Abstract
CD36, a fatty acid scavenger receptor expressed in tumors, is associated with a poor prognosis in several cancers. Our previous research demonstrated the involvement of CD36 in the proliferation and migration of oral squamous cell carcinoma (OSCC) cells. However, the clinical significance of CD36 expression in OSCC remains unclear. The purpose of this study was to evaluate the association between CD36 expression and the clinicopathological characteristics of OSCC patients. Immunohistochemical expression of CD36 was quantified using the H-score, and its association with clinicopathological characteristics was evaluated in 55 OSCC patients. The mean H-score for membrane-associated CD36 expression was 84.8. CD36 expression was significantly correlated with tumor stage, mode of invasion, differentiation, and recurrence of OSCC cells. Moreover, elevated CD36 expression was significantly correlated with a high rate of relapse. Univariate and multivariate analyses showed that CD36 expression was an independent risk factor for relapse. Moreover, The Cancer Genome Atlas (TCGA) dataset analysis revealed that CD36 expression may coexist with transcriptional activation of β-oxidation-related and epithelial–mesenchymal transition (EMT)-related pathways. These findings suggest that CD36 might serve as a predictive biomarker for OSCC malignancy and recurrence.
1. Introduction
Oral cancer, categorized as a type of head and neck cancer, ranks as the sixteenth most common malignancy worldwide [1]. Over 90% of oral cancers are squamous cell carcinomas [2]. Recent advancements in novel treatment strategies, such as molecular targeted therapy and immunotherapy, have shown significant promise in oral cancer treatment [3]. However, their clinical efficacy remains unsatisfactory in some patients because of drug resistance or acquired tolerance; therefore, the identification of alternative targetable molecules and the development of new treatment strategies are imperative.
CD36, a fatty acid membrane-associated receptor of approximately 80 kDa, binds to ligands such as fatty acids and lipoproteins [4]. CD36 is abundantly expressed in adipocytes, hepatocytes, and macrophages and is strongly associated with fat metabolism, taste perception, and the development of metabolic diseases such as diabetes and obesity [4]. Moreover, recent studies have revealed that the expression of CD36 in tumor cells may correlate with treatment outcomes and prognosis in various tumor types, including esophageal squamous cell carcinoma, lung squamous cell carcinoma, bladder cancer, luminal A breast cancer, and glioblastoma [5,6,7,8], suggesting that this molecule contributes to tumor progression.
Our previous study demonstrated that CD36 plays a role in the proliferation and migration of oral squamous cell carcinoma (OSCC), suggesting its potential as a novel therapeutic target for oral cancer [9]. However, the correlation between CD36 expression and the clinicopathological characteristics of OSCC remains unclear. Therefore, this study aimed to evaluate the association between CD36 expression and the clinicopathological characteristics of OSCC. Moreover, CD36 has been reported to be biologically associated with β-oxidation and epithelial–mesenchymal transition (EMT) [10,11]. Hence, we aimed to elucidate correlation with CD36 expression and genes related to fatty acid β-oxidation (PPARA, ACADL, TXNIP) and EMT (ZEB1, ARG1) using The Cancer Genome Atlas (TCGA) dataset analysis.
2. Results
2.1. Patients and Characteristics
The clinicopathological characteristics of patients are presented in Table 1. Of the 55 patients, 26 were male and 29 were female, with a median age of 73.0 years (range, 31–92 years). The median follow-up time for survivors was 22.0 months (7–152 months).

Table 1.
Correlation between CD36 expression and clinicopathological characteristics in patients with OSCC.
