Trichostatin A-Induced Epigenetic Modifications and Their Influence on the Development of Porcine Cloned Embryos Derived from Bone Marrow–Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
2.1. Effect of TSA on the Developmental Capacity of NT Embryos Derived from Bone Marrow–MSCs (NT-BMSC)
2.2. Ability of TSA to Improve Development-Related Genes in NT-BMSC Embryos
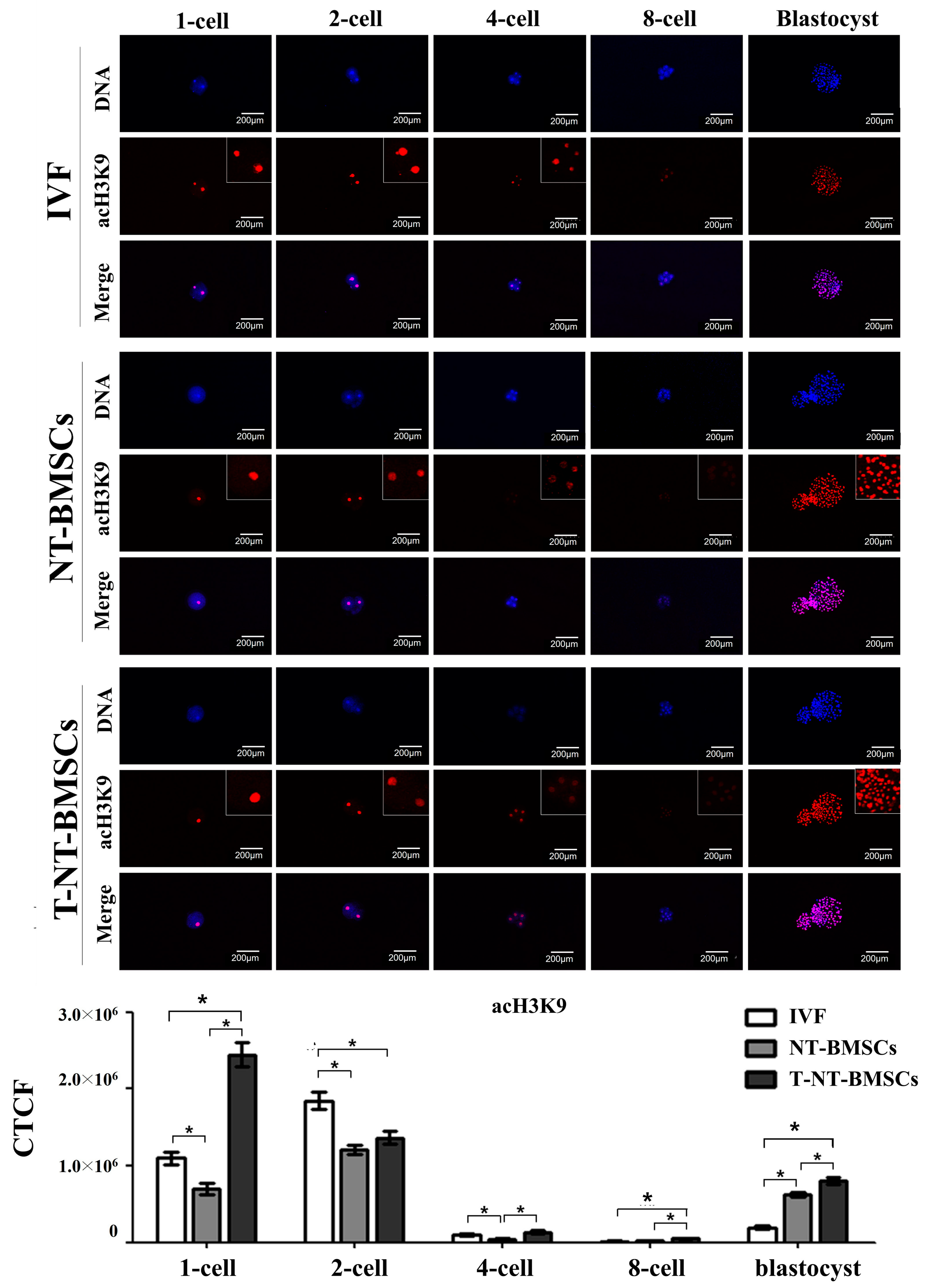
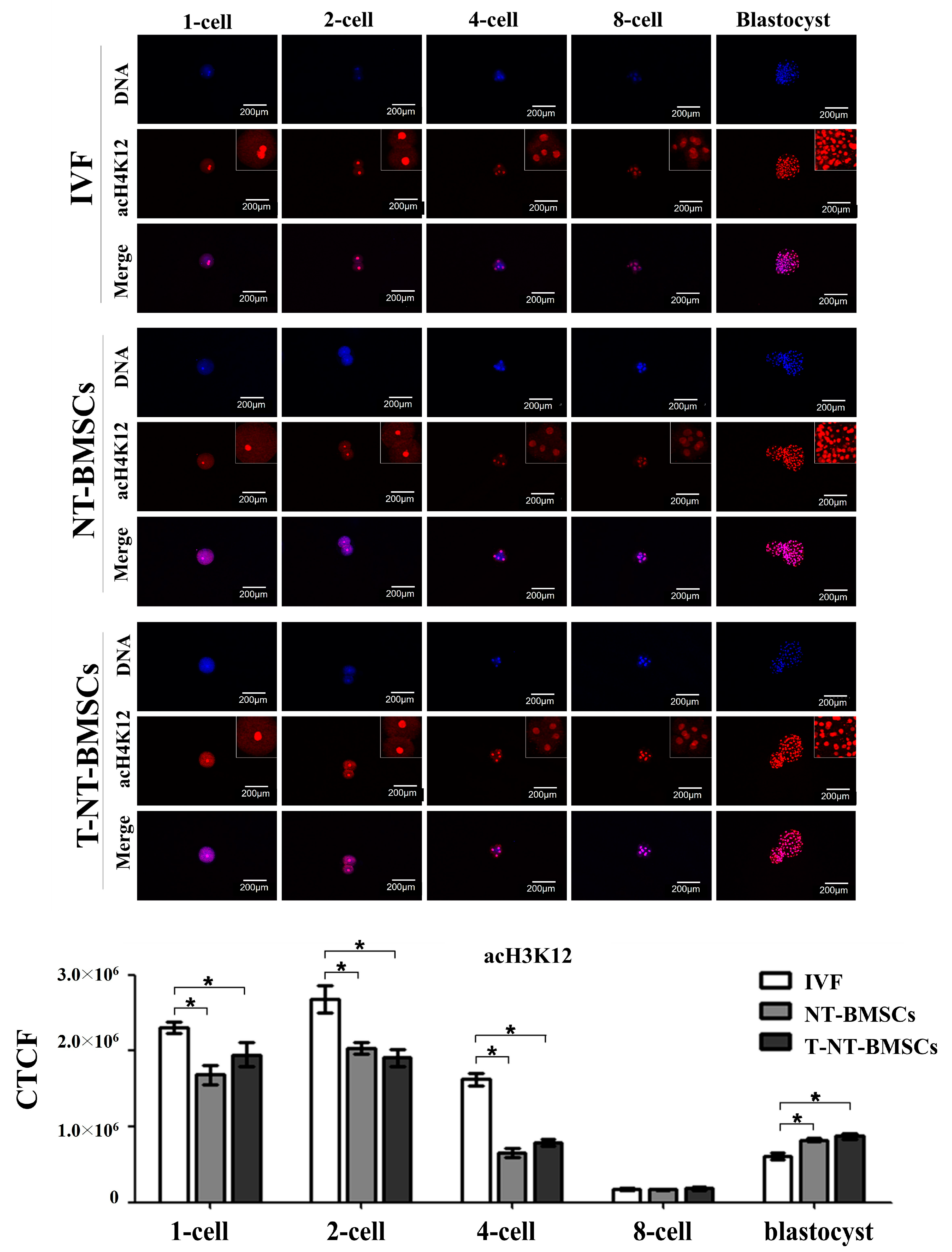
2.3. Effect of TSA Treatment on Histone Acetylation Status in NT-BMSC Embryos
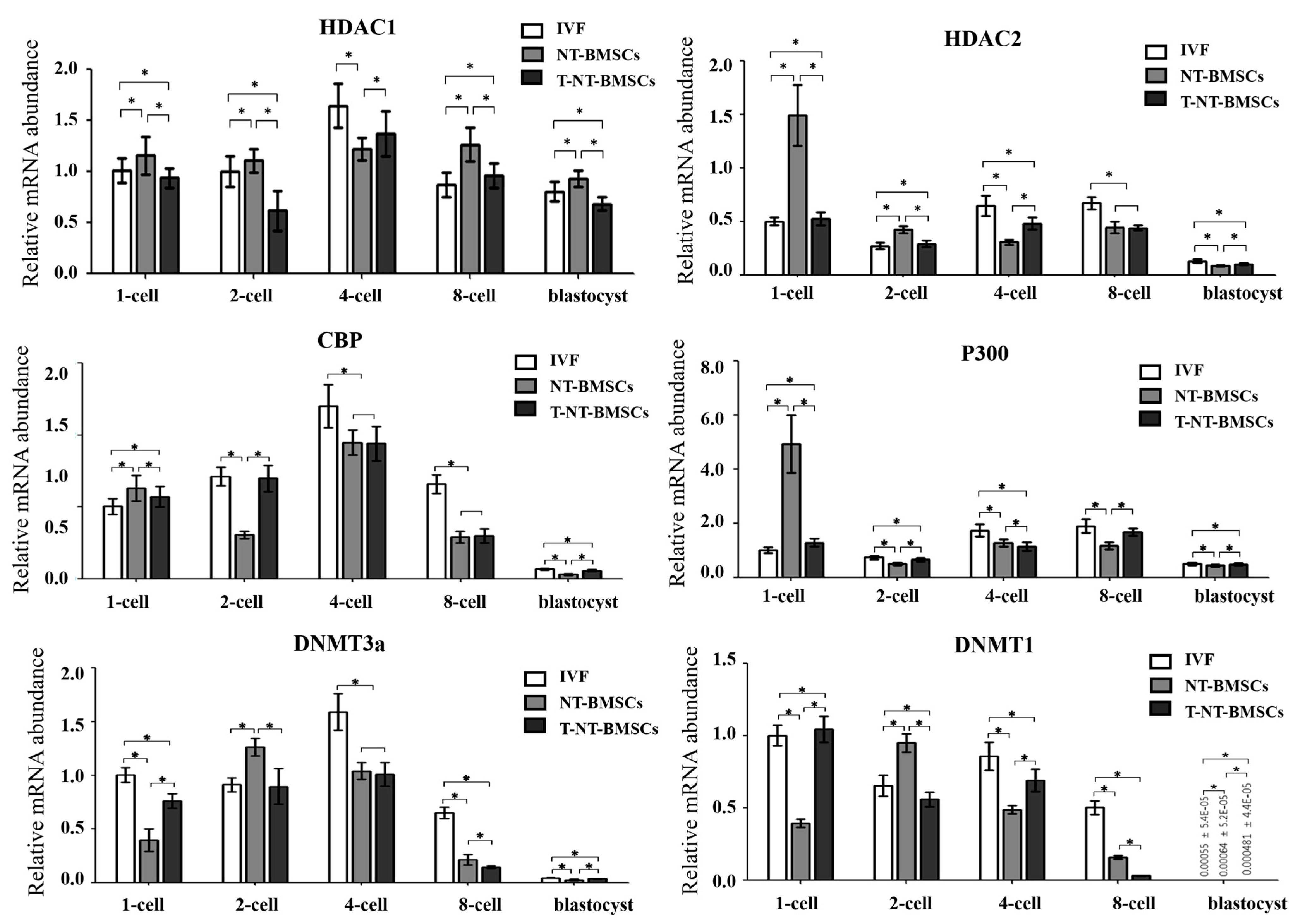
2.4. TSA Enhances the Expression of Genes Related to Histone Acetylation and DNA Methylation in NT-BMSC Embryos
3. Discussion
4. Materials and Methods
4.1. Chemicals and Media
4.2. Preparation of Mesenchymal Stem Cells as Donor Cells
4.3. Oocyte Collection and In Vitro Maturation (IVM)
4.4. Production of IVF Embryos
4.5. Production of NT Embryos Derived from BM-MSCs
4.6. In Vitro Culture of Embryos
4.7. Treatment of TSA in NT Embryos
4.8. Embryo Collection for Assessment
4.9. Total Cell Number in Blastocysts
4.10. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
4.11. Immunofluorescence Staining of Embryos
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vajta, G.; Zhang, Y.; Macháty, Z. Somatic cell nuclear transfer in pigs: Recent achievements and future possibilities. Reprod. Fertil. Dev. 2007, 19, 403–423. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.-W.; Cheong, H.-T.; Greenstein, J.L.; IM, G.-S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of alpha -1,3-Galactosyltransferase Knockout Pigs by Nuclear Transfer Cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef]
- Ahn, K.S.; Won, J.Y.; Heo, S.Y.; Kang, J.H.; Yang, H.S.; Shim, H. Transgenesis and nuclear transfer using porcine embryonic germ cells. Cloning Stem Cells. 2007, 9, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Bai, J.; Duan, Q.; Costa, M.; Dai, W. Covalent modifications of histones during mitosis and meiosis. Cell Cycle 2009, 8, 3688–3694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, X.; Li, Y.; Li, G.-P.; Yang, D.; Yue, Y.; Wang, L.; Li, K.; Xin, P.; Bou, S.; Yu, H. Trichostatin A improved epigenetic modifications of transfected cells but did not improve subsequent cloned embryo development. Anim. Biotechnol. 2008, 19, 211–224. [Google Scholar] [CrossRef]
