Abstract
Vascular calcification (VC) is a pathological process involving the deposition of mineral salts within the vascular wall, representing a significant risk factor for the development and progression of cardiovascular disease. The gut microbiota refers to the diverse microbial ecosystem inhabiting the gastrointestinal tract, including bacteria, fungi, viruses, and other microorganisms. This community exhibits considerable variability in both population density and taxonomic composition, with current estimates indicating approximately 1013–1014 microorganisms residing in the human gut. Recent studies suggest that metabolites produced by the gut microbiota may influence the pathogenesis of VC through the gut–vascular axis. This review consolidates current findings on the molecular mechanisms driving VC and examines the potential contribution of gut microbiota dysbiosis to vascular pathology. Particular attention is given to the functional roles of microbial metabolites such as short-chain fatty acids (SCFAs), trimethylamine N-oxide (TMAO), lipopolysaccharide (LPS), uremic toxins, secondary bile acids, and vitamin K in modulating calcific processes. In addition, current limitations in the existing literature are outlined, and potential therapeutic approaches, including probiotic use, prebiotic interventions, and targeted dietary strategies, are discussed in the context of their relevance for future clinical management of VC.
1. Introduction
Vascular calcification (VC), defined by the pathological accumulation of calcium ions and hydroxyapatite within the arterial wall [1], is closely associated with aging and smoking. It frequently accompanies cardiovascular disorders, including atherosclerosis, hypertension, diabetes mellitus, and chronic kidney disease (CKD)-associated vasculopathy [2,3]. Both thoracic aortic calcification and coronary artery calcification exhibit age-dependent prevalence patterns, posing significant health concerns for middle-aged and older populations [4]. Despite its clinical relevance, current medical practice lacks effective therapeutic strategies capable of preventing or reversing VC, partly due to the unavailability of definitive diagnostic biomarkers [5]. As such, elucidating the underlying mechanisms and identifying viable treatment approaches remain critical priorities.
The human microbiome, often regarded as a “second genome,” significantly influences host physiological processes. It encompasses diverse microbial taxa, including Actinobacteria, Bacteroidetes, Firmicutes, Clostridium, and Proteus, which inhabit various body sites such as the gastrointestinal tract, skin, oral cavity, and nasal passages [6]. Increasing evidence indicates that the gut microbiota plays an important role in modulating health and disease states, including vascular conditions [7]. VC is no exception. Recent findings suggest that gut microbiota and its metabolic products contribute to the development and progression of VC. However, most existing research has emphasized alterations in microbial composition among individuals with VC or examined the effects of individual gut-derived metabolites on calcification. A comprehensive account of the mechanisms by which gut microbiota influences VC remains lacking. This review aims to summarize how gut microbiota and key metabolites affect VC, assess evidence strength, and suggest research and clinical priorities.
2. Vascular Calcification
2.1. Classification and Cellular Mechanisms Involved in VC
Arterial calcification is generally categorized into two forms: medial calcification and intimal calcification. Medial calcification, commonly associated with CKD, is characterized by widespread mineral deposition along the elastic lamina of the arterial media, resulting in reduced vascular elasticity [8]. This pathological stiffening contributes to decreased diastolic blood pressure, increased systolic blood pressure and pulse pressure, and elevated cardiac afterload, thereby exacerbating cardiovascular complications [9]. In contrast, intimal calcification frequently occurs in advanced stages of type 2 diabetes mellitus (T2DM) [10]. As a shared pathophysiological mechanism in cardiovascular and metabolic diseases, arterial calcification in these contexts is closely linked to metabolic disturbances, including dysregulation of glucose and lipid metabolism, alongside inflammatory responses and impaired autophagy [11].
Among the key cellular contributors to VC are vascular smooth muscle cells (VSMCs), which undergo pathological transformation under mineralizing conditions. This includes endothelial-to-mesenchymal transition and phenotypic switching toward an osteoblast-like profile, thereby promoting calcific deposition [12]. These cells upregulate transcription factors involved in osteogenesis and chondrogenesis, facilitating extracellular matrix mineralization. Concurrently, contractile protein expression is diminished, accelerating the calcification process [13].
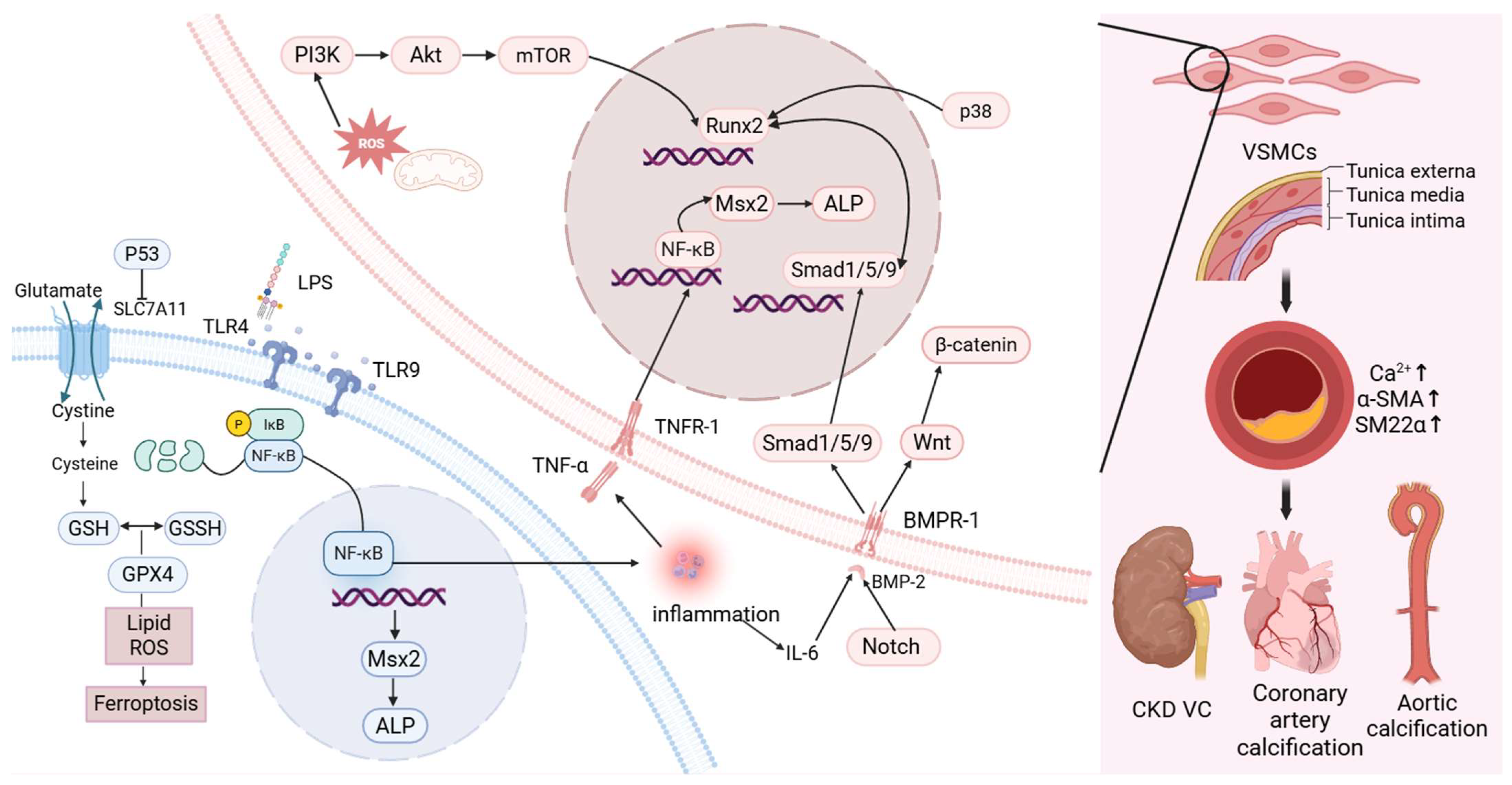
Calcified VSMCs exhibit elevated expression of pro-calcific mediators such as bone morphogenetic protein 2 (BMP-2), Runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), osteopontin, osteocalcin (OCN), receptor activator of nuclear factor kappa-Β ligand, and Msh homeobox 2 (MSX2) [14]. This is accompanied by a marked reduction in smooth muscle markers, including α-smooth muscle actin (α-SMA) and SM22α [15]. Additionally, the calcification process is modulated by inhibitors such as pyrophosphate and matrix Gla protein (MGP) [16].
BMP-2, a member of the TGF-β superfamily, is the most significant regulatory factor involved in the physiological ossification and repair of bones, osteogenic differentiation and osteoblast proliferation [17]. Runx2, formerly designated as Cbfa1/Pebp2αA/AML3, is essential for osteoblast lineage commitment [18], It also exerts transcriptional control over targets such as X-C motif chemokine receptor 1, further supporting osteogenic differentiation [19]. BMP-2 transmits its signal via BMPR-I, activating Smad1 and Smad5 as intracellular effectors [20,21]. This signaling cascade inhibits VSMC phenotypic conversion, thereby mitigating VC progression [22]. Studies of aortic valve calcification have shown that Smad1/5 activation may also involve the bidirectional regulation of p38 signaling [23]. Conversely, other findings suggest that p38 and JNK pathways may promote the expression of osteogenic genes such as Runx2 [22]. Runx2 functions as a key downstream effector of the BMP pathway, with Smad proteins directly interacting with the Runx2 transcriptional complex [24].
VC is closely associated with chronic, nonspecific inflammation. Interleukin (IL)-6 has been shown to regulate the BMP-2–Wnt/β-catenin signaling cascade, thereby promoting VC [25]. This regulation may be related to the subsequent activation of Runx2 [26]. A study has shown that β-catenin increases the mRNA and protein levels of Runx2 [27]. TNF-α, primarily secreted by macrophages, directly induces MSX2 expression via the NF-κB pathway, which subsequently increases the expression of ALP—a key enzyme involved in mineralization in VSMCs—thus contributing to VC [28,29]. In addition, NF-κB signaling plays a central role in the upregulation of calcifying mediators, including BMP-2 and Runx2 [30]. IL-18 promotes calcification and osteogenic differentiation of VSMCs through activation of the transient receptor potential melastatin 7 channel [31]. IL-1β acts as a major driver of calcium deposition in VSMCs by initiating osteogenic transcriptional programs. Elevated IL-1β expression has been observed in calcified coronary arteries of patients [32]. Interestingly, activation of Smad2/3 and Smad4 has been reported to inhibit the production of pro-inflammatory cytokines such as TNF-α while enhancing the expression of anti-inflammatory cytokines such as IL-10 [33].
The Notch signaling pathway, comprising a family of highly conserved cell surface receptors encoded by the Notch gene, regulates several processes in normal cell morphogenesis, including differentiation of pluripotent progenitor cells, apoptosis, and proliferation [34]. Studies have shown that lipoprotein(a) [Lp(a)] induces VC through both the Notch1–NF-κB and Notch1–BMP2–Smad1/5/9 pathways. NF-κB silencing attenuates Lp(a)-induced VC [35]. Moreover, miR-34a promotes aortic calcification by upregulating Runx2 via Notch1 [36], indicating that the Notch signaling pathway may serve as a regulatory target in VC.