The primary sites included the tongue (n = 32, 58%), mandibular gingiva (n = 10, 18%), maxillary gingiva (n = 9, 16%), floor of the mouth (n = 2, 4%), buccal mucosa (n = 1, 2%), and lips (n = 1, 2%). Based on the Union for International Cancer Control (UICC) TNM classification criteria for oral cavity cancer, 8th edition [12], 22 (40%) patients were diagnosed with stage I, 17 (30%) with stage II, 8 (15%) with stage III, and 8 (15%) with stage IV. Histopathologically, OSCCs were classified as well differentiated (n = 26, 47%), moderately differentiated (n = 20, 36%), or poorly differentiated (n = 9, 16%), based on the World Health Organization classification [13]. The mode of invasion at the invasive front of the tumor was classified based on the criteria of Yamamoto et al. as grade 1 (n = 3, 5%), grade 2 (n = 16, 29%), grade 3 (n = 21, 38%), and grade 4C/4D (n = 15, 27%) [14]. Lymphovascular invasion was observed in 8 (15%), and perineural invasion in 3 (5%). Representative membrane-associated CD36 and H-E-stained photographs of the mode of invasion classification are shown in Figure 1. The mean H-scores for CD36 expression were 84.8 ± 46.9.
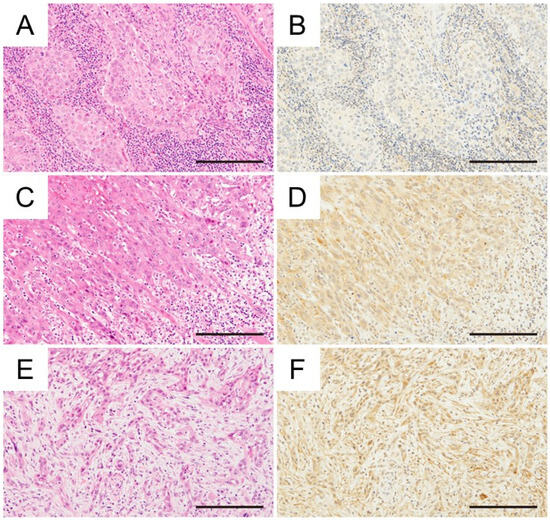
Figure 1.
Immunohistochemical investigation between the mode of invasion and CD36 expression in OSCC (H&E staining, immunohistochemical CD36 staining) (A,B) Grade 3, tongue cancer, H-score: 77.0. (C,D) Grade 4C, tongue cancer, H-score: 125.6. (E,F) Grade 4D, tongue cancer, H-score: 135.2. Magnification ×400, scale bar: 200 μm.
2.2. Correlations Between CD36 Expression and the Clinicopathological Characteristics of Patients with OSCC
The results from the analysis of the correlations between CD36 expression and the clinicopathological characteristics of patients with OSCC are presented in Table 1. High CD36 expression was significantly associated with the primary site (p = 0.0011), tumor stage (p = 0.026), differentiation (p = 0.013), mode of invasion (p = 0.0034), and recurrence (p = 0.0004).
2.3. Correlation Between CD36 Expression and Survival in Patients with OSCC After Treatment
The patient population was divided into two groups based on the median H-score (79.5) and analyzed according to CD36 expression, respectively. Overall survival (OS) rates showed no significant difference (p = 0.42, Figure 2). However, relapse-free survival (DFS) rates were significantly lower in patients with high CD36 expression than in those with low CD36 expression (p = 0.0002, Figure 3). Univariate analysis identified several significant prognostic factors for poor overall survival, including the T factor (p = 0.018), N factor (p = 0.048), tumor stage (p = 0.028), and mode of invasion (p = 0.04) (Table 2). CD36 expression was not relevant to overall survival. For relapse, significant prognostic factors included the T factor (p = 0.0014), N factor (p = 0.017), tumor stage (p = 0.016), mode of invasion (p < 0.0001), and CD36 expression (p = 0.0008) (Table 3). Moreover, multivariate analysis showed that the mode of invasion (p = 0.04, HR = 10.02, 95%CI = 1.48–197.7) was an independent risk factor for poor overall survival, whereas the mode of invasion (p = 0.04, HR = 2.62, 95%CI = 1.07–6.86) and CD36 expression (p = 0.036, HR = 3.29, 95%CI = 1.14–10.93) were independent risk factors for relapse (Table 2 and Table 3).
