Abstract
In mammalian sperm, the regulation of intracellular calcium (Ca2+) is essential for fertility. Semen processing for assisted reproduction may disturb Ca2+ homeostasis. This study aimed to investigate whether chilling boar sperm to 5 °C and subsequent storage affect the function of intracellular Ca2+ stores. Semen was stored in BTS-extender at 5 °C or 17 °C (control) for up to five days. Fluo-4/AM-loaded aliquots were incubated in Ca2+-free Tyrode’s medium at 38 °C. Sperm preserved at 17 °C had higher free intracellular Ca2+ levels compared with those stored at 5 °C (p < 0.05). However, there was no difference between storage groups in Ca2+ levels during incubation at 38 °C. Thimerosal, a sensitizer of Ca2+ channels, was added, and changes in the free intracellular Ca2+ concentration were monitored in viable acrosome-intact sperm by continuous flow cytometry. There was no effect of storage temperature on the kinetic response to thimerosal at days 1 and 3. At day 5, the relative increase in Ca2+ was higher in 5 °C-stored samples after 3 min of incubation. At 60 and 120 min of incubation, the thimerosal response was no longer influenced by the storage temperature or storage duration. In conclusion, chilling and storage do not affect the release dynamics of free Ca2+ from intracellular stores in viable boar sperm after rewarming.
1. Introduction
Boar semen is typically stored in a liquid state at 16 to 18 °C to avoid any chilling injury associated with lower temperatures. Triggered by the increasing prevalence of antimicrobial resistance arising from the routine use of antibiotic additives in semen extenders, cold storage of boar semen at 5 °C has recently been introduced as a preservation method that permits the omission of antibiotics. Despite the high performance of preserved semen in vitro and in vivo (reviewed by Waberski & Luther, [1]), there remain concerns that chilling injury could harm boar spermatozoa, particularly with subsequent long-term storage.
Pig breeding is an economically important agribusiness sector [2], in which even minor, sublethal sperm damage may compromise the performance and profitability of traded semen. Consequently, there is a need to further explore possible subtle effects of chilling on aspects of sperm function that are essential for fertilization.
A key component of sperm function is the ability to regulate free cytosolic calcium (Ca2+). Calcium serves as a crucial second messenger in physiological processes in mature sperm (reviewed in Mata-Martinez, et al. [3]). The capability for intracellular Ca2+ storage and release is central to capacitation, hyperactivation, and the acrosome reaction [4,5,6]. Sperm organelles and membranous compartments that can serve as calcium reservoirs include the acrosome, mitochondria, and calreticulin-containing vesicles of the redundant nuclear envelope [5,7]. All of those are present in boar spermatozoa, like in the sperm of other mammalian species.
To elicit the appropriate spatio-temporal oscillations in cytosolic Ca2+ levels, free intracellular Ca2+ is tightly regulated in sperm. The regulatory machinery includes a variety of Ca2+ transporters, e.g., Ca2+-ATPases and Na+-Ca2+ exchangers [8,9]; Ca2+ channels in the plasma membrane [10]; Ca2+-binding structures in the cytoplasm (calmodulin; [11]); and intracellular Ca2+ stores in the acrosome, the redundant nuclear envelope, and mitochondria [11,12,13].
These stores play a central role in regulating intracellular Ca2+ concentration and are operated mainly by sarcoplasmic–endoplasmic reticulum ATPases that are located in the acrosome and midpiece of boar spermatozoa [9] and by channels that mobilize stored Ca2+, such as those gated by inositol 1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR) [12,13,14] (as reviewed by Mata-Martinez, et al. [3]).
It is well known that semen freezing or cooling followed by storage at temperatures above 0 °C disturbs intracellular Ca2+ homeostasis, Ca2+ signaling, and ultimately sperm function [15,16,17]. These phenomena are mainly attributed to increased leakiness to Ca2+ ions at the plasma membrane, allowing entry of extracellular Ca2+. The effects of cooling on the regulation of cytosolic Ca2+ levels by intracellular stores are less clear, particularly in sperm that survive chilling stress.
Therefore, the aim of this study was to investigate whether chilling and storage impair the functionality of intracellular Ca2+ stores in viable acrosome-intact boar spermatozoa after rewarming. To this end, a moderate chilling injury was provoked by the rapid cooling of semen in a short-term extender before storage at 5 °C, whereas samples stored at 17 °C served as the control. The effects of chilling and storage on basal, free intracellular Ca2+ levels and on the kinetic response of viable acrosome-intact sperm to thimerosal (sodium ethylmercurithiosalicylate), a sensitizer of IP3R- and RyR-gated Ca2+ channels, were studied.
2. Results
2.1. Effects of Storage Temperature and Time on Motility Parameters, Viability, and Acrosome Integrity
Motility (% total motile sperm) was significantly influenced by the semen storage temperature (p < 0.05, Table 1). On day 1 (d 1) of storage, motility in samples stored at 5 °C was significantly lower compared with the RT samples (d 0) and samples stored at 17 °C (p < 0.05). Samples stored at 17 °C did not show a decline in motility compared to the RT samples until day 3 of storage. Storage time had no effect on sperm motility at either temperature (p > 0.05). The mean values for velocity, linearity, beat-cross frequency, and amplitude of lateral head displacement of progressively motile sperm were not influenced by the storage temperature or time (p > 0.05). A storage temperature of 5 °C reduced the viable acrosome-intact sperm population on day 1 of storage compared with the RT samples (d 0) and samples stored at 17 °C (p < 0.05). Prolonged storage up to day 5 (d 5) had no effect on the percentage of viable acrosome-intact sperm stored at 5 °C or 17 °C (Table 1). In summary, semen stored in BTS at 5 °C showed a moderate reduction in sperm motility and membrane integrity, indicating a suitable condition for subsequent investigation of chilling injury in this study.

Table 1.
Motility characteristics and viability for boar spermatozoa at the day of semen dilution (Day 0) and during storage at 17 °C or 5 °C for up to five days. All data are the means and standard deviations (mean ± SD). Different letters (a,b) within a column indicate significant differences (p < 0.05; n = 6 boars).
