabłońska, E.; Sewastianik, T.; Komar, D.; Dębek, S.; Garbicz, F.; Wojtas, M.; Szydłowski, M.; Polak, A.; et al. SIRT1 and HSP90α feed-forward circuit safeguards chromosome segregation integrity in diffuse large B cell lymphomas. Cell Death Dis. 2023, 14, 667. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Mishra, A.K.; Silva, A.; Ren, W.; Pan, Z.; Wang, J.H. Chemotherapy-induced differential cell cycle arrest in B-cell lymphomas affects their sensitivity to Wee1 inhibition. Haematologica 2018, 103, 466–476. [Google Scholar] [CrossRef]
- Falgàs, A.; Pallarès, V.; Unzueta, U.; Núñez, Y.; Sierra, J.; Gallardo, A.; Alba-Castellón, L.; Mangues, M.A.; Álamo, P.; Villaverde, A.; et al. Specific Cytotoxic Effect of an Auristatin Nanoconjugate Towards CXCR4(+) Diffuse Large B-Cell Lymphoma Cells. Int. J. Nanomed. 2021, 16, 1869–1888. [Google Scholar] [CrossRef]
- Wang, Y.; Tsukamoto, Y.; Hori, M.; Iha, H. Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL). Int. J. Mol. Sci. 2024, 25, 7156. [Google Scholar] [CrossRef]
- He, T.; Geng, J.; Hou, C.; Li, H.; Zhang, H.; Zhao, P.; He, P.; Lu, X. Predictive biomarkers and molecular subtypes in DLBCL: Insights from PCD gene expression and machine learning. Discov. Oncol. 2025, 16, 542. [Google Scholar] [CrossRef]
- Guan, M.; Zhao, H.; Zhang, Q.; Li, L.; Wang, X.; Tang, B. A novel anoikis-related signature predicts prognosis risk and treatment responsiveness in diffuse large B-cell lymphoma. Expert. Rev. Mol. Diagn 2024, 24, 439–457. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Zhang, J.; Zhou, W.; Ma, J.; Zhao, X. Targeting PARP1 As an Effective Treatment for Diffuse Large B-Cell Lymphoma with High Level of γH2AX. Blood 2018, 132, 5799. [Google Scholar] [CrossRef]
- Xu, X.; Sun, B.; Zhao, C. Poly (ADP-Ribose) polymerase 1 and parthanatos in neurological diseases: From pathogenesis to therapeutic opportunities. Neurobiol. Dis. 2023, 187, 106314. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. Mechanisms of alkaliptosis. Front. Cell Dev. Biol. 2023, 11, 1213995. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, Y.; Xue, L.; Zhang, J.; Qiao, Y.; Jin, Q.; Li, H. Expression of Keap1 and Nrf2 in diffuse large B-cell lymphoma and its clinical significance. Exp. Ther. Med. 2018, 16, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jin, W.; Tong, L.; Lin, N.; Zhang, L.; Zhao, J. Therapeutic strategies of targeting non-apoptotic regulated cell death (RCD) with small-molecule compounds in cancer. Acta Pharm. Sin. B 2024, 14, 2815–2853. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Xiang, X.; Long, X.; Chen, Z.; Huang, X.; Yang, J.; Li, W. Cuproptosis correlates with immunosuppressive tumor microenvironment based on pan-cancer multiomics and single-cell sequencing analysis. Mol. Cancer 2023, 22, 59. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.