Chronic Thromboembolic Pulmonary Disease: Right Ventricular Function and Pulmonary Hemodynamics in a 4-Year Follow-Up
Abstract
1. Introduction
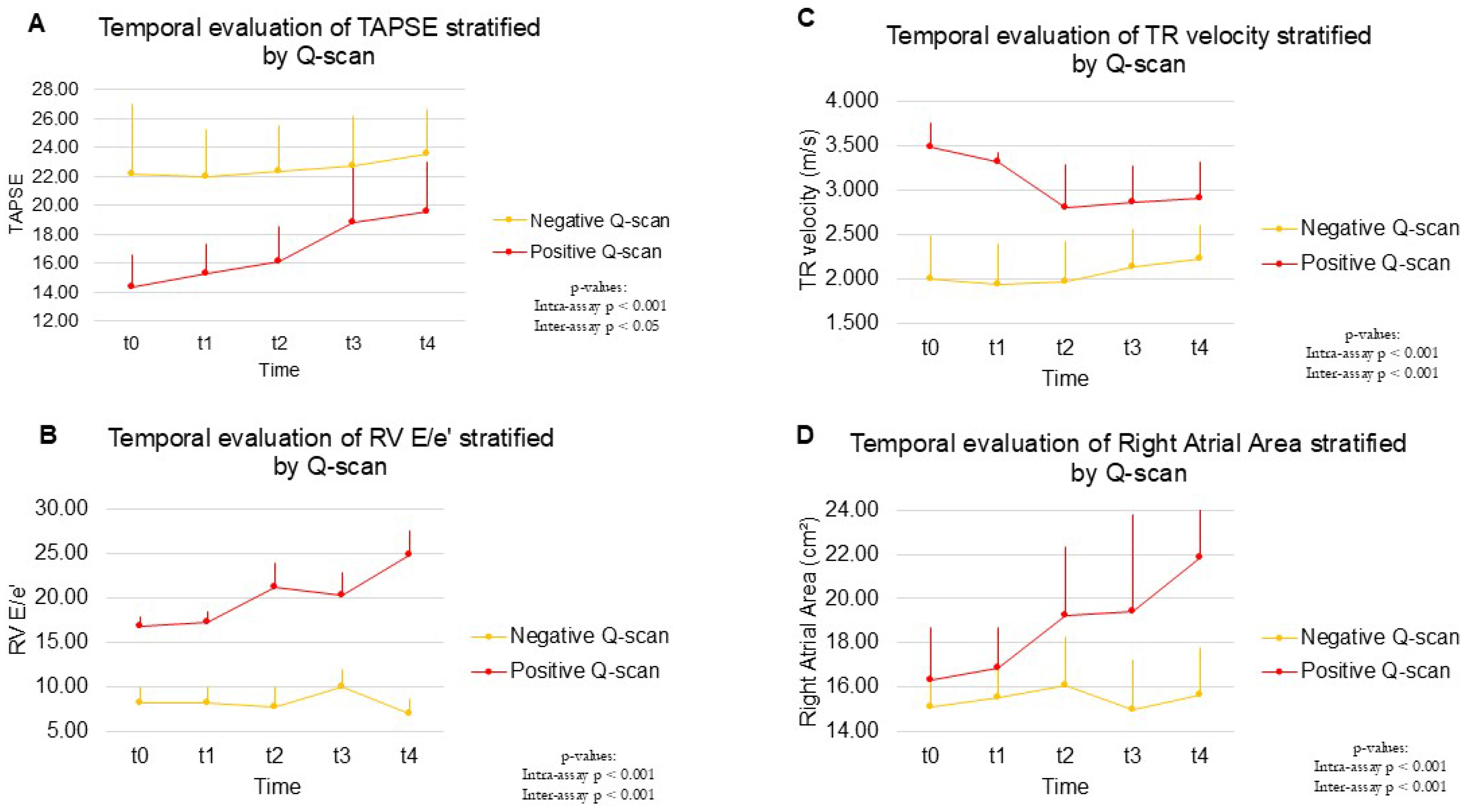
2. Results
2.1. Patient Recruitment and Population Characteristics
2.2. Baseline Assessment
2.3. Four-Month Follow-Up
2.3.1. Clinical and CPET Parameters at 4 Months
2.3.2. Resting and Exercise Echocardiography
2.4. Long-Term Follow-Up
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Data Collection
- Extent of thrombotic load of PE on Q-scan (number of segments with perfusion defect) and contrast-enhanced CT pulmonary angiography at admission (mild, sub-massive, or massive PE) according to the American Heart Association definitions [11];
- Presence of thrombophilia (limited to tests not affected by anticoagulation, namely factor V Leiden, prothrombin variant, anti-phospholipid, and anti-beta-2-glycoprotein antibodies);
- Cardiovascular risk factors;
- Anthropometric and demographic parameters;
- Anticoagulation treatment in the hospital and at discharge.
