Abstract
The peptidyl-prolyl isomerase A (PPIA), also known as cyclophilin A (CYPA), is involved in multiple steps of the HIV-1 replication cycle. CYPA regulates the restriction of many host factors by interacting with the CYPA-binding loop on the HIV-1 capsid (CA) surface. TRIM5 (tripartite motif protein 5) in primates is a key species-specific restriction factor defining the HIV-1 pandemic. The incomplete adaptation of HIV-1 to humans is due to the different utilization of CYPA by pandemic and non-pandemic HIV-1. The enzymatic activity of CYPA on the viral core is likely an important reason for regulating the TRIM5 restriction activity. Thus, the HIV-1 capsid and its CYPA interaction may serve as new targets for future anti-AIDS therapeutic agents. This article will describe the species-specificity of the restriction factor TRIM5, understand the role of CYPA in regulating restriction factors in retroviral infection, and discuss important future research issues.
1. Introduction
Human immunodeficiency virus 1 (HIV-1), a lentivirus, is the result of successful cross-species transmissions of simian immunodeficiency viruses (SIVs) from chimpanzees and gorillas to humans [1,2]. HIV-1 is subdivided into four groups, HIV-1 M and N, which evolved from the adaptation of the SIV of the central African chimpanzee Pan troglodytes troglodytes (SIVcpzPtt) in humans, and HIV-1 P and O, which originated from the western lowland gorilla (Gorilla gorilla gorilla) lentivirus (SIVgor) [3,4] (Figure 1). In contrast, HIV-2 is the result of multiple cross-species transmissions of sooty mangabey SIV (SIVsmm) [5]. HIV-1 M is the primary cause of the pandemic of AIDS worldwide [2]. Although antiretroviral therapy (ART) has played a positive role in the prevention and treatment of HIV-1 infection, it does not cure the virus infection, and drug resistance can evolve [6,7]. As a result, novel compounds that are effective against drug-resistant HIV-1 strains and novel therapeutic classes that target unexplored viral sites must be developed. A better understanding of the intracellular interactions of HIV-1 may facilitate the identification of new treatment targets. Herein, we provide details of the HIV-1 capsid structure and CYPA-binding elements within this structure and how HIV-1 M escapes the restriction of human (hu) tripartite motif 5 alpha (TRIM5α) through CYPA binding. We then describe the restriction of huTRIM5α to HIV-1 non-M, which has been reported to be regulated by the CYPA-binding loop of the capsid. TRIM5 plays an important role in protecting cells from cross-species transmission of retroviruses, so understanding the detailed mechanism by which CYPA regulates TRIM5’s restriction of HIV-1 will help inspire the development of new methods to inhibit HIV-1 infection.
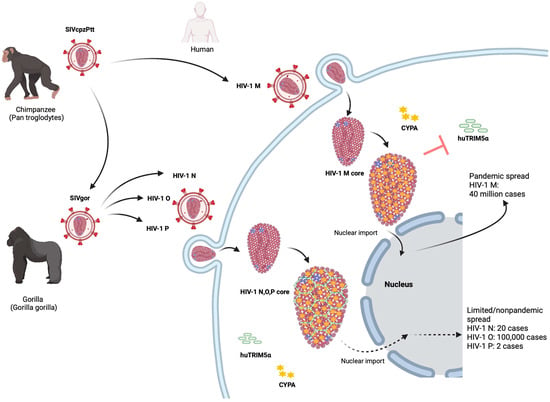
Figure 1.
The origin and epidemiology of HIV-1. Chimpanzees acquired lentivirus infections from preyed monkeys. SIVcpzPtt from western chimpanzees was transmitted to humans and evolved into the global HIV-1 group M and the rare HIV-1 group N. SIVcpzPtt also spread to gorillas and evolved into SIVgor. SIVgor invaded humans and evolved into HIV-1 group O and rare cases of the HIV-1 group P. After HIV-1 M invades cells, CYPA binding to the capsid (CA) can protect the viral core of HIV-1 M from human TRIM5α to successfully infect host cells. TRIM5α can form a hexagonal lattice on the viral core of HIV-1, thereby inhibiting early cell infection. In contrast to HIV-1 M, the non-pandemic HIV-1 cannot use their CYPA CA interaction to protect their core against human TRIM5α.
