Cocaine Differentially Affects Mitochondrial Function Depending on Exposure Time
Abstract
1. Introduction
2. Results
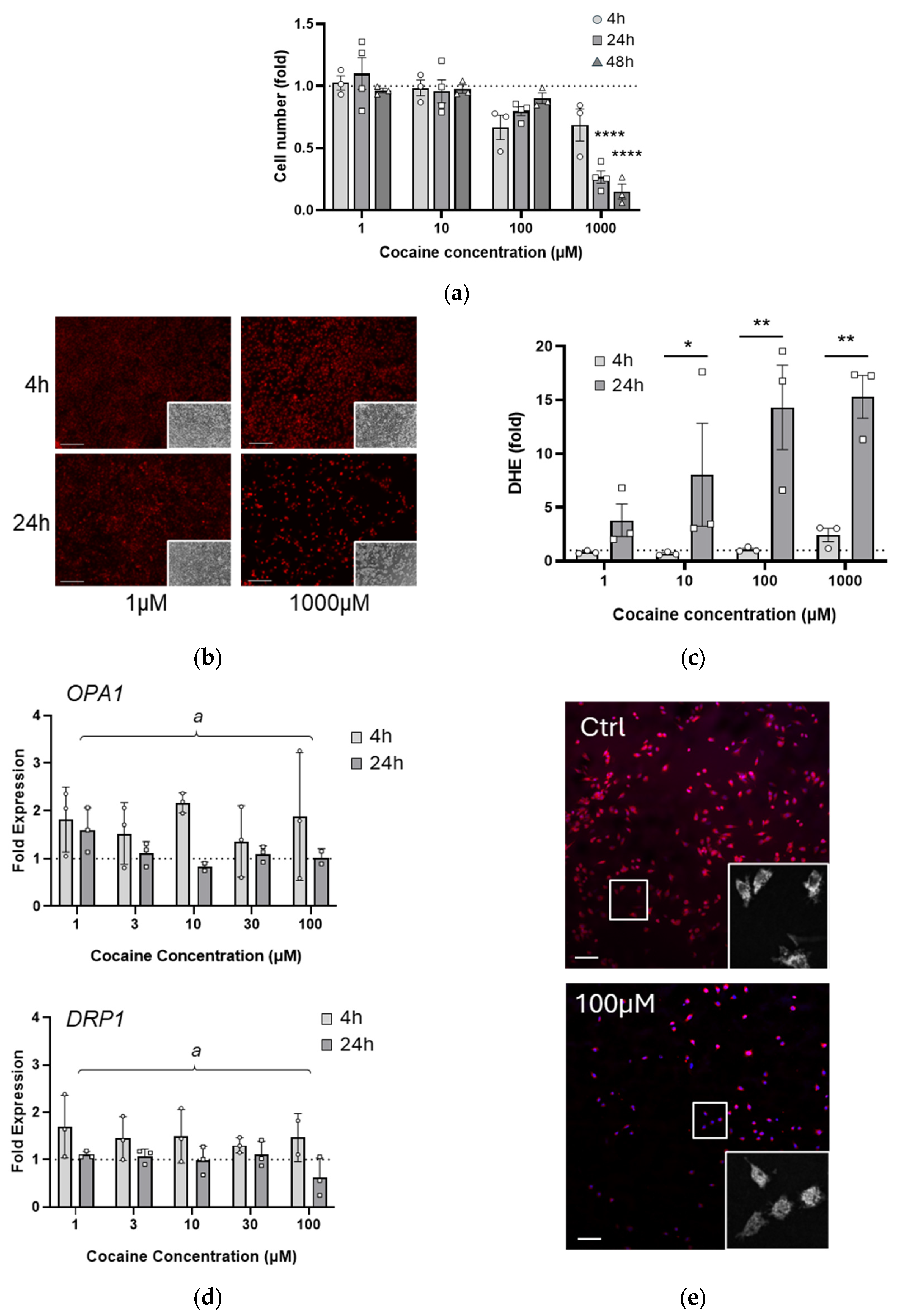
2.1. A Single Exposure to Cocaine Rapidly Alters Cell Health and Mitochondrial Morphology
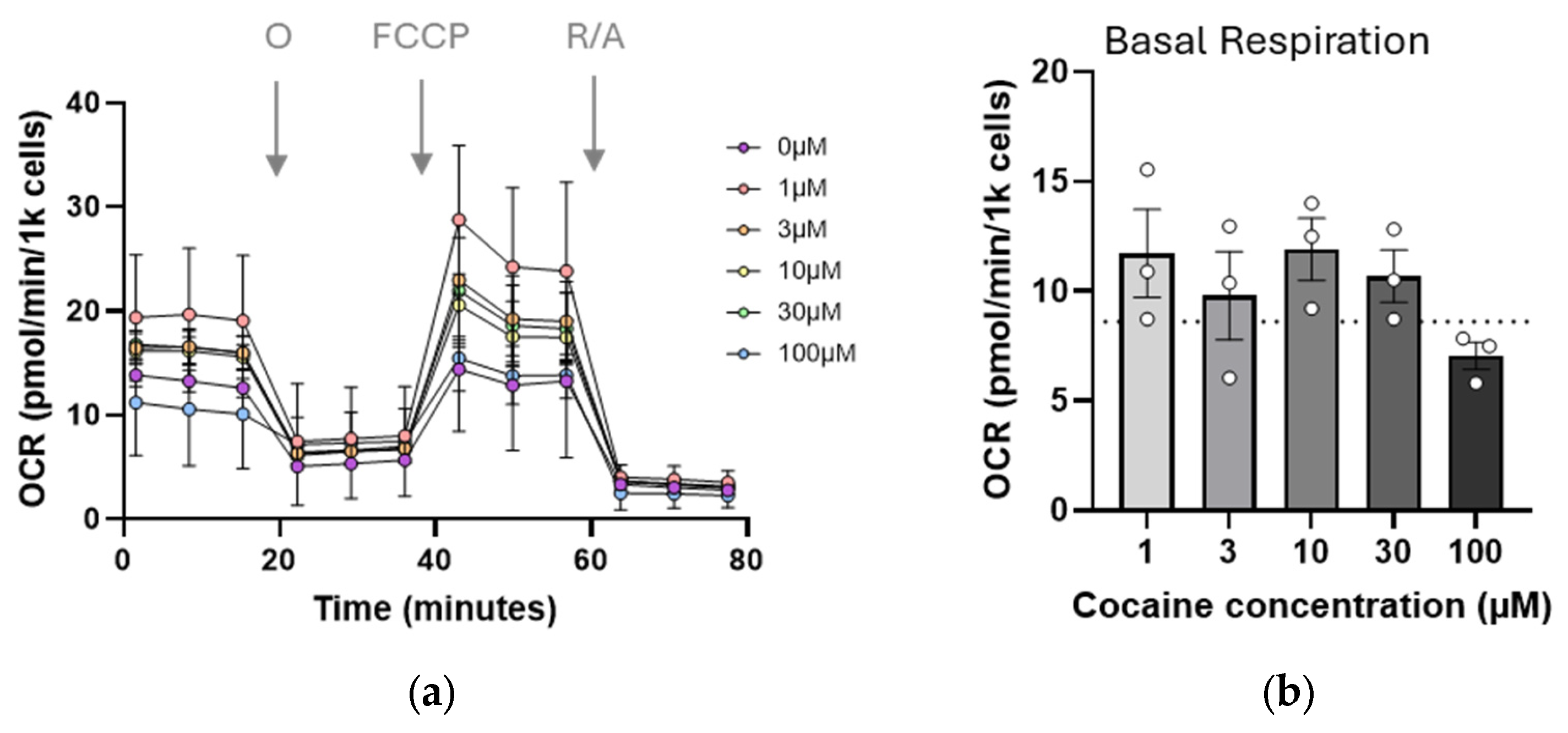
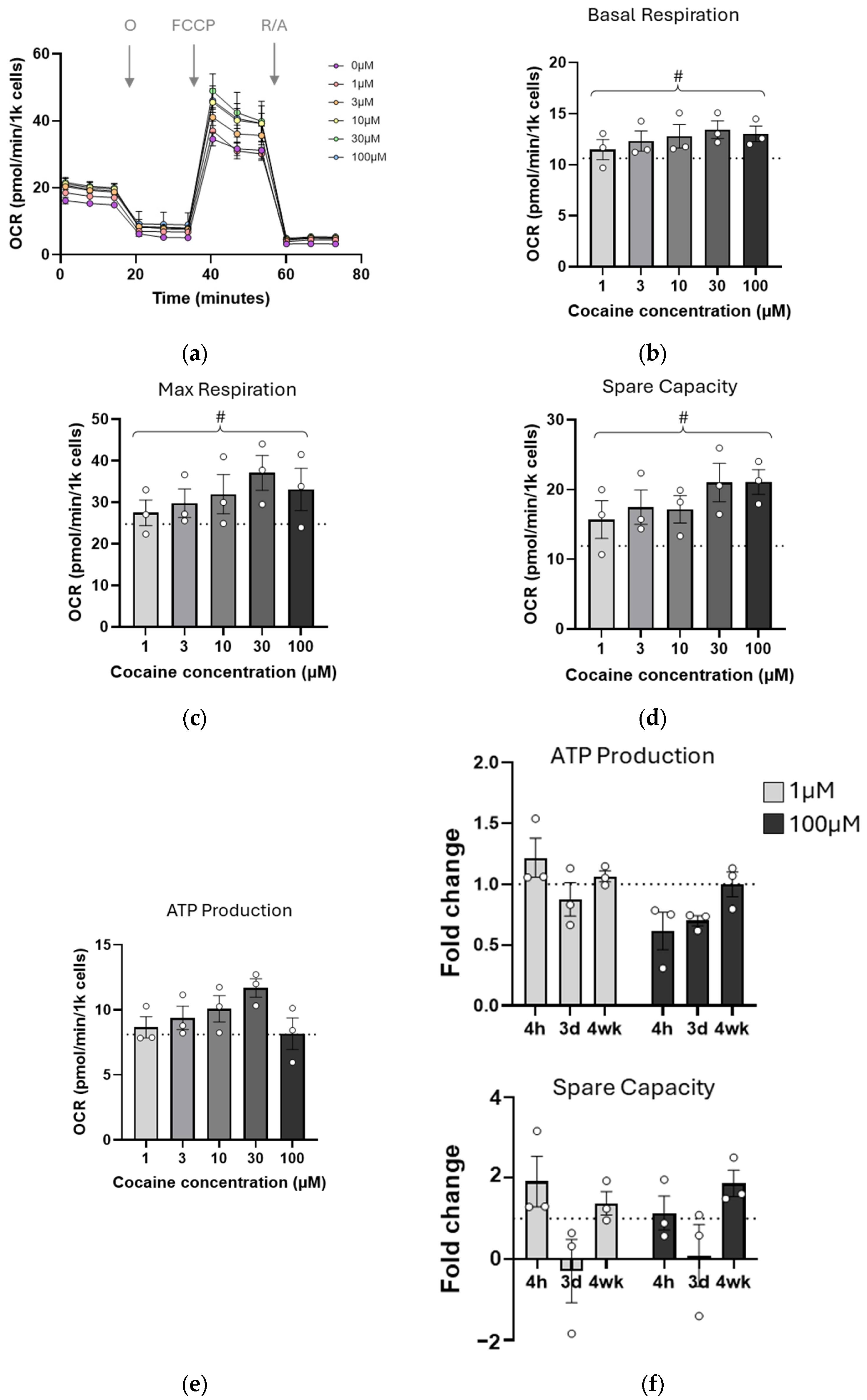
2.2. Mitochondrial Function Is Transiently Impaired by Single Cocaine Exposure
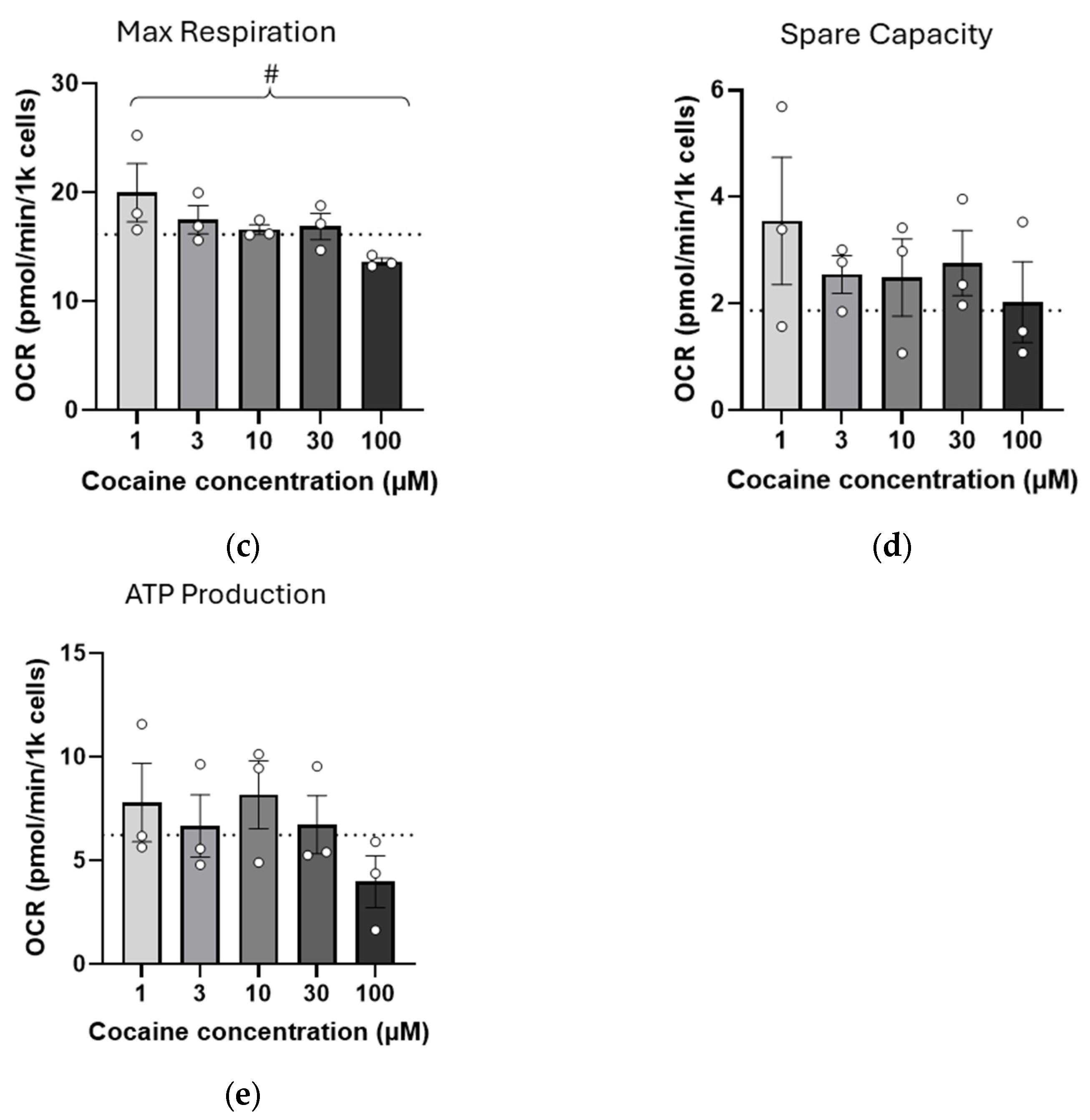
2.3. Repeated (Three-Day) Exposure to Cocaine Reduces Mitochondrial Reserve
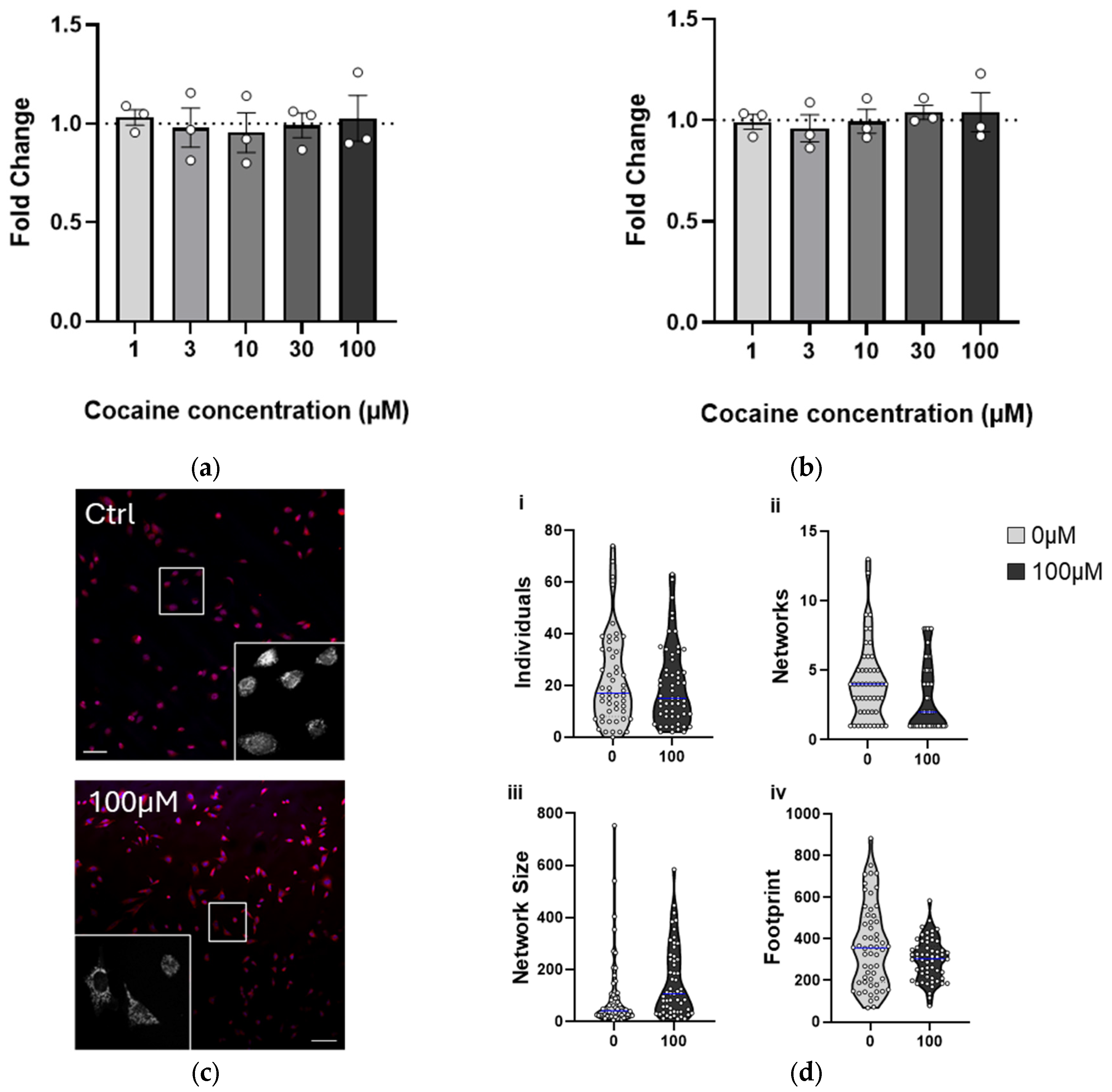
2.4. Chronic (4-Week) Exposure to Cocaine Results in Adaptation, Recovering Cell Health
3. Discussion
4. Limitations of the Study
5. Materials and Methods
5.1. Cell Culture and Cocaine Treatment
5.2. ROS Analysis
5.3. Nuclear and Mitochondrial Imaging
5.4. Quantitative Real-Time PCR
5.5. Oxygen Consumption Rate (OCR)
5.6. Statistics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- UKGov. United Kingdom Drug Situation 2019: Focal Point Annual Report. Available online: https://www.gov.uk/government/publications/united-kingdom-drug-situation-focal-point-annual-report (accessed on 7 August 2024).
- UNODC. World Drug Report 2022; United Nations Office on Drugs and Crime: Vienna, Austria, 2022. [Google Scholar]
- Morton, W.A. Cocaine and Psychiatric Symptoms. Prim. Care Companion J. Clin. Psychiatry 1999, 1, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Juarez, B.; Morel, C.; Walker, D.M.; Cahill, M.E.; Ribeiro, E.; Roman-Ortiz, C.; Ramakrishnan, C.; Deisseroth, K.; Han, M.-H.; et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017, 8, 13877. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, N.; Marchetti, D. Cocaine and its metabolites in the placenta: A systematic review of the literature. Reprod. Toxicol. 2012, 33, 1–14. [Google Scholar] [CrossRef]
