Characterization of Circulating Vesicles of Complicated and Uncomplicated Systemic Sclerosis Patients and Their Role in Vascular Dysfunction
Abstract
1. Introduction
2. Results
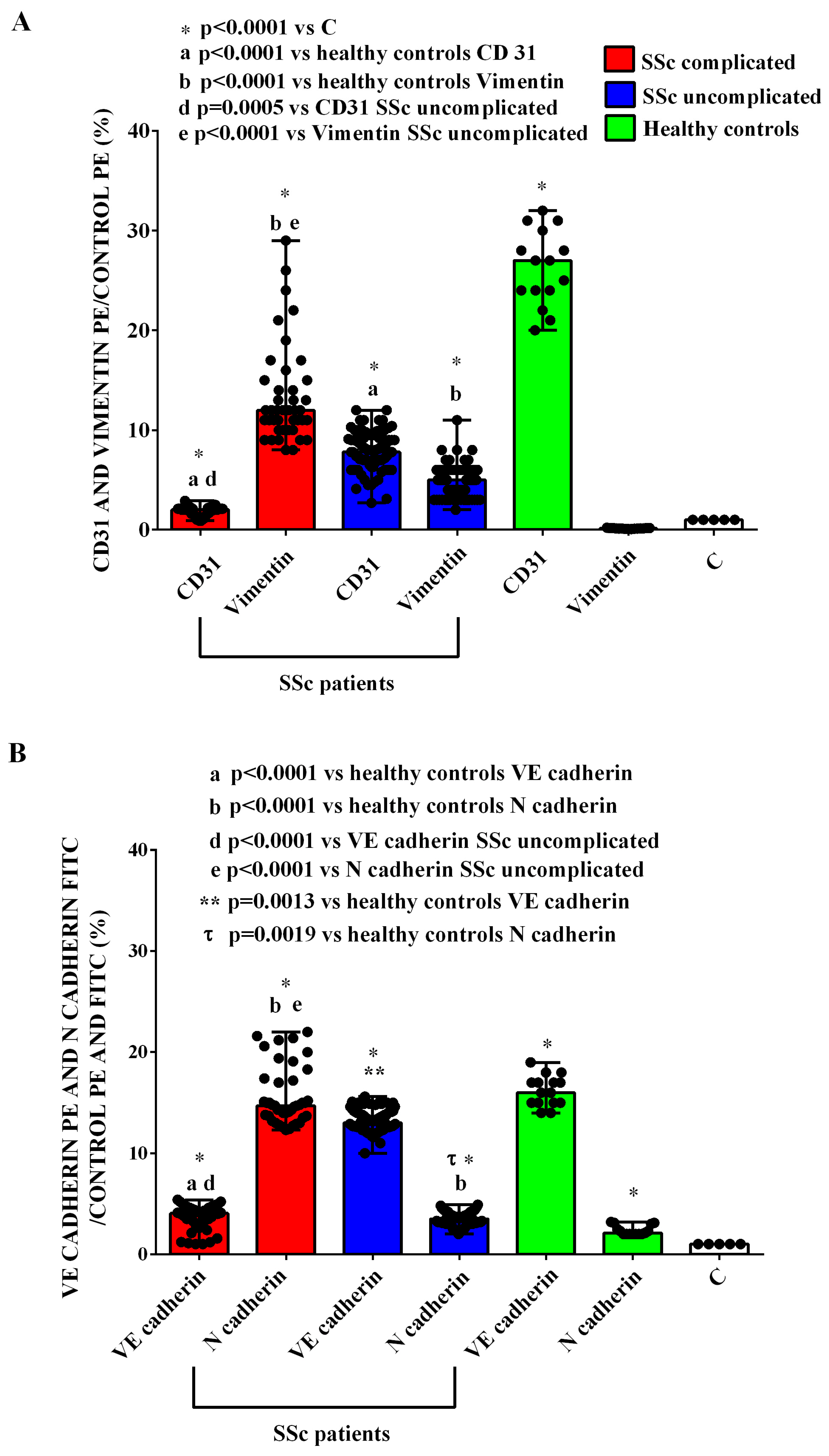
2.1. EVs Characterization
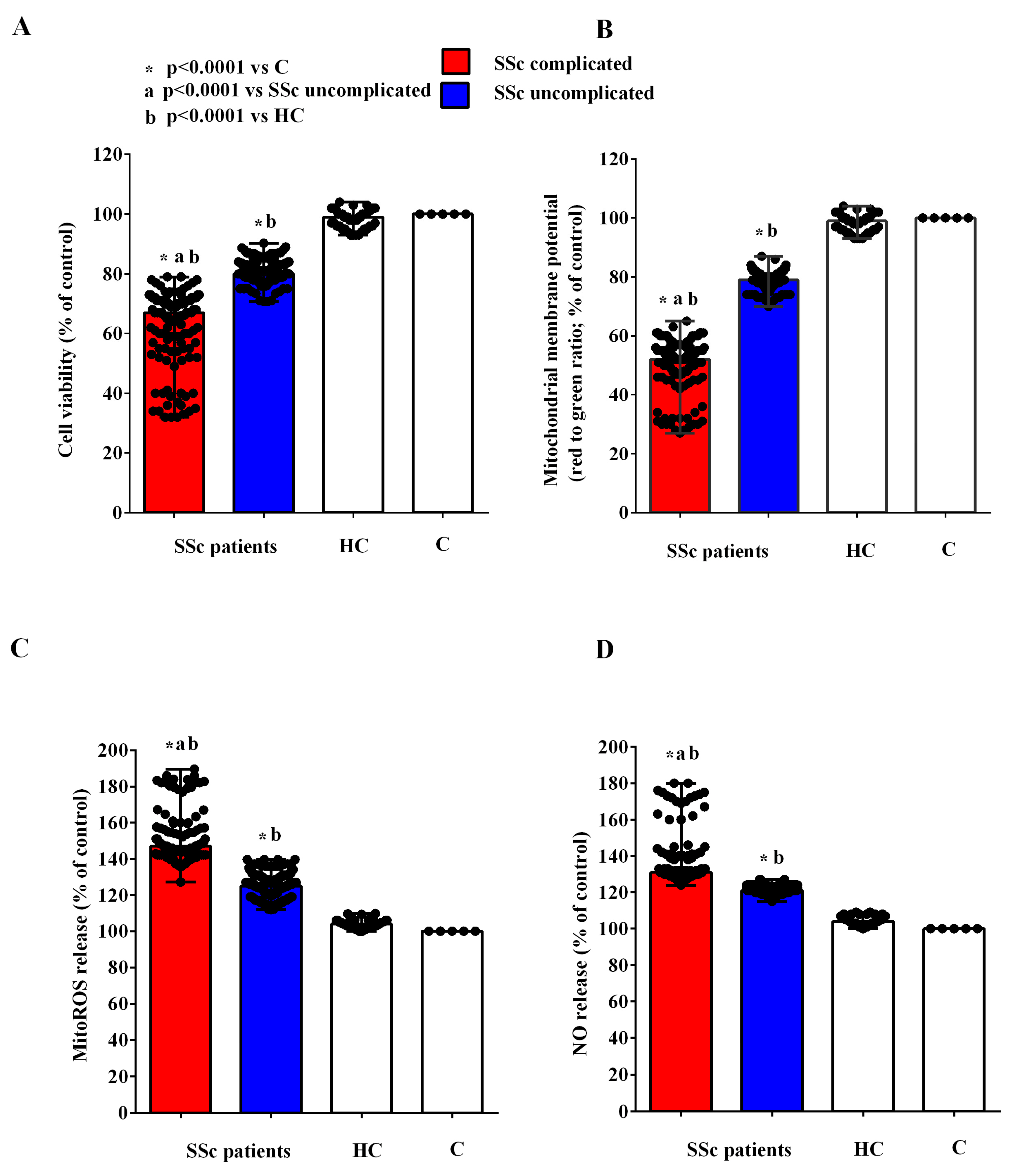
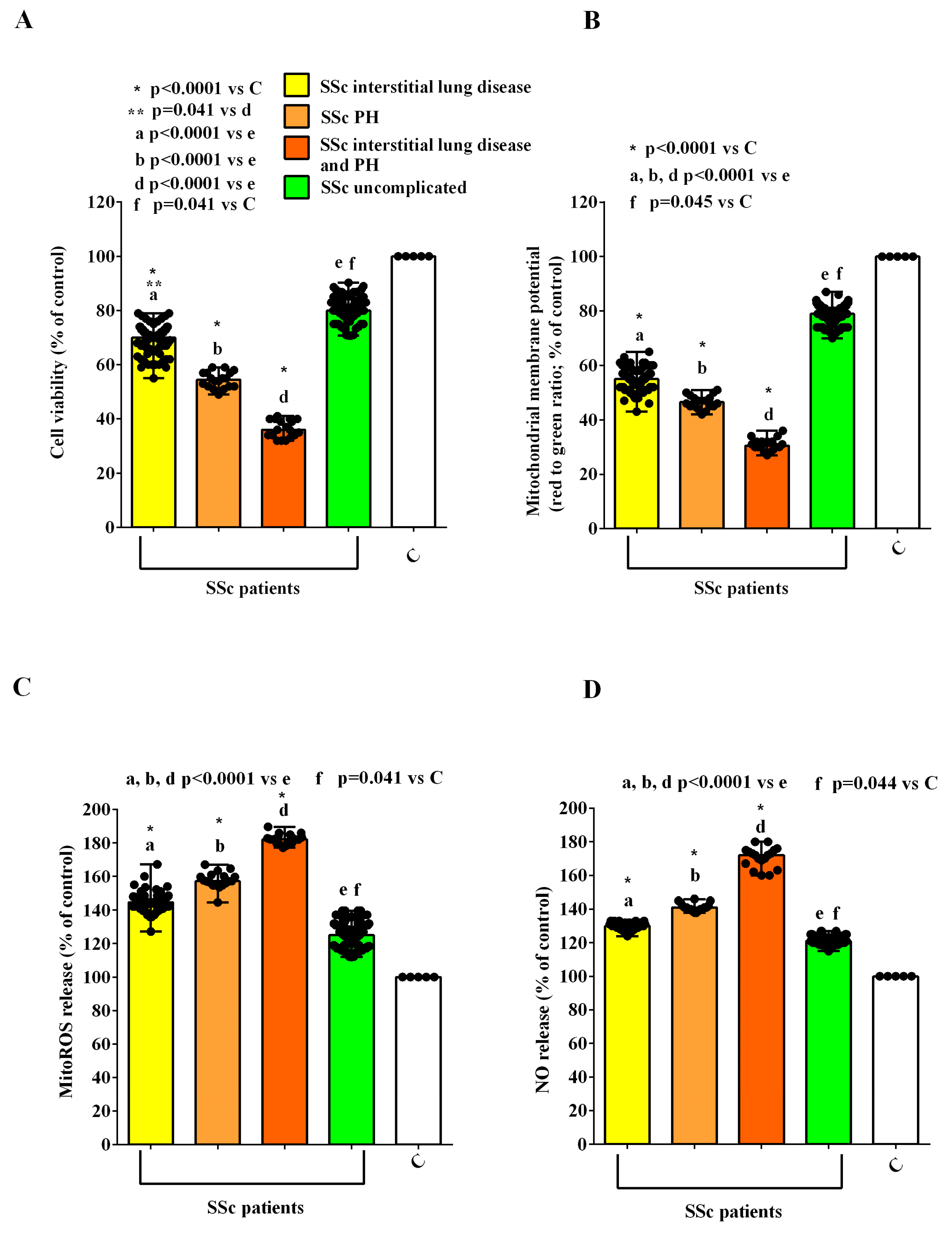
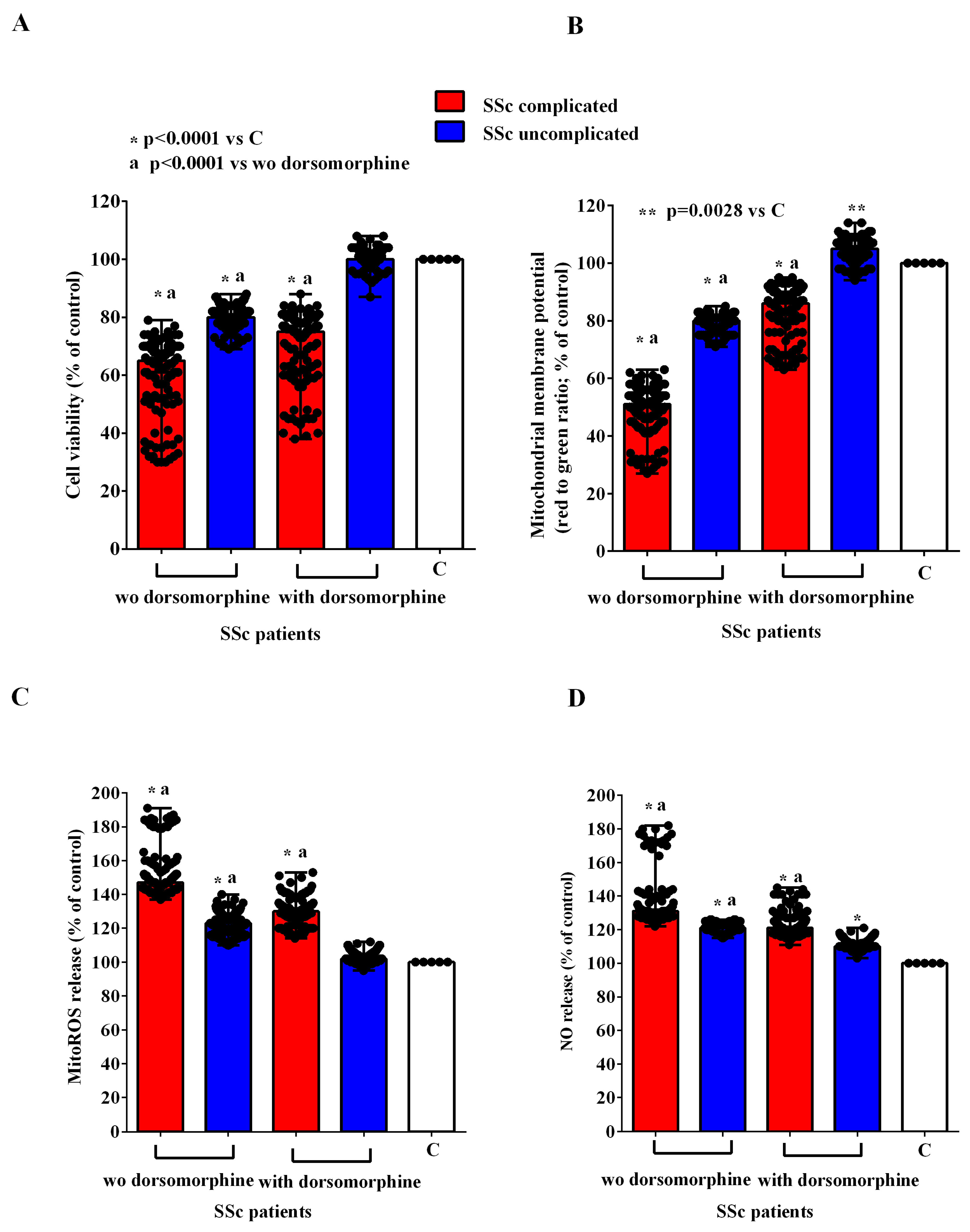
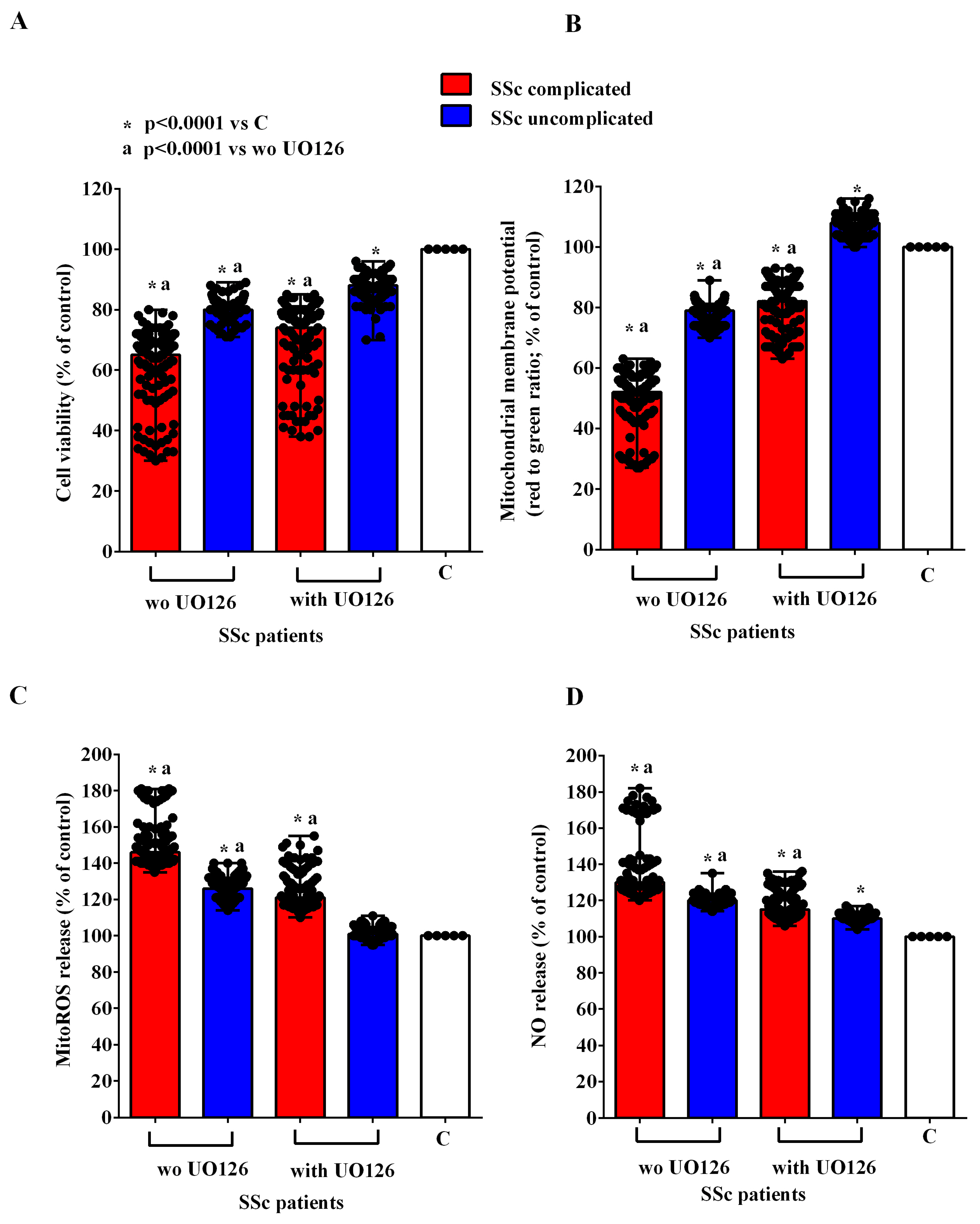
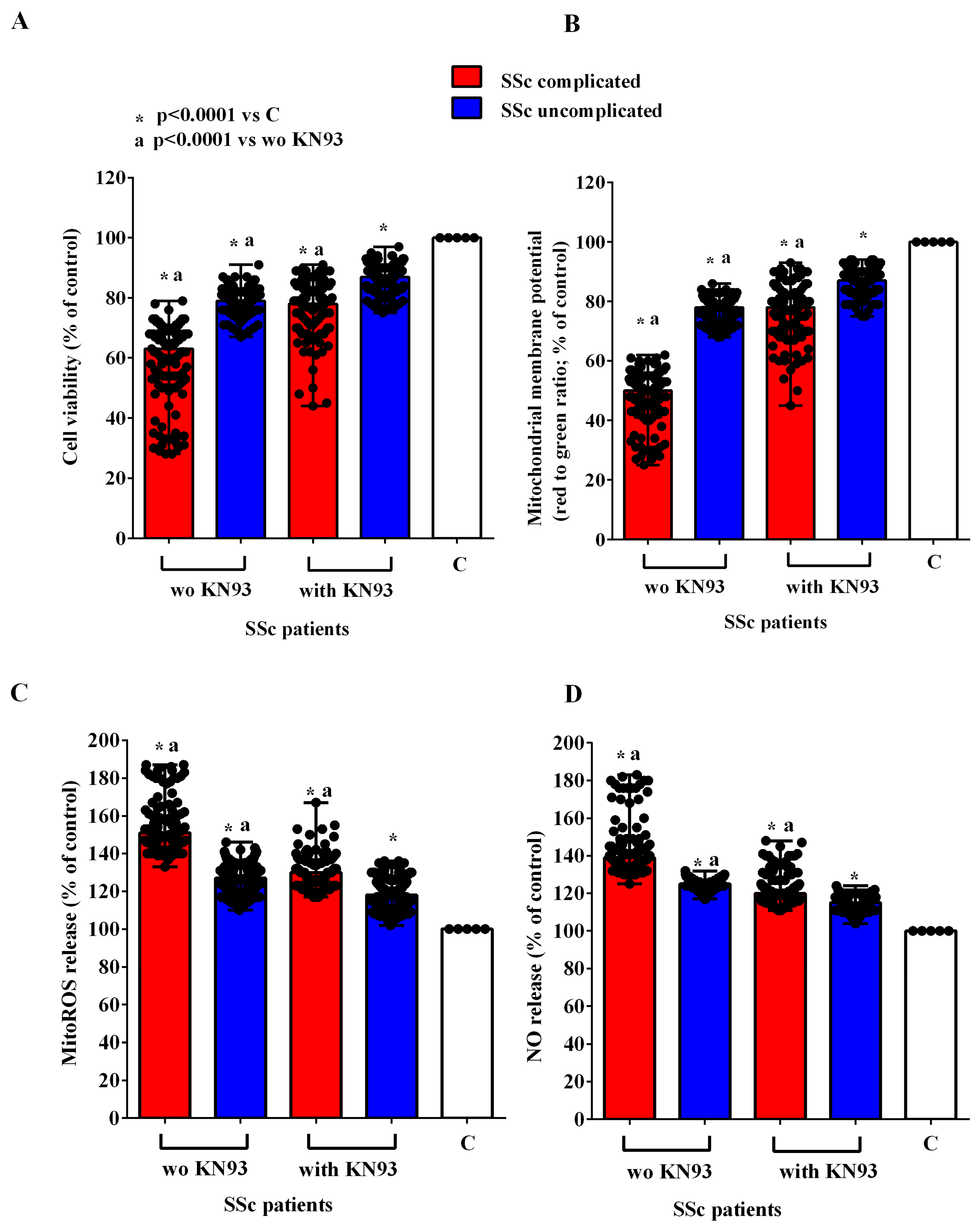
2.2. Effects of EVs on HUVECs
2.3. Effects of EVs on C2C12
3. Discussion
4. Materials and Methods
4.1. Plasma Sampling
4.2. EVs Isolation
4.3. EVs Characterization
4.4. MACSPlex Exosome Kit Analysis
4.5. HUVECs
4.6. Cell Viability Evaluation
4.7. Mitochondrial Membrane Potential Evaluation
4.8. MitoROS Release
4.9. NO Release
4.10. EndMT Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colaci, M.; Zanoli, L.; Lo Gullo, A.; Sambataro, D.; Sambataro, G.; Aprile, M.L.; Castellino, P.; Malatino, L. The Impaired Elasticity of Large Arteries in Systemic Sclerosis Patients. J. Clin. Med. 2022, 11, 3256. [Google Scholar] [CrossRef]
- Edigin, E.; Ojemolon, P.E.; Eseaton, P.O.; Jamal, S.; Shaka, H.; Akuna, E.; Asemota, I.R.; Manadan, A. Systemic Sclerosis Is Associated with Increased Inpatient Mortality in Patients Admitted for Acute Coronary Syndrome: Analysis of the National Inpatient Sample. J. Clin. Rheumatol. 2022, 28, E110–E117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lefebvre, F.; Hoa, S.; Hudson, M. Mortality and Morbidity in Scleroderma Renal Crisis: A Systematic Literature Review. J. Scleroderma Relat. Disord. 2021, 6, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Fischer, A. Update on Morbidity and Mortality in Systemic Sclerosis–Related Interstitial Lung Disease. J. Scleroderma Relat. Disord. 2021, 6, 11–20. [Google Scholar] [CrossRef]
- De Almeida, C.S.; Porel, T.; Mounié, M.; Alric, L.; Astudillo, L.; Huart, A.; Lairez, O.; Michaud, M.; Prévot, G.; Ribes, D.; et al. Sine Scleroderma, Limited Cutaneous, and Diffused Cutaneous Systemic Sclerosis Survival and Predictors of Mortality. Arthritis Res. Ther. 2021, 23, 295. [Google Scholar] [CrossRef]