4.2. Resting Echocardiography
4.3. Exercise Echocardiography
4.4. Cardiopulmonary Exercise Testing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| CPET | cardiopulmonary exercise test |
| CTEPD | chronic thromboembolic pulmonary disease |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| ESE | exercise stress echocardiography |
| Ex-PH | exercise-induced pulmonary hypertension |
| mPAP | mean pulmonary arterial pressure |
| PAPs | systolic pulmonary artery pressure |
| PE | pulmonary thromboembolism |
| PPES | post-PE syndrome |
| Q-scan | perfusion scan |
| RHC | right heart catheterization |
| RV | right ventricle |
| TTE | transthoracic echocardiography |
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Kim, N.H.; D’Armini, A.M.; Delcroix, M.; Jais, X.; Jevnikar, M.; Madani, M.M.; Matsubara, H.; Palazzini, M.; Wiedenroth, C.B.; Simonneau, G.; et al. Chronic thromboembolic pulmonary disease. Eur. Respir. J. 2024, 64, 2401294. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.T.; Andersson, T.; Carlberg, B.; Johansson, L.; Söderberg, S. Electrocardiographic abnormalities and NT-proBNP levels at long-term follow-up of patients with dyspnea after pulmonary embolism. Scand. Cardiovasc. J. 2024, 58, 2373090. [Google Scholar] [CrossRef]
- Dzikowska-Diduch, O.; Kostrubiec, M.; Kurnicka, K.; Lichodziejewska, B.; Pacho, S.; Miroszewska, A.; Bródka, K.; Skowrońska, M.; Łabyk, A.; Roik, M.; et al. The post-pulmonary syndrome-results of echocardiographic driven follow up after acute pulmonary embolism. Thromb. Res. 2020, 186, 30–35. [Google Scholar] [CrossRef]
- Dzikowska-Diduch, O.; Kurnicka, K.; Lichodziejewska, B.; Dudzik-Niewiadomska, I.; Machowski, M.; Roik, M.; Wiśniewska, M.; Siwiec, J.; Staniszewska, I.M.; Pruszczyk, P. Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism. J. Clin. Med. 2022, 11, 7369. [Google Scholar] [CrossRef]
- Nilsson, L.T.; Andersson, T.; Larsen, F.; Lang, I.M.; Liv, P.; Söderberg, S. Dyspnea after pulmonary embolism: A nation-wide population-based case–control study. Pulm. Circ. 2021, 11, 20458940211046830. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, K.; Kalantari, S.; Mohammadi, M.; Farrashi, M.; Kaviani, R.; Farmani, D.; Naghshbandi, M.; Moosavi, J.; Mohebbi, B.; Bakhshandeh, H.; et al. 3-year quality of life, functional performance, and long-term survival after acute pulmonary embolism; A prospective study. Pulm. Circ. 2024, 14, e70012. [Google Scholar] [CrossRef]
- Madonna, R.; Alberti, M.; Biondi, F.; Morganti, R.; Badagliacca, R.; Vizza, C.D.; De Caterina, R. Chronic thromboembolic pulmonary disease: Association with exercise-induced pulmonary hypertension and right ventricle adaptation over time. Eur. J. Intern. Med. 2024, 123, 120–126. [Google Scholar] [CrossRef]