Reactive oxygen species (ROS) are partially reduced oxygen derivatives generated in biological systems. These include oxygen radicals such as superoxide anion (O2·−) and hydroxyl radicals (·OH), as well as non-radical oxidants such as hydrogen peroxide (H2O2) and singlet oxygen (1O2). Excessive oxidative or reductive stress resulting from ROS imbalance can cause severe cellular injury and inappropriate cell death, potentially leading to organ dysfunction or systemic failure [37]. Mitochondrial dysfunction contributes to elevated ROS production and inflammation, which may induce osteogenic transdifferentiation and apoptosis of VSMCs, thereby promoting VC [38]. Additional studies suggest that ROS activate Runx2 via the PI3K/AKT/mTOR signaling pathway, further contributing to vascular mineralization [14].
Inflammatory responses associated with TGF-β1 signaling also influence calcification, particularly in CKD models and cultured human aortic smooth muscle cells (HASMCs) maintained in osteogenic medium. Upon TGF-β1 binding to its receptor TGFBR1, Smad2/3 becomes phosphorylated, facilitating its association with Smad4. This complex plays a central role in downstream signal transduction. Downregulation of Smad4 has been linked to the development of inflammation-related vascular pathology [33].
Ferroptosis represents a distinct form of regulated cell death, separate from apoptosis, necrosis, and autophagy [39]. It is primarily characterized by extensive lipid peroxidation of cellular membranes, which results in toxic ROS accumulation [40,41]. Seto et al. reported that iron supplementation attenuates VC by suppressing the osteogenic transdifferentiation of phosphate transporters and VSMCs [42]. In CKD contexts, inhibition of the SLC7A11/GSH/GPX4 axis promotes ferroptosis in VSMCs, thereby facilitating VC [43]. Moreover, suppression of p53 in VSMCs leads to reduced SLC7A11 expression, diminished glutathione biosynthesis, increased ROS deposition, and ferroptotic cell death, all contributing to calcification [44].
Collectively, these findings indicate that inflammatory mediators, such as IL-1β, NF-κB, IL-6, and TNF-α, as well as signaling cascades involving ROS and ferroptosis, are integral to the pathogenesis of VC. The underlying mechanisms are multifactorial and highly interconnected.
2.2. Intracranial Artery Calcification, Carotid Artery Calcification and Vertebral Artery Calcification
Intracranial artery calcification (IAC), a hallmark radiographic feature of intracranial arteriosclerosis on CT scans, is an established risk factor for stroke [45] and dementia [46]. Current studies have shown that there are three patterns of intracranial arterial calcification, including intimal calcification, medial calcifications (characterized by mineralization of the internal elastic lamina) [47], and adventitial calcification. These calcification types demonstrate differential clinical manifestations. Intimal calcification is an atherosclerotic lesion, usually related to arterial stenosis. In contrast, autopsy findings reveal that medial calcification in the intracranial internal carotid artery (iICA) exhibits histologically non-atherosclerotic characteristics, associated with arterial stiffening, diminished arterial compliance and limited distensibility [48]. This can result in an increase in pulse wave velocity and pulse pressure, subsequently leading to chronic damage to brain tissues [49]. This distinct pathophysiology underlies both the slower progression of cerebral artery calcification compared to coronary counterparts and a temporal dissociation spanning approximately two decades between the peak incidence of cardiovascular diseases (such as coronary heart disease and myocardial infarction) and cerebrovascular diseases (often stroke).
Asymptomatic mineralization of carotid and vertebral arteries and their intracranial continuations is a common incidental discovery on CT imaging, reflecting distinct pathophysiological processes from coronary calcification [50]. In pediatric period, internal carotid artery (ICA) calcification predominantly localizes to the supraclinoid segment and carotid siphon as non-atherosclerotic internal elastic lamina deposits, while vertebral artery calcification preferentially involves the V4 segment [51]. In adults, both ICA siphon and vertebral artery calcification volumes increase with age, with the highest prevalence in Asian populations [52].
2.3. Related Manifestations and Specific Molecular Mechanisms of VC
From a macroscopic perspective, the development of VC is complex, with VSMCs undergoing osteogenic differentiation under conditions of mineral imbalance and various external stimuli. At the molecular level, the NF-κB signaling pathway plays a central role in VC pathogenesis. It facilitates the activation of several downstream calcification pathways, most notably the BMP2–Smad1/5–Runx2 axis and the NF-κB–Msx2–ALP cascade, by stimulating the release of pro-inflammatory cytokines. Additionally, ferroptosis-related mechanisms and ROS-induced cellular injury also contribute to the progression of VC. While detailed exploration of these molecular interactions falls beyond the scope of this review, the present work focuses on assessing whether gut microbiota-derived metabolites can modulate VC through these or related mechanisms (Figure 1).
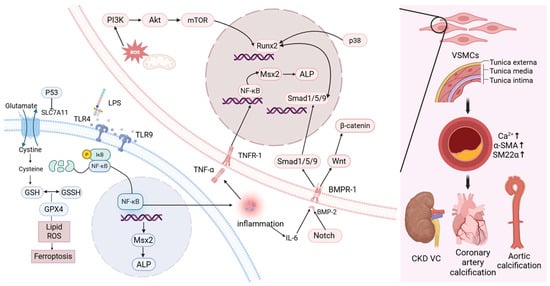
Figure 1.
The related manifestations of VC and the corresponding cellular and molecular pathways. (Created in BioRender. c, X. (2026) https://BioRender.com/yae1im8).
3. The Changes in the Intestinal Flora of Patients with Various Types of Vascular Calcification
VC is recognized as a major contributor to cardiovascular morbidity and mortality [53]. It is highly prevalent in patients with CKD and is frequently observed as a complication in those with end-stage renal disease [54,55]. Studies have demonstrated that the gut microbiota of individuals with VC, particularly VC secondary to CKD, differs markedly from that of healthy controls. Hao et al. reported a significant increase in the abundance of Prevotella copri in CKD rats with aortic calcification. Administration of P. copri to the aortas of CKD rats led to reduced expression of the contractile marker α-SMA, alongside increased expression of osteogenic markers Runx2 and BMP2 [56], suggesting its involvement in promoting VC.
In advanced CKD, dialysis is routinely employed to maintain physiological stability. Bao et al. examined the relationship between gut microbiota and VC in hemodialysis (HD) patients using abdominal aortic calcification (AAC) scores. Their findings indicated that patients with higher AAC scores exhibited significantly reduced overall fecal microbial diversity. In this group, Proteobacteria were more abundant, while Bacteroidetes and Firmicutes were notably less represented. At the genus level, Escherichia-Shigella, Ruminococcus gnavus group, and Lactobacillus were enriched in patients with high AAC scores. Conversely, Ruminococcus and Lachnospiraceae_NK4A136_group were more prevalent in individuals with low AAC scores. Among these taxa, Escherichia-Shigella had the greatest influence on VC progression in HD patients [57]. Merino-Ribas et al. conducted a study on CKD patients undergoing peritoneal dialysis (PD) and observed distinct microbial signatures associated with VC. In patients with VC, there was an increased abundance of Cutibacterium, Pajaroellobacter, Devosia, and Hyphomicrobium, while Pelomonas was relatively depleted. Changes were also noted in the abundance of Coprobacter, Coprococcus 3, Lactobacillus, and Eubacterium eligens group [58].
Yu et al. reported that Adlercreutzia and Alistipes were negatively correlated with VC, with the increased abundance of Adlercreutzia potentially linked to enhanced production of SCFAs [59]. Liu et al. examined differences in the gut microbiome of patients with high and low aortic arch calcification (AoAC) scores [60], noting distinct microbial community structures among those with lower scores. Specifically, greater abundance was observed for Agathobacter, Eubacterium coprostanoligenes group, Ruminococcaceae UCG-002, Barnesiella, Butyricimonas, Oscillibacter, Ruminococcaceae DTU089, and Oxalobacter. By contrast, the relative abundance of Bacilli was elevated in the high AoAC group [61].
In a study of Japanese men, Okami et al. investigated gut microbiota and CAC. They found that the Firmicutes-to-Bacteroidetes ratio, as well as the relative abundance of Lactobacillus, positively correlated with CAC scores and the presence of CAD. Furthermore, a positive association was identified between Enterobacteriaceae and CAC, while the genus Blautia appeared protective against CAD but showed no significant association with CAC progression [62]. Sun et al. further observed that increased abundance of Acinetobacter species in the bloodstream correlated positively with calcification of the kidneys and thoracic aorta, promoting Ca2+ precipitation in VSMCs [63]. Similarly, Yan et al. demonstrated, through metabolomic analysis, that compared to controls, patients with VC exhibited reduced Firmicutes-to-Bacteroidetes ratios, alongside decreased abundance of Firmicutes, Muribaculaceae, and Alloprevotella. Conversely, Bacteroidetes, Proteobacteria, Bifidobacterium, and Escherichia-Shigella were significantly enriched in the VC group [64]. The summary of gut microbiota changes associated with VC is shown in Table 1.

Table 1.
Alterations in gut microbiota associated with vascular calcification.
Collectively, these studies demonstrate a correlation between microbial alterations and VC. However, whether such changes represent causal factors or secondary phenomena remains unresolved. Moreover, microbial patterns associated with VC vary across species and treatment conditions. These inconsistencies may reflect the inherent diversity of microbial communities, differences in experimental approaches, or species-specific responses. Larger cohort-based investigations are required to clarify the causal relationship between gut microbiota and VC.