- Pokeerbux, M.R.; Giovannelli, J.; Dauchet, L.; Mouthon, L.; Agard, C.; Lega, J.C.; Allanore, Y.; Jego, P.; Bienvenu, B.; Berthier, S.; et al. Survival and Prognosis Factors in Systemic Sclerosis: Data of a French Multicenter Cohort, Systematic Review, and Meta-Analysis of the Literature. Arthritis Res. Ther. 2019, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Noviani, M.; Chellamuthu, V.R.; Albani, S.; Low, A.H.L. The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity. Int. J. Mol. Sci. 2023, 24, 14287. [Google Scholar] [CrossRef]
- Zanin-Silva, D.C.; Santana-Gonçalves, M.; Kawashima-Vasconcelos, M.Y.; Oliveira, M.C. Management of Endothelial Dysfunction in Systemic Sclerosis: Current and Developing Strategies. Front. Med. 2021, 8, 788250. [Google Scholar] [CrossRef]
- Ramahi, A.; Altorok, N.; Kahaleh, B. Epigenetics and Systemic Sclerosis: An Answer to Disease Onset and Evolution? Eur. J. Rheumatol. 2020, 7, 147–156. [Google Scholar] [CrossRef]
- Patnaik, E.; Lyons, M.; Tran, K.; Pattanaik, D. Endothelial Dysfunction in Systemic Sclerosis. Int. J. Mol. Sci. 2023, 24, 14385. [Google Scholar] [CrossRef]
- Jimenez, S.A. Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. ISRN Rheumatol. 2013, 2013, 83594. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A.; Piera-Velazquez, S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of Systemic Sclerosis associated pulmonary fibrosis and pulmonary arterial hypertension. Myth or reality? Matrix Biol. 2016, 51, 26–36. [Google Scholar] [CrossRef]
- Thuan, D.T.B.; Zayed, H.; Eid, A.H.; Abou-Saleh, H.; Nasrallah, G.K.; Mangoni, A.A.; Pintus, G. A Potential Link Between Oxidative Stress and Endothelial-to-Mesenchymal Transition in Systemic Sclerosis. Front. Immunol. 2018, 9, 1985. [Google Scholar] [CrossRef]
- Tang, H.; Babicheva, A.; McDermott, K.M.; Gu, Y.; Ayon, R.J.; Song, S.; Wang, Z.; Gupta, A.; Zhou, T.; Sun, X.; et al. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L256–L275. [Google Scholar]
- Karatas, A.; Oz, B.; Celik, C.; Akar, Z.A.; Akkoc, R.F.; Etem, E.O.; Dagli, A.F.; Koca, S.S. Tofacitinib and metformin reduce the dermal thickness and fibrosis in mouse model of systemic sclerosis. Sci. Rep. 2022, 12, 2553. [Google Scholar] [CrossRef]
- Simon, M.; Lücht, C.; Hosp, I.; Zhao, H.; Wu, D.; Heidecke, H.; Witowski, J.; Budde, K.; Riemekasten, G.; Catar, R. Autoantibodies from Patients with Scleroderma Renal Crisis Promote PAR-1 Receptor Activation and IL-6 Production in Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11793. [Google Scholar] [CrossRef]