- Dhayyat, A.; Hilde, J.M.; Jervan, Ø.; Rashid, D.; Gleditsch, J.; Stavem, K.; Ghanima, W.; Steine, K. Exercise pulmonary hypertension in chronic thromboembolic pulmonary disease: A right heart catheterization study. Pulm. Circ. 2024, 14, e70018. [Google Scholar] [CrossRef]
- de Groot, M.R.; Turkstra, F.; van Marwijk Kooy, M.; Oostdijk, A.H.; van Beek, E.J.; Buller, H.R. Value of chest X-ray combined with perfusion scan versus ventilation/perfusion scan in acute pulmonary embolism. Thromb. Haemost. 2000, 83, 412–415. [Google Scholar] [PubMed]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef]
- Klok, F.A.; Delcroix, M.; Bogaard, H.J. Chronic thromboembolic pulmonary hypertension from the persecutive of patients with pulmonary embolism. J. Thromb. Haemost. 2018, 16, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; Albanese, P.; Biasiolo, A.; Pegoraro, C.; Iliceto, S.; et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N. Engl. J. Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Delcroix, M.; Bogaard, H.J. Chronic throLuijten, D.; Talerico, R.; Barco, S.; Cannegieter, S.C.; Delcroix, M.; Ende-Verhaar, Y.M.; Huisman, M.V.; Konstantinidis, S.; Mairuhu, A.T.A.; van Mens, T.E.; et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: An updated systematic review and meta-analysis. Eur. Respir. J. 2023, 62, 2300449. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, N.; Kozak, M.; Jug, B. Post-acute pre-discharge echocardiography in the long-term prognostic assessment of pulmonary thrombembolism. Sci. Rep. 2021, 11, 2450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Argiento, P.; Chesler, N.; Mulè, M.; D’Alto, M.; Bossone, E.; Unger, P.; Naeije, R. Exercise stress echocardiography for the study of the pulmonary circulation. Eur. Respir. J. 2010, 35, 1273–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saito, Y.; Obokata, M.; Harada, T.; Kagami, K.; Sorimachi, H.; Yuasa, N.; Kato, T.; Wada, N.; Okumura, Y.; Ishii, H. Disproportionate exercise-induced pulmonary hypertension in relation to cardiac output in heart failure with preserved ejection fraction: A non-invasive echocardiographic study. Eur. J. Heart Fail 2023, 25, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Yamada, H.; Hotchi, J.; Bando, M.; Nishio, S.; Hirata, Y.; Ise, T.; Yamaguchi, K.; Yagi, S.; Soeki, T.; et al. Prediction of Future Overt Pulmonary Hypertension by 6-Min Walk Stress Echocardiography in Patients with Connective Tissue Disease. J. Am. Coll. Cardiol. 2015, 66, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Falter, M.; Bekhuis, Y.; L’Hoyes, W.; Milani, M.; Hoedemakers, S.; Soens, L.; Moura-Ferreira, S.; Dhont, S.; Pauwels, R.; Jacobs, A.; et al. Exercise Echocardiography for Risk Stratification in Unexplained Dyspnea: The Incremental Value of the Mean Pulmonary Artery Pressure/Slope. J. Am. Soc. Echocardiogr. 