Abstract
Natural products are a valuable source of bioactive compounds with established roles in oncology. Their structural diversity and ability to target multiple cancer-related pathways make them promising candidates for anticancer drug development. Increasing preclinical and clinical data highlight their potential not only to exert direct antitumor effects but also to enhance patient tolerance to conventional therapies by reducing side effects and improving treatment adherence. The Mediterranean region, known for its biodiversity and traditional dietary habits, provides a rich array of natural compounds with documented health benefits. Key Mediterranean natural plant products (MNPPs), including bioactives from olive oil, onion, citrus fruits, chili pepper and grapes, exhibit antioxidant, anti-inflammatory, and anti-proliferative properties. This review focuses on the molecular mechanisms of selected MNPPs, such as polyphenols, flavonoids, alkaloids, terpenes, organosulfur and furanocoumarin compounds, which modulate oxidative stress, inflammation, apoptosis, and tumor progression. Evidence from in vitro and in vivo studies supports their role in cancer prevention and as adjuvants in therapy. While further clinical research is needed, these findings suggest that incorporating MNPPs into therapeutic regimens could offer low-toxicity, multi-targeted support in oncology, improving both outcomes and quality of life in cancer patients.
1. Introduction
Cancer remains a leading cause of death and disability worldwide, despite major advancements in early detection, treatment, and prevention. Globally, cancer is characterized by uncontrolled cell proliferation, tumor heterogeneity, and invasive malignancies that result in high mortality and social burden. According to the GLOBOCAN 2024 database, an estimated 19.96 million new cancer cases and nearly 9.7 million cancer-related deaths occurred in 2022 [1]. The rise in cancer is associated with population and socioeconomic growth, pollution, aging, unhealthy diet, and lifestyle [2,3]. These underscore the global urgency for more effective and less toxic treatment strategies.
Current standard treatments—such as surgery, chemotherapy, and radiotherapy—have improved outcomes, particularly for early-stage malignancies. However, they remain limited by severe side effects, variable efficacy in advanced disease, and the eventual development of resistance [4]. Adverse effects such as fatigue, myelosuppression, nausea, vomiting, neuropathy, cardiotoxicity, and alopecia often reduce patients’ quality of life and lead to treatment discontinuation [5,6]. Furthermore, the high development costs and low success rates of synthetic chemotherapeutics exacerbate the need for alternative and more sustainable approaches [7].
Recent decades have witnessed the evolution of cancer therapy through combination regimens, targeted therapies, immunotherapy, and personalized medicine based on molecular profiling. These strategies have significantly prolonged survival in certain cancer types. For instance, identifying tumor-specific biomarkers has enabled more tailored treatment decisions and enhanced patient outcomes [8,9]. Nevertheless, chemotherapy remains the cornerstone for many advanced-stage malignancies, despite its systemic toxicity and limitations [10]. Moreover, drug resistance—such as resistance to paclitaxel and cisplatin—is a major clinical challenge that contributes to relapse and poor prognosis [11]. Mechanisms of resistance include genetic mutations, activation of alternative signaling pathways, tumor heterogeneity, and immune evasion [12].
In this context, plant-derived natural products have gained growing attention for their potential to provide effective anticancer agents with lower toxicity and the ability to target multiple molecular pathways [13,14], as well as being considered safer and with low or no toxicity compared to synthetic drugs [15]. Phytochemicals have long served as a foundation in oncology drug development, exemplified by compounds like vinblastine, vincristine (from Camptotheca acuminata), paclitaxel (Taxus brevifolia), camptothecin (Camptotheca acuminata), and etoposide (Podophyllum peltatum), all of which have been successfully integrated into clinical oncology [16,17,18,19].
Beyond the well-established therapeutic agents, recent investigations have increasingly examined the anticancer properties of dietary phytochemicals, many of which come from plants traditionally included in the Mediterranean Diet (MD). This eating pattern has drawn considerable interest from the scientific and medical communities in recent decades. The MD is a nutrient-rich, predominantly plant-based regimen that prioritizes seasonal fruits and vegetables, as well as other plant-derived staples. It also incorporates moderate consumption of fish, poultry, and fermented dairy products, with extra virgin olive oil serving as the main source of beneficial fats. Conversely, the intake of processed red meat and refined sugars is markedly restricted [20].
Extensive research has characterized a wide array of bioactive phytochemicals present in plants typical of the Mediterranean region, many of which exhibit significant anticancer activity. Among the most extensively investigated compounds are curcumin (from Curcuma longa), resveratrol (from Vitis vinifera), epigallocatechin gallate (EGCG) (from green tea), capsaicin (from chili pepper), oleocanthal, hydroxytyrosol, and oleuropein (from olive oil), bergapten (from Citrus bergamia) and onionin A (from onion), all of which modulate key hallmarks of cancer such as oxidative stress, angiogenesis, apoptosis, and metastasis [21,22,23]. Several of these compounds also exhibit chemopreventive properties, modulating DNA repair mechanisms, cell cycle regulation, and inflammatory signaling [24]. Moreover, a number of plant-derived compounds have demonstrated the capacity to reverse or prevent multidrug resistance (MDR), further enhancing their therapeutic value [25,26]. This mechanistic evidence is consistent with the robust epidemiological association between adherence to the MD and reduced cancer incidence.
Interestingly, core plant-based foods integral to the MD, including Olea europaea (olive), Allium sativum (garlic), Allium cepa (onion), Vitis vinifera (grapes), Citrus (oranges, bergamot, lemon), Solanum lycopersicum (tomato), Capsicum annuum (pepper), and herbs such as Origanum vulgare (oregano) and Salvia rosmarinus (rosemary), contain a wealth of polyphenols, flavonoids, terpenoids, carotenoids, and sulfur compounds with demonstrated antioxidant and anticancer properties [27,28,29,30]. While herbal compounds present exciting opportunities for cancer therapy and prevention, challenges remain. These include variability in phytochemical composition, the need for rigorous standardization and clinical validation, and potential herb–drug interactions [31,32]. Moreover, it is important to note that the role of antioxidants in cancer is complex and highly context-dependent. In normal tissues, antioxidants reduce inflammation and DNA damage, thereby exerting chemopreventive effects. However, in established tumors, antioxidants can have dual effects. At low concentrations, they may promote cancer cell survival by maintaining redox homeostasis. At higher concentrations or under specific microenvironmental conditions, some antioxidants paradoxically act as pro-oxidants, increasing ROS to cytotoxic levels and inducing apoptosis [33]. These contrasting effects depend on dose, cell type, and tumor microenvironment, making the therapeutic use of antioxidants in oncology a double-edged sword [34,35]. Further preclinical and translational studies are essential to clarify mechanisms of action, optimize bioavailability, and evaluate safety profiles in well-designed clinical trials [36]. While previous literature has explored the anticancer properties of various Mediterranean natural compounds, including phytochemicals and agri-food by-products, these works often focus on individual diseases, isolated compounds, or specific molecular pathways. In contrast, the present review analyzes the antioxidant and antitumoral potential of Mediterranean food products across multiple tumor contexts, thus providing a broader, more translationally oriented perspective.
2. Literature Search Strategy
2.1. Study Design
This narrative review is based on a structured but nonsystematic literature search performed in PubMed and Google Scholar. The search focused on studies investigating the anticancer potential of five MNPPs and their major bioactive compounds: oleocanthal, hydroxytyrosol, oleuropein (olive), S-allyl cysteine, onionin A, quercetin (onion), bergapten (bergamot), capsaicin (chili pepper), and resveratrol (grape).
Although this work does not constitute a systematic review and is therefore not intended to follow PRISMA guidelines, elements inspired by the PRISMA, such as explicit reporting of search strategies, eligibility criteria, and study selection, were incorporated to improve transparency, clarity, and reproducibility.
2.2. Study Selection
The literature search was conducted in iterative phases from May to September 2025. Keyword combinations were tailored to the known biological activities of each compound. Additional articles were identified through reference lists and citation tracking.
For each plant-based compound, the following generic search strings were used:
- “[compound name]” AND (“anticancer” OR “cancer” OR “tumor” OR “treatment”) to retrieve foundational data on each compound’s ability to affect cancer cell growth and viability.
- “[compound name]” AND (“antioxidant” OR “oxidative stress”) AND (“cancer”) to identify evidence on redox modulation and molecular pathways associated with tumor initiation/progression.
- “[compound name]” AND “in vivo” AND (“cancer” OR “tumor” OR “xenograft” OR “animal model”) to assess available animal models.
- “[compound name]” AND (“preclinical” OR “clinical trial”) AND (“cancer” OR “tumor”) to retrieve translational studies or early-phase human trials, if available.
- “[compound name]” AND (“chemotherapy” OR “chemosensitizer” OR “combination therapy”) AND (“cancer”) to explore interactions with standard anticancer treatments and potential chemosensitizing effects.
2.3. Eligibility Criteria
Inclusion Criteria:
- Original research articles
- In vitro, in vivo, or clinical studies
- Published in English
- Relevance to anticancer mechanisms or biological activities of MNPPs.
Exclusion criteria:
- Studies not related to cancer
- Non-original papers (e.g., editorials, commentaries)
- Non-English publications
- Studies lacking mechanistic or biological relevance.
2.4. Selection Process
The authors manually screened titles and abstracts. The full texts of potentially eligible papers were evaluated based on their relevance to molecular mechanisms, antioxidant responses, and antitumor activities. As this is a narrative review, no quantitative synthesis or formal PRISMA flow diagram was conducted. However, the selection process adhered to principles of transparency and methodological clarity.
3. Natural Plant Products from Mediterranean Diet
The Mediterranean Basin is one of the world’s major biodiversity hotspots. It is home to over 25,000 plant species, around half of which are endemic [37,38]. This exceptional richness of flora stems from a complex biogeographical history, diverse microclimates and long-standing interactions between plants and human activity [39]. Exposure to seasonal drought, intense solar radiation and nutrient-poor soils has favored the evolution of specialized secondary metabolic pathways [40]. Consequently, Mediterranean plants exhibit distinctive phytochemical profiles, rich in phenolic acids, flavonoids, terpenoids, alkaloids, carotenoids, and dietary fiber [41,42]. Many of these compounds exhibit antioxidant, anti-inflammatory, antimicrobial and anticancer properties [42,43].
These bioactive compounds play a key role in the protective effects of the MD, which has been linked to a lower incidence of various cancers, including those affecting the digestive tract, breast, and lungs [44].