2.2. Effect of Storage Temperature and Time on Baseline Free Intracellular Ca2+ Levels During Incubation at 38 °C
Changes in the free intracellular Ca2+ levels due to the storage temperature and storage time were evaluated from baseline values for the fluorescence intensity of Fluo-4 in control samples after 3, 60, and 120 min of incubation at 38 °C in Ca2+-free Tyrode’s medium (Figure 1). The gating strategy considered exclusively viable spermatozoa with intact acrosomes. The storage temperature had a significant effect (F(1, 5) = 31.240, p < 0.01) on the free intracellular Ca2+ levels in viable acrosome-intact boar sperm, and the storage time also had a significant effect (F(2, 10) = 10.538, p < 0.01). At 3 min, samples stored at 17 °C had consistently higher free intracellular Ca2+ levels than those samples stored at 5 °C (p < 0.05). The effects of storage temperature (F(1, 5) = 7.882, p < 0.05) and storage time (F(2, 10) = 4.221, p < 0.05) were weaker after incubation for 60 min and absent after 120 min (p > 0.05). At 60 and 120 min of incubation at 38 °C, there was no difference in intracellular Ca2+ levels between the samples stored at 17 °C and 5 °C. In summary, viable acrosome-intact boar spermatozoa are able to maintain low intracellular free Ca2+ after chilling. During rewarming to body temperature, intracellular Ca2+ levels are similarly regulated in chilled and control sperm.
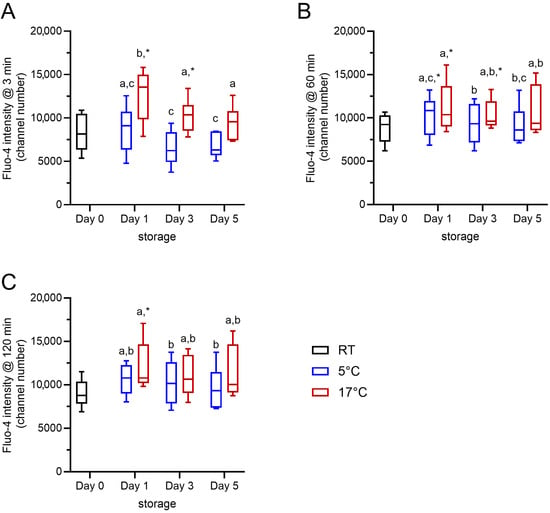
Figure 1.
Intracellular Ca2+ levels for samples at RT (day 0) and subsequently stored at 17 °C (red) or 5 °C (blue) for up to 5 days (n = 6 boars). Baseline values of the average fluorescence intensity for Fluo-4, i.e., the free intracellular Ca2+ level, in viable acrosome-intact sperm after 3 min (A), 60 min (B), and 120 min (C) incubation at 38 °C have been plotted. Storage temperature (17 °C vs. 5 °C) and storage time (day 1 vs. day 3 vs. day 5) had a significant impact on the intracellular Ca2+ levels (p < 0.05). Different small letters (a–c) indicate significant differences (p < 0.05). An asterisk indicates significant differences between a given storage time by temperature combination and the reference point, i.e., the sample on the day of dilution (day 0; p < 0.05).
2.3. Dose–Response Effect of Thimerosal on Intracellular Ca2+ Concentration
The free intracellular addition of thimerosal induced a rapid and dose-dependent rise in intracellular Ca2+ concentration in all samples, whereas no change was observed in the control samples. Changes in intracellular Ca2+ levels were reported as relative changes, with the baseline value serving as the reference point for each measurement. The gating strategy considered exclusively viable spermatozoa with intact acrosomes.
Representative dose–response curves are shown in Figure 2A. A concentration of 100 µM thimerosal was selected for subsequent experiments because it produced the most pronounced changes at all evaluated time points (1, 3, and 5 min) after addition (Figure 2B). In summary, a thimerosal concentration of 100 µM was identified for studies of Ca2+ release from intracellular stores.
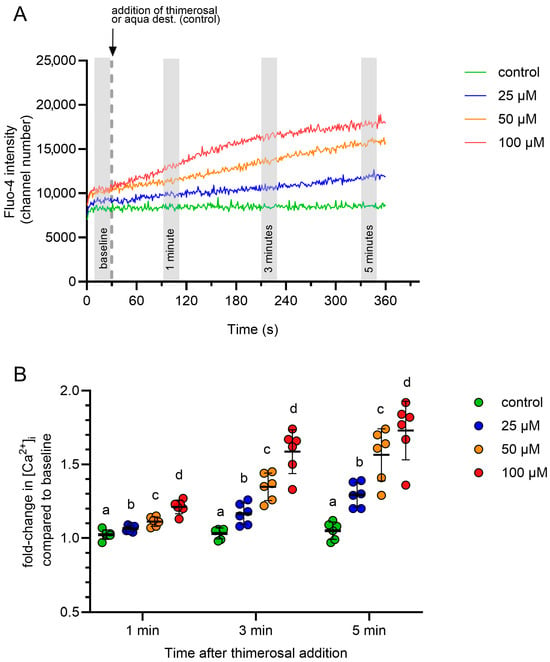
Figure 2.
(A) Line graph illustrating the change in average fluorescence intensity for Fluo-4 in viable acrosome-intact spermatozoa to increasing concentrations of thimerosal or a solvent control (aqua dest.). Each line is the average of data from six independent measurements (n = 6 boars). Average fluorescence intensities for Fluo-4 for each boar were calculated for 20 s intervals directly before the addition of thimerosal (baseline), and 1 min, 3 min, and 5 min after the addition of thimerosal (shaded areas). (B) Relative changes in fluorescence intensity after the addition of thimerosal or aqua dest. (control) were calculated in relation to the baseline values of each curve. Different small letters (a–d) indicate significant differences between treatments at a given time point (p < 0.05; mean ± SD, n = 6 boars).