- Lee, D.Y.; Hayes, J.J.; Pruss, D.; Wolffe, A.P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 1993, 72, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Zakhartchenko, V.; Stojkovic, M.; Peters, A.; Jenuwein, T.; Wolf, E.; Reik, W.; Dean, W. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr. Biol. 2003, 13, 1116–1121. [Google Scholar] [CrossRef]
- Senda, S.; Wakayama, T.; Arai, Y.; Yamazaki, Y.; Ohgane, J.; Tanaka, S.; Hattori, N.; Yanagimachi, R.; Shiota, K. DNA methylation errors in cloned mice disappear with advancement of aging. Cloning Stem Cells. 2007, 9, 293–302. [Google Scholar] [CrossRef]
- Geiman, T.M.; Robertson, K.D. Chromatin remodeling, histone modifications and DNA methylation-How does it all fit together? J. Cell. Biochem. 2002, 87, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Hao, J.; Wang, L.; Jouneau, A.; Zhou, Q. Pluripotency maintenance in mouse somatic cell nuclear transfer embryos and its improvement by treatment with the histone deacetylase inhibitor TSA. Cell. Reprogram. 2011, 13, 47–56. [Google Scholar] [CrossRef]
- Iager, A.E.; Ragina, N.P.; Ross, P.J.; Beyhan, Z.; Cunniff, K.; Rodriguez, R.M.; Cibelli, J.B. Trichostatin A improves histone acetylation in bovine somatic cell nuclear transfer early embryos. Cloning Stem Cells 2008, 10, 371–379. [Google Scholar] [CrossRef]
- Kishigami, S.; Mizutani, E.; Ohta, H.; Hikichi, T.; Thuan, N.V.; Wakayama, S.; Bui, H.-T.; Wakayama, T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 2006, 340, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Das, Z.C.; Gupta, M.K.; Uhm, S.J.; Lee, H.T. Increasing histone acetylation of cloned embryos, but not donor cells, by sodium butyrate improves their in vitro development in pigs. Cell. Reprogram. 2010, 12, 95–104. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Li, Y.; Wang, L.; Xiong, X.; Su, J.; Zhang, Y. Valproic acid improves the in vitro development competence of bovine somatic cell nuclear transfer embryos. Cell. Reprogram. 