Abstract
The aim of this study was to investigate the effects on the tibia, liver, and gut, and on performance, when supplementing nursery pigs with different levels of rare earth–chitosan chelate (RECC). A total of 80 piglets, weaned at 7.67 ± 0.09 kg, were randomly assigned to groups RECC0 (RECC, 0 mg/kg diet), RECC200 (RECC, 200 mg/kg diet), RECC400 (RECC, 400 mg/kg diet), and RECC600 (RECC, 600 mg/kg diet), with four replicates in each group and five pigs per replicate during a 28 d experiment. Samples of the left hind tibia, serum, and feces were collected for analysis. The results indicated that, compared to pigs from group RECC0, pigs from group RECC200 presented with the following: a longer trabecular perimeter (p < 0.05), a larger trabecular area (p < 0.01), a higher trabecular number (p < 0.05), a smaller degree of trabecular separation (p < 0.01), and a lower number of osteoclasts (p < 0.01) in the tibia; higher abundances of beneficial fecal bacteria such as g_Prevotellaceae_NK3B31_group, g_UCG_005, g_Rikenellaceae_RC9_gut_group, g_Acetitomaculum, g_Glutamicibacter, g_Frisingicoccus, and g_Alistipes; higher (p < 0.01) serum levels of IgM, IgA, IgG, and IL-10; a lower (p < 0.01) serum concentration of TNF-α; a higher (p < 0.05) average daily gain and feed conversion ratio; and a lower (p < 0.01) incidence of diarrhea. The dietary addition of RECC contributes to improvements in tibia quality, gut health, and performance in nursery pigs.
1. Introduction
In recent years, the incidence of limb bone diseases in intensive pig farms has been increasing, and the clinical symptoms mainly include lameness, instability, or paralysis [1]. It has been reported that osteomalacia mainly manifests as abnormal walking, decreased weight-bearing capacity, skeletal deformation, or pain due to insufficient bone mineralization [2]. Osteoporosis is a bone metabolism disorder characterized by decreased bone mass, imbalanced bone tissue absorption and formation, abnormal bone trabeculae, increased bone fragility, and decreased weight-bearing capacity, making bones prone to fractures [3].
A number of factors influence bone health, performance, and disease resistance in animals [4,5,6], and nutrition can influence these parameters in pigs by regulating the absorption of dietary nutrients and the composition of the gut microbiota [7,8,9]. Low dietary Ca intake results in bone loss by increasing vitamin D receptor expression in mice osteoclasts [10] or affecting mesenchymal stem cell activity in piglets [11]. The administration of phytase to gilts and barrows significantly reduces the cortical wall thickness and index of metacarpal bones by affecting the intake of calcium and phosphorus [12]. Supplementing growing gilts with copper, manganese, and zinc enhances the strength and density of bone [13].
Rare earth elements, especially lanthanum and cerium, are also used as safe feed additives to promote animal health, performance, and bone quality [14]. Bone formation is a complex process that occurs through the regulation of osteogenesis, osteoclastogenesis, and angiogenesis by nutrients [15]. Trivalent lanthanum can increase cortical bone density and bone volume, prevent the differentiation and maturation of osteoclasts, directly stimulate bone formation, and inhibit osteolysis by orchestrating the balance between osteoblasts and osteoclasts [16,17]. Local bone angiogenesis can accelerate bone development and fracture healing [18,19], and treating bone marrow mesenchymal stem cells with lanthanum or cerium ions was found to significantly promote angiogenesis [20]. Moreover, the addition of CeO to the diet of broilers improved tibia quality by increasing bone strength and osteocalcin gene expression [21].
The gut microbiota can also influence bone health [22,23,24] because gut dysbiosis can lead to bone diseases due to abnormal nutrient absorption and systemic inflammation [25,26,27]. It has been reported that chitosan can also improve animal health and performance, modulate gut microbiota [28], and induce bone formation [29]. Rare earth–chitosan chelates (RECC) are feed additives produced by chelating lanthanum and cerium ions from rare earth elements with chitosan. Previous experiments indicated that the dietary inclusion of RECC in laying hens at 100 mg/kg reduced the levels of interleukin 2 (IL-2) and tumor necrosis factor-alpha (TNF-α) [30]. In addition, the supplementation of RECC in the diet of sows during the perinatal period increased the relative abundances of Ruminococcaceae and Christensenellaceae but inhibited the growth of pathogenic Proteobacteria and Campylobacter and elevated serum levels of the growth hormone and insulin-like growth factor-1 [31].
The tibia is one of the most important bones in pigs, playing a crucial role in weight-bearing and walking [32]. However, the effects of RECC on the bone quality of nursery pigs have not been reported to date. The aim of this study was to investigate the effects of RECC on tibia quality, fecal microbiota, performance, immunity, and inflammation in nursery pigs, providing technical support for RECC addition into the diet of nursery pigs.
2. Results
2.1. Effects of Dietary RECC Addition on Performance of Nursery Pigs
The results in Table 1 show that nursery pigs from group RECC200 had a higher final live bodyweight (p < 0.01) and a higher average daily gain (p < 0.05) than nursery pigs from groups RECC0, RECC400, and RECC600, respectively. Meanwhile, nursery pigs from group RECC200 also had a lower feed-to-gain ratio (p < 0.05) and a lower incidence of diarrhea (p < 0.01) than nursery pigs from groups RECC0 and RECC600, respectively. As the levels of RECC increased, the final live body weight and average daily gain decreased, but the feed-to-gain ratio and the incidence of diarrhea increased, respectively.

Table 1.
Performance of nursery pigs supplemented with different RECC levels.
2.2. Effects of Dietary RECC Addition on Tibia Microarchitecture of Nursery Pigs
The dietary addition of RECC to nursery pigs improved the trabecular bone microarchitecture (Figure 1) and decreased the number of osteoclasts (Figure 2). The results for the tibia microarchitecture, shown in Table 2, indicated that the dietary addition of RECC increased the trabecular perimeter, trabecular area, and trabecular number, but decreased the trabecular separation and osteoclast numbers. Groups RECC200 and RECC400 presented a longer trabecular perimeter (p < 0.05), a larger trabecular area (p < 0.01), a higher trabecular number (p < 0.05), a smaller degree of trabecular separation (p < 0.01), and a lower number of osteoclasts (p < 0.01) than group RECC0, respectively.
