Abstract
Tuberculosis (TB), a human and animal disease caused by Mycobacterium tuberculosis (M.tb), has the highest global mortality rate after coronavirus disease 2019 (COVID-19) and poses a major public health threat globally. Since 1890, vaccine candidates for various forms of TB have been developed for different age groups, but these vaccine candidates have not provided intended protection in adolescents and adults in clinical trials. To help prevent and control the spread of TB, the development of a safe and effective TB vaccine is imperative. The MTB39A protein and the molecular adjuvant MTB32C protein were expressed by an insect-baculovirus expression system, and the recombinant baculovirus surface-displayed particles were evaluated for their immunogenicity in BALB/c mice and calves. The results showed that the rvAc-71CA/rvAc-MTB39A recombinant baculovirus surface-displayed particles exhibited good immunogenicity in mice and calves and could be further developed as potential candidates.
1. Introduction
Tuberculosis (TB) is a chronic infectious disease that primarily affects the lungs (pulmonary TB) but can also manifest in other parts of the body (extrapulmonary TB) [1]. When humans and cows with TB cough, droplets are discharged into the air, which can be inhaled by healthy people and cows to cause infection. According to the Global TB Report 2023, there are approximately 10 million new cases each year, and approximately one in four people worldwide have latent TB infection (LTBI), of which 5–10% will develop active TB, resulting in 1.3 million deaths. The incidence of human tuberculosis caused by Mycobacterium bovis accounts for 5–10% [2]. Although bovine tuberculosis has been controlled in many developed countries, it still threatens people’s health in many developing countries, posing a major public threat in many parts of the world [3].
Vaccination remains the primary means of preventing and controlling TB. Bacillus Calmette–Guerin (BCG) is the most time-tested and widely used TB vaccine and remains the only licensed TB vaccine; thus, BCG provides protection for kids, but its efficacy has been reduced in adults [4]. However, BCG has significant limitations, especially being age-dependent, that is, the inability to provide adequate protection against TB for adolescents, adults, and adult cattle [5,6]. Subunit vaccine candidates are designed to augment BCG-triggered responses and mainly include viral vector or recombinant protein-adjuvanted vaccines, of which recombinant protein-adjuvanted subunit vaccines are more attractive due to their excellent safety profile, well-defined molecular composition, and absence of an immune response generated by the body against the vector [7].
Members of a specific glycine-rich gene family, the PE/PPE gene family, are encoded in the M.tb genome, accounting for approximately 10% of the genomic coding region and including a total of 168 genes. The members of this family play key roles in the virulence, subcellular localization, and immune escape of M.tb and the host cell fate [8]. Among these members, MTB39A (PPE18), encoded by the Rv1196 gene, is able to inhibit macrophage-mediated presentation of MHC class II antigens in a dose-dependent manner [9,10,11]. Previous studies have shown that the C-terminal structural domain of MTB32A (MTB32C), which is recognized by IFN-γ-secreting CD8+ T cells [12], produces strong cellular responses in pre- and post-exposure mouse models of M.tb, making it an ideal candidate vaccine antigen. M.tb infections are a major source of anti-infective immunity, and CD4+ T cells—particularly the Th1 and Th17 subtypes—are essential for this defense. Th1 cells secrete IFN-γ (interferon-γ) and other cytokines that stimulate macrophages and aid in the destruction of intracellular pathogens, whereas Th17 cells aid in the recruitment of neutrophils and fortify mucosal defense by secreting IL-17 [13]. Ag85A and ESAT-6, two M.tb components, have been proven in vaccine experiments to be efficacious in eliciting CD4 T cell responses, namely Th1 and Th17 responses. In order to enhance protection against M.tb, Ag85A and ESAT-6 are frequently utilized in vaccine formulation to trigger a particular CD4 T cell immunological response [14].
Insect baculovirus expression systems can express some simple glycosylated proteins at high levels, are simple to operate, and can be used for vaccine development, particularly regarding adjuvant activity and safety. In this study, the MTB39A protein from M.tb was successfully expressed and localized using the baculovirus surface display system, and the resulting recombinant baculovirus surface-displayed particles were evaluated in mice and calves. The results indicated that the baculovirus surface display system can be applied as an effective method for the development of M.tb subunit vaccines.
2. Results
2.1. Construction and Characterization of MTB32C-MTB39A Recombinant Vector
Expression plasmid pFastBac dual-MTB32C-MTB39A-mCherry (pFastBac dual-71CA-mCherry, Figure 1) was constructed, and MTB32C and MTB39A were identified by gene synthesis followed by sequencing. PCR was performed to validate the bacmid using the M13 universal primer (Figure 2A,B), and full-length gels are presented in Supplementary Figure S1. The recombinant baculovirus display particles were named as rvAc-71CA-mCherry, and the supernatant of the infected cells was collected for virus preparation. The purified and concentrated virus (107 PFU/mL) was stored at −80 °C.
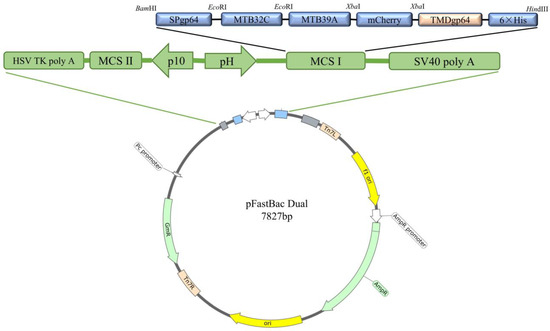
Figure 1.
Schematic presentation of the recombinant vector pFastBac dual-71CA-mCherry.