2012, 14, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ross, J.W.; Hao, Y.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol. Reprod. 2009, 81, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.G.; Coppola, G.; Perrault, S.D.; Rho, G.J.; Betts, D.H.; King, W.A. S-adenosylhomocysteine treatment of adult female fibroblasts alters X-chromosome inactivation and improves in vitro embryo development after somatic cell nuclear transfer. Reproduction 2008, 135, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hai, T.; Wang, Y.; Guo, R.; Li, W.; Wang, L.; Zhou, Q. Epigenetic reprogramming, gene expression and in vitro development of porcine SCNT embryos are significantly improved by a histone deacetylase inhibitor-m-carboxycinnamic acid bishydroxamide (CBHA). Protein Cell 2014, 5, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Li, Y.; Li, R.; Li, Q.; Wu, Y.; Quan, F.; Liu, J.; Guo, Z.; Zhang, Y. Oxamflatin significantly improves nuclear reprogramming, blastocyst quality and in vitro development of bovine SCNT embryos. PLoS ONE 2011, 6, e23805. [Google Scholar] [CrossRef]
- Li, J.; Svarcova, O.; Villemoes, K.; Kragh, P.M.; Schmidt, M.; Bøgh, I.B.; Zhang, Y.; Du, Y.; Lin, L.; Purup, S.; et al. High in vitro development after somatic cell nuclear transfer and trichostatin A treatment of reconstructed porcine embryos. Theriogenology 2008, 70, 800–808. [Google Scholar] [CrossRef]
- Matoba, S.; Shikata, D.; Shirai, F.; Tatebe, T.; Hirose, M.; Nakata, A.; Watanabe, N.; Hasegawa, A.; Ito, A.; Yoshida, M.; et al. Reduction of H3K9 methylation by G9a inhibitors improves the development of mouse SCNT embryos. Stem Cell Rep. 2024, 19, 906–921. [Google Scholar] [CrossRef]
- Peng, C.; Lv, Z.; Hai, T.; Dai, X.; Zhou, Q. Differential effects of trichostatin A on mouse embryogenesis and development. Reproduction 2021, 162, 83–94. [Google Scholar] [CrossRef]
- Kang, H.; Roh, S. Extended exposure to trichostatin A after activation alters the expression of genes important for early development in nuclear transfer murine embryos. J. Vet. Med. Sci. 2011, 73, 623–631. [Google Scholar] [CrossRef]