2.4. Localization of Free Intracellular Ca2+ Signals
The localization and quantification of signal intensities in viable acrosome-intact sperm treated with 100 µM thimerosal, and the control (aqua dest.), are shown in Figure 3A,B. Thimerosal increased the overall fluorescence intensity of Fluo-4 compared to the control condition (Figure 3C). Calcium signals were visible throughout the whole midpiece and principal piece during live imaging—the highest signal intensities after thimerosal application were observed on the head base and the proximal midpiece. Due to an out-of-focus position of the tail during image acquisition, calcium signals were not visualized in the distal half of the midpiece and alongside the principal and end piece of the sperm tail.
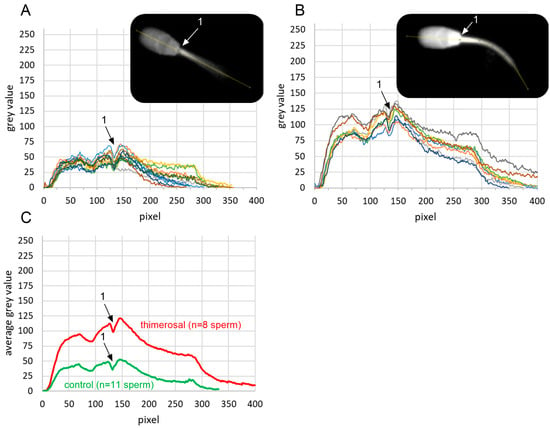
Figure 3.
Localization and quantification of free intracellular calcium levels in viable boar spermatozoa on the day of semen collection, before and after exposure to thimerosal. Fluo-4 signal intensities in viable, i.e., propidium iodide-negative spermatozoa, were quantified in the longitudinal axis of the sperm head and anterior midpiece (yellow line in gray-scale images inserted in (A,B)) in ImageJ. Intensity values were plotted for control (aqua dest.; (A); n = 11 spermatozoa) and exposure to 100 µM thimerosal for 5 min ((B); n = 8 spermatozoa). Each colored line represents the intensity profile for an individual spermatozoon. Intensity profiles were aligned based on a characteristic local minimum in fluorescence intensity at the junction between the sperm head and tail, indicated by position ‘1’ in the images and plots. Average gray values for both groups are shown in (C).
2.5. Effects of Storage Temperature and Time on Thimerosal-Induced Release of Ca2+ from Intracellular Stores
An effect of storage temperature on Ca2+ release from intracellular stores induced by 100 µM thimerosal was absent on day 1 (d1) of storage (p > 0.05; Figure 4A–C). At all timepoints, the gating strategy considered exclusively viable spermatozoa with intact acrosomes. A significant effect of storage temperature was noted on day 5 (d5) of storage after 5 min of incubation at 38 °C (F(1, 5) = 6.691, p < 0.05; Figure 4G). The relative change in [Ca2+]i for sperm stored at 5 °C tended to be higher 1 min (p = 0.060) and 3 min (p = 0.080) after the thimerosal addition, and was significantly higher 5 min after the thimerosal addition. A similar trend was observed after 60 min of incubation at 38 °C on day 3 (F(1, 5) = 5.955, p = 0.059; Figure 4E) and day 5 (F(1, 5) = 4.497, p = 0.087; Figure 4H). At both time points, samples stored at 5 °C tended to show a greater amplitude in the initial increase in intracellular Ca2+ levels, i.e., 1 min after thimerosal addition (Figure 4E: p = 0.079; Figure 4H: p = 0.095). In addition, the maximum increase in intracellular Ca2+ levels at 3 min (Figure 4E: p = 0.066; Figure 4H: p = 0.103) and 5 min (Figure 4E: p = 0.064; Figure 4H: p = 0.081) after the addition of thimerosal tended to be higher. After 120 min of incubation, changes in free intracellular Ca2+ levels showed non-significant differences between the samples stored at 17 °C and at 5 °C, regardless of the semen storage time (Figure 4C,F,I).
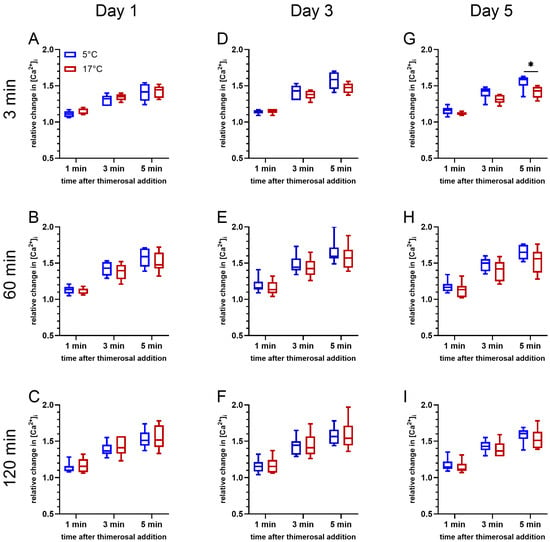
Figure 4.
Changes in free intracellular Ca2+ levels in viable acrosome-intact spermatozoa after 3 min (A,D,G), 60 min (B,E,H), and 120 min (C,F,I) of incubation at 38 °C for samples stored at 17 °C or 5 °C for up to five days (n = 6 boars). Changes in fluorescence intensity for Fluo-4 (F1) in relation to a reference point (F0), i.e., the baseline fluorescence intensities before addition of 100 µM thimerosal have been calculated (relative intracellular Ca2+ level = F1/F0). An asterisk indicates significant differences between the samples stored at different temperatures (p < 0.05).
The aforementioned data focus on evaluating the effects of the storage temperature and storage time. However, it should be noted that Ca2+-release dynamics from intracellular stores after the thimerosal addition consistently showed a lower amplitude for the samples stored at 17 °C after 3 min of pre-incubation on all days of storage (d 1, d 3, and d 5), compared with freshly diluted samples on the day of semen collection (d 0; Supplemental Figure S1). For samples stored at 5 °C, this was evident only at d 1. After 60 or 120 min of pre-incubation, no differences in release dynamics between the stored and fresh samples were evident (Supplemental Figures S2 and S3). In summary, after chilling and storage, viable acrosome-intact sperm maintained their ability for Ca2+ release from intracellular stores during rewarming to body temperature.