Table 1 summarizes key foods from MD, their principal bioactive compounds, and the antitumoral mechanisms documented in major cancer models.

Table 1.
Anticancer Properties of Key Bioactive Compounds Found in Major Mediterranean Plants.
In this narrative review, we focused on five selected plant species (olive, onion, citrus fruits, chili pepper, and grapes) that are not only emblematic of the broader Mediterranean basin but also represent the specific agro-ecological and culinary tradition of Southern Italy.
Furthermore, when compared to the same species growing outside the Mediterranean basin, the Mediterranean populations of the five plant species discussed in this review show a marked increase in phytochemicals, reflecting their adaptations to enhanced photoprotection and oxidative stress management. Table 2 provides an overview of the phytochemical classes and key bioactive molecules that distinguish the selected Mediterranean plant species from non-Mediterranean ones.

Table 2.
Comparison of Five Selected Mediterranean Plant Species with Their Non-Mediterranean Counterparts.
3.1. Olive Tree Bioactives in Cancer Therapy
Scientific literature reports benefits from olive trees in ancient times, in particular in 1854, when Daniel Hanbury first described that a decoction of olive leaves efficiently reduced malaria-associated fever [70]. Since then, numerous pharmacological studies have highlighted the therapeutic potential of olive-derived extracts, including their ability to alleviate arrhythmias, enhance blood circulation, lower blood pressure, and reduce cancer [71,72].
The extra virgin olive oil (EVOO) from the olive tree is the main fat source in the MD and through mechanical pressing, it provides additional bioactive components, notably oleocanthal, hydroxytyrosol, oleuropein, tyrosol, and monounsaturated fatty acids like oleic acid [73]. These compounds exhibit potent antioxidant activity by scavenging reactive oxygen species (ROS), while also inducing apoptosis in cancer cells and inhibiting inflammation [46]. Large cohort studies such as the EPIC (European Prospective Investigation into Cancer and Nutrition) have shown inverse associations between olive oil intake and the incidence of breast and colorectal cancers [74]. Specifically, EVOO polyphenols can inhibit matrix metalloproteinases (MMPs) and cyclooxygenase-2 (COX-2), which are associated with cancer cell invasion and metastasis. The antitumoral effects of EVOO have been observed in breast, colon, and prostate cancer models [45].
The fruit pulp of the olive is a rich source of polyphenols such as oleuropein and hydroxytyrosol, both of which exhibit potent antioxidant, anti-inflammatory, and anti-proliferative activities (at concentrations ranging from 50 to 100 μmol/L, in HL60 cells, while no effect on apoptosis was observed in freshly isolated human lymphocytes and polymorphonuclear cells). The leaves of the olive tree also contain high levels of oleuropein, along with verbascoside and various flavonoids, contributing to the plant’s therapeutic potential. It has been reported that olive leaf extract reduced cell proliferation of human lymphoblastic leukemia cell line [75]. It was also effective in reducing cell viability of pancreatic and bladder cancer cell lines, blocking cell cycle progression [76,77].
Further in vitro studies have demonstrated that oleuropein inhibits cancer cell proliferation, induces apoptosis, and arrests the cell cycle in multiple cancer cell lines, including breast, colon, and prostate cancers [78,79]. Its antioxidant properties also help reduce oxidative DNA damage, a key factor in carcinogenesis [80]. In breast cancer, oleuropein inhibits estradiol-induced ERK1/2 activation in MCF-7 cells, reducing hormone-driven proliferation [81]. It also induces autophagy and suppresses migration and invasion through the modulation of p62, Beclin-1 and LC3 II/I expression [82]. In both MCF-7 and MDA-MB-231 cells, it increases ROS levels and promotes apoptosis by inhibiting Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling cascade [83]. In vivo, combined treatment with oleuropein (50 mg/kg), and doxorubicin (2.5 mg/kg) in breast cancer xenografts significantly reduced tumor volume and downregulated NF-κB, Bcl-2 and survivin, enhancing apoptosis more effectively than either compound alone [84]. It has shown efficacy in colorectal, thyroid and lung cancer models [78,85,86,87].
Despite oleuropein being determined in plasma, it undergoes extensive metabolism to hydroxytyrosol and other products [88].
Oleocanthal, a phenolic aldehyde unique to EVOO, has anticancer activity in various tumor cell lines, especially breast cancer, melanoma, and multiple myeloma cells. In breast cancer (MCF-7, MDA-MB-231 and BT-474) inhibits proliferation, migration and G1/S cell cycle progression through suppression of Hepatocyte Growth Factor (HGF)-induced c-Met activation, without affecting non-tumorous cells (MCF10). The IC50 values for (-)-oleocanthal treatment in HGF-supplemented media were 10.9, 20.1 and 25.4 µM in MDA-MB-231, MCF-7 and BT-474 breast cancer cells, respectively. However, the IC50 values for (-)-oleocanthal treatment in HGF-free media were 16.2, 40.8 and 58.4 µM in MDA-MB-231, MCF-7 and BT-474 breast cancer cells, respectively. These results indicate that (-)-oleocanthal treatment inhibits HGF-dependent MDA-MB-231, MCF-7 and BT-474 cell growth in a dose- and time-responsive manner compared to cells in the vehicle-treated control groups [89]. In melanoma cells (501Mel and A375), it reduces viability in a dose-dependent manner through COX inhibition and downregulation of Bcl-2, ERK1/2 and AKT signaling [90,91]. The downregulation of the latter pathway is implicated in BRAF inhibitor resistance in melanoma, suggesting a potential chemosensitizing role for oleocanthal in resistant tumors [92]. Oleocanthal (0.2, 2 and 20 μM) works as a COX-1 and COX-2 inhibitor, mirroring the anti-inflammatory effect of ibuprofen, and has been shown to selectively induce apoptosis in human cancer cells by disrupting lysosomal membranes [93]. Moreover, oleocanthal (60 µM) potentiates chemosensitivity in gastric cancer cells resistant to cisplatin, 5-fluorouracil, and paclitaxel in vitro [94]. Combined with lapatinib, a Human Epidermal Growth Factor Receptor 2 (HER2)/EFR inhibitor, it reduces proliferation of HER2-positive breast cancer cells (BT-474, SKBR-3), reducing lapatinib dose (using 12 µM oleocanthal with 30 nM Lapatinib in BT-474 cells and 15 µM with 60 nM in SKBR-3) [95]. In breast cancer cells with HER2 overexpression, it synergized with trastuzumab, enhancing cancer cell death and targeting HER2 and FAS signaling pathways. Its ability to potentiate chemotherapy is also reported in other cell lines, like gastric cancer cells, liver, and colon [94,96]. In vivo studies using xenograft mouse models have confirmed the tumor-suppressive effects of both oleuropein and oleocanthal, leading to reduced tumor volume and angiogenesis [21]. However, due to the limited pharmacokinetic data in humans, oleocanthal is difficult to detect in plasma following administration, suggesting that its biological effects may instead be mediated by its metabolites [97].
Hydroxytyrosol, a phenolic compound, is rapidly absorbed and over 90% is excreted with the urine; its absorption strongly depends on the food matrix, with oily matrices like EVOO enhancing bioavailability compared to aqueous vehicles [98,99]. It has chemotherapeutic effects through modulation of several oncogenic pathways, including growth factor receptors (Epidermal Growth Factor Receptor, EGFR; Insulin-like Growth Factor 1 Receptor, IGF-1R) [85,100,101], receptor-associated proteins [102,103], and interleukin signaling [104]. It inhibits cyclin D1, leading to G1/S cell cycle arrest, particularly in MCF-7, MDA-MB-21 breast cancer cells, CaCo2 colon cancer cells and TPC-1, FB-2, and WRO thyroid cancer cell lines at a concentration of 324 µM [103,105,106]. It can reduce ROS generation, leading to apoptotic cell death and mitochondrial dysfunction, as shown in colon (DLD1) and prostate (PC3) cancer models [107,108]. Hydroxytyrosol selectively inhibits prostate cancer cell proliferation (LNCaP and C4-2) through downregulation of Cyclins D1/E and CDKs 2/4 and upregulation of p21WAF1/p27KIP1, leading to G1 arrest. Apoptosis is promoted via caspase activation, Poly (ADP-ribose) Polymerase (PARP) cleavage and increased BAX/Bcl-2 ratio (at increasing concentrations of 100, 200 and 300 µM) [109]. It also inhibits AKT/Signal Transducer and Activator of Transcription 3 (STAT3)/NF-κB signaling in androgen-sensitive prostate cancers [110,111]. In colorectal adenocarcinoma cells (HT-29, WiDr, CaCo2), it significantly downregulated EGFR expression via ubiquitin-mediated proteasomal and lysosomal degradation [101]. Additionally, hydroxytyrosol has entered early-phase clinical trials for its antioxidant and cardioprotective effects, with emerging interest in its potential adjunct role in cancer prevention and therapy [112].
3.2. Antitumor Effects of Onion-Derived Bioactive Compounds
The Mediterranean diet is rich in vegetables, and among the most commonly used is the onion. In particular, the Tropea onion stands out as one of the most renowned varieties, both for its flavor and its health properties. The outer layers and red onion varieties are rich in flavonoids, in particular quercetin, organosulfur compounds, such as S-allyl cysteine and onionin A, and other phenolic constituents. These bioactive molecules exert anti-proliferative, pro-apoptotic, antioxidant, and anti-inflammatory effects, making them promising adjuvants in cancer therapy.
In vitro, quercetin-rich extracts and quercetin glucosides inhibit proliferation in diverse cancer cell lines, including breast (MCF-7), colon (HT-29), prostate (PC-3), liver (HepG2), and adrenocortical carcinoma (H295R, SW-13). After ingestion, it is rapidly absorbed and extensively metabolized into conjugated forms such as quercetin-3′-sulfate and quercetin-3′-glucuronide, which appear in plasma within 1–2 h and are excreted within 4–8 h. Its absorption is enhanced when consumed as onion peel extract and in the presence of dietary fats. Conjugated metabolites, rather than the aglycone, predominate systemically; therefore, aglycone-centric mechanisms observed in vitro do not directly mirror plasma exposure. However, several processes allow partial translation in vivo. Quercetin conjugates have the capacity to undergo site-specific deconjugation in β-glucuronidase-rich tissues, such as those involved in detoxification and repair (liver, kidney, intestinal epithelium, spleen, lymph nodes, thymus, ovary, and preputial gland), thereby regenerating the aglycone locally at biologically relevant levels. It has been demonstrated that certain conjugates exhibit intrinsic bioactivity, encompassing effects on redox balance, endothelial function, and cell signaling. Conjugated species may enter cells via dedicated transporters and be deconjugated intracellularly, yielding transient intracellular aglycone pools. Collectively, these mechanisms support the possibility that aglycone-based anti-proliferative effects can still occur in vivo, but through metabolite-dependent and tissue-specific activation, rather than direct systemic exposure [113].