- Wang, E.; Wang, H.; Chakrabarti, S. Endothelial-to-mesenchymal transition: An underappreciated mediator of diabetic complications. Front Endocrinol. 2023, 14, 1050540. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.; Corallo, C.; Chirico, C.; Brazzi, A.; Marinetti, A.; Fioravanti, A.; Valenti, R.; Nuti, R.; Pecetti, G. Pulmonary arterial hypertension in systemic sclerosis: Diagnosis and treatment according to the European Society of Cardiology and European Respiratory Society 2015 guidelines. J. Scleroderma Relat. Disord. 2019, 4, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kuryliszyn-Moskal, A.; Klimiuk, P.A.; Sierakowski, S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: Relationship to organ systemic involvement. Clin. Rheumatol. 2004, 24, 111–116. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Capece, D.; Zazzeroni, F.; Liakouli, V.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Pecetti, G.; et al. The Endothelial-Mesenchymal Transition in Systemic Sclerosis Is Induced by Endothelin-1 and Transforming Growth Factor and May Be Blocked by Macitentan, a Dual Endothelin-1 Receptor Antagonist. J. Rheumatol. 2015, 42, 1808–1816. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Z.; Wang, Y.; Yang, J.; Wan, W.; Zou, H.; Yang, X. Progress in research on mesenchymal stem cells and their extracellular vesicles for treating fibrosis in systemic sclerosis. Clin. Exp. Med. 2023, 23, 2997–3009. [Google Scholar] [CrossRef]
- Colletti, M.; Galardi, A.; De Santis, M.; Guidelli, G.M.; Di Giannatale, A.; Di Luigi, L.; Antinozzi, C. Exosomes in Systemic Sclerosis: Messengers Between Immune, Vascular and Fibrotic Components? Int. J. Mol. Sci. 2019, 20, 4337. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Yaici, A.; de Groote, P.; Montani, D.; Sitbon, O.; Launay, D.; Gressin, V.; Guillevin, L.; Clerson, P.; Simonneau, G.; et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: Clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011, 63, 3522–3530. [Google Scholar] [CrossRef]