3. Discussion
Understanding the functional alterations in viable spermatozoa is essential for elucidating the impact of preservation-related stressors on sperm fertilizing capacity. The present study shows that chilling and storage do not have a pronounced effect on the kinetics of modulator-induced Ca2+ release, mediated by inositol 1,4,5-trisphosphate receptors (IP3Rs), from intracellular stores in boar spermatozoa.
It is well established, and confirmed in the present study, that subsets of the heterogeneous sperm population are particularly sensitive to chilling injury, exhibiting a loss of motility and membrane integrity, especially when rapidly cooled and stored in the basic short-term semen extender used here. Surviving spermatozoa constitute a more resistant population, which, nonetheless, may experience sublethal damage. This could affect essential steps in fertilization, many of which are tightly regulated by cytosolic Ca2+.
After ejaculation, and before activation in the female reproductive tract, cytosolic Ca2+ concentrations in functionally intact spermatozoa are maintained at low levels (<100 nM; Ho, et al. [18]). Increased cytosolic Ca2+ concentrations in freshly ejaculated or stored semen samples are associated with membrane destabilization and premature stages of capacitation, caused by either the uptake of extracellular Ca2+ or release from intracellular stores [17,19]. To examine alterations in the mobilization of internal Ca2+ stores, in the present study, all steps of semen processing (i.e., dilution, chilling, storage, and rewarming) were performed in a Ca2+-free, non-capacitating environment. Baseline levels of free intracellular Ca2+ were lower in viable acrosome-intact spermatozoa from chilled samples (5 °C) than in the control fresh samples (day 0, RT) and in the samples stored at the conventional temperature (17 °C). In contrast, an earlier fluorometric study reported a significant increase in internal Ca2+ immediately after chilling boar spermatozoa in an essentially Ca2+-free PBS (without an added Ca2+ chelator), which was subsequently reversed after incubation at 25 °C for 150 min [17]. In the present study, Ca2+ concentrations were studied exclusively in the subpopulation of plasma membrane-intact, non-capacitated spermatozoa showing no signs of acrosomal exocytosis. It is suggested that the cell population surviving the chilling injury (approximately 50% in the present study) possesses a highly efficient internal regulatory system that allows them to maintain low cytosolic Ca2+ concentrations in the absence of extracellular Ca2+.
Storage duration exerted less stress on the Ca2+-regulatory system than relatively rapid cooling in a non-cold-shock protective semen extender. Baseline fluorescence intensities for the Ca2+ probe (before addition of thimerosal) indicate that the effects of temperature and storage on intracellular Ca2+ levels became less pronounced with continued rewarming of the samples to body temperature. By the end of incubation (120 min), the preceding storage temperature no longer influenced the cytosolic Ca2+ levels, indicating the high efficiency of internal Ca2+-regulatory mechanisms in restoring Ca2+ homeostasis. We suggest that warming reactivates ATP-dependent Ca2+-regulatory units, which promote Ca2+ uptake into intracellular stores. Calcium transport from the cytosol into stores normally occurs against the electrochemical gradient and, therefore, requires energy. Typically, this is achieved by ATPase pumps such as the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and secretory pathway Ca2+-ATPases (SPCA). Additionally, Ca2+ exchangers may be involved [5]. In boar semen stored for three days at 5 or 17 °C, the ATP content and energy charge were found to be highly correlated with sperm viability, suggesting that surviving cells maintain efficient energy metabolism that allows them to reconstitute Ca2+ regulation. Additionally, the previous study showed that the ATP content and energy charge in sperm rewarmed to 38 °C after storage for 24 h at 5 °C did not differ from the sperm that were stored at 17 °C, indicating a high resilience of energy metabolism to chilling stress that was provoked by rapid cooling in a basic short-term semen extender [20].
Mobilization of Ca2+ from intracellular stores is a key event in capacitation, hyperactivation, and the acrosome reaction [5,21]. A main objective, therefore, was to investigate whether semen chilling and storage affect the function of thimerosal-sensitive intracellular Ca2+ stores in viable boar spermatozoa. Thimerosal stimulates Ca2+ flux through IP3Rs and RyRs in a similar manner, probably by targeting a highly conserved sequence containing two cysteine residues near the carboxyl terminus of these receptors [22]. Treatment of spermatozoa with thimerosal stimulates the release of Ca2+ from intracellular stores [23]. In many cell types, IP3Rs and ryanodine receptors (RyRs) represent the two principal intracellular Ca2+ channels responsible for releasing stored Ca2+ [12]. In boar spermatozoa, type 1 IP3Rs (IP3R1) are present in the connecting piece and the acrosome [24], and it was also reported that IP3R1 is located in the neck region and, at a lower density, along the axonemal membrane [11]. The presence of RyRs in boar spermatozoa remains unknown. They have been detected in mature rodents [25] and human spermatozoa [14], but not in bovine spermatozoa [12].
