Abstract
Type 2 diabetes mellitus (T2DM) is one of the most widespread chronic diseases globally, with its prevalence expected to rise significantly in the years ahead. Previous studies on risk stratification for T2DM identify certain biomarkers, including glycated hemoglobin (HbA1c), oral glucose tolerance testing (OGTT), fructosamine, and glycated albumin, as key indicators for predicting the onset and progression of T2DM. However, these traditional markers have been shown to lack sensitivity and specificity and their results are difficult to analyze due to non-standardized interpretation criteria, posing significant challenges to an accurate and definitive diagnosis. The strict measures of these traditional markers may not catch gradual increases in blood sugar levels during the early stages of diabetes evolution, as these might still fall within acceptable glycemic parameters. Recent advancements in research have suggested novel micro ribonucleic acid (miRNA) as circulatory molecules that can facilitate the early detection of prediabetic conditions in high-risk groups and potentially enable prevention of the progression to T2DM. This capability makes them a very powerful tool for potentially improving population health, enhancing outcomes for many patients, and reducing the overall burden of T2DM. These promising biomarkers are small, noncoding RNA involved in the regulation of many cellular functions that have a hand in the metabolic activities of cells, making them a very useful and relevant biomarker to explore for the diagnosis and risk stratification of T2DM. This review analyzes the current literature, outlining the occurrence of miRNAs in prediabetic and diabetic individuals and their implications in predicting dysglycemic disorders.
1. Introduction
Across the spectrum of diabetes, two primary forms are the most prevalent: type 1 diabetes (T1DM) and type 2 diabetes (T2DM). T1DM is an autoimmune disorder characterized by the self-destruction of pancreatic β-cells and is relatively less prevalent than T2DM [1]. Conversely, T2DM is marked by hyperglycemia caused by the loss of β-cells through apoptosis, leaving a residual population of dysfunctional β-cells. Over time, these dysfunctional β-cells cannot counteract the effects of increasing insulin resistance, leading to inadequate insulin secretion and elevated blood glucose levels [2,3,4]. Prolonged hyperglycemia can lead to severe diabetes-related complications, specifically microvascular complications, such as retinopathy, neuropathy, and nephropathy [5,6], or macrovascular complications, such as atherosclerosis, in the vessels supplying the heart, brain, limbs, and other organs.
Before the onset of fully developed T2DM, many individuals pass through an intermediary stage known as prediabetes. Prediabetes is the stage of abnormal glucose homeostasis where blood glucose levels surpass normal glycemic parameters but remain below the diagnostic threshold [7]. Prediabetes is widespread, particularly among older and obese demographics, often persisting for years before the full clinical condition develops [8].
Addressing this early stage is crucial because although pharmacological interventions can slow the progression of T2DM, they do not inherently restore normal metabolic function, leaving patients vulnerable to microvascular complications [9]. Lifestyle modifications represent a non-pharmacological approach well-integrated into clinical medicine for T2DM management [10,11]. Unfortunately, the long-term sustainability of these modifications is compromised by various factors, with patient non-compliance being a prominent issue [12]. Given the growing public health concern of T2DM, with the International Diabetes Federation predicting an increase in prevalence to 400 million cases by 2040, early detection and effective management strategies are essential [13,14].
Diagnosing T2DM heavily relies on biomarkers, which are objective and quantifiable measures showing the biological and pathological processes occurring within organisms [15]. Conventional biomarkers have undergone extensive research and have become established in clinical medicine as standard diagnostic tools [16,17]. However, emerging biomarkers, including proteomic and micro ribonucleic acid (miRNA) markers, have yet to be validated in clinical settings.
Epigenetic mechanisms, including DNA methylation, histone modification, and ncRNAs, regulate gene expression without altering the underlying DNA sequence. Epigenetics represents a rapidly emerging field of research with significant implications for understanding the heritability and progression of various diseases, including T2DM [18]. Among the various aspects of epigenetics, noncoding RNAs (ncRNA) are increasingly recognized for their pivotal role in orchestrating cellular function. MiRNAs, a subset of ncRNAs, are particularly significant due to their capacity to regulate gene expression post-transcriptionally [19,20]. MiRNAs’ ability to regulate genes allows them to control several biological and pathophysiological processes, including cell death and proliferation [21]. The dysregulation of miRNA can disrupt signaling pathways, as is observed in T2DM, resulting in disease development and progression through interference with the critical insulin signaling pathways [22].
This literary analysis first examines well-established traditional biomarkers and then considers more recently identified novel miRNA biomarkers closely associated with T2DM and their potential application in clinical medicine to facilitate the early detection of prediabetic and T2DM patients. Various microRNAs have been reported to be associated with diabetes. The miRNAs selected for this paper were reported in greater frequency in diabetic patients (miR-375 and -126, 7a, and Let-7) and diabetic complications (miR-373, -181B, -21, -192, 133b, 342-3p, and -30), highlighting their potential diagnostic value in diabetes.
2. Traditional Biomarkers
2.1. HbA1c
Hemoglobin A1c (HbA1c), an essential component in clinical diagnostics, is formed by gradually incorporating glucose molecules into the amino-terminal group of the hemoglobin beta subunit within the circulatory system [23]. The HbA1c level indicates an individual’s glycemic control over the preceding three months [24]. The criteria outlined by the American Diabetes Association (ADA) and the National Institute for Health and Care Excellence (NICE) in the United Kingdom report HbA1c values of ≥6.5% (48 mmol/L) for diabetes and ≥5.7% to 6.4% (39 to 46 mmol/L) for prediabetes [1,25]. HbA1c eliminates the need for fasting, offering a more patient-friendly assessment process [26]. It also has a lower biological variability and is unaffected by short-term disturbances, thus ensuring a stable pre-analytical phase [26,27]. On the other hand, HbA1c may not be optimal for early diabetes detection [28]. Particularly during the prediabetic phase, during which blood glucose levels experience minor elevations, HbA1c may remain <5.5% (37 mmol/L), potentially failing to detect subtle changes in glucose levels accurately based on ADA guidelines [29,30]. Additionally, clinical conditions that reduce (iron deficiency anemia) or promote red blood cell turnover (splenomegaly) can introduce inaccuracies in HbA1c measurements [31,32,33]. Other states, including severe hyperbilirubinemia (>20 mg/dL) and severe hypertriglyceridemia (>1750 mg/dL), can also contribute to inaccurate elevations in HbA1c levels [34].
2.2. Fructosamine
Fructosamine is an alternative method for monitoring glycemic control in T2DM and holds potential for the diagnosis of prediabetes [35]. This biomarker results from the glycation of circulatory proteins, including albumin, globulins, and lipoproteins. Increased glucose concentration in the blood causes increased binding to serum proteins, with a greater affinity for albumin [36]. Fructosamine can be used alongside HbA1c in T2DM because it reflects the glucose profile for 2 to 3 weeks before the test [36,37]. Fructosamine, however, has limitations. Conditions affecting albumin levels, such as liver or kidney disease, can alter fructosamine results [35]. These conditions lead to a rapid protein turnover, resulting in falsely low fructosamine levels [38]. Furthermore, fructosamine does not account for individual variations in albumin metabolism, a process influenced by age, sex, and environmental variables [39,40].