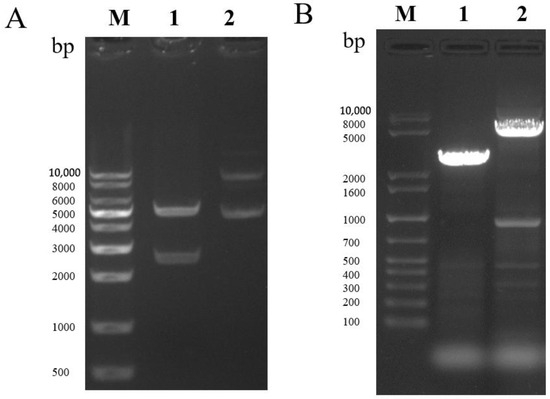
Figure 2.
(A) Double digest of the recombinant vector pFastBac dual-71CA-mCherry. Lane 1 shows the double digest BamHI-HindIII (2589–5238 bp), and Lane 2 shows the recombinant vector plasmid. (B) PCR identification of recombinant bacmid-71CA-mCherry. Lane 1 shows the pFastBac dual empty vector, and Lane 2 shows Bacmid-71CA-mCherry.
2.2. Localization of Antigenic Expression in Recombinant Baculovirus Surface Display Particles rvAc-71CA-mCherry
At 72, 96, and 120 h after transfection, the expression of red fluorescent protein was assessed by immunofluorescence identification and transmission electron microscopy. The results showed that the nuclei of both rvAc-71CA-mCherry and wild-type viruses showed blue fluorescence and could be stained by DAPI, while only cells infected with rvAc-71CA-mCherry produced green fluorescence signals. It was demonstrated that the protein expressed by cells infected with rvAc-71CA-mCherry could successfully locate on the surface of the cell membrane (Figure 3 and Figure 4). Immunocolloidal gold labeling of recombinant baculovirus revealed that the morphology of the fluorescent recombinant virus was basically similar to that of the wild-type baculovirus; both were rod-shaped, and the particle size was about 60 nm × 300 nm. On the surface of recombinant viruses incubated with mCherry, colloidal gold particles with a diameter of about 10 nm were gathered in the capsule region, but no colloidal gold particles were attached to the surface of wild-type viruses. The fluorescent protein that proves the success of the recombinant virus is displayed on its surface (Figure 5).
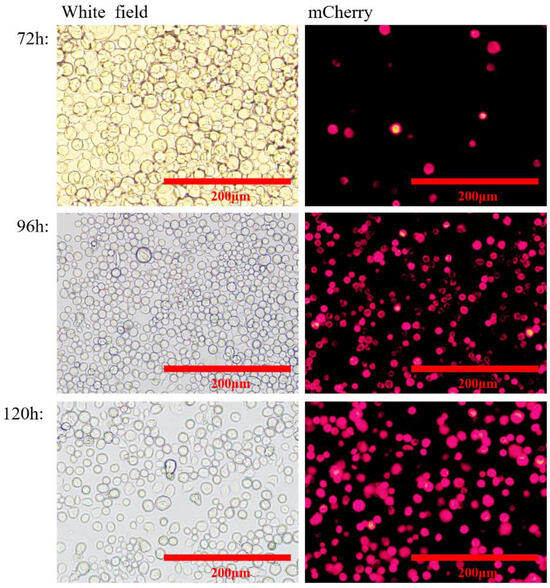
Figure 3.
Visualization of rvAc-71CA-mCherry-infected Sf9 cells through mCherry fluorescence.
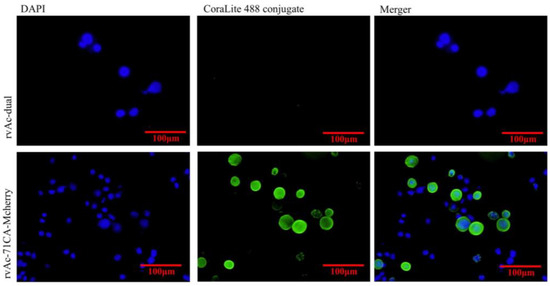
Figure 4.
Indirect immunofluorescence of recombinant virus- and wild-type virus-infected cells.
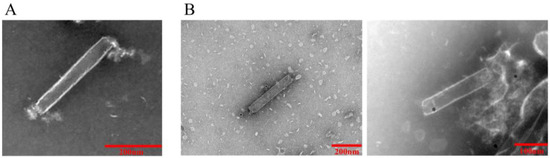
Figure 5.
Electron micrograph of recombinant baculovirus rvAc-71CA-mCherry. (A) rvAc-dual was incubated with anti-mCherry monoclonal antibody. (B) rvAc-71CA-mCherry was incubated with anti-mCherry monoclonal antibody.
2.3. rvAc-71CA/rvAc-MTB39A Induces Immune Responses in Mice
The fluorescent protein and/or MTB32C protein was excised to obtain the corresponding recombinant baculovirus-displayed nanoparticles rvAc-71CA/rvAc-MTB39A, and the expression of viral antigens was detected by Western blotting with an anti-6×His (Figure 6), and full-length blots are presented in Supplementary Figure S2. To assess the humoral immune response induced by both nanoparticles in mice, the IgG levels were measured by indirect enzyme-linked immunosorbent assay (ELISA), and the results are shown in Figure 7A. At 35 and 42 DAI, the serum anti-MTB39A IgG levels of the rvAc-71CA/rvAc-MTB39A group were significantly higher (p < 0.001) than those of the two negative control and BCG groups.
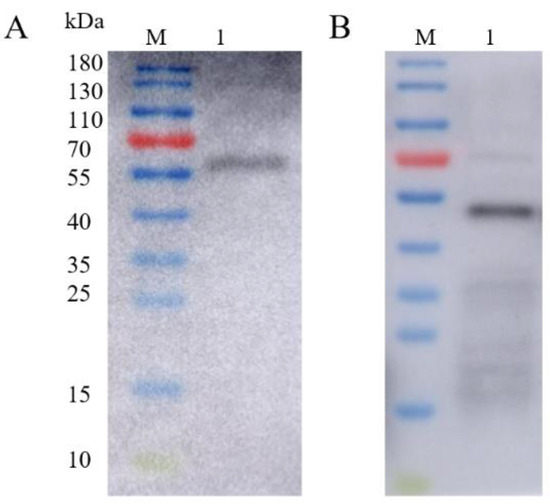
Figure 6.