4. The Role of Gut Microbiota and Its Metabolites in the Development of Vascular Calcification
4.1. Short-Chain Fatty Acids
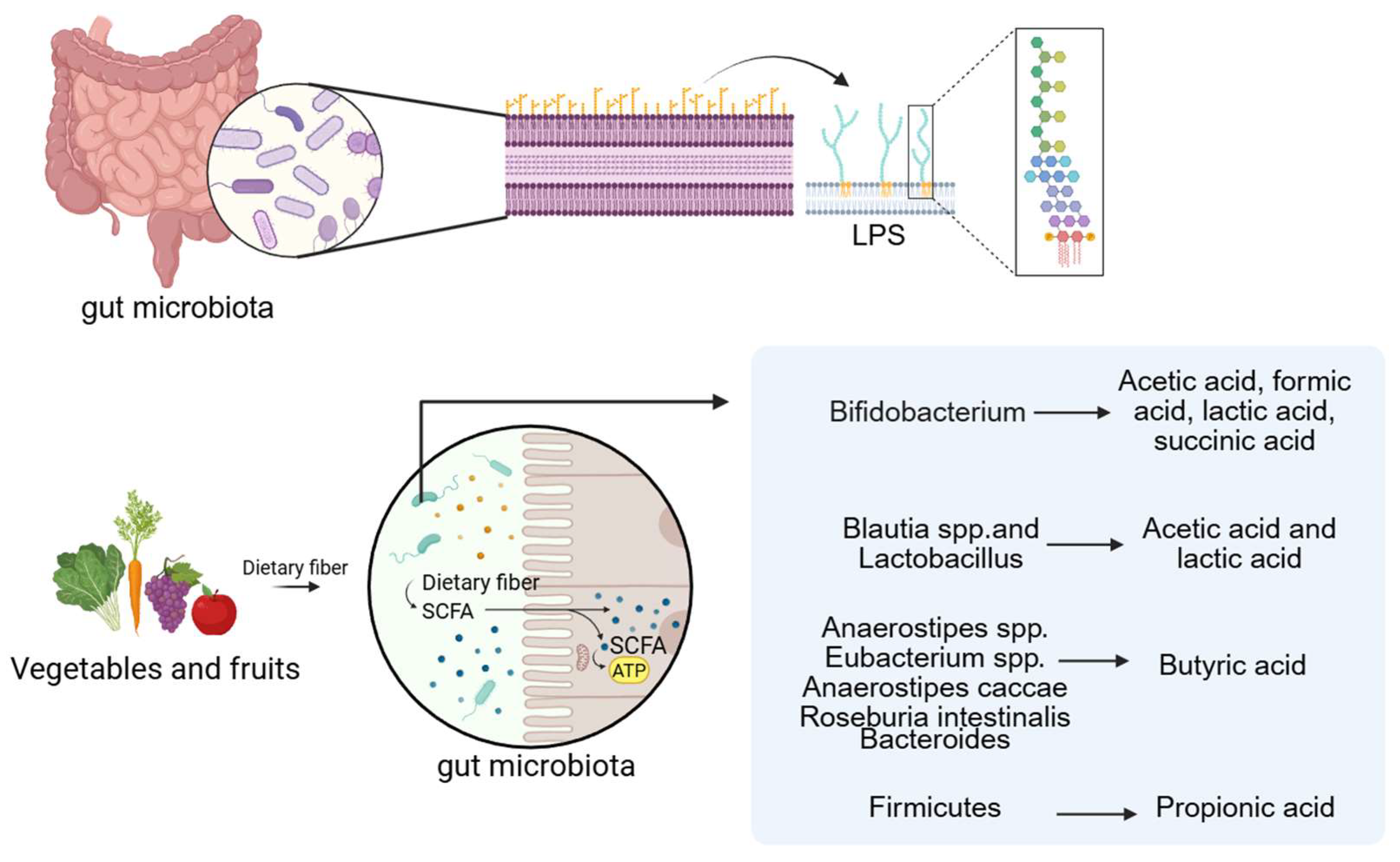
Fatty acids are generally classified into short-chain, medium-chain, and long-chain forms based on carbon chain length. Among these, acetic acid, propionic acid, and butyric acid are common Short-Chain Fatty Acids (SCFAs). They are the end products of dietary fiber fermentation by gut microbiota under anaerobic conditions and are typically present at high concentrations in the proximal colon [65,66]. A wide range of gut microbes participate in SCFA production, with members of the Bacteroidetes and Firmicutes phyla serving as primary contributors [67]. For example, Bifidobacterium species produce acetic acid, formic acid, lactic acid, and succinic acid; Blautia and Lactobacillus species generate acetic and lactic acid; and butyrate can be derived from Anaerostipes, Eubacterium, Anaerostipes caccae, and Roseburia intestinalis [68]. Propionate is primarily synthesized by Bacteroidetes via the succinate pathway, while butyrate production in Firmicutes occurs mainly through the acetyl-CoA pathway. Ruminococcus, a Gram-positive anaerobe belonging to the Firmicutes phylum, ferments polysaccharides to yield SCFAs [69,70,71]. The Bacteroidetes phylum also contributes to SCFA biosynthesis through metabolic transformations involving the tricarboxylic acid cycle, pyruvate metabolism, and starch and sucrose utilization. Consequently, reduced Bacteroidetes abundance may impair SCFA production [72,73]. Other SCFA-producing taxa include Butyricimonas [74] and Bani [75] are also intestinal microorganisms that produce SCFAs.
SCFAs contribute to intestinal barrier integrity, regulate glucose and lipid metabolism, modulate immune and inflammatory responses, and influence blood pressure homeostasis. Increased SCFA availability is therefore considered beneficial for cardiovascular and metabolic health [76]. Yan et al. reported that Muribaculaceae and Akkermansia showed positive correlations with SCFA levels in plasma and feces, whereas Escherichia-Shigella, Bilophila, and Parasutterella exhibited negative correlations. Propionate has been shown to attenuate VC in rats with vitamin D3 plus nicotine -induced disease by reducing inflammatory activity and protecting the intestinal mucosa through enhanced SCFAs generation. In this context, Akkermansia played a key role in fostering beneficial microbial populations that promote SCFA synthesis [64]. Moreover, acetic acid, propionic acid, and butyric acid can activate host receptors, suppress pro-inflammatory mediators such as TNF-α, IL-4, IL-5, IL-17a, and IL-6, and thereby mitigate VC [64,77].
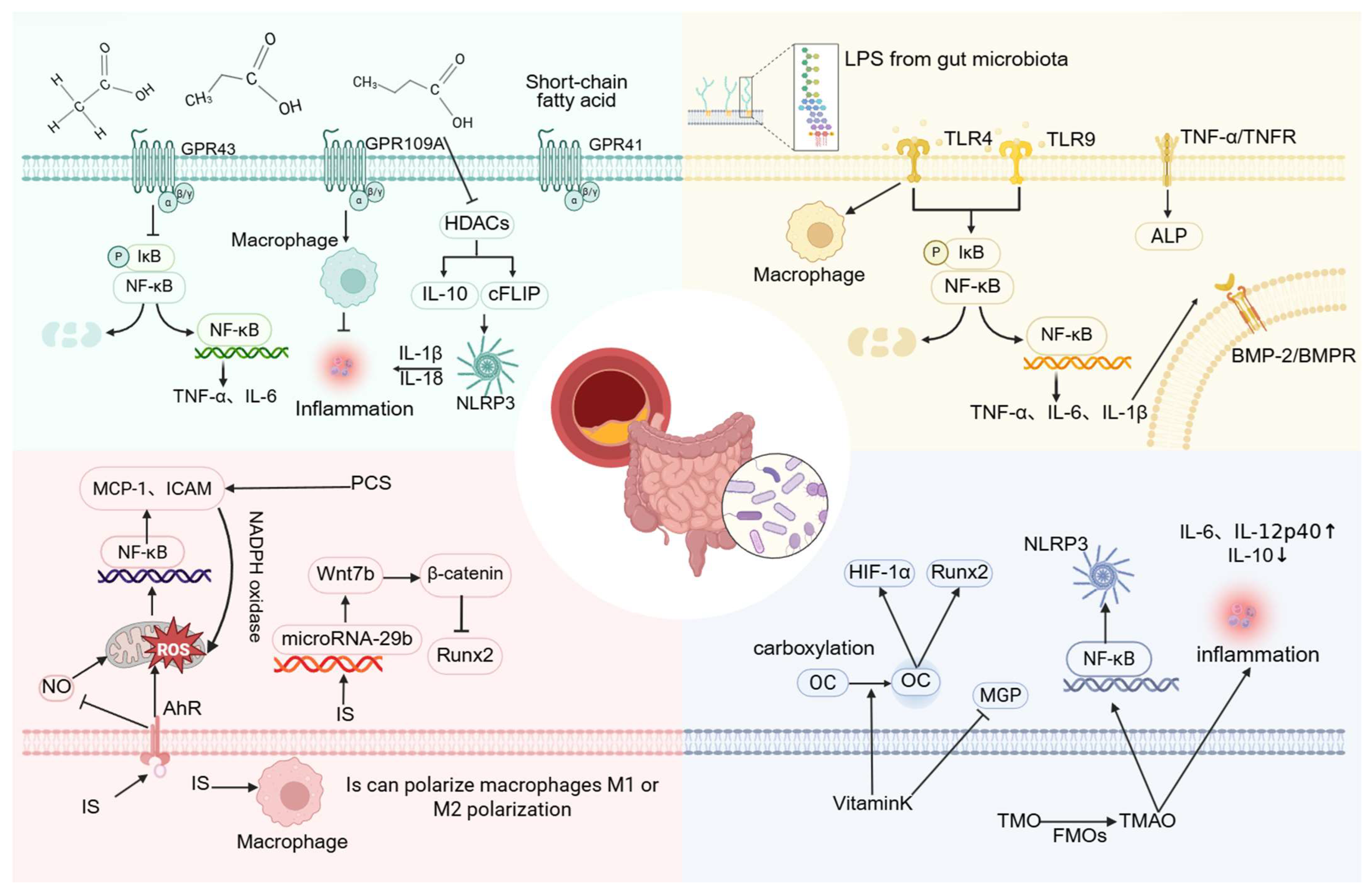
Studies have confirmed that acetic acid, propionic acid, and butyric acid mediate signal transduction through surface-expressed free fatty acid receptors or via G protein-coupled receptors (GPCRs) such as GPR41, GPR43, and GPR109A, subsequently activating downstream signaling cascades. Both GPR41 and GPR43 respond to acetate, propionate, and butyrate, whereas GPR109A is primarily activated by butyrate [68]. Current research on SCFAs has largely emphasized their capacity to regulate inflammatory processes, which may underlie their protective role against VC. Zeng et al. reported that antibiotic treatment can enhance VC by reducing serum SCFA concentrations. They further demonstrated that acetate derived from gut microbiota attenuates VC [78], likely through inhibition of VSMCs osteogenic transformation. This mechanism may involve GPR43 signaling, which suppresses the NF-κB pathway and decreases the release of TNF-α and IL-6 [79]. In intestinal epithelial cells, GPR43 can also activate the NLRP3 inflammasome and increase IL-18 production [80]. Whether VSMCs employ a comparable mechanism remains to be established.
SCFAs additionally interact with GPR109A, modulating macrophage activity and suppressing inflammatory responses [67]. Butyric acid and propionic acid inhibit histone deacetylase (HDAC) activity, thereby exerting anti-inflammatory effects on intestinal epithelial cells, macrophages, and dendritic cells [66]. Their influence on bone metabolism may be mediated through regulatory T cells (Tregs), which are known to suppress osteoclastogenesis and enhance bone mass [81]. Regulation of Tregs by SCFAs may therefore represent another pathway through which these metabolites influence VC development. Notably, SCFAs may also exert pro-inflammatory effects under certain conditions. Studies indicate that when activated via Toll-like receptors (TLRs), SCFAs can regulate inflammation by inhibiting HDAC activity, reducing IL-10 expression, and downregulating the cell-type Fas-associated death domain-like IL-1β converting enzyme inhibitor protein. These changes promote NLRP3 inflammasome activation and IL-1β release, thereby intensifying inflammatory responses [82] and potentially accelerating VC.
4.2. Lipopolysaccharide
Lipopolysaccharide (LPS), the major pro-inflammatory component of the Gram-negative bacterial cell envelope, is recognized by TLRs, thereby initiating downstream signaling that promotes pro-inflammatory cytokine synthesis [83]. This inflammatory response may be further amplified in the context of VC. For example, LPS derived from P. copri binds to TLR4 and markedly induces osteogenic differentiation and calcification of VSMCs by activating the NF-κB pathway, concomitant with increased expression of TNF-α, IL-1β, and IL-6 [56].
Su et al. reported that oxidized low-density lipoprotein enhances BMP-2 expression in human coronary artery endothelial cells through both TLR2 and TLR4. This effect involves ERK1/2 and NF-κB signaling cascades [84]. Similarly, Zhao et al. demonstrated that LPS activates TLR4 and TLR9, promoting calcification and osteoblast differentiation in HASMCs. Inhibition of the TLR9/NF-κB/BMP-2 axis was shown to attenuate VC associated with chronic renal failure [85], These findings suggest that NF-κB-mediated regulation of BMP-2 expression may occur through pro-inflammatory mediators such as IL-6 and TNF-α. Notably, TNF-α itself is a potent stimulator of vascular cell mineralization [86]. In conditioned medium, LPS activates monocytes and macrophages through TNF-α, which in turn enhances ALP activity and osteogenic differentiation of calcifying vascular cells [87].