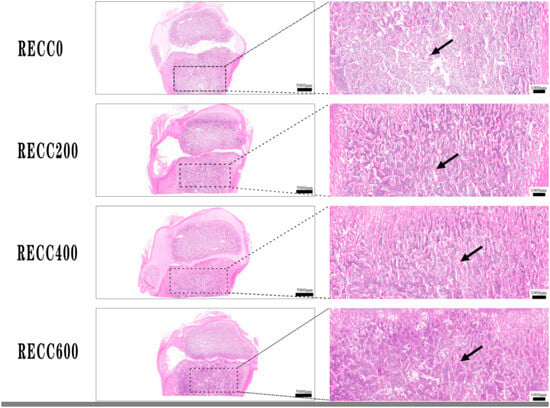
Figure 1.
HE staining of trabecular bone in proximal tibia. Red irregular tissues indicated with black arrow.
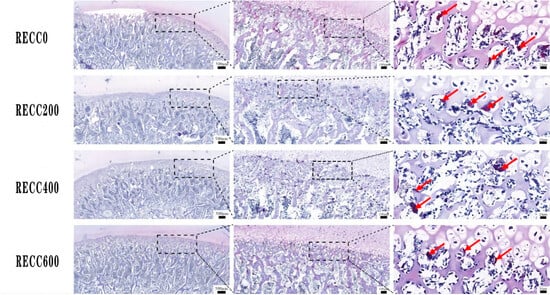
Figure 2.
TRAP staining of osteoclasts in proximal tibia. TRAP-positive cells (dark red) indicated with red arrow.

Table 2.
Effects of RECC supplementation on trabecular bone and osteoclasts in tibia.
2.3. Effects of Dietary RECC Addition on Fecal Bacterial Composition of Nursery Pigs
The composition of fecal bacteria at the phylum and genus levels is presented in Figure 3. Firmicutes and Bacteroidota were the dominant phyla in the fecal samples of the four groups, but group RECC200 had a higher relative abundance of Euryarchaeota than the other three groups (Figure 3a). Dietary RECC addition increased the relative abundance of Lactobacillus but decreased the relative abundance of Megasphaera (Figure 3b). The alpha diversity results are listed in Table 3; the data indicated that groups RECC400 and RECC600 had lower indices of Ace, Chao 1, and Shannon than groups RECC0 and RECC200 (p < 0.05), respectively. There was no significant difference between groups RECC0 and RECC200 in terms of the alpha diversity of fecal bacteria (p > 0.05). Linear discriminant analysis Effect Size (LEfSe) analysis (LDA score ≥ 3) was used to identify the characteristic bacterial taxa of each group (Figure 4), and the results showed that the numbers of significantly different microbiota in groups RECC0, RECC200, RECC400, and RECC600 were 15, 10, 1, and 3, respectively. At the genus level, the characteristic bacteria were g_Megasphaera, g_Corynebacterium, g_Ignavigranum, g_TM7a, and g_Unidentified_Weeksellaceae in group RECC0; g_Prevotellaceae_NK3B31_group, g_UCG_005, g_Rikenellaceae_RC9_gut_group, g_Acetitomaculum, g_Glutamicibacter, g_Frisingicoccus, and g_Alistipes in group RECC200; g_Eubacterium_ruminantium_group in group RECC400; and g_Dorea in group RECC600.
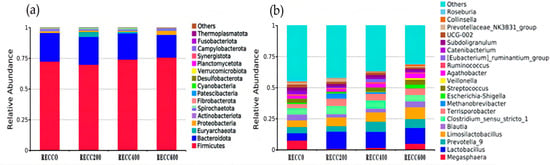
Figure 3.
The composition of fecal bacteria at (a) the phylum level and (b) the genus level. The color-coded bar plot shows the average bacterial distribution in each group.

Table 3.
Alpha diversity of fecal bacteria in different groups.
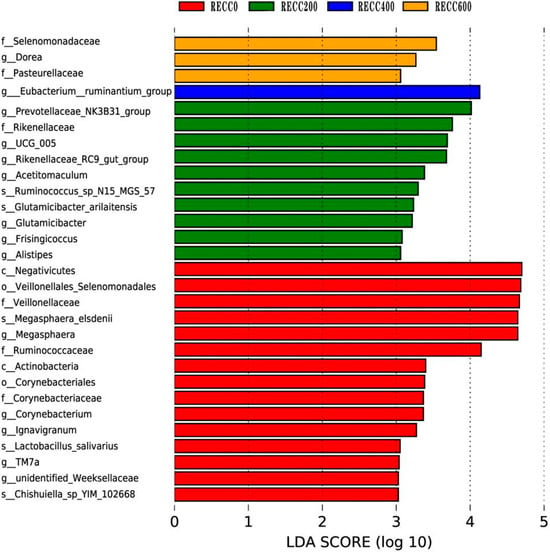
Figure 4.
The differential bacteria among groups based on linear discriminant analysis (LDA) Effect Size (LEfSe). Each plot shows taxa with significant differences in abundance among groups, with the LDA score (log 10) indicating the effect size. Red, green, blue, and orange bars represent a higher abundance of bacteria at different taxonomic levels in groups RECC0, RECC200, RECC400, and RECC600, respectively.
2.4. Effects of Dietary RECC Addition on Hepatic Histomorphology of Nursery Pigs
Figure 5 indicates that the nursery pigs from group RECC200 had less liver damage (lymphocytic infiltration, hepatocyte hydropic degeneration, or hepatic sinusoidal dilatation) than those from group RECC0.