- Richardson, G.A.; De Genna, N.M.; Willford, J.A.; Goldschmidt, L. Pathways from prenatal cocaine exposure to adult substance use and behavior. Neurotoxicol. Teratol. 2024, 102, 107335. [Google Scholar] [CrossRef]
- Grewen, K.; Burchinal, M.; Vachet, C.; Gouttard, S.; Gilmore, J.H.; Lin, W.; Johns, J.; Elam, M.; Gerig, G. Prenatal cocaine effects on brain structure in early infancy. NeuroImage 2014, 101, 114–123. [Google Scholar] [CrossRef]
- Kampman, K.M. The treatment of cocaine use disorder. Sci. Adv. 2019, 5, eaax1532. [Google Scholar] [CrossRef]
- Solimini, R.; Rotolo, M.C.; Pellegrini, M.; Minutillo, A.; Pacifici, R.; Busardo, F.P.; Zaami, S. Adulteration Practices of Psychoactive Illicit Drugs: An Updated Review. Curr. Pharm. Biotechnol. 2017, 18, 524–530. [Google Scholar] [CrossRef]
- Zimmer, B.A.; Dobrin, C.V.; Roberts, D.C. Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 2741–2749. [Google Scholar] [CrossRef]
- Heard, K.; Palmer, R.; Zahniser, N.R. Mechanisms of acute cocaine toxicity. Open Pharmacol. J. 2008, 2, 70–78. [Google Scholar] [CrossRef]
- Barnett, G.; Hawks, R.; Resnick, R. Cocaine pharmacokinetics in humans. J. Ethnopharmacol. 1981, 3, 353–366. [Google Scholar] [CrossRef]
- Thornton, C.; Grad, E.; Yaka, R. The role of mitochondria in cocaine addiction. Biochem. J. 2021, 478, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Verma, V. Classic Studies on the Interaction of Cocaine and the Dopamine Transporter. Clin. Psychopharmacol. Neurosci. 2015, 13, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Volkow, N.D.; Pan, Y.; Du, C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J. Neurosci. 2013, 33, 15827–15836. [Google Scholar] [CrossRef] [PubMed]
- Beuming, T.; Kniazeff, J.; Bergmann, M.L.; Shi, L.; Gracia, L.; Raniszewska, K.; Newman, A.H.; Javitch, J.A.; Weinstein, H.; Gether, U.; et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat. Neurosci. 2008, 11, 780–789. [Google Scholar] [CrossRef]