Mechanistic studies in vitro show quercetin induces G1 or G2/M cell cycle arrest; activates apoptosis by modulating BAX/Bcl-2 ratio, activating caspases and p53, and causing mitochondrial membrane depolarization; and reduces ROS in treated cells. It also inhibits the Phosphatidyl Inositol 3-Kinase (PI3K)/AKT and MAPK/Extracellular signal-Regulated Kinase (ERK) signaling pathways involved in tumor progression and chemoresistance [114]. Moreover, in multidrug-resistant human breast cancer cells (MCF-7/ADR), quercetin (10 μM) sensitizes cancer cells to chemotherapeutics like cisplatin and doxorubicin by interfering with drug-resistance pathways, such as downregulation of P-glycoprotein. In vivo, quercetin suppresses tumor growth and angiogenesis in murine models; it reduces oxidative DNA damage and enhances the activity of detoxification enzymes (e.g., glutathione S transferase), contributing to its chemopreventive potential [115]. Quercetin (30 μmol/L) enhances the therapeutic efficacy of temozolomide in glioblastoma models (U87 and U251) by promoting apoptosis and autophagy [116].
Onionin A is a sulfur-containing compound uniquely isolated from onion bulbs that lacks pharmacokinetic characterization in both humans and animals, limiting current understanding of its systemic availability and therapeutic potential. In vitro, it inhibits proliferation and induces G2/M cell cycle arrest in ovarian, liver and pancreatic cancer cell lines [114] and suppresses STAT3 activation, which is commonly involved in tumor growth and immune evasion. Moreover, it reduces the population of myeloid-derived suppressor cells (MDSCs), thus reversing tumor-mediated immune suppression [117]. In murine models of ovarian cancer, onionin A reduced tumor burden and prolonged survival time and inhibited tumor angiogenesis and macrophage infiltration [118]. It has been reported that onionin A potentiates the antitumor effects of paclitaxel in ovarian cancer cells by enhancing tumor cell sensitivity and modulating the tumor microenvironment [117].
S-allyl cysteine and other organosulfur compounds, instead, act as ROS scavengers, reducing oxidative stress in normal cells and protecting healthy tissue by neutralizing ROS [119]. In contrast, they promote ROS-mediated apoptosis in cancer cells, leading to DNA damage, mitochondrial dysfunction, and activation of apoptotic pathways through Jun-N-Kinase (JNK) signaling [120]. These compounds modulate cell cycle regulatory proteins by downregulating cyclin D1 and upregulating the CDK inhibitor p21WAF1, resulting in cell cycle arrest in cancer cells, like colon, bladder, and lung (effects were observed at a concentration of 300 μmol/L) [118,120,121]. S-allyl cysteine interferes with carcinogen activation via cytochrome P450 enzyme inhibition in animal models [122].
3.3. Anticancer Potential of Bergamot-Derived Bergapten
Citrus bergamia, commonly known as bergamot, is a small citrus tree believed to be a hybrid between Citrus aurantium (bitter orange) and Citrus limon (lemon). It is predominantly cultivated in the coastal areas of Calabria, in Southern Italy, where the unique climatic conditions favor the production of high-quality fruits rich in aromatic compounds. In addition to its widespread use in the perfume and food industries through its essential oil, bergamot holds a notable place in the Mediterranean diet, where it is consumed in the form of juices, extracts, and traditional preparations [123]. This dietary inclusion contributes to the overall antioxidant and anti-inflammatory properties associated with Mediterranean nutritional patterns.
Bergamot contains a variety of biologically active secondary metabolites, including flavonoids and furanocoumarins, among which bergapten (5-methoxypsoralen, 5-MOP) is one of the most studied [124,125]. Belonging to the linear furanocoumarin subclass, bergapten is characterized by a coumarin core fused with a furan ring and a methoxy substituent, conferring distinctive photoreactive and lipophilic properties.
Pharmacokinetic studies have reported good absorption of bergapten after oral administration, with absolute bioavailability ranging from approximately 70% to 94% in animal models. Interestingly, in vitro analysis performed on Caco-2 cells highlighted a moderate intestinal permeability, while in vivo studies reported rapid absorption and wide tissue distribution, particularly in the liver, heart, brain, testis, uterus, and skin. Notably, it has been observed that bergapten is also able to cross the blood–brain barrier, suggesting potential central nervous system activity. After oral administration, bergapten is mainly metabolized in the liver and excreted via feces, with the production of multiple metabolites through oxidation and conjugation pathways. Importantly, it has been shown that bergapten inhibited several CYP450 enzymes (e.g., CYP3A4, CYP1A1, CYP1A2), influencing drug–drug interactions and oral bioavailability of co-administered compounds [123].
It has been extensively investigated for its anti-inflammatory, antioxidant, and cytoprotective activities in various experimental models involving oxidative stress, immune responses, and tissue injury.
More recently, the potential of bergapten in oncological research has attracted growing attention, particularly due to its ability to modulate key cellular processes such as apoptosis, pyroptosis, ferroptosis, and mitochondrial function at concentrations of 5, 10, and 20 μM [126]. These pleiotropic effects, combined with its well-documented photosensitizing capacity, make bergapten a promising candidate for cancer prevention and therapy, especially within the context of photodynamic and photochemotherapeutic approaches [127,128,129]. Supporting this potential, studies have shown that ultraviolet-activated bergapten can significantly disrupt survival signaling in breast cancer cells, enhancing cell death through the modulation of critical regulatory pathways. This highlights the compound’s ability to trigger DNA damage and apoptosis in malignant cells, further reinforcing its therapeutic relevance in light-based anticancer strategies. Several other studies and reviews have further emphasized the potential of bergapten and related furocoumarins as natural photosensitizers, highlighting their ability to generate ROS, intercalate with DNA upon UV exposure, and trigger cell death mechanisms in various cancer models. The relevance of this compound in both photodynamic applications and diet-related cancer risk modulation has also been increasingly recognized in recent pharmacological and oncological literature [130,131,132].
Taken together, these findings delineate the well-established photoactivated anticancer actions of bergapten, which depend on UV-induced DNA intercalation, ROS generation, and apoptotic signaling. However, because these mechanisms depend on light exposure, any potential clinical application would require careful control of UV dose, treatment timing, and phototoxicity risk. The latter remains a known concern for linear furocoumarins. Sunlight exposure can significantly impact both therapeutic and adverse effects and must therefore be considered in translational settings [130,133].
Unlike the UV-dependent pathways described above, a growing body of evidence shows that bergapten has non-photoactivated and UV-independent anticancer effects. These activities occur in the absence of light stimulation, bypassing the phototoxicity concerns associated with its photosensitizing properties.
In human breast cancer cells, Panno et al. provided early evidence that bergapten can induce apoptosis without the need for UV activation. The compound was shown to enhance the gene expression of p53, a key tumor suppressor involved in DNA repair and cell cycle arrest, thereby promoting programmed cell death [134]. This photo-independent effect broadens the potential therapeutic use of bergapten beyond phototherapy settings. Building on this, subsequent studies have deepened this perspective. Bergapten has been shown to activate autophagy in breast cancer cells by upregulating Phosphatase and TENsin homolog (PTEN) expression, contributing to the suppression of oncogenic PI3K/AKT signaling [135]. In parallel, another investigation demonstrated that bergapten (5, 10, 20 and 50 μM) triggers metabolic reprogramming in breast cancer cells, shifting them away from a glycolytic phenotype and potentially sensitizing them to further therapeutic interventions [136]. Moreover, bergapten was also found to trigger estrogen receptor (ER) depletion through SMAD4-mediated ubiquitination, further undermining key survival mechanisms in hormone-responsive breast tumor cells [137].
Beyond breast cancer, the anticancer activity of non-UV-activated bergapten extends to multiple tumor cell lines. In colorectal cancer cells, bergapten (30 and 50 μM) induces G1 cell cycle arrest and a pro-apoptotic cascade involving the p53/p21WAF1/PTEN axis, highlighting its ability to modulate crucial checkpoints of cell proliferation and survival [138]. A 2023 study investigating the non-UV-activated form of bergapten in several tumor cell lines, including osteosarcoma (Saos 2/HOS), colorectal carcinoma (HT-29/SW 620), and multiple myeloma (RPMI 8226/U266), reported dose-dependent cytotoxicity, with Saos 2 cells being particularly sensitive (IC50 ~40 μM). The mechanism involved G2 phase cell cycle arrest, mitochondrial membrane depolarization, and activation of caspase 9 and 3, along with an increased BAX/Bcl-2 ratio and decreased AKT phosphorylation. Notably, bergapten showed limited toxicity toward normal fibroblasts and osteoblasts, indicating selectivity for cancer cells. The HT-29 (colorectal adenocarcinoma cells) (IC50 = 332.4 μM), SW 620 (metastatic colorectal carcinoma) (IC50 = 354.5 μM), and HOS (osteosarcoma cell line) (IC50 = 257.5 μM) cells were characterized by moderate sensitivity, while RPMI8226 (multiple myeloma) (IC50 = 1272 μM) and U266 (multiple myeloma) (IC50 = 1190 μM) were particularly drug-resistant [139]. In non-small cell lung cancer (NSCLC), bergapten has been shown to induce G1 arrest associated with p53-mediated signaling, and network pharmacology combined with molecular docking further elucidated its interaction with multiple targets involved in NSCLC progression [140]. Interestingly, in hepatocellular carcinoma, bergapten was reported to inhibit liver carcinogenesis through modulation of LXR/PI3K/AKT and IDOL/LDLR pathways both in vitro and in vivo, suggesting a role in tumor metabolism and cholesterol regulation [141]. Additionally, in pancreatic cancer models, novel bergapten derivatives were designed, synthesized, and biologically evaluated, revealing potent antitumor activity and supporting structural optimization for therapeutic use [142]. In a separate 2025 study, bergapten (10 μM/mL and 15 μM/mL) was shown to inhibit proliferation of human papillary thyroid cancer cells via modulation of the GSK-3β, PI3K, and AKT pathways, along with apoptosis induction [143].