- Michalska-Jakubus, M.; Kowal-Bielecka, O.; Smith, V.; Cutolo, M.; Krasowska, D. Plasma endothelial microparticles reflect the extent of capillaroscopic alterations and correlate with the severity of skin involvement in systemic sclerosis. Microvasc. Res. 2017, 110, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, S.; Distler, J.H.; Jüngel, A.; Huscher, D.; Huber, L.C.; Michel, B.A.; Gay, R.E.; Pisetsky, D.S.; Gay, S.; Matucci-Cerinic, M.; et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 2008, 58, 2845–2853. [Google Scholar] [CrossRef]
- Wu, Y.J.; Hua, C.C.; Chen, J.Y.; Chang, Y.W.; Tseng, J.C. The role of endothelial microparticles in autoimmune disease patients with Raynaud’s phenomenon. J. Microbiol. Immunol. Infect. 2017, 50, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Lammi, M.R.; Saketkoo, L.A.; Okpechi, S.C.; Ghonim, M.A.; Wyczechowska, D.; Bauer, N.; Pyakurel, K.; Saito, S.; deBoisblanc, B.P.; Boulares, A.H. Microparticles in systemic sclerosis: Potential pro-inflammatory mediators and pulmonary hypertension biomarkers. Respirology 2019, 24, 675–683. [Google Scholar] [CrossRef]
- Gabsi, A.; Heim, X.; Dlala, A.; Gati, A.; Sakhri, H.; Abidi, A.; Amri, S.; Neili, B.; Leroyer, A.S.; Bertaud, A.; et al. TH17 cells expressing CD146 are significantly increased in patients with Systemic sclerosis. Sci. Rep. 2019, 9, 17721. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence That Systemic Sclerosis Is a Vascular Disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Manetti, M. Recent Insights into Cellular and Molecular Mechanisms of Defective Angiogenesis in Systemic Sclerosis. Biomedicines 2024, 12, 1331. [Google Scholar] [CrossRef]
- Fukasawa, T.; Yoshizaki-Ogawa, A.; Enomoto, A.; Yamashita, T.; Miyagawa, K.; Sato, S.; Yoshizaki, A. Single cell analysis in systemic sclerosis—A systematic review. Immunol. Med. 2024, 47, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, E.V.; Shayakhmetova, R.U.; Gerasimova, D.A.; Popkova, T.V.; Ananyeva, L.P. Systemic Sclerosis and Atherosclerosis: Potential Cellular Biomarkers and Mechanisms. Front Biosci. Schol. Ed. 2023, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Piera-Velazquez, S.; Rosenbloom, J.; Jimenez, S.A. Existing and novel biomarkers for precision medicine in systemic sclerosis. Nat. Rev. Rheumatol. 2018, 14, 421–432. [Google Scholar] [CrossRef]