Nascent HIV-1 particles assemble at the infected cell’s plasma membrane and bud as immature virions with a membrane envelope [8]. During the initiation of virion maturation, the Gag-Pol polyprotein is hydrolyzed into reverse transcriptase (RT), integrase (IN), and protease (PR) enzymes, where the PR cleaves the Gag precursor protein into three viral structural proteins: matrix (MA), nucleocapsid (NC), and capsid (CA) proteins [9,10]. Gag cleavage results in a rearrangement of the internal structure of the virus to form the cone-shaped core from CA proteins. The MA forms a spherical shell that binds to the viral membrane and recruits the viral envelope glycoproteins (Env glycoproteins) [11,12]. Inside the MA lattice lies the viral core. The core is composed of the mature CA organized in 250 hexamers and 12 pentamers and the viral ribonucleoprotein complex consisting of two copies of the plus-stranded viral RNA genome along with NC, RT, and IN [13,14,15,16]. It has been shown that the core is the key structure of HIV-1 and plays a crucial role in almost every step during the early phase of the viral replication cycle, namely intracellular trafficking after viral fusion, nuclear import, uncoating, and integration of the viral genome into host chromatin [17,18,19] (Figure 2).
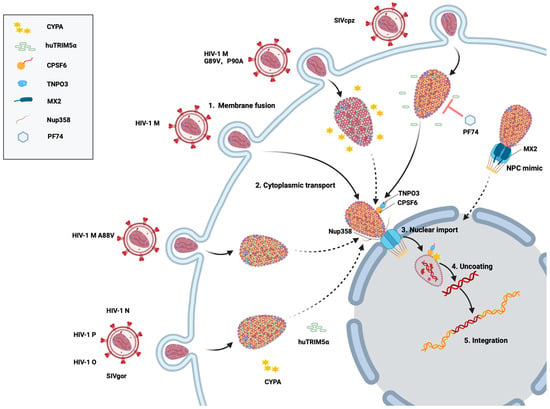
Figure 2.
Schematic of HIV-1 M and SIVcpz cell infection. After virus–cell membrane fusion (step 1), the viral core is transported into the cytoplasm and interacts with CYPA and TRIM5α (step 2). Binding of the core with cellular proteins (e.g., CPSF6 and NUP358) promotes transport through the nuclear pore complex (NPC) (step 3). In the nucleus, after the reverse transcription of the viral RNA, the uncoating releases the viral DNA (step 4) and DNA integration can take place (step 5). SIVcpz remains mostly unaffected by the small-molecule inhibitor PF74 during invasion, whereas HIV-1 M is inhibited by PF74 under the same conditions. HIV-1 N, P, O, and SIVgor are restricted by TRIM5α. HIV-1 M capsid mutants G89V and P90A prevent CYPA from binding to the capsid, allowing huTRIM5 α to exert its restriction effect. The CA mutation HIV-1 M A88V alters the CYPA interaction with the CYPA-binding loop, shifting the isomerization pattern of its proline residues from trans to cis. This conformational change allows the recognition of the HIV-1 capsid by huTRIM5α, restoring its ability to restrict HIV-1 infection. Cytoplasmic MX2 recruits FG-Nups and importins to form biomolecular condensates that mimic nuclear pores and disrupt the nuclear targeting of HIV-1. Broken lines indicate impaired and restricted steps.
To coordinate the early stages of the HIV-1 replication cycle, the core critically depends on host factors [20,21]. Cyclophilin A (CYPA and PPIA), an example of the several interactors of the core [22,23,24,25,26], is a member of the cyclophilin family of immunophilins with ubiquitous cellular distribution [27,28,29]. Many cyclophilins possess the peptidyl/prolyl trans-cis isomerase (PPIase) activity that catalyzes the isomerization of the peptide bond upstream of proline residues in proteins [30,31]. Although this review focuses on CYPA, cyclophilin B (CYPB), another member of the cyclophilin family encoded by the human PPIB gene, has been shown to regulate HIV-1 nuclear import [32]. Among the eighteen different cyclophilin isoenzymes in humans, CYPA, the most abundant member of the cyclophilin family and the major player in cellular PPIase activity, is encoded by the PPIA gene located on chromosome 7 [33]. CYPA is an intracellular binding partner for the small-molecule immunosuppressant cyclosporin A (CsA) and participates in many biological processes, such as supporting the translation of intrinsically disordered proteins, protein folding, post-translational modifications, protein transport, the assembly of essential cellular protein complexes, and cell signaling [34,35,36]. In addition, CYPA plays a critical role in homologous recombination DNA repair following replication fork stalling, and its inhibition by CsA renders some cancer cell lines highly sensitive to cell death [37].