- Zheng, F.; Zhan, C.G. Modeling of pharmacokinetics of cocaine in human reveals the feasibility for development of enzyme therapies for drugs of abuse. PLoS Comput. Biol. 2012, 8, e1002610. [Google Scholar] [CrossRef]
- Zheng, F.; Jin, Z.; Deng, J.; Chen, X.; Zheng, X.; Wang, G.; Kim, K.; Shang, L.; Zhou, Z.; Zhan, C.-G. Development of a Highly Efficient Long-Acting Cocaine Hydrolase Entity to Accelerate Cocaine Metabolism. Bioconjugate Chem. 2022, 33, 1340–1349. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Eliaz, R.E.; McCann, D.J.; Loupe, P.S.; Eyal, E.; Blatt, K.; Cohen-Barak, O.; Hallak, H.; Chiang, N.; Gyaw, S. Placebo-controlled evaluation of a bioengineered, cocaine-metabolizing fusion protein, TV-1380 (AlbuBChE), in the treatment of cocaine dependence. Drug Alcohol. Depend. 2016, 166, 13–20. [Google Scholar] [CrossRef]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef]
- Kuzawa, C.W.; Chugani, H.T.; Grossman, L.I.; Lipovich, L.; Muzik, O.; Hof, P.R.; Wildman, D.E.; Sherwood, C.C.; Leonard, W.R.; Lange, N. Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. USA 2014, 111, 13010–13015. [Google Scholar] [CrossRef]
- Misgeld, T.; Schwarz, T.L. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017, 96, 651–666. [Google Scholar] [CrossRef]
- Menzies, R.A.; Gold, P.H. The Turnover of Mitochondria in a Variety of Tissues of Young Adult and Aged Rats. J. Biol. Chem. 1971, 246, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, H.G.; Langer, T. The Good and the Bad of Mitochondrial Breakups. Trends Cell Biol. 2019, 29, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Engeln, M.; Schiefer, C.; Patton, M.H.; Martin, J.A.; Werner, C.T.; Riggs, L.M.; Francis, T.C.; McGlincy, M.; Evans, B.; et al. Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron 2017, 96, 1327–1341.e6. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A.; Kotarska, A.; Frankowska, M.; Jastrzebska, J.; Wydra, K.; Miszkiel, J.; Przegalinski, E.; Filip, M. The Alterations in Mitochondrial DNA Copy Number and Nuclear-Encoded Mitochondrial Genes in Rat Brain Structures after Cocaine Self-Administration. Mol. Neurobiol. 2017, 54, 7460–7470. [Google Scholar] [CrossRef][Green Version]