Taken together, these observations indicate that LPS contributes to VC by activating TLR signaling, engaging the NF-κB pathway, and inducing pro-inflammatory cytokine production. Additionally, LPS promotes TNF-α-mediated upregulation of ALP, thereby intensifying VC.
Of further significance, the macrophage murine cell line RAW 264.7 treated with lipopolysaccharide (LPS-EK) derived from Proteobacteria species such as Escherichia coli, Vibrio cholerae, and Helicobacter pylori reduces macrophage-derived extracellular vesicles (EVs). EVs exposed to LPS-EK are enriched in pro-inflammatory cytokines as well as CAD-related proteins, including plasminogen activator inhibitor-1 (PAI-1) and serum amyloid A-3 protein (SAA3). These vesicles activate TLR4, with SAA3 specifically inducing pro-inflammatory cytokine release and osteogenic differentiation of mesenchymal stem cells via TLR4 signaling [88]. This mechanism may represent another pathway by which LPS contributes to VC (Figure 2).
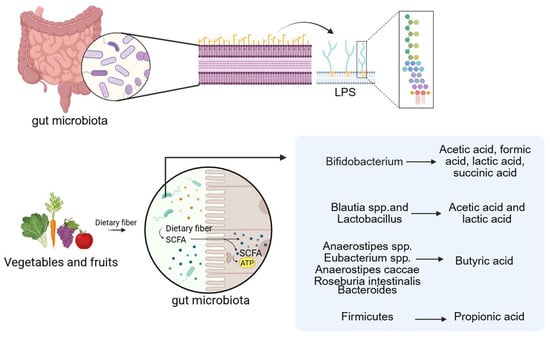
Figure 2.
LPS and SCFAs are the related metabolites of the gut microbiota. (Created in BioRender. c, X. (2026) https://BioRender.com/wqxcil4).
4.3. Uremic Toxin from End-Stage Renal Disease
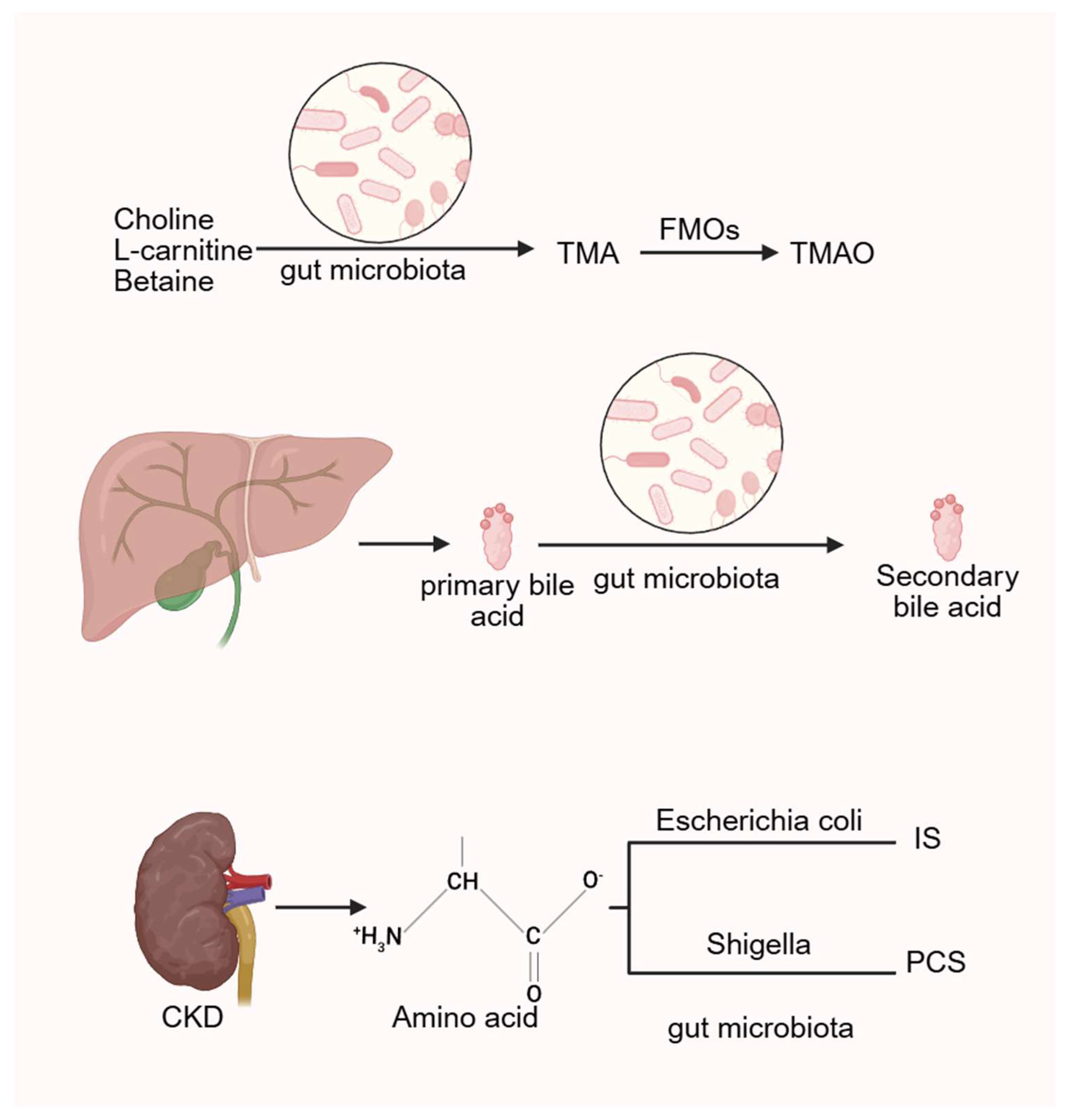
CKD is associated with intestinal dysbiosis and impaired nutrient absorption, leading to increased production of uremic toxins derived from microbial metabolism. These include trimethylamine N-oxide (TMAO), p-cresol sulfate (PCS), indoxyl sulfate (IS), and indole-3-acetic acid (3-IAA). Such toxins elevate oxidative stress and sustain chronic systemic inflammation, thereby amplifying the risk of CKD-related cardiovascular disease (CVD) [89,90]. In CKD rat models, Eggerthella lenta and Fusobacterium nucleatum enhance the generation of uremic toxins and accelerate disease progression [91]. IS and PCS, both products of amino acid metabolism, involve intestinal Escherichia coli and Shigella, indicating that increased abundance of Escherichia-Shigella is linked to VC [57]. Among these toxins, IS plays a particularly important role in promoting VC. Barreto et al. demonstrated a positive correlation between serum IS, PCS, and VC in CKD patients [92]. Similarly, Opdebeeck et al. reported that chronic exposure of CKD rats to IS and PCS activated inflammatory and coagulation pathways, thereby accelerating VC progression [93].
The aryl hydrocarbon receptor (AhR), abundantly expressed in vascular tissues, is critically involved in vascular pathology [94]. During interactions between gut microbiota and host cells, tryptophan metabolites and SCFAs are generated, both of which can activate AhR [95]. AhR, an intracellular receptor originally identified for dioxins and dioxin-like compounds, also serves as a receptor for IS [96]. Experimental studies have shown that AhR agonists such as IS and hexachlorobenzene can induce hypertension and vascular dysfunction in mice [97,98]. IS-mediated AhR activation stimulates ROS production, contributing to oxidative stress, inflammation, and endothelial cell senescence [99,100,101]. This pathway also activates NF-κB in endothelial cells, upregulating vascular adhesion molecules such as ICAM-1 and MCP-1, thereby increasing susceptibility to VC and atherosclerosis [102].
In addition, IS reduces nitric oxide production while enhancing ROS generation, leading to progressive endothelial injury [103]. AhR dysregulation further promotes MAPK/NF-κB signaling, triggering inflammation without activating the NLRP3 inflammasome. This may explain the persistence of chronic, low-grade inflammation induced by IS in CKD patients [104]. Collectively, these findings suggest that gut microbiota-derived metabolites, including tryptophan derivatives and SCFAs, may activate AhR, drive ROS production, and trigger inflammatory pathways in vascular endothelial cells. Such processes likely contribute to the initiation and progression of VC.
IS has also been shown to regulate Wnt7b/β-catenin signaling via microRNA-29b in HASMCs, thereby reducing intracellular RUNX2 levels and attenuating VC [105]. Sun et al. further demonstrated that IS exposure activates the renal renin–angiotensin–aldosterone system (RAAS), with increased expression of renin, angiotensinogen, and AT1R proteins. Their work also revealed that IS stimulates RAAS activation through the TGF-β pathway [106]. This pathway may represent a mechanistic link between IS and VC, although further validation is required.
Another uremic toxin, PCS, promotes endothelial dysfunction by upregulating TNF-α, MCP-1, and ICAM-1, thereby contributing to atherosclerosis. This effect is mediated by the induction of NADPH oxidase activity, leading to ROS generation [107,108].
Emerging evidence also suggests that macrophage polarization may play a role in VC progression. Chen et al. reported that pravastatin enhances the activity of Bacteroides by promoting ArsR expression, which in turn increases production of 3,4,5-trimethoxycinnamic acid (TMCA). TMCA was shown to promote VC in type 2 diabetes by inducing macrophage polarization toward the pro-inflammatory M1 phenotype [109]. Conversely, Bacteroides fragilis has been implicated in vesicle-mediated activation of STING signaling, Trib1, and Mef2d genes, driving macrophage polarization toward the M2 phenotype and thereby facilitating VC [110]. IS has similarly been associated with skewing macrophages toward the M1 phenotype, while also possessing the capacity to induce M2 polarization. This dual modulatory effect may contribute to vascular remodeling in atherosclerotic lesions among CKD patients [111]. Whether IS actively promotes VC through macrophage polarization, however, remains to be clarified.
4.4. Trimethylamine N-oxide
Studies have shown that administration of metronidazole, which alters gut microbiota composition, leads to a marked reduction in serum TMAO levels [112], suggesting a strong association between TMAO production and microbial activity. In humans, compounds such as choline, L-carnitine, and betaine are metabolized by intestinal microbiota into trimethylamine (TMA), which is subsequently oxidized in the liver by flavin-containing monooxygenases (FMOs) into TMAO [113]. Multiple international cohort studies have demonstrated that elevated TMAO levels increase the risk of CVD and promote atherosclerosis [114,115], as well as raising the likelihood of heart failure [116,117].
In vitro experiments involving mouse bone marrow-derived macrophages revealed that TMAO enhances the secretion of pro-inflammatory cytokines IL-6 and IL-12p40 while suppressing the anti-inflammatory cytokine IL-10. It also upregulates several inflammation-related genes, including Nr4a3, H-Q5, H2-Q6, Irf3, Irf5, and Acod1 [112]. Ma et al. demonstrated that berberine can attenuate atherosclerosis by reducing microbial TMA production from choline, acting through vitamin-like modulation of gut metabolism [118].
TMAO has also been shown to promote VC by activating the NLRP3 inflammasome, enhancing NF-κB signaling, and inducing osteogenic differentiation in VSMCs [119]. In both VSMCs and endothelial cells, TMAO activates MAPK signaling pathways, contributing to inflammatory gene expression and promoting leukocyte adhesion [120]. Some studies suggest that high-choline diets elevate serum TMAO levels and facilitate NF-κB activation through endoplasmic reticulum–mitochondrial stress, promoting osteogenic responses in human aortic valves. However, this mechanism has not yet been investigated in VSMCs [121]. TMAO has also been implicated in exacerbating renal damage by increasing Smad3 phosphorylation, a key regulator of TGF-β signaling [122].