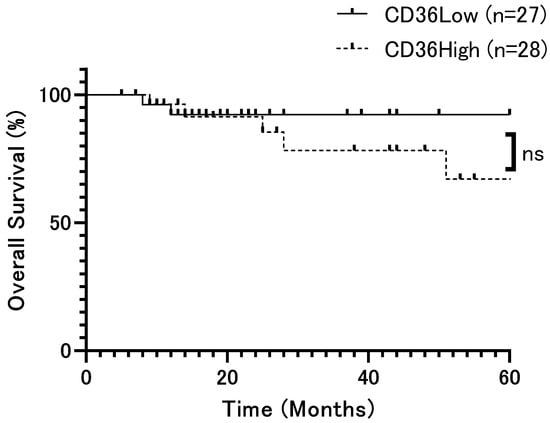
Figure 2.
Overall survival rate of patients with OSCC according to CD36 expression (p = 0.42). Overall survival rates showed no significant difference. The x-axis represents time (months), and the y-axis represents overall survival (%). ns: not significant.
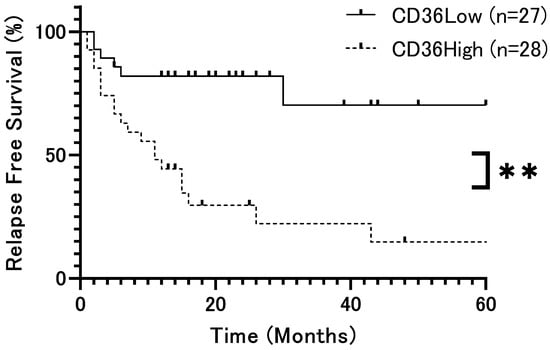
Figure 3.
Relapse-free survival rate of patients with OSCC according to CD36 expression (p = 0.0002). Relapse-free survival rates were significantly lower in patients with high CD36 expression than in those with low CD36 expression. The x-axis represents time (months), and the y-axis represents relapse-free survival (%). **: p < 0.01.

Table 2.
Results of univariate and multivariate analyses of clinicopathological factors affecting overall survival rates following surgery.

Table 3.
Results of univariate and multivariate analyses of clinicopathological factors affecting relapse-free survival rates following surgery.
2.4. Correlation Between CD36 Expression and Related Molecular Profiles in Head and Neck Cancer from the TCGA Datasets
To further explore the molecular context of CD36, we analyzed RNA-seq data from 288 patients with head and neck cancer in TCGA Head and Neck Squamous Cell Carcinoma (HNSC) cohort. Genes related to fatty acid β-oxidation (PPARA, ACADL, TXNIP) and EMT (ZEB1, ARG1) were selected a priori because β-oxidation and EMT have been reported to be biologically associated with CD36 [10,15,16,17,18,19,20].
Spearman’s rank correlation analysis revealed modest but statistically significant positive associations between CD36 and several genes: ACADL (ρ = 0.29, p < 0.0001), ARG1 (ρ = 0.41, p < 0.0001), ZEB1 (ρ = 0.32, p < 0.0001), PPARA (ρ = 0.29, p < 0.0001), and TXNIP (ρ = 0.26, p < 0.0001) (Figure 4 and Figure 5).
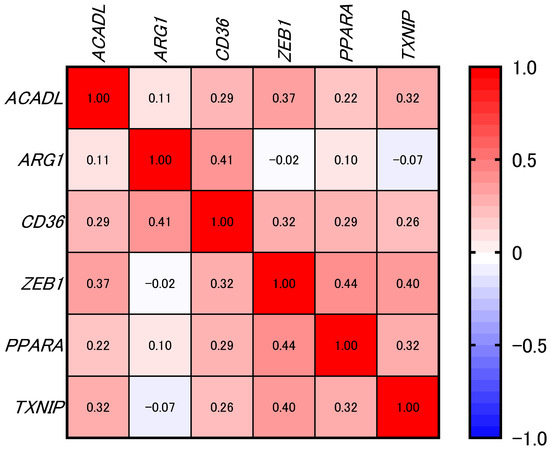
Figure 4.
Heatmap showing the correlations between CD36 and selected genes of interest (not filtered by significance) in samples from the TCGA-HNSC dataset (n = 288). The numbers inside the cells of the heatmap represent correlation coefficients. The color key on the right shows the range of Spearman’s rank correlation coefficients (ρ), with red indicating values close to 1 (positive correlations) and blue indicating values close to −1 (negative correlations). Correlation was calculated using Spearman’s rank correlation test.