CYPA inhibits or promotes HIV-1 infection in a cell-specific manner and is intricately involved in many steps of the viral replication cycle, from cytoplasmic trafficking to genome integration [26,38,39,40]. The molecular mechanisms by which cyclophilins regulate HIV-1 infection are complex and not yet fully understood. Recent studies have shown that CYPA could promote the interaction of the viral core with host factors, including cleavage and polyadenylation specificity factor 6 (CPSF6) [38], SAD1 and UNC84 domain containing (SUN) 1 [41], SUN2 [42], simian TRIM5α [43,44,45,46], and myxovirus resistance 2 (MX2) [47,48,49]. CYPA has an opposite role in the context of human TRIM5α: CYPA protects HIV-1 M against human TRIM5α restriction, while the Old World monkey TRIM5α variant needs CYPA for anti-HIV-1 activity [44,45,50,51].
2. Capsid Structure and CYPA Binding Sites
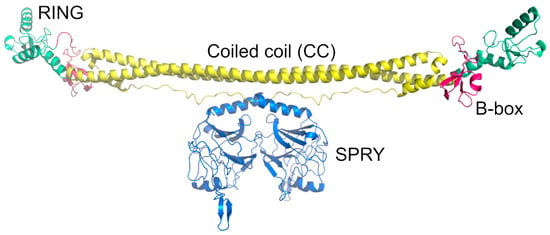
The mature HIV-1 core is composed of around 250 capsid hexamers and 12 capsid pentamers, with seven pentamers and five pentamers distributed at both ends of the core (Figure 3A,B) [11,52]. The pentamer provides a downward inclination angle for the surface of the originally regular hexamer honeycomb, inducing the bending of the closed shell to form a unique conical capsid structure [52]. The HIV-1 capsid (CA) is a 231-residue protein that folds into two largely α-helical domains—an N-terminal domain (CA-NTD) and a C-terminal domain (CA-CTD)—connected by a short linker [53,54] (Figure 3C). The CA-NTD consists of seven α-helices and a characteristic extended CYPA-binding loop [55] (Figure 3C). The CA-CTD is made up of four α-helices, a short 310 helix, and the main homology region (MHR), a highly conserved element needed for viral replication in all ortho-retroviruses, a subfamily of retroviridae that includes lentiviruses [54,56]. The NTD stands atop the CTD, facing the external environment and arranged into hexamers and pentamers, while the CTD forms the surface, facing the interior of the cone (Figure 3B). The hexameric and pentameric oligomers are formed by NTD-NTD and NTD-CTD interactions [13,57], while the CTD interacts to form homodimers and homotrimers that connect the lattice [58,59].
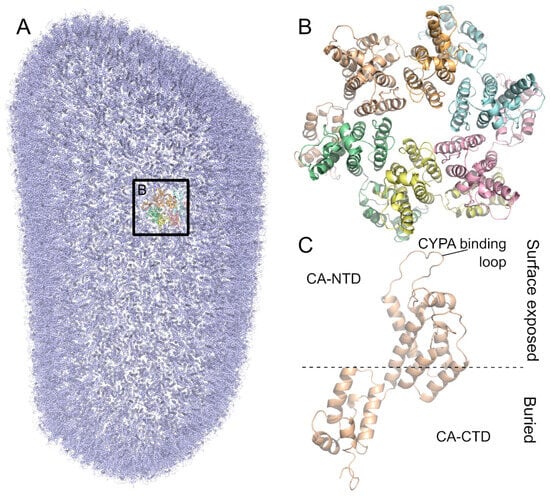
Figure 3.