2025, 38, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Klin, B.; Zlotkevich, L.; Horne, T.; Livshitz, G.; Efrati, Y.; Vinograd, I. A selective approach to the treatment of acute scrotum in children. Pediatr. Surg. Int. 1996, 11, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Sostman, H.D.; Miniati, M.; Gottschalk, A.; Matta, F.; Stein, P.D.; Pistolesi, M. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. J. Nucl. Med. 2008, 49, 1741–1748. [Google Scholar] [CrossRef]
- Chemla, D.; Castelain, V.; Humbert, M. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 2004, 126, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in nonischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Vaidy, A.; Vahdatpour, C.A.; Mazurek, J. Exercise Testing in Patients with Pulmonary Hypertension. J. Clin. Med. 2024, 13, 795. [Google Scholar] [CrossRef] [PubMed]

| Clinical Features | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| Sex | 0.064 | ||
| Female | 8 | 23 | |
| Male | 12 | 12 | |
| Age | 59.40 (10.80) | 67.26 (18.68) | 0.053 |
| BMI | 26.76 (4.69) | 26.86 (5.01) | 0.94 |
| Smoking history | 0.839 | ||
| None or quit > 20 years | 10 | 18 | |
| Quit < 20 years | 3 | 7 | |
| Yes | 7 | 10 | |
| SAH | 0.325 | ||
| Absent | 18 | 28 | |
| Present | 2 | 7 | |
| Dyslipidemia | 0.234 | ||
| Absent | 18 | 27 | |
| Present | 2 | 8 | |
| Diabetes Mellitus | 0.624 | ||
| Absent | 19 | 32 | |
| Present | 1 | 3 | |
| Vascular events | 0.009 | ||
| Absent | 11 | 7 | |
| SVT + DVT | 0 | 11 | |
| PE | 3 | 8 | |
| DVT + PE | 6 | 9 | |
| Thyroid disease | 0.276 | ||
| Absent | 20 | 33 | |
| Hypothyroidism | 0 | 2 | |
| COPD | 0.475 | ||
| Absent | 19 | 31 | |
| Present | 1 | 4 | |
| Oncologic history | 0.082 | ||
| No | 17 | 22 | |
| Yes | 3 | 13 | |
| Known thrombophilia | 0.057 | ||
| No | 18 | 35 | |
| Yes | 2 | 0 | |
| Hematologic diseases | 0.446 | ||
| Absent | 20 | 34 | |
| Notspecified | 0 | 1 | |
| Family history of PAH | 0.276 | ||
| No | 20 | 33 | |
| Yes | 0 | 2 | |
| AoCo bypass | 0.446 | ||
| No | 20 | 34 | |
| Yes | 0 | 1 | |
| Valve surgery | 0.276 | ||
| No | 20 | 33 | |
| Yes | 0 | 2 | |
| Single-vessel CAD | 0.182 | ||
| No | 19 | 35 | |
| Yes | 1 | 0 | |
| Three-vessel CAD | 0.446 | ||
| No | 20 | 34 | |
| Yes | 0 | 1 | |
| Family history of CAD | 0.556 | ||
| No | 18 | 33 | |
| Yes | 2 | 2 | |
| Beta-blockers | 0.335 | ||
| No | 18 | 28 | |
| Yes | 2 | 7 | |
| Antiarrhythmics | 0.446 | ||
| No | 20 | 34 | |
| Yes | 0 | 1 | |
| Sartans | 0.425 | ||
| No | 19 | 31 | |
| Yes | 1 | 4 | |
| ACE inhibitors | 0.425 | ||
| No | 19 | 31 | |
| Yes | 1 | 4 | |
| Calcium channel blockers | 0.276 | ||
| No | 20 | 33 | |
| Yes | 0 | 2 | |
| Diuretics | 0.556 | ||
| No | 18 | 33 | |
| Yes | 2 | 2 | |
| Lipid-lowering drugs | 0.425 | ||
| No | 19 | 31 | |
| Yes | 1 | 4 | |
| Antidiabetic drugs | 0.425 | ||
| No | 19 | 31 | |