2.3. Glycated Albumin
Glycated albumin (GA) is a product of the non-enzymatic glycation of albumin that occurs in the bloodstream. This process results from the reaction between glucose and albumin, in which glucose binds to the amino groups of albumin, forming a ketoamine [41]. The rate of albumin glycation depends on blood glucose levels and the duration of albumin’s presence in the bloodstream [42]. The reference ranges for normal albumin levels in an adult’s blood range from 3.5 to 5.5 g per deciliter (g/dL) [43]. Ongoing studies have suggested that GA levels above 16–17% may indicate poor glycemic control, but these values can vary depending on the individual’s health status [44,45,46]. Despite its clinical utility, glycated albumin has limitations that warrant consideration. Factors such as hypoalbuminemia, liver disease, and inflammatory conditions can influence glycated albumin measurements [47,48,49]. Additionally, the need for standardized reference ranges for glycated albumin poses challenges in interpreting results consistently across different laboratories and patient populations.
2.4. Oral Glucose Tolerance Test
The Oral Glucose Tolerance Test (OGTT) ascertains whether a patient’s body can effectively use and store glucose [50]. It is often utilized to test for conditions such as diabetes mellitus or insulin resistance and may assess pancreatic β-cell function impairment [51,52]. An OGTT begins with an initial measurement of the patient’s fasting plasma glucose (FPG) level. The reference ranges for FPG levels are as follows: non-diabetic < 100mg/dL (<5.6 mmol/L), prediabetic 100–125 mg/dL (5.6–6.9 mmol/L), and diabetic ≥ 126 mg/dL (≥7 mmol/L). Ideally, the test is performed when the patient’s pretest FPG levels are between 100 and 125 mg/dL (5.6–6.9 mmol/L) [53,54]. Blood glucose ranges and interpretations following the OGTT are outlined in Table 1. Impaired glucose tolerance is considered when the 2 h 75 g OGTT yields range between 140 and 199 mg/dL [53,55].
While the OGTT is a valuable diagnostic tool, it has limitations. OGTT procedures are expensive and often time-consuming. Additionally, the preparations expected from the patient, such as diet, activity, and stress level, can affect the results [55]. Furthermore, OGTT results may be difficult to interpret in cases where the readings are borderline [52]. In 20% of cases, OGTT results may be categorized as non-diagnostic due to significant differences in glucose concentrations between the samples [56].

Table 1.
miRNA expression and targets in type 2 diabetes mellitus.
Table 1.
miRNA expression and targets in type 2 diabetes mellitus.
| Biomarker | Expression | Target | Clinical Value | Reference |
|---|---|---|---|---|
| miRNA-375 | Up-/Downregulated | MTPN, PDK1 | Dysregulated levels can be detected early compared to traditional biomarkers in serum of high-risk groups. Can be utilized for investigative procedures for early detection of diabetes and prediabetes in high-risk groups. | [57,58,59] |
| miRNA-126 | Downregulated | IRS-1 | Investigative utility—may be used as part of screening tool for early detection of diabetes, as well as microvascular and retinal complications. Can further monitor levels to observe response to treatment. | [22] |
| miRNA-7a | Upregulated | IRS-2 | Increased expression observed in the serum aids in identifying dysregulated insulin signaling and may be used as an investigative tool to detect and monitor response to treatment. | [60,61] |
| Let-7 | Upregulated | IGF1R, INSR, IRS-2 | Increased levels in the plasma suggest impaired/decreased insulin secretion, a key factor in the pathogenesis of T2DM. Potential to aid in early detection of diabetes in clinic. | [62] |
3. MicroRNA
MicroRNAs are small noncoding RNA about 22 nucleotides long and endogenously expressed [63]. MicroRNAs can regulate cellular function by suppressing gene expression and have been associated with metabolic activities, cellular development, proliferation, and apoptosis [64].
These molecules are part of an epigenetic regulation network, in which several miRNAs, including low-expressed miRNAs, will interact with the same UTR (3′UTR) and regulate gene expression [65,66]. The interaction of the miRNAs with the 3′UTR of the mRNA target can lead to degradation and blocked translation of the target mRNA [64,67].
MiRNA biogenesis can occur through canonical and non-canonical pathways and primarily involves the co-transcription of RNA polymerase II/III proteins [68]. Sequences from the DNA are transcribed into primary miRNAs, with further transcription into precursor miRNAs, and eventually mature into the miRNA form [68]. Many identified miRNAs are primarily transcribed from intragenic introns but may also be intergenic and processed independently [69,70].
Moreover, the pathological expression of miRNAs can play a role in various disease development and progression, including autoimmune conditions such as T1DM and T2DM [71,72]. MiRNAs often occur in high levels in body fluids such as saliva, urine, and plasma and offer biological and chemical stability, thus allowing for miRNA detection in the clinical setting as biomarkers of various diseases [73].
4. MicroRNAs Role in T2DM
Specific miRNAs are suggested to regulate protein-coding genes in the insulin signaling pathway and further mediate insulin-regulated glucose homeostasis [74]. Alteration of miRNA expression can impact glucose metabolism and insulin secretion, further changing the target tissue’s response to insulin and leading to insulin resistance [75]. Insulin resistance is characteristic of T2DM and is seen in response to abnormalities in the signaling pathway due to the dysregulation of key proteins [76,77].
Various miRNAs implicated in glucose homeostasis were shown to be expressed in pancreatic β-cells, skeletal muscles, and serum [78,79,80]. MiRNAs expressed in pancreatic β-cells are suggested to be critical in the regulation and proliferation of β-cell mass [81]. Additionally, miRNAs regulate genes, and the pathological expression of miRNAs will target specific protein-coding genes in the insulin pathway, as seen in Table 2, impacting β-cell survival and insulin secretion [73,82].

Table 2.
MicroRNAs and their expressions in complications of type 2 diabetes mellitus.
4.1. miRNA-375
MicroRNA-375 is a circulatory noncoding RNA expressed in glucagon-secreting alpha cells and insulin-secreting β-cells of pancreatic islets [90,91]. MiRNA-375 has a significant role in insulin homeostasis; recent studies have shown that miRNA-375 can repress glucose-induced insulin secretion by targeting the myotrophin gene (Mtpn), which aids in insulin secretion through exocytosis, and the 3′-phosphoinositide-dependent kinase-1 gene (PDK1), which activates the phosphatidylinositol 3-kinase pathway (PIK3), resulting in β-cell dysfunction and decreased insulin secretion [57,58,92]. The dysregulation of miRNA-375 can affect these target genes and cause the inhibition or overexpression of their pathways [93,94].
Profile studies of miRNA-375 in type 2 diabetic, prediabetic, and non-diabetic patient samples were conducted [59]. Due to miRNA being a circulatory biomarker, blood samples are a sufficient and noninvasive method to collect, isolate, and determine miRNA-375 levels [59].
In T2DM, progressive β-cell dysfunction leading to β-cell destruction is an essential pathophysiological characteristic of disease progression [95,96]. The upregulation of miRNA-375 in T2DM corresponds with decreased β-cell levels [97].
Recent experimental research has observed an increased prevalence of miRNA-375 expression in high-risk patient groups for T2DM onset and those with first-degree relatives with T2DM [59,98]. Wu and colleagues similarly underline the overexpression of miRNA-375 in at-risk patients and their relatives with diabetes [59]. The results from the studies suggest that miRNA-375 is a reliable biomarker for predicting and detecting prediabetes and T2DM [59].
Moreover, the upregulation of miRNA-375 has shown a positive association with glycemic control values, including OGTT, FPG, and HbA1c, offering substantial diagnostic ability for prediabetes and T2DM [59].