Western blot identification of recombinant baculovirus surface-displayed particles. (A) M: 10–180 kDa; Lane 1: Western blot identification of rvAc-71CA (63.5 kDa). (B) M: 10–180 kDa; Lane 1: Western blot identification of rvAc-MTB39A (50.5 kDa).
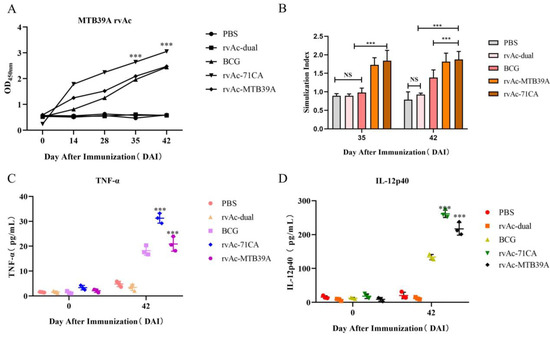
Figure 7.
Analysis of immunogenicity in mice. (A) Analysis of the IgG response induced by the MTB39A recombinant protein in mice measured by indirect ELISA. The y-axis indicates the optical density (OD450) values of serum samples collected from each group at 0, 14, 28, 35, and 42 DAI. *** p < 0.001, significantly different from the PBS, rvAc-dual, and BCG groups (Bonferroni test). (B) Results of the lymphocyte proliferation assay. The y-axis represents the stimulation of splenic lymphocyte samples collected at 35 and 42 DAI. ns, no significant difference; *** p < 0.001, significantly different (Bonferroni test). (C,D) Quantitative analysis of the IL-12p40 and TNF-α levels in the serum of immunized mice. All analyses were performed in triplicate, and the error bars show the standard deviations (SDs).
At 35 and 42 DAI, the splenocyte stimulation index was significantly higher in the rvAc-71CA/rvAc-MTB39A group than in the PBS, rvAc-dual, and BCG groups (p < 0.001; Figure 7B). The stimulation level observed with knife bean protein A (positive control for lymphocyte proliferation assay) was considered effective. The IL-12p40 and TNF-α levels in mouse serum were determined at 0 and 42 DAI. The rvAc-71CA/rvAc-MTB39A group exhibited significantly higher levels of IL-12p40 and TNF-α than the other three groups (p < 0.001; Figure 7C,D). The serum IL-12p40 and TNF-α levels of mice immunized with recombinant baculovirus surface-displayed nanoparticles of rvAc-71CA at 42 DAI were 261.27 ± 6.32 pg/mL and 31.25 ± 1.15 pg/mL, respectively, and the serum IL-12p40 and TNF-α levels of mice immunized with recombinant baculovirus surface-displayed nanoparticles of rvAc-MTB39A at 42 DAI were 216.93 ± 10.74 pg/mL and 20.89 ± 1.68 pg/mL, respectively. The results showed that the recombinant baculovirus induced cellular immunity in mice.
2.4. Evaluation of rvAc-71CA/rvAc-MTB39A Nanoparticle Vaccine-Activated T-Cell Responses and Safety in Mice
IFN-γ and IL-4 will induce the proliferation of CD4+ and CD8+ T cells, which mediates adaptive immunity to eradicate the infected cells. To examine the expression of IFN-γ and IL-4, we isolated lymphocytes from the spleens of immunized mice. The rvAc-MTB39A group had a higher CD4+/CD8+ ratio than rvAc-71CA, suggesting that the rvAc-MTB39A vaccine group elicited immunity primarily from CD4+ T cells, and rvAc-71CA elicited immunity primarily from CD8+ T cells (Figure 8A,B). As shown in Figure 8C–G, the rvAc-71CA immunized group secreted higher levels of IFN-γ+ and IL-4+ cells, inducing stronger cellular immune responses by T cells.
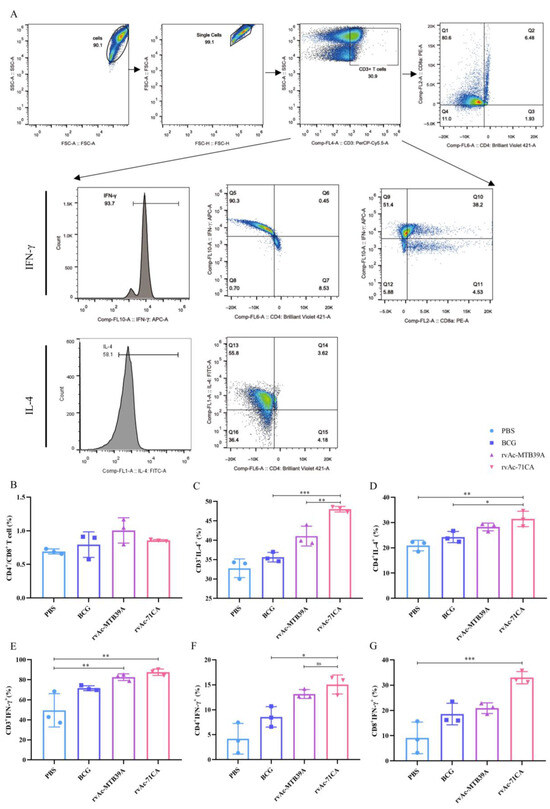
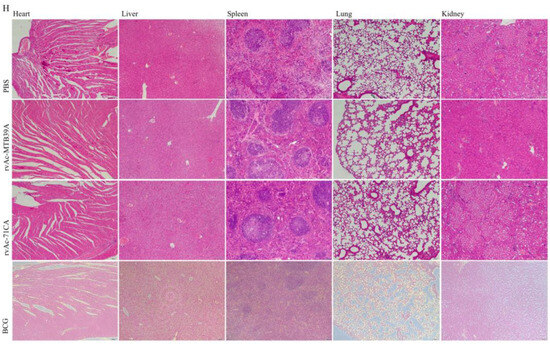
Figure 8.