- Cao, P.; Li, H.; Zuo, Y.; Nashun, B. Characterization of DNA methylation patterns and mining of epigenetic markers during genomic reprogramming in SCNT embryos. Front. Cell Dev. Biol. 2020, 2, 570107. [Google Scholar] [CrossRef]
- Olivera, R.; Moro, L.N.; Jordan, R.; Pallarols, N.; Guglielminetti, A.; Luzzani, C.; Miriuka, S.G.; Vichera, G. Bone marrow mesenchymal stem cells as nuclear donors improve viability and health of cloned horses. Stem Cells Cloning 2018, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Kang, E.J.; Maeng, G.H.; Kim, M.J.; Park, J.K.; Kim, T.S.; Hyun, S.H.; Lee, E.S.; Rho, G.J. Developmental ability of miniature pig embryos cloned with mesenchymal stem cells. J. Reprod. Dev. 2010, 56, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, W.J.; Jeon, R.H.; Lee, Y.M.; Jang, S.J.; Lee, S.L.; Jeon, B.G.; Ock, S.A.; King, W.A.; Rho, G.J. Development and gene expression of porcine cloned embryos derived from bone marrow stem cells with overexpressing Oct4 and Sox2. Cell. Reprogram. 2014, 16, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 2002, 12, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Wee, G.; Koo, D.-B.; Song, B.-S.; Kim, J.-S.; Kang, M.-J.; Moon, S.-J.; Kang, Y.-K.; Lee, K.-K.; Han, Y.-M. Inheritable histone H4 acetylation of somatic chromatins in cloned embryos. J. Biol. Chem. 2006, 281, 6048–6057. [Google Scholar] [CrossRef]
- Shi, L.-H.; Miao, Y.-L.; Ouyang, Y.-C.; Huang, J.-C.; Lei, Z.-L.; Yang, J.-W.; Han, Z.-M.; Song, X.-F.; Sun, Q.-Y.; Chen, D.-Y. Trichostatin A (TSA) improves the development of rabbit-rabbit intraspecies cloned embryos, but not rabbit-human interspecies cloned embryos. Dev. Dyn. 2008, 237, 640–648. [Google Scholar] [CrossRef]
- Cervera, R.P.; Martí-Gutiérrez, N.; Escorihuela, E.; Moreno, R.; Stojkovic, M. Trichostatin A affects histone acetylation and gene expression in porcine somatic cell nucleus transfer embryos. Theriogenology 2009, 72, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Matsukawa, K.; Mizutani, E.; Fukunari, K.; Kaneda, M.; Watanabe, S.; Takahashi, S. Treatment with a histone deacetylase