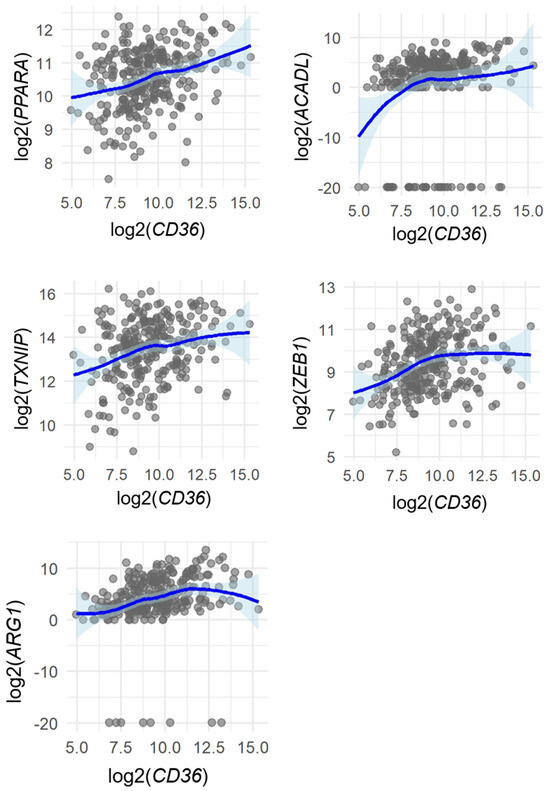
Figure 5.
Spearman’s rank correlations between CD36 and selected genes (PPARA, ACADL, TXNIP, ZEB1, and ARG1) in samples from the TCGA-HNSC dataset (n = 288). Each dot represents one sample. LOESS (locally weighted regression) curves with 95% confidence intervals are shown to visualize overall trends without assuming linearity. Correlation strength and p-values were determined using Spearman’s rank correlation coefficient (ρ).
These results suggest that CD36 expression may coexist with transcriptional activation of β-oxidation-related and EMT-related pathways, although the correlations were modest and do not imply causation.
3. Discussion
CD36 has been shown to play a significant role in various pro-tumor functions, including the regulation of proliferation, metastasis, resistance to chemotherapy or radiotherapy, and angiogenesis [21,22]. Moreover, recent clinical studies have shown a correlation between high CD36 expression and a poor prognosis in several cancers, including esophageal squamous cell carcinoma, gastric cancer, lung squamous cell carcinoma, bladder cancer, luminal A breast cancer, glioblastoma, and acute myeloid leukemia [5,6,7,8,21,23]. However, the correlation between CD36 expression and the clinicopathological characteristics of oral cancer remains unclear. The purpose of this study was to examine the clinicopathological role of CD36 in OSCC. Consequently, high CD36 expression was significantly correlated with high malignancy in oral cancer.
Our previous study highlighted the biological activity of CD36, including its involvement in the proliferation and migration of OSCC cells [9]. More recently, we demonstrated that selective inhibition of CD36 can induce antitumor immunomodulatory effects in a mouse model of oral cancer [24]. Furthermore, the regulatory function of CD36 in EMT has been demonstrated in hepatocellular carcinoma and cervical cancer cells [11,20]. Taken together, in OSCC, CD36 is correlated to high malignancy and may be associated with EMT and become a potential diagnostic biomarker or therapeutic target.
In this clinicopathological study, high levels of CD36 immunohistochemical expression were significantly associated with poor histological differentiation and high-grade 4C/4D mode of invasion. Moreover, patients with high levels of CD36 expression showed a high rate of recurrence. Multivariate analysis revealed high CD36 expression and high-grade 4C/4D mode of invasion as a significant prognostic factor for relapse. A significant difference in relapse-free survival was observed, but not in overall survival. This may be explained by the fact that most patients had early-stage cancer, few patients relapsed and subsequently died after additional treatment, and the follow-up period was relatively short.