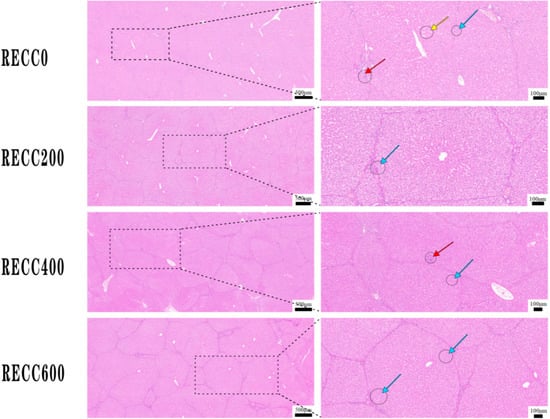
Figure 5.
Alterations in histomorphology in livers of nursery pigs supplemented with RECC. Red arrow: liver lymphocytic infiltration, blue arrow: hepatocyte hydropic degeneration, yellow arrow: hepatic sinusoidal dilatation.
2.5. Effects of Dietary RECC Addition on Jejunal Histomorphology of Nursery Pigs
The data in Table 4 indicate that the nursery pigs from group RECC200 had longer (p < 0.01) jejunal villi than those from groups RECC0, RECC400, and RECC600, respectively. The nursery pigs from groups RECC200, RECC400, and RECC600 had deeper (p < 0.01) crypts than those from group RECC0. The villus height/crypt depth ratio in the jejunum significantly increased (p < 0.05) when comparing nursery pigs from group RECC200 to nursery pigs from group RECC400.

Table 4.
Jejunal histomorphology of nursery pigs supplemented with different RECC levels.
2.6. Effects of Dietary RECC Addition on Immunoglobins and Cytokines in Serum of Nursery Pigs
The supplementation of RECC to nursery pigs increased the serum levels of IgM, IgA, IgG, and IL-10 and decreased the serum concentrations of IL-2 and TNF-α (Table 5). Compared to the nursery pigs from groups RECC0 and RECC600, those from groups RECC200 and RECC400 had higher (p < 0.01) levels of IgM and IgA in serum, respectively. The nursery pigs from groups RECC200, RECC400, and RECC600 had higher (p < 0.01) levels of IgG and IL-10 in serum than the pigs from group RECC0, respectively. The level of TNF-α was lower (p < 0.01) in the serum of nursery pigs from group RECC200 than in that of the pigs from groups RECC0, RECC400, and RECC600, respectively.

Table 5.
Serum immunoglobins and cytokines of nursery pigs supplemented with different amounts of RECC.
3. Discussion
The parameters (trabecular number, thickness, and spacing) of the trabecular bone and the number of osteoclasts are often used to assess bone quality in animals [33]. Increased trabecular spacing results in the loss of bone density [34,35]. Osteoclasts are a kind of mobile cell with multiple nuclei that attach to the bone surface to break down and absorb the organic and inorganic components of bones; an increase in the number of osteoclasts can lead to the deterioration of bone density [36]. The results of this study showed that the dietary addition of RECC reduced the number of osteoclasts in the tibia of nursery pigs. It is well known that many factors can exert influences on bone quality in animals, and many researchers are currently examining the effects of rare earth elements, rare earth–chitosan chelate, and the gut microbiota on the bone quality of commercial and breeding animals. Prior studies have reported that cerium ions can increase the trabecular thickness and decrease the trabecular spacing of rat skulls [20], and the addition of La(NO3)3 to bone marrow mesenchymal stem cells from the tibia of mice was found to significantly increase trabecular thickness compared to the untreated group [37]. Lanthanum trivalent ions can inhibit the formation of osteoclasts [17,37,38]. In this study, the dietary addition of RECC at 200 or 400 mg/kg to the diet of nursery pigs significantly increased the trabecular number and area and decreased the trabecular spacing and number of osteoclasts in the tibia. Bone loss can be reduced by inhibiting osteoclastogenesis through the blocking of IL-21 expression [37]. The results of this study also indicated that dietary RECC supplementation reduced the number of osteoclasts in the tibia, decreased serum TNF-α levels, and increased serum IL-10 levels. It has been reported that decreased TNF-α levels and increased IL-10 levels may improve bone quality by suppressing osteoclast formation [39,40]; one possible pathway through which IL-10 suppresses osteoclast formation may be through the regulation of E2 on bone metabolism by enhancing regulatory T cell production. Bone development and health are also affected by the gut microbiota [41,42]. Some Lactobacillus spp., including L. reuteri, L. rhamnosus, and L. paracasei, can prevent bone loss by decreasing osteoclastogenesis and bone resorption in mouse models [43,44]. Oral CeO2 administration significantly decreased the abundance of Lactobacillus in the gut of rats [45,46], but dietary lanthanum and cerium supplementation increased fecal Lactobacillus in pigs [47]. Short-chain fatty-acid-producing bacteria play vital roles in reducing bone loss and improving bone mineral density [48]. Among these, Alistipes are usually considered one of the protective factors for bone mineral density [48] as they increase mineral absorption [49,50]. Previous results are further validated by the data obtained in this study because the nursery pigs supplemented with RECC had better tibia quality and higher relative abundances of g_Lactobacillus and g_Alistipes.