From a clinical perspective, this dual behavior highlights an important distinction. While photoactivated bergapten shows promise for controlled photodynamic applications, its intrinsic photoreactivity poses a risk of phototoxicity, especially when exposed to natural sunlight. Conversely, non-photoactivated mechanisms may offer therapeutic opportunities with a potentially safer profile, provided light exposure is managed properly. Understanding this dichotomy is crucial for future translational and clinical research.
These findings suggest a multi-pathway, multi-tumor effect for bergapten, reinforcing its potential as a natural anticancer agent.
In conclusion, bergapten emerges as a multifunctional, diet-derived anticancer agent capable of affecting diverse tumor cell lines through mechanisms such as p53/PTEN axis activation, autophagy induction, ER degradation, metabolic interference, and signaling pathway modulation, with or without UV activation. These properties support its promising role in cancer prevention and therapy, particularly within the context of the Mediterranean diet.
3.4. Role of Capsaicin in Cancer Therapy
The chili pepper is rich in capsaicin, which is the main pungent alkaloid. It is a member of a family of compounds that includes dihydrocapsaicin, homocapsaicin, homodihydrocapsaicin, nordihydrocapsaicin, capsaicin esters, dihydrocapsaicin ester, nordihydrocapsaicin esters, capsanthin-β-D-glucoside and dihydrocapsanthin-β-D-glucoside [144]. Capsaicin has been demonstrated to exert analgesic, antioxidant, cardioprotective, anticancer and thermogenic effects, and it can promote weight loss [145]. The majority of these effects are mediated by the TRPV1 receptor (Transient Receptor Potential Cation Channel Subfamily V member 1), to which capsaicin binds specifically [146]. A number of studies have reported that the half-life of capsaicin is relatively short in various organs, including liver, kidney, intestine, lung and blood [147]. However, the administration of capsaicin is associated with different side effects, including gastrointestinal discomfort, such as stomach pain, irritation, gastrointestinal cramps, nausea, and vomiting. Nevertheless, two strategies have been investigated to overcome these effects. Firstly, the encapsulation of capsaicin in drug delivery systems and, secondly, the use of non-pungent capsaicin analogs, which preserve the antitumor activities [148]. Al-Samydai et al., to enhance capsaicin’s pharmacokinetic properties, loaded the molecules into nanoliposomes (IC50 of 21.52 ± 10.90 μmole) model and demonstrated that this system enhanced stability, selectivity and safety compared to the capsaicin alone (IC50 of 515.55 ± 207.8 μmole), as well as higher anticancer activity, inducing apoptosis in a dose-dependent manner in various cancer cell lines (MCF7, MDA-MB 231, K562, PANC1 and A375) [149].
In recent years, capsaicin has garnered growing scientific attention for its anticancer and chemopreventive potential [150]. Extensive research has demonstrated that capsaicin can disrupt several key pathways involved in cancer development and progression. These include the induction of apoptosis and cell cycle arrest [52], as well as the activation of autophagic processes [151]. Mechanistically, capsaicin has been shown to promote the generation of reactive oxygen species (ROS) and modulate critical signaling cascades, such as NF-κB, STAT3, MAPK, PI3K-AKT, Hedgehog, and β-catenin, while also enhancing the activation of ASK1 [152].
In lung cancer cell lines, capsaicin exerts anti-proliferative and pro-apoptotic effects. It inhibits proliferation, induces apoptosis and autophagy, and suppresses angiogenesis. Notably, it (50 µM) activates the E2F4 pathway in small cell lung cancer (NSCLC) [153], promotes mutant p53 degradation via autophagy (IC50 of 200 μM) [154], and triggers apoptosis through the TRPV6-calcium-calpain axis [155]. Moreover, capsaicin (at concentrations of 1 and 10 μM) induces G0/G1 cell cycle arrest and apoptosis by suppressing HER2, EGFR, ERK, and cyclin D1 while increasing p27KIP1 and caspase activity [156]. Moreover, it promotes apoptosis via both caspase-dependent and -independent pathways [157] and activates autophagy through p38 MAPK signaling in malignant and normal breast epithelial cells [158]. Additionally, capsaicin (200 μM) triggers mitochondrial-mediated apoptosis and PARP cleavage in MCF-7 and BT-20 cells [159].
In colorectal cancer cells, the role of capsaicin remains controversial. Some studies report tumor-promoting effects via ROS generation, AKT/mTOR and STAT3 pathway activation, and tNOX upregulation (at 100 µM for SW480 and CT-26 cell lines and at 12.5 µM for HCT116 cell line) [160], while others demonstrate its antitumor action via PPARγ activation [161], ROS-mediated mitochondrial disruption [162], nitric oxide induction (100 μM of capsaicin and/or 50 μM of resveratrol) [163], and p53 stabilization [164]. It also possesses immunomodulatory activity, affecting cytokine production in co-cultures of colon cancer cells and PBMCs. 200 µM of capsaicin suppresses TNF-α, IL-1β, IFN-γ, IL-10, and IL-1ra while stimulating IL-6 at lower concentrations, suggesting dose-dependent immune modulation [165].
In stomach cancer, capsaicin exhibits concentration-dependent effects: low intake may offer protection, while high intake has been associated with increased cancer risk, particularly in genetically predisposed individuals infected with Helicobacter pylori [166]. Mechanistically, it promotes apoptosis and proliferation arrest via cytochrome c release, p53 activation, and MAPK signaling in human gastric cancer cells [167] and inhibits migration by downregulating tNOX and POU3F2 in human gastric carcinoma cells [168]. It also attenuates Helicobacter pylori-induced inflammation, suggesting a potential role in the chemoprevention of gastritis-associated gastric cancer. Capsaicin exerts anticancer effects through both TRPV1-dependent and TRPV1-independent mechanisms. In the TRPV1-dependent pathway, capsaicin activates the TRPV1 channel, inducing Ca2+ influx, ROS generation, ER stress, autophagy, and JNK activation, leading to apoptosis [169,170,171]. Conversely, low-dose TRPV1 activation may stimulate PI3K/AKT signaling and promote proliferation in androgen-sensitive prostate cancer cells [150]. In TRPV1-independent mechanisms, capsaicin directly disrupts mitochondrial function, increases ceramide accumulation, and inhibits AKT, NF-κB, and ERCC1 signaling, enhancing sensitivity to chemo- and radiotherapy [172,173,174].
In prostate cancer cells, capsaicin exerts anti-proliferative and pro-apoptotic effects through both TRPV1-dependent and -independent pathways. These effects involve ROS production, endoplasmic reticulum stress, ceramide accumulation, autophagy induction, and JNK activation [169,170,171]. Its action is dose- and androgen-sensitivity dependent: low doses may stimulate proliferation in androgen-sensitive cells via PI3K-TRPV1 signaling, while higher concentrations inhibit growth independently of TRPV1 [175].
One of the most promising aspects of capsaicin’s pharmacological profile is its ability to enhance the efficacy of conventional chemotherapeutic agents. A growing body of evidence supports the notion that capsaicin can sensitize cancer cells to various anticancer drugs, leading to synergistic anticancer effects both in vitro and in vivo.
Capsaicin has demonstrated synergistic effects with various chemotherapeutic agents across multiple tumor cell lines. In cholangiocarcinoma and gastric cancer, it enhanced the cytotoxicity of 5-fluorouracil (5-FU) by inhibiting autophagy via the PI3K/AKT/mTOR pathway and reducing 5-FU’s IC50. The IC50 of QBC939 cells for 5-FU in combination with capsaicin (20, 40, 80 μM) was significantly decreased from 126 μM to 35 μM, and significant synergy with capsaicin was found at 40 μM [176]. In SCLC, this compound potentiated camptothecin-induced apoptosis through intracellular Ca2+ elevation and calpain activation [177]. Co-treatment with cisplatin (DDP) in osteosarcoma and gastric cancer cell lines increased apoptosis and suppressed NF-κB signaling [178,179], while capsaicin also sensitized bladder cancer cells to pirarubicin by inducing cell cycle arrest via TRPV1 activation [180]. In B-cell lymphoma, this compound restored etoposide sensitivity by enhancing CD40-mediated apoptosis [181]. While capsaicin generally acts as a chemosensitizer, one study noted that it promoted resistance in bladder cancer to mitomycin C, gemcitabine, and doxorubicin via Hedgehog-dependent EMT and autophagy (IC50 of 300 μM) [182]. However, combinations of capsaicin with other dietary compounds improved gemcitabine efficacy in preclinical in vivo model of pancreatic cancer [183]. In hepatocellular carcinoma cell lines, capsaicin synergizes with sorafenib to inhibit proliferation and induce apoptosis through TRPV1-independent mechanisms by potentiating ERK signaling [174]. Capsaicin (25, 50 and 100 μM) also boosted the anticancer activity of erlotinib in NSCLC by suppressing ERCC1 and AKT signaling [172]. Finally, capsaicin (1–10 µM) sensitized prostate cancer cells to radiation therapy (RT) by inhibiting NF-κB, with no added toxicity in normal tissues. Clinical observation supports its role in prolonging PSA [173].
Collectively, these findings underscore the potential of capsaicin as a valuable adjuvant in cancer therapy. Its natural origin and ability to augment the effectiveness of standard treatments suggest that dietary phytochemicals could play a supportive role in oncology. Nevertheless, the precise molecular mechanisms driving capsaicin-induced drug sensitization remain to be fully elucidated and warrant further investigation.
3.5. Resveratrol in Cancer Therapy
Wine is traditionally regarded as one of the components of the Mediterranean diet. Several studies have highlighted how the moderate consumption of wine, particularly red wine during meals, can exert beneficial effects on human health, especially on the cardiovascular system. This is due in part to the presence of bioactive compounds, named polyphenols, which are abundant in red wine and are known for their antioxidant, anti-inflammatory, and cardioprotective properties [184].
Polyphenols in wine are broadly classified into two main groups: flavonoids (including anthocyanins, flavanols, and flavonols) and non-flavonoids. The latter category includes phenolic acids, lignans, and stilbenes, among which the most studied and biologically relevant is resveratrol [185]. Particularly, resveratrol is predominantly found in the skin of red grapes (Vitis vinifera), where it acts as phytoalexin, a compound produced by grapevines in response to environmental stress and pathogenic invasion, which significantly increases its amount [186]. Although resveratrol concentration in wine is relatively low, usually only a few milligrams per liter, the repeated intake through moderate, regular wine consumption could contribute to its observed biological effects.