The possible mechanism by which CD36 affects recurrence is the cancellation of dormancy for cancer cells due to oxidative stress. Increased fatty acid storage due to high CD36 expression promotes β-oxidation, which results in increased reactive oxygen species (ROS) and oxidative stress [25]. Oxidative stress is a candidate factor for recurrence of dormant cancer cells [26]. High expression of CD36 may be indicator of recurrence in OSCC. For instance, in papillary thyroid cancer, high expression of CD36 on macrophages increases the recurrence rate [27]. It has also been reported that high expression of CD36 contributes to an increased recurrence rate in pancreatic ductal adenocarcinoma and acute myeloid leukemia [23,28]. On the other hand, in this study, high-grade 4C/4D mode of invasion was also associated with recurrence. Previous studies have shown that the high-grade 4C/4D mode of tumor invasion is associated with high malignancy and metastasis [14,29]. Phenotypic alterations like cell scattering occur via EMT; therefore, the mode of invasion may be related to EMT [30].
Through EMT, epithelial markers such as E-cadherin are downregulated, while mesenchymal markers and cellular motility are enhanced, leading to increased local invasion and changes in the pattern of infiltration, such as cord-like or diffuse invasion corresponding to grade 4C/4D mode of invasion [31,32]. These features are considered factors contributing to an increased risk of local recurrence or metastasis after surgical resection. However, EMT and histological dedifferentiation are distinct concepts. This is consistent with the results of the multivariate analysis of this study.
To further understand the molecular context of CD36, we performed correlation analyses using TCGA data. Interestingly, this analysis revealed that PPARA, ACADL, TXNIP, ZEB1, and ARG1 showed a significant positive correlation with CD36 expression. PPARA and ACADL are related to β-oxidation [15,16]. TXNIP is involved in the production of ROS [17]. These molecules are significantly correlated with CD36, and high expression of CD36 may increase β-oxidation and ROS production, which may contribute to the recurrence of dormant cancer cells. Moreover, amplification of ZEB1 or ARG1 is relevant to EMT or metastasis [18,19]. Correlation analysis suggests that EMT-related molecules such as ZEB1 and ARG1 may be co-expressed with CD36. These findings indicate that high CD36 expression is associated with EMT. Additionally, high CD36 expression may be correlated to poor histological differentiation and an aggressive invasion pattern (grade 4C/4D mode of invasion). Further studies are required to clarify the underlying causal mechanisms.
This study has several limitations. First, the sample size was relatively small and restricted to surgically resectable cases, which may limit the generalizability of the findings. Second, the TCGA analysis was based solely on mRNA expression and did not include protein-level validation. Although CD36 expression showed positive correlations with EMT-related genes such as ZEB1 and ARG1, these results only indicate possible transcriptional co-expression and do not establish a causal relationship. Therefore, functional studies are required to clarify whether CD36 directly regulates EMT or invasion pathways in OSCC.
In conclusion, our results demonstrated that CD36 is associated with OSCC malignancy and may serve as a novel diagnostic biomarker. However, no association with overall survival was observed, suggesting that CD36 may have limited significance as a prognostic predictor. The possibility that CD36 expression is involved in the mechanism of recurrence is clinically important and provides evidence that will serve as a basis for future functional studies and consideration as a therapeutic target.
4. Materials and Methods
4.1. Patients
This study included 55 patients who underwent surgical treatment for OSCC at the Department of Oral and Maxillofacial Surgery, University of Toyama Hospital, between April 2009 and March 2024. The inclusion criterion was a cytological or histopathological diagnosis of OSCC, and inclusion was also limited by sample availability. No predefined exclusion criteria were applied. Nevertheless, none of the tissue specimens showed positive surgical margins, and no patients in our cohort had received preoperative chemotherapy or radiotherapy or had immunosuppression or other conditions likely to substantially affect survival or molecular profiles. Medical records were retrospectively examined for age, sex, body mass index (BMI), primary site, tumor stage, T factor, N factor, differentiation, mode of invasion, lymphovascular invasion, perineural invasion and recurrence. The tumor extent and the histopathological grading were classified based on the UICC TNM classification criteria for oral cavity cancer, 8th edition [12]. The mode of invasion at the invasive front of the tumor was classified according to Yamamoto et al. [14]. This study was conducted in accordance with the ethical guidelines outlined in the Declaration of Helsinki and was approved by Research Ethics Committee for Clinical and Epidemiological Research of Toyama University (Approval No. R2018168; Approval Date 19 April 2019). Owing to the retrospective, observational nature of this study, informed consent was obtained using an opt-in/opt-out approach.