- Mouawad, J.E.; Sanderson, M.; Sharma, S.; Helke, K.L.; Pilewski, J.M.; Nadig, S.N.; Feghali-Bostwick, C. Role of Extracellular Vesicles in the Propagation of Lung Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Shemiakova, T.; Ivanova, E.; Grechko, A.V.; Gerasimova, E.V.; Sobenin, I.A.; Orekhov, A.N. Mitochondrial Dysfunction and DNA Damage in the Context of Pathogenesis of Atherosclerosis. Biomedicines 2020, 8, 166. [Google Scholar] [CrossRef]
- Prajapat, S.K.; Maharana, K.C.; Singh, S. Mitochondrial dysfunction in the pathogenesis of endothelial dysfunction. Mol. Cell Biochem. 2024, 479, 1999–2016. [Google Scholar] [CrossRef]
- Rongvaux, A. Innate immunity and tolerance toward mitochondria. Mitochondrion. Mitochondrion 2018, 41, 14–20. [Google Scholar] [CrossRef]
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS expression in vascular diseases. Front Biosci. Landmark Ed. 2021, 26, 85–101. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Liaudet, L.; Vassalli, G.; Pacher, P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. Landmark Ed. 2009, 14, 4809–4814. [Google Scholar] [CrossRef]
- Diers, A.R.; Broniowska, K.A.; Hogg, N. Nitrosative stress and redox-cycling agents synergize to cause mitochondrial dysfunction and cell death in endothelial cells. Redox Biol. 2013, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Vomero, M.; Navarini, L.; Dolo, V.; Cipriani, P.; Giacomelli, R. Endothelial-to-mesenchymal transition in systemic sclerosis. Clin. Exp. Immunol. 2021, 205, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.S.; Palisoc, P.J.; Flavahan, N.A.; Khanna, D. Dissecting the Cellular Mechanism of Prostacyclin Analog Iloprost in Reversing Vascular Dysfunction in Scleroderma. Arthritis Rheumatol. 2021, 73, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.M.; Rokni, M.; Mahmoudi, M.; Farhadi, E. Ras family signaling pathway in immunopathogenesis of inflammatory rheumatic diseases. Front. Immunol. 2023, 14, 1151246. [Google Scholar] [CrossRef]
- Moser, B.; Poetsch, F.; Estepa, M.; Luong, T.T.D.; Pieske, B.; Lang, F.; Alesutan, I.; Voelkl, J. Increased β-adrenergic stimulation augments vascular smooth muscle cell calcification via PKA/CREB signalling. Pflugers Arch. 2021, 473, 1899–1910. [Google Scholar] [CrossRef]
- Saddouk, F.Z.; Ginnan, R.; Singer, H.A. Ca2+/Calmodulin-Dependent Protein Kinase II in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 171–202. [Google Scholar]