In the present study, the addition of thiomersal to a Ca2+-free, non-capacitating medium induced a dose-dependent increase in internal Ca2+ within seconds. Thimerosal was used at a concentration of 100 µM to illustrate the relative change in cytosolic Ca2+, although a lower concentration (25 µM) was also effective. Hyperactivated motility has been induced using different concentrations of thimerosal, such as 20–100 µM, in bulls [12], 25 µM in boar [26], 5 µM in humans [27], and 50 µM in mice [28,29]. In our study, the kinetics of Ca2+ release revealed a dose-dependent relative increase in internal Ca2+ over 5 min following the addition of thimerosal. Storage reduced spermatozoa’s initial response to thimerosal regardless of the temperature. However, rewarming the samples prior to a prolonged thimerosal challenge abolished the storage effect, supporting the idea of an active, energy- and/or ion flux-dependent restorative mechanism of intracellular Ca2+ homeostasis. Notably, hypothermic semen storage at 5 °C, compared with 17 °C, prior to rewarming, did not affect thimerosal-induced Ca2+ mobilization. Likewise, the level of the response to thimerosal in spermatozoa stored at 5 °C and 17 °C was similar, regardless of the duration of pre-incubation at 38 °C. Together, these observations point to an efficient and robust activation mechanism of IP3 receptors that enables well-controlled Ca2+ release in spermatozoa that have survived chilling stress. Whether chilling affects the upstream components of the phosphoinositide signaling pathway, leading to the generation of IP3, or interferes with the formation or function of receptor-mediated activators, was not the subject of the present study. The activity of IP3R- and RyR-gated channels is modulated by several factors, including Ca2+, magnesium, ATP, and post-translational modifications [5]. Altered mobilization of internal Ca2+ stores due to chilling-induced imbalances in these components cannot be ruled out. Nonetheless, the observed high resilience of viable sperm after chilling to 5 °C is in agreement with previous observations in boar semen doses that were subjected to stress from storage [30] and transport-related vibrations [31,32]. In agreement with the results of the present study, chilled sperm maintain their functional integrity for capacitation and associated mitochondrial activity [33], both of which rely on the dynamic regulation of intracellular Ca2+.
In conclusion, chilling and storage do not affect the function of thimerosal-sensitive intracellular Ca2+ channels in viable acrosome-intact boar spermatozoa and thus confirm the safety for 5 °C storage protocols with respect to Ca2+ regulation. From a practical perspective, semen storage at 5 °C is advantageous for a reduction in bacterial growth. The present study revealed that spermatozoa surviving the initial chilling stress maintain their ability to regulate Ca2+ homeostasis by intracellular stores. Furthermore, besides enabling the omission of antibiotics from the semen extender, cold-stored sperm may even offer better long-term preservation than boar semen stored at 17 °C due to a higher stress resilience in the surviving sperm population.
4. Materials and Methods
4.1. Chemicals and Reagents
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich (Steinheim, Germany), Merck (Darmstadt, Germany), and Roth (Karlsruhe, Germany). Propidium iodide (PI) and Hoechst 33342 were obtained from Axxora (Lörrach, Germany). Fluo-4/AM and PNA-Alexa FluorTM 647 were obtained from Invitrogen (Thermo Fisher Scientific, Rockford, IL, USA). Thimerosal was obtained from Sigma-Aldrich (Steinheim, Germany).
4.2. Semen Collection and Processing
Semen (sperm-rich and sperm-poor fractions) was collected by trained personnel from a total of six mature, clinically healthy boars that were housed at the Unit for Reproductive Medicine, University of Veterinary Medicine Hannover, Germany. The boars, aged 12 months to 5 years, belonged to the breeds Piétrain, German Large White, and crossbred animals. Housing conditions and any handling of the boars were performed in accordance with the European Commission Directive for Pig Welfare and were approved by the Institutional Animal Welfare Committee of the University of Veterinary Medicine Hannover. Semen was collected using the “gloved-hand” technique and filtered through gauze to remove the gel fraction. Immediately after collection, the semen samples were transferred to the laboratory in insulated boxes. Only normospermic ejaculates were used for the experiments, defined as ejaculates with a volume ≥ 100 mL, concentration ≥ 160 × 106 sperm/mL, ≥70% motile sperm, ≤25% morphologically abnormal sperm, and ≤15% sperm with cytoplasmic droplets.
All semen samples were extended to a concentration of 20 × 106 sperm/mL with the pre-warmed (32 °C) short-term extender Beltsville Thawing Solution (BTS: 176.61 mM glucose, 20.4 mM trisodium citrate dihydrate, 15.47 mM NaHCO3, 10.73 mM KCl, 3.49 mM Na2-EDTA and 260 µg/mL gentamicin sulfate (SERVA, Heidelberg, Germany); 300 ± 5 mOsmol/kg; pH 7.0 at 22 °C).
Extended semen samples were kept at room temperature (RT, 21 ± 1 °C) for 120 min (d 0), after which a subsample was analyzed, and the remainder of the samples were moved to a 17 °C storage unit. Samples designated for final storage at 5 °C were held for 120 min at 17 °C before being transferred to a 5 °C storage unit.
4.3. Experimental Design
In Experiment 1, a dose–response of viable intact sperm to four different concentrations of thimerosal, 0 µM (control), 25 µM, 50 µM, and 100 µM, in freshly diluted semen, was studied. In Experiment 2, the extended semen was analyzed for sperm motility, viability, acrosome integrity, and baseline free intracellular Ca2+ levels in viable acrosome-intact sperm. In addition, changes in free intracellular Ca2+ levels in viable acrosome-intact spermatozoa upon stimulation with 100 µM thimerosal after 3 min, 60 min, and 120 min incubations at 38 °C, reflecting the body temperature in the pig’s female tract, were investigated. Analysis was performed on semen stored at 17 °C and 5 °C at four different time points after dilution: at 2 h (d 0), 24 h (d 1), 72 h (d 3), and 120 h (d 5).
4.4. Assessment of Sperm Motility
Diluted semen samples were incubated for 30 min at 38 °C in a water bath and assessed by computer-assisted sperm analysis (CASA) using AndroVision version 9.1 (Minitube, Tiefenbach, Germany), as described in Henning et al. [34]. Five successive fields in the central axis of a Leja chamber (Leja Products B.V., Nieuw-Vennep, The Netherlands) were recorded as a video (0.8 s, 60 frames per second). The AndroVision software considered sperm to be motile when their amplitude of lateral head displacement (ALH) exceeded 1.0 µm, and their curvilinear velocity (VCL) exceeded 24.0 µm/s.