| Yes | 1 | 4 | |
| Temporary PE risk factors | 0.022 | ||
| No | 19 | 24 | |
| Yes | 1 | 11 | |
| Permanent PE risk factors | 0.356 | ||
| No | 15 | 22 | |
| Yes | 5 | 13 | |
| Unprovoked PE | 0.012 | ||
| No | 5 | 21 | |
| Yes | 15 | 14 | |
| Estroprogestin use | 0.036 | ||
| No | 19 | 25 | |
| Yes | 1 | 10 |
| Clinical Features | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| HR (bpm) | 67.05 (10.25) | 91.06 (22.43) | <0.001 |
| SBP (mmHg) | 118.45 (13.57) | 110.26 (17.86) | 0.062 |
| DBP (mmHg) | 76.10 (6.21) | 67.74 (14.92) | 0.006 |
| NT-proBNP | 633.55 (2309.90) | 5187.31 (8088.77) | 0.003 |
| WHO functional class | <0.001 | ||
| 1 | 12 | 6 | |
| 2 | 5 | 5 | |
| 3 | 2 | 24 | |
| 4 | 1 | 0 | |
| Heart failure | 0.006 | ||
| No | 14 | 11 | |
| Yes | 6 | 24 | |
| Syncope | 0.047 | ||
| No | 18 | 23 | |
| Yes | 2 | 12 | |
| Resting PH probability | <0.001 | ||
| Low | 17 | 0 | |
| Intermediate | 3 | 1 | |
| High | 0 | 34 | |
| PESI score at admission | <0.001 | ||
| Low | 16 | 9 | |
| Intermediate | 2 | 7 | |
| High | 2 | 19 | |
| Fibrinolysis | 0.001 | ||
| No | 20 | 21 | |
| Yes | 0 | 14 | |
| NOAC | 0.239 | ||
| No | 7 | 18 | |
| Yes | 13 | 17 | |
| IVC filter | 0.182 | ||
| No | 19 | 35 | |
| Yes | 1 | 0 | |
| Heparin | 0.003 | ||
| No | 19 | 20 | |
| Yes | 1 | 15 | |
| AngioCT acute phase | <0.001 | ||
| Mild | 14 | 6 | |
| Submassive | 4 | 6 | |
| Massive | 2 | 23 | |
| Tricuspid regurgitation | 0.262 | ||
| No | 4 | 12 | |
| Yes | 16 | 23 | |
| Mitral regurgitation | 0.714 | ||
| No | 7 | 14 | |
| Yes | 13 | 21 | |
| Aortic regurgitation | 0.116 | ||
| No | 20 | 31 | |
| Yes | 0 | 4 | |
| Aortic stenosis | 0.446 | ||
| No | 20 | 34 | |
| Yes | 0 | 1 |
| TTE Findings | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| LVEDD | 46.50 (7.56) | 50.11 (6.58) | 0.069 |
| LVESD | 27.61 (8.95) | 29.94 (6.64) | 0.326 |
| LVEDV | 117.05 (13.93) | 119.49 (30.20) | 0.685 |
| LVESV | 50.65 (18.48) | 41.80 (16.16) | 0.069 |
| LV mass | 135.15 (15.15) | 156.51 (28.57) | 0.003 |
| LAD | 40.40 (9.49) | 43.29 (4.81) | 0.216 |
| LAV | 25.55 (11.54) | 46.83 (10.42) | <0.001 |
| LVEF | 55.95 (13.26) | 65.00 (8.19) | 0.010 |
| FwSV LVOT | 80.95 (10.32) | 69.17 (7.61) | 0.009 |
| MR | 1.25 (0.85) | 1.06 (0.94) | 0.452 |
| AO disease | 0.25 (0.44) | 0.77 (0.91) | 0.006 |
| LV E/A | 1.18 (0.46) | 0.98 (0.44) | 0.083 |
| LV E/e’ | 14.25 (1.25) | 6.63 (2.18) | <0.001 |
| RD1 | 35.15 (7.56) | 53.31 (3.64) | <0.001 |
| RD2 | 29.30 (7.96) | 51.26 (5.38) | <0.001 |
| RD3 | 21.35 (4.90) | 49.37 (6.02) | <0.001 |
| RVOTprox | 24.70 (5.87) | 44.37 (4.87) | <0.001 |
| RVOTdist | 23.65 (5.46) | 43.31 (5.60) | <0.001 |
| Eccentricity index | 1.05 (0.12) | 0.67 (0.16) | <0.001 |
| RV/LV diameter ratio | 0.89 (0.11) | 0.77 (0.13) | <0.001 |
| TAPSE | 22.10 (4.85) | 14.29 (2.28) | <0.001 |
| FAC | 50.70 (10.63) | 31.34 (5.65) | <0.001 |