The HIV-1 capsid is arranged in a conical lattice of hexamers and pentamers of the CA protein. (A) Model of the full HIV-1 M capsid based on PDB ID 3J3Q [60]. All monomers are colored in blue except for one hexamer highlighted by a box. (B) Close-up of the HIV-1 M capsid hexamer. Monomers are colored differently. (C) CA protein monomer shown from the side. The CA-NTD is on the surface exposed side. The CYPA-binding loop is part of the CA-NTD. The CA-CTD is buried in the inside of the capsid.
During infection by retroviruses such as HIV-1, many host factors bind to the viral core through several residues, particularly at the N-terminal domain [19,61,62,63,64]. CYPA has been shown to bind directly to the capsid CYPA-binding loop. Experiments involving the depletion of CYPA in cells or the inhibition of capsid binding by the use of CsA provided important information about the relevance of the interaction between CYPA and the HIV-1 capsid [39,65,66,67]. X-ray crystallography, molecular dynamics (MD) simulations, cryo-electron microscopy (cryoEM), and cryo-electron tomography (cryoET) have enabled the detailed visualization of the binding of the CA by CYPA [68]. The crystal structure of CYPA in complex with the CA-NTD shows that CA residues A88-G89-P90 and the configuration of the CYPA-binding loop are important for the binding interaction (Figure 4A–C) [55,69]. Mutations in the CA CYPA-binding loop directly affect viral replication [70].
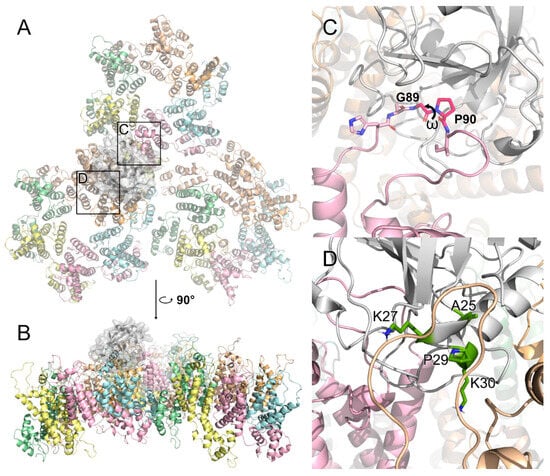
Figure 4.
CYPA binds on the surface of the HIV-1 capsid. (A) The binding effect of CYPA, shown in white, on the solvent-exposed parts of CA hexamers. The region of the canonical binding site is marked with box C. The region of the putative non-canonical binding site is marked with box D. This model is based on PDB ID 6Y9X [71]. (B) Side view on CYPA binding on the surface. (C) CYPA binds to the classical CYPA-binding loop. Residues G89 and P90 are indicated, as well as the ω dihedral between them. The trans/cis isomerization of this angle is catalyzed by CYPA. (D) One of the secondary binding sites of CYPA to the dimer interface. Residues A25, K27, P29, and K30, deemed crucial for the interaction, are colored green.
Interestingly, cryoEM and MD simulations recently showed that CYPA recognizes not just the CA protomer but three different monomers belonging to adjacent hexamers simultaneously [71]. One of the monomers interfaces with CYPA via the classical CYPA-binding loop (Figure 4A–C), but the other two interactions take place in two binding modes at nonclassical sites: one in which CYPA binds to the dimer interface (Figure 4D), and the other in which one CYPA binds above the dimer interface and another CYPA binds above the trimer interface [71,72,73,74,75]. However, it has been reported that residue mutations at one of the secondary binding sites of CYPA (A25, K27, P29, and K30; Figure 4D) did not affect the affinity of CYPA for the CA lattice [76]. Therefore, CYPA binds to the HIV-1 capsid through both classical and nonclassical sites, with mutations at secondary binding sites not affecting its overall affinity, suggesting flexible and compensatory binding mechanisms.
There is early evidence that CYPA binding to the core of incoming viruses is responsible for their effects on infection [66,69,77]. Some studies suggest that CYPA uses its PPIase activity to catalyze the trans-cis isomerization of the CYPA-binding loop, especially binding to the capsid domain of dimerized Gag, to promote the depolymerization of HIV-1 capsid complexes [78,79,80]. Recent studies have demonstrated that the interaction between CYPA and the HIV-1 capsid promotes viral infection in human cells by shielding the incoming viral capsid from the host restriction factor TRIM5α [44]. Interestingly, the A88V mutation in the capsid protein plays a pivotal role in this process. This mutation alters the CYPA-mediated isomerization pattern of proline residues in the CYPA-binding loop, shifting it from trans to cis, which allows huTRIM5α to restrict the virus. Thus, the A88V residue highlights the intricate balance between viral adaptation and host restriction mechanisms [44,45,50].