- Pati, S.; Angel, P.; Drake, R.R.; Wagner, J.J.; Cummings, B.S. Lipidomic changes in the rat hippocampus following cocaine conditioning, extinction, and reinstatement of drug-seeking. Brain Behav. 2019, 9, e01451. [Google Scholar] [CrossRef]
- Campbell, R.R.; Chen, S.; Beardwood, J.H.; López, A.J.; Pham, L.V.; Keiser, A.M.; Childs, J.E.; Matheos, D.P.; Swarup, V.; Baldi, P.; et al. Cocaine induces paradigm-specific changes to the transcriptome within the ventral tegmental area. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2021, 46, 1768–1779. [Google Scholar] [CrossRef]
- Cole, S.L.; Chandra, R.; Harris, M.; Patel, I.; Wang, T.; Kim, H.; Jensen, L.; Russo, S.J.; Turecki, G.; Gancarz-Kausch, A.M.; et al. Cocaine-induced neuron subtype mitochondrial dynamics through Egr3 transcriptional regulation. Mol. Brain 2021, 14, 101. [Google Scholar] [CrossRef]
- Funakoshi, T.; Furukawa, M.; Aki, T.; Uemura, K. Repeated exposure of cocaine alters mitochondrial dynamics in mouse neuroblastoma Neuro2a. Neurotoxicology 2019, 75, 70–77. [Google Scholar] [CrossRef]
- Thangaraj, A.; Periyasamy, P.; Guo, M.L.; Chivero, E.T.; Callen, S.; Buch, S. Mitigation of cocaine-mediated mitochondrial damage, defective mitophagy and microglial activation by superoxide dismutase mimetics. Autophagy 2020, 16, 289–312. [Google Scholar] [CrossRef]
- Sivalingam, K.; Cirino, T.J.; McLaughlin, J.P.; Samikkannu, T. HIV-Tat and Cocaine Impact Brain Energy Metabolism: Redox Modification and Mitochondrial Biogenesis Influence NRF Transcription-Mediated Neurodegeneration. Mol. Neurobiol. 2021, 58, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Cunha-Oliveira, T. Role of Mitochondria on the Neurological Effects of Cocaine. In The Neuroscience of Cocaine; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 1, pp. 205–218. [Google Scholar]