Chemically, resveratrol is known as 3,4′,5-trihydroxy-trans-stilbene, consisting of two aromatic rings connected by a double bond and bearing three hydroxyl groups that are crucial to its biological activity [187]. The double bond enables isomerization under UV irradiation, with the trans-isomer being not only more abundant than the cis-isomer but also more biologically active. This structural configuration underlies its antioxidant, anti-inflammatory, cardioprotective, neuroprotective, and anticancer effects [187]. In the context of cancer prevention and therapy, resveratrol has emerged as one of the principal bioactive compounds contributing to the potential health benefits of wine.
Extensive in vitro research has highlighted the important role of resveratrol in the early stages of carcinogenesis, largely due to its strong antioxidant activity. Interestingly, a dual antioxidant effect of resveratrol has been reported, depending on cellular context. In cancer cells, it has been shown to prevent oxidative stress-induced DNA damage by disrupting redox homeostasis, leading to a reduction in intracellular reactive oxygen species (ROS) [188]. More specifically, resveratrol enhances both the expression and activity of key antioxidant enzymes, including superoxide dismutase (SOD), catalase, and glutathione peroxidase, resulting in mitochondrial accumulation of hydrogen peroxide and the induction of apoptosis in tumor cells. Additionally, resveratrol inhibits the accumulation of hypoxia-inducible factor-1α (HIF-1α), suppressing glycolytic metabolism and interfering with the metabolic reprogramming that sustains tumor growth. On the contrary, in normal cells, a protective role of resveratrol has been reported by mildly upregulating antioxidant defense systems and reducing oxidative stress, thereby preserving cellular integrity [188].
It also modulates different signaling pathways involved in cell proliferation, apoptosis, angiogenesis, and metastasis. For instance, resveratrol activates the tumor suppressor p53, inhibits NF-κB and STAT3 signaling, and interferes with the PI3K/AKT and MAPK pathways. These molecular effects have been observed in many cancer cell lines, such as breast, prostate, colon, lung, and glioblastoma [189,190]. In breast cancer cells, resveratrol was shown to inhibit estrogen receptor α (ERα) gene expression via phosphorylation of the NF-Y/p53/Sin3/HDAC1 complex, mediated by p38MAPK/CK2 signaling, including MCF-7 tamoxifen-resistant cells. IC50 values for viability were in the 30–45 μM range [191]. In glioblastoma, the combination of resveratrol with Notch inhibitors induced cell death by shifting the balance from autophagy to apoptosis. Resveratrol activated ERK1/2 at low concentrations (1 pM-10 μM), but at higher concentrations (50–100 μM), it inhibited MAPK in human neuroblastoma SH-SY5Y cells [192]
In vivo studies corroborate the in vitro findings, showing that resveratrol can inhibit tumor growth, reduce metastatic spread, and improve survival in animal models of cancer (0.5, 5, or 50 mg/kg BW; oral gavage) [193]. In mouse models, it has demonstrated the ability to reduce tumor volume in breast and colon cancer and to limit metastasis in prostate and lung cancers. These effects are associated with its regulation of angiogenesis, modulation of the tumor microenvironment, and enhancement of immune response [194].
Despite its promising preclinical profile, translating resveratrol into clinical therapy remains challenging. Although topical application of the compound has proven effective in inhibiting tumor formation in animal models of skin cancer, its therapeutic use against other tumor types is limited by the need for oral administration. Indeed, clinical trials have confirmed that resveratrol’s therapeutic potential is hampered by poor pharmacokinetic profile and low bioavailability after oral consumption. Resveratrol undergoes rapid metabolism in the intestine and liver, resulting in low plasma concentrations and limited systemic exposure [195].
To overcome this limitation, numerous efforts have been made to identify strategies capable of enhancing the bioavailability of resveratrol following oral administration. Among these, the use of delivery systems such as nanoformulations, liposomes, solid lipid nanoparticles, and micellar systems has shown particular promise, as they can improve the compound’s solubility, stability, and absorption, thereby increasing its systemic availability and therapeutic potential [187]. One promising formulation is JOTROL™, a micellar 10% resveratrol solubilization formulation that is thought to increase bioavailability of resveratrol via lymphatic system absorption by exploiting lymphatic distribution to bypass first-pass metabolism and enhance systemic bioavailability. Early clinical data indicate that JOTROL™ achieves significantly higher plasma levels of resveratrol compared to conventional formulations [195]. Additionally, co-administration with bioenhancers, use of prodrugs, and structural analog development are under investigation as means to optimize its therapeutic use [196,197].
Moreover, several studies have demonstrated that resveratrol can enhance the efficacy of conventional chemotherapeutic agents such as 5-fluorouracil, doxorubicin, temozolomide and cisplatin by promoting apoptosis and inhibiting tumor cell proliferation. At the same time, resveratrol exerts protective effects against drug-induced toxicity in healthy tissues, particularly in the heart, highlighting its potential as an adjuvant in cancer therapy (5-FU alone (200 μg/mL), resveratrol alone (200 μg/mL), and 5-FU in combination with resveratrol (200 μg/L + 50 μmol/L)) [198,199,200,201].
In conclusion, resveratrol represents a compelling candidate for cancer chemoprevention and adjunctive therapy, owing to its pleiotropic actions on cancer-related pathways. However, to fully realize its clinical potential, further work is needed to address the challenges of bioavailability and to validate its efficacy in well-designed, large-scale human trials [202].
4. Conclusions
Natural compounds are generally less toxic or even non-toxic to normal cells while exhibiting selective activity against cancer cells. They present a favorable safety profile that, coupled with the richness of phytochemical diversity, allows them to modulate multiple oncogenic signaling pathways simultaneously. Increasing evidence supports their role not only in cancer prevention but also as adjuvant to conventional chemotherapy, enhancing therapeutic efficacy, reducing drug resistance, and mitigating adverse side effects. Many phytochemicals act by interfering with key molecular hallmarks of carcinogenesis, such as sustaining proliferative signaling (e.g., via EGFR or PI3K/AKT/mTOR pathways), evading apoptosis (e.g., through BAX/Bcl-2 ratio modulation), inducing autophagy or ferroptosis, inhibiting angiogenesis, and preventing metastasis (e.g., through EMT suppression). Others restore immune surveillance, regulate oxidative stress, and influence the tumor microenvironment. These mechanisms make natural compounds particularly promising as multi-targeted therapeutic agents, complementing standard anticancer treatments. Notably, many of these compounds, such as oleocanthal, oleuropein, quercetin, onionin A, capsaicin, bergapten, and resveratrol, exhibit potent antioxidant activity, reducing oxidative stress, thus contributing to cancer prevention by maintaining genomic stability and limiting inflammation-induced tumor initiation. However, this same antioxidant action must be evaluated carefully in the therapeutic context, as it may interfere with ROS-dependent cancer treatments (Figure 1). This paradox is called the “antioxidant double-edged sword” [203]. A major advantage of these natural compounds lies in their abundance in common plants; they can be easily extracted from readily available plants, which has a considerable effect on reducing the cost of production. Despite their promising potential in cancer therapy and prevention, several challenges remain. These include variability in phytochemical composition, the need for rigorous standardization and clinical validation, and the risk of herb–drug interactions. Therefore, further preclinical and translational studies are essential to elucidate their mechanisms of action and improve bioavailability. Due to low solubility, poor absorption, or rapid metabolism, their therapeutic effectiveness is currently limited. In addition, it is important to evaluate safety profiles in well-designed clinical trials.
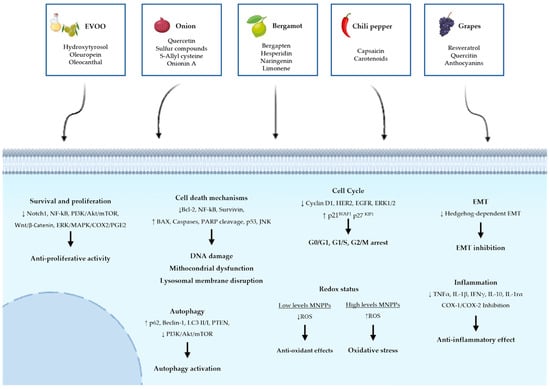
Figure 1.
Common Molecular Mechanisms underlying the antitumor effects of five Selected Mediterranean Natural Plant-Derived Compounds. The figure summarizes the effects of selected Mediterranean plant extracts on cancer-related cellular pathways. Arrows indicate the upregulation (↑) or downregulation (↓) of key molecular markers and pathways.
In conclusion, it is evident that a diet abundant in vegetables, fruit, olive oil, and herbs, like the Mediterranean diet, associated with physical activity and a reduced intake of sugar and alcohol, may play a role not only in preventing but also in supporting the treatment of malignancies. The integration of nutritional strategies and phytochemicals with conventional therapeutic modalities may offer a more comprehensive and efficacious approach to contemporary oncology.