4. CYPA Modulates Interactions Between Host Factors and Retroviruses
The positive effects of the CYPA-CA interactions on HIV-1 replication in human cells are reversed in some Old World monkey cells. The molecular mechanism by which CYPA affects the resistance of HIV-1 M to TRIM5α in human cells is not fully understood and may involve changes in the trans/cis isomerization activity and core dynamics of CYPA [50]. In contrast, the depletion or inactivation of CYPA relieves HIV-1 from the restriction by the rhTRIM5α protein [66,173]. Surprisingly, TRIM5α restriction-dependent CYPA-CA interaction is a common but not universal phenotype in lentiviruses. For example, most lentiviruses that are restricted by TRIM5α are regulated by a CYPA interaction with the CA. The exceptions are SIVmac and EIAV, as well as some retroviruses from non-primates, such as MLV, which are TRIM5α-restricted without a CYPA interaction [117,174,175,176]. It is worth noting that CYPA-CA binding does not only regulate the restrictive role of TRIM5α; it also affects some other restriction factors in the early stages of the viral infection [40,62,177]. As we described above (Section 3), the proposed CA interaction surface involves the CYPA-binding loop. Thus, it is discussed whether CYPA binding “blocks” the binding of huTRIM5α by introducing competition or whether the enzymatic activity of CYPA changes the conformation of the TRIM5α binding site in the CA.
4.1. CYPA Regulates the Anti HIV-1 Activity of TRIM5α
In Old World monkey cells, TRIM5α-mediated HIV-1 restriction is promoted through the effects of free CYPA in the target cells [51,178,179]. Similar findings were obtained if rhTRIM5α was ectopically expressed in feline or canine cells [51,173,180]. In the first experiments, HIV-1 variants with mutations in the CYPA-binding loop that prevent the binding of CYPA as G89A or P90A showed escape from rhTRIM5α restriction [51,178]. However, follow-up studies showed that HIV-1 with these mutations is restricted by rhTRIM5α similar to wild-type HIV-1 [173,180,181,182]. Interestingly, for SIVagmTAN, unlike HIV-1, CYPA does not enhance the rhTRIM5α restriction against the virus, even though the CA of SIVagmTAN does bind CYPA [182,183,184]. By exchanging regions of the CA between SIVagmTAN and HIV-1, the determinants of this phenotype were found to center on the loop between helix 4/5 (loop 4–5) and 6/7 (loop 6–7), which is the CYPA-binding loop [184]. This suggests that for some of the anti-HIV-1 activity of some TRIM5α proteins, the interaction of CYPA with other cellular components may be more critical than its direct binding to the core.
Recently, it was reported that CYPA offers protection against the restrictive effect of huTRIM5α on HIV-1 by binding to the viral core [44,45] (Figure 1). Disruption of the CA-CYPA interaction by capsid mutations (P90A or G89V in the CYPA-binding loop) or CsA treatment significantly enhanced the affinity of huTRIM5α to the HIV-1 core and the antiviral activity [45]. The reason why non-pandemic HIV-1s (N, O, and P) did not spread widely in humans is unknown, with the restricting role of huTRIM5α being one of the possible reasons [50,160]. Although the observed affinity of non-pandemic HIV-1 capsids for CYPA is similar to that of HIV-1 M, huTRIM5α exhibits significant restrictions [50] (Figure 1). Thus, the CYPA interaction with non-pandemic HIV-1s does not protect strongly against TRIM5α [50]. It has been reported that capsid residues 50 and 88 are important for TRIM5α’s capacity to restrict HIV [50,160]. Moreover, in the CYPA-binding loop, valine for HIV-1 N and HIV-1 P capsid proteins or methionine for HIV-1 O located at capsid residue 88, rather than HIV-1 M alanine, can affect the trans/cis isomerization on G89-P90 peptide in the CYPA-binding loop of the HIV-1 capsid, likely indirectly influencing binding or sensitivity to huTRIM5α [50]. In HIV-1 M, in contrast to non-pandemic HIV-1s, bound CYPA has a predicted trans/cis isomerization activity on the capsid G89-P90 peptide that likely changes the binding strength or antiviral action of huTRIM5α through allosteric ways, which supports early assumptions [80,185,186]. Based on our model, HIV-1 M affects huTRIM5α binding by producing a higher proportion of cis conformations in the CYPA-binding loop through a faster CYPA-mediated isomerization process. This possibility was verified by performing potential of mean force (PMF) calculations on the isomerization reaction in the presence of CYPA, which revealed that the isomerization barrier of HIV-1 M was reduced the most compared to other HIVs [50]. Accordingly, the kinetics of the trans/cis isomerization of the CYPA-binding loop mediated by CYPA enzymatic activity on the viral core may determine the sensitivity to huTRIM5α.