inhibitor after nuclear transfer improves the preimplantation development of cloned bovine embryos. J. Reprod. Dev. 2011, 57, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Mohapatra, S.K.; Singh, M.K.; Singla, S.K.; Chauhan, M.S.; Manik, R.S. Effects of epigenetic modifier on the developmental competence and quantitative expression of genes in male and female buffalo (Bubalus bubalis) cloned embryos. Zygote 2023, 31, 129–139. [Google Scholar] [CrossRef]
- Boiani, M.; Eckardt, S.; Schöler, H.R.; McLaughlin, K.J. Oct4 distribution and level in mouse clones: Consequences for pluripotency. Genes Dev. 2002, 16, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Bortvin, A.; Eggan, K.; Skaletsky, H.; Akutsu, H.; Berry, D.L.; Yanagimachi, R.; Page, D.C.; Jaenisch, R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 2003, 130, 1673–1680. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Park, C.H.; Kim, H.S.; Jeong, Y.W.; Lee, J.Y.; Park, S.W.; Lee, S.Y.; Hyun, S.H.; Kim, Y.W.; Shin, T.; et al. Effects of Trichostatin A on in vitro development of porcine embryos derived from somatic cell nuclear transfer. Asian-Australas. J. Anim. Sci. 2013, 26, 1680–1688. [Google Scholar] [CrossRef]
- De Sousa, P.A.; Dobrinsky, J.R.; Zhu, J.; Archibald, A.L.; Ainslie, A.; Bosma, W.; Bowering, J.; Bracken, J.; Ferrier, P.M.; Fletcher, J.; et al. Somatic cell nuclear transfer in the pig: Control of pronuclear formation and integration with improved methods for activation and maintenance of pregnancy. Biol. Reprod. 2002, 66, 642–650. [Google Scholar] [CrossRef]
- Cui, X.-S.; Xu, Y.-N.; Shen, X.-H.; Zhang, L.-Q.; Zhang, J.-B.; Kim, N.-H. Trichostatin A modulates apoptotic-related gene expression and improves embryo viability in cloned bovine embryos. Cell. Reprogram. 2011, 13, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhu, K.; Liu, Z.; Song, Z.; Huang, Y.; Zhao, H.; Chen, Y.; He, Z.; Mo, D.; Cong, P. Improvement of porcine cloning efficiency by trichostatin A through early-stage induction of embryo apoptosis. Theriogenology 2013, 79, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Rollo, C.; Li, Y.; Jin, X.L.; O’Neill, C. Histone 3 lysine 9 acetylation is a biomarker of the effects of culture on zygotes. Reproduction 2017, 154, 375–385. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, H.Y.; Guo, Q.; Li, X.C.; Zhang, Y.C.; Zhang, G.L.; Xing, X.X.; Xuan, M.F.; Luo, Q.R.; Yin, X.J.; et al. PCI-24781 can improve in vitro and in vivo developmental capacity of pig somatic cell nuclear transfer embryos. Biotechnol. Lett. 2016, 38, 1433–1441. [Google Scholar] [CrossRef]
- Yamanaka, K.; Sugimura, S.; Wakai, T.; Kawahara, M.; Sato, E. Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J. Reprod. Dev. 2009, 55, 638–644. [Google Scholar] [CrossRef]
- Huan, Y.; Wang, H.; Wu, Z.; Zhang, J.; Zhu, J.; Liu, Z.; He, H. Epigenetic modification of cloned embryos improves NANOG reprogramming in pigs. Cell. Reprogram. 2015, 17, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hah, Y.S.; Ock, S.A.; Lee, J.H.; Jeon, R.H.; Park, J.S.; Lee, S.I.; Rho, N.Y.; Rho, G.J.; Lee, S.L. Cell source-dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp. Cell Res. 2015, 333, 273–288. [Google Scholar] [CrossRef]



| Group | No. of Reconstructed Embryos | Day 2 No. of Cleaved Embryos (Mean% ± SEM) | Day 7 Blastocysts | |
|---|---|---|---|---|
| No. of Blastocysts (Mean% ± SEM) | No. of Total Cells (Mean ± SEM) | |||
| 1 IVF | 440 | 267 (59.2 ± 10.5) a | 66 (14.2 ± 2.3) a | 61.5 ± 5.9 |
| 2 NT-BMSC | 347 | 249 (71.3 ± 4.6) b | 48 (13.7 ± 1.9) a | 61.6 ± 4.7 |
| 3 T-NT-BMSC | 325 | 227 (69.0 ± 2.4) b | 105 (32.5 ± 1.1) b | 66.4 ± 6.8 |
| Gene | Primer Sequence (5′-3′) | GenBank Accession Number |
|---|---|---|
| OCT4 | F: AGT CCC AGG ACA TCA AAG CG R: CCT TCC CAA AGA GAA CCC CC | NM_001113060.1 |
| SOX2 | F: CGC AGA CCT ACA TGA ACG R: TCG GAC TTG ACC ACT GAG | NM_001123197.1 |
| NANOG | F: CGA AGC ATC CAT TTC CAG CG R: TCG AGG GTC TCA GCA GAT GA | NM_001129971.1 |
| CDX2 | F: TCT TCC CTG CAA GGC TCG GT R: GAC GGT GGG GTT TAG CAC GC | NM_001278769.1 |
| IGF2R | F: CGC TCT CTG CCT CTA GCA GT R: CCT ACA CCC CAA GTC TGG AA | XM_013992438.1 |
| HDAC1 | F: CCT GGA CAC GGA GAT CCC TA R: GGG CAG CAT TCT CAG GTT CT | XM_013999116.1 |