4.2. Immunohistochemistry
Immunohistochemical analysis of paraffin-embedded samples was performed. The thickness of the sections was 4 μm. For antigen retrieval, the sections were immersed in citric acid buffer (pH 6.0) and heated at 121 °C for 10 min. The deparaffinized and rehydrated sections were immersed in 0.3% hydrogen peroxide in methanol for 10 min to block endogenous peroxidase activity. To prevent nonspecific binding of the antibodies, the sections were immersed in a blocking solution (Nacalai Tesque, Kyoto, Japan) for 15 min. Subsequently, the sections were incubated with the primary antibody (anti-CD36 rabbit monoclonal antibody, 1:100; clone EPR6573; Abcam, Cambridge, UK) overnight at 4 °C. After incubation, the sections were immersed in phosphoric acid thrice for 3 min. Peroxidase reactions were developed using a 3,3’-diaminobenzidine tetrahydrochloride substrate solution (DAKO, Glostrup, Denmark), and the sections were counterstained with hematoxylin. The same primary antibody was used as a positive control.
4.3. Quantification of CD36 Expression with Immunoreactivity Scoring
CD36 expression was quantified based on H-score [33]. Immunohistochemically stained specimens were captured to create virtual slides, and immunoreactivity was analyzed using Aperio analysis software (Leica Biosystems, Tokyo, Japan) at ×20 magnification with objective lens. Expression intensity was categorized into four stages: no expression, 0; weak, 1+; moderate, 2+; and strong, 3+. The distribution of expression was presented as a percentage of the number of cells (numerator) relative to the total number of cells in the analysis area (denominator). The H-score was calculated using the following formula: H-score (100−300) = (% staining area of “1+”) × 1 + (% staining area of “2+”) × 2 + (% staining area of “3+”) × 3.
4.4. Statistical Analysis
Comparisons were made using the Mann–Whitney U test or Kruskal–Wallis test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Correlations were analyzed using Spearman’s rank correlation. Survival curves were generated using the Kaplan–Meier method, and differences between overall survival curves were evaluated using the log-rank test. Univariate and multivariate analyses were performed using Cox’s proportional hazards model. Differences were considered statistically significant at p < 0.05. All statistical analyses were performed using GraphPad Prism software v.9 (GraphPad Software, Boston, MA, USA).
4.5. Acquisition and Analysis of TCGA Data
A total of 288 RNA-seq data from the TCGA-HNSC cohort were obtained via the Genomic Data Commons (GDC) portal [34]. TCGA data were accessed on October 14, 2025. As a breakdown, oral cavity, oropharyngeal, laryngeal/hypopharyngeal and unknown samples included 172, 16, 59, and 41 cases, respectively. Gene-level expression (STAR-counts workflow) was normalized, log2-transformed, and analyzed using R software. Spearman’s rank correlation coefficients (ρ) were calculated to assess monotonic relationships between CD36 and candidate genes related to fatty acid β-oxidation (PPARA, ACADL, TXNIP) or EMT/metastasis (ZEB1, ARG1). Scatter plots were visualized using LOESS smoothing with 95% confidence intervals to illustrate overall expression trends.
Author Contributions
K.S.: Conceptualization, data curation, methodology, formal analysis, and writing—review and editing. K.T.: Conceptualization, data curation, methodology, project administration, and writing—original draft, and writing—review and editing. M.Y.: Conceptualization, methodology, visualization, and writing—review and editing. J.-i.T.: Conceptualization, methodology, and writing—review and editing. S.-i.Y.: Conceptualization, methodology, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number JP24K19990.
Institutional Review Board Statement
This study was conducted in accordance with the ethical guidelines outlined in the Declaration of Helsinki and was approved by Research Ethics Committee for Clinical and Epidemiological Research of Toyama University (Approval No. R2018168; Approval Date 19 April 2019).