Nevertheless, TMAO has been shown to increase calcium release from endoplasmic reticulum stores in platelets, promoting aggregation and thrombosis [123]. Overall, the role of TMAO in promoting atherosclerosis is well established, whereas its direct involvement in VC remains under investigation (Figure 3).
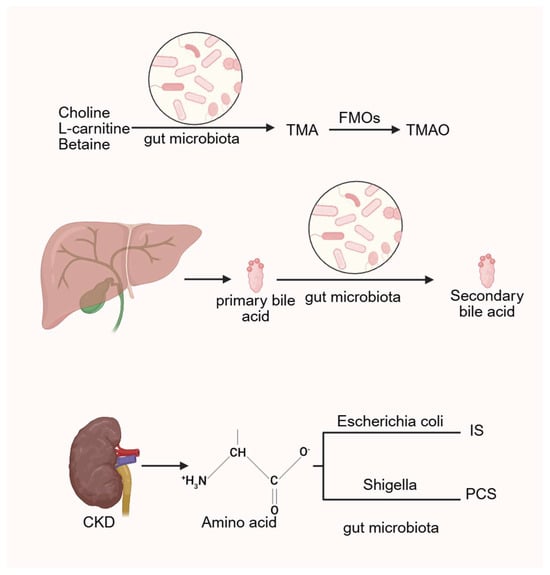
Figure 3.
The gut microbiota generates TMAO, bile acids and related uremic toxins by regulating related metabolic processes. (Created in BioRender. c, X. (2026) https://BioRender.com/wz2634r).
4.5. Other Related Mechanisms
Vitamin K2 (VK2) is synthesized through the metabolic activity of gut microorganisms, with approximately 50% of the body’s vitamin K originating from bacterial decomposition in the intestine. Key contributing genera include Clostridium and Lactobacillus [124]. These microbes also contribute to the maintenance of intestinal microbial homeostasis and possess coagulation-supporting properties that help prevent bleeding [125]. VK2 has been shown to modulate the gut microbiota composition, alleviate inflammation, and reduce oxidative stress in both the gastrointestinal and central nervous systems [126].
OCN, the most abundant non-collagenous protein in bone, plays an important role in both physiological and pathological calcification [127]. OCN can promote VC by activating hypoxia-inducible factor 1-alpha (HIF-1α), thereby enhancing mineralization in VSMCs, or by stimulating the Runx2 signaling pathway [128]. VK2 facilitates the carboxylation of OCN, thereby reducing aberrant calcium deposition in blood vessels. In addition, VK2 activates MGP, a known inhibitor of arterial wall calcification. Once phosphorylated, MGP acquires the capacity to bind calcium and hydroxyapatite, reducing vascular deposition of Ca2+ and mitigating VC risk [129].
Oxalobacter formigenes has been identified as a key organism in the intestinal degradation of oxalate and may lower the risk of calcium oxalate kidney stone formation [130]. Oxalate can induce reactive oxygen species, driving oxidative stress, inflammation, and damage to vascular endothelial cells [131]. A significant association between calcium oxalate stones and AAC has also been reported [132]. However, the precise mechanisms by which oxalate contributes to aortic calcification remain unclear and warrant further investigation.
Bile acids are cholesterol-derived molecules primarily synthesized in the liver. Their signaling functions are mediated through various receptors, including the nuclear receptor farnesoid X receptor (FXR) and the membrane-bound Takeda G protein-coupled receptor 5, also known as the G protein-coupled bile acid receptor 1. In the gut, primary bile acids undergo microbial 7α-dehydroxylation to form secondary bile acids, with deoxycholic acid (DCA) being a principal product. This transformation is primarily facilitated by a limited number of anaerobic bacteria, such as Clostridium, via bile salt hydrolases (BSHs) [133].
DCA has been shown to activate the transferrin receptor–acyl-CoA synthetase long-chain family member 4 pathway, inducing ferroptosis [134]. In CKD, free DCA aggravates VC by inducing activating transcription factor 4 via endoplasmic reticulum stress. Conversely, FXR-specific agonists have been reported to reduce circulating bile acids, including DCA, in murine models, thereby preventing CKD-associated medial layer and atherosclerotic calcification [135]. Although elevated serum DCA levels are often observed in patients with advanced CKD and appear to be associated with CAC, cross-sectional analyses have not established a definitive correlation [136].
Lithocholic acid, another secondary bile acid produced by intestinal bacteria, serves as an agonist of the vitamin D receptor. Hashimoto et al. found that lithocholic acid enhances calcium and phosphate absorption in a vitamin D receptor–claudin-3 dependent manner. They also reported reduced serum levels of 1,25(OH)2 vitamin D in CKD patients [137]. These findings support the hypothesis that lithocholic acid may contribute to the development of VC, particularly in patients with impaired vitamin D metabolism. Therefore, the interplay between gut microbiota-derived bile acids and host signaling pathways likely plays a critical role in VC pathogenesis.
Additionally, recent research suggests that multiple microbial species may modulate metabolite levels to influence VC progression. For instance, the microbial strain gKLE1615 has been implicated in promoting VC by reducing the methionine-to-phosphate ratio. Key pathways involved include GDP-L-fucose biosynthesis, 5-aminoimidazole ribonucleotide biosynthesis, L-glutamine degradation, the S-adenosyl-L-methionine cycle, and thiazole biosynthesis [138] (Figure 4).
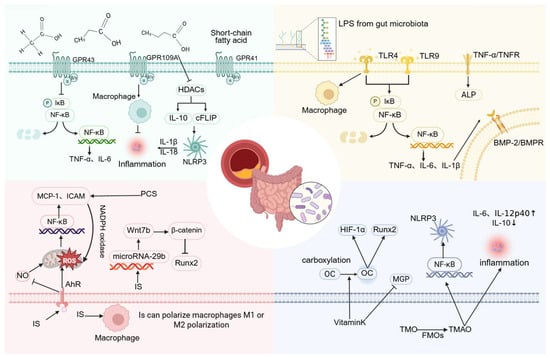
Figure 4.
The molecular mechanisms by which various gut microbiota-related metabolites regulate vascular calcification. (Created in BioRender. c, X. (2026) https://BioRender.com/nap4i75).
5. Methods and Measures for Preventing Vascular Calcification Through Modulation of Gut Microbiota
5.1. Dietary Modulation for VC Prevention
Dietary habits play an essential role in mitigating CVD risk. Experimental studies have shown that increased dietary fiber intake enhances SCFA production via microbial fermentation, while simultaneously increasing the abundance of Firmicutes and the relative proportion of Proteobacteria, particularly Enterobacteriaceae. In contrast, reduced fiber consumption leads to decreased production of indole-3-acetic acid from tryptophan, a change associated with diminished abundance of Clostridium species [139]. This reduction may lower AhR activation, potentially contributing to VC progression.
There is evidence suggesting that a high-choline diet promotes the microbial conversion of TMA, which is subsequently oxidized to TMAO in the liver. Conversely, a high-fiber diet facilitates the microbial conversion of cholesterol into bile acids, which then activates liver FMOs to enhance TMA oxidation and TMAO production [140]. Bile acids further stimulate FXR within the FMO gene family, promoting hepatic TMAO generation. Among dietary sources, TMAO derived from fish has the most substantial effect on circulating TMAO levels. Additionally, excessive caloric intake (>1000 kcal/day) and high-fat consumption (55% fat) over a period of four weeks have been shown to elevate fasting plasma TMAO concentrations [122].
Collectively, these findings suggest that high-choline diets may exacerbate VC by increasing gut-derived TMAO. In contrast, dietary fiber exhibits a dual effect: it may reduce VC through increased SCFA production but also contribute to TMAO elevation, depending on fiber type and intake patterns.
Interestingly, the choline analog 3,3-dimethylbutanol (DMB) has been shown to inhibit choline TMA lyase activity, thereby lowering systemic TMAO levels and attenuating choline-induced atherosclerosis. DMB is a naturally occurring compound found in certain foods such as balsamic vinegar, red wine, cold-pressed extra virgin olive oil, and grape seed oil [141].
5.2. Probiotics, Prebiotics, Synbiotics, and Aerobic Exercise in the Regulation of Vascular Calcification
Probiotic microorganisms influence a range of physiological processes. Their functions include pathogen defense, immune modulation, mineral absorption, and the regulation of metabolic activity [142]. Common probiotic strains belong to the genera Lactobacillus, Bifidobacterium, and Streptococcus, along with Saccharomyces [143]. Lactobacillus and Bifidobacterium have been shown to reduce levels of inflammatory mediators such as IL-1β in murine models of inflammatory bowel disease, contributing to the maintenance of intestinal barrier integrity [144,145]. Through suppression of systemic inflammation, these strains may help alleviate VC.
However, certain Lactobacillus strains have been reported to translocate across impaired intestinal barriers and colonize internal organs such as the liver, potentially inducing inflammatory responses [146] that could contribute to VC. In another study, researchers from China and Sweden identified Eubacterium eligens (E. eligens) as a promising microbial target for treating atherosclerosis and VC [147]. Based on this, transplantation of beneficial bacteria may represent a viable therapeutic strategy.
Innovative approaches, such as the development of bioinspired wrinkled microspheres loaded with probiotics (CSM@5), have been shown to significantly lower serum phosphorus levels while modulating gut microbiota composition. This intervention led to increased beneficial bacteria and decreased harmful species, ultimately improving VC outcomes [148].
Prebiotics, such as inulin, have been found to elevate SCFA levels and alter gut microbiota by increasing the abundance of specific bacterial genera, although without significant effects on plasma cholesterol or atherosclerosis progression [149]. In CKD models, synbiotic supplementation—including Sialobacter LBR228, Bifidobacterium BFS309, fructooligosaccharides, and chitosan oligosaccharides—has been effective in alleviating hyperphosphatemia and secondary hyperparathyroidism (SHPT), and in reducing VC induced by calcium deposition secondary to SHPT [150]. Resveratrol, a plant-derived antitoxin with prebiotic properties, reduces plasma TMAO levels in ApoE−/− mice by reshaping the intestinal microbiota. This compound increases Lactobacillus and Bifidobacterium abundance while promoting bile acid synthesis [151].
Physical activity may also influence VC through modulation of the gut microbiota. In athletes, the relative abundance of Akkermansia has been found to be significantly higher in those with lower body mass index, potentially promoting SCFA production and reducing VC risk. Long-term exercise has been associated with increased proportions of the Firmicutes phylum and Ruminococcaceae family, and decreased abundance of the Lactobacillaceae family, Bacteroides genus, and Lactobacillus genus [152]. These exercise-induced shifts in microbial composition may represent an additional pathway for VC prevention (Figure 5). The elderly elite athletes (aged 60–80) who mainly engaged in endurance training had significantly higher maximum oxygen uptake (VO2max) and total bone mineral deposition compared to their sedentary counterparts of the same age. A detailed analysis revealed a significant negative correlation between VO2max and coronary artery calcification damage, and a significant positive correlation with the VC inhibitor fetuin-A, suggesting that long-term exercise can simultaneously inhibit coronary artery calcification and increase bone mineral content [153].