However, limitations have stalled the incorporation of miRNA-375 into clinical medicine. Supplemental research on larger sample groups considering different demographics is required to validate miRNA-375 as an appropriate biomarker in high-risk groups [59,99]. In addition, further studies of human sample groups are necessary to evaluate whether the dedifferentiation of β-cells is induced by pathologic miRNA-375 upregulation during the development of T2DM [81,100].
4.2. miRNA-126
MicroRNA-126 is a small noncoding molecule that is found abundantly in endothelial cells [101]. The function of miRNA-126 includes binding to the vascular endothelial growth factor A (VEGF-A) messenger molecule to regulate and inhibit the VEGF molecule [87,102]. Furthermore, miRNA-126 is critical in angiogenic cell maintenance, regulation, and apoptosis [103]. Pathological downregulation can lead to uncontrolled angiogenesis, which is implicated in T2DM microvascular complications [104]. MicroRNA-126 is a circulatory marker that can be an effective noninvasive tool in detecting conditions of diabetic vascular injury and hyperglycemia [105,106].
In a recent study published by Liu et al., researchers assessed the level of circulating miRNA-126 in four patient groups: newly diagnosed T2DM patients, patients with impaired glucose tolerance (IGT), patients with impaired fasting glucose (IFG), and a control group of healthy individuals. The results indicate a significant decrease in miRNA-126 levels for the T2DM, IGT, and FGT groups compared to their healthy counterparts [107]. The study concluded that a reduction in the expression of miRNA-126 is associated with increased glucose and suppressed insulin secretion [107]. Zampetaki and colleagues similarly noted a significant decrease in miRNA-126 in T2DM patients [72]. Additionally, they detected a gradual decline in miRNA-126 within a two-year follow-up period in patients who presented with normal glucose tolerance and progressed to T2DM [72]. The data collected from these studies underline the viability of miRNA-126 as a predictive tool in the early diagnosis of prediabetes and T2DM.
Additionally, miRNA-126 can be further utilized as a biomarker for diabetic retinopathy [108]. The downregulation of miRNA-126 in endothelial cells reduced the inhibition of VEGF. The increased VEGF molecules are an important factor in the development of retinopathy, contributing to retinal neovascularization [108].
While miRNA-126 has significant prognostic and diagnostic value in prediabetes, T2DM, and microvascular complications, more studies in larger cohorts are still required to clearly define its clinical utility.
4.3. miRNA-7a
MicroRNA-7a is highly expressed in the pancreatic islet cells and has been suggested to play an essential role in maintaining β-cell mass and the process of insulin secretion [109]. Previous studies have shown miRNA-7a to be co-expressed with transcriptional factors, including RNA binding protein, and neuronal cells are involved in regulating its expression [110,111].
Prior research on the presence of miRNA-7 in diabetic patients and those with microvascular complications compared to healthy controls has suggested an overexpression of serum miRNA-7 [60,112]. One study showed an increase in serum miRNA-7 of 401 ± 34 fmol/L and 501 ± 82 fmol/L (p < 0.001) in patients with T2DM and T2DM with complications, respectively, with the level in the control subjects being much lower at 176 ± 17 fmol/L [60].
The upregulation of miRNA-7 dysregulates the insulin signaling pathway by repressing the expression of specific genes, such as the insulin receptor gene (INSR) and insulin receptor substrate 2 (IRS-2) [109]. However, additional in vivo work found miRNA-7 to be decreased in diabetic mice. Ji et al. investigated the role of miRNA-7 in diabetic retinopathy, with the results showing lower levels of miRNA-7 in the endothelial cells (EC) and retinal pericytes (RP) of diabetic mice and increased levels of IRS-2 [113]. Cao and colleagues considered the role of miRNA in the expression of PI3K, the protein kinase B (AKT) pathway, and the VEGF protein through miRNA-7 mimics and found that the overexpression of miRNA-7 downregulates the expression of PI3K, AKT, and the VEGF protein, thereby downregulating retinal cell proliferation [114].
Currently, there are conflicting studies on miRNA-7 expression and its associated effect on diabetes and its complications. While an association with the pathway of T2DM diabetes exists because miRNA-7 plays a regulatory role in insulin secretion, further studies are required to validate the implications of miRNA-7 in the development of T2DM before it can be usefully integrated into clinical medicine
4.4. Let-7
Let-7 was one of the first miRNAs to be discovered; it has 12 conserved isoforms and is found in a wide array of organisms [115]. Current research has observed the dysregulation of Let-7 in cancer; Let-7 serves as a tumor suppressor by inhibiting the expression of oncogenes [61,116].
Recent studies have investigated the function of Let-7 miRNA in the pancreatic β cells of mice [117]. Let-7 was found to be a crucial miRNA in regulating insulin secretion and β cell proliferation. Furthermore, the overexpression of Let-7 is suggested to be associated with decreased insulin secretion because of the inhibition of β cell proliferation and decreased cyclin D1 and D2 [117,118]. Additional studies conducted on patients with T2DM and control subjects have reported an increased level of serum Let-7b in the T2DM patient group (p < 0.05) [118].
Let-7 is further suggested to be associated with retinal complications. The relevant literature has shown Let-7 to be expressed in endothelial and retinal cells. Studies conducted in diabetic mice showed an upregulation of Let-7 to cause non-proliferative diabetic retinopathy [119,120]. Current studies suggest that Let-7 is a robust biomarker that has the potential to aid in the early detection of T2DM and associated complications such as diabetic retinopathy.
5. miRNAs in T2DM-Associated Complications
Characterized by β cell dysfunction/destruction and insulin resistance, the progression of T2DM can result in various microvascular and macrovascular complications. The pathological expression of miRNAs, which is highlighted in Table 2, is suggested to be implicated in the pathophysiology of vascular complications, such as diabetic retinopathy (DR) and diabetic nephropathy (DN) (Figure 1) [121].
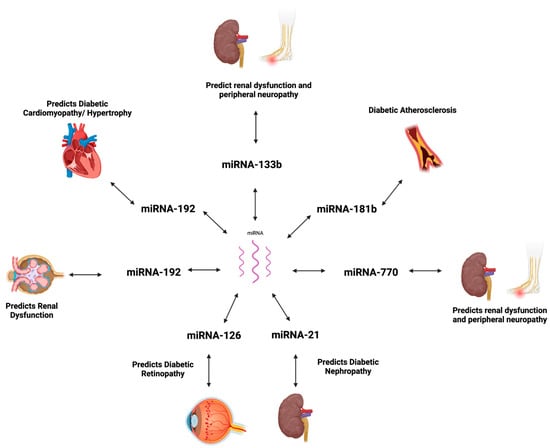
Figure 1.
MicroRNA expression in diabetic complications. Created using BioRender.com [122].
5.1. Diabetic Cardiomyopathy
Diabetic cardiomyopathy is a leading complication in diabetic patients. The disease process leading to DC is the result of persistent hyperglycemia in the setting of insulin resistance in cardiac cells, which pushes the myocardial cells to utilize fatty acids, leading to the accumulation of lipids [62]. Eventually, the deposition of lipid intermediates causes myocardial fibrosis and dysfunction [62]. Hypertrophy of the cardiomyocyte in the setting of high glucose has been shown to activate the mitogen-activated protein kinase (MAPK), a signaling pathway that is implicated in inflammation, fibrosis, and the dysfunction of the myocardium [83,123]. MiRNA-373 expression is suggested to be regulated by p38 MAPK, where the inhibition of the signaling pathway results in decreased expression of miRNA-373 [124].