Informed Consent Statement
Owing to the retrospective, observational nature of this study, informed consent was obtained using an opt-in/opt-out approach.
Data Availability Statement
The dataset is available at https://doi.org/10.6084/m9.figshare.28254308.v1.
Acknowledgments
Pathological contents were supported by Pathology Institute Corp. (Toyama city, Toyama, Japan). We would like to thank Editage (www.editage.com) for English language editing.
Conflicts of Interest
The authors declare no conflicts of interest for this article.
Abbreviations
The following abbreviations are used in this manuscript:
| OSCC | Oral squamous cell carcinoma |
| EMT | Epithelial–mesenchymal transition |
| ROS | Reactive oxygen species |
References
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Kujan, O.; van Schaijik, B.; Farah, C.S. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Cancers 2020, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal 2009, 2, re3. [Google Scholar] [CrossRef]
- Hale, J.S.; Otvos, B.; Sinyuk, M.; Alvarado, A.G.; Hitomi, M.; Stoltz, K.; Wu, Q.; Flavahan, W.; Levison, B.; Johansen, M.L.; et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells 2014, 32, 1746–1758. [Google Scholar] [CrossRef]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Watt, M.J.; Clark, A.K.; Selth, L.A.; Haynes, V.R.; Lister, N.; Rebello, R.; Porter, L.H.; Niranjan, B.; Whitby, S.T.; Lo, J.; et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 2019, 11, eaau5758. [Google Scholar] [CrossRef]
- Yoshida, T.; Yokobori, T.; Saito, H.; Kuriyama, K.; Kumakura, Y.; Honjo, H.; Hara, K.; Sakai, M.; Miyazaki, T.; Obinata, H.; et al. CD36 Expression Is Associated with Cancer Aggressiveness and Energy Source in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2021, 28, 1217–1227. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomihara, K.; Yamazaki, M.; Heshiki, W.; Moniruzzaman, R.; Sekido, K.; Tachinami, H.; Ikeda, A.; Imaue, S.; Fujiwara, K.; et al. CD36 expression on oral squamous cell carcinoma cells correlates with enhanced proliferation and migratory activity. Oral Dis. 2020, 26, 745–755. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Nath, A.; Li, I.; Roberts, L.R.; Chan, C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci. Rep. 2015, 5, 14752. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Yamamoto, E.; Kohama, G.; Sunakawa, H.; Iwai, M.; Hiratsuka, H. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer 1983, 51, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.N.; Patel, D.P.; Velenosi, T.J.; Kim, D.; Yan, T.; Yue, J.; Li, G.; Krausz, K.W.; Gonzalez, F.J. Extrahepatic PPARα modulates fatty acid oxidation and attenuates fasting-induced hepatosteatosis in mice. J. Lipid Res. 2018, 59, 2140–2152. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Tan, X.Y.; Pan, Y.X.; Zhang, L.H.; Chen, Q.L. Fatty Acid β-Oxidation Is Essential in Leptin-Mediated Oocytes Maturation of Yellow Catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2018, 19, 1457. [Google Scholar] [CrossRef]
- Li, X.; Rong, Y.; Zhang, M.; Wang, X.L.; LeMaire, S.A.; Coselli, J.S.; Zhang, Y.; Shen, Y.H. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem. Biophys. Res. Commun. 2009, 381, 660–665. [Google Scholar] [CrossRef]
- Krebs, A.M.; Mitschke, J.; Lasierra Losada, M.; Schmalhofer, O.; Boerries, M.; Busch, H.; Boettcher, M.; Mougiakakos, D.; Reichardt, W.; Bronsert, P.; et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017, 19, 518–529. [Google Scholar] [CrossRef]