The gut microbiota can not only affect the quality of bones but is also involved in disease resistance and performance in animals [51]. Previous studies reported that Citrobacter rodentium infection can induce colitis, and g_Prevotellaceae-NK3B31-group can reduce infection by inhibiting the growth of Citrobacter rodentium [52]. The reduction in gut inflammation results in the improvement of disease resistance capacity and performance, a process that is further verified by the results of this study. Feeding g_Rikenellaceae_RC9_gut_group to pigs can increase the feed conversion ratio and reduce backfat thickness [53]. g_Acetitomaculum often generate volatile fatty acids by fermenting glucose and other carbohydrates [54,55,56] and show a strong positive correlation with the levels of IL-10 [57]. g_Alistipes are short-chain fatty-acid-producing bacteria [58,59] that can decrease the inflammatory response and liver diseases [49,50,60]. g_Eubacterium_ruminantium_group can reduce inflammation by producing short-chain fatty acids [61,62,63], improving gut histomorphology [64]. g_Dorea can exert anti-inflammatory effects by producing short-chain fatty acids (acetate, propionate, and butyrate) through the fermentation of starch, cellulose, and hemicellulose in the gut [65,66,67]. In addition, g_Dorea have positive genetic correlations with backfat thickness [53,68]; backfat thickness can affect both the value of the carcass and the reproductive traits of sows [69], so the genetic control of g_Dorea has major economic importance for breeding sows. The results of this study indicated that the nursery pigs treated with a 200 mg/kg diet of RECC had higher relative abundances of g_Prevotellaceae_NK3B31_group, g_Rikenellaceae_RC9_gut_group, g_Alistipes, and g_Acetitomaculum than those without RECC treatment, contributing to the improved performance and disease resistance outcomes among nursery pigs. The data obtained in this study also indicated that the nursery pigs from group RECC0 had high levels of IL-2 and TNF-α, high diarrhea incidence, a poor feed conversion ratio, and low levels of IL-10, which might be attributed to the high fecal numbers of g_Megasphaera, g_Corynebacterium, g_Ignavigranum, and g_TM7. It is reported that g_Megasphaera can reduce the feed conversion ratio [70]. g_Corynebacterium has a positive correlation with pro-inflammatory cytokines and ferroptosis, but a negative correlation with IL-10 [71]. g_Ignavigranum are inflammation-producing bacteria identified in the milk of cows infected with mastitis [72]. g_TM7 are also associated with inflammatory bowel diseases, which are characterized by high diarrhea incidence and pro-inflammatory cytokines [73].
It is well known that increasing the content of IgA, IgG, and IgM can improve disease resistance in animals. The addition of dietary chitosan increased the content of IgA, IgG, and IgM in the serum of Huoyan geese [74]. In this study, the data demonstrated that dietary RECC addition also increased the concentration of IgA, IgG, and IgM in the serum of nursery pigs. The morphological and functional integrity of the gut mucosa is important for nutrient absorption and the performance of livestock [75]. The addition of rare-earth-containing lanthanum and cerium to the diet at levels from 0 to 600 mg/kg had no significant impact on the jejunal histomorphology of hens during the late laying stage [76]. However, the addition of dietary lanthanum significantly improved the intestinal morphology of finishing pigs [44], and the addition of a rare earth element (Azomite) to the diet at 2.5–5.0 g/kg also significantly elevated the intestinal villus height of tilapia [77]. The results of this study indicated that supplementing nursery pigs with RECC at 200 mg/kg diet significantly improved jejunal histomorphology; this is possibly attributed to decreased inflammation in the gut.
4. Materials and Methods
4.1. Materials
Chitosan is a naturally occurring linear polysaccharide of glucosamine and N-acetylglucosamine that is obtained through the deacetylation of chitin. It exhibits stable coordination with rare earth ions through hydrogen or salt bonds due to the presence of numerous hydroxyl, amino, and N-acetylamino groups in the chitosan molecular chain. RECC is a novel feed additive formed via coordination chelation between rare earth ions and the amino and hydroxyl groups on chitosan molecules through electrochemical processes.
The RECC product used in this study was provided by Jiangxi Weiting Industrial Co., Ltd. (Jiangxi, China). It contains 30% RECC and 70% carriers (chitosan, montmorillonite, and calcium carbonate), and the concentrations of lanthanum and cerium in this RECC product are 5.12% and 3.26%, respectively.
4.2. Animal Experimental Design
A total of 80 crossbred piglets (Duroc × Landrace × Yorkshire) weaned at 26 days with an average bodyweight of 7.67 ± 0.09 kg were randomly assigned to group RECC0 (RECC, 0 mg/kg diet), group RECC200 (RECC, 200 mg/kg diet), group RECC400 (RECC, 400 mg/kg diet), or group RECC600 (RECC, 600 mg/kg diet). Each group included 20 piglets (10♂, 10♀) with four replicates (pens), and each pen had five piglets.
4.3. Feed Preparation and Animal Feeding
The commercial diet for the nursery pigs was purchased from a feed factory. The nutrient levels of this commercial diet were analyzed and are listed as follows (on a dry matter basis): dry matter, 89.10%; crude protein, 17.40%; ether extract, 4.70%; crude fiber, 1.80%; crude ash, 6.00%; calcium, 0.75%; and total phosphorus, 0.68%. The test feed offered to the nursery pigs in each group was prepared as follows. First, the daily requirements for the RECC product and commercial diet for nursery pigs in each treatment group were calculated and weighed. Second, the RECC product was dissolved in tap water at a ratio of 1:100 (weight/weight) and was then mixed well with the feed in a small feed mixer.
All piglets were kept in concrete-floored pens. The room temperature was set at 28 °C for the first week and then gradually reduced by 1 °C to 25 °C for the fourth week. The humidity in the room was 63.6%, and ventilation was provided by an exhaust fan with a diameter of 70 cm. The test feed was artificially placed into the feed trough, and all nursery piglets had free access to the test feed and water throughout the 28-day experiment.
4.4. Data Collection and Calculation
All piglets were weighed at the beginning and the end of experiment, respectively, and the average daily gain (ADG) was calculated as follows: ADG (g/d) = (final bodyweight − initial bodyweight)/experimental days. The feed offered and refused were also recorded each day, and the average daily feed intake (ADFI) was calculated with the formula ADFI (g/d) = (feed offered − feed refusal)/experimental days. The number of piglets with diarrhea symptoms was recorded every day, and the diarrhea incidence (%) was calculated as ([number of piglets with diarrhea within a treatment]/[number of piglets × total experimental days]) × 100, where the “number of piglets with diarrhea” was the total number of piglets with diarrhea observed each day [78].
4.5. Sample Collection and Preparation
Fecal samples were collected from the anus of each nursery pig on days 13 and 26, respectively. After the collection at each time point, fecal samples from the same treatment group were mixed, placed into sterile plastic tubes with 3 replicates, and stored in liquid nitrogen for 16S rDNA sequencing.
After 12 h of fasting, a total of eight piglets were randomly selected from each group (1♂ and 1♀ per pen) on day 28 to collect blood samples from the precaval vein into nonheparinized vacuum tubes, and after standing for one hour at room temperature (22 °C), all blood samples were centrifuged at 3000× g for 10 min at 4 °C. The supernatant of each vacuum tube was pipetted into new sterile EP tubes as aliquots, and the serum samples were stored at −20 °C until analysis.