- Cunha-Oliveira, T.; Rego, A.C.; Morgadinho, M.T.; Macedo, T.; Oliveira, C.R. Differential cytotoxic responses of PC12 cells chronically exposed to psychostimulants or to hydrogen peroxide. Toxicology 2006, 217, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Goodman, C.B. Effects of chronic cocaine in rat C6 astroglial cells. Int. J. Mol. Med. 2012, 30, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Beiser, T.; Yaka, R. The Role of Oxidative Stress in Cocaine Addiction. J. Neurol. Neuromedicine 2019, 4, 17–21. [Google Scholar] [CrossRef]
- Karch, S.B.; Stephens, B.; Ho, C.H. Relating cocaine blood concentrations to toxicity--an autopsy study of 99 cases. J. Forensic Sci. 1998, 43, 41–45. [Google Scholar] [CrossRef]
- Mittleman, R.E.; Wetli, C.V. Death caused by recreational cocaine use. An update. JAMA 1984, 252, 1889–1893. [Google Scholar] [CrossRef]
- Peretti, F.J.; Isenschmid, D.S.; Levine, B.; Caplan, Y.H.; Smialek, J.E. Cocaine fatality: An unexplained blood concentration in a fatal overdose. Forensic Sci. Int. 1990, 48, 135–138. [Google Scholar] [CrossRef]
- Shang, E.H.; Zhdanova, I.V. The circadian system is a target and modulator of prenatal cocaine effects. PLoS ONE 2007, 2, e587. [Google Scholar] [CrossRef]
- López Patiño, M.A.; Yu, L.; Yamamoto, B.K.; Zhdanova, I.V. Gender differences in zebrafish responses to cocaine withdrawal. Physiol. Behav. 2008, 95, 36–47. [Google Scholar] [CrossRef]
- Riley, E.; Maymi, V.; Pawlyszyn, S.; Yu, L.; Zhdanova, I.V. Prenatal cocaine exposure disrupts the dopaminergic system and its postnatal responses to cocaine. Genes. Brain Behav. 2018, 17, e12436. [Google Scholar] [CrossRef]