- Hofmann, L.; Harasymczuk, M.; Huber, D.; Szczepanski, M.J.; Dworacki, G.; Whiteside, T.L.; Theodoraki, M.N. Arginase-1 in Plasma-Derived Exosomes as Marker of Metastasis in Patients with Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 5449. [Google Scholar] [CrossRef]
- Deng, M.; Cai, X.; Long, L.; Xie, L.; Ma, H.; Zhou, Y.; Liu, S.; Zeng, C. CD36 promotes the epithelial-mesenchymal transition and metastasis in cervical cancer by interacting with TGF-β. J. Transl. Med. 2019, 17, 352. [Google Scholar] [CrossRef]
- Aoki, T.; Kinoshita, J.; Munesue, S.; Hamabe-Horiike, T.; Yamaguchi, T.; Nakamura, Y.; Okamoto, K.; Moriyama, H.; Nakamura, K.; Harada, S.; et al. Hypoxia-Induced CD36 Expression in Gastric Cancer Cells Promotes Peritoneal Metastasis via Fatty Acid Uptake. Ann. Surg. Oncol. 2023, 30, 3125–3136. [Google Scholar] [CrossRef]
- Guerrero-Rodríguez, S.L.; Mata-Cruz, C.; Pérez-Tapia, S.M.; Velasco-Velázquez, M.A. Role of CD36 in cancer progression, stemness, and targeting. Front. Cell Dev. Biol. 2022, 10, 1079076. [Google Scholar] [CrossRef]
- Farge, T.; Nakhle, J.; Lagarde, D.; Cognet, G.; Polley, N.; Castellano, R.; Nicolau, M.L.; Bosc, C.; Sabatier, M.; Sahal, A.; et al. CD36 Drives Metastasis and Relapse in Acute Myeloid Leukemia. Cancer Res. 2023, 83, 2824–2838. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, M.; Tachinami, H.; Takatsuka, D.; Yonesi, A.; Sakurai, K.; Rasul, M.I.; Imaue, S.; Yamada, S.I.; Ruslin, M.; Yamazaki, M.; et al. Targeting CD36-Mediated Lipid Metabolism by Selective Inhibitor-Augmented Antitumor Immune Responses in Oral Cancer. Int. J. Mol. Sci. 2024, 25, 9438. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology 2016, 157, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Havas, K.M.; Milchevskaya, V.; Radic, K.; Alladin, A.; Kafkia, E.; Garcia, M.; Stolte, J.; Klaus, B.; Rotmensz, N.; Gibson, T.J.; et al. Metabolic shifts in residual breast cancer drive tumor recurrence. J. Clin. Investig. 2017, 127, 2091–2105. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Tian, W.; Yang, Y.; Yin, Y.; Qiu, Y.; Wang, W.; Li, Y.; Zhang, G.; Zhao, X.; et al. CD36+ Proinflammatory Macrophages Interact with ZCCHC12+ Tumor Cells in Papillary Thyroid Cancer Promoting Tumor Progression and Recurrence. Cancer Immunol. Res. 2024, 12, 1621–1639. [Google Scholar] [CrossRef]
- Kubo, M.; Gotoh, K.; Eguchi, H.; Kobayashi, S.; Iwagami, Y.; Tomimaru, Y.; Akita, H.; Asaoka, T.; Noda, T.; Takeda, Y.; et al. Impact of CD36 on Chemoresistance in Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 610–619. [Google Scholar] [CrossRef]
- Shimizu, S.; Miyazaki, A.; Sonoda, T.; Koike, K.; Ogi, K.; Kobayashi, J.I.; Kaneko, T.; Igarashi, T.; Ueda, M.; Dehari, H.; et al. Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma: With special reference to the mode of invasion and worst pattern of invasion. PLoS ONE 2018, 13, e0195451. [Google Scholar] [CrossRef]
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef]
- Kaihara, T.; Kusaka, T.; Kawamata, H.; Oda, Y.; Fujii, S.; Morita, K.; Imura, J.; Fujimori, T. Decreased expression of E-cadherin and Yamamoto-Kohama’s mode of invasion highly correlates with lymph node metastasis in esophageal squamous cell carcinoma. Pathobiology 2001, 69, 172–178. [Google Scholar] [CrossRef]
- Li, D.; Xia, L.; Huang, P.; Wang, Z.; Guo, Q.; Huang, C.; Leng, W.; Qin, S. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Prolif. 2023, 56, e13423. [Google Scholar] [CrossRef]
- McCarty, K.S., Jr.; Miller, L.S.; Cox, E.B.; Konrath, J.; McCarty, K.S., Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).