At the end of the experiment, a total of 4 piglets (2♂, 2♀) with approximately average body weights were selected from each group (1 piglet/pen) and bled after electrical stunning. After opening the abdominal cavity, the liver and intestine were taken out. The liver samples were collected from the inferior margin of the right lobe, and jejunal segments of approximately 2 cm in length were removed from the middle of the jejunum and flushed with normal saline until the digesta was thoroughly removed. The left proximal tibia was harvested and the upper part of the tibia was halved longitudinally in two parts with a saw. The samples of liver, jejunum, and tibia were then immediately fixed with 4% paraformaldehyde solution for 24 h. After 24 h fixation, the tibia samples were decalcified in a 10% ethylenediaminetetraacetic acid (EDTA) solution at a pH of 7.4 at room temperature for 5 weeks.
4.6. Tissue Staining
The treated tibia, liver, and jejunum samples were then dehydrated in ethanol solution with different concentrations, embedded in paraffin, and longitudinally cut into sections with a thickness of 5 μm. After deparaffinization and rehydration, sections of the liver, jejunum, and tibia were subjected to staining with hematoxylin and eosin (HE) kits (Solarbio, Beijing, China) according to the manufacturer’s protocols. In addition, the tibia sections were also stained with tartrate-resistant acid phosphatase (TRAP) kits (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions to detect the osteoclasts. TRAP-positive multinucleated cells were considered to be osteoclasts [37].
After staining, the HE- and TRAP-stained slides were scanned using a Pannoramic DESK/MIDI/250/1000 digital scanner (3DHISTECH Ltd., Budapest, Hungary). The target images were captured with Case Viewer software 2.2 (3DHISTECH Ltd., Budapest, Hungary). The Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA) was used to measure the villus height and crypt depth (randomly measuring 5 villi and 5 crypts per section) of the jejunum, the histomorphometry parameters, and the osteoclast numbers of the tibia, respectively.
4.7. Fecal Bacteria Sequencing
The DNA of fecal bacteria obtained from each sample was extracted using DNeasy PowerSoil Kits (Qiagen, Hilden, Germany) according to the manufacturer’s protocols. The qualified DNA samples were amplified by targeting the V3-V4 region using the primers 343F (5′-TACGGRAGGCAGCAG-3′) and 798R (5′-AGGGTATCTAATCCT-3′). PCR reactions were performed in a mixture containing 15 µL of Phusion ➅ High-Fidelity PCR Master Mix, 0.2 µM of the primers, and 10 ng of the template DNA with the following conditions: initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, elongation at 72 °C for 30 s, and finally, 72 °C for 5 min.
The PCR products were extracted from 2% agarose gels and purified using Vazyme DNA clean beads. After purification, the PCR products were quantified with a Qubit 3.0 Fluorometer (Invitrogen, Waltham, MA, USA) to generate amplicon libraries. The libraries were pooled in equimolar amounts and paired-end-sequenced (PE250) at Novogene Co., Ltd. (Beijing, China) on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA). Bioinformatics analysis was performed according to a previously published method [79]. The raw sequencing data were deposited in the Sequence Read Archive of the National Center for Biotechnology Information under the BioProject ID PRJNA1056486.
4.8. Serum Sample Analysis
The concentrations of immunoglobin (Ig)A, IgG, IgM, interleukin (IL)-10, IL-2, and tumor necrosis factor-alpha (TNF-α) of the serum samples were tested using enzyme-linked immunosorbent assay kits purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China).
4.9. Statistical Analysis
The data were analyzed using the Prism Software 5.0 (GraphPad Software, Inc., San Diego, CA, USA), and normal distribution was determined using the Shapiro–Wilk test. The statistical significance (p < 0.05) was evaluated via one-way ANOVA and Tukey’s multiple comparisons, and the results are presented as means with the standard error of the mean.
5. Conclusions
Supplementing RECC into the diet at a level of 200 mg/kg contributes to a higher trabecular perimeter, trabecular area, and trabecular number; a lower degree of trabecular separation and a lower osteoclast number; and higher abundances of the beneficial bacteria Prevotellaceae_NK3B31_group, UCG_005, Rikenellaceae_RC9_gut_group, Acetitomaculum, Glutamicibacter, Frisingicoccus, and Alistipes, thus improving the disease resistance capacity and performance of nursery pigs. The findings of this study provide novel insights into improving bone quality, health, and performance in nursery pigs via the administration of RECC to alter the gut microbiota; however, the underlying mechanisms remain unclear and should be explored further in subsequent studies.
