From Development, Disease, and Decline: A Review of What Defines an Osteoclast Progenitor
Abstract
1. Introduction
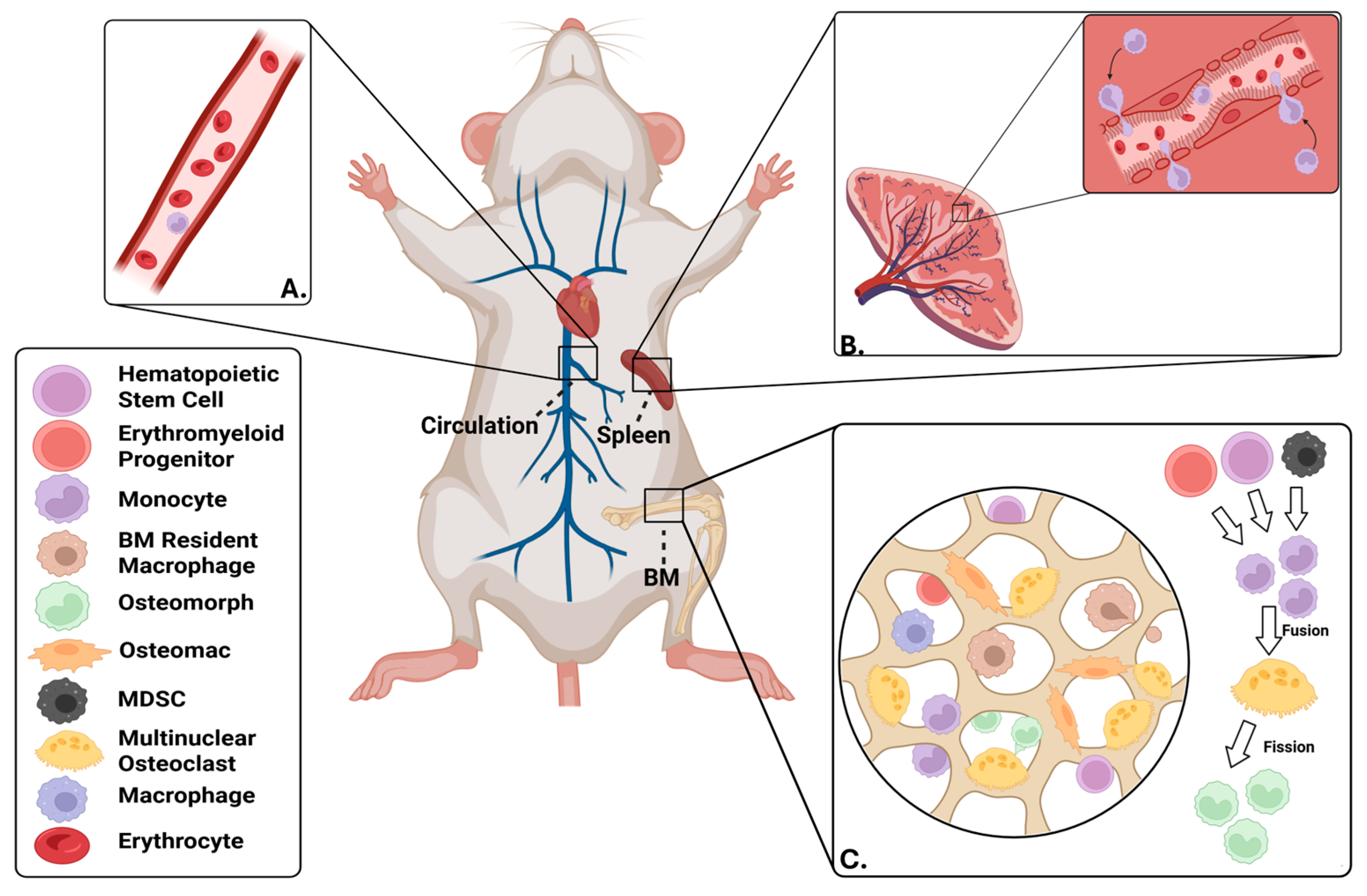
2. Sources of Osteoclast Progenitors
2.1. Developmental Origins of Osteoclast Progenitors
2.2. Bone Marrow-Resident OCPs
2.3. OCPs Within the Circulation
2.4. Non-Canonical Osteoclast Progenitors
3. Defining Surface Markers of Osteoclast Progenitors
4. OCP Changes During Disease, Injury, and Age-Associated Bone Loss
4.1. Changes to Osteoclast Progenitors with Advanced Age
4.2. Osteoclast Progenitors Participating in Bone Healing Following Injury
4.3. Osteoclast Progenitors in Rheumatoid Arthritis
4.4. Osteoclast Progenitors in Periodontitis
4.5. Impacts of Trained Immunity on Osteoclast Progenitors
4.6. Osteoclast Progenitors in Osteoarthritis (OA)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarafrazi, N.; Wambogo, E.A.; Shepherd, J.A. Osteoporosis or Low Bone Mass in Older Adults: United States, 2017–2018. NCHS Data Brief 2021, 1–8. [Google Scholar] [PubMed]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Kats, A.M.; Boyd, C.M.; Diem, S.J.; Schousboe, J.T.; Taylor, B.C.; Bauer, D.C.; Stone, K.L.; Langsetmo, L.; Study of Osteoporotic Fractures Research, G. Association of Disease Definition, Comorbidity Burden, and Prognosis With Hip Fracture Probability Among Late-Life Women. JAMA Intern. Med. 2019, 179, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Sing, C.W.; Lin, T.C.; Bartholomew, S.; Bell, J.S.; Bennett, C.; Beyene, K.; Bosco-Levy, P.; Bradbury, B.D.; Chan, A.H.Y.; Chandran, M.; et al. Global Epidemiology of Hip Fractures: Secular Trends in Incidence Rate, Post-Fracture Treatment, and All-Cause Mortality. J. Bone Miner. Res. 2023, 38, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.I.; Switzer, J.A. AAOS Clinical Practice Guideline Summary: Management of Hip Fractures in Older Adults. J. Am. Acad. Orthop. Surg. 2022, 30, e1291–e1296. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hodges, N.A.; Sussman, E.M.; Stegemann, J.P. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials 2021, 278, 121127. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coury, F.; Peyruchaud, O.; Machuca-Gayet, I. Osteoimmunology of Bone Loss in Inflammatory Rheumatic Diseases. Front. Immunol. 2019, 10, 679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lukac, N.; Katavic, V.; Novak, S.; Sucur, A.; Filipovic, M.; Kalajzic, I.; Grcevic, D.; Kovacic, N. What do we know about bone morphogenetic proteins and osteochondroprogenitors in inflammatory conditions? Bone 2020, 137, 115403. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Graves, D.T. Impact of the host response and osteoblast lineage cells on periodontal disease. Front. Immunol. 2022, 13, 998244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
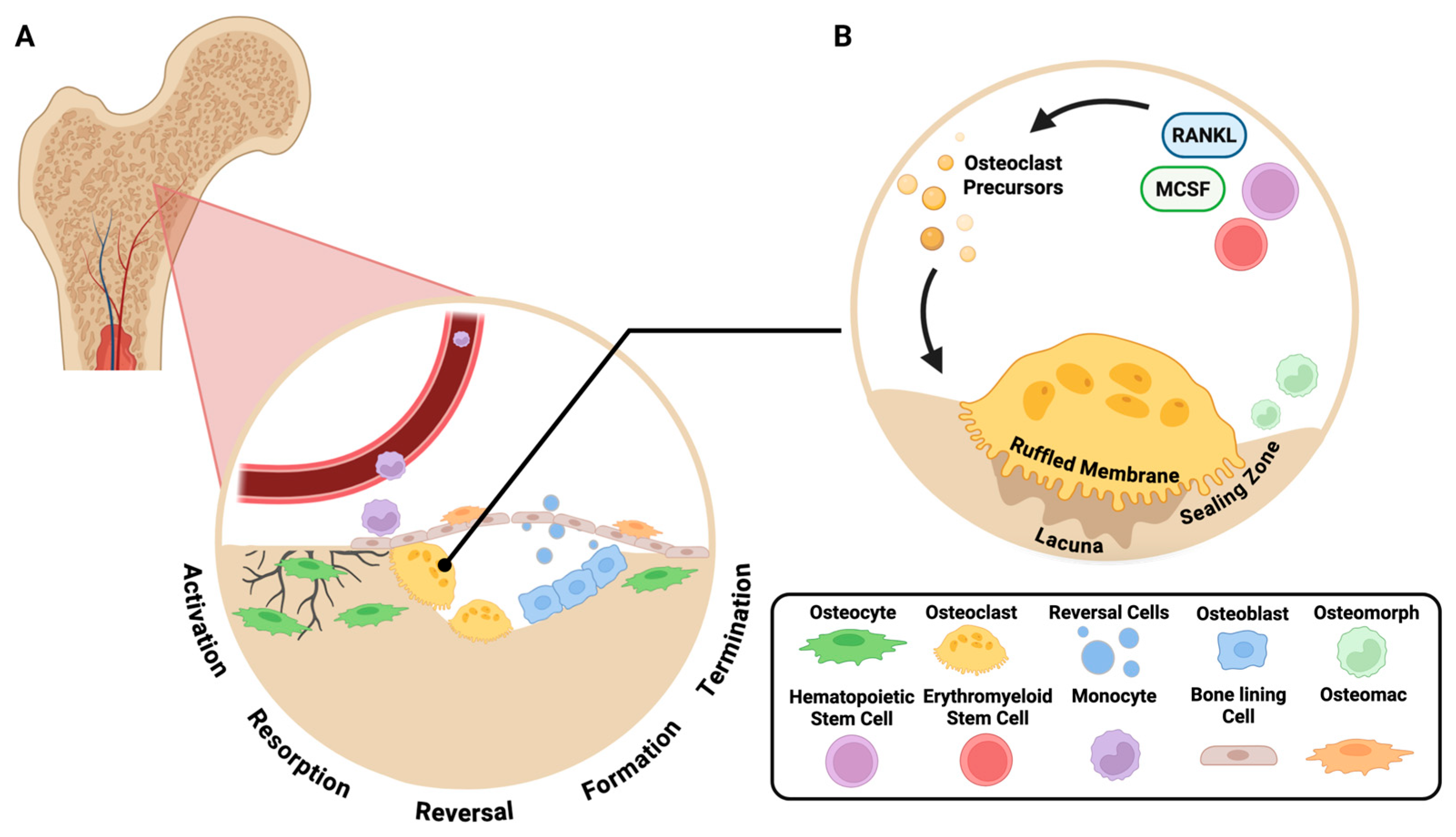
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weivoda, M.M.; Bradley, E.W. Macrophages and Bone Remodeling. J. Bone Miner. Res. 2023, 38, 359–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Nemoto, Y.L.; Oikawa, T.; Takano, K.; Fujiwara, T.K.; Tsujita, K.; Itoh, T. Mechanical control of osteoclast fusion by membrane-cortex attachment and BAR proteins. J. Cell. Biol. 2025, 224, e202411024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med. J. 2016, 52, 12–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011, 343, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Delaisse, J.M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347 e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, M.K.; Raggatt, L.J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Matsuo, K.; Fox, S.W.; Fuller, K.; Gibson, F.M.; Draycott, G.; Wani, M.R.; Bayley, K.E.; Wong, B.R.; Choi, Y.; et al. Osteoclast lineage commitment of bone marrow precursors through expression of membrane-bound TRANCE. Bone 2000, 27, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.P.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Aguas, A.P.; Barbosa, J.N. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials 2015, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ballet, J.J.; Griscelli, C.; Coutris, C.; Milhaud, G.; Maroteaux, P. Bone-marrow transplantation in osteopetrosis. Lancet 1977, 2, 1137. [Google Scholar] [CrossRef] [PubMed]
- Coccia, P.F.; Krivit, W.; Cervenka, J.; Clawson, C.; Kersey, J.H.; Kim, T.H.; Nesbit, M.E.; Ramsay, N.K.; Warkentin, P.I.; Teitelbaum, S.L.; et al. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N. Engl. J. Med. 1980, 302, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Sorell, M.; Kapoor, N.; Kirkpatrick, D.; Rosen, J.F.; Chaganti, R.S.; Lopez, C.; Dupont, B.; Pollack, M.S.; Terrin, B.N.; Harris, M.B.; et al. Marrow transplantation for juvenile osteopetrosis. Am. J. Med. 1981, 70, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Ash, P.; Loutit, J.F.; Townsend, K.M. Osteoclasts derived from haematopoietic stem cells. Nature 1980, 283, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Frattini, A.; Blair, H.C.; Sacco, M.G.; Cerisoli, F.; Faggioli, F.; Cato, E.M.; Pangrazio, A.; Musio, A.; Rucci, F.; Sobacchi, C.; et al. Rescue of ATPa3-deficient murine malignant osteopetrosis by hematopoietic stem cell transplantation in utero. Proc. Natl. Acad. Sci. USA 2005, 102, 14629–14634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, D.G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 1975, 190, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Jacome-Galarza, C.E.; Percin, G.I.; Muller, J.T.; Mass, E.; Lazarov, T.; Eitler, J.; Rauner, M.; Yadav, V.K.; Crozet, L.; Bohm, M.; et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 2019, 568, 541–545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiang, Q.; Li, L.; Ji, W.; Gawlitta, D.; Walboomers, X.F.; van den Beucken, J. Beyond resorption: Osteoclasts as drivers of bone formation. Cell Regen. 2024, 13, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yahara, Y.; Barrientos, T.; Tang, Y.J.; Puviindran, V.; Nadesan, P.; Zhang, H.; Gibson, J.R.; Gregory, S.G.; Diao, Y.; Xiang, Y.; et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020, 22, 49–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yahara, Y.; Ma, X.; Gracia, L.; Alman, B.A. Monocyte/Macrophage Lineage Cells From Fetal Erythromyeloid Progenitors Orchestrate Bone Remodeling and Repair. Front. Cell Dev. Biol. 2021, 9, 622035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yahara, Y.; Nguyen, T.; Ishikawa, K.; Kamei, K.; Alman, B.A. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development 2022, 149, dev199908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Novak, S.; Roeder, E.; Kalinowski, J.; Jastrzebski, S.; Aguila, H.L.; Lee, S.K.; Kalajzic, I.; Lorenzo, J.A. Osteoclasts Derive Predominantly from Bone Marrow-Resident CX3CR1(+) Precursor Cells in Homeostasis, whereas Circulating CX3CR1(+) Cells Contribute to Osteoclast Development during Fracture Repair. J. Immunol. 2020, 204, 868–878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alle, Q.; Le Borgne, E.; Bensadoun, P.; Lemey, C.; Bechir, N.; Gabanou, M.; Estermann, F.; Bertrand-Gaday, C.; Pessemesse, L.; Toupet, K.; et al. A single short reprogramming early in life initiates and propagates an epigenetically related mechanism improving fitness and promoting an increased healthy lifespan. Aging Cell 2022, 21, e13714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macip, C.C.; Hasan, R.; Hoznek, V.; Kim, J.; Lu, Y.R.; Metzger LEt Sethna, S.; Davidsohn, N. Gene Therapy-Mediated Partial Reprogramming Extends Lifespan and Reverses Age-Related Changes in Aged Mice. Cell. Reprogram. 2024, 26, 24–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pessina, P.; Di Stefano, B. Early Life Reprogramming-Based Treatment Promotes Longevity. Cell. Reprogram. 2023, 25, 9–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Root, S.H.; Aguila, H.L. Novel population of human monocyte and osteoclast progenitors from pluripotent stem cells and peripheral blood. Blood Adv. 2021, 5, 4435–4446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flanagan, A.M.; Massey, H.M. Generating human osteoclasts in vitro from bone marrow and peripheral blood. Methods Mol. Med. 2003, 80, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, C.; Gran, D.E.; Lee, S.K.; Lorenzo, J.A.; Aguila, H.L. Identification of multiple osteoclast precursor populations in murine bone marrow. J. Bone Miner. Res. 2006, 21, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kukita, T.; MacDonald, B.R.; Bird, A.; Mundy, G.R.; McManus, L.M.; Miller, M.; Boyde, A.; Jones, S.J.; Roodman, G.D. Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J. Clin. Investig. 1989, 83, 543–550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, Y.; Zijl, S.; Wang, L.; de Groot, D.C.; van Tol, M.J.; Lankester, A.C.; Borst, J. Identification of the Common Origins of Osteoclasts, Macrophages, and Dendritic Cells in Human Hematopoiesis. Stem Cell Rep. 2015, 4, 984–994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mbalaviele, G.; Jaiswal, N.; Meng, A.; Cheng, L.; Van Den Bos, C.; Thiede, M. Human mesenchymal stem cells promote human osteoclast differentiation from CD34+ bone marrow hematopoietic progenitors. Endocrinology 1999, 140, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Qin, Y.; Xia, Y.; Han, J.; Yuan, R.; Sun, J.; Xu, R.; Jiang, J.X.; Greenblatt, M.B.; Zhao, B. Bone marrow Adipoq-lineage progenitors are a major cellular source of M-CSF that dominates bone marrow macrophage development, osteoclastogenesis, and bone mass. eLife 2023, 12, e82118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, J.; He, Q.; Wang, H.; Yao, L.; Duffy, M.; Guo, H.; Braun, C.; Zhou, Y.; Liang, Q.; Lin, Y.; et al. Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice. Bone Res. 2025, 13, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, L.; Lu, J.; He, Q.; Wang, H.; Yao, L.; Duffy, M.; Guo, H.; Braun, C.; Lin, Y.; Zhou, Y.; et al. Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice. Res Sq. 2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, L.; Lu, J.; Fang, J.; Yao, L.; Yu, W.; Gui, T.; Duffy, M.; Holdreith, N.; Bautista, C.A.; Huang, X.; et al. Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone. eLife 2023, 12, e82112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flegar, D.; Filipovic, M.; Sucur, A.; Markotic, A.; Lukac, N.; Sisl, D.; Ikic Matijasevic, M.; Jajic, Z.; Kelava, T.; Katavic, V.; et al. Preventive CCL2/CCR2 Axis Blockade Suppresses Osteoclast Activity in a Mouse Model of Rheumatoid Arthritis by Reducing Homing of CCR2(hi) Osteoclast Progenitors to the Affected Bone. Front. Immunol. 2021, 12, 767231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petitprez, V.; Royer, B.; Desoutter, J.; Guiheneuf, E.; Rigolle, A.; Marolleau, J.P.; Kamel, S.; Guillaume, N. CD14+ CD16+ monocytes rather than CD14+ CD51/61+ monocytes are a potential cytological marker of circulating osteoclast precursors in multiple myeloma. A preliminary study. Int. J. Lab. Hematol. 2015, 37, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.G.; Henriksen, K.; Schaller, S.; Henriksen, D.B.; Nielsen, F.C.; Dziegiel, M.H.; Karsdal, M.A. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J. Bone Miner. Metab. 2007, 25, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xu, L.; Zhu, H.; Bai, M.; Li, X.; Zhao, Z.; Zhong, H.; Cheng, G.; Li, X.; Hu, F.; et al. CD14(+)CD16(-) monocytes are the main precursors of osteoclasts in rheumatoid arthritis via expressing Tyro3TK. Arthritis Res. Ther. 2020, 22, 221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawant, A.; Deshane, J.; Jules, J.; Lee, C.M.; Harris, B.A.; Feng, X.; Ponnazhagan, S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013, 73, 672–682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishii, M.; Kikuta, J.; Shimazu, Y.; Meier-Schellersheim, M.; Germain, R.N. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 2010, 207, 2793–2798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacome-Galarza, C.E.; Lee, S.K.; Lorenzo, J.A.; Aguila, H.L. Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J. Bone Miner. Res. 2013, 28, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, M.Y.; Lottsfeldt, J.L.; Fevold, K.L. Identification and characterization of osteoclast progenitors by clonal analysis of hematopoietic cells. Blood 1992, 80, 1710–1716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamichi, Y.; Mizoguchi, T.; Arai, A.; Kobayashi, Y.; Sato, M.; Penninger, J.M.; Yasuda, H.; Kato, S.; DeLuca, H.F.; Suda, T.; et al. Spleen serves as a reservoir of osteoclast precursors through vitamin D-induced IL-34 expression in osteopetrotic op/op mice. Proc. Natl. Acad. Sci. USA 2012, 109, 10006–10011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotani, M.; Kikuta, J.; Klauschen, F.; Chino, T.; Kobayashi, Y.; Yasuda, H.; Tamai, K.; Miyawaki, A.; Kanagawa, O.; Tomura, M.; et al. Systemic circulation and bone recruitment of osteoclast precursors tracked by using fluorescent imaging techniques. J. Immunol. 2013, 190, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.D.; Vermeer, J.A.; Bloemen, V.; Stap, J.; Everts, V. Osteoclast fusion and fission. Calcif. Tissue Int. 2012, 90, 515–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamad, S.F.; Gunawan, A.; Blosser, R.; Childress, P.; Aguilar-Perez, A.; Ghosh, J.; Hong, J.M.; Liu, J.; Kanagasabapathy, D.; Kacena, M.A.; et al. Neonatal Osteomacs and Bone Marrow Macrophages Differ in Phenotypic Marker Expression and Function. J. Bone Miner. Res. 2021, 36, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Soki, F.N.; Koh, A.J.; Eber, M.R.; Entezami, P.; Park, S.I.; van Rooijen, N.; McCauley, L.K. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc. Natl. Acad. Sci. USA 2014, 111, 1545–1550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batoon, L.; Millard, S.M.; Raggatt, L.J.; Wu, A.C.; Kaur, S.; Sun, L.W.H.; Williams, K.; Sandrock, C.; Ng, P.Y.; Irvine, K.M.; et al. Osteal macrophages support osteoclast-mediated resorption and contribute to bone pathology in a postmenopausal osteoporosis mouse model. J. Bone Miner. Res. 2021, 36, 2214–2228. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Chang, M.K.; Maylin, E.R.; Kohler, T.; Muller, R.; Wu, A.C.; Van Rooijen, N.; Sweet, M.J.; Hume, D.A.; Raggatt, L.J.; et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 2011, 26, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Jaimes, K.; Walton, B.; Tung, P.; Smalling, R.W. An Unusual Cause of Hypoxia: Ventricular Septal Defect, Pulmonary Artery Atresia, and Major Aortopulmonary Collaterals Diagnosed in the Adult Cardiac Catheterization Lab. Case Rep. Cardiol. 2020, 2020, 4726529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, R.T.; Patel, S.R.; Lin, E.; Butler, B.R.; Lake, J.G.; Newberry, R.D.; Williams, I.R. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J. Immunol. 2007, 178, 5659–5667. [Google Scholar] [CrossRef] [PubMed]
- Sigl, V.; Jones, L.P.; Penninger, J.M. RANKL/RANK: From bone loss to the prevention of breast cancer. Open Biol. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renema, N.; Navet, B.; Heymann, M.F.; Lezot, F.; Heymann, D. RANK-RANKL signalling in cancer. Biosci Rep. 2016, 36, 160230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katavic, V.; Grcevic, D.; Lee, S.K.; Kalinowski, J.; Jastrzebski, S.; Dougall, W.; Anderson, D.; Puddington, L.; Aguila, H.L.; Lorenzo, J.A. The surface antigen CD45R identifies a population of estrogen-regulated murine marrow cells that contain osteoclast precursors. Bone 2003, 32, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.F.; Hsu, L.Y.; Niemi, E.C.; Weiss, A.; Aliprantis, A.O.; Nakamura, M.C. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J. Clin. Investig. 2012, 122, 4592–4605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, X.; Li, Z.; Zhao, Y.; Katz, J.; Michalek, S.M.; Feng, X.; Li, Y.; Zhang, P. Enhanced dual function of osteoclast precursors following calvarial Porphyromonas gingivalis infection. J. Periodontal Res. 2020, 55, 410–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikic Matijasevic, M.; Flegar, D.; Kovacic, N.; Katavic, V.; Kelava, T.; Sucur, A.; Ivcevic, S.; Cvija, H.; Lazic Mosler, E.; Kalajzic, I.; et al. Increased chemotaxis and activity of circulatory myeloid progenitor cells may contribute to enhanced osteoclastogenesis and bone loss in the C57BL/6 mouse model of collagen-induced arthritis. Clin. Exp. Immunol. 2016, 186, 321–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moerings, B.G.J.; de Graaff, P.; Furber, M.; Witkamp, R.F.; Debets, R.; Mes, J.J.; van Bergenhenegouwen, J.; Govers, C. Continuous Exposure to Non-Soluble beta-Glucans Induces Trained Immunity in M-CSF-Differentiated Macrophages. Front. Immunol. 2021, 12, 672796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, T.; Arai, F.; Ohneda, O.; Takagi, K.; Anderson, D.M.; Suda, T. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood 2000, 96, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Muto, A.; Udagawa, N.; Arai, A.; Yamashita, T.; Hosoya, A.; Ninomiya, T.; Nakamura, H.; Yamamoto, Y.; Kinugawa, S.; et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J. Cell Biol. 2009, 184, 541–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puchner, A.; Saferding, V.; Bonelli, M.; Mikami, Y.; Hofmann, M.; Brunner, J.S.; Caldera, M.; Goncalves-Alves, E.; Binder, N.B.; Fischer, A.; et al. Non-classical monocytes as mediators of tissue destruction in arthritis. Ann. Rheum. Dis. 2018, 77, 1490–1497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J. Med. 2011, 60, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, Y.; Lee, M.Y. Isolation and characterization of murine clonogenic osteoclast progenitors by cell surface phenotype analysis. Blood 1998, 91, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Palomero, J.; Grabowska, J.; Wang, L.; de Rink, I.; van Helvert, L.; Borst, J. Macrophages and osteoclasts stem from a bipotent progenitor downstream of a macrophage/osteoclast/dendritic cell progenitor. Blood Adv. 2017, 1, 1993–2006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beerman, I.; Maloney, W.J.; Weissmann, I.L.; Rossi, D.J. Stem cells and the aging hematopoietic system. Curr. Opin. Immunol. 2010, 22, 500–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jevon, M.; Sabokbar, A.; Fujikawa, Y.; Hirayama, T.; Neale, S.D.; Wass, J.; Athanasou, N.A. Gender- and age-related differences in osteoclast formation from circulating precursors. J. Endocrinol. 2002, 172, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Chang, J.; Iyer, S.; Han, L.; Campisi, J.; Manolagas, S.C.; Zhou, D.; Almeida, M. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell 2019, 18, e12923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koshihara, Y.; Suematsu, A.; Feng, D.; Okawara, R.; Ishibashi, H.; Yamamoto, S. Osteoclastogenic potential of bone marrow cells increases with age in elderly women with fracture. Mech. Ageing Dev. 2002, 123, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.M.J.; Delaisse, J.M.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Soe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, F.L.; Stone, M.D.; Turton, J.; McCabe, L.R.; Wang, E.C.Y.; Williams, A.S. Oestrogen-deficiency induces bone loss by modulating CD14(+) monocyte and CD4(+) T cell DR3 expression and serum TL1A levels. BMC Musculoskelet. Disord. 2019, 20, 326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaneko, K.; Tsai, J.; Menez, D.; Oh, B.; Suh, A.J.; Bae, S.; Mizuno, M.; Umemoto, A.; Giannopoulou, E.; Fujii, T.; et al. Cellular signatures in human blood track bone mineral density in postmenopausal women. JCI Insight 2024, 9, e178977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clowes, J.A.; Eghbali-Fatourechi, G.Z.; McCready, L.; Oursler, M.J.; Khosla, S.; Riggs, B.L. Estrogen action on bone marrow osteoclast lineage cells of postmenopausal women in vivo. Osteoporos. Int. 2009, 20, 761–769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Kirkwood, K.L.; Zhang, L.; Thiyagarajan, R.; Seldeen, K.L.; Troen, B.R. Myeloid-Derived Suppressor Cells at the Intersection of Inflammaging and Bone Fragility. Immunol. Investig. 2018, 47, 844–854. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salas, A.; Ponnusamy, S.; Senkal, C.E.; Meyers-Needham, M.; Selvam, S.P.; Saddoughi, S.A.; Apohan, E.; Sentelle, R.D.; Smith, C.; Gault, C.R.; et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 2011, 117, 5941–5952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiyagarajan, R.; Zhang, L.; Glover, O.D.; Kwack, K.H.; Ahmed, S.; Murray, E.; Yellapu, N.K.; Bard, J.; Seldeen, K.L.; Rosario, S.R.; et al. Age-related increase of CD38 directs osteoclastogenic potential of monocytic myeloid-derived suppressor cells through mitochondrial dysfunction in male mice. Aging Cell 2024, 23, e14298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perkins, S.L.; Gibbons, R.; Kling, S.; Kahn, A.J. Age-related bone loss in mice is associated with an increased osteoclast progenitor pool. Bone 1994, 15, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Wronski, T.J.; Iwaniec, U.; Phleger, L.; Kurimoto, P.; Boudignon, B.; Halloran, B.P. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J. Bone Miner. Res. 2005, 20, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Marecic, O.; McArdle, A.; Sinha, R.; Gulati, G.S.; Tong, X.; Wang, Y.; Steininger, H.M.; Hoover, M.Y.; Koepke, L.S.; et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 2021, 597, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Schell, H.; Lienau, J.; Epari, D.R.; Seebeck, P.; Exner, C.; Muchow, S.; Bragulla, H.; Haas, N.P.; Duda, G.N. Osteoclastic activity begins early and increases over the course of bone healing. Bone 2006, 38, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.; Chatani, M.; Takano, Y.; Kudo, A. In-vivo imaging of the fracture healing in medaka revealed two types of osteoclasts before and after the callus formation by osteoblasts. Dev. Biol. 2014, 394, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Geurtzen, K.; Lopez-Delgado, A.C.; Duseja, A.; Kurzyukova, A.; Knopf, F. Laser-mediated osteoblast ablation triggers a pro-osteogenic inflammatory response regulated by reactive oxygen species and glucocorticoid signaling in zebrafish. Development 2022, 149, dev199803. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walia, B.; Lingenheld, E.; Duong, L.; Sanjay, A.; Drissi, H. A novel role for cathepsin K in periosteal osteoclast precursors during fracture repair. Ann. N. Y. Acad. Sci. 2018, 1415, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Riquelme-Guzman, C.; Tsai, S.L.; Carreon Paz, K.; Nguyen, C.; Oriola, D.; Schuez, M.; Brugues, J.; Currie, J.D.; Sandoval-Guzman, T. Osteoclast-mediated resorption primes the skeleton for successful integration during axolotl limb regeneration. Elife 2022, 11, e79966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biguetti, C.C.; Cavalla, F.; Silveira, E.M.; Fonseca, A.C.; Vieira, A.E.; Tabanez, A.P.; Rodrigues, D.C.; Trombone, A.P.F.; Garlet, G.P. Oral implant osseointegration model in C57Bl/6 mice: Microtomographic, histological, histomorphometric and molecular characterization. J. Appl. Oral Sci. 2018, 26, e20170601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Futami, T.; Fujii, N.; Ohnishi, H.; Taguchi, N.; Kusakari, H.; Ohshima, H.; Maeda, T. Tissue response to titanium implants in the rat maxilla: Ultrastructural and histochemical observations of the bone-titanium interface. J. Periodontol. 2000, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lenneras, M.; Palmquist, A.; Norlindh, B.; Emanuelsson, L.; Thomsen, P.; Omar, O. Oxidized Titanium Implants Enhance Osseointegration via Mechanisms Involving RANK/RANKL/OPG Regulation. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. 2), e486–e500. [Google Scholar] [CrossRef] [PubMed]
- Makishi, S.; Watanabe, T.; Saito, K.; Ohshima, H. Effect of Hydroxyapatite/beta-Tricalcium Phosphate on Osseointegration after Implantation into Mouse Maxilla. Int. J. Mol. Sci. 2023, 24, 3124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirakura, M.; Fujii, N.; Ohnishi, H.; Taguchi, Y.; Ohshima, H.; Nomura, S.; Maeda, T. Tissue response to titanium implantation in the rat maxilla, with special reference to the effects of surface conditions on bone formation. Clin. Oral. Implant. Res. 2003, 14, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Slaets, E.; Carmeliet, G.; Naert, I.; Duyck, J. Early cellular responses in cortical bone healing around unloaded titanium implants: An animal study. J. Periodontol. 2006, 77, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Childs, L.M.; Paschalis, E.P.; Xing, L.; Dougall, W.C.; Anderson, D.; Boskey, A.L.; Puzas, J.E.; Rosier, R.N.; O’Keefe, R.J.; Boyce, B.F.; et al. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J. Bone Miner. Res. 2002, 17, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, T.H.; Chong, A.C.; Bai, L.; Yu, H.; Gong, W.; Wooley, P.H.; Yang, S.Y. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther. 2010, 17, 1262–1269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agemura, T.; Hasegawa, T.; Yari, S.; Kikuta, J.; Ishii, M. Arthritis-associated osteoclastogenic macrophages (AtoMs) participate in pathological bone erosion in rheumatoid arthritis. Immunol. Med. 2022, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Updating the pathophysiology of arthritic bone destruction: Identifying and visualizing pathological osteoclasts in pannus. Immunol. Med. 2021, 44, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kikuta, J.; Sudo, T.; Matsuura, Y.; Matsui, T.; Simmons, S.; Ebina, K.; Hirao, M.; Okuzaki, D.; Yoshida, Y.; et al. Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat Immunol. 2019, 20, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.; Flegar, D.; Anicic, S.; Sisl, D.; Kelava, T.; Kovacic, N.; Sucur, A.; Grcevic, D. Transcriptome profiling of osteoclast subsets associated with arthritis: A pathogenic role of CCR2(hi) osteoclast progenitors. Front. Immunol. 2022, 13, 994035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sucur, A.; Jajic, Z.; Artukovic, M.; Matijasevic, M.I.; Anic, B.; Flegar, D.; Markotic, A.; Kelava, T.; Ivcevic, S.; Kovacic, N.; et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res. Ther. 2017, 19, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Z.; Li, P.; Zhang, Q.; Schwarz, E.M.; Keng, P.; Arbini, A.; Boyce, B.F.; Xing, L. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J. Biol. Chem. 2006, 281, 11846–11855. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Inoue, K.; Du, Y.; Baker, S.J.; Premkumar Reddy, E.; Greenblatt, M.B.; Zhao, B. TGFbeta reprograms TNF stimulation of macrophages towards a non-canonical pathway driving inflammatory osteoclastogenesis. Nat. Commun. 2022, 13, 3920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Li, Z.; Su, L.; Ballesteros-Tato, A.; Katz, J.; Michalek, S.M.; Feng, X.; Zhang, P. Frontline Science: Characterization and regulation of osteoclast precursors following chronic Porphyromonas gingivalis infection. J. Leukoc. Biol. 2020, 108, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, B.; Tuter, G.; Serdar, M.; Akdemir, P.; Uygur, C.; Firatli, E.; Bal, B. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J. Periodontol. 2005, 76, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- de Vries, T.J.; El Bakkali, I.; Kamradt, T.; Schett, G.; Jansen, I.D.C.; D’Amelio, P. What Are the Peripheral Blood Determinants for Increased Osteoclast Formation in the Various Inflammatory Diseases Associated with Bone Loss? Front. Immunol. 2019, 10, 505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durstberger, G.; Nguyen, P.Q.; Hohensinner, V.; Pietschmann, P.; Rausch-Fan, X.; Andrukhov, O. Effect of Enamel Matrix Derivatives on Osteoclast Formation from PBMC of Periodontitis Patients and Healthy Individuals after Interaction with Activated Endothelial Cells. Medicina 2021, 57, 269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrera, B.S.; Bastos, A.S.; Coimbra, L.S.; Teixeira, S.A.; Rossa, C.; Van Dyke, T.E., Jr.; Muscara, M.N.; Spolidorio, L.C. Peripheral blood mononuclear phagocytes from patients with chronic periodontitis are primed for osteoclast formation. J. Periodontol. 2014, 85, e72–e81. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mahendra, J.; Samuel, S.; Govindraj, J.; Loganathan, T.; Vashum, Y.; Mahendra, L.; Krishnamoorthy, T. Platelet-rich fibrin/biphasic calcium phosphate impairs osteoclast differentiation and promotes apoptosis by the intrinsic mitochondrial pathway in chronic periodontitis. J. Periodontol. 2019, 90, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Tjoa, S.T.; de Vries, T.J.; Schoenmaker, T.; Kelder, A.; Loos, B.G.; Everts, V. Formation of osteoclast-like cells from peripheral blood of periodontitis patients occurs without supplementation of macrophage colony-stimulating factor. J. Clin. Periodontol. 2008, 35, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, N.R.; Belluomo, R.; Kruyt, M.C.; Gawlitta, D.; Joosten, L.A.B.; Weinans, H.; Croes, M. Trained innate immunity modulates osteoblast and osteoclast differentiation. Stem Cell Rev. Rep. 2024, 20, 1121–1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meirow, Y.; Jovanovic, M.; Zur, Y.; Habib, J.; Colombo, D.F.; Twaik, N.; Ashkenazi-Preiser, H.; Ben-Meir, K.; Mikula, I.; Jr Reuven, O.; et al. Specific inflammatory osteoclast precursors induced during chronic inflammation give rise to highly active osteoclasts associated with inflammatory bone loss. Bone Res. 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fanucchi, S.; Dominguez-Andres, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Leeming, D.J.; Dam, E.B.; Henriksen, K.; Alexandersen, P.; Pastoureau, P.; Altman, R.D.; Christiansen, C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr. Cartil. 2008, 16, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Siebelt, M.; Waarsing, J.H.; Groen, H.C.; Muller, C.; Koelewijn, S.J.; de Blois, E.; Verhaar, J.A.; de Jong, M.; Weinans, H. Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone 2014, 66, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight 2020, 5, e135446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, D.A.; McWilliams, D.F.; Turley, M.J.; Dixon, M.R.; Franses, R.E.; Mapp, P.I.; Wilson, D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 2010, 49, 1852–1861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Wang, L.; Cui, J.; Wang, S.; Han, Y.; Shao, H.; Wang, C.; Hu, Y.; Li, X.; Zhou, Q.; et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 2023, 9, eabo7868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimak, M.J.; Kim, G.; Karkache, I.Y.; Vu, E.K.; Chavez, E.; Manser, J.C.; Patterson, E.; Basak, A.; Vu, K.C.; Mitchell, S.; et al. From Development, Disease, and Decline: A Review of What Defines an Osteoclast Progenitor. Int. J. Mol. Sci. 2025, 26, 10619. https://doi.org/10.3390/ijms262110619
Shimak MJ, Kim G, Karkache IY, Vu EK, Chavez E, Manser JC, Patterson E, Basak A, Vu KC, Mitchell S, et al. From Development, Disease, and Decline: A Review of What Defines an Osteoclast Progenitor. International Journal of Molecular Sciences. 2025; 26(21):10619. https://doi.org/10.3390/ijms262110619
Chicago/Turabian StyleShimak, Mitchell J., Grant Kim, Ismael Y. Karkache, Elizabeth K. Vu, Emily Chavez, Joseph C. Manser, Emily Patterson, Archisha Basak, Keng Cha Vu, Samuel Mitchell, and et al. 2025. "From Development, Disease, and Decline: A Review of What Defines an Osteoclast Progenitor" International Journal of Molecular Sciences 26, no. 21: 10619. https://doi.org/10.3390/ijms262110619
APA StyleShimak, M. J., Kim, G., Karkache, I. Y., Vu, E. K., Chavez, E., Manser, J. C., Patterson, E., Basak, A., Vu, K. C., Mitchell, S., Koroth, J., & Bradley, E. W. (2025). From Development, Disease, and Decline: A Review of What Defines an Osteoclast Progenitor. International Journal of Molecular Sciences, 26(21), 10619. https://doi.org/10.3390/ijms262110619