4.5. Sample Incubation and Flow Cytometry
Aliquots of diluted semen were incubated with 2 µM of the Ca2+-sensitive probe Fluo-4/AM and 0.375 μg/mL Hoechst 33342 for 30 min at RT. Subsequently, 10 µL of stained sperm were added to 1990 µL of pre-warmed (38 °C), Ca2+-free Tyrode’s medium that was supplemented with Hoechst 33342 (final concentration: 0.6 µg/mL), PI (2 µg/mL), and PNA-Alexa Fluor™ 647 (3 µg/mL). The Ca2+-free Tyrode’s medium consisted of 112 mM NaCl, 3.1 mM KCl, 1 mM Na2-EGTA, 0.4 mM MgSO4, 5 mM glucose, 0.3 mM KH2PO4, 20 mM HEPES, 21.6 mM sodium lactate, 1 mM sodium pyruvate, 3 mg/mL of bovine serum albumin (BSA; Cohn’s Fraction V, fatty acid free, Sigma-Aldrich, Steinheim, Germany), 100 µg/mL of gentamicin sulfate and 20 µg/mL of phenol red with a final osmolality of 300 ± 5 mOsmol/kg. The pH was adjusted to 7.4 at 38 °C using NaOH. Subsequently, the samples were incubated in a metal heating block at 38 °C for 3 min, 60 min, or 120 min, respectively. After each incubation time, the samples were assessed on a CytoFlex flow cytometer (Beckman Coulter, Krefeld, Germany) that was controlled by “CytExpert” software (version 2.3, Beckman Coulter). Laser lines for excitation were at 405 nm (80 mW), 488 nm (50 mW), and 638 nm (50 mW), respectively. Emission filters for the detection of blue fluorescence (450/45 nm; Hoechst 33342), green fluorescence (525/40 nm, Fluo-4), orange fluorescence (585/42 nm; propidium iodide), and red fluorescence (660/10 nm; PNA-Alexa FluorTM 647) were used. A silicone tubing (length: 12 cm; inner diameter: 0.3 mm; order no. 14164, Reichelt Chemietechnik, Heidelberg, Germany) was placed on the sample pick-up probe and inserted into the sample tube. This setup made it possible to keep the sample tube in the metal heating block at 38 °C during the assessment and add compounds at designated time points.
The samples were read at a flow rate of 100 to 200 events per second for a total period of six minutes (360 s). The first 30 s of the recording served to assess the baseline values for the fluorescence intensity of Fluo-4 in viable acrosome-intact single sperm before thimerosal at a final concentration of 25 µM, 50 µM, or 100 µM was added to the sample. Control samples were run with the addition of the solvent only, i.e., distilled water. Data from the second 10 to 30 of the baseline reading were used to assess the percentage of viable acrosome-intact sperm as a basic measure of sperm quality.
The gating strategy started by defining a logical gate for DNA-containing events (Hoechst 33342-positive) with a forward scatter signal in the size range of single sperm. By this, debris and agglutinated sperm were excluded from the evaluation. Next, the population of viable sperm with intact acrosomes (PI-negative and PNA-Alexa FluorTM 647-negative) was defined. An overlap of emission spectra for PI and PNA-Alexa FluorTM 647 was corrected after the acquisition by mathematical compensation. Values for the Fluo-4 fluorescence intensity in viable acrosome-intact single sperm were exported to an Excel file, and the average free intracellular Ca2+ concentration, i.e., Fluo-4 fluorescence intensity, for viable acrosome-intact single sperm was calculated by Kaluza software (Version 2.1, Beckman Coulter) for each second of the measurement.
A representative flow cytometry plot for the changes in the free intracellular Ca2+ concentration in viable acrosome-intact single sperm upon addition of thimerosal or aqua dest. are given in Figure 5A,B. Each dot in the density plots represents a single spermatozoon with its fluorescence intensity for Fluo-4 (y-axis) at a given time (x-axis). The fluorescence intensities of 100 to 200 spermatozoa per second were averaged and plotted in Figure 5C,D. The zig-zag pattern corresponds to slight variations in the average fluorescence intensity of the analyzed subsample of sperm at each second. In order to minimize a putative “sampling bias” on the interpretation of the data, intracellular Ca2+ levels of spermatozoa were determined by averaging the Fluo-4 fluorescence intensity for 20 s at four different periods, i.e., before (baseline) and 1, 3, and 5 min after the addition of thimerosal or aqua dest. (Figure 5E).
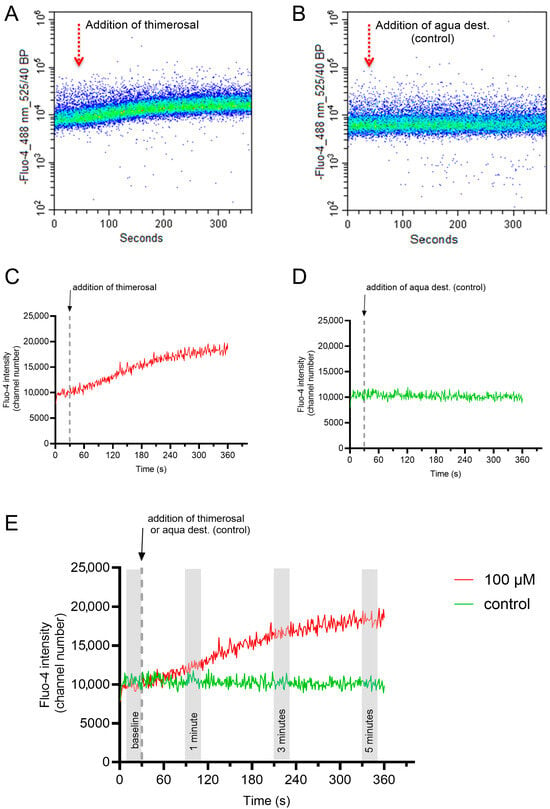
Figure 5.