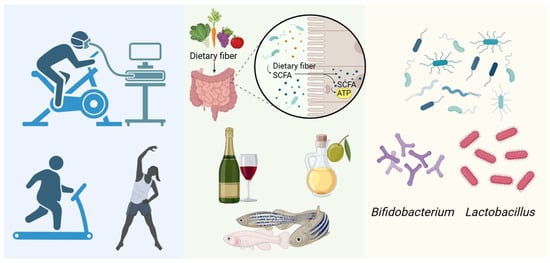
Figure 5.
Relieving vascular calcification through the gut microbiota. (Created in BioRender. c, X. (2026) https://BioRender.com/kouu89w).
The elderly of the same age group who have lifelong exercise habits have better vascular elasticity compared to those without exercise habits. This indicates that establishing good exercise habits during youth has significant benefits for resisting VC in old age, maintaining vascular compliance and maintaining good vascular function [154].
6. Conclusions
The gut microbiota and its metabolites play a central role in the initiation and progression of VC. Numerous studies have demonstrated that dysbiosis of the gut microbiota influences VC through a range of complex signaling pathways and physiological mechanisms. As described in this review, specific microbial taxa may modulate VC by participating in metabolic pathways such as the LPS/TLR/NF-κB axis, which promotes inflammatory responses; the Notch/BMP2/Smad signaling cascade, which mediates vascular mineralization; and the TNF-α pathway, which induces Msx2 expression and subsequently enhances ALP activity, promoting calcification.
Key microbial metabolites, including IS, SCFAs, TMAO, and VK2, are involved in these regulatory mechanisms. These metabolites may activate signaling pathways that contribute to VC, although the precise nature of their interactions remains to be fully elucidated. In addition to dietary and probiotic interventions, other strategies such as fecal microbiota transplantation and antibiotic administration have also shown potential in modulating VC [64]. However, most evidence supporting these interventions is derived from animal models, and robust clinical validation is lacking.
Several studies have suggested that both gut microbiota and their metabolic products may influence aortic valve calcification. Although these processes share overlapping molecular mechanisms with VC, it is still unclear whether the effects observed in valve tissues are directly applicable to vascular wall calcification, particularly in the context of CKD or T2DM.
Despite considerable progress, many challenges remain. The complexity of gut microbial ecosystems and the dynamic gut environment limit our current understanding of the interactions between specific microbial species and their metabolites. Additionally, substantial inter-individual variability in gut microbiota composition and function complicates efforts to determine how these differences influence VC susceptibility and progression. Most existing research in this field is based on animal and in vitro models, and high-quality clinical data, especially randomized controlled trials, are still lacking.
Future research should aim to elucidate the molecular mechanisms through which gut microbiota and their metabolites regulate VC, integrating multi-pathway and multi-omics approaches. Such efforts could facilitate the development of novel therapeutic strategies targeting the gut microbiota for the prevention and treatment of VC.
Author Contributions
X.-Y.C.: Writing—original draft, Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Visualization. A.-Y.Z.: Writing—original draft, Conceptualization. K.-F.L.: Investigation, Software, Validation, Visualization, Writing—review and editing. Y.-W.B.: Data curation, Investigation, Software, Writing—review and editing. G.-W.X.: Conceptualization, Formal Analysis, Investigation, Validation, Visualization, Writing—review and editing. C.-Y.Z.: Software, Validation. X.-Y.M.: Investigation, Visualization. Y.-Y.Z.: Software, Validation. H.-J.S.: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing—original draft, Writing—review and editing. X.-X.Z. Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
work was funded and supported by the National Natural Science Foundation of China (82300414, 82370364, 81800340 and 82170424), Jiangsu Natural Science Foundation (BK20240204, BK20231049 and BK20191138), the Fundamental Research Funds for the Central Universities (JUSRP124036).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed in this review. All data discussed are from published studies available through PubMed search.
Acknowledgments
For scientific drawing, I used BioRender.com software. Thank you, Biorender, for providing the drawing software. The charts created with BioRender in this document are licensed for use.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef]
- Chang, J.R.; Guo, J.; Wang, Y.; Hou, Y.L.; Lu, W.W.; Zhang, J.S.; Yu, Y.R.; Xu, M.J.; Liu, X.Y.; Wang, X.J.; et al. Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho. Kidney Int. 2016, 89, 586–600. [Google Scholar] [CrossRef]
- Lessmann, N.; de Jong, P.A.; Celeng, C.; Takx, R.A.P.; Viergever, M.A.; van Ginneken, B.; Išgum, I. Sex Differences in Coronary Artery and Thoracic Aorta Calcification and Their Association with Cardiovascular Mortality in Heavy Smokers. JACC Cardiovasc. Imaging 2019, 12, 1808–1817. [Google Scholar] [CrossRef]
- Kälsch, H.; Lehmann, N.; Möhlenkamp, S.; Hammer, C.; Mahabadi, A.A.; Moebus, S.; Schmermund, A.; Stang, A.; Bauer, M.; Jöckel, K.H.; et al. Prevalence of thoracic aortic calcification and its relationship to cardiovascular risk factors and coronary calcification in an unselected population-based cohort: The Heinz Nixdorf Recall Study. Int. J. Cardiovasc. Imaging 2013, 29, 207–216. [Google Scholar] [CrossRef]
- Pan, W.; Jie, W.; Huang, H. Vascular calcification: Molecular mechanisms and therapeutic interventions. MedComm 2023, 4, e200. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The human microbiome: Our second genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Blanton, L.V.; DiGiulio, D.B.; Relman, D.A.; Lebrilla, C.B.; Mills, D.A.; Gordon, J.I. A microbial perspective of human developmental biology. Nature 2016, 535, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Wu, H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arter. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M. Mechanical principles in arterial disease. Hypertension 1995, 26, 2–9. [Google Scholar] [CrossRef]
- Niskanen, L.; Siitonen, O.; Suhonen, M.; Uusitupa, M.I. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care 1994, 17, 1252–1256. [Google Scholar] [CrossRef]
- Rogers, M.A.; Aikawa, E. Cardiovascular calcification: Artificial intelligence and big data accelerate mechanistic discovery. Nat. Rev. Cardiol. 2019, 16, 261–274. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Evrard, S.; Delanaye, P.; Kamel, S.; Cristol, J.P.; Cavalier, E. Vascular calcification: From pathophysiology to biomarkers. Clin. Chim. Acta 2015, 438, 401–414. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification-New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Chavkin, N.W.; Chia, J.J.; Crouthamel, M.H.; Giachelli, C.M. Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp. Cell Res. 2015, 333, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Cheng, C.W.; Tsai, Y.S.; Huang, H.S. Crosstalk between Renal and Vascular Calcium Signaling: The Link between Nephrolithiasis and Vascular Calcification. Int. J. Mol. Sci. 2021, 22, 3590. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, Y.J.; Kim, H.J.; Park, H.D.; Kang, A.R.; Kyung, H.M.; Sung, J.H.; Wozney, J.M.; Kim, H.J.; Ryoo, H.M. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 2003, 278, 34387–34394. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.H.; Peng, P.; Qiu, Z.H.; Di, C.X.; Chen, X.F.; Wang, N.N.; Chen, F.; He, Y.W.; Liu, Z.B.; et al. RUNX2 Phase Separation Mediates Long-Range Regulation Between Osteoporosis-Susceptibility Variant and XCR1 to Promote Osteoblast Differentiation. Adv. Sci. 2025, 12, e2413561. [Google Scholar] [CrossRef]
- Namiki, M.; Akiyama, S.; Katagiri, T.; Suzuki, A.; Ueno, N.; Yamaji, N.; Rosen, V.; Wozney, J.M.; Suda, T. A kinase domain-truncated type I receptor blocks bone morphogenetic protein-2-induced signal transduction in C2C12 myoblasts. J. Biol. Chem. 1997, 272, 22046–22052. [Google Scholar] [CrossRef]
- Nishimura, R.; Kato, Y.; Chen, D.; Harris, S.E.; Mundy, G.R.; Yoneda, T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J. Biol. Chem. 1998, 273, 1872–1879. [Google Scholar] [CrossRef]
- Orth-Alampour, S.; Gayrard, N.; Salem, S.; Bhargava, S.; Jankowski, V.; Jover, B.; Notarnicola, C.; Noels, H.; van der Vorst, E.P.C.; Kuppe, C.; et al. Prevention of vascular calcification by the endogenous chromogranin A-derived mediator that inhibits osteogenic transdifferentiation. Basic Res. Cardiol. 2021, 116, 57. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Du, A.; Zhang, S.; Deng, M.; Zhao, R.; Lu, Y.; Ji, Y.; Shao, Y.; Sun, W.; et al. SPARC-related modular calcium binding 1 regulates aortic valve calcification by disrupting BMPR-II/p-p38 signalling. Cardiovasc. Res. 2022, 118, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chang, Q.; Xin, M.; Wang, Q.; Li, H.; Qian, J. Endogenous bone morphogenetic protein 2 plays a role in vascular smooth muscle cell calcification induced by interleukin 6 in vitro. Int. J. Immunopathol. Pharmacol. 2017, 30, 227–237. [Google Scholar] [CrossRef]
- Zeng, D.; Umar, M.; Zhu, Z.; Pan, H.; Lu, W.W.; Xiao, G.; Chen, Y.; Tong, L.; Chen, D. Development of novel osteoarthritis therapy by targeting AMPK-β-catenin-Runx2 signaling. Genes Dis. 2025, 12, 101247. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, M.; Ma, J.; Liu, Z.; Zhang, Z.; Chen, M.; Zhao, Y.; Li, H.; Wang, S.; Wei, X.; et al. Moscatilin inhibits vascular calcification by activating IL13RA2-dependent inhibition of STAT3 and attenuating the WNT3/β-catenin signalling pathway. J. Adv. Res. 2025, 68, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Woo, K.M.; Ryoo, H.M.; Baek, J.H. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem. Biophys. Res. Commun. 2010, 391, 1087–1092. [Google Scholar] [CrossRef]
- Rotondi, S.; Pasquali, M.; Tartaglione, L.; Muci, M.L.; Mandanici, G.; Leonangeli, C.; Sales, S.; Farcomeni, A.; Mazzaferro, S. Soluble α -Klotho Serum Levels in Chronic Kidney Disease. Int. J. Endocrinol. 2015, 2015, 872193. [Google Scholar] [CrossRef]
- Liu, X.; Qian, F.; Fan, Q.; Lin, L.; He, M.; Li, P.; Cai, H.; Ma, L.; Cheng, X.; Yang, X. NF-κB activation impedes the transdifferentiation of hypertrophic chondrocytes at the growth plate of mouse embryos in diabetic pregnancy. J. Orthop. Transl. 2021, 31, 52–61. [Google Scholar] [CrossRef]