- Grossini, E.; Smirne, C.; Venkatesan, S.; Tonello, S.; D’Onghia, D.; Minisini, R.; Cantaluppi, V.; Sainaghi, P.P.; Comi, C.; Tanzi, A.; et al. Plasma Pattern of Extracellular Vesicles Isolated from Hepatitis C Virus Patients and Their Effects on Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 10197. [Google Scholar] [CrossRef]
- Rakshit, T.; Pal, S. Extracellular Vesicles for Drug Delivery and Theranostics In Vivo. JACS Au 2024, 18, 318–327. [Google Scholar] [CrossRef]
- Cras, A.; Farge, D.; Carmoi, T.; Lataillade, J.J.; Wang, D.D.; Sun, L. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res. Ther. 2015, 17, 301. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Sarvar, D.P.; Effatpanah, H.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal stromal cell-derived extracellular vesicles: Novel approach in hematopoietic stem cell transplantation. Stem Cell Res. Ther. 2022, 13, 202. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Sallustio, F.; Bruno, S.; Merlotti, G.; Quaglia, M.; Grandaliano, G.; Pontrelli, P.; Thurman, J.M.; Camussi, G.; et al. Extracellular vesicles derived from patients with antibody-mediated rejection induce tu-bular senescence and endothelial to mesenchymal transition in renal cells. Am. J. Transpl. 2022, 22, 2139–2157. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Koch, B.; Morikawa, K.; Suda, G.; Sakamoto, N.; Rueschenbaum, S.; Akhras, S.; Dietz, J.; Hildt, E.; Zeuzem, S.; et al. Macrophage-Derived Extracellular Vesicles Induce Long-Lasting Immunity Against Hepatitis C Virus Which Is Blunted by Polyunsaturated Fatty Acids. Front. Immunol. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Esposito, T.; Viretto, M.; Venkatesan, S.; Licari, I.; Surico, D.; Della Corte, F.; Castello, L.; Bruno, S.; Quaglia, M.; et al. Circulating Extracellular Vesicles in Subarachnoid Hemorrhage Patients: Characterization and Cellular Effects. Int. J. Mol. Sci. 2023, 24, 14913. [Google Scholar] [CrossRef]
- Cavallari, C.; Dellepiane, S.; Fonsato, V.; Medica, D.; Marengo, M.; Migliori, M.; Quercia, A.D.; Pitino, A.; Formica, M.; Panichi, V.; et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J. Immunol. 2019, 202, 2372–2383. [Google Scholar] [CrossRef]