Author Contributions
Conceptualization, Y.H. and W.L.; methodology, S.H., W.S., P.W. and H.W.; software, S.H., W.S. and P.W.; validation, S.H., W.S. and P.W.; formal analysis, S.H.; investigation, S.H.; resources, Y.H.; data curation, S.H.; writing—original draft preparation, Y.H. and S.H.; writing—review and editing, Y.H.; visualization, S.H.; supervision, Y.H.; project administration, Y.H.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangxi Modern Agricultural Research Collaborative Innovation Project (JXXTCX2016003-02).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Jiangxi Agricultural University (JXAULL-2024-10-02).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RECC | Rare earth–chitosan chelate |
| IgM | Immunoglobulin M |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IL-10 | Interleukin-10 |
| IL-2 | Interleukin-2 |
| TNF-α | Tumor necrosis factor-α |
| Tb.Pm | Trabecular perimeter |
| Tb.Ar | Trabecular bone area |
| Tb.Th | Trabecular thickness |
| Tb.N | Trabecular number |
| Tb.Sp | Trabecular separation |
| N.Oc | Osteoclast number |
| ADG | Average daily gain |
| ADFI | Average daily feed intake |
References
- Boudon, A.; Karhapää, M.; Siljander-Rasi, H.; Cantaloube, E.; Brossard, L.; Le Floc’h, N.; Meunier-Salaün, M.C. Effect of moderate forced physical activity on behaviour, lameness and osteochondrosis in growing pigs from two divergent lines selected for feed efficiency. Animal 2022, 1, 100010. [Google Scholar] [CrossRef]
- Cianferotti, L. Osteomalacia is not a single disease. Int. J. Mol. Sci. 2022, 23, 14896. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, E.; Ballanti, P. Osteoporosis-bone remodeling and animal models. Toxicol. Pathol. 2014, 42, 957–969. [Google Scholar] [CrossRef]
- Regmi, P.; Nelson, N.; Haut, R.C.; Orth, M.W.; Karcher, D.M. Influence of age and housing systems on properties of tibia and humerus of Lohmann White hens(1): Bone properties of laying hens in commercial housing systems. Poult. Sci. 2017, 96, 3755–3762. [Google Scholar] [CrossRef]
- Osiak-Wicha, C.; Tomaszewska, E.; Muszyński, S.; Flis, M.; Świetlicki, M.; Arciszewski, M.B. Comparative analysis of morphometric, densitometric, and mechanical properties of skeletal locomotor elements in three duck species (Anatidae: Anatinae). Animals 2024, 14, 2191. [Google Scholar] [CrossRef]
- Hinić-Frlog, S.; Motani, R. Relationship between osteology and aquatic locomotion in birds: Determining modes of locomotion in extinct ornithurae. J. Evol. Biol. 2010, 23, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, M.M.J.; Millet, S.; Aluwé, M.; Janssens, G.P.J. Impact of nutrition on lameness and claw health in sows. Livest. Sci. 2013, 156, 24–35. [Google Scholar] [CrossRef]
- Hasan, M.; Oster, M.; Reyer, H.; Wimmers, K.; Fischer, D.C. Efficacy of dietary vitamin D3 and 25(OH)D3 on reproductive capacities, growth performance, immunity and bone development in pigs. Br. J. Nutr. 2023, 130, 1298–1307. [Google Scholar] [CrossRef]
- Floradin, P.; Pomar, C.; Létourneau-Montminy, M.P.; Schlegel, P. Development of the mineralisation of individual bones and bone regions in replacement gilts according to dietary calcium and phosphorus. Animal 2024, 18, 101241. [Google Scholar] [CrossRef]
- Starczak, Y.; Reinke, D.C.; Barratt, K.R.; Russell, P.K.; Clarke, M.V.; Davey, R.A.; Atkins, G.J.; Anderson, P.H. Vitamin D receptor expression in mature osteoclasts reduces bone loss due to low dietary calcium intake in male mice. J. Steroid Biochem. Mol. Biol. 2021, 210, 105857. [Google Scholar] [CrossRef]
- Mahajan, A.; Alexander, L.S.; Seabolt, B.S.; Catrambone, D.E.; McClung, J.P.; Odle, J.; Pfeiler, T.W.; Loboa, E.G.; Stahl, C.H. Dietary calcium restriction affects mesenchymal stem cell activity and bone development in neonatal pigs. J. Nutr. 2011, 141, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Vötterl, J.C.; Klinsoda, J.; Koger, S.; Hennig-Pauka, I.; Verhovsek, D.; Metzler-Zebeli, B.U. Available phosphorus levels modulate gene expression related to intestinal calcium and phosphorus absorption and bone parameters differently in gilts and barrows. Anim. Biosci. 2023, 36, 740–752. [Google Scholar] [CrossRef]
- Fabà, L.; Gasa, J.; Tokach, M.D.; Font-i-Furnols, M.; Vilarrasa, E.; Solà-Oriol, D. Effects of additional organic micro-minerals and methionine on carcass composition, gait score, bone characteristics, and osteochondrosis in replacement gilts of different growth rate. Anim. Feed Sci. Technol. 2019, 256, 114262. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Mohamed, E.; Abd El-Hack, M.E.; Khafaga, A.F.; Noreldin, A.E.; Arif, M.; Chaudhry, M.T.; Losacco, C.; Abdeen, A.; Abdel-Daim, M.M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lantha. Sci. Total Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef]
- Wang, Z.R.; Zhang, Y.L.; Chen, S.S.; Qu, Y.; Tang, M.C.; Wang, W.Y.; Li, W.C.; Gu, L.S. Multifunctional CeO2 nanozymes for mitigating high-glucose induced senescence and enhancing bone regeneration in type 2 diabetes mellitus. Chem. Eng. J. 2024, 485, 149842. [Google Scholar] [CrossRef]
- Fumoto, T.; Ito, M.; Ikeda, K. Lanthanum carbonate stimulates bone formation in a rat model of renal insufficiency with low bone turnover. J. Bone Miner. Metab. 2014, 32, 484–493. [Google Scholar] [CrossRef]
- Jiang, C.; Shang, J.Y.; Li, Z.; Qin, A.; Ouyang, Z.X.; Qu, X.H.; Li, H.W.; Tian, B.; Wang, W.G.; Wu, C.L.; et al. Lanthanum chloride attenuates osteoclast formation and function via the downregulation of Rankl-induced Nf-kappab and Nfatc1 activities. J. Cell Physiol. 2016, 231, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef]