| RV E/A | 1.41 (0.26) | 0.63 (0.18) | <0.001 |
| RV E/e’ | 8.20 (1.70) | 16.77 (1.14) | <0.001 |
| TR | 1.60 (0.60) | 1.26 (0.74) | 0.084 |
| sPAP | 27.45 (10.11) | 44.74 (2.60) | <0.001 |
| mPAP | 18.65 (6.27) | 29.00 (1.64) | <0.001 |
| TR velocity | 2.01 (0.49) | 3.49 (0.27) | <0.001 |
| RVOT AT | 114.55 (12.52) | 61.31 (10.61) | <0.001 |
| IVC diameter | 16.30 (1.08) | 24.49 (3.17) | <0.001 |
| Right atrial area | 15.50 (2.67) | 16.31 (2.32) | 0.241 |
| TAPSE/sPAP | 0.98 (0.42) | 0.32 (0.06) | <0.001 |
| IVC collapsibility | 0.178 | ||
| No | 0 | 3 | |
| Yes | 20 | 32 |
| Clinical Features | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| WHO Functional Class | 0.013 | ||
| 1 | 10 | 5 | |
| 2 | 6 | 14 | |
| 3 | 4 | 16 | |
| Heart failure | 0.194 | ||
| No | 19 | 29 | |
| Yes | 1 | 6 | |
| CPET compatible with ex-PH | <0.001 | ||
| No | 17 | 4 | |
| Yes | 0 | 24 | |
| Resting PH probability | <0.001 | ||
| 1 | 19 | 0 | |
| 2 | 1 | 35 | |
| ESE compatible with ex-PH | <0.001 | ||
| No | 16 | 0 | |
| Yes | 0 | 24 | |
| HR (bpm) | 76.2 (14.7) | 72.7 (13.2) | 0.377 |
| SBP (mmHg) | 126.6 (14.2) | 128.0 (16.7) | 0.747 |
| DBP (mmHg) | 75.7 (7.3) | 75.4 (9.6) | 0.919 |
| NT-proBNP | 94.7 (134.4) | 1256.2 (562.5) | <0.001 |
| N. seg defect by P scan | 0.5 (0.9) | 2.9 (1.1) | <0.001 |
| 6MWT distance | 684.6 (52.1) | 469.2 (146.6) | <0.001 |
| CPET Findings | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| CPET compatible with ex-PH | <0.001 | ||
| No | 17 | 4 | |
| Yes | 0 | 24 | |
| Peak VO2 | 23.25 (3.98) | 15.75 (4.27) | <0.001 |
| VE/VCO2 slope | 18.00 (4.19) | 34.00 (6.52) | <0.001 |
| Peak O2 pulse | 23.62 (5.38) | 8.81 (3.49) | <0.001 |
| VD/VT | 0.87 (0.64) | 0.24 (0.12) | <0.001 |
| HR/VO2 slope | 2.90 (1.73) | 6.72 (2.81) | <0.001 |
| PetCO2 | 6.39 (8.94) | 6.21 (3.40) | 0.921 |
| TTE Findings | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| LVEDD | 46.60 (8.17) | 52.20 (7.21) | 0.011 |
| LVESD | 28.75 (7.45) | 32.23 (7.14) | 0.093 |
| LVEDV | 117.65 (20.26) | 120.94 (27.32) | 0.641 |
| LVESV | 47.50 (10.90) | 42.89 (15.38) | 0.243 |
| LV mass | 136.05 (14.71) | 158.00 (26.05) | 0.001 |
| LAD | 38.35 (11.17) | 44.26 (4.83) | 0.034 |
| LAV | 40.40 (9.21) | 47.14 (9.28) | 0.012 |
| LVEF | 59.30 (6.68) | 64.14 (7.65) | 0.022 |
| FwSV LVOT | 72.87 (10.27) | 66.46 (5.69) | 0.036 |
| MR | 1.25 (0.91) | 1.09 (0.92) | 0.525 |
| AO disease | 0.25 (0.55) | 0.80 (0.90) | 0.007 |
| LV E/A | 1.04 (0.40) | 0.92 (0.38) | 0.269 |
| LV E/e’ | 11.30 (2.25) | 7.31 (1.98) | <0.001 |
| RD1 | 30.45 (7.04) | 46.63 (5.13) | <0.001 |
| RD2 | 27.55 (6.12) | 44.77 (6.02) | <0.001 |
| RD3 | 22.80 (4.61) | 42.77 (6.32) | <0.001 |
| RVOT prox | 26.30 (2.87) | 39.26 (5.54) | <0.001 |
| RVOT dist | 25.70 (3.66) | 38.97 (5.99) | <0.001 |
| Eccentricity index | 1.14 (0.09) | 0.65 (0.13) | <0.001 |