Author Contributions
A.E.L., G.D.N. and F.G. summarized the literature, conceived the structure of the text, and wrote the manuscript. L.M. and F.D.A. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MUR ex 60%.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation of this manuscript, the authors used GPT-5.2 for assistance in revising the text for clarity and language, as well as for generating the table content. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MNPPs | Mediterranean Natural Plant Products |
| EGCG | EpiGalloCatechin Gallate |
| MDR | MultiDrug Resistance |
| MD | Mediterranean Diet |
| EVOO | Extra Virgin Olive Oil |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| MMPs | Matrix MetalloProteinases |
| COX2 | CycloOXygenase-2 |
| HGF | Hepatocyte Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| IGF-1R | Insulin-like Growth Factor 1 Receptor |
| PARP | Poly (ADP-ribose) Polymerase |
| MUFA | Monounsaturated Fatty Acid |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| CDK1 | Cyclin Dependent Kinase 1 |
| CDK2 | Cyclin Dependent Kinase 2 |
| SOD | Superoxide Dismutase |
| JNK | Jun-N-Kinase |
| ROS | Reactive Oxygen Species |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| PI3K | PhosphatidylInositol 3-Kinase |
| ERK | Extracellular Signal-Regulated Kinase |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| MDSCs | Myeloid-Derived Suppressor Cells |
| PTEN | Phosphatase and TENsin homolog |
| ER | Estrogen Receptor |
| NSCLC | Non-Small Cell Lung Cancer |
| TRPV1 | Transient Receptor Potential Cation Channel Subfamily V member 1 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cho, W.C. Molecular connections of aging and cancer. Aging Dis. 2017, 8, 685. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: London, UK, 2008; Volume 2. [Google Scholar]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, Z.; Liu, X.; Wang, T.; Wang, Y.; Ye, F.; Su, J.; Yao, X.; Xiong, L.; Yang, D.-H. Natural products for combating multidrug resistance in cancer. Pharmacol. Res. 2024, 202, 107099. [Google Scholar] [CrossRef]
- Elbadawi, M.; Efferth, T. In Vivo and Clinical Studies of Natural Products Targeting the Hallmarks of Cancer. In Natural Products as Sources of Novel Drugs; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Shrivastava, B.; Apte, K.; Navale, S. Medicinal plants as potential source of anticancer agents: A review. J. Pharmacogn. Phytochem. 2016, 5, 291–295. [Google Scholar]
- Hande, K. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.L. The discovery of the vinca alkaloids—Chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990, 68, 1344–1351. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.A.; Palmer, K.H.; McPhail, A.A.; Sim, G. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Trajkovska-Broach, A.; Trajkovska Petkoska, A. Mediterranean herbs, spices, and medicinal plants—Natural remedies and rich sources of bioactive compounds. JSFA Rep. 2023, 3, 4–12. [Google Scholar] [CrossRef]
- Araújo, J.R.; Gonçalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 2011, 31, 77–87. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X. Green tea and cancer prevention. Nutr. Cancer 2010, 62, 931–937. [Google Scholar] [CrossRef]
- Sharma, M.; Kashyap, N.; Verma, M.; Devi, B.; Prashar, A.; Sinha, S. Role of natural products in cancer management: A comprehensive review. J. Ethnopharmacol. Toxicol. 2024, 2, 1–18. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E. Multidrug resistance in cancer: Understanding molecular mechanisms, immunoprevention and therapeutic approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Jia, H.R.; Duan, Q.Y.; Wu, F.G. Nanomedicines for combating multidrug resistance of cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1715. [Google Scholar] [CrossRef]
- Ben-Arye, E.; Schiff, E.; Zollman, C.; Heusser, P.; Mountford, P.; Frenkel, M.; Bar-Sela, G.; Lavie, O. Integrating complementary medicine in supportive cancer care models across four continents. Med. Oncol. 2013, 30, 511. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front. Pharmacol. 2017, 8, 420. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; Vela, V.L.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Watson, L.; Ernst, E. Herb–drug interactions: An overview of systematic reviews. Br. J. Clin. Pharmacol. 2013, 75, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free. Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2016, 8, 15996. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Lopez-Alvarado, J.; Farris, E. Ecology and evolution of plants in the Mediterranean basin: Perspectives and challenges. Plants 2022, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, L.; Debono, K.; Grech, F.; Bellia, A.F.; Pace, G.; Lanfranco, S. Topographic complexity is a principal driver of plant endemism in mediterranean islands. Plants 2024, 13, 546. [Google Scholar] [CrossRef]
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Vargas, P. Mediterranean biomes: Evolution of their vegetation, floras, and climate. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- La Scala, S.; Naselli, F.; Quatrini, P.; Gallo, G.; Caradonna, F. Drought-adapted mediterranean diet plants: A source of bioactive molecules able to give nutrigenomic effects per sè or to obtain functional foods. Int. J. Mol. Sci. 2024, 25, 2235. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Menicucci, F.; Brunetti, C.; dos Santos Nascimento, L.B.; Pasquini, D.; Alderotti, F.; Detti, C.; Ferrini, F.; Gori, A. Unlocking the Hidden Potential of Rosemary (Salvia rosmarinus Spenn.): New Insights into Phenolics, Terpenes, and Antioxidants of Mediterranean Cultivars. Plants 2024, 13, 3395. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean lavenders from section Stoechas: An undervalued source of secondary metabolites with pharmacological potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef]
- Tramice, A.; Vella, F.M.; Carra, A.; Carimi, F.; Pignone, D.; Laratta, B. Phytochemical study on Mediterranean Calendula maritima (Guss.). Eur. Food Res. Technol. 2025, 251, 1–9. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean diet: A review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Murakami, S.; Lefebvre, V.; de Crombrugghe, B. Potent Inhibition of the Master Chondrogenic FactorSox9 Gene by Interleukin-1 and Tumor Necrosis Factor-α. J. Biol. Chem. 2000, 275, 3687–3692. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.-H. Anticancer properties of capsaicin against human cancer. Anticancer. Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Horvathova, M.; Orszaghova, Z.; Laubertova, L.; Vavakova, M.; Sabaka, P.; Rohdewald, P.; Durackova, Z.; Muchova, J. Effect of the French oak wood extract Robuvit on markers of oxidative stress and activity of antioxidant enzymes in healthy volunteers: A pilot study. Oxidative Med. Cell. Longev. 2014, 2014, 639868. [Google Scholar] [CrossRef]
- Karkabounas, S.; Kostoula, O.; Daskalou, T.; Veltsistas, P.; Karamouzis, M.; Zelovitis, I.; Metsios, A.; Lekkas, P.; Evangelou, A.; Kotsis, N. Anticarcinogenic and antiplatelet effects of carvacrol. Exp. Oncol. 2006, 28, 121–125. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–75. [Google Scholar]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Maio, I.D.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Epriliati, I.; Ginjom, I.R. Bioavailability of phytochemicals. Phytochem. A Glob. Perspect. Their Role Nutr. Health 2012, 21, 401–428. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Pimentel Junior, A.; Borges, C.V.; Rodrigues, J.D.; Figueira, R.; Moura, M.F.; Minatel, I.O.; Nunes, A.; Lima, G.P.P.; Tecchio, M.A. Polyphenolic profile and antioxidant activity of whole grape juices from Vitis labrusca and Brazilian hybrid grapes in two training systems. Antioxidants 2024, 13, 1132. [Google Scholar] [CrossRef]
- Russo, M.; Torre, G.; Carnovale, C.; Bonaccorsi, I.; Mondello, L.; Dugo, P. A new HPLC method developed for the analysis of oxygen heterocyclic compounds in Citrus essential oils. J. Essent. Oil Res. 2012, 24, 119–129. [Google Scholar] [CrossRef]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S. Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. 2022, 29, 31025–31041. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Cho, M.L.; Kim, D.-B.; Shin, G.-H.; Lee, J.-H.; Lee, J.S.; Park, S.-O.; Lee, S.-J.; Shin, H.M.; Lee, O.-H. The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Howard, L.; Talcott, S.; Brenes, C.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.S.; Fernandes, C.C.; Santos, L.S.; DEUS, I.P.B.D.; SOUSA, T.L.D.; Miranda, M.L.D. Ethanolic extract from Capsicum chinense Jacq. ripe fruits: Phenolic compounds, antioxidant activity and development of biodegradable films. Food Sci. Technol. 2020, 41, 497–504. [Google Scholar]
- Hanbury, D. On the febrifuge properties of the olive (Olea europea, L.). Pharm. J. Prov. Trans. 1854, 13, 353–354. [Google Scholar]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Long-term impact of mediterranean diet on cardiovascular disease prevention: A systematic review and meta-analysis of randomized controlled trials. Curr. Probl. Cardiol. 2024, 49, 102509. [Google Scholar]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The antioxidant and anti-proliferative activity of the Lebanese Olea europaea extract. Plant Foods Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Mosca, L.; Puca, R.; Mangino, G.; Rossi, A.; Lendaro, E. Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells. Oncol. Rep. 2016, 36, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical properties and anti-proliferative activity of Olea europaea L. leaf extracts against pancreatic cancer cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar] [CrossRef]
- Rizzo, M.; Ventrice, D.; Giannetto, F.; Cirinnà, S.; Santagati, N.A.; Procopio, A.; Mollace, V.; Muscoli, C. Antioxidant activity of oleuropein and semisynthetic acetyl-derivatives determined by measuring malondialdehyde in rat brain. J. Pharm. Pharmacol. 2017, 69, 1502–1512. [Google Scholar] [CrossRef]
- Sirianni, R.; Chimento, A.; De Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Ando, S.; Maggiolini, M. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Xie, J.; Zhang, Z.; Zhu, J.; Jiang, S.; Shen, W.-J.; Wu, B.; Ding, T.; Wang, S.-L. Hydroxytyrosol and oleuropein inhibit migration and invasion via induction of autophagy in ER-positive breast cancer cell lines (MCF7 and T47D). Nutr. Cancer 2021, 73, 350–360. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor–negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic anti-breast-cancer effects of combined treatment with oleuropein and doxorubicin in vivo. Altern. Ther. Health Med. 2019, 25, 17–24. [Google Scholar]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; de la Lastra, C.A. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef]