4.2. CYPA in the TRIM5α Restriction of Other Retroviruses
In addition to effectively restricting the human lentiviruses HIV-1 and HIV-2, rhTRIM5α can also restrict retroviruses from other species (Figure 7) [175]. The rhTRIM5α restriction of HIV-1 is regulated by CYPA, and the ancestor of HIV-1, SIVcpz, is subject to the same restriction mechanism [176]. In contrast, CYPA does not enhance the rhTRIM5α restriction of SIVagmTAN, although the CA of this virus, like HIV-1, does bind CYPA [66,182,184]. However, the capsid of rhesus monkey SIV (SIVmac) does not interact with CYPA, and rhTRIM5α cannot restrict the closely related SIVmac, but this does not mean that it completely escapes TRIM5α, as shown by its sensitivity to distantly related TRIM5α from the New World squirrel monkey [174]. Squirrel monkey TRIM5α blocks SIVmac infection after DNA synthesis and is not saturable with restriction-sensitive virus-like particles [174]. HIV-2, largely confined to West Africa, is less replicative, less transmissible, and less pathogenic [2,187]. HIV-2 has a weak but specific affinity for CYPA and is restricted by rhTRIM5α [156]. rhTRIM5α also effectively blocks FIV infection, but it is unknown whether its restriction is related to CYPA, to which the FIV capsid can similarly bind [182]. Finally, rhTRIM5α is restrictive to EIAV and N-MLV but not B-MLV, which are both not CYPA-regulated [112,138,188,189,190,191]. Therefore, TRIM5α dependence on CYPA-CA interactions for restriction function is a common but not universal phenotype in retrovirus restriction.
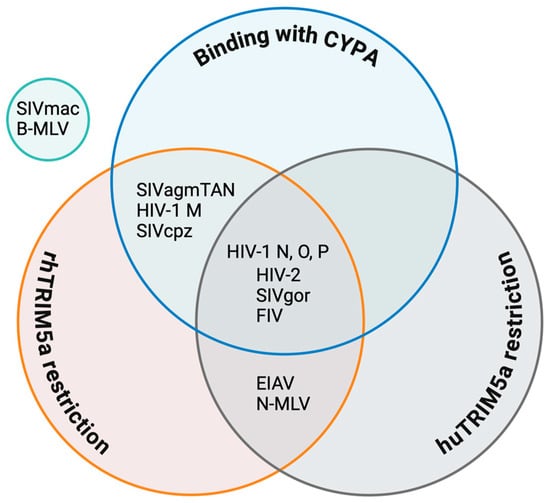
Figure 7.
Venn diagram of the regulatory roles of TRIM5α and CYPA in HIV and other retroviruses. Data sources for individual viruses are as follows: HIV-1 M [50,175]; HIV-1 N, O, and P, which are restricted by huTRIM5α [50], as well as by rhTRIM5α, according to Twizerimana et al. (unpublished); HIV-2 [2,156,187]; SIVcpz [176]; SIVagmTAN [182,184,192]; SIVmac [139,174]; SIVgor [50]; FIV [182,193]; EIAV [138]; N-MLV [138,168]; and B-MLV [168].
huTRIM5α has slightly different restrictions on primate retroviruses than rhTRIM5α, but is otherwise similar. SIVcpz is protected by CYPA to escape huTRIM5α, as is HIV-1 M, whereas SIVgor, the progenitor virus of HIV-1 O and HIV-1 P, is restricted by huTRIM5α, similarly to both descendant viruses. In all these viruses, residue 88 in the CYPA-binding loop plays a determining function [50] (Figure 2). HIV-2 cores show high susceptibility to huTRIM5α, with a less clear dependence on CYPA [187].