| RV/LV diameter ratio | 0.85 (0.10) | 0.71 (0.12) | <0.001 |
| TAPSE | 22.00 (3.26) | 15.29 (2.01) | <0.001 |
| FAC | 56.60 (10.56) | 32.94 (5.26) | <0.001 |
| RV E/A | 1.61 (0.25) | 0.65 (0.14) | <0.001 |
| RV E/e’ | 8.10 (1.83) | 17.23 (1.24) | <0.001 |
| TR | 1.45 (0.69) | 1.23 (0.73) | 0.274 |
| sPAP | 24.10 (6.24) | 43.29 (2.18) | <0.001 |
| mPAP | 16.12 (4.30) | 28.00 (2.14) | <0.001 |
| TR velocity | 1.94 (0.45) | 3.32 (0.10) | <0.001 |
| RVOT AT | 118.00 (12.49) | 65.86 (9.54) | <0.001 |
| VCI diameter | 17.25 (0.72) | 19.71 (4.06) | 0.001 |
| Right atrial area | 15.90 (2.20) | 16.89 (1.76) | 0.074 |
| TAPSE/sPAP | 1.01 (0.42) | 0.35 (0.05) | <0.001 |
| IVC collapsibility | 0.116 | ||
| No | 20 | 31 | |
| Yes | |||
| ΔmPAP/CO | 2.09 (0.36) | 6.40 (1.28) | <0.001 |
| Clinical Features | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| WHO Functional Class | <0.001 | ||
| 1 | 18 | 7 | |
| 2 | 2 | 12 | |
| 3 | 0 | 16 | |
| Heart failure | 0.178 | ||
| No | 20 | 32 | |
| Yes | 0 | 3 | |
| Resting PH probability | <0.001 | ||
| 1 | 19 | 9 | |
| 2 | 1 | 25 | |
| 3 | 0 | 1 | |
| HR (bpm) | 74.15 (7.95) | 76.29 (8.29) | 0.355 |
| SBP (mmHg) | 131.20 (8.28) | 134.63 (9.59) | 0.186 |
| DBP (mmHg) | 73.15 (4.25) | 77.91 (5.69) | <0.001 |
| TTE Findings | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| LVEDD | 46.10 (6.73) | 47.40 (8.42) | 0.557 |
| LVESD | 28.80 (6.06) | 30.91 (6.67) | 0.248 |
| LVEDV | 111.00 (24.03) | 124.54 (27.65) | 0.073 |
| LVESV | 43.25 (12.96) | 43.20 (15.85) | 0.990 |
| LV mass | 162.45 (47.74) | 157.46 (22.40) | 0.663 |
| LAD | 39.50 (9.07) | 42.31 (5.75) | 0.164 |
| LAV | 39.00 (7.69) | 47.94 (9.64) | 0.001 |
| LVEF | 60.40 (8.98) | 64.74 (9.96) | 0.113 |
| FwSV LVOT | 67.50 (11.94) | 69.00 (6.89) | 0.611 |
| MR | 1.25 (0.85) | 1.11 (0.93) | 0.594 |
| AO disease | 0.20 (0.52) | 0.80 (0.96) | 0.004 |
| E wave | 0.73 (0.31) | 0.65 (0.12) | 0.309 |
| LV E/e′ | 13.80 (1.54) | 7.10 (2.24) | <0.001 |
| RD1 | 31.30 (5.22) | 39.43 (11.82) | 0.001 |
| RD2 | 29.25 (4.62) | 36.31 (10.77) | 0.001 |
| RD3 | 27.10 (4.47) | 32.80 (11.50) | 0.012 |
| RVOT prox | 23.95 (4.25) | 32.89 (8.49) | <0.001 |
| RVOT dist | 23.00 (4.22) | 33.06 (8.26) | <0.001 |
| Eccentricity index | 1.13 (0.08) | 1.03 (0.29) | 0.078 |
| RV/LV diameter ratio | 0.85 (0.08) | 0.78 (0.13) | 0.016 |
| TAPSE | 23.50 (3.07) | 19.57 (3.42) | <0.001 |
| FAC | 59.40 (5.09) | 37.40 (8.98) | <0.001 |
| RV E/A | 1.56 (0.24) | 0.72 (0.23) | <0.001 |
| RV E/e’ | 6.90 (1.74) | 24.86 (2.64) | <0.001 |
| TR | 1.35 (0.75) | 1.20 (0.76) | 0.481 |
| sPAP | 21.05 (4.62) | 39.46 (7.07) | <0.001 |
| mPAP | 14.75 (2.81) | 25.89 (4.39) | <0.001 |
| TR velocity | 2.23 (0.39) | 2.92 (0.40) | <0.001 |
| RVOT AT | 118.75 (9.48) | 85.71 (25.45) | <0.001 |
| IVC diameter | 16.40 (1.47) | 21.14 (3.19) | <0.001 |