Evidence shows miRNA-373 to have protective effects against hyperglycemia-induced cardiac downregulating of the myocyte enhancer factor 2C (MEF2C) gene, which codes for the hypertrophic protein [124]. Experiments with diabetic mice revealed downregulated levels of miRNA-373 in the cardiac tissue, which is suggested to be a result of oxidative stress on cardiomyocytes induced by persistent hyperglycemia. Thus, investigating the expression of miR-373 in cardiomyocytes can help in the early detection of cardiac dysfunction and further facilitate early treatment in high-risk groups [124].
5.2. Atherosclerosis
Cardiovascular disease and atherosclerosis are significant complications of DM. The progression to atherosclerosis is suggested to be mediated by mechanisms including dyslipidemia, leading to lipid accumulation and the production and accumulation of advanced glycation end products in the setting of hyperglycemia [125,126]. Atherosclerotic plaques consist of macrophages, which induce inflammation and plaque formation. Diabetes-associated atherosclerosis can further be studied using miR-181b, which is suggested to regulate macrophage accumulation that leads to plaque formation [84]. Sun et al. suggested increased miR-181b to have protective effects against atherosclerosis by targeting PHLPP2—a phosphatase that inhibits proliferation cell proliferation [127,128]. An experiment using the endothelial cells from the epididymal white adipose tissue of insulin-resistant mice showed downregulated mIR-181b, allowing for the accumulation of macrophages [128].
5.3. Diabetic Nephropathy
DN is a common complication of DM and a leading cause of renal failure [129]. The disease progression of DN is a result of oxidative stress and the release of proinflammatory and fibrotic mediators because of hyperglycemia. Eventually, the release of these compounds causes functional and structural changes to the renal system, leading to interstitial fibrosis and glomerulosclerosis [130]. Significant tissue damage is often present by the time microalbuminuria is detected [129]. Current diagnostic methods, including urine microalbuminuria, may not be adequate in providing an accurate progression of the disease. MiRNA-21 regulates transforming growth factor-β (TGF-β) signaling and is a potential biomarker for DN progression. The overexpression of miRNA-21 may target and suppress mothers against decapentaplegic homolog family member 7 (SMAD7), an inhibitor of TGF-β, ultimately producing fibrotic and inflammatory markers. Identifying increased levels of miR-21 from renal tubular epithelial cell samples in high-risk groups can aid in the easy detection of the potential onset of diabetic nephropathy and guide treatment [131,132]. Moreover, prior research highlights that the upregulation of miRNA-192 targets the repressors ZEB1 and ZEB2 and promotes the expression of Col1a2 and Col4a1 fibrotic genes [133]. A recent publication identified dysregulated urinary exosomal miRNA-133b, -342-3p, and -30a-5p as viable biomarkers in the pathogenesis of DN, which can also be used as early diagnostic markers [134]. A meta-analysis on the biomarkers of DN reports downregulated urinary values of miRNA-126 and miRNA-770 in diabetic patients, suggesting their involvement in renal disease [135].
5.4. Diabetic Retinopathy
DR represents a severe consequence of T2DM and a significant cause of blindness in adults between the ages of 20 and 75 years [136]. DR is a progressive disease, with the early stages characterized as non-proliferative (NPDR) and preventable [137]. The late stage is proliferative (PDR) and may subsequently lead to blindness [138]. MiRNA-126 downregulation and reduced VEGF inhibition contribute to the development of retinal damage and DR [106,108]. Additional research outlines the upregulation of miRNA-21 to promote tumor angiogenesis and downstream production of VEGF molecules, contributing to retinal neovascularization and DR [85,139].
6. Conclusions
This review of the existing literature underlines the pragmatic benefits of using specific and sensitive microRNA biomarkers in predicting the onset of prediabetes and T2DM during their early stages when β cell function can still be preserved with lifestyle interventions.
While traditional tools for screening and diagnosing dysglycemia, such as HbA1c, OGTT, and GA, are proven to be beneficial, they have limitations and may underestimate the risk of disease progression or even fail to result in a diagnosis. This review highlights the associations of miRNA biomarkers in predicting and diagnosing prediabetic patients and the progression of T2DM.
The dysregulation of specific miRNAs is often seen in prediabetic and T2DM patients and is consistent with diabetic risk factors and glycemic control values; furthermore, miRNAs can be used to detect associated vascular complications, such as diabetic retinopathy or nephropathy, at an early stage. Therefore, while the dysregulation of these miRNA biomarkers has shown potential in early detection of dysglycemic status, future studies on larger cohorts should focus on comparative analysis and assess their utility in identifying dysglycemic conditions and improving clinical outcomes.
Moving forward, technological advancements have paved the way for newer methods to predict T2DM and its complications. Novel techniques, such as bioinformatics, are currently being explored to identify specific genes and pathways associated with T2DM, as well as proteins that may be expressed or play a role in the pathogenesis of the condition [89].
Researchers have used advanced software and computerized analyses to process and identify microarray data from control and affected patient samples from tissues containing beta cells to isolate and pinpoint specific factors, such as genes, microRNA, and transcription factors, directly linked to the development and progression of T2DM [89]. Another emerging field of research related to microRNA is that of precision medicine. The identification of specific microRNA in individuals will help tailor treatment specific to each case and create a broader spectrum of treatment options and algorithms [120]. Network medicine is another domain that directly relates to identifying disease genes and their interactions to help identify disease pathways and predict other disease genes [140]. Its main aim is to create a diseasome, which is a map of a disease [140]. The map displays multiple diseases as a “node”, and the connections between them display how different pathologies may be related through shared molecular or cellular mechanisms [140]. This methodology is becoming highly relevant for drug design and disease classification, which can further help to create better therapeutics for the complexities and comorbidities associated with T2DM [140].
Finally, another fascinating developing realm is artificial intelligence or machine learning models. Researchers have been using these technologies to enhance the miRNA detection techniques and help to identify comorbidities that may present along with T2DM [141]. With T2DM being such a complex and multifactorial disease, primitive methods have proven to be inaccurate and lack the sensitivity and specificity required for the adequate diagnosis and therapeutics of the disease. In this current age of new technologies, microRNAs have streamlined the process of detection and made it more efficient to predict the development of the disease and its complications. The newer technologies being explored only widen the horizons to create more precise and effective diagnostics and treatments for this highly prevalent disease.
Author Contributions
S.A., H.A., and M.A.K. researched the literature and wrote the manuscript. A.E.B. provided supervision and edited the manuscript. A.E.B. is the guarantor of this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors provided their consent for publication.
Data Availability Statement
No new data were generated in the writing of this review article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47 (Suppl. 1), S5–S10.