- Koh, N.; Fujimori, T.; Nishiguchi, S.; Tamori, A.; Shiomi, S.; Nakatani, T.; Sugimura, K.; Kishimoto, T.; Kinoshita, S.; Kuroki, T.; et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001, 280, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Ceneri, N.; Zhao, L.; Young, B.D.; Healy, A.; Coskun, S.; Vasavada, H.; Yarovinsky, T.O.; Ike, K.; Pardi, R.; Qin, L.; et al. Rac2 Modulates Atherosclerotic Calcification by Regulating Macrophage Interleukin-1β Production. Arter. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 328–340. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Sun, Z.; Liu, R.; Zhu, Y.; Yi, J.; Li, Y.; Hu, M.; Wang, D. Structural characterization and osteogenic differentiation-promoting activity of polysaccharide purified from Chroogomphus rutilus. Carbohydr. Polym. 2024, 328, 121709. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.M.; Liu, H.H.; Xu, R.X.; Zhu, C.G.; Guo, Y.L.; Wu, N.Q.; Dong, Q.; Cui, C.J.; Li, J.J. Lipoprotein (a)-mediated vascular calcification: Population-based and in vitro studies. Metabolism 2022, 127, 154960. [Google Scholar] [CrossRef]
- Toshima, T.; Watanabe, T.; Narumi, T.; Otaki, Y.; Shishido, T.; Aono, T.; Goto, J.; Watanabe, K.; Sugai, T.; Takahashi, T.; et al. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling. Cardiovasc. Res. 2020, 116, 983–994. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Pongsuwan, K.; Kusirisin, P.; Narongkiattikhun, P.; Chattipakorn, S.C.; Chattipakorn, N. Mitochondria and vascular calcification in chronic kidney disease: Lessons learned from the past to improve future therapy. J. Cell Physiol. 2022, 237, 4369–4396. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Hamada, C.; Tomino, Y. Suppressive effects of iron overloading on vascular calcification in uremic rats. J. Nephrol. 2014, 27, 135–142. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef]
- Ma, W.Q.; Sun, X.J.; Zhu, Y.; Liu, N.F. Metformin attenuates hyperlipidaemia-associated vascular calcification through anti-ferroptotic effects. Free Radic. Biol. Med. 2021, 165, 229–242. [Google Scholar] [CrossRef]
- van den Beukel, T.C.; van der Toorn, J.E.; Vernooij, M.W.; Kavousi, M.; Akyildiz, A.C.; de Jong, P.A.; van der Lugt, A.; Ikram, M.K.; Bos, D. Morphological Subtypes of Intracranial Internal Carotid Artery Arteriosclerosis and the Risk of Stroke. Stroke 2022, 53, 1339–1347. [Google Scholar] [CrossRef]
- van den Beukel, T.C.; Wolters, F.J.; Siebert, U.; Spiering, W.; Ikram, M.A.; Vernooij, M.W.; de Jong, P.A.; Bos, D. Intracranial arteriosclerosis and the risk of dementia: A population-based cohort study. Alzheimers Dement. 2024, 20, 869–879. [Google Scholar] [CrossRef]
- Yang, W.J.; Zheng, L.; Wu, X.H.; Huang, Z.Q.; Niu, C.B.; Zhao, H.L.; Leung, T.W.; Wong, L.K.; Chen, X.Y. Postmortem Study Exploring Distribution and Patterns of Intracranial Artery Calcification. Stroke 2018, 49, 2767–2769. [Google Scholar] [CrossRef]
- Kockelkoren, R.; Vos, A.; Van Hecke, W.; Vink, A.; Bleys, R.L.; Verdoorn, D.; Mali, W.P.; Hendrikse, J.; Koek, H.L.; de Jong, P.A.; et al. Computed Tomographic Distinction of Intimal and Medial Calcification in the Intracranial Internal Carotid Artery. PLoS ONE 2017, 12, e0168360. [Google Scholar] [CrossRef]
- Golüke, N.M.S.; de Brouwer, E.J.M.; de Jonghe, A.; Claus, J.J.; Staekenborg, S.S.; Emmelot-Vonk, M.H.; de Jong, P.A.; Koek, H.L. Intracranial artery calcifications: Risk factors and association with cardiovascular disease and cognitive function. J. Neuroradiol. 2022, 49, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bartstra, J.W.; van den Beukel, T.C.; Van Hecke, W.; Mali, W.; Spiering, W.; Koek, H.L.; Hendrikse, J.; de Jong, P.A.; den Harder, A.M. Intracranial Arterial Calcification: Prevalence, Risk Factors, and Consequences: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Blackham, A.; Jones, B. Incidental internal carotid artery calcifications on temporal bone CT in children. Pediatr. Radiol. 2007, 37, 141–144. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, X.Y.; Wang, L.J.; Wong, K.S. Intracranial Artery Calcification and Its Clinical Significance. J. Clin. Neurol. 2016, 12, 253–261. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Hsu, B.G.; Tsai, J.P. Vascular calcification of chronic kidney disease: A brief review. Tzu Chi Med. J. 2021, 33, 34–41. [Google Scholar] [CrossRef]
- Sharma, S.; Sarnak, M.J. Epidemiology: The global burden of reduced GFR: ESRD, CVD and mortality. Nat. Rev. Nephrol. 2017, 13, 447–448. [Google Scholar] [CrossRef]
- Hao, Q.Y.; Yan, J.; Wei, J.T.; Zeng, Y.H.; Feng, L.Y.; Que, D.D.; Li, S.C.; Guo, J.B.; Fan, Y.; Ding, Y.F.; et al. Prevotella copri promotes vascular calcification via lipopolysaccharide through activation of NF-κB signaling pathway. Gut Microbes 2024, 16, 2351532. [Google Scholar] [CrossRef]
- Bao, W.H.; Yang, W.L.; Su, C.Y.; Lu, X.H.; He, L.; Zhang, A.H. Relationship between gut microbiota and vascular calcification in hemodialysis patients. Ren. Fail. 2023, 45, 2148538. [Google Scholar] [CrossRef] [PubMed]
- Merino-Ribas, A.; Araujo, R.; Pereira, L.; Campos, J.; Barreiros, L.; Segundo, M.A.; Silva, N.; Costa, C.; Quelhas-Santos, J.; Trindade, F.; et al. Vascular Calcification and the Gut and Blood Microbiome in Chronic Kidney Disease Patients on Peritoneal Dialysis: A Pilot Study. Biomolecules 2022, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishida, H.; Matsuda, N.; Fujiu, A.; Matsuda, A.; Ito, K.; Ando, Y.; Nitta, K. Simple evaluation of aortic arch calcification by chest radiography in hemodialysis patients. Hemodial. Int. 2009, 13, 301–306. [Google Scholar] [CrossRef]
- Liu, Y.H.; Peng, P.; Hung, W.C.; Wu, P.H.; Kao, C.Y.; Wu, P.Y.; Huang, J.C.; Hung, C.H.; Su, H.M.; Chen, S.C.; et al. Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 5673. [Google Scholar] [CrossRef] [PubMed]
- Okami, Y.; Arima, H.; Kondo, K.; Hexun, Z.; Yano, Y.; Kadota, A.; Torii, S.; Hisamatsu, T.; Fujiyoshi, A.; Kadowaki, S.; et al. The gut microbiota and coronary artery calcification in Japanese men. Am. Heart J. 2024, 267, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Fang, Y.; Zheng, J.; Shi, G.; Guo, J.; Zhang, X.; Zhang, R. Role of symbiotic microbiota dysbiosis in the progression of chronic kidney disease accompanied with vascular calcification. Front. Pharmacol. 2023, 14, 1306125. [Google Scholar] [CrossRef]
- Yan, J.; Pan, Y.; Shao, W.; Wang, C.; Wang, R.; He, Y.; Zhang, M.; Wang, Y.; Li, T.; Wang, Z.; et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome 2022, 10, 195. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Louis, P. Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat. Rev. Microbiol. 2025, 23, 635–651. [Google Scholar] [CrossRef]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Diet Quality and Protein-Bound Uraemic Toxins: Investigation of Novel Risk Factors and the Role of Microbiome in Chronic Kidney Disease. J. Ren. Nutr. 2022, 32, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kakuta, M.; Hasegawa, T.; Yamaguchi, R.; Uchino, E.; Murashita, K.; Nakaji, S.; Imoto, S.; Yanagita, M.; Okuno, Y. Metagenomic profiling of gut microbiome in early chronic kidney disease. Nephrol. Dial. Transpl. Transplant. 2021, 36, 1675–1684. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Hu, J.; Chen, Y.; Dong, B.; Wang, Y. A comparative study of acarbose, vildagliptin and saxagliptin intended for better efficacy and safety on type 2 diabetes mellitus treatment. Life Sci. 2021, 274, 119069. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Y.; Liu, Y.F.; Zeng, Z.L.; Zhao, Z.B.; Yan, X.L.; Zheng, J.; Chen, W.H.; Wang, Z.X.; Xie, H.; Liu, J.H. Antibiotic-induced gut microbiota disruption promotes vascular calcification by reducing short-chain fatty acid acetate. Mol. Med. 2024, 30, 130. [Google Scholar] [CrossRef]
- Ang, Z.; Er, J.Z.; Ding, J.L. The short-chain fatty acid receptor GPR43 is transcriptionally regulated by XBP1 in human monocytes. Sci. Rep. 2015, 5, 8134. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Wang, W.; Dernst, A.; Martin, B.; Lorenzi, L.; Cadefau-Fabregat, M.; Phulphagar, K.; Wagener, A.; Budden, C.; Stair, N.; Wagner, T.; et al. Butyrate and propionate are microbial danger signals that activate the NLRP3 inflammasome in human macrophages upon TLR stimulation. Cell Rep. 2024, 43, 114736. [Google Scholar] [CrossRef]
- Hajjar, A.M.; Ernst, R.K.; Tsai, J.H.; Wilson, C.B.; Miller, S.I. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 2002, 3, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ao, L.; Shi, Y.; Johnson, T.R.; Fullerton, D.A.; Meng, X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J. Biol. Chem. 2011, 286, 12213–12220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, Y.; Cui, L.Y.; Tang, W.; Liu, B.; Zheng, J.J.; Si, W.Z.; Wang, X.; Xu, M.J. Suppression of Gut Bacterial Translocation Ameliorates Vascular Calcification through Inhibiting Toll-Like Receptor 9-Mediated BMP-2 Expression. Oxid. Med. Cell Longev. 2019, 2019, 3415682. [Google Scholar] [CrossRef]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Patel, J.; Territo, M.; Saini, T.; Parhami, F.; Demer, L.L. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 2002, 105, 650–655. [Google Scholar] [CrossRef]
- Yaker, L.; Tebani, A.; Lesueur, C.; Dias, C.; Jung, V.; Bekri, S.; Guerrera, I.C.; Kamel, S.; Ausseil, J.; Boullier, A. Extracellular Vesicles From LPS-Treated Macrophages Aggravate Smooth Muscle Cell Calcification by Propagating Inflammation and Oxidative Stress. Front. Cell Dev. Biol. 2022, 10, 823450. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Simões-Silva, L.; Pestana, M.; Araujo, R.; Soares-Silva, I.J. The Role of the Gut Microbiome on Chronic Kidney Disease. Adv. Appl. Microbiol. 2016, 96, 65–94. [Google Scholar] [CrossRef]
- Shimizu, H.; Hirose, Y.; Nishijima, F.; Tsubakihara, Y.; Miyazaki, H. ROS and PDGF-beta [corrected] receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am. J. Physiol. Cell Physiol. 2009, 297, C389–C396. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, Z.; Luo, X. Roles of xenobiotic receptors in vascular pathophysiology. Circ. J. 2014, 78, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Perdew, G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes 2020, 12, 1859812. [Google Scholar] [CrossRef]
- Ito, S.; Osaka, M.; Edamatsu, T.; Itoh, Y.; Yoshida, M. Crucial Role of the Aryl Hydrocarbon Receptor (AhR) in Indoxyl Sulfate-Induced Vascular Inflammation. J. Atheroscler. Thromb. 2016, 23, 960–975. [Google Scholar] [CrossRef]