- Kamenaga, T.; Kuroda, Y.; Nagai, K.; Tsubosaka, M.; Takashima, Y.; Kikuchi, K.; Fujita, M.; Ikuta, K.; Anjiki, K.; Maeda, T.; et al. Cryopreserved human adipose-derived stromal vascular fraction maintains fracture healing potential via angiogenesis and osteogenesis in an immuno-deficient rat model. Stem Cell Res. Ther. 2021, 12, 110. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhou, Z.Z.; Hou, M.Z.; Xia, X.W.; Liu, Y.; Zhao, Z.J.; Wu, Y.B.; Deng, Y.G.; Zhang, Y.J.; He, F.; et al. Capturing cerium ions via hydrogel microspheres promotes vascularization for bone regeneration. Mater. Today Bio 2024, 25, 100956. [Google Scholar] [CrossRef]
- Sabir, P.S.; Abbas, K.A. Effect of strontium ranelate and cerium oxide addition in the diet on bone quality and expression level of osteocalcin and alkaline phosphatase genes in broiler chicken. Vet. Med. Sci. 2023, 9, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef]
- David Yatsonsky, I.; Pan, K.; Shendge, V.B.; Liu, J.; Ebraheim, N.A. Linkage of microbiota and osteoporosis: A mini literature review. World J. Orthop. 2019, 10, 123–127. [Google Scholar]
- Lu, L.Y.; Chen, X.X.; Liu, Y.; Yu, X.J. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Zhang, C.L.; Liu, Z.Y.; Li, C.; Ren, Z.G. Gut microbiota and bone diseases: A growing partnership. Front. Microbiol. 2022, 13, 877776. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, C.L.; Cao, X.J.; Feng, B.; Li, X.L. Intestinal population in host with metabolic syndrome during administration of chitosan and its derivatives. Molecules 2020, 25, 5857. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, S.G.; Lim, S.C.; Ong, J.L. Osteogenic activity of the mixture of chitosan and particulate dentin. J. Biomed. Mater. Res. 2008, 87, 618–623. [Google Scholar] [CrossRef]
- Lu, X.X.; Chang, X.Y.; Zhang, H.J.; Wang, J.; Qiu, K.; Wu, S.G. Effects of dietary rare earth chitosan chelate on performance, egg quality, immune and antioxidant capacity, and intestinal digestive enzyme activity of laying hens. Polymers 2023, 15, 1600. [Google Scholar] [CrossRef]
- Xiong, Y.; Pang, J.M.; Lv, L.K.; Wu, Y.J.; Li, N.; Huang, S.M.; Feng, Z.; Ren, Y.; Wang, J.J. Effects of maternal supplementation with rare earth elements during late gestation and lactation on performances, health, and fecal microbiota of the sows and their offspring. Animals 2019, 9, 738. [Google Scholar] [CrossRef]
- Feng, S.F.; Du, X.Y.; Wang, C.; Ye, D.D.; Ma, G.J.; Zhao, S.H.; Wang, H.Y.; Liu, X.D. Identification of mRNAs related to tibial cartilage development of Yorkshire piglets. Biomed Res. Int. 2019, 2019, 2365416. [Google Scholar] [CrossRef] [PubMed]
- Bioletto, F.; Sibilla, M.; Berton, A.M.; Prencipe, N.; Varaldo, E.; Maiorino, F.; Cuboni, D.; Pusterla, A.; Gasco, V.; Grottoli, S.; et al. Mild hyponatremia is not associated with degradation of trabecular bone microarchitecture despite bone mass loss. J. Clin. Endocrinol. Metab. 2024, 12, dgae234. [Google Scholar] [CrossRef]
- Bisazza, K.T.; Nelson, B.B.; Sikes, K.J.; Nakamura, L.; Easley, J.T. Computed tomography provides improved quantification of trabecular lumbar spine bone loss compared to dual-energy X-ray absorptiometry in ovariectomized sheep. JBMR Plus 2023, 7, e10807. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.L.; Xu, P.; Zhong, Y.H.; Zhou, Z.G.; Zhang, W.C. Interleukin-21 knockout reduces bone loss in ovariectomized mice by inhibiting osteoclastogenesis. Biosci. Biotechnol. Biochem. 2023, 87, 1265–1273. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yao, H.; Tjahjono, A.W.; Xiang, L.; Li, K.; Tang, J.H.; Gao, Y.X. Si-Zhi Wan regulates osteoclast autophagy in osteoporosis through the AMPK signaling pathway to attenuate osteoclastogenesis. J. Pharm. Pharmacol. 2024, 76, 236–244. [Google Scholar] [CrossRef]
- Zou, D.D.; Lin, R.L.; Han, Y.; Jia, J.; Zhou, G.Q.; Zhang, H.S.; Ge, K. Lanthanum promoting bone formation by regulating osteogenesis, osteoclastogenesis and angiogenesis. J. Rare Earths 2024, 42, 621–628. [Google Scholar] [CrossRef]
- Xu, W.; Wei, K.; Lin, Z.; Wu, T.; Li, G.; Wang, L. Storage and release of rare earth elements in microsphere-based scaffolds for enhancing osteogenesis. Sci. Rep. 2022, 12, 6383. [Google Scholar] [CrossRef]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological reaction in TNF-alpha-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013, 2013, 181849. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Frey, B.; Hess, A.; Zwerina, J.; Luther, J.; Nimmerjahn, F.; Engelke, K.; Kollias, G.; Hünig, T.; Schett, G.; et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J. Immunol. 2010, 184, 7238–7246. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Bone remodeling and the microbiome. Cold Spring Harb. Perspect. Med. 2018, 8, a031203. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R.; Probiotic, L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Bubnov, R.; Babenko, L.; Lazarenko, L.; Kryvtsova, M.; Shcherbakov, O.; Zholobak, N.; Golubnitschaja, O.; Spivak, M. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J. 2019, 10, 317–335. [Google Scholar] [CrossRef]
- Ye, Q.R.; Jia, D.T.; Ji, J.; Liu, Y.; Wu, G. Effects of nano-cerium dioxide on intestinal microflora in rats by oral subchronic exposure. PLoS ONE 2024, 19, e0298917. [Google Scholar] [CrossRef]
- Cai, L.; Nyachoti, C.M.; Kim, I.H. Impact of rare earth element enriched yeast on growth performance, nutrient digestibility, blood profile, and fecal microflora in finishing pigs. Can. J. Anim. Sci. 2018, 98, 347–353. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.J.; Tang, G.J.; Deng, P.; Qin, Y.Y.; Han, J.L.; Wang, S.L.; Sun, X.J.; Li, D.X.; Chen, Z.J. The causal relationship between gut microbiota and bone mineral density: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1268935. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Wang, T.F.; Guan, K.F.; Su, Q.J.; Wang, X.T.; Yan, Z.Q.; Kuang, K.L.; Wang, Y.; Zhang, Q.D.; Zhou, X.; Liu, B. Change of gut microbiota in PRRSV-resistant pigs and PRRSV-susceptible pigs from Tongcheng pigs and Large white pigs crossed population upon PRRSV infection. Animals 2022, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Wu, B.; He, T.S.; Xie, L.; Liu, Z.P. Dock2 affects the host susceptibility to Citrobacter rodentium infection through regulating gut microbiota. Gut Pathog. 2021, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Aliakbari, A.; Zemb, O.; Billon, Y.; Barilly, C.; Ahn, I.; Riquet, J.; Gilbert, H. Genetic relationships between feed efficiency and gut microbiome in pig lines selected for residual feed intake. J. Anim. Breed. Genet. 2021, 138, 491–507. [Google Scholar] [CrossRef]