- Grossini, E.; Venkatesan, S.; Ola Pour, M.M.; Ferrante, D.; Surico, D.; Vaschetto, R.; Cantaluppi, V.; Pirisi, M. Chromogranin B Protects Human Umbilical Endothelial Cells against Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 10296. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Venkatesan, S.; Pour, M.M.O.; Conti, A.; Concina, D.; Opizzi, A.; Sanguedolce, A.; Rinaldi, C.; Russotto, S.; Gramaglia, C.M.; et al. Beneficial effects of a combined lifestyle intervention for older people in a long-term-care facility on redox balance and endothelial function. Heliyon 2024, 10, e35850. [Google Scholar] [CrossRef]
- Grossini, E.; De Marchi, F.; Venkatesan, S.; Mele, A.; Ferrante, D.; Mazzini, L. Effects of Acetyl-L-Carnitine on Oxidative Stress in Amyotrophic Lateral Sclerosis Patients: Evaluation on Plasma Markers and Members of the Neurovascular Unit. Antioxidants 2023, 12, 1887. [Google Scholar] [CrossRef]
- Grossini, E.; Aquino, C.I.; Venkatesan, S.; Troìa, L.; Tizzoni, E.; Fumagalli, F.; Ferrante, D.; Vaschetto, R.; Remorgida, V.; Surico, D. Plasma Redox Balance in Advanced-Maternal-Age Pregnant Women and Effects of Plasma on Umbilical Cord Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 4869. [Google Scholar] [CrossRef]
- Medica, D.; Franzin, R.; Stasi, A.; Castellano, G.; Migliori, M.; Panichi, V.; Figliolini, F.; Gesualdo, L.; Camussi, G.; Cantaluppi, V. Extracellular Vesicles Derived from Endothelial Progenitor Cells Protect Human Glomerular Endothelial Cells and Podocytes from Complement- and Cytokine-Mediated Injury. Cells 2021, 10, 1675. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Medica, D.; Mannari, C.; Stiaccini, G.; Figliolini, F.; Dellepiane, S.; Quercia, A.D.; Migliori, M.; Panichi, V.; Giovannini, L.; et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol. Dial. Transplant 2015, 30, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca 2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Molinari, C.; Sigaudo, L.; Biella, M.; Mary, D.A.; Vacca, G. Calcium handling in porcine coronary endothelial cells by gastrin-17. J. Mol. Endocrinol. 2013, 50, 243–253. [Google Scholar] [CrossRef] [PubMed]
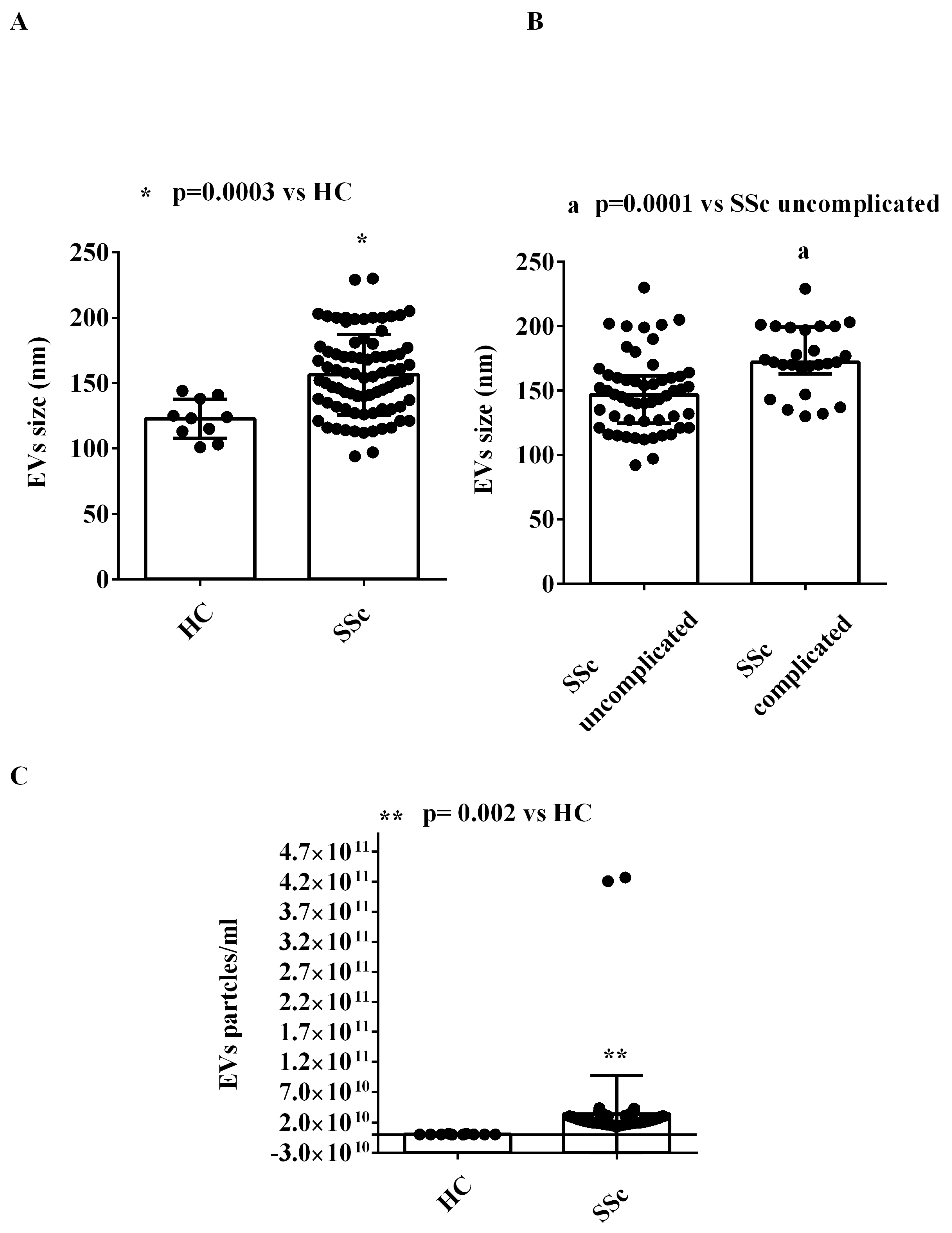
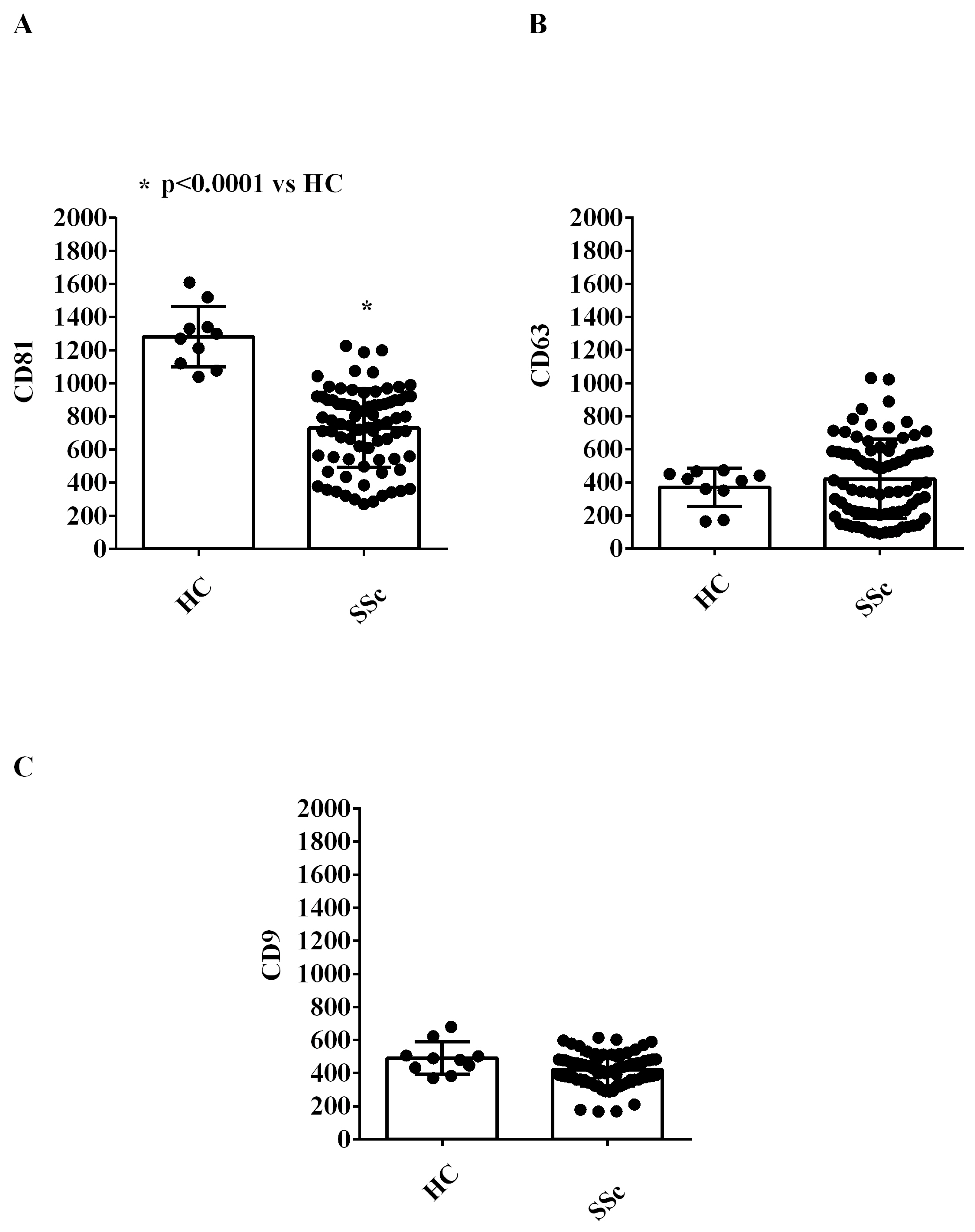
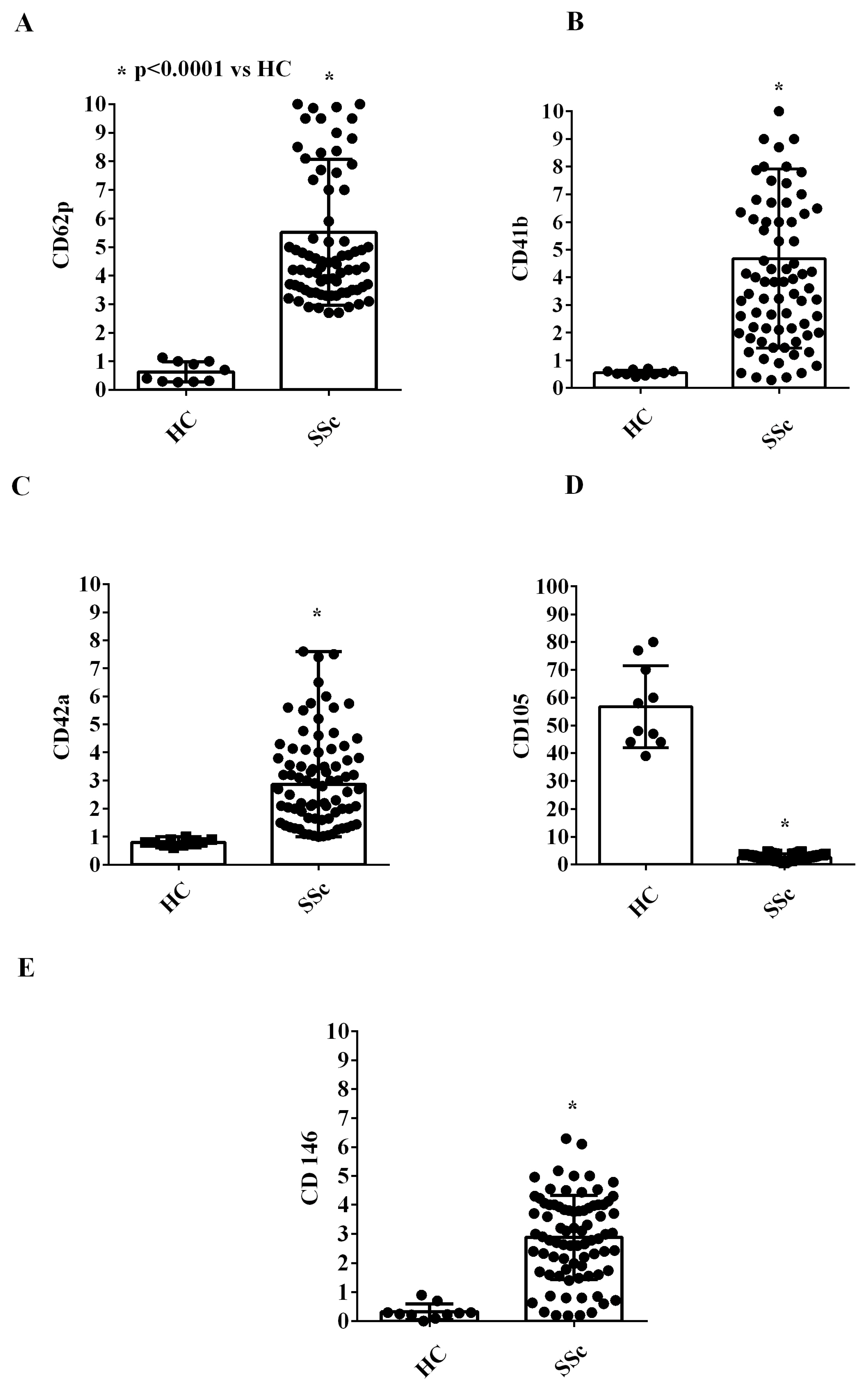
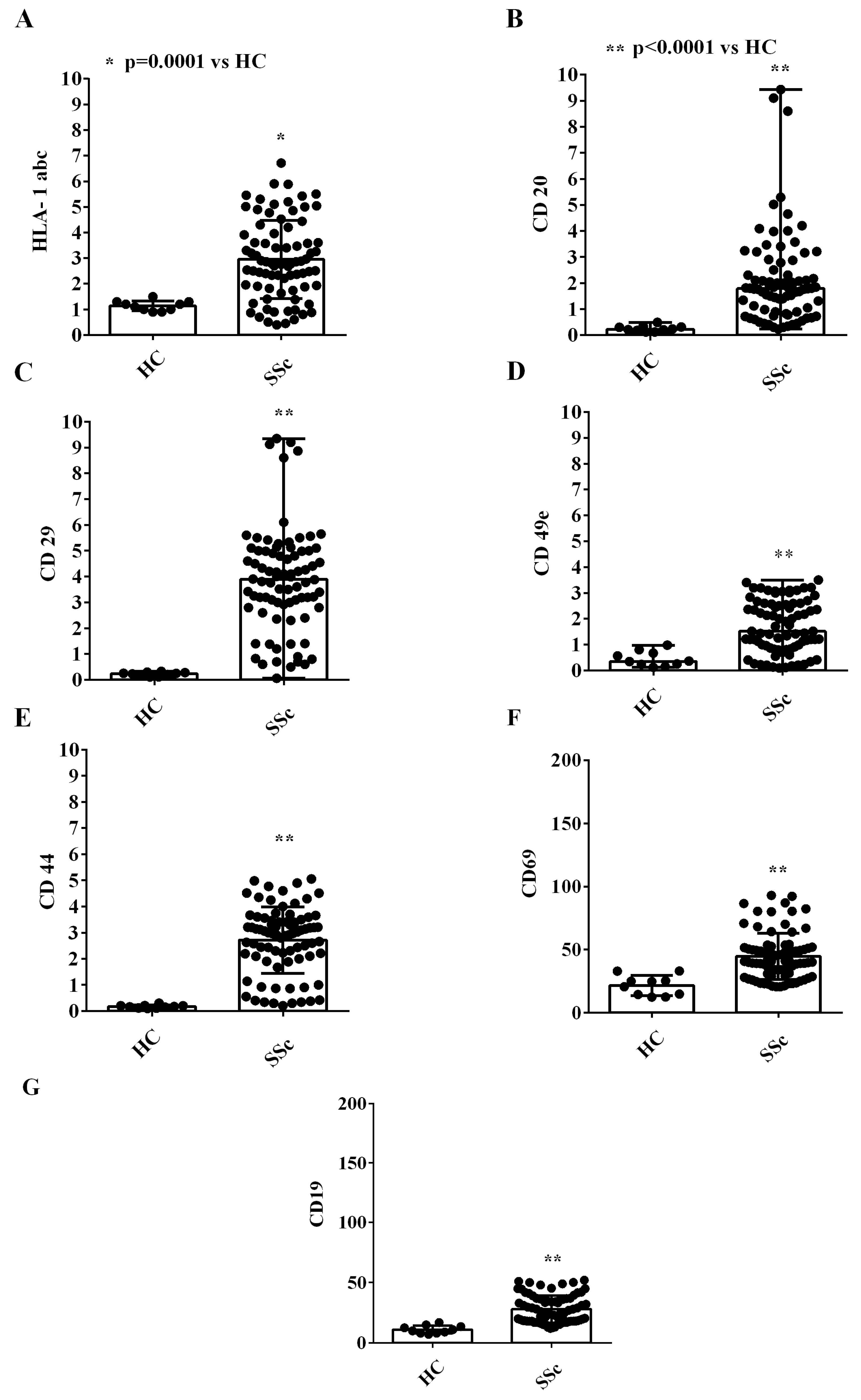


| SSc Complicated (n = 13) | SSc Uncomplicated (n = 27) | p Value | |
|---|---|---|---|
| Age (years) | 68.5 ± 9.2 | 61.6 ± 8.7 | =0.01 |
| Females/males | 12/13 | 23/27 | >0.05 |
| BMI (kg/m2) | 22.6 ± 2.9 | 23.2 ± 4.8 | >0.05 |