- Mezza, T.; Cinti, F.; Cefalo, C.M.A.; Pontecorvi, A.; Kulkarni, R.N.; Giaccari, A. β-Cell Fate in Human Insulin Resistance and Type 2 Diabetes: A Perspective on Islet Plasticity. Diabetes 2019, 68, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Aghaei Zarch, S.M.; Dehghan Tezerjani, M.; Talebi, M.; Vahidi Mehrjardi, M.Y. Molecular biomarkers in diabetes mellitus (DM). Med. J. Islam. Repub. Iran 2020, 34, 28. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Agarwal, R. Pathogenesis of Diabetic Nephropathy. In Chronic Kidney Disease and Type 2 Diabetes; ADA Clinical Compendia Series; American Diabetes Association: Arlington, VA, USA, 2021; pp. 2–7. [Google Scholar]
- Ahmed, A.; Majeed, S.; Obaid, H.; Al-Hmmamy, S. Biochemistry and Molecular Cell Biology of Diabetic Complications. Syst. Rev. Pharm. 2020, 11, 850–860. [Google Scholar]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhtaresh, H.A.; Al-Kafaji, G. Evaluation of Two-Diabetes Related microRNAs Suitability as Earlier Blood Biomarkers for Detecting Prediabetes and type 2 Diabetes Mellitus. J. Clin. Med. 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ohkuma, T.; Cooper, M.; Harrap, S.; Mancia, G.; Poulter, N.; Wang, J.-G.; Zoungas, S.; Woodward, M.; Chalmers, J. Effects of Intensive Glycemic Control on Clinical Outcomes Among Patients With Type 2 Diabetes With Different Levels of Cardiovascular Risk and Hemoglobin A1c in the ADVANCE Trial. Diabetes Care 2020, 43, 1293–1299. [Google Scholar] [CrossRef]
- Antwi, J. Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes. Curr. Nutr. Rep. 2023, 12, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef]
- Su, J.; Luo, Y.; Hu, S.; Tang, L.; Ouyang, S. Advances in Research on Type 2 Diabetes Mellitus Targets and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 13381. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Schwarz, P.E. Preventing Diabetes: Early Versus Late Preventive Interventions. Diabetes Care 2016, 39 (Suppl. 2), S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sato Imuro, S.E.; Sabharwal, A.; Bevier, W.; Kerr, D. Evaluating HbA(1c)-to-average glucose conversion with patient-specific kinetic models for diverse populations. Sci. Rep. 2024, 14, 22098. [Google Scholar] [CrossRef]
- Cs, L.; Aw, T.-C. HbA1c in the diagnosis and management of diabetes mellitus: An update. Diabetes Updates 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Ling, C.; Bacos, K.; Rönn, T. Epigenetics of type 2 diabetes mellitus and weight change—A tool for precision medicine? Nat. Rev. Endocrinol. 2022, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Panni, S.; Pizzolotto, R. Integrated Analysis of microRNA Targets Reveals New Insights into Transcriptional–Post-Transcriptional Regulatory Cross-Talk. Biology 2025, 14, 43. [Google Scholar] [CrossRef]
- Vaghf, A.; Khansarinejad, B.; Ghaznavi-Rad, E.; Mondanizadeh, M. The role of microRNAs in diseases and related signaling pathways. Mol. Biol. Rep. 2022, 49, 6789–6801. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, H.; Si, H.; Li, X.; Ding, X.; Sheng, Q.; Chen, P.; Zhang, H. Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta Diabetol. 2014, 51, 823–831. [Google Scholar] [CrossRef]
- Nigi, L.; Grieco, G.E.; Ventriglia, G.; Brusco, N.; Mancarella, F.; Formichi, C.; Dotta, F.; Sebastiani, G. MicroRNAs as Regulators of Insulin Signaling: Research Updates and Potential Therapeutic Perspectives in Type 2 Diabetes. Int. J. Mol. Sci. 2018, 19, 3705. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hng, T.-M. HbA1c: More than just a number. Aust. J. Gen. Pract. 2021, 50, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Mukonda, E.; van der Westhuizen, D.J.; Dave, J.A.; Cleary, S.; Hannan, L.; Rusch, J.A.; Lesosky, M. Understanding the relationship between the frequency of HbA1c monitoring, HbA1c changes over time, and the achievement of targets: A retrospective cohort study. BMC Endocr. Disord. 2025, 25, 3. [Google Scholar] [CrossRef]
- NICE. Type 2 Diabetes in Adults: Management: NICE. 2022. Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 30 July 2024).
- Hussain, N. Implications of using HBA1(C) as a diagnostic marker for diabetes. Diabetol. Int. 2016, 7, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasamurthy, L. Evolution in Diagnosis and Classification of Diabetes. J. Diabetes Mellit. 2021, 11, 200–207. [Google Scholar] [CrossRef]
- Hellgren, M.; Hjörleifsdottir Steiner, K.; Bennet, L. Haemoglobin A1c as a screening tool for type 2 diabetes and prediabetes in populations of Swedish and Middle-East ancestry. Prim. Care Diabetes 2017, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, J.; Akin, S.; Karagaoglu, E.; Gurlek, A. The performance of hemoglobin A1c against fasting plasma glucose and oral glucose tolerance test in detecting prediabetes and diabetes. J. Res. Med. Sci. 2014, 19, 1051–1057. [Google Scholar]
- Hong, J.W.; Ku, C.R.; Noh, J.H.; Ko, K.S.; Rhee, B.D.; Kim, D.-J. Association Between the Presence of Iron Deficiency Anemia and Hemoglobin A1c in Korean Adults: The 2011–2012 Korea National Health and Nutrition Examination Survey. Medicine 2015, 94, e825. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, B.A.; Salamatullah, H.K.; Alsharm, F.S.; Baljoon, J.M.; Abukhodair, A.O.; Ahmed, M.E.; Malaikah, H.; Radi, S. The effect of different types of anemia on HbA1c levels in non-diabetics. BMC Endocr. Disord. 2023, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Seker, R.; Sahin, E.A.; Sener, A. HbA1c levels in iron deficiency anemia cases grouped according to hemoglobin levels. Ann. Med. Res. 2024, 31, 622–626. [Google Scholar] [CrossRef]
- Cavagnolli, G.; Pimentel, A.L.; Freitas, P.A.C.; Gross, J.L.; Camargo, J.L. Factors affecting A1C in non-diabetic individuals: Review and meta-analysis. Clin. Chim. Acta 2015, 445, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.N.; Brady, S.; Monteiro, D.; Nathan, Y.; Crook, M. Unexpected high HbA1c results due to an unusual haemoglobin variant: An important clinical lesson. Postgrad. Med. J. 2022, 98, 331–332. [Google Scholar] [CrossRef]
- Nansseu, J.R.N.; Fokom-Domgue, J.; Noubiap, J.J.N.; Balti, E.V.; Sobngwi, E.; Kengne, A.P. Fructosamine measurement for diabetes mellitus diagnosis and monitoring: A systematic review and meta-analysis protocol. BMJ Open 2015, 5, e007689. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E. Alternative biomarkers for assessing glycemic control in diabetes: Fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.T.K.; Cukier, P.; Damascena, A.S.; Batista, R.L.; de Azevedo Correa, F.; Zanatta Kawahara, E.; Minanni, C.A.; Hoff, A.O.; Nery, M. Fructosamine and glycated hemoglobin as biomarkers of glycemic control in people with type 2 diabetes mellitus and cancer (GlicoOnco study). Clinics 2023, 78, 100240. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, S.H.; Bhavsar, R.; Kaur, R.; Kim, P.; Sachmechi, I. A Case Report of Fructosamine’s Unreliability as a Glycemic Control Assessment Tool in Nephrotic Syndrome. Cureus 2017, 9, e1694. [Google Scholar] [CrossRef] [PubMed]