Representative flow cytometry plot for the changes in the free intracellular Ca2+ concentration in viable acrosome-intact sperm upon addition of 100 µM thimerosal or the solvent (aqua dest; control). Data were gated to restrict the analysis to single, viable, and acrosome-intact sperm (Hoechst 33342-positive, PI-negative, and PNA-Alexa FluorTM 647-negative). The flow cytometry plots show the change in fluorescence intensity for Fluo-4 based on the single sperm data during the assessment (A,B). The corresponding graph depicts the average change in fluorescence intensity for each second of the assessment (C,D). For systematic comparisons of the response curves, fluorescence intensities over 20 s (Seconds 10 to 30) were averaged to represent the baseline Ca2+ values, as well as the Ca2+ levels at 1 min (Seconds 80 to 100), 3 min (Seconds 200 to 220), and 5 min (Seconds 320 to 340) after the addition of thimerosal/aqua dest were determined (E).
4.6. Imaging of Free Intracellular Calcium in Viable Boar Spermatozoa
Exemplary imaging of spermatozoa was performed on samples on the day of semen collection (d 0) for three boars. Aliquots of diluted semen in Ca2+-free Tyrodes’s medium supplemented with PI and Fluo-4/AM, as described above, were placed on pre-warmed (38 °C) glass slides, covered with pre-warmed cover slips (18 × 18 mm), and imaged at 1000× magnification with immersion oil on the heated stage (38 °C) of an Olympus BX 60 microscope (Olympus, Hamburg, Germany) with phase contrast optics, a mercury lamp, and a filter for 460–490 nm excitation and 515 nm long pass emission. Images were continuously acquired by a Gryphax NAOS color camera and Gryphax software, version 2.2.0.1234, (both Jenoptik AG, Jena, Germany) at 30 frames per second in fluorescence mode and saved as mpeg4 files. Individual frames were extracted from the video files for analysis using ImageJ, version 1.48v [35]. Frames were chosen, in which the entire head and anterior midpiece were in focus. Color channels (RGB) were separated, and the gray-scale image corresponding to the green channel was analyzed. A segmented line was drawn in the central axis of the sperm head and tail. Gray values for each pixel along the line were determined by ImageJ and exported for further processing.
4.7. Statistical Analysis
Data were analyzed using the statistical package SAS Enterprise Guide for Windows (version 7.1, SAS Institute Inc., Cary, NC, USA). Data from all assessments are presented as the mean ± SD (standard deviation). Data were tested for normal distribution with the Shapiro–Wilk test (PROC UNIVARIATE), and for equality of variances, with Mauchly’s test for sphericity. One-way and two-way repeated-measure ANOVAs were performed to assess the effects of storage temperature, storage time, and thimerosal concentration on the changes in intracellular Ca2+ concentration at defined time points after the thimerosal addition. The global test was followed by either two-sided Dunnett t-tests to identify after which time of storage the response differed from the response recorded in fresh diluted samples, or by a paired Student’s t-test to compare the samples stored at 5 °C and 17 °C, or the samples exposed to different thimerosal concentrations. The probability value of p < 0.05 was considered statistically significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms27031248/s1.
Author Contributions
Conceptualization, H.H. and D.W.; methodology, H.H.; software, D.W.; validation, H.H. and A.-M.L.; formal analysis, D.H.B.; investigation, D.H.B.; resources, D.W.; data curation, D.H.B. and H.H.; writing—original draft preparation, D.H.B.; writing—review and editing, H.H., D.W. and A.-M.L.; visualization, D.H.B., H.H. and A.-M.L.; supervision, D.W. and H.H.; project administration, D.W.; funding acquisition, D.H.B. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Open Access Publication Fund of the University of Veterinary Medicine Hannover, Foundation.
Institutional Review Board Statement
The animal study protocol was performed in accordance with the European Commission Directive for Pig Welfare and was approved by the Institutional Animal Welfare Committee of the University of Veterinary Medicine Hannover (approval number: TVO-2023-V-31; approval date: 21 June 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
Doanh Huy Bui was supported by a PhD scholarship from the Ministry of Agriculture and Rural Development (Vietnam) under the Agriculture and Fisheries Biotechnology Program (TS-2015, BNN) and a DAAD-MOET scholarship (no. 91613874; Germany).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waberski, D.; Luther, A.M. Boar semen storage at 5 degrees C for the reduction of antibiotic use in pig insemination: Pathways from science into practice. Anim. Reprod. Sci. 2024, 269, 107486. [Google Scholar] [CrossRef]
- Knox, R.V. Worldwide perspective for swine production and reproduction for the next 20 years. Theriogenology 2025, 234, 24–33. [Google Scholar] [CrossRef]
- Mata-Martinez, E.; Sanchez-Cardenas, C.; Chavez, J.C.; Guerrero, A.; Trevino, C.L.; Corkidi, G.; Montoya, F.; Hernandez-Herrera, P.; Buffone, M.G.; Balestrini, P.A.; et al. Role of calcium oscillations in sperm physiology. Biosystems 2021, 209, 104524. [Google Scholar] [CrossRef]
- Hong, C.Y.; Chiang, B.N.; Turner, P. Calcium ion is the key regulator of human sperm function. Lancet 1984, 2, 1449–1451. [Google Scholar] [CrossRef]
- Costello, S.; Michelangeli, F.; Nash, K.; Lefievre, L.; Morris, J.; Machado-Oliveira, G.; Barratt, C.; Kirkman-Brown, J.; Publicover, S. Ca2+-stores in sperm: Their identities and functions. Reproduction 2009, 138, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Suarez, S.S. Hyperactivation of mammalian spermatozoa: Function and regulation. Reproduction 2001, 122, 519–526. [Google Scholar] [CrossRef]