- Badisa, R.B.; Wi, S.; Jones, Z.; Mazzio, E.; Zhou, Y.; Rosenberg, J.T.; Latinwo, L.M.; Grant, S.C.; Goodman, C.B. Cellular and molecular responses to acute cocaine treatment in neuronal-like N2a cells: Potential mechanism for its resistance in cell death. Cell Death Discov. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Curel, C.J.M.; Nobeli, I.; Thornton, C. Leflunomide Treatment Does Not Protect Neural Cells following Oxygen-Glucose Deprivation (OGD) In Vitro. Cells 2024, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Winhusen, T.; Storkson, J.M.; Lewis, D.; Pariza, M.W.; Somoza, E.; Somoza, V. Total antioxidant capacity is significantly lower in cocaine-dependent and methamphetamine-dependent patients relative to normal controls: Results from a preliminary study. Hum. Psychopharmacol. 2014, 29, 537–543. [Google Scholar] [CrossRef]
- Numa, R.; Kohen, R.; Poltyrev, T.; Yaka, R. Tempol diminishes cocaine-induced oxidative damage and attenuates the development and expression of behavioral sensitization. Neuroscience 2008, 155, 649–658. [Google Scholar] [CrossRef]
- Numa, R.; Baron, M.; Kohen, R.; Yaka, R. Tempol attenuates cocaine-induced death of PC12 cells through decreased oxidative damage. Eur. J. Pharmacol. 2011, 650, 157–162. [Google Scholar] [CrossRef]
- Beiser, T.; Numa, R.; Kohen, R.; Yaka, R. Chronic treatment with Tempol during acquisition or withdrawal from CPP abolishes the expression of cocaine reward and diminishes oxidative damage. Sci. Rep. 2017, 7, 11162. [Google Scholar] [CrossRef]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Q.; Mash, D.C.; Goldman, D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc. Natl. Acad. Sci. USA 2011, 108, 6626–6631. [Google Scholar] [CrossRef]
- Mews, P.; Cunningham, A.M.; Scarpa, J.; Ramakrishnan, A.; Hicks, E.M.; Bolnick, S.; Garamszegi, S.; Shen, L.; Mash, D.C.; Nestler, E.J. Convergent abnormalities in striatal gene networks in human cocaine use disorder and mouse cocaine administration models. Sci. Adv. 2023, 9, eadd8946. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Ernst, C.; Mash, D.; Turecki, G. DNA Methylation Dynamics and Cocaine in the Brain: Progress and Prospects. Genes 2017, 8, 138. [Google Scholar] [CrossRef]