- Malmström, H.; Walldius, G.; Grill, V.; Jungner, I.; Gudbjörnsdottir, S.; Hammar, N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies—Cross-sectional and longitudinal experience from the AMORIS cohort. PLoS ONE 2014, 9, e111463. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Loomis, S.J.; Venkataraghavan, S.; Zhang, J.; Tin, A.; Yu, B.; Chatterjee, N.; Selvin, E.; Duggal, P. Characterizing Common and Rare Variations in Nontraditional Glycemic Biomarkers Using Multivariate Approaches on Multiancestry ARIC Study. Diabetes 2024, 73, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.C.; Ehlert, L.R.; Camargo, J.L. Glycated albumin: A potential biomarker in diabetes. Arch. Endocrinol. Metab. 2017, 61, 296–304. [Google Scholar] [CrossRef]
- Kumari, N.; Vaishnav, M.; Srikanta, S.; Krishnaswamy, P.; Bhat, N. Exploring Glycated Sites in Human Serum Albumin: Impact of Sample Processing Techniques on Detection and Analysis. Anal. Methods 2024, 16, 5239–5247. [Google Scholar] [CrossRef] [PubMed]
- Moman, R.N.; Gupta, N.; Varacallo, M. Physiology, Albumin; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fang, M.; Daya, N.; Coresh, J.; Christenson, R.H.; Selvin, E. Glycated Albumin for the Diagnosis of Diabetes in US Adults. Clin. Chem. 2022, 68, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Matsha, T.E.; Korf, M.; Erasmus, R.T.; Hoffmann, M.; Mapfumo, C.; Smit, F.; Zemlin, A.E. Reference interval determination for glycated albumin in defined subgroups of a South African population. Ann. Clin. Biochem. 2019, 56, 480–487. [Google Scholar] [CrossRef]
- Freitas, P.A.C.; Hernandez, M.K.; Camargo, J.L. Factors associated with glycated albumin in adults without diabetes. Med. Pharm. Rep. 2021, 94, 170–175. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chume, F.C.; Schiavenin, L.G.; Freitas, P.A.C.; Pimentel, A.L.; Camargo, J.L. The usefulness of glycated albumin in patients with diabetes and renal disease: A scoping review. J. Lab. Precis. Med. 2022, 7, 12. [Google Scholar] [CrossRef]
- Tang, M.; Berg, A.H.; Zheng, H.; Rhee, E.P.; Allegretti, A.S.; Nigwekar, S.U.; Karumanchi, S.A.; Lash, J.P.; Kalim, S. Glycated Albumin and Adverse Clinical Outcomes in Patients With CKD: A Prospective Cohort Study. Am. J. Kidney Dis. 2024, 84, 329–338. [Google Scholar] [CrossRef]
- Jagannathan, R.; Neves, J.S.; Dorcely, B.; Chung, S.T.; Tamura, K.; Rhee, M.; Bergman, M. The Oral Glucose Tolerance Test: 100 Years Later. Diabetes Metab. Syndr. Obes. 2020, 13, 3787–3805. [Google Scholar] [CrossRef] [PubMed]
- Thewjitcharoen, Y.; Jones Elizabeth, A.; Butadej, S.; Nakasatien, S.; Chotwanvirat, P.; Wanothayaroj, E.; Krittiyawong, S.; Himathongkam, T.; Himathongkam, T. Performance of HbA1c versus oral glucose tolerance test (OGTT) as a screening tool to diagnose dysglycemic status in high-risk Thai patients. BMC Endocr. Disord. 2019, 19, 23. [Google Scholar] [CrossRef]
- Eyth, E.; Basit, H.; Swift, C.J. Glucose Tolerance Test; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47 (Suppl. 1), S20–S42. [Google Scholar]
- Rao, S.S.; Disraeli, P.; McGregor, T. Impaired glucose tolerance and impaired fasting glucose. Am. Fam. Physician 2004, 69, 1961–1968. [Google Scholar] [PubMed]
- Ligthart, S.; van Herpt, T.T.W.; Leening, M.J.G.; Kavousi, M.; Hofman, A.; Stricker, B.H.C.; van Hoek, M.; Sijbrands, E.J.G.; Franco, O.H.; Dehghan, A. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: A prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 44–51. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. 1), S17–S38. [Google Scholar] [CrossRef]
- Kaur, P.; Kotru, S.; Singh, S.; Behera, B.S.; Munshi, A. Role of miRNAs in the pathogenesis of T2DM, insulin secretion, insulin resistance, and β cell dysfunction: The story so far. J. Physiol. Biochem. 2020, 76, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Raina, S.; Chugh, J.; Sharma, S. MIRNAS: Early Prognostic Biomarkers for Type 2 Diabetes Mellitus? Biomark. Med. 2015, 9, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Man, B.; Li, D. Assessing MicroRNA-375 Levels in Type 2 Diabetes Mellitus (T2DM) Patients and Their First-Degree Relatives with T2DM. Diabetes Metab. Syndr. Obes. 2021, 14, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wang, J.; Wang, J.; Wu, J.; Song, J.; Zhang, C.-Y.; Zhang, C.; Wang, C.; Wang, J.-J. Increased serum miR-7 is a promising biomarker for type 2 diabetes mellitus and its microvascular complications. Diabetes Res. Clin. Pract. 2017, 130, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, L.L.; Wen, X.; Liu, B.; Huang, J.; Wang, J.H.; Wei, Y.Q. Oncogenic and tumor suppressive roles of microRNAs in apoptosis and autophagy. Apoptosis 2014, 19, 1177–1189. [Google Scholar] [CrossRef]
- Huo, J.-L.; Feng, Q.; Pan, S.; Fu, W.-J.; Liu, Z.; Liu, Z. Diabetic cardiomyopathy: Early diagnostic biomarkers, pathogenetic mechanisms, and therapeutic interventions. Cell Death Discov. 2023, 9, 256. [Google Scholar] [CrossRef]
- Hu, X.; Yin, G.; Zhang, Y.; Zhu, L.; Huang, H.; Lv, K. Recent advances in the functional explorations of nuclear microRNAs. Front. Immunol. 2023, 14, 1097491. [Google Scholar] [CrossRef]
- Pordzik, J.; Pisarz, K.; De Rosa, S.; Jones, A.D.; Eyileten, C.; Indolfi, C.; Malek, L.; Postula, M. The Potential Role of Platelet-Related microRNAs in the Development of Cardiovascular Events in High-Risk Populations, Including Diabetic Patients: A Review. Front. Endocrinol. 2018, 9, 74. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Gupta, A. MicroRNAs: Potential biomarkers for diagnosis and prognosis of different cancers. Transl. Cancer Res. 2020, 9, 5798–5818. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar]
- Steiman-Shimony, A.; Shtrikman, O.; Margalit, H. Assessing the functional association of intronic miRNAs with their host genes. RNA 2018, 24, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Talukder, A.; Cha, M.; Li, X.; Hu, H. Computational annotation of miRNA transcription start sites. Brief. Bioinform. 2021, 22, 380–392. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Czlonkowska, A.; Postula, M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke—A Comprehensive Review and Bioinformatic Analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Pordzik, J.; Jakubik, D.; Jarosz-Popek, J.; Wicik, Z.; Eyileten, C.; De Rosa, S.; Indolfi, C.; Siller-Matula, J.M.; Czajka, P.; Postula, M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Cardiovasc. Diabetol. 2019, 18, 113. [Google Scholar] [CrossRef]