T cell proliferation and safety evaluation of mice immunized with nanoparticle vaccines. (A) Flow cytometer gate strategy. (B) Percentage of CD4+/CD8+ T cells in splenic lymphocytes of immunized mice, (C) CD3+IL-4+ T cells, (D) CD4+IL-4+, (E) CD3+IFN-γ+, (F) CD4+IFN-γ+, and (G) CD8+IFN-γ+. (H) Haematoxylin–eosin (HE) staining of tissue sections of major organs (10×, n = 3). The data are shown as the means ± SDs and analyzed by two-way ANOVA using the Bonferroni test. ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001.
The safety of a vaccine plays a very important role in its clinical application. We examined the H&E-stained sections of the heart, liver, spleen, lungs, and kidneys of each group at 42 days and found no nodules or necrosis, among other features. Some tissues were then collected for H&E staining, and the results of microscopic examination are shown in the figure (Figure 8H). Tissue sections of the selected organs did not show any obvious pathological changes, such as congestion, bruising, necrosis, and inflammatory exudation, and had good biocompatibility.
2.5. rvAc-71CA/rvAc-MTB39A Induced Immune Response in Calves
As a zoonotic pathogen, TB infection of cattle poses a dire challenge not only to the animal husbandry industry but also to human health. Therefore, we also investigated the immunogenicity of the recombinant subunit vaccine in calves and analyzed the cytokine levels in the peripheral blood of calves (Figure 9A,B). After 30 d of immunization, calves in the rvAc-71CA/rvAc-MTB39A group were detected in vivo with IgG levels of (60,098.53 ± 2224.81 pg/mL, 52,269.23 ± 1674.35 pg/mL), 60 d IgG levels of (61,182.76 ± 447.33 pg/mL, 60,654.567 ± 430.86 pg/mL), and 90d IgG levels were (62,625.83 ± 3393.89 pg/mL, 60,279.03 ± 3417.33 pg/mL), which were not significantly different from BCG and BCG protein. The levels of TNF-α (462.42 ± 27.84 pg/mL, 407.73 ± 111.73 pg/mL) and IFN-γ (242.92 ± 29.15 pg/mL, 413.09 ± 22.82 pg/mL) in the peripheral blood were significantly higher than those in the PBS group (p < 0.001, Figure 9C–E), indicating that the rvAc-71CA subunit vaccine elicited significant humoral immune response and the Th1-type cellular immune response in calves.
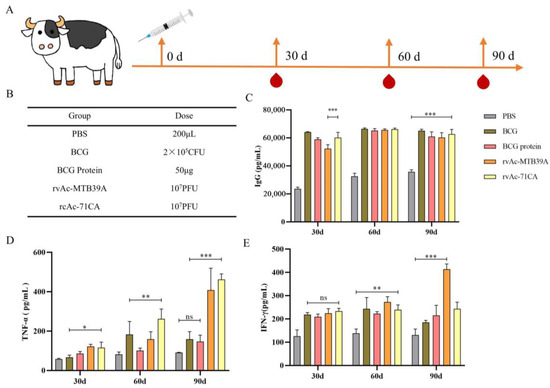
Figure 9.
Immunogenicity analysis of calves. (A) Calf immunization strategy; (B) calf immunization groups and doses; (C) IgG immune response; (D) quantitative analysis of cytokine TNF-α levels; and (E) quantitative analysis of cytokine IFN-γ levels. ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001, significant difference (Bonferroni test).
3. Discussion
As the number of TB cases and the comorbidity of TB and HIV increase, the only approved vaccine, BCG, is insufficient to achieve TB elimination in this century [15,16], and new prevention and control measures are urgently needed [17], among which the development of new and effective TB vaccines is an important means of preventing TB [18]. In this way, the immune system of M.tb-infected individuals can establish immune homeostasis, inhibit immune damage, enhance immunity, or clear M.tb [19]. M72/AS01E is a tuberculosis vaccine candidate designed to prevent the progression of latent tuberculosis infection to active tuberculosis. The vaccine consists of the recombinant protein antigen M72 (MTB39A and MTB32A) together with the AS01E adjuvant system, which is an immune-enhancing adjuvant. Clinical trials have demonstrated that M72/AS01E shows some protection in healthy adults as well as in people with latent TB infection, particularly in preventing TB progression. The vaccine offers a potential new tool in the global response to the TB epidemic, but its long-term efficacy and broad applicability remain to be validated in further clinical studies. The first vaccine candidate with protective efficacy against tuberculosis in clinical phase IIb trials was the modified Ankara vaccine (MVA), a viral vector vaccine expressing M.tb Ag85 complex antigens; however, the T-cell activation and elevation of the blood monocyte/lymphocyte ratios induced by this vaccine increase the risk of developing TB, and vaccination failed to produce any protective effect [20,21]. Subunit vaccines benefit from having a novel adjuvant with potent T-cell stimulation ability and no harmful side effects, and one such vaccine was designed to enhance the BCG-triggered response [22,23]. Among these, subunit vaccines that specifically target important Mycobacterium TB antigens like Ag85A/B and ESAT-6 can elicit particular immune responses. These vaccinations are intended to be administered in conjunction with BCG in order to boost immunological effectiveness and give recipients of the BCG vaccination extra protection.