- Correia, J.; Michelangeli, F.; Publicover, S. Regulation and roles of Ca2+ stores in human sperm. Reproduction 2015, 150, R65–R76. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.; Wootton, L.; Michelangeli, F.; Lefievre, L.; Barratt, C.; Publicover, S. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca2+]i signals in human spermatozoa. J. Cell Sci. 2005, 118, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Garriga, F.; Martinez-Hernandez, J.; Parra-Balaguer, A.; Llavanera, M.; Yeste, M. The Sarcoplasmic/Endoplasmic reticulum Ca2+-ATPase (SERCA) is present in pig sperm and modulates their physiology over liquid preservation. Sci. Rep. 2025, 15, 4184. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Alvarez-Rodriguez, M.; Rodriguez-Martinez, H. The CatSper channel modulates boar sperm motility during capacitation. Reprod. Biol. 2017, 17, 69–78. [Google Scholar] [CrossRef]
- Gugssa, A.; Williams, A.; Tilahun, R.; Eckberg, W.; Anderson, W.; Ahluwalia, B.; Kaul, L.; Broomfield, D. Localization of guanylate cyclase receptor, inositol trisphosphate receptor, and calmodulin in boar spermatozoa. Int. J. Biol. 2010, 2, 13–18. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 2001, 65, 1606–1615. [Google Scholar] [CrossRef]
- Walensky, L.D.; Snyder, S.H. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 1995, 130, 857–869. [Google Scholar] [CrossRef]
- Harper, C.V.; Barratt, C.L.; Publicover, S.J. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca2+]i oscillations and cyclical transitions in flagellar beating. J. Biol. Chem. 2004, 279, 46315–46325. [Google Scholar] [CrossRef]
- Robertson, L.; Bailey, J.L.; Buhr, M.M. Effects of cold shock and phospholipase A2 on intact boar spermatozoa and sperm head plasma membranes. Mol. Reprod. Dev. 1990, 26, 143–149. [Google Scholar] [CrossRef] [PubMed]
- White, I.G. Lipids and calcium uptake of sperm in relation to cold shock and preservation: A review. Reprod. Fertil. Dev. 1993, 5, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Buhr, M.M. Regulation of internal Ca2+ by chilled bull and boar spermatozoa. Cryobiology 1995, 32, 259–269. [Google Scholar] [CrossRef]
- Ho, H.C.; Granish, K.A.; Suarez, S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002, 250, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Watson, P.F. Comparison of the capacitation-like state of cooled boar spermatozoa with true capacitation. Reproduction 2001, 122, 889–898. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Wallner, U.; Schmicke, M.; Waberski, D.; Henning, H. Energy metabolic state in hypothermically stored boar spermatozoa using a revised protocol for efficient ATP extraction. Biol. Open 2016, 5, 1743–1751. [Google Scholar] [CrossRef]
- Darszon, A.; Acevedo, J.J.; Galindo, B.E.; Hernández-González, E.O.; Nishigaki, T.; Treviño, C.L.; Wood, C.; Beltrán, C. Sperm channel diversity and functional multiplicity. Reproduction 2006, 131, 977–988. [Google Scholar] [CrossRef]
- Kaplin, A.I.; Ferris, C.D.; Voglmaier, S.M.; Snyder, S.H. Purified Reconstituted Inositol 1,4,5-Trisphosphate Receptors—Thiol Reagents Act Directly on Receptor Protein. J. Biol. Chem. 1994, 269, 28972–28978. [Google Scholar] [CrossRef]
- Chang, H.; Suarez, S.S. Two distinct Ca2+ signaling pathways modulate sperm flagellar beating patterns in mice. Biol. Reprod. 2011, 85, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Harayama, H.; Murase, T.; Miyake, M. A cyclic adenosine 3′,5′-monophosphate stimulates phospholipase Cgamma1-calcium signaling via the activation of tyrosine kinase in boar spermatozoa. J. Androl. 2005, 26, 732–740. [Google Scholar] [CrossRef]
- Trevino, C.L.; Santi, C.M.; Beltran, C.; Hernandez-Cruz, A.; Darszon, A.; Lomeli, H. Localisation of inositol trisphosphate and ryanodine receptors during mouse spermatogenesis: Possible functional implications. Zygote 1998, 6, 159–172. [Google Scholar] [CrossRef]
- Otsuka, N.; Harayama, H. Characterization of extracellular Ca2+-dependent full-type hyperactivation in ejaculated boar spermatozoa preincubated with a cAMP analog. Mol. Reprod. Dev. 2017, 84, 1203–1217. [Google Scholar] [CrossRef]
- Alasmari, W.; Costello, S.; Correia, J.; Oxenham, S.K.; Morris, J.; Fernandes, L.; Ramalho-Santos, J.; Kirkman-Brown, J.; Michelangeli, F.; Publicover, S.; et al. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J. Biol. Chem. 2013, 288, 6248–6258. [Google Scholar] [CrossRef]
- Marquez, B.; Ignotz, G.; Suarez, S.S. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 2007, 303, 214–221. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Zhou, G.-B.; Zeng, Y.; Li, J.-J.; Zhang, Q.-J.; Hou, Y.-P.; Zhu, S.-E. Impact on hyperactivated motility of cryopreserved mouse sperm from pretreatment with Thimerosal. Asian J. Anim. Vet. Adv. 2011, 6, 1052–1060. [Google Scholar] [CrossRef]
- Schmid, S.; Henning, H.; Oldenhof, H.; Wolkers, W.F.; Petrunkina, A.M.; Waberski, D. The specific response to capacitating stimuli is a sensitive indicator of chilling injury in hypothermically stored boar spermatozoa. Andrology 2013, 1, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, A.F.; Luther, A.M.; Jakop, U.; Schulze, M.; Bortolozzo, F.P.; Waberski, D. Factors influencing the response of spermatozoa to agitation stress: Implications for transport of extended boar semen. Theriogenology 2021, 175, 54–60. [Google Scholar] [CrossRef]
- Hensel, B.; Henneberg, S.; Riesenbeck, A.; Jung, M.; Schulze, M. Effects of vibrations during boar semen transport: Low-temperature transport as a new management tool. Anim. Reprod. Sci. 2024, 261, 107413. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, H.; Henning, H.; Luther, A.M.; Rohn, K.; Waberski, D. Assessment of chilling injury in hypothermic stored boar spermatozoa by multicolor flow cytometry. Cytom. Part A 2021, 99, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Henning, H.H.W.; Batz-Schott, J.; Grünther, B.; Le Thi, X.; Waberski, D. Fluorescent labelling of boar spermatozoa for quantitative studies on competitive sperm–oviduct binding. Reprod. Fertil. Dev. 2019, 31, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.