- Huć, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef]
- Romero Caimi, G.; Gorzalczany, S.; Bonazzola, P.; Deza, Z.; Rosón, M.I.; Alvarez, L.; Castilla, R. Angiotensin II type 1 receptor is involved in hypertension and vascular alterations caused by environmental toxicant hexachlorobenzene. Toxicol. Rep. 2021, 8, 1599–1606. [Google Scholar] [CrossRef]
- Salminen, A. Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: Role of tryptophan metabolites generated by gut host-microbiota. J. Mol. Med. 2023, 101, 201–222. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Ha, Y.; Tilton, R.G.; Zhang, W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed. Res. Int. 2014, 2014, 902842. [Google Scholar] [CrossRef]
- Fujii, H.; Nakai, K.; Fukagawa, M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther. Apher. Dial. 2011, 15, 125–128. [Google Scholar] [CrossRef]
- Pedruzzi, L.M.; Stockler-Pinto, M.B.; Leite, M., Jr.; Mafra, D. Nrf2-keap1 system versus NF-κB: The good and the evil in chronic kidney disease? Biochimie 2012, 94, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Tumur, Z.; Niwa, T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Yamamoto, S.; Ito, T.; Sato, Y.; Matsuo, K.; Takahashi, Y.; Kaneko, Y.; Goto, S.; Kazama, J.J.; Gejyo, F.; et al. Indoxyl Sulfate Promotes Macrophage IL-1β Production by Activating Aryl Hydrocarbon Receptor/NF-κ/MAPK Cascades, but the NLRP3 inflammasome Was Not Activated. Toxins 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Shen, Z.; Gu, Y.; Xu, L.; Hu, J.; Zhang, X.; Ding, X. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol. Lett. 2018, 284, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef]
- Jing, Y.J.; Ni, J.W.; Ding, F.H.; Fang, Y.H.; Wang, X.Q.; Wang, H.B.; Chen, X.N.; Chen, N.; Zhan, W.W.; Lu, L.; et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE-/- mice. Kidney Int. 2016, 89, 439–449. [Google Scholar] [CrossRef]
- Han, H.; Zhu, J.; Zhu, Z.; Ni, J.; Du, R.; Dai, Y.; Chen, Y.; Wu, Z.; Lu, L.; Zhang, R. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J. Am. Heart Assoc. 2015, 4, e001852. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Z.F.; He, Y.Q.; Li, A.Q.; Gao, Y.; Pan, Q.W.; Cao, J.S. Pravastatin promotes type 2 diabetes vascular calcification through activating intestinal Bacteroides fragilis to induce macrophage M1 polarization. J. Diabetes 2024, 16, e13514. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Z.; He, Y.; Gao, Y.; Ouyang, S.; Wang, L.; Liu, J.; Cao, J. Bacteroides Fragilis Exacerbates T2D Vascular Calcification by Secreting Extracellular Vesicles to Induce M2 Macrophages. Adv. Sci. 2025, 12, e2410495. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Yamamoto, S.; Yoshida, S.; Narita, I. Indoxyl Sulfate-Induced Macrophage Toxicity and Therapeutic Strategies in Uremic Atherosclerosis. Toxins 2024, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Mirji, G.; Worth, A.; Bhat, S.A.; El Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.Y.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Schuett, K.; Kleber, M.E.; Scharnagl, H.; Lorkowski, S.; März, W.; Niessner, A.; Marx, N.; Meinitzer, A. Trimethylamine-N-oxide and Heart Failure with Reduced Versus Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 3202–3204. [Google Scholar] [CrossRef]
- Ma, S.R.; Tong, Q.; Lin, Y.; Pan, L.B.; Fu, J.; Peng, R.; Zhang, X.F.; Zhao, Z.X.; Li, Y.; Yu, J.B.; et al. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Target. Ther. 2022, 7, 207. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arter. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, Q.; Xiong, Z.; Xian, G.; Liu, Z.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; et al. Trimethylamine N-oxide induces osteogenic responses in human aortic valve interstitial cells in vitro and aggravates aortic valve lesions in mice. Cardiovasc. Res. 2022, 118, 2018–2030. [Google Scholar] [CrossRef]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef]
- Cui, W.; Hao, M.; Yang, X.; Yin, C.; Chu, B. Gut microbial metabolism in ferroptosis and colorectal cancer. Trends Cell Biol. 2025, 35, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Shampo, M.A.; Kyle, R.A. Henrik Dam--Discoverer of Vitamin K. Mayo Clin. Proc. 1998, 73, 46. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mazumder, P.M.; Sarkar, S.R.; Saha, R.; Chatterjee, A.; Sarkar, B.; Banerjee, S. Neuroprotective effect of Vitamin K2 against gut dysbiosis associated cognitive decline. Physiol. Behav. 2023, 269, 114252. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, A.; Rais, Y.; Monsonego-Ornan, E. Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arter. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e55–e71. [Google Scholar] [CrossRef]
- Wallin, R.; Cain, D.; Hutson, S.M.; Sane, D.C.; Loeser, R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2). Thromb. Haemost. 2000, 84, 1039–1044. [Google Scholar] [CrossRef]
- Kaufman, D.W.; Kelly, J.P.; Curhan, G.C.; Anderson, T.E.; Dretler, S.P.; Preminger, G.M.; Cave, D.R. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol. 2008, 19, 1197–1203. [Google Scholar] [CrossRef]
- Asokan, D.; Kalaiselvi, P.; Varalakshmi, P. Modulatory effect of the 23-kD calcium oxalate monohydrate binding protein on calcium oxalate stone formation during oxalate stress. Nephron Physiol. 2004, 97, 23–30. [Google Scholar] [CrossRef]
- Daniel, S.L.; Moradi, L.; Paiste, H.; Wood, K.D.; Assimos, D.G.; Holmes, R.P.; Nazzal, L.; Hatch, M.; Knight, J. Forty Years of Oxalobacter formigenes, a Gutsy Oxalate-Degrading Specialist. Appl. Environ. Microbiol. 2021, 87, e0054421. [Google Scholar] [CrossRef]
- Lee, M.H.; Nuccio, S.P.; Mohanty, I.; Hagey, L.R.; Dorrestein, P.C.; Chu, H.; Raffatellu, M. How bile acids and the microbiota interact to shape host immunity. Nat. Rev. Immunol. 2024, 24, 798–809. [Google Scholar] [CrossRef]
- Liu, S.; Gao, Z.; He, W.; Wu, Y.; Liu, J.; Zhang, S.; Yan, L.; Mao, S.; Shi, X.; Fan, W.; et al. The gut microbiota metabolite glycochenodeoxycholate activates TFR-ACSL4-mediated ferroptosis to promote the development of environmental toxin-linked MAFLD. Free Radic. Biol. Med. 2022, 193, 213–226. [Google Scholar] [CrossRef]
- Miyazaki-Anzai, S.; Masuda, M.; Shiozaki, Y.; Keenan, A.L.; Chonchol, M.; Kremoser, C.; Miyazaki, M. Free Deoxycholic Acid Exacerbates Vascular Calcification in CKD through ER Stress-Mediated ATF4 Activation. Kidney360 2021, 2, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Jovanovich, A.; Cai, X.; Frazier, R.; Bundy, J.D.; He, J.; Rao, P.; Lora, C.; Dobre, M.; Go, A.; Shafi, T.; et al. Deoxycholic Acid and Coronary Artery Calcification in the Chronic Renal Insufficiency Cohort. J. Am. Heart Assoc. 2022, 11, e022891. [Google Scholar] [CrossRef]
- Hashimoto, N.; Matsui, I.; Ishizuka, S.; Inoue, K.; Matsumoto, A.; Shimada, K.; Hori, S.; Lee, D.G.; Yasuda, S.; Katsuma, Y.; et al. Lithocholic acid increases intestinal phosphate and calcium absorption in a vitamin D receptor dependent but transcellular pathway independent manner. Kidney Int. 2020, 97, 1164–1180. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Ma, J.; Zhu, H.; Qi, Y.; Jin, Y.; Zhang, M.; Yin, L.; Luo, M.; Chen, S.; Xie, C.; et al. Blood metabolites mediate causal inference studies on the effect of gut microbiota on the risk of vascular calcification. J. Adv. Res. 2025, 79, 619–631. [Google Scholar] [CrossRef]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 2021, 29, 394–407. [Google Scholar] [CrossRef]
- Koay, Y.C.; Chen, Y.C.; Wali, J.A.; Luk, A.W.S.; Li, M.; Doma, H.; Reimark, R.; Zaldivia, M.T.K.; Habtom, H.T.; Franks, A.E.; et al. Plasma levels of trimethylamine-N-oxide can be increased with ‘healthy’ and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc. Res. 2021, 117, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, J.; Sun, X.; Zhang, Z.; Sun, Y.; Jin, Y.; Wu, T. Targeting the human gut microbiome: A comparative review of probiotics, prebiotics, synbiotics, and postbiotics. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Shi, J.; Xie, Q.; Yue, Y.; Chen, Q.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Gut microbiota modulation and anti-inflammatory properties of mixed lactobacilli in dextran sodium sulfate-induced colitis in mice. Food Funct. 2021, 12, 5130–5143. [Google Scholar] [CrossRef]
- Li, M.; Ding, J.; Stanton, C.; Ross, R.P.; Zhao, J.; Yang, B.; Chen, W. Bifidobacterium longum subsp. infantis FJSYZ1M3 ameliorates DSS-induced colitis by maintaining the intestinal barrier, regulating inflammatory cytokines, and modifying gut microbiota. Food Funct. 2023, 14, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Fine, R.L.; Mubiru, D.L.; Kriegel, M.A. Friend or foe? Lactobacillus in the context of autoimmune disease. Adv. Immunol. 2020, 146, 29–56. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Liu, X.; Cheng, L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. Faseb J. 2020, 34, 14166–14181. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, Y.; Yu, L.; Cao, L.; Liu, S.; Zhong, L.; Wang, G.; Qiu, X.; Hou, H. Biomimetic wrinkled prebiotic microspheres with enhanced intestinal retention for hyperphosphatemia and vascular calcification. Sci. Adv. 2025, 11, eads5286. [Google Scholar] [CrossRef] [PubMed]
- Hoving, L.R.; Katiraei, S.; Pronk, A.; Heijink, M.; Vonk, K.K.D.; Amghar-El Bouazzaoui, F.; Vermeulen, R.; Drinkwaard, L.; Giera, M.; van Harmelen, V.; et al. The prebiotic inulin modulates gut microbiota but does not ameliorate atherosclerosis in hypercholesterolemic APOE*3-Leiden.CETP mice. Sci. Rep. 2018, 8, 16515. [Google Scholar] [CrossRef]
- Anegkamol, W.; Bowonsomsarit, W.; Taweevisit, M.; Tumwasorn, S.; Thongsricome, T.; Kaewwongse, M.; Pitchyangkura, R.; Tosukhowong, P.; Chuaypen, N.; Dissayabutra, T. Synbiotics as a novel therapeutic approach for hyperphosphatemia and hyperparathyroidism in chronic kidney disease rats. Sci. Rep. 2025, 15, 7493. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Wilund, K.R.; Tomayko, E.J.; Evans, E.M.; Kim, K.; Ishaque, M.R.; Fernhall, B. Physical activity, coronary artery calcium, and bone mineral density in elderly men and women: A preliminary investigation. Metabolism 2008, 57, 584–591. [Google Scholar] [CrossRef]
- Shibata, S.; Levine, B.D. Effect of exercise training on biologic vascular age in healthy seniors. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1340–H1346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.