However, in this study, BCG was not used for initial immunization, and the subunit vaccine was used for booster immunization. We hope that the prepared subunit vaccine can achieve the initial immunization effect of replacing BCG, with a view to providing an experimental basis for the development of a new type of tuberculosis vaccine. Recent years have seen some advancements in the study of subunit vaccines; these include the H56 and ID93 vaccines, which have shown promise in preclinical and early-stage clinical studies for inducing a robust T cell response [24]. The subunit vaccine produced in this study will eventually integrate protection tests and immunogenicity data to further measure the vaccine’s protective impact. This will help to guarantee that the vaccination is effective in preventing tuberculosis and will allow us to better assess the vaccine’s clinical application potential [25].Baculovirus expression vector systems (BEVSs) have the advantages of a high level of exogenous gene expression, high biosafety, and a short replication time [26,27,28]. BEVSs have been widely used in biopharmaceuticals, genetic engineering, and vaccine research and production, and the production of HPV and influenza virus vaccines using this system has shown good results [29,30].
MTB39A protein and MTB32A protein can enhance phagocytic activity, and the C-terminal structural domain MTB32C can produce a strong CD8 T-cell immune response, which are candidate vaccine antigens [31]. In this study, we chose to use the baculovirus expression system with the PE/PPE protein family members, MTB39A protein and MTB32C protein, in order to achieve similar or better immunization effects than the M72 vaccine. And to provide a reference for vaccines prepared by such systems.
In this study, the M.tb protein MTB32C-MTB39A(71CA) was successfully expressed by a BEVS. The expression of nanoparticles labelled with mCherry could be directly observed under an inverted microscope (Figure 3), demonstrating that they were displayed on the plasma membrane of Sf9 cells (Figure 4) and on the vesicle membrane of the virus (Figure 5). These results indicate that the recombinant baculovirus displaying the mCherry-tagged MTB39A protein was correctly assembled and able to infect Sf9 cells. The immunogenicity of the recombinant protein MTB39A was verified in both mice and calves. In mouse immunization experiments, rvAc-71CA induced a robust IgG response in the rvAc-dual and rvAc-MTB39A groups at 35 and 42 DAI. This result suggests that rvAc-71CA can induce the development of strong humoral immunity against the MTB39A antigen in mice (Figure 7A). The lower antibody levels in the rvAc-MTB39A group compared with the rvAc-71CA group possibly occurred because the MTB32C protein enhances the immunogenicity of the MTB39A protein.
In cellular immunity, both CD4+ T and CD8+ T cells are central to the defense against M. tb infection, and their content and CD4+ T/CD8+ T cell ratios are commonly used to measure the state of cellular immunity in the body [32]. Lymphocyte proliferation and cytokine production after antigenic stimulation are also important indicators of immune effects. The results of the lymphocyte proliferation assay and flow cytometry showed that rvAc-71CA/rvAc-MTB39A induced strong cellular immunity against the MTB39A antigen (Figure 7B) and increased the serum levels of IL-12p40 and TNF-α in BALB/c mice (Figure 7C,D). The expression of CD4+IFN-γ+, CD8+IFN-γ+, and CD4+IL-4+ T cells in the rvAc-71CA immunized group was higher than that in the rv-MTB39A group, suggesting that the MTB32C protein elicits a strong CD8+ T-cell immune response, which is able to participate in the killing of infected cells (Figure 8A–G) and that the nanoparticle vaccine exhibited good biocompatibility (Figure 8H). In the calf immunization assay (Figure 9A,B), rvAc-71CA/rvAc-MTB39A significantly increased the levels of serum antibody IgG (Figure 9C) and the cytokines TNF-α and IFN-γ (Figure 9D,E), which resulted in the host being able to resist M.tb [33].
4. Materials and Methods
4.1. Construction of the MTB32C-MTB39A Gene and Expression Vector
Based on studies of the MTB39A protein and MTB32A protein, we selected the gene sequence corresponding to the MTB39A protein of M.tb H37Rv (GenBank ID: NC_000962.3) and intercepted the MTB32A sequence (GenBank ID: MTCI418B.07) of the 396-bp C segment (MTB32C). The MTB39A and MTB32C gene sequences were designed to encode the fusion protein MTB32C-MTB39A with a His tag at the C-terminus, referred to as 71CA. mCherry was used as the protein tag for visualization in assays. The sequence SPgp64 (GenBank AFO10080.1) was inserted upstream of the target protein. The downstream tag protein was inserted into TMDgp64 (GenBank CAA24524.1). The fusion gene fragment SPgp64-71CA-mCherry-TMDgp64-6×His was then inserted into the pFastBac dual vector to generate pFastBac dual-71CA-mCherry (Table 1).

Table 1.
Antigens selected in this experiment.
4.2. Culture of Grassland Nightshade Moth (Sf9) Cells and Amplification of Recombinant Baculovirus
Insect Sf9 cells were cultured at 27 °C and used for gene expression. The recombinant plasmid pFastBac dual-71CA-mCherry and the plasmid pFastBac dual (negative control) were transformed into DH10 Bac, and the positive colonies obtained were amplified with M13 universal primers. The positive bacilli were named Bacmid-71CA-mCherry, and the negative bacilli were named Bacmid-dual. Sf9 cells in good growth condition were spread into 6-well plates, ensuring that cell confluence was around 80%. To prepare the transfection mixture, 95 μL of Sf900 medium and 5 μL of TransIT®-LT1 Transfection Reagent (Mirus) were added [34]. Into a 1.5 mL ep tube, 1 μg of Bacmid was added, the volume was made up to 100 μL with the medium, and the sample was incubated for 5 min at room temperature. Finally, the diluted Bacmid was gently added into the transfection reagent, blown and mixed, and left for 20 min at room temperature. The diluted Bacmid was gently added to the transfection reagent; it was blown and mixed and kept at room temperature for 20 min. Then, 200 μL of transfection mixture was placed drop by drop into each well of pre-layered Sf9 cells and cultivated in the cell culture incubator at 27 °C. The viruses produced by the transfected virus were of the P0 generation.