- Sekar, D.; Venugopal, B.; Sekar, P.; Ramalingam, K. Role of microRNA 21 in diabetes and associated/related diseases. Gene 2016, 582, 14–18. [Google Scholar] [CrossRef]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xing, W.; Xie, L. Regulatory Roles of MicroRNAs in Diabetes. Int. J. Mol. Sci. 2016, 17, 1729. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Katsiki, N.; Behnam, B.; Iranpanah, H.; Sahebkar, A. MicroRNAs and type 2 diabetes mellitus: Molecular mechanisms and the effect of antidiabetic drug treatment. Metabolism 2018, 87, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L. The small RNA miR-375—A pancreatic islet abundant miRNA with multiple roles in endocrine beta cell function. Mol. Cell. Endocrinol. 2017, 456, 95–101. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huang, H.Y.; Shrestha, S.; Chou, C.H.; Chen, Y.H.; Chen, C.R.; Hong, H.C.; Li, J.; Chang, Y.A.; Chiew, M.Y.; et al. Multi-omics profiling reveals microRNA-mediated insulin signaling networks. BMC Bioinform. 2020, 21 (Suppl. 13), 389. [Google Scholar] [CrossRef]
- Macvanin, M.T.; Gluvic, Z.; Radovanovic, J.; Essack, M.; Gao, X.; Isenovic, E.R. Diabetic cardiomyopathy: The role of microRNAs and long non-coding RNAs. Front. Endocrinol. 2023, 14, 1124613. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, X.; Shan, P.F. MicroRNAs and Cardiovascular Disease in Diabetes Mellitus. Biomed. Res. Int. 2017, 2017, 4080364. [Google Scholar] [CrossRef]
- Jiang, Q.; Lyu, X.-M.; Yuan, Y.; Wang, L. Plasma miR-21 expression: An indicator for the severity of Type 2 diabetes with diabetic retinopathy. Biosci. Rep. 2017, 37, BSR20160589. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, C.; Lv, C.; Wu, C.; Wang, Q. The Expression of miR-192 and Its Significance in Diabetic Nephropathy Patients with Different Urine Albumin Creatinine Ratio. J. Diabetes Res. 2016, 2016, 6789402. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.Z.; Liu, B.; Li, Y.T.; Fang, L.; Zhang, Y.; Li, Y.G.; Meng, S. MicroRNA-126 protects against vascular injury by promoting homing and maintaining stemness of late outgrowth endothelial progenitor cells. Stem Cell Res. Ther. 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Maxwell, A.P.; Simpson, D.A.; McKay, G.J. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci. Rep. 2019, 9, 10900. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Li, G.; Lu, H. Identification of potential markers for type 2 diabetes mellitus via bioinformatics analysis. Mol. Med. Rep. 2020, 22, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; Macdonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef]
- Li, X. miR-375, a microRNA related to diabetes. Gene 2014, 533, 1–4. [Google Scholar] [CrossRef]
- Dumortier, O.; Fabris, G.; Pisani, D.F.; Casamento, V.; Gautier, N.; Hinault, C.; Lebrun, P.; Duranton, C.; Tauc, M.; Dalle, S.; et al. microRNA-375 regulates glucose metabolism-related signaling for insulin secretion. J. Endocrinol. 2020, 244, 189–200. [Google Scholar] [CrossRef]
- Zhu, H.; Leung, S.W. Identification of microRNA biomarkers in type 2 diabetes: A meta-analysis of controlled profiling studies. Diabetologia 2015, 58, 900–911. [Google Scholar] [CrossRef]
- Drokow, E.K.; Sun, K.; Ahmed, H.A.W.; Akpabla, G.S.; Song, J.; Shi, M. Circulating microRNA as diagnostic biomarkers for haematological cancers: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 4313–4326. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Dhawan, S.; Hoang, J.; Cory, M.; Zeng, K.; Fritsch, H.; Meier, J.J.; Rizza, R.A.; Butler, P.C. β-Cell Deficit in Obese Type 2 Diabetes, a Minor Role of β-Cell Dedifferentiation and Degranulation. J. Clin. Endocrinol. Metab. 2016, 101, 523–532. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Q.; Xing, X.; Yuan, T.; Li, P. Clinical research progress on β-cell dysfunction in T2DM development in the Chinese population. Rev. Endocr. Metab. Disord. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, G.M.; Azoz, N.M.A.; El Zohne, R.A.; Abdellatif, H.; Saleem, T.H.; Emam, W.A.; Mohammed, A.R.; Mohamed, S.A.; Muhammed, A.A.; Abd el-Rady, N.M.; et al. Dysregulated miRNA-375, IL-17, TGF-β, and Microminerals Are Associated with Calpain-10 SNP 19 in Diabetic Patients: Correlation with Diabetic Nephropathy Stages. Int. J. Mol. Sci. 2023, 24, 17446. [Google Scholar] [CrossRef] [PubMed]
- Sangali, P.; Abdullahi, S.; Nosrati, M.; Khosravi-Asrami, O.F.; Mahrooz, A.; Bagheri, A. Altered expression of miR-375 and miR-541 in type 2 diabetes patients with and without coronary artery disease (CAD): The potential of miR-375 as a CAD biomarker. J. Diabetes Metab. Disord. 2024, 23, 1101–1106. [Google Scholar] [CrossRef]
- Chang, X.; Li, S.; Li, J.; Yin, L.; Zhou, T.; Zhang, C.; Chen, X.; Sun, K. Ethnic differences in microRNA-375 expression level and DNA methylation status in type 2 diabetes of Han and Kazak populations. J. Diabetes Res. 2014, 2014, 761938. [Google Scholar] [CrossRef]
- Nathan, G.; Kredo-Russo, S.; Geiger, T.; Lenz, A.; Kaspi, H.; Hornstein, E.; Efrat, S. MiR-375 Promotes Redifferentiation of Adult Human β Cells Expanded In Vitro. PLoS ONE 2015, 10, e0122108. [Google Scholar] [CrossRef]
- Liao, L.; Tang, Y.; Zhou, Y.; Meng, X.; Li, B.; Zhang, X. MicroRNA-126 (MiR-126): Key roles in related diseases. J. Physiol. Biochem. 2024, 80, 277–286. [Google Scholar] [CrossRef]
- Ye, P.; Liu, J.; He, F.; Xu, W.; Yao, K. Hypoxia-induced deregulation of miR-126 and its regulative effect on VEGF and MMP-9 expression. Int. J. Med. Sci. 2014, 11, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nammian, P.; Razban, V.; Tabei Mohammad Bagher, S.; Asadi-Yousefabad, S.-L. MicroRNA-126: Dual Role in Angiogenesis Dependent Diseases. Curr. Pharm. Des. 2020, 26, 4883–4893. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1α which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Sci. Rep. 2019, 9, 7381. [Google Scholar] [CrossRef]
- Monfared, Y.K.; Mirzaii-Dizgah, M.-R.; Khodabandehloo, E.; Sarookhani, M.R.; Hashemipour, S.; Mirzaii-Dizgah, I. Salivary microRNA-126 and 135a: A potentially non-invasive diagnostic biomarkers of type- 2 diabetes. J. Diabetes Metab. Disord. 2021, 20, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Rezk, N.A.; Sabbah, N.A.; Saad, M.S.S. Role of MicroRNA 126 in screening, diagnosis, and prognosis of diabetic patients in Egypt. IUBMB Life 2016, 68, 452–458. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The Role of Circulating MicroRNA-126 (miR-126): A Novel Biomarker for Screening Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.L.; An, M.X.; Liu, Y.L.; Xu, H.C.; Lu, Z.Q. MicroRNA-126: A promising novel biomarker in peripheral blood for diabetic retinopathy. Int. J. Ophthalmol. 2017, 10, 530–534. [Google Scholar]