| SSc phenotype (lcSSc vs. dcSSc) | lcSSc: 8/13 dcSSc: 5/13 | lcSSc: 21/27 dcSSc: 6/27 | >0.05 |
| Overlap autoimmune diseases | 2/13 (myositis, Sjogren) | 3/27 (polymyositis, Sjogren, primary biliary cholangitis) | >0.05 |
| WHO (1, 2, 3, 4) | 1: 4/13 2:7/13 3:2/13 | 1: 19/27 2:8/27 | WHO 1 = 0.038 WHO 2 > 0.05 |
| C | HC | SSc Complicated | SSc Uncomplicated | |
|---|---|---|---|---|
| CD31 | 1 | 27 (20–32) * | 2 (0.9–2.9) *a | 7.8 (2.7–12) *a |
| Vimentin | 1 | 0.15 (0.07–0.21) | 12 (8–29) *b | 5 (2–11) *b |
| VE-cadherin | 1 | 15 (12–19) * | 4.05 (1–5.4) *d | 13.1 (11.6–16) *d |
| N-cadherin | 1 | 2.3 (2–4) * | 14.7 (12.3–22) *e | 3.5 (2–4.9) *e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grossini, E.; Bellan, M.; Venkatesan, S.; Ola Pour, M.M.; Mennuni, M.; D’Amario, D.; Bruno, S.; Ferrante, D.; Capello, D.; Sainaghi, P.P.; et al. Characterization of Circulating Vesicles of Complicated and Uncomplicated Systemic Sclerosis Patients and Their Role in Vascular Dysfunction. Int. J. Mol. Sci. 2025, 26, 2380. https://doi.org/10.3390/ijms26062380
Grossini E, Bellan M, Venkatesan S, Ola Pour MM, Mennuni M, D’Amario D, Bruno S, Ferrante D, Capello D, Sainaghi PP, et al. Characterization of Circulating Vesicles of Complicated and Uncomplicated Systemic Sclerosis Patients and Their Role in Vascular Dysfunction. International Journal of Molecular Sciences. 2025; 26(6):2380. https://doi.org/10.3390/ijms26062380
Chicago/Turabian StyleGrossini, Elena, Mattia Bellan, Sakthipriyan Venkatesan, Mohammad Mostafa Ola Pour, Marco Mennuni, Domenico D’Amario, Stefania Bruno, Daniela Ferrante, Daniela Capello, Pier Paolo Sainaghi, and et al. 2025. "Characterization of Circulating Vesicles of Complicated and Uncomplicated Systemic Sclerosis Patients and Their Role in Vascular Dysfunction" International Journal of Molecular Sciences 26, no. 6: 2380. https://doi.org/10.3390/ijms26062380
APA StyleGrossini, E., Bellan, M., Venkatesan, S., Ola Pour, M. M., Mennuni, M., D’Amario, D., Bruno, S., Ferrante, D., Capello, D., Sainaghi, P. P., Pirisi, M., & Patti, G. (2025). Characterization of Circulating Vesicles of Complicated and Uncomplicated Systemic Sclerosis Patients and Their Role in Vascular Dysfunction. International Journal of Molecular Sciences, 26(6), 2380. https://doi.org/10.3390/ijms26062380