| HDAC2 | F: TGC AGA GAT TTA ATG TTG GA R: GTT GTC GGT TTA ACT TCA CA | XM_001925318.5 |
| DNMT1 | F: AGA ATT ATC AGA GGA GGG CTA CC R: CAT TCA CTT CCC GAC TGA AAG C | NM_001032355 |
| DNMT3A | F: CTG AGA AGC CCA AG TCA AG R: CAG CAG ATG GTG CAG TAG GA | NM_001123197.1 |
| CBP | F: GTC TGG CTC ATG TTC AAC AAC G R: GTA CTT TCG TCC ACA GCA ATA CC | XM_003354647 |
| P300 | F: TTT CTT CCT CAG GCT CAG TTC C R: AGG CAT TAT AGG AGA GTT CAC AGG | XM_001929213 |
| SDHA | F: CAC ACG CTT TCC TAT GTC GAT G R: TGG CAC AGT CAG CTT CAT TC | XM_003362140.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-C.; Lee, W.-J.; Son, Y.-B.; Jin, Y.B.; Lee, H.-J.; Bok, E.; Lee, S.; Lee, S.-Y.; Jo, C.-H.; Kim, T.-S.; et al. Trichostatin A-Induced Epigenetic Modifications and Their Influence on the Development of Porcine Cloned Embryos Derived from Bone Marrow–Mesenchymal Stem Cells. Int. J. Mol. Sci. 2025, 26, 2359. https://doi.org/10.3390/ijms26052359
Lee S-C, Lee W-J, Son Y-B, Jin YB, Lee H-J, Bok E, Lee S, Lee S-Y, Jo C-H, Kim T-S, et al. Trichostatin A-Induced Epigenetic Modifications and Their Influence on the Development of Porcine Cloned Embryos Derived from Bone Marrow–Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2025; 26(5):2359. https://doi.org/10.3390/ijms26052359
Chicago/Turabian StyleLee, Seung-Chan, Won-Jae Lee, Young-Bum Son, Yeung Bae Jin, Hyeon-Jeong Lee, Eunyeong Bok, Sangyeob Lee, Sang-Yun Lee, Chan-Hee Jo, Tae-Seok Kim, and et al. 2025. "Trichostatin A-Induced Epigenetic Modifications and Their Influence on the Development of Porcine Cloned Embryos Derived from Bone Marrow–Mesenchymal Stem Cells" International Journal of Molecular Sciences 26, no. 5: 2359. https://doi.org/10.3390/ijms26052359
APA StyleLee, S.-C., Lee, W.-J., Son, Y.-B., Jin, Y. B., Lee, H.-J., Bok, E., Lee, S., Lee, S.-Y., Jo, C.-H., Kim, T.-S., Hong, C.-Y., Kang, S.-Y., Rho, G.-J., Choe, Y.-H., & Lee, S.-L. (2025). Trichostatin A-Induced Epigenetic Modifications and Their Influence on the Development of Porcine Cloned Embryos Derived from Bone Marrow–Mesenchymal Stem Cells. International Journal of Molecular Sciences, 26(5), 2359. https://doi.org/10.3390/ijms26052359