- Hua, C.F.; Tian, J.; Tian, P.; Cong, R.; Luo, Y.W.; Geng, Y.; Tao, S.Y.; Ni, Y.D.; Zhao, R.Q. Feeding a high concentration diet induces unhealthy alterations in the composition and metabolism of ruminal microbiota and host response in a goat model. Front. Microbiol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Gualdrón-Duarte, L.B.; Allen, M.A. Effects of acetic acid or sodium acetate infused into the rumen or abomasum on feeding behavior and metabolic response of cows in the postpartum period. J. Dairy Sci. 2018, 101, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, G.; Liu, Z.; Wu, P.; Yu, Z.; Wang, J. Repeated inoculation with fresh rumen fluid before or during weaning modulates the microbiota composition and co-occurrence of the rumen and colon of lambs. BMC Microbiol. 2020, 20, 29. [Google Scholar] [CrossRef]
- Lin, A.L.; Yan, X.X.; Wang, H.Y.; Su, Y.; Zhu, W.Y. Effects of lactic acid bacteria-fermented formula milk supplementation on ileal microbiota, transcriptomic profile, and mucosal immunity in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 113. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Xiao, K.P.; Jiang, F.; Wang, H.C.; Tang, D.Z.; Liu, D.; Liu, B.; Liu, Y.S.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef]
- Rao, Z.Y.; Li, Y.; Yang, X.P.; Guo, Y.P.; Zhang, W.; Wang, Z.X. Diet xylo-oligosaccharide supplementation improves growth performance, immune function, and intestinal health of broilers. Anim. Nutr. 2024, 17, 165–176. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Smith, P.; Howitt, M.; Panikov, N.; Michaud, M.; Gallini, C.; Bohlooly-Y., M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Wang, H.Y.; Xia, P.K.; Lu, Z.Y.; Su, Y.; Zhu, W.Y. Metabolome-microbiome responses of growing pigs induced by time-restricted feeding. Front. Vet. Sci. 2021, 8, 681202. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.; Cotter, P. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.N.; Ma, Y.; Xiong, K.; Wang, Y.R.; Liu, Y.J.; Sun, Y.; Yang, Y.X.; Ma, A.G. Ameliorating effects of vitamin K2 on dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Mol. Sci. 2023, 24, 2986. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functionalinteractions between the gut microbiota and host metabolism. Nature 2012, 489, 242249. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Siegerstetter, S.C.; Magowan, E.; Lawlor, P.G.; O’Connell, N.E.; Zebeli, Q. Fecal microbiota transplant from highly feed efficient donors affects cecal physiology and microbiota in low-and high-feed efficient chickens. Front. Microbiol. 2019, 10, 1576. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Roongsitthichai, A.; Tummaruk, P. Importance of backfat thickness to reproductive performance in female pigs. Thai J. Vet. Med. 2014, 44, 171–178. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, Y.; Yang, T.; Ao, H.; Chen, S.K.; Xing, K.; Zhang, F.X.; Zhao, X.T.; Liu, J.F.; Wang, C.D. Differences in gut microbiota composition in finishing Landrace pigs with low and high feed conversion ratios. Antonie Leeuwenhoek 2018, 111, 1673–1685. [Google Scholar] [CrossRef]
- Zhou, Z.; Niu, H.J.; Bian, M.; Zhu, C.S. Kidney tea [Orthosiphon aristatus (Blume) Miq.] improves diabetic nephropathy via regulating gut microbiota and ferroptosis. Front. Pharmacol. 2024, 15, 1392123. [Google Scholar] [CrossRef]
- Kusumawati, A.; Mustopa, A.Z.; Wibawan, I.W.T.; Setiyono, A.; Sudarwanto, M.B. Metagenomic analysis of pathogen mastitis in cow’s milk from Cicurug, Sukabumi, West Java, Indonesia. Earth Environ. Sci. 2021, 762, 012064. [Google Scholar] [CrossRef]
- Kuehbacher, T.; Rehman, A.; Lepage, P.; Hellmig, S.; lsch, U.R.F.; Schreiber, S.; Ott, S.J. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J. Med. Microbiol. 2008, 57, 1569–1576. [Google Scholar] [CrossRef]
- Miao, Z.G.; Zhao, W.X.; Guo, L.P.; Wang, S.; Zhang, J.Z. Effects of dietary supplementation of chitosan on immune function in growing Huoyan geese. Poult. Sci. 2020, 99, 95–100. [Google Scholar] [CrossRef]
- Zeng, Y.D.; Wang, Z.R.; Zou, T.D.; Chen, J.; Li, G.H.; Zheng, L.Z.; Li, S.; You, J.M. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Yan, L.W.; Cao, X.H.; Luo, Y.M.; Peng, X.T.; Wang, Z.R.; Zou, T.D.; Chen, J.; You, J.M. Effects of rare earth as feed additive on production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota in late-phase laying hens. Front. Sustain. Food Syst. 2023, 7, 1155543. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, X.Q.; Xu, Z.; Yao, W.X.; Leng, X.J. Dietary Azomite, a natural trace mineral complex, improved the growth, immunity response, intestine health and resistance against bacterial infection in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 108, 53–62. [Google Scholar] [CrossRef]
- Sun, P.; Li, D.; Li, Z.; Dong, B.; Wang, F. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J. Nutr. Biochem. 2008, 19, 627–633. [Google Scholar] [CrossRef]
- Zhou, H.M.; Wu, H.D.; Chen, Y.X.; Zou, W.J.; Lu, W.; He, Y.Y. Administration of all-trans retinoic acid to pregnant sows alters gut bacterial community of neonatal piglets with different hoxa1 genotypes. Front. Microbiol. 2021, 12, 712212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).