- Doke, M.; Jeganathan, V.; McLaughlin, J.P.; Samikkannu, T. HIV-1 Tat and cocaine impact mitochondrial epigenetics: Effects on DNA methylation. Epigenetics 2020, 16, 980–999. [Google Scholar] [CrossRef] [PubMed]
- Samikkannu, T.; Atluri, V.S.; Nair, M.P. HIV and Cocaine Impact Glial Metabolism: Energy Sensor AMP-activated protein kinase Role in Mitochondrial Biogenesis and Epigenetic Remodeling. Sci. Rep. 2016, 6, 31784. [Google Scholar] [CrossRef] [PubMed]
- Anier, K.; Malinovskaja, K.; Aonurm-Helm, A.; Zharkovsky, A.; Kalda, A. DNA Methylation Regulates Cocaine-Induced Behavioral Sensitization in Mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 2450–2461. [Google Scholar] [CrossRef]
- Engmann, O.; Labonté, B.; Mitchell, A.; Bashtrykov, P.; Calipari, E.S.; Rosenbluh, C.; Loh, Y.-H.E.; Walker, D.M.; Burek, D.; Hamilton, P.J.; et al. Cocaine-Induced Chromatin Modifications Associate with Increased Expression and Three-Dimensional Looping of Auts2. Biol. Psychiatry 2017, 82, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Chalecka, M.; Kazberuk, A.; Palka, J.; Surazynski, A. P5C as an Interface of Proline Interconvertible Amino Acids and Its Role in Regulation of Cell Survival and Apoptosis. Int. J. Mol. Sci. 2021, 22, 11763. [Google Scholar] [CrossRef]
- Dash, S.; Dash, C.; Pandhare, J. Activation of proline metabolism maintains ATP levels during cocaine-induced polyADP-ribosylation. Amino Acids 2021, 53, 1903–1915. [Google Scholar] [CrossRef]
- Calipari, E.S.; Beveridge, T.J.; Jones, S.R.; Porrino, L.J. Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur. J. Neurosci. 2013, 38, 3749–3757. [Google Scholar] [CrossRef]