4.3. CYPA and Nuclear Entry Host Factors
The nuclear import of the viral core during infection depends on the CA protein. In addition to CYPA, several host factors are discussed as important players in this step, pro-viral factors such as nucleoporine proteins (NUPs) and CPSF6 or antiviral proteins such as MX2.
CPSF6 is a nuclear protein consisting of an N-terminal RNA recognition motif (RRM) domain, a middle proline-rich domain (PRD), and a C-terminal Arg/Ser-like domain (RSLD), in which the PRD mediates binding to HIV-1 CA [63,194,195,196,197,198]. CPSF6 binds to the HIV-1 capsid at the interface between the two CA monomers defined by helices 3, 4, and 5 (Figure 8), and the disruption of binding is mediated by changes in residues F321 in the PRD or by mutations in the CA, including residues 57, 70, 74, 77, and 105 [63,177,199,200,201]. The RSLD in CPSF6 is directly bound by β-karyopherin transportin 3 (TNPO3), which influences the nuclear import of the protein (Figure 2) [195,196]. Accordingly, the truncation of the C-terminus of CPSF6 (e.g., a truncation at residue 358, CPSF6-358) leads to the localization of the protein to the cytoplasm and inhibits HIV-1 infection by binding to the capsid and sequestering it from the nucleus [196,200,202,203]. HIV-1’s ancestor SIVcpzPtt shows a similar restriction mechanism to CPSF6-358, but the related SIVcpzPts is not inhibited [62]. Moreover, CPSF6 has been shown to contribute to HIV-1 trafficking to nuclear speckles. Although most established cell lines do not experience an overall drop in HIV-1 infection due to decreased CPSF6 expression or capsid binding to CPSF6, viral DNA integration is misdirected outside of gene-dense, transcriptionally active, host chromatin-to-heterochromatic, lamina-associated areas [26,177,204]. Recent data suggest that CYPA binding to the HIV-1 core prevents untimely binding of CPSF6 to the capsid in the cytoplasm. The inhibition of this binding leads to the formation of higher-order CPSF6 complexes, which disrupt HIV-1 capsid assemblies in vitro, alter capsid trafficking, and reduce infectivity [38].
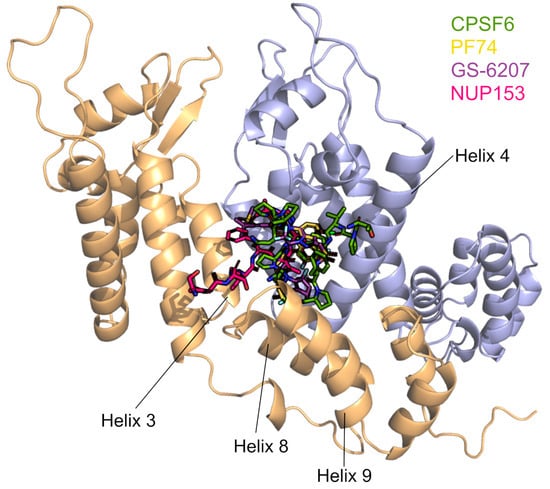
Figure 8.
CPSF6, PF74, LEN/GS-6207, and NUP153 share a binding site in the interface of two CA monomers. The binding site is formed by Helixes 3, 4, 8, and 9. The binding positions of the four ligands are taken from PDB IDs 4WYM, 4XFZ, 6V2F, and 5TSV. The CYPA-binding loops point to the top of the figure.
The N74D HIV-1 CA mutant lost binding to CPSF6 and, interestingly, to CYPA [205]. The loss of CYPA protection in the N74D virus causes inhibition via human TRIM5α [206]. This mutation has also been identified as a resistance mutation against lenacapavir, a capsid-targeting antiviral [207]. Additionally, HIV-1 N74D capsid mutant viruses are restricted by the TRIM5-analog TRIM34 [208,209]. TRIM34-restricted lentiviral capsid activity is dependent on TRIM5α, but TRIM5α restriction is not TRIM34-dependent because TRIM34 knockdown has little effect on CYPA-binding-deficient P90A capsid mutant viruses [208,209]. This suggests that N74D impacts the “conformation” of the viral core, which leads to both a loss of CYPA binding and a newly formed interface for a TRIM34 complex.