The rvAc-71CA/rvAc-MTB39A viruses were implanted into suspension-cultured Sf9 cells at a density of roughly 2 × 106 cells/mL with MOI = 0.1. Following 72 h of infection, the suspension was collected by centrifugation at 2500 rpm for 5 min at 4 °C, cell debris was removed by centrifugation at 10,000 rpm for 30 min, and virus particles were recovered by ultracentrifugation at 35,000 rpm for 2 h. The virus particles were recovered via ultracentrifugation at 35,000 rpm. The virus particles were added to a gradient sucrose solution containing 20–40–60% sucrose, ultracentrifuged at 35,000 rpm for 2 h at 4 °C, and the turbid zone between the 40–60% sucrose solution was collected, ultracentrifuged again to remove sucrose, then solved with PBS and stored at -80 °C.
4.3. Indirect Immunofluorescence and Immunocolloidal Gold Electron Microscopy
To visualize the location of fusion protein 71CA in infected Sf9 cells, recombinant baculovirus surface-displayed particles rvAc-71CA-mCherry and rvAc-dual (MOI of infection plural = 0.5) were infected into Sf9 cells until cytopathic lesions appeared, supernatants were discarded, and cells were fixed with 4% paraformaldehyde (Solarbio, Beijing, China), 0.2% Triton X-100 (Solarbio, China), Permeabilization was performed, 5% BSA blocking solution was blocked for 1 h, mouse anti-His monoclonal antibody (1:500 dilution; Abcam, Shanghai, China) was incubated overnight, PBS was washed 3 times, CoraLite 488 coupled goat anti-mouse IgG (secondary antibody, 1:500 dilution; Proteintech, Wuhan, China) was incubated, and nuclei were stained with DAPI. Fluorescence images were taken with a laser confocal microscope (Leica TCS SPE, Wetzlar, Germany).
To determine the location of the fusion protein 71CA in the baculovirus envelope, rvAc-dual and rvAc-71CA-mCherry were adsorbed in a 200-mesh copper mesh for 5 min, air-dried, washed with 0.01 mol/L PBS for 2 min, blocked with 1% BSA for 1 h, and incubated with a mouse anti-mCherry monoclonal antibody (1:1000 dilution) for 2 h at room temperature (Abmart, Shanghai, China). The secondary antibody, protein-A-gold coupled with colloidal gold particles, was incubated at room temperature for 1 h. After washing, the sample-loaded copper mesh was naturally dried, dyed with 3% phosphotungstic acid solution for 5 min, and photographed at 80 kV in a transmission electron microscope (Hitachi HC-1, Tokyo, Japan).
4.4. Vector and Western Blot Identification for Animal Immunization Experiments
To obtain proteins without a fluorescent tag and MTB32C, two vectors, pFastBac dual-71CA (with the tag protein mCherry removed) and pFastBac dual-MTB39A (with the mCherry and MTB32C proteins removed), were produced. The recombinant baculovirus displaying rvAc-71CA/rvAc-MTB39A was obtained using the method described above, and rvAc-dual served as a negative control. The baculovirus rvAc-71CA/rvAc-MTB39A was identified by protein blotting using the following antibodies: mouse anti-His monoclonal antibody (1:5000, Abcam) and HRP-conjugated AffiniPure goat anti-mouse IgG (H + L) (1:5000, Proteintech).
4.5. Mouse Vaccination
Seventy-five 6–8-week-old healthy female BALB/c mice were purchased from Beijing Vitonda Biotechnology Co., Ltd., Beijing, China (certificate of conformity No. SCXK Jing 2021-0006) and randomly divided into five groups of 15 mice each (Table 2). Immunization was performed by subcutaneous injection at 2-week intervals, and the BCG and experimental dose was based on relevant studies [35,36]. Serum samples were collected at 0, 14, 28, 35, and 42 days after immunization (DAI), and three mice in each group were sacrificed at 35 and 42 DAI. Spleens were isolated for lymphocyte proliferation assays, and mouse experiments were performed in accordance with the recommendations of the Experimental Ethics Committee of the Experimental Animal Center of Ningxia Medical University (IACUC-NYLAC-2021-126).

Table 2.
Vaccination strategy in mice.
4.6. Analysis of the Serum IgG Antibody Levels
The enzyme plate was coated with MTB39A protein at a concentration of 1 μg/mL (0.05 mol/L carbonate buffer, pH 9.6) overnight at 4 °C, incubated with 5%BSA at 37 °C for 2h, and then the added serum samples were collected by 0, 14, 28, 35, and 42 DAI (1:100 dilution), incubated for 1h, and washed with PBST 3 times. Then, the samples were incubated with enzyme-labeled goat anti-mouse IgG secondary antibody for 1h (1:100 dilution; Proteintech). Finally, the reaction was stopped using 2M H2SO4 after 30min of TMB color (Solarbio, China) development solution. All the samples were analyzed in triplicate, and the absorbance was determined with a microplate reader (EnSpire, Waltham, MA, USA) at OD450 nm.
4.7. Lymphocyte Proliferation Test
rvAc-71CA/rvAc-MTB39A, 100 μL of RPMI-1640 (negative control, Gibco, NY, USA), and knife bean protein A (5 μg/mL; Solarbio, Beijing, China, positive control) were used as stimulants at 106 PFUs/mL. Splenic lymphocyte proliferation was detected using the MTT assay (5 mg/mL; Solarbio Beijing, China) by determining the OD490 nm. The cellular immune effect was reflected by the stimulation index (SI) of the splenic lymphocyte proliferation assay: SI = mean OD490 value of the stimulated wells/mean OD490 value of the negative control wells.