| Right atrial area | 15.67 (2.09) | 21.66 (3.52) | <0.001 |
| TAPSE/sPAP | 1.15 (0.31) | 0.53 (0.18) | <0001 |
| IVC collapsibility | 0.276 | ||
| No | 0 | 2 | |
| Yes | 20 | 33 |
| ΔTTE Findings (t1–t4) | Q-Scan Negative (Mean ± SD or n/%) | Q-Scan Positive (Mean ± SD or n/%) | p-Value |
|---|---|---|---|
| WHO Functional Class | −20.83 (40.78)% | 8.57 (49.90)% | 0.029 |
| LVEF | 2.69 (17.06)% | 1.36 (12.71)% | 0.074 |
| RD1 | 4.79 (13.02) mm | −15.86 (22.03) mm | <0.001 |
| RD2 | 7.95 (13.66) mm | −18.57 (23.46) mm | <0.001 |
| RD3 | 21.69 (22.60) mm | −22.85 (26.01) mm | <0.001 |
| RVOT prox | −8.59 (14.33) mm | −16.51 (15.83) mm | 0.071 |
| RVOT dist | −10.13 (14.41) mm | −14.78 (17.73) mm | 0.296 |
| Eccentricity index | −0.69 (4.57) | 65.38 (62.47) | <0.001 |
| RV/LV diameter ratio | 0.46 (5.76) | 11.66 (20.85) | 0.005 |
| TAPSE | 9.03 (22.81) mm | 30.46 (29.73) mm | 0.007 |
| FAC | 8.25 (21.26) | 13.89 (21.71) | 0.354 |
| RV E/A | 13.74 (36.72) | −2.14 (12.88) | 0.025 |
| RV E/e’ | −13.11 (18.97) | 45.04 (19.26) | <0.001 |
| TR | −5.56 (23.57) mL | −6.90 (34.65) mL | 0.886 |
| sPAP | −7.46 (26.03) mmHg | −8.79 (16.09) mmHg | 0.838 |
| mPAP | 0.79 (47.20) mmHg | −7.17 (16.08) mmHg | 0.473 |
| TR velocity | 17.75 (17.34) m/s | −12.07 (11.86) m/S | <0.001 |
| RVOT AT | 1.45 (11.10) s | 29.76 (33.32) s | <0.001 |
| IVC diameter | −4.78 (9.21) mm | 10.45 (22.64) mm | 0.001 |
| Right atrial area | 2.33 (17.38) mm2 | 29.20 (22.95) mm2 | <0.001 |
| TAPSE/sPAP | 28.72 (63.69) mm/mmHg | 55.45 (61.82) mm/mmHg | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madonna, R.; Tocci, G.; Biondi, F.; Cipollini, V.; Morganti, R.; De Caterina, R. Chronic Thromboembolic Pulmonary Disease: Right Ventricular Function and Pulmonary Hemodynamics in a 4-Year Follow-Up. Int. J. Mol. Sci. 2025, 26, 10617. https://doi.org/10.3390/ijms262110617
Madonna R, Tocci G, Biondi F, Cipollini V, Morganti R, De Caterina R. Chronic Thromboembolic Pulmonary Disease: Right Ventricular Function and Pulmonary Hemodynamics in a 4-Year Follow-Up. International Journal of Molecular Sciences. 2025; 26(21):10617. https://doi.org/10.3390/ijms262110617
Chicago/Turabian StyleMadonna, Rosalinda, Giorgia Tocci, Filippo Biondi, Viola Cipollini, Riccardo Morganti, and Raffaele De Caterina. 2025. "Chronic Thromboembolic Pulmonary Disease: Right Ventricular Function and Pulmonary Hemodynamics in a 4-Year Follow-Up" International Journal of Molecular Sciences 26, no. 21: 10617. https://doi.org/10.3390/ijms262110617
APA StyleMadonna, R., Tocci, G., Biondi, F., Cipollini, V., Morganti, R., & De Caterina, R. (2025). Chronic Thromboembolic Pulmonary Disease: Right Ventricular Function and Pulmonary Hemodynamics in a 4-Year Follow-Up. International Journal of Molecular Sciences, 26(21), 10617. https://doi.org/10.3390/ijms262110617