- Mao, W.; Shi, H.; Chen, X.; Yin, Y.; Yang, T.; Ge, M.; Luo, M.; Chen, D.; Qian, X. Anti-proliferation and migration effects of oleuropein on human A549 lung carcinoma cells. Lat. Am. J. Pharm 2012, 31, 1217–1221. [Google Scholar]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.J.; Jambrak, A.R.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive phenolics as c-Met inhibitors:(-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C. Cytotoxic activity of oleocanthal isolated from virgin olive oil on human melanoma cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.; Lago, F.; Smith Iii, A.; Gualillo, O. Oleocanthal inhibits proliferation and MIP-1α expression in human multiple myeloma cells. Curr. Med. Chem. 2013, 20, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, V.W.; Wood, E.; Fedorenko, I.V.; Paraiso, K.H.; Haarberg, H.E.; Chen, Y.; Xiang, Y.; Sarnaik, A.; Gibney, G.T.; Sondak, V.K. Evaluating melanoma drug response and therapeutic escape with quantitative proteomics. Mol. Cell. Proteom. 2014, 13, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- LeGendre, O.; Breslin, P.A.; Foster, D.A. (-)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization. Mol. Cell. Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef]
- Peri, S.; Ruzzolini, J.; Urciuoli, S.; Versienti, G.; Biagioni, A.; Andreucci, E.; Peppicelli, S.; Bianchini, F.; Bottari, A.; Calorini, L. An oleocanthal-enriched EVO oil extract induces the ROS production in gastric cancer cells and potentiates the effect of chemotherapy. Antioxidants 2022, 11, 1762. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.Y.; Akl, M.R.; Ayoub, N.M.; Goda, A.A.; Mohyeldin, M.M.; Nagumalli, S.K.; Hananeh, W.M.; Liu, Y.-Y.; Meyer, S.A. (−)-Oleocanthal combined with lapatinib treatment synergized against HER-2 positive breast cancer in vitro and in vivo. Nutrients 2019, 11, 412. [Google Scholar] [CrossRef]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; Mccubrey, J.A.; Montalto, G. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Karampetsou, K.V.; Koutsoni, O.S.; Skaltsounis, A.-L.; Dotsika, E.; Halabalaki, M. Pharmacokinetics and Metabolism Investigation of Oleocanthal. J. Nat. Prod. 2023, 87, 530–543. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Di Renzo, L.; Smeriglio, A.; Ingegneri, M.; Gualtieri, P.; Trombetta, D. The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics. Pharmaceutics 2023, 15, 743. [Google Scholar] [CrossRef]
- Lamy, S.; Ouanouki, A.; Béliveau, R.; Desrosiers, R.R. Olive oil compounds inhibit vascular endothelial growth factor receptor-2 phosphorylation. Exp. Cell Res. 2014, 322, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519–529. [Google Scholar] [CrossRef]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V. Oleuropein and hydroxytyrosol activate GPER/GPR 30-dependent pathways leading to apoptosis of ER-negative SKBR 3 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 478–489. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Goswami, M.; Kalen, A.L.; Lafin, J.T.; Goswami, P.C. Hydroxytyrosol inhibits chemokine CC motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age 2014, 36, 1213–1224. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; Messa, C.; Perri, E.; Notarnicola, M. Antiproliferative, antioxidant and anti-inflammatory effects of hydroxytyrosol on human hepatoma HepG2 and Hep3B cell lines. Anticancer. Res. 2012, 32, 5371–5377. [Google Scholar] [PubMed]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessì, M.A.; Spencer, J.P. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar] [CrossRef]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol induces apoptosis and cell cycle arrest and suppresses multiple oncogenic signaling pathways in prostate cancer cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Suman, S.; Alatassi, H.; Ankem, M.; Damodaran, C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016, 7, e2111. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yamashita, H.; Yu, X.; Wang, J.; Franco, O.E.; Wang, Y.; Hayward, S.W.; Matusik, R.J. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene 2015, 34, 3700–3710. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.; Panici, P.B.; De Vincenzo, R.; Bonanno, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rumi, C.; Cianfriglia, M. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.-p.; Li, R.-j.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological Properties of Allium cepa, preclinical and clinical evidences; a review. Iran. J. Pharm. Res. IJPR 2021, 20, 107. [Google Scholar]
- Ho, J.N.; Kang, M.; Lee, S.; Oh, J.J.; Hong, S.K.; Lee, S.E.; Byun, S.S. Anticancer effect of S-allyl-L-cysteine via induction of apoptosis in human bladder cancer cells. Oncol. Lett. 2018, 15, 623–629. [Google Scholar] [CrossRef]
- Wargovich, M.J. Diallylsulfide and allylmethylsulfide are uniquely effective among organosulfur compounds in inhibiting CYP2E1 protein in animal models. J. Nutr. 2006, 136, 832S–834S. [Google Scholar] [CrossRef]
- Xiao, D.; Pinto, J.T.; Gundersen, G.G.; Weinstein, I.B. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol. Cancer Ther. 2005, 4, 1388–1398. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaya, Y.; Notoya, N.; Nishizuka, M. Cycloalliin, an Organosulfur Compound in Garlic, Inhibits EMT and Invasion of the A549 Non-Small Cell Lung Cancer Cell Line. BPB Rep. 2024, 7, 101–105. [Google Scholar] [CrossRef]
- Davenport, D.M.; Wargovich, M.J. Modulation of cytochrome P450 enzymes by organosulfur compounds from garlic. Food Chem. Toxicol. 2005, 43, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.R.; Choi, R.-Y.; Lee, H.-I.; Lee, M.-K. Methoxsalen and bergapten prevent diabetes-induced osteoporosis by the suppression of osteoclastogenic gene expression in mice. Int. J. Mol. Sci. 2019, 20, 1298. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.; Ilari, S.; Giancotti, L.A.; Morabito, C.; Malafoglia, V.; Gliozzi, M.; Palma, E.; Salvemini, D.; Muscoli, C. The protective role of bergamot polyphenolic fraction on several animal models of pain. PharmaNutrition 2016, 4, S35–S40. [Google Scholar] [CrossRef]
- Luo, T.; Jia, X.; Feng, W.-d.; Wang, J.-y.; Xie, F.; Kong, L.-d.; Wang, X.-j.; Lian, R.; Liu, X.; Chu, Y.-j. Bergapten inhibits NLRP3 inflammasome activation and pyroptosis via promoting mitophagy. Acta Pharmacol. Sin. 2023, 44, 1867–1878. [Google Scholar] [CrossRef]
- Aidoo, D.B.; Konja, D.; Henneh, I.T.; Ekor, M. Protective effect of bergapten against human erythrocyte hemolysis and protein denaturation in vitro. Int. J. Inflamm. 2021, 2021, 1279359. [Google Scholar] [CrossRef]
- Singh, G.; Singh, A.; Singh, P.; Bhatti, R. Bergapten ameliorates vincristine-induced peripheral neuropathy by inhibition of inflammatory cytokines and NFκB signaling. ACS Chem. Neurosci. 2019, 10, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Sun, Z.-C.; Wang, L.; Xian, C.; Lin, R.; Zhuo, G.; Wang, H.; Fang, Y.; Liu, Y.; Yang, R. Inhalable and bioactive lipid-nanomedicine based on bergapten for targeted acute lung injury therapy via orchestrating macrophage polarization. Bioact. Mater. 2025, 43, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.; Taghavi, E.; Ikram, M. An overview of potential natural photosensitizers in cancer photodynamic therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, R.; Jaswal, V.S.; Nepovimova, E.; Chaudhary, A.; Kuca, K. Psoralen: A biologically important coumarin with emerging applications. Mini Rev. Med. Chem. 2020, 20, 1838–1845. [Google Scholar] [CrossRef]
- Melough, M. Relationship between Dietary Furocoumarins and Melanoma Risk. Doctoral Dissertations, University of Connecticut, Storrs, CT, USA, 2019. [Google Scholar]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some natural photosensitizers and their medicinal properties for use in photodynamic therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Palma, M.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Ando, S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S. Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef]
- Santoro, M.; Guido, C.; De Amicis, F.; Sisci, D.; Cione, E.; Dolce, V.; Dona, A.; Panno, M.L.; Aquila, S. Bergapten induces metabolic reprogramming in breast cancer cells. Oncol. Rep. 2016, 35, 568–576. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Rizza, P.; Pellegrino, M.; Zito, D.; Giordano, C.; Mauro, L.; Catalano, S.; Aquila, S.; Sisci, D. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res. Treat. 2012, 136, 443–455. [Google Scholar] [CrossRef]
- Lin, C.P.; Lin, C.S.; Lin, H.H.; Li, K.T.; Kao, S.H.; Tsao, S.M. Bergapten induces G1 arrest and pro-apoptotic cascade in colorectal cancer cells associating with p53/p21/PTEN axis. Environ. Toxicol. 2019, 34, 303–311. [Google Scholar] [CrossRef]
- Bartnik, M.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Zdzisińska, B. Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumor Cells and the Insight into the Molecular Mechanism of Its Action. Int. J. Mol. Sci. 2023, 24, 15555. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-R.; Lin, C.-S.; Lin, H.-H.; Shieh, P.-C.; Kao, S.-H. Bergapten induces G1 arrest of non-small cell lung cancer cells, associated with the p53-mediated cascade. Mol. Med. Rep. 2019, 19, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, S.P.; Bose, P.; Sunita, P.; Siddique, M.U.M.; Lapenna, A. Bergapten inhibits liver carcinogenesis by modulating LXR/PI3K/Akt and IDOL/LDLR pathways. Biomed. Pharmacother. 2018, 108, 297–308. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.-R.; Wang, N.; Wang, X.-R.; Ma, J.-Y.; Yang, J.-L. Design, synthesis, and biological evaluation of bergapten derivatives as potent anti-pancreatic cancer agents. J. Asian Nat. Prod. Res. 2025, 1–17. [Google Scholar] [CrossRef]
- Li, J.; Hussain, S.A.; Daddam, J.R.; Sun, M. Bergapten attenuates human papillary thyroid cancer cell proliferation by triggering apoptosis and the GSK-3β, P13K and AKT pathways. Adv. Clin. Exp. Med. 2025, 34, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Higashiguchi, F.; Nakamura, H.; Hayashi, H.; Kometani, T. Purification and structure determination of glucosides of capsaicin and dihydrocapsaicin from various Capsicum fruits. J. Agric. Food Chem. 2006, 54, 5948–5953. [Google Scholar] [CrossRef]
- Cunha, M.R.; Tavares, M.T.; Fernandes, T.B.; Parise-Filho, R. Peppers: A “Hot” natural source for antitumor compounds. Molecules 2021, 26, 1521. [Google Scholar] [CrossRef]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.-D.; Caruntu, A.; Scheau, A.-E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M. The effects of capsaicin on gastrointestinal cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Sugrue, A.M.; Conley, K.B.; Modi, K.J.; Light, R.S.; Cox, A.J.; Bender, C.R.; Miles, S.L.; Denning, K.L.; Finch, P.T. Anti-cancer activity of capsaicin and its analogs in gynecological cancers. Adv. Cancer Res. 2024, 164, 241–281. [Google Scholar]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.; Azzam, H.; Aburjai, T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef]