To enter the nucleus, HIV capsid proteins make direct contact with several nucleoporine proteins. For the NUPs NUP35 and POM121, interaction with the viral core was shown to be dependent on CYPA binding to a CA [205]. The antiviral activity of the cellular MX2 protein is also regulated by the CA-CYPA interaction. MX2 is an interferon-induced GTPase consisting of three domains (the stalk domain, the GTPase domain, and the bundling signaling element (BSE) domain). Its unstructured N-terminal domain (NTD) is critical for its restriction activity [210,211]. MX2 is a strong inhibitory factor for various retroviruses, such as HIV-1, SIV, EIAV, and FIV, blocking virus replication by binding to the capsid, and in turn preventing uncoating, nuclear import of the viral core, and viral DNA integration [212,213,214,215,216]. MX2 is reported to target the HIV-1 capsid by recognizing a negatively charged pocket defined by 12 glutamate residues (three copies of E71, E75, E212, and E213) created at three hexamer intersections [211,217]. It is thought that the R11-R12-R13 sequence on the MX2 NTD binds to this negative hexamer pocket, creating electrostatic attraction between the two proteins [217]. Nucleoporins mediate the antiviral activity of MX2 in a CA-CYPA-dependent manner, and the elimination of GTPase activity alters the dependence on specific NUPs for MX2 activity [215,218]. Temporal and spatial details of the MX2-mediated HIV-1 inhibition showed that MX2 assembles phenylalanine-glycine (FG)-rich nucleoporins into cytoplasmic biomolecular condensates that likely act as nuclear pore decoys that interact with incoming capsids to trap them or induce premature viral genome release [219] (Figure 2). HIV-1 CA mutant viruses (G89V and P90A) defective in CYPA binding, shRNA-mediated CYPA depletion, or CsA treatment have been reported to result in insensitivity to MX2, suggesting that the antiviral activity of MX2 is affected by the CYPA-CA interaction [47,48,49,215,216,220]. Interestingly, HIV-1 N74D reduced its sensitivity to MX2 [218]. Another study suggests that the role of CPSF6 in nuclear imports depends on MX2, and the cooperative relationship between the two affects the NUP358-mediated nuclear import of HIV-1 cores and viral replication [214]. While these data may suggest that the nuclear import pathways are important for the MX2 restriction, the failed interaction of CYPA with the N74D virus supports an essential role of CYPA for the antiviral activity of MX2 [49].
4.4. CYPA and Capsid Inhibitors
Since the HIV-1 CA plays essential roles in HIV-1 replication and is highly conserved, it is a desirable target for therapeutic intervention. Recently, many novel HIV-1 capsid inhibitors have been successfully reported [221,222,223,224,225]. Most of these inhibitors can inhibit or accelerate capsid disassembly or inhibit assembly, and some of them can inhibit reverse transcription, but most of them are not currently fully approved for clinical use [226,227,228,229]. Capsid residues that are required for interactions with such inhibitors have been extensively looked at [62,229,230]. Furthermore, the influence of other host interactors of capsids on some capsid inhibitors has been highlighted [62,63,201,231,232]. These drugs can also inhibit the nuclear import and transport of the HIV-1 replication complex, possibly by competitively blocking the binding of host factors such as CPSF6 and NUP153 and/or by altering CA core stability [201,233,234].
The small-molecule capsid inhibitor PF-3450074 (PF74) has been extensively studied, and susceptibility to SIV and HIV capsids was recently reported to be modulated by CYPA, as CYPA knockout or inhibition with CsA resulted in altered inhibitor’s activity (Figure 2) [62,231,235]. The potent inhibitor lenacapavir (LEN/GS-6207) is the first and only capsid inhibitor to be approved for clinical use. Researchers are excited that the injectable LEN used as pre-exposure prophylaxis protects people for six months with each shot and thus has the potential to reduce global infection rates [236]. Molecular docking studies showed that LEN, PF74, CPSF6, and NUP153 share the same binding site located between the capