Unraveling the Pathophysiology of Irritable Bowel Syndrome: Mechanisms and Insights
Abstract
1. Introduction
2. Materials and Methods
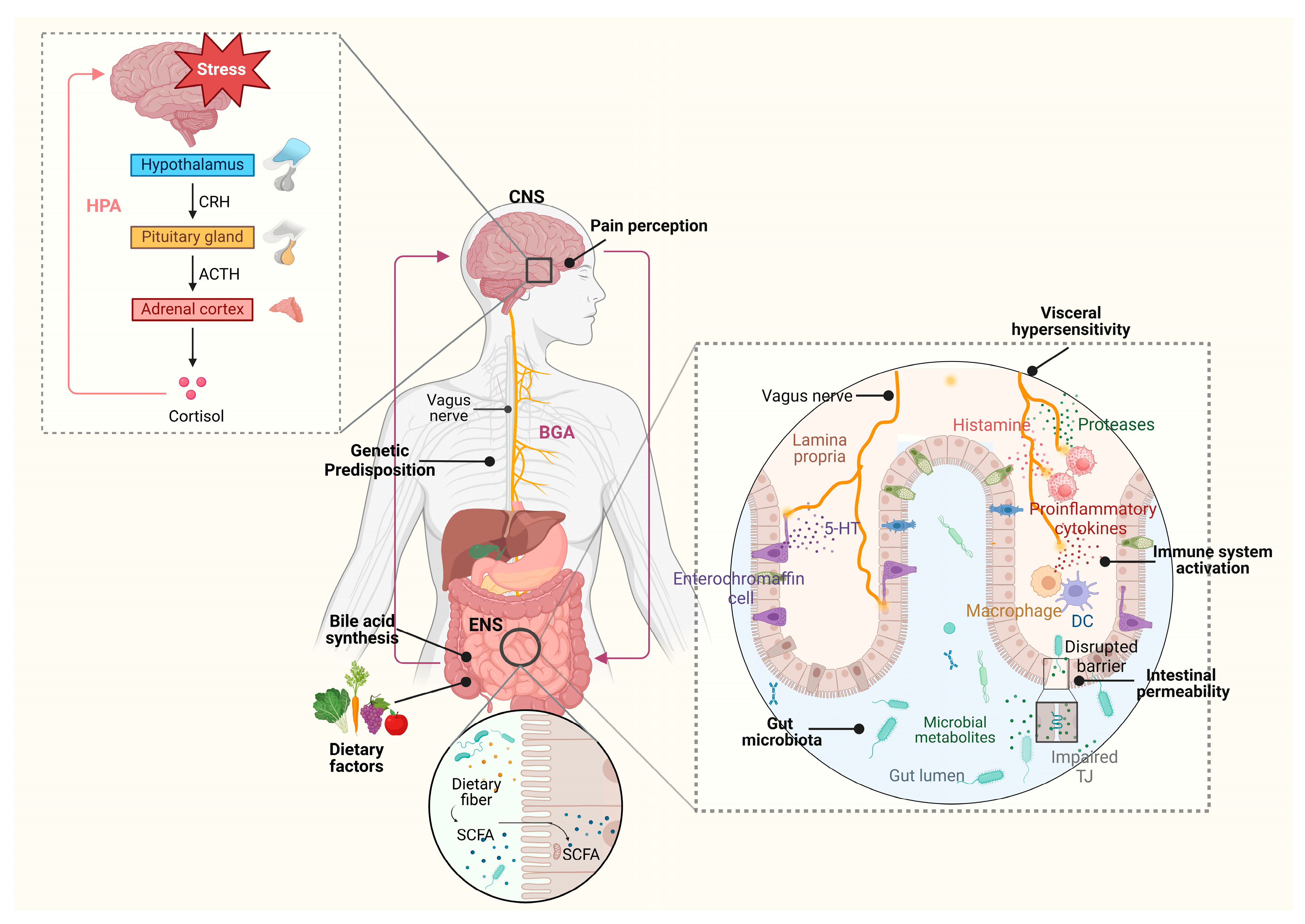
3. The Brain–Gut Axis in IBS: From Neuroendocrine Regulation to Clinical Manifestations
4. HPA Axis Dysregulation in IBS Pathophysiology
5. Mucosal and Systemic Immune Dysregulation: Linking Inflammation to Symptoms
6. Barrier Dysfunction and Epithelial Integrity: Molecular Mechanisms and Clinical Implications
7. Serotonin and Bile Acid Dysregulation: Converging Pathways in IBS Pathophysiology
8. Genetic and Epigenetic Contributions in IBS Pathophysiology: Mechanistic Insights and Clinical Implications
9. Gut Microbiome and Metabolites: Integrating Microbial Functions with Clinical Outcomes
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Camilleri, M.; Zhernakova, A.; Bozzarelli, I.; D’Amato, M. Genetics of irritable bowel syndrome: Shifting gear via biobank-scale studies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 689–702. [Google Scholar] [CrossRef]
- Kamp, E.J.; Kane, J.S.; Ford, A.C. Irritable Bowel Syndrome and Microscopic Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 659–668.e1; quiz e654–655. [Google Scholar] [CrossRef]
- Irvine, A.J.; Chey, W.D.; Ford, A.C. Screening for Celiac Disease in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 65–76. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Yiannakou, Y.; Houghton, L.A.; Ford, A.C. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin. Gastroenterol. Hepatol. 2020, 18, 392–398.e392. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Early Life Stress and Gut Microbiome Dysbiosis: A Narrative Review. Stresses 2025, 5, 38. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef]
- Marano, G.; Traversi, G.; Pola, R.; Gasbarrini, A.; Gaetani, E.; Mazza, M. Irritable Bowel Syndrome: A Hallmark of Psychological Distress in Women? Life 2025, 15, 277. [Google Scholar] [CrossRef]
- Pasta, A.; Formisano, E.; Calabrese, F.; Plaz Torres, M.C.; Bodini, G.; Marabotto, E.; Pisciotta, L.; Giannini, E.G.; Furnari, M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients 2024, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Mahurkar-Joshi, S.; Chang, L. Epigenetic Mechanisms in Irritable Bowel Syndrome. Front. Psychiatry 2020, 11, 805. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Triantos, C. Microbiome Shifts and Their Impact on Gut Physiology in Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 12395. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Y.N.; Alqifari, S.F.; Alshehri, K.; Alhowiti, A.; Mirghani, H.; Alrasheed, T.; Aljohani, F.; Alghamdi, A.; Hetta, H.F. Microbiome Gut-Brain-Axis: Impact on Brain Development and Mental Health. Mol. Neurobiol. 2025, 62, 10813–10833. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Jiang, A.J.; Wang, X.Y.; Wang, H.; Guan, Y.Y.; Li, F.; Shen, G.M. Uncovering the pathophysiology of irritable bowel syndrome by exploring the gut-brain axis: A narrative review. Ann. Transl. Med. 2021, 9, 1187. [Google Scholar] [CrossRef]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279.E2. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Karakan, T.; Ozkul, C.; Küpeli Akkol, E.; Bilici, S.; Sobarzo-Sánchez, E.; Capasso, R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Bacalbasa, N.; Gheorghe, F.; Diaconu, C.C. The Latest Data Concerning the Etiology and Pathogenesis of Irritable Bowel Syndrome. J. Clin. Med. 2024, 13, 5124. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e775. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Padhy, S.K.; Sahoo, S.; Mahajan, S.; Sinha, S.K. Irritable bowel syndrome: Is it “irritable brain” or “irritable bowel”? J. Neurosci. Rural Pract. 2015, 6, 568–577. [Google Scholar] [CrossRef][Green Version]
- Staudacher, H.M.; Mikocka-Walus, A.; Ford, A.C. Common mental disorders in irritable bowel syndrome: Pathophysiology, management, and considerations for future randomised controlled trials. Lancet Gastroenterol. Hepatol. 2021, 6, 401–410. [Google Scholar] [CrossRef]
- Pastras, P.; Aggeletopoulou, I. Impact of Enteric Nervous Cells on Irritable Bowel Syndrome: Potential Treatment Options. Microorganisms 2024, 12, 2036. [Google Scholar] [CrossRef]
- Thijssen, A.Y.; Mujagic, Z.; Jonkers, D.M.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Clemens, C.H.; Conchillo, J.M.; Kruimel, J.W.; Masclee, A.A. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 43, 272–282. [Google Scholar] [CrossRef]
- Fadgyas-Stanculete, M.; Buga, A.M.; Popa-Wagner, A.; Dumitrascu, D.L. The relationship between irritable bowel syndrome and psychiatric disorders: From molecular changes to clinical manifestations. J. Mol. Psychiatry 2014, 2, 4. [Google Scholar] [CrossRef]
- Tooth, D.; Garsed, K.; Singh, G.; Marciani, L.; Lam, C.; Fordham, I.; Fields, A.; Banwait, R.; Lingaya, M.; Layfield, R.; et al. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: Origin and effect of gut transit. Gut 2014, 63, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.; Roka, R.; Ferrier, L.; Leveque, M.; Eutamene, H.; Cartier, C.; Ait-Belgnaoui, A.; Rosztoczy, A.; Izbeki, F.; Fioramonti, J. Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008, 57, 591–599. [Google Scholar] [CrossRef]
- Beatty, J.K.; Bhargava, A.; Buret, A.G. Post-infectious irritable bowel syndrome: Mechanistic insights into chronic disturbances following enteric infection. World J. Gastroenterol. 2014, 20, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Gwee, K.A. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: The missing link. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 435–441. [Google Scholar] [CrossRef]
- Cremon, C.; Stanghellini, V.; Pallotti, F.; Fogacci, E.; Bellacosa, L.; Morselli-Labate, A.M.; Paccapelo, A.; Di Nardo, G.; Cogliandro, R.F.; De Giorgio, R.; et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology 2014, 147, 69–77. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.; Schurman, J.V.; Colombo, J.M.; Friesen, C.A. The relationship between mucosal inflammatory cells, specific symptoms, and psychological functioning in youth with irritable bowel syndrome. Sci. Rep. 2020, 10, 11988. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.L.; Talley, N.J.; Keely, S. Immune responses in the irritable bowel syndromes: Time to consider the small intestine. BMC Med. 2022, 20, 115. [Google Scholar] [CrossRef]
- Törnblom, H.; Lindberg, G.; Nyberg, B.; Veress, B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 2002, 123, 1972–1979. [Google Scholar] [CrossRef]
- Mearin, F.; Perelló, A.; Balboa, A.; Perona, M.; Sans, M.; Salas, A.; Angulo, S.; Lloreta, J.; Benasayag, R.; García-Gonzalez, M.A.; et al. Pathogenic mechanisms of postinfectious functional gastrointestinal disorders: Results 3 years after gastroenteritis. Scand. J. Gastroenterol. 2009, 44, 1173–1185. [Google Scholar] [CrossRef]
- Hasler, W.L.; Grabauskas, G.; Singh, P.; Owyang, C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14339. [Google Scholar] [CrossRef]
- Salvo-Romero, E.; Martínez, C.; Lobo, B.; Rodiño-Janeiro, B.K.; Pigrau, M.; Sánchez-Chardi, A.D.; González-Castro, A.M.; Fortea, M.; Pardo-Camacho, C.; Nieto, A.; et al. Overexpression of corticotropin-releasing factor in intestinal mucosal eosinophils is associated with clinical severity in Diarrhea-Predominant Irritable Bowel Syndrome. Sci. Rep. 2020, 10, 20706. [Google Scholar] [CrossRef]
- Bashashati, M.; Rezaei, N.; Shafieyoun, A.; McKernan, D.P.; Chang, L.; Öhman, L.; Quigley, E.M.; Schmulson, M.; Sharkey, K.A.; Simrén, M. Cytokine imbalance in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2014, 26, 1036–1048. [Google Scholar] [CrossRef]
- Mitselou, A.; Grammeniatis, V.; Varouktsi, A.; Papadatos, S.S.; Katsanos, K.; Galani, V. Proinflammatory cytokines in irritable bowel syndrome: A comparison with inflammatory bowel disease. Intest. Res. 2020, 18, 115–120. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, P.; Kumar, A. Targeted therapy of irritable bowel syndrome with anti-inflammatory cytokines. Clin. J. Gastroenterol. 2022, 15, 1–10. [Google Scholar] [CrossRef]
- Vicario, M.; González-Castro, A.M.; Martínez, C.; Lobo, B.; Pigrau, M.; Guilarte, M.; de Torres, I.; Mosquera, J.L.; Fortea, M.; Sevillano-Aguilera, C.; et al. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut 2015, 64, 1379–1388. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, X.; Li, L.; Lin, L.; Zuo, X.; Cong, Y.; Li, Y. Increased Ileal Immunoglobulin A Production and Immunoglobulin A-Coated Bacteria in Diarrhea-Predominant Irritable Bowel Syndrome. Clin. Transl. Gastroenterol. 2020, 11, e00146. [Google Scholar] [CrossRef]
- Hughes, P.A.; Harrington, A.M.; Castro, J.; Liebregts, T.; Adam, B.; Grasby, D.J.; Isaacs, N.J.; Maldeniya, L.; Martin, C.M.; Persson, J.; et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 2013, 62, 1456–1465. [Google Scholar] [CrossRef]
- Hughes, P.A.; Moretta, M.; Lim, A.; Grasby, D.J.; Bird, D.; Brierley, S.M.; Liebregts, T.; Adam, B.; Blackshaw, L.A.; Holtmann, G.; et al. Immune derived opioidergic inhibition of viscerosensory afferents is decreased in Irritable Bowel Syndrome patients. Brain Behav. Immun. 2014, 42, 191–203. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef]
- Rettura, F.; Lambiase, C.; Tedeschi, R.; Grosso, A.; Cancelli, L.; Ricchiuti, A.; Bottari, A.; Giacomelli, L.; de Bortoli, N.; Bellini, M. Mucoprotectants and gut barrier: Mechanisms of action and clinical applications in IBS. Is there a possible role? Front. Pharmacol. 2025, 16, 1538791. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients with Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated with Immunoglobulin E. Gastroenterology 2019, 157, 109–118.e105. [Google Scholar] [CrossRef]
- Rodiño-Janeiro, B.K.; Martínez, C.; Fortea, M.; Lobo, B.; Pigrau, M.; Nieto, A.; González-Castro, A.M.; Salvo-Romero, E.; Guagnozzi, D.; Pardo-Camacho, C.; et al. Decreased TESK1-mediated cofilin 1 phosphorylation in the jejunum of IBS-D patients may explain increased female predisposition to epithelial dysfunction. Sci. Rep. 2018, 8, 2255. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.; Anand, P.; Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008, 57, 923–929. [Google Scholar] [CrossRef]
- Lacy, B.E.; Rosenbaum, D.; Edelstein, S.; Kozuka, K.; Williams, L.A.; Kunkel, D.C. Intestinal Permeability, Irritable Bowel Syndrome with Constipation, and the Role of Sodium-Hydrogen Exchanger Isoform 3 (NHE3). Clin. Exp. Gastroenterol. 2024, 17, 173–183. [Google Scholar] [CrossRef]
- Mallardi, D.; Maqoud, F.; Guido, D.; Aloisio, M.; Linsalata, M.; Russo, F. Mapping Research Trends on Intestinal Permeability in Irritable Bowel Syndrome with a Focus on Nutrition: A Bibliometric Analysis. Nutrients 2025, 17, 1064. [Google Scholar] [CrossRef]
- Singh, P.; Silvester, J.; Chen, X.; Xu, H.; Sawhney, V.; Rangan, V. Serum zonulin is elevated in IBS and correlates with stool frequency in IBS-D. United Eur. Gastroenterol. J. 2019, 7, 709–715. [Google Scholar] [CrossRef]
- Bertiaux-Vandaële, N.; Youmba, S.B.; Belmonte, L.; Lecleire, S.; Antonietti, M.; Gourcerol, G.; Leroi, A.M.; Déchelotte, P.; Ménard, J.F.; Ducrotté, P.; et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 2011, 106, 2165–2173. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Ao, H. Corticosterone effects induced by stress and immunity and inflammation: Mechanisms of communication. Front. Endocrinol. 2025, 16, 1448750. [Google Scholar] [CrossRef]
- Shaikh, S.D.; Sun, N.; Canakis, A. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e116. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Oh, J.S. Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1160. [Google Scholar] [CrossRef]
- Bohórquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Terry, N.; Margolis, K.G. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef]
- Crowell, M.D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 2004, 141, 1285–1293. [Google Scholar] [CrossRef]
- Sadeghi, A.; Biglari, M.; Nasseri Moghaddam, S. Post-infectious Irritable Bowel Syndrome: A Narrative Review. Middle East. J. Dig. Dis. 2019, 11, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, S.P.; Jenkins, D.; Neal, K.R.; Spiller, R.C. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003, 125, 1651–1659. [Google Scholar] [CrossRef]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 2006, 130, 34–43. [Google Scholar] [CrossRef]
- Foley, S.; Garsed, K.; Singh, G.; Duroudier, N.P.; Swan, C.; Hall, I.P.; Zaitoun, A.; Bennett, A.; Marsden, C.; Holmes, G.; et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology 2011, 140, 1434–1443.e1431. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Duan, Z.J.; Wang, L.X.; Yang, D.; Zhao, G.; Zhang, L. The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: A meta-analysis of 25 studies. BMC Gastroenterol. 2014, 14, 23. [Google Scholar] [CrossRef]
- Fritz, N.; Berens, S.; Dong, Y.; Martínez, C.; Schmitteckert, S.; Houghton, L.A.; Goebel-Stengel, M.; Wahl, V.; Kabisch, M.; Götze, D.; et al. The serotonin receptor 3E variant is a risk factor for female IBS-D. J. Mol. Med. 2022, 100, 1617–1627. [Google Scholar] [CrossRef]
- Grzesiak, M.; Beszłej, J.A.; Waszczuk, E.; Szechiński, M.; Szewczuk-Bogusławska, M.; Frydecka, D.; Dobosz, T.; Jonkisz, A.; Lebioda, A.; Małodobra, M.; et al. Serotonin-Related Gene Variants in Patients with Irritable Bowel Syndrome and Depressive or Anxiety Disorders. Gastroenterol. Res. Pract. 2017, 2017, 4290430. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Di Sabatino, A.; Cremon, C.; Giuffrida, P.; Fiorentino, M.; Altimari, A.; Bellacosa, L.; Stanghellini, V.; Barbara, G. Interferon-γ is increased in the gut of patients with irritable bowel syndrome and modulates serotonin metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G439–G447. [Google Scholar] [CrossRef]
- Cremon, C.; Carini, G.; Wang, B.; Vasina, V.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Grundy, D.; Tonini, M.; De Ponti, F.; et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 1290–1298. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Troost, F.J.; Jonkers, D.M.; van Eijk, H.M.; Dekker, J.; Buurman, W.A.; Masclee, A.A. Visceral hypersensitivity in irritable bowel syndrome: Evidence for involvement of serotonin metabolism—A preliminary study. Neurogastroenterol. Motil. 2015, 27, 1127–1137. [Google Scholar] [CrossRef]
- Gros, M.; Gros, B. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, A.; Arasaradnam, R. Bile acid diarrhoea: Pathophysiology, diagnosis and management. Frontline Gastroenterol. 2021, 12, 500–507. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef]
- Sinha, J.; Chen, F.; Miloh, T.; Burns, R.C.; Yu, Z.; Shneider, B.L. beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G996–G1003. [Google Scholar] [CrossRef]
- Wang, L.X.; Frey, M.R.; Kohli, R. The Role of FGF19 and MALRD1 in Enterohepatic Bile Acid Signaling. Front. Endocrinol. 2022, 12, 799648. [Google Scholar] [CrossRef]
- Triantis, V.; Saeland, E.; Bijl, N.; Oude-Elferink, R.P.; Jansen, P.L. Glycosylation of fibroblast growth factor receptor 4 is a key regulator of fibroblast growth factor 19-mediated down-regulation of cytochrome P450 7A1. Hepatology 2010, 52, 656–666. [Google Scholar] [CrossRef]
- Aziz, I.; Mumtaz, S.; Bholah, H.; Chowdhury, F.U.; Sanders, D.S.; Ford, A.C. High Prevalence of Idiopathic Bile Acid Diarrhea Among Patients With Diarrhea-Predominant Irritable Bowel Syndrome Based on Rome III Criteria. Clin. Gastroenterol. Hepatol. 2015, 13, 1650–1655.e1652. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Camilleri, M.; Carlson, P.J.; Guicciardi, M.E.; Burton, D.; McKinzie, S.; Rao, A.S.; Zinsmeister, A.R.; Gores, G.J. A Klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology 2011, 140, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275.e1271. [Google Scholar] [CrossRef]
- Camilleri, M.; Busciglio, I.; Acosta, A.; Shin, A.; Carlson, P.; Burton, D.; Ryks, M.; Rhoten, D.; Lamsam, J.; Lueke, A.; et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am. J. Gastroenterol. 2014, 109, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Wong, B.S.; Camilleri, M.; Odunsi-Shiyanbade, S.T.; McKinzie, S.; Ryks, M.; Burton, D.; Carlson, P.; Lamsam, J.; Singh, R.; et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: A pharmacodynamic and pharmacogenetic analysis. Gastroenterology 2010, 139, 1549–1558, 1558.e1541. [Google Scholar] [CrossRef]
- Dior, M.; Delagrèverie, H.; Duboc, H.; Jouet, P.; Coffin, B.; Brot, L.; Humbert, L.; Trugnan, G.; Seksik, P.; Sokol, H.; et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 1330–1340. [Google Scholar] [CrossRef]
- Min, Y.W.; Rezaie, A.; Pimentel, M. Bile Acid and Gut Microbiota in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 549–561. [Google Scholar] [CrossRef]
- Saito, Y.A. The role of genetics in IBS. Gastroenterol. Clin. N. Am. 2011, 40, 45–67. [Google Scholar] [CrossRef]
- Henström, M.; D’Amato, M. Genetics of irritable bowel syndrome. Mol. Cell. Pediatr. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Eijsbouts, C.; Zheng, T.; Kennedy, N.A.; Bonfiglio, F.; Anderson, C.A.; Moutsianas, L.; Holliday, J.; Shi, J.; Shringarpure, S.; Agee, M.; et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat. Genet. 2021, 53, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, F.; Zheng, T.; Garcia-Etxebarria, K.; Hadizadeh, F.; Bujanda, L.; Bresso, F.; Agreus, L.; Andreasson, A.; Dlugosz, A.; Lindberg, G.; et al. Female-Specific Association Between Variants on Chromosome 9 and Self-Reported Diagnosis of Irritable Bowel Syndrome. Gastroenterology 2018, 155, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef]
- Husein, D.M.; Rizk, S.; Hoter, A.; Wanes, D.; D’Amato, M.; Naim, H.Y. Severe pathogenic variants of intestinal sucrase-isomaltase interact avidly with the wild type enzyme and negatively impact its function and trafficking. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166523. [Google Scholar] [CrossRef]
- Zamfir-Taranu, A.; Löscher, B.S.; Franke, A.; Bonfiglio, F.; Ohlsson, B.; D’Amato, M. Sucrase-isomaltase hypomorphic variant Val15Phe affects the response to a sucrose challenge test in patients with IBS. Gut 2025, in press. [Google Scholar] [CrossRef]
- Strege, P.R.; Mazzone, A.; Bernard, C.E.; Neshatian, L.; Gibbons, S.J.; Saito, Y.A.; Tester, D.J.; Calvert, M.L.; Mayer, E.A.; Chang, L.; et al. Irritable bowel syndrome patients have SCN5A channelopathies that lead to decreased Na(V)1.5 current and mechanosensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G494–G503. [Google Scholar] [CrossRef]
- Verstraelen, T.E.; Ter Bekke, R.M.; Volders, P.G.; Masclee, A.A.; Kruimel, J.W. The role of the SCN5A-encoded channelopathy in irritable bowel syndrome and other gastrointestinal disorders. Neurogastroenterol. Motil. 2015, 27, 906–913. [Google Scholar] [CrossRef]
- Grozić, A.; Coker, K.; Dussik, C.M.; Sabir, M.S.; Sabir, Z.; Bradley, A.; Zhang, L.; Park, J.; Yale, S.; Kaneko, I.; et al. Identification of putative transcriptomic biomarkers in irritable bowel syndrome (IBS): Differential gene expression and regulation of TPH1 and SERT by vitamin D. PLoS ONE 2022, 17, e0275683. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, T.; Grabauskas, G.; Owyang, C. Mucosal Serotonin Reuptake Transporter Expression in Irritable Bowel Syndrome Is Modulated by Gut Microbiota Via Mast Cell-Prostaglandin E2. Gastroenterology 2022, 162, 1962–1974.e1966. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, G.; Drokhlyansky, E.; Anand, S.; Fiskin, E.; Subramanian, A.; Slyper, M.; Wang, J.; Van Wittenberghe, N.; Rouhana, J.M.; Waldman, J.; et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science 2022, 376, eabl4290. [Google Scholar] [CrossRef]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Weaver, K.R.; Mustapic, M.; Kapogiannis, D.; Henderson, W.A. Neuronal-enriched extracellular vesicles in individuals with IBS: A pilot study of COMT and BDNF. Neurogastroenterol. Motil. 2022, 34, e14257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Murray, G.K.; Byrne, E.M.; Sidorenko, J.; Visscher, P.M.; Wray, N.R. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun. 2021, 12, 1146. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Rühlemann, M.C.; Hermes, B.M.; Bang, C.; Doms, S.; Moitinho-Silva, L.; Thingholm, L.B.; Frost, F.; Degenhardt, F.; Wittig, M.; Kässens, J.; et al. Genome-wide association study in 8,956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat. Genet. 2021, 53, 147–155. [Google Scholar] [CrossRef]
- Dothel, G.; Barbaro, M.R.; Di Vito, A.; Ravegnini, G.; Gorini, F.; Monesmith, S.; Coschina, E.; Benuzzi, E.; Fuschi, D.; Palombo, M.; et al. New insights into irritable bowel syndrome pathophysiological mechanisms: Contribution of epigenetics. J. Gastroenterol. 2023, 58, 605–621. [Google Scholar] [CrossRef]
- Hong, S.; Zheng, G.; Wiley, J.W. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology 2015, 148, 148–157.e147. [Google Scholar] [CrossRef]
- Kano, M.; Muratsubaki, T.; Van Oudenhove, L.; Morishita, J.; Yoshizawa, M.; Kohno, K.; Yagihashi, M.; Tanaka, Y.; Mugikura, S.; Dupont, P.; et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci. Rep. 2017, 7, 12425. [Google Scholar] [CrossRef]
- Jacenik, D.; Cygankiewicz, A.I.; Fichna, J.; Mokrowiecka, A.; Małecka-Panas, E.; Krajewska, W.M. Estrogen signaling deregulation related with local immune response modulation in irritable bowel syndrome. Mol. Cell Endocrinol. 2018, 471, 89–96. [Google Scholar] [CrossRef]
- Cao, D.Y.; Bai, G.; Ji, Y.; Traub, R.J. Epigenetic upregulation of metabotropic glutamate receptor 2 in the spinal cord attenuates oestrogen-induced visceral hypersensitivity. Gut 2015, 64, 1913–1920. [Google Scholar] [CrossRef]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Nohesara, S.; Mostafavi Abdolmaleky, H.; Pirani, A.; Pettinato, G.; Thiagalingam, S. The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention. Nutrients 2025, 17, 1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Sugi, Y.; Narabayashi, H.; Kobayakawa, T.; Nakanishi, Y.; Tsuda, M.; Hosono, A.; Kaminogawa, S.; Hanazawa, S.; Takahashi, K. Commensal microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. J. Biol. Chem. 2017, 292, 15426–15433. [Google Scholar] [CrossRef] [PubMed]
- Zimberlin, C.D.; Lancini, C.; Sno, R.; Rosekrans, S.L.; McLean, C.M.; Vlaming, H.; van den Brink, G.R.; Bots, M.; Medema, J.P.; Dannenberg, J.H. HDAC1 and HDAC2 collectively regulate intestinal stem cell homeostasis. FASEB J. 2015, 29, 2070–2080. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, L.; Chen, C.; Ma, Y.; Ma, Y. The role of miRNA in IBS pathogenesis, diagnosis and therapy: The latest thought. Dig. Liver Dis. 2024, 56, 1433–1441. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Larson, S.; Basra, S.; Merwat, S.; Tan, A.; Croce, C.; Verne, G.N. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2016, 65, 797–805. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Han, J.; Fasina, Y.; Harrison, S.H. Connecting gut microbiomes and short chain fatty acids with the serotonergic system and behavior in Gallus gallus and other avian species. Front. Physiol. 2022, 13, 1035538. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Talley, N.J.; Veldhuyzen van Zanten, S.J.; Vakil, N.B.; Simel, D.L.; Moayyedi, P. Will the history and physical examination help establish that irritable bowel syndrome is causing this patient’s lower gastrointestinal tract symptoms? JAMA 2008, 300, 1793–1805. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Y. The microbiota-gut-brain axis and central nervous system diseases: From mechanisms of pathogenesis to therapeutic strategies. Front. Microbiol. 2025, 16, 1583562. [Google Scholar] [CrossRef]
- Tang, Y.; Du, J.; Wu, H.; Wang, M.; Liu, S.; Tao, F. Potential Therapeutic Effects of Short-Chain Fatty Acids on Chronic Pain. Curr. Neuropharmacol. 2024, 22, 191–203. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Alharbi, A.A.; Alsharef, S.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; El Amin, H. Gut Microbiome as a Target of Intervention in Inflammatory Bowel Disease Pathogenesis and Therapy. Immuno 2024, 4, 400–425. [Google Scholar] [CrossRef]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 33, e13983. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients with Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef]
- Luck, B.; Horvath, T.D.; Engevik, K.A.; Ruan, W.; Haidacher, S.J.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; Haag, A.M.; Versalovic, J. Neurotransmitter profiles are altered in the gut and brain of mice mono-associated with Bifidobacterium dentium. Biomolecules 2021, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Li, M.; Li, L.X.; Sun, Y.Y.; Zhang, W.X.; Zhao, D.Y.; Li, Y.Q. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil. 2018, 30, e13227. [Google Scholar] [CrossRef]
- Jiang, M.; Incarnato, D.; Modderman, R.; Lazaro, A.A.; Jonkers, I.H.; Bianchi, F.; van den Bogaart, G. Low butyrate concentrations exert anti-inflammatory and high concentrations exert pro-inflammatory effects on macrophages. J. Nutr. Biochem. 2025, 144, 109962. [Google Scholar] [CrossRef]
- Ju, X.; Jiang, Z.; Ma, J.; Yang, D. Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1727. [Google Scholar] [CrossRef]
- Edogawa, S.; Edwinson, A.L.; Peters, S.A.; Chikkamenahalli, L.L.; Sundt, W.; Graves, S.; Gurunathan, S.V.; Breen-Lyles, M.; Johnson, S.; Dyer, R.; et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut 2020, 69, 62–73. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef]
- Kanazawa, M.; Miyamoto, K.; Kano, M.; Inooka, K.; Oka, K.; Takahashi, M.; Mano, N.; Fukudo, S. Effects of a Protease Inhibitor Camostat Mesilate on Gut Microbial Function in Patients with Irritable Bowel Syndrome: A Pilot Randomized Placebo-Controlled Study. Digestion 2025, 106, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C. Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef] [PubMed]
- Schaus, S.R.; Vasconcelos Pereira, G.; Luis, A.S.; Madlambayan, E.; Terrapon, N.; Ostrowski, M.P.; Jin, C.; Henrissat, B.; Hansson, G.C.; Martens, E.C. Ruminococcus torques is a keystone degrader of intestinal mucin glycoprotein, releasing oligosaccharides used by Bacteroides thetaiotaomicron. mBio 2024, 15, e0003924. [Google Scholar] [CrossRef]
- Arora, T.; Vanslette, A.M.; Hjorth, S.A.; Bäckhed, F. Microbial regulation of enteroendocrine cells. Med 2021, 2, 553–570. [Google Scholar] [CrossRef]
- Masse, K.E.; Lu, V.B. Short-chain fatty acids, secondary bile acids and indoles: Gut microbial metabolites with effects on enteroendocrine cell function and their potential as therapies for metabolic disease. Front. Endocrinol. 2023, 14, 1169624. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Ghoshal, U.; Shukla, R.; Srivastava, D.; Ghoshal, U.C. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver 2016, 10, 932–938. [Google Scholar] [CrossRef]
- Linden, D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal 2014, 20, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Gillilland, M., 3rd; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Fallah, F.; Pormohammad, A.; Taghipour, A.; Safari, H.; Chirani, A.S.; Sabour, S.; Alizadeh-Sani, M.; Azimi, T. The possible role of bacteria, viruses, and parasites in initiation and exacerbation of irritable bowel syndrome. J. Cell Physiol. 2019, 234, 8550–8569. [Google Scholar] [CrossRef] [PubMed]
- Callahan, S.M.; Dolislager, C.G.; Johnson, J.G. The Host Cellular Immune Response to Infection by Campylobacter Spp. and Its Role in Disease. Infect. Immun. 2021, 89, e0011621. [Google Scholar] [CrossRef]
- Imbrea, A.-M.; Balta, I.; Dumitrescu, G.; McCleery, D.; Pet, I.; Iancu, T.; Stef, L.; Corcionivoschi, N.; Liliana, P.-C. Exploring the Contribution of Campylobacter jejuni to Post-Infectious Irritable Bowel Syndrome: A Literature Review. Appl. Sci. 2024, 14, 3373. [Google Scholar] [CrossRef]
- Patel, S.; McCormick, B.A. Mucosal Inflammatory Response to Salmonella typhimurium Infection. Front. Immunol. 2014, 5, 311. [Google Scholar] [CrossRef]
| Molecular Mechanism Involved | IBS-D (Diarrhea-Predominant) | IBS-C (Constipation-Predominant) | Not Subtype-Specific |
|---|---|---|---|
| Serotonin signaling | ↑ Postprandial 5-HT release; ↓ Platelet 5-HT uptake; ↓ Duodenal SERT mRNA; ↑ Enterochromaffin cells; HTR3E rs56109847 variant; IFN-γ–induced ↓ SERT | ↓ Postprandial 5-HT release; 5-HTTLPR LL genotype linked to IBS-C (East Asian cohorts) | NR |
| Bile acid metabolism | ↑ Colonic bile acids (impaired FGF-19 feedback); FGF-4 and klotho-β variants; idiopathic bile acid diarrhea (~20%) | NR | NR |
| Immune activation | ↑ Mast cells, eosinophils, lymphocytes; ↑ IL-6, IL-8, TNFα; ↑ Mucosal B cells and IgA-coated bacteria; ↑ CRF in mucosal eosinophils | Mild mucosal immune activation | ↓ β-Endorphin release from immune cells |
| Barrier integrity | ↑ Intestinal permeability; ↓ ZO-1, occludin, claudin-1; ↑ zonulin; TESK1/CFL downregulation | ↑ Intestinal permeability (4–25%); ↑ Mast-cell activation; ↑ TRPV1 fibers | NR |
| Microbial alterations | ↑ E. coli; ↓ Leptum, Bifidobacterium spp. | ↑ Methanobrevibacter smithii (methane producers) | ↑ Fecal bile acids; ↑ Fecal proteases; disrupted Firmicutes/Bacteroidetes ratio; altered SCFAs/indoles; impaired epithelial integrity |
| Metabolites and neurotransmitters | ↑ Butyrate, tryptamine; ↑ motility (dose-dependent effects) | ↑ Methane → slowed transit | Altered SCFAs, tryptamine, indoles, and GABA signaling |
| Genetic/epigenetic influences | HTR3E rs56109847; SERT variants; IFN-γ–induced ↓ SERT expression; ↓ miR-199a/b expression → ↑ visceral hypersensitivity | 5-HTTLPR LL genotype; SCN5A polymorphisms | Immune-related gene variants within the HLA region |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggeletopoulou, I.; Papantoniou, K.; Pastras, P.; Triantos, C. Unraveling the Pathophysiology of Irritable Bowel Syndrome: Mechanisms and Insights. Int. J. Mol. Sci. 2025, 26, 10598. https://doi.org/10.3390/ijms262110598
Aggeletopoulou I, Papantoniou K, Pastras P, Triantos C. Unraveling the Pathophysiology of Irritable Bowel Syndrome: Mechanisms and Insights. International Journal of Molecular Sciences. 2025; 26(21):10598. https://doi.org/10.3390/ijms262110598
Chicago/Turabian StyleAggeletopoulou, Ioanna, Konstantinos Papantoniou, Ploutarchos Pastras, and Christos Triantos. 2025. "Unraveling the Pathophysiology of Irritable Bowel Syndrome: Mechanisms and Insights" International Journal of Molecular Sciences 26, no. 21: 10598. https://doi.org/10.3390/ijms262110598
APA StyleAggeletopoulou, I., Papantoniou, K., Pastras, P., & Triantos, C. (2025). Unraveling the Pathophysiology of Irritable Bowel Syndrome: Mechanisms and Insights. International Journal of Molecular Sciences, 26(21), 10598. https://doi.org/10.3390/ijms262110598