- Patowary, P.; Pathak, M.P.; Zaman, K.; Raju, P.; Chattopadhyay, P. Research progress of capsaicin responses to various pharmacological challenges. Biomed. Pharmacother. 2017, 96, 1501–1512. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, Y.S.; Lim, S.C.; Hou, Y.F.; Chang, I.Y.; You, H.J. Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner. Autophagy 2008, 4, 1009–1019. [Google Scholar] [CrossRef]
- Ranjan, A.; Fofaria, N.M.; Kim, S.-H.; Srivastava, S.K. Modulation of signal transduction pathways by natural compounds in cancer. Chin. J. Nat. Med. 2015, 13, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef]
- Garufi, A.; Pistritto, G.; Cirone, M.; D’Orazi, G. Reactivation of mutant p53 by capsaicin, the major constituent of peppers. J. Exp. Clin. Cancer Res. 2016, 35, 136. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef]
- Thoennissen, N.; O’kelly, J.; Lu, D.; Iwanski, G.; La, D.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and-negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef]
- Chou, C.-C.; Wu, Y.-C.; Wang, Y.-F.; Chou, M.-J.; Kuo, S.-J.; Chen, D.-R. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol. Rep. 2009, 21, 665–671. [Google Scholar] [PubMed]
- Choi, C.-H.; Jung, Y.-K.; Oh, S.-H. Autophagy induction by capsaicin in malignant human breast cells is modulated by p38 and extracellular signal-regulated mitogen-activated protein kinases and retards cell death by suppressing endoplasmic reticulum stress-mediated apoptosis. Mol. Pharmacol. 2010, 78, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Chen, S.; Chien, S.; Kuo, S.; Tsai, H.; Chen, D. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum. Exp. Toxicol. 2011, 30, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, T.; Xu, G.; Luo, B.; Chen, Y.; Zhang, T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 2013, 60, 364–372. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, W.-H.; Park, J.-Y.; Kang, J.-H.; Kim, M.-O.; Kawada, T.; Yoo, H.; Han, I.-S.; Yu, R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor γ in HT-29 human colon cancer cells. J. Med. Food 2004, 7, 267–273. [Google Scholar] [CrossRef]
- Yang, K.; Pyo, J.; Kim, G.-Y.; Yu, R.; Han, I.; Ju, S.; Kim, W.; Kim, B.-S. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell. Mol. Biol. Lett. 2009, 14, 497–510. [Google Scholar] [CrossRef]
- Kim, M.Y.; Trudel, L.J.; Wogan, G.N. Apoptosis induced by capsaicin and resveratrol in colon carcinoma cells requires nitric oxide production and caspase activation. Anticancer. Res. 2009, 29, 3733–3740. [Google Scholar]
- Jin, J.; Lin, G.; Huang, H.; Xu, D.; Yu, H.; Ma, X.; Zhu, L.; Ma, D.; Jiang, H. Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. Int. J. Biol. Sci. 2014, 10, 285. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, M. Capsaicin modulates the immune cross talk between human mononuclears and cells from two colon carcinoma lines. Nutr. Cancer 2017, 69, 14–20. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; Camargo, M.C.; Schneider, B.G.; Sicinschi, L.A.; Hernández-Ramírez, R.U.; Correa, P.; Cebrian, M.E. Capsaicin consumption, Helicobacter pylori CagA status and IL1B-31C > T genotypes: A host and environment interaction in gastric cancer. Food Chem. Toxicol. 2012, 50, 2118–2122. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.-Y.; Lee, S.-M.; Jun, C.-H.; Cho, S.-B.; Park, C.-H.; Joo, Y.-E.; Kim, H.-S.; Choi, S.-K.; Rew, J.-S. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol. Med. Rep. 2014, 9, 499–502. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lee, Y.H.; Chen, H.Y.; Yeh, C.A.; Chueh, P.J.; Lin, Y.-M.J. Capsaicin inhibited aggressive phenotypes through downregulation of tumor-associated NADH oxidase (tNOX) by POU domain transcription factor POU3F2. Molecules 2016, 21, 733. [Google Scholar] [CrossRef]
- Ramos-Torres, Á.; Bort, A.; Morell, C.; Rodríguez-Henche, N.; Díaz-Laviada, I. The pepper’s natural ingredient capsaicin induces autophagy blockage in prostate cancer cells. Oncotarget 2015, 7, 1569. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Martínez-Botas, J.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Lasunción, M.A.; Díaz-Laviada, I. Induction of the endoplasmic reticulum stress protein GADD153/CHOP by capsaicin in prostate PC-3 cells: A microarray study. Biochem. Biophys. Res. Commun. 2008, 372, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Chiloeches, A.; Díaz-Laviada, I. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis 2007, 12, 2013–2024. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ko, J.-C.; Yen, T.-C.; Chen, T.-Y.; Lin, Y.-C.; Ma, P.-F.; Lin, Y.-W. Capsaicin enhances erlotinib-induced cytotoxicity via AKT inactivation and excision repair cross-complementary 1 (ERCC1) down-regulation in human lung cancer cells. Toxicol. Res. 2019, 8, 459–470. [Google Scholar] [CrossRef]
- Venier, N.A.; Colquhoun, A.J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate 2015, 75, 113–125. [Google Scholar] [CrossRef]
- Zhang, S.-s.; Ni, Y.-h.; Zhao, C.-r.; Qiao, Z.; Yu, H.-x.; Wang, L.-y.; Sun, J.-y.; Du, C.; Zhang, J.-h.; Dong, L.-y. Capsaicin enhances the antitumor activity of sorafenib in hepatocellular carcinoma cells and mouse xenograft tumors through increased ERK signaling. Acta Pharmacol. Sin. 2018, 39, 438–448. [Google Scholar] [CrossRef]
- Mori, A.; Lehmann, S.R.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef]
- Hong, Z.-F.; Zhao, W.-X.; Yin, Z.-Y.; Xie, C.-R.; Xu, Y.-P.; Chi, X.-Q.; Zhang, S.; Wang, X.-M. Capsaicin enhances the drug sensitivity of cholangiocarcinoma through the inhibition of chemotherapeutic-induced autophagy. PLoS ONE 2015, 10, e0121538. [Google Scholar] [CrossRef]
- Friedman, J.R.; Perry, H.E.; Brown, K.C.; Gao, Y.; Lin, J.; Stevenson, C.D.; Hurley, J.D.; Nolan, N.A.; Akers, A.T.; Chen, Y.C. Capsaicin synergizes with camptothecin to induce increased apoptosis in human small cell lung cancers via the calpain pathway. Biochem. Pharmacol. 2017, 129, 54–66. [Google Scholar] [CrossRef]
- Huh, H.-C.; Lee, S.-Y.; Lee, S.-K.; Park, N.H.; Han, I.-S. Capsaicin induces apoptosis of cisplatin-resistant stomach cancer cells by causing degradation of cisplatin-inducible Aurora-A protein. Nutr. Cancer 2011, 63, 1095–1103. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Yu, C.; Zhao, G.; Zhou, J.; Zhang, G.; Li, M.; Jiang, D.; Quan, Z.; Zhang, Y. Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts. J. Exp. Clin. Cancer Res. 2018, 37, 251. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L. Capsaicin enhances anti-proliferation efficacy of pirarubicin via activating TRPV1 and inhibiting PCNA nuclear translocation in 5637 cells. Mol. Med. Rep. 2016, 13, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.T.; Hickman, J.A.; Dive, C. Epigenetic determinants of resistance to etoposide regulation of Bcl-XL and Bax by tumor microenvironmental factors. J. Natl. Cancer Inst. 2000, 92, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Morelli, M.B.; Nabissi, M.; Cardinali, C.; Santoni, M.; Gismondi, A.; Santoni, G. Capsaicin triggers autophagic cell survival which drives epithelial mesenchymal transition and chemoresistance in bladder cancer cells in an Hedgehog-dependent manner. Oncotarget 2016, 7, 50180. [Google Scholar] [CrossRef] [PubMed]
- Vendrely, V.; Peuchant, E.; Buscail, E.; Moranvillier, I.; Rousseau, B.; Bedel, A.; Brillac, A.; de Verneuil, H.; Moreau-Gaudry, F.; Dabernat, S. Resveratrol and capsaicin used together as food complements reduce tumor growth and rescue full efficiency of low dose gemcitabine in a pancreatic cancer model. Cancer Lett. 2017, 390, 91–102. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M. Wine, polyphenols, and Mediterranean diets. What else is there to say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC analysis of phenols in negroamaro and primitivo red wines from Salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef]
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. In Proceedings of the International Conference on Efficient Production and Processing (ICEPP-2021), Virtual, 19–21 November 2021; E3S Web of Conferences. EDP Sciences: Les Ulis Cedex A, France, 2021; p. 01063. [Google Scholar]
- Robertson, I.; Hau, T.W.; Sami, F.; Ali, M.S.; Badgujar, V.; Murtuja, S.; Hasnain, M.S.; Khan, A.; Majeed, S.; Ansari, M.T. The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential. Int. J. Pharm. 2022, 618, 121605. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, K.; Cheng, L.; Yan, B.; Qian, W.; Cao, J.; Li, J.; Wu, E.; Ma, Q.; Yang, W. Resveratrol and cancer treatment: Updates. Ann. New York Acad. Sci. 2017, 1403, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, N.; Beretta, G.L. Resveratrol and prostate cancer: The power of phytochemicals. Curr. Med. Chem. 2021, 28, 4845–4862. [Google Scholar] [CrossRef]
- De Amicis, F.; Giordano, F.; Vivacqua, A.; Pellegrino, M.; Panno, M.L.; Tramontano, D.; Fuqua, S.A.; Ando, S. Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor α gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J. 2011, 25, 3695. [Google Scholar] [CrossRef]
- Giordano, F.; Montalto, F.I.; Panno, M.L.; Andò, S.; De Amicis, F. A Notch inhibitor plus Resveratrol induced blockade of autophagy drives glioblastoma cell death by promoting a switch to apoptosis. Am. J. Cancer Res. 2021, 11, 5933. [Google Scholar]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kemper, C.; Behnam, D.; Brothers, S.; Wahlestedt, C.; Volmar, C.-H.; Bennett, D.; Hayward, M. Safety and pharmacokinetics of a highly bioavailable resveratrol preparation (JOTROL TM). AAPS Open 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Bagherian, M.; Torabi, S.M.; Sharifzadeh, S.O.; Hushmandi, K.; Fives, K.R.; Khan, H. Resveratrol augments doxorubicin and cisplatin chemotherapy: A novel therapeutic strategy. Curr. Mol. Pharmacol. 2023, 16, 280–306. [Google Scholar]
- Carlson, L.J.; Cote, B.; Alani, A.W.; Rao, D.A. Polymeric micellar co-delivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. J. Pharm. Sci. 2014, 103, 2315–2322. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lee, C.-C.; Shih, Y.-L.; Lin, T.-Y.; Wang, S.-H.; Lin, Y.-F.; Shih, C.-M. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free. Radic. Biol. Med. 2012, 52, 377–391. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Dong, Y.; Lin, S.; Guan, W.; Song, J. Resveratrol as a cardioprotective adjuvant for 5-fluorouracil in the treatment of gastric cancer cells. Braz. J. Med. Biol. Res. 2024, 57, e13537. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Silvestrini, A.; Mancini, A. The Double-Edged Sword of Total Antioxidant Capacity: Clinical Significance and Personal Experience. Antioxidants 2024, 13, 933. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).