4.8. Flow Cytometry
Splenocytes were isolated 42 days after immunization, inoculated into 6-well plates at 1 × 106 cells per well, and stimulated in vitro with 5 μg/mL of rvAc-71CA and rvAc-MTB39A for 12 h. The cells were centrifuged at 1000 rpm for 5min, collected, and then used for surface staining (Proteintech, Wuhan, China): CoraLite®Plus 405 Anti-Mouse CD4 (GK1.5, Proteintech, Wuhan, China)), PE Anti-Mouse CD3 (17A2, Proteintech, Wuhan, China)), and CoraLite®Plus 705 Anti-Mouse CD8a (53-6.7, Proteintech, Wuhan, China)). After permeabilization of the cells with complete surface staining, the intracellular cytokine staining antibodies used were CoraLite®Plus 488 Anti-Mouse IL-4 (11B11, Proteintech, Wuhan, China)) and CoraLite®Plus 647 Anti-Mouse IFN-γ (XMG1.2, Proteintech, Wuhan, China)). The cells were then detected with a SONY MA900 and analyzed using FlowJo 10.0 (Tree Star Inc., Ashland, OR, USA) software.
4.9. H&E Staining
The hearts, livers, spleens, lungs, and kidneys of mice in each group at 42 d were taken, fixed in 4% paraformaldehyde, and after paraffin sectioning, the nuclei of the cells were stained with hematoxylin, the cytoplasm was stained with eosin, and finally dehydrated and sealed, and under an inverted microscope, the images were captured.
4.10. Immunization Strategy and Cytokine Detection in Calves
Fifteen calves at 21 days of age were randomly divided into five groups of three calves each. The first immunization was administered via a subcutaneous injection, and the doses of the commercial vaccine BCG and cleaved whole BCG protein (whole bacterial lysate) were based on relevant research literature. The recombinant baculovirus group, the BCG, and the BCG protein all require only one injection and no booster immunization. Blood was collected intravenously 30, 60, and 90 days after the initial immunization, and serum was collected by centrifugation. The serum antibody levels of IgG and the levels of the cytokines TNF-α and IFN-γ were detected by indirect ELISA. The calf experiments were performed according to the recommendations of the Experimental Ethics Committee of the Experimental Animal Center of Ningxia University.
4.11. Statistical Analysis
The statistical analyses were performed using GraphPad Prism® Version 6 (GraphPad Software, San Diego, CA, USA) software. The immunoreactivity of the groups was compared by Bonferroni’s test. The data are expressed as the means ± SDs, and p < 0.05 was considered to indicate a significant difference.
5. Conclusions
This report describes the production, identification, and immunogenicity of recombinant baculoviruses displaying MTB32C and MTB39A fusion antigenic proteins. The results show that this recombinant baculovirus is able to induce both humoral and cellular effects in mice and calves. In addition, it represents a potential vaccine candidate against MTB32C and MTB39A.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26020797/s1.
Author Contributions
P.W. and G.Z. wrote the original draft and performed the experiments. L.J. participated in experiments. S.Z. and W.G. crunched the numbers. Y.L. and Z.W. provided the initial idea, designed the research, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number U22A20525), the Key Research and Development Program of Ningxia Hui Autonomous Region (grant number: 2023BCF01038), the Key Research and Development Program of Ningxia Hui Autonomous Region (grant number 2021BEF02028), the Nanjing University-Ningxia University Collaborative Project (grant number 2017BN04), and the Ningxia Natural Science Foundation (2022AAC03077).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Experimental Animal Welfare Ethics Committee of the Center for Experimental Animals of Ningxia Medical University (IACUC-NYLAC-2021-126, 2023.5.4).
Informed Consent Statement
Data is contained within the article and Supplementary Materials.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank Yulong He of Zhejiang Sci-Tech University for his help with the cell studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed]
- Thoen, C.; Lobue, P.; de Kantor, I. The importance of Mycobacterium bovis as a zoonosis. Vet. Microbiol. 2006, 112, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Global Tuberculosis Report. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (accessed on 2 May 2024).
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.E.; Netea, M.G.; Mandalakas, A.M. 100 years of Mycobacterium bovis bacille Calmette-Guerin. Lancet Infect. Dis. 2022, 22, e2–e12. [Google Scholar] [CrossRef] [PubMed]
- Gela, A.; Murphy, M.; Rodo, M.; Hadley, K.; Hanekom, W.A.; Boom, W.H.; Johnson, J.L.; Hoft, D.F.; Joosten, S.A.; Ottenhoff, T.H.M.; et al. Effects of BCG vaccination on donor unrestricted T cells in two prospective cohort studies. EBioMedicine 2022, 76, 103839. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Kumari, A.; Das, G.; Dwivedi, V.P. Tuberculosis vaccine: A journey from BCG to present. Life Sci. 2020, 252, 117594. [Google Scholar] [CrossRef]
- Fruth, U.; Young, D. Prospects for new TB vaccines: Stop TB Working Group on TB Vaccine Development. Int. J. Tuberc. Lung Dis. 2004, 8, 151–155. [Google Scholar] [PubMed]
- Kohli, S.; Singh, Y.; Sharma, K.; Mittal, A.; Ehtesham, N.Z.; Hasnain, S.E. Comparative genomic and proteomic analyses of PE/PPE multigene family of Mycobacterium tuberculosis H(3)(7)Rv and H(3)(7)Ra reveal novel and interesting differences with implications in virulence. Nucleic Acids Res. 2012, 40, 7113–7122. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.M.; Mahaffey, S.B.; Kruh, N.A.; Dobos, K.M. Proteomic definition of the cell wall of Mycobacterium tuberculosis. J. Proteome Res. 2010, 9, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Dolasia, K.; Nazar, F.; Mukhopadhyay, S. Mycobacterium tuberculosis PPE18 protein inhibits MHC class II antigen presentation and B cell response in mice. Eur. J. Immunol. 2021, 51, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.H.; Ahmed, A.; Kumar, S.; Sharma, P.; Mukhopadhyay, S. Role of PPE18 protein in intracellular survival and pathogenicity of Mycobacterium tuberculosis in mice. PLoS ONE 2012, 7, e52601. [Google Scholar] [CrossRef]