- Fernández-de Frutos, M.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell. Biol. 2019, 39, e00170-19. [Google Scholar] [CrossRef]
- Horsham, J.L.; Ganda, C.; Kalinowski, F.C.; Brown, R.A.M.; Epis, M.R.; Leedman, P.J. MicroRNA-7: A miRNA with expanding roles in development and disease. Int. J. Biochem. Cell Biol. 2015, 69, 215–224. [Google Scholar] [CrossRef]
- Zhang, H.M.; Kuang, S.; Xiong, X.; Gao, T.; Liu, C.; Guo, A.Y. Transcription factor and microRNA co-regulatory loops: Important regulatory motifs in biological processes and diseases. Brief. Bioinform. 2015, 16, 45–58. [Google Scholar] [CrossRef]
- Xu, H.; Guo, S.; Li, W.; Yu, P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci. Rep. 2015, 5, 12453. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Luo, J.; Su, T.; Chen, C.; Su, Y. miR-7a Targets Insulin Receptor Substrate-2 Gene and Suppresses Viability and Invasion of Cells in Diabetic Retinopathy Mice via PI3K-Akt-VEGF Pathway. Diabetes Metab. Syndr. Obes. 2021, 14, 719–728. [Google Scholar] [CrossRef]
- Cao, Y.L.; Liu, D.J.; Zhang, H.G. MiR-7 regulates the PI3K/AKT/VEGF pathway of retinal capillary endothelial cell and retinal pericytes in diabetic rat model through IRS-1 and inhibits cell proliferation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4427–4430. [Google Scholar] [PubMed]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. A Regulator of Metabolic Reprogramming: MicroRNA Let-7. Transl. Oncol. 2019, 12, 1005–1013. [Google Scholar] [CrossRef]
- Katayama, M.; Sjögren Rasmus, J.O.; Egan, B.; Krook, A. miRNA let-7 expression is regulated by glucose and TNF-α by a remote upstream promoter. Biochem. J. 2015, 472, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Fan, L.; Shan, A.; Wang, W.; Ning, G.; Cao, Y.; Jiang, X. Let7b-5p inhibits insulin secretion and decreases pancreatic β-cell mass in mice. Mol. Cell. Endocrinol. 2022, 540, 111506. [Google Scholar] [CrossRef]
- Zhou, Q.; Frost, R.J.A.; Anderson, C.; Zhao, F.; Ma, J.; Yu, B.; Wang, S. let-7 Contributes to Diabetic Retinopathy but Represses Pathological Ocular Angiogenesis. Mol. Cell. Biol. 2017, 37, e00001-17. [Google Scholar] [CrossRef] [PubMed]
- Angelescu, M.A.; Andronic, O.; Dima, S.O.; Popescu, I.; Meivar-Levy, I.; Ferber, S.; Lixandru, D. miRNAs as Biomarkers in Diabetes: Moving towards Precision Medicine. Int. J. Mol. Sci. 2022, 23, 12843. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; El-Mahdy, H.A.; Eldeib, M.G.; Doghish, A.S. miRNAs as cornerstones in diabetic microvascular complications. Mol. Genet. Metab. 2023, 138, 106978. [Google Scholar] [CrossRef] [PubMed]
- Adnan, H. MicroRNA Expression in Diabetic Complications [Internet]. 2025. Available online: https://BioRender.com/y62j230 (accessed on 11 January 2025).
- Wang, S.; Ding, L.; Ji, H.; Xu, Z.; Liu, Q.; Zheng, Y. The Role of p38 MAPK in the Development of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2016, 17, 1037. [Google Scholar] [CrossRef]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Krecke, K.N.; Bapat, A.S.; Yang, T.; Lopresti, M.W.; Mashek, D.G.; Kelekar, A. Phosphatase PHLPP2 regulates the cellular response to metabolic stress through AMPK. Cell Death Dis. 2021, 12, 904. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, J.; Zhang, Y.; Kang, S.; Belkin, N.; Wara, A.K.; Icli, B.; Hamburg, N.M.; Li, D.; Feinberg, M.W. MicroRNA-181b Improves Glucose Homeostasis and Insulin Sensitivity by Regulating Endothelial Function in White Adipose Tissue. Circ. Res. 2016, 118, 810–821. [Google Scholar] [CrossRef]
- Simpson, K.; Wonnacott, A.; Fraser, D.J.; Bowen, T. MicroRNAs in Diabetic Nephropathy: From Biomarkers to Therapy. Curr. Diabetes Rep. 2016, 16, 35. [Google Scholar] [CrossRef]
- Gallagher, H.; Suckling, R.J. Diabetic nephropathy: Where are we on the journey from pathophysiology to treatment? Diabetes Obes. Metab. 2016, 18, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gan, H.; Zhang, H.; Tang, W.; Sun, Y.; Tang, X.; Kong, D.; Zhou, J.; Wang, Y.; Zhu, Y. MicroRNA-21 inhibits SMAD7 expression through a target sequence in the 3′ untranslated region and inhibits proliferation of renal tubular epithelial cells. Mol. Med. Rep. 2014, 10, 707–712. [Google Scholar] [CrossRef]
- Dhas, Y.; Arshad, N.; Biswas, N.; Jones, L.D.; Ashili, S. MicroRNA-21 Silencing in Diabetic Nephropathy: Insights on Therapeutic Strategies. Biomedicines 2023, 11, 2583. [Google Scholar] [CrossRef]
- Kato, M.; Natarajan, R. MicroRNAs in diabetic nephropathy: Functions, biomarkers, and therapeutic targets. Ann. N. Y. Acad. Sci. 2015, 1353, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Bekhet, M.M. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed. Pharmacother. 2016, 83, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Moon, S.; Lee, K.; Park Ie, B.; Lee Dae, H.; Nam, S. Urinary and Blood MicroRNA-126 and -770 are Potential Noninvasive Biomarker Candidates for Diabetic Nephropathy: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 46, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.T.; Zhang, J.F. Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural Regen. Res. 2023, 18, 976–982. [Google Scholar] [PubMed]
- Lima, V.C.; Cavalieri, G.C.; Lima, M.C.; Nazario, N.O.; Lima, G.C. Risk factors for diabetic retinopathy: A case-control study. Int. J. Retin. Vitr. 2016, 2, 21. [Google Scholar] [CrossRef]
- Corcóstegui, B.; Durán, S.; González-Albarrán, M.O.; Hernández, C.; Ruiz-Moreno, J.M.; Salvador, J.; Udaondo, P.; Simó, R. Update on Diagnosis and Treatment of Diabetic Retinopathy: A Consensus Guideline of the Working Group of Ocular Health (Spanish Society of Diabetes and Spanish Vitreous and Retina Society). J. Ophthalmol. 2017, 2017, 8234186. [Google Scholar] [CrossRef]
- Rezazadeh-Gavgani, E.; Oladghaffari, M.; Bahramian, S.; Majidazar, R.; Dolati, S. MicroRNA-21: A critical underestimated molecule in diabetic retinopathy. Gene 2023, 859, 147212. [Google Scholar] [CrossRef] [PubMed]
- Woerner, J.; Sriram, V.; Nam, Y.; Verma, A.; Kim, D. Uncovering genetic associations in the human diseasome using an endophenotype-augmented disease network. Bioinformatics 2024, 40, btae126. [Google Scholar] [CrossRef]
- Alamro, H.; Bajic, V.; Macvanin, M.T.; Isenovic, E.R.; Gojobori, T.; Essack, M.; Gao, X. Type 2 Diabetes Mellitus and its comorbidity, Alzheimer’s disease: Identifying critical microRNA using machine learning. Front. Endocrinol. 2023, 13, 1084656. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).