- Gozzi, A.; Tessari, M.; Dacome, L.; Agosta, F.; Lepore, S.; Lanzoni, A.; Cristofori, P.; Pich, E.M.; Corsi, M.; Bifone, A. Neuroimaging Evidence of Altered Fronto-Cortical and Striatal Function after Prolonged Cocaine Self-Administration in the Rat. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 2431–2440. [Google Scholar] [CrossRef]
- Nicolas, C.; Tauber, C.; Lepelletier, F.-X.; Chalon, S.; Belujon, P.; Galineau, L.; Solinas, M. Longitudinal Changes in Brain Metabolic Activity after Withdrawal from Escalation of Cocaine Self-Administration. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 1981–1990. [Google Scholar] [CrossRef]
- Nezhyva, M.; Shahen-Zoabi, S.; Kabirova, M.; Bentov-Arava, E.; Shalev, O.; Andrén, P.E.; Thornton, C.; Yaka, R.; Margulis, K.; Jansson, E.T. Spatial multiomic insights into acute cocaine exposure. PNAS Nexus 2024, 3, pgae458. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattad, S.; Bryant, G.; Shmuel, M.; Smith, H.L.; Yaka, R.; Thornton, C. Cocaine Differentially Affects Mitochondrial Function Depending on Exposure Time. Int. J. Mol. Sci. 2025, 26, 2131. https://doi.org/10.3390/ijms26052131
Wattad S, Bryant G, Shmuel M, Smith HL, Yaka R, Thornton C. Cocaine Differentially Affects Mitochondrial Function Depending on Exposure Time. International Journal of Molecular Sciences. 2025; 26(5):2131. https://doi.org/10.3390/ijms26052131
Chicago/Turabian StyleWattad, Sahar, Gabriella Bryant, Miriam Shmuel, Hannah L. Smith, Rami Yaka, and Claire Thornton. 2025. "Cocaine Differentially Affects Mitochondrial Function Depending on Exposure Time" International Journal of Molecular Sciences 26, no. 5: 2131. https://doi.org/10.3390/ijms26052131
APA StyleWattad, S., Bryant, G., Shmuel, M., Smith, H. L., Yaka, R., & Thornton, C. (2025). Cocaine Differentially Affects Mitochondrial Function Depending on Exposure Time. International Journal of Molecular Sciences, 26(5), 2131. https://doi.org/10.3390/ijms26052131