- Skeiky, Y.A.; Alderson, M.R.; Ovendale, P.J.; Guderian, J.A.; Brandt, L.; Dillon, D.C.; Campos-Neto, A.; Lobet, Y.; Dalemans, W.; Orme, I.M.; et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 2004, 172, 7618–7628. [Google Scholar] [CrossRef] [PubMed]
- Tishon, A.; Lewicki, H.; Rall, G.; Von Herrath, M.; Oldstone, M.B. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology 1995, 212, 244–250. [Google Scholar] [CrossRef]
- Smith, D.J. Prospects in caries vaccine development. J. Dent. Res. 2012, 91, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Eisenhut, M.; Harris, R.J.; Rodrigues, L.C.; Sridhar, S.; Habermann, S.; Snell, L.; Mangtani, P.; Adetifa, I.; Lalvani, A.; et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ 2014, 349, g4643. [Google Scholar] [CrossRef] [PubMed]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef]
- Mendelsohn, S.C.; Fiore-Gartland, A.; Penn-Nicholson, A.; Mulenga, H.; Mbandi, S.K.; Borate, B.; Hadley, K.; Hikuam, C.; Musvosvi, M.; Bilek, N.; et al. Validation of a host blood transcriptomic biomarker for pulmonary tuberculosis in people living with HIV: A prospective diagnostic and prognostic accuracy study. Lancet Glob. Health 2021, 9, e841–e853. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022, 13, 830497. [Google Scholar] [CrossRef] [PubMed]
- Rijnink, W.F.; Ottenhoff, T.H.M.; Joosten, S.A. B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front. Immunol. 2021, 12, 640168. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Griffiths, K.L.; Poyntz, H.C.; Harrington-Kandt, R.; Dicks, M.D.; Stockdale, L.; Betts, G.; McShane, H. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine 2015, 33, 6800–6808. [Google Scholar] [CrossRef]
- Hawkridge, T.; Scriba, T.J.; Gelderbloem, S.; Smit, E.; Tameris, M.; Moyo, S.; Lang, T.; Veldsman, A.; Hatherill, M.; Merwe, L.; et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J. Infect. Dis. 2008, 198, 544–552. [Google Scholar] [CrossRef]
- Ullah, I.; Bibi, S.; Ul Haq, I.; Safia; Ullah, K.; Ge, L.; Shi, X.; Bin, M.; Niu, H.; Tian, J.; et al. The Systematic Review and Meta-Analysis on the Immunogenicity and Safety of the Tuberculosis Subunit Vaccines M72/AS01E and MVA85A. Front. Immunol. 2020, 11, 1806. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Kaufmann, S.H. Tuberculosis vaccines: Time to think about the next generation. Semin. Immunol. 2013, 25, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Schrager, L.K.; Harris, R.C.; Vekemans, J. Research and development of new tuberculosis vaccines: A review. F1000Research 2019, 7, 1732. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.R. Recombinant expression systems in the pharmaceutical industry. Appl. Microbiol. Biotechnol. 2004, 65, 363–372. [Google Scholar] [CrossRef] [PubMed]
- van Loo, N.D.; Fortunati, E.; Ehlert, E.; Rabelink, M.; Grosveld, F.; Scholte, B.J. Baculovirus infection of nondividing mammalian cells: Mechanisms of entry and nuclear transport of capsids. J. Virol. 2001, 75, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chung, Y.C.; Hu, Y.C. Update on baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev. Vaccines 2014, 13, 1501–1521. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kawasaki, M.; Hariguchi, N.; Hirota, K.; Matsumoto, M. A baculovirus dual expression system-based malaria vaccine induces strong protection against Plasmodium berghei sporozoite challenge in mice. Infect. Immun. 2009, 77, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.C.; Lu, H.R.; Ross, T.M. Hemagglutinin displayed baculovirus protects against highly pathogenic influenza. Vaccine 2010, 28, 6821–6831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Feng, Y.; Li, L.; Ye, X.M.; Wang, J.L.; Wang, Q.; Li, P.C.; Li, N.; Zheng, X.H.; Gao, X.; et al. Immunization with an adenovirus-vectored TB vaccine containing Ag85A-Mtb32 effectively alleviates allergic asthma. J. Mol. Med. 2018, 96, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Wu, Y.; Behar, S.M. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J. Immunol. 2008, 181, 8595–8603. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lao, S.; Yang, B.; Yu, S.; Zhang, Y.; Wu, C. Mycobacterium tuberculosis-Specific IL-21+IFN-gamma+CD4+ T Cells Are Regulated by IL-12. PLoS ONE 2016, 11, e0147356. [Google Scholar]
- Yao, L.; Sun, J.; Xu, H.; Kan, Y.; Zhang, X.; Yan, H.C. A novel economic method for high throughput production of recombinant baculovirus by infecting insect cells with Bacmid-containing diminopimelate-auxotrophic Escherichia coli. J. Biotechnol. 2010, 145, 23–29. [Google Scholar] [CrossRef]
- Gunasena, M.; Shukla, R.K.; Yao, N.; Rosas Mejia, O.; Powell, M.D.; Oestreich, K.J.; Aceves-Sanchez, M.J.; Flores-Valdez, M.A.; Liyanage, N.P.M.; Robinson, R.T. Evaluation of early innate and adaptive immune responses to the TB vaccine Mycobacterium bovis BCG and vaccine candidate BCGDeltaBCG1419c. Sci. Rep. 2022, 12, 12377. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, G.; Zheng, W.; Shu, J.; Chen, J.; Yang, F.; Wu, Y.; He, Y. Development of a Combined Genetic Engineering Vaccine for Porcine Circovirus Type 2 and Mycoplasma Hyopneumoniae by a Baculovirus Expression System. Int. J. Mol. Sci. 2019, 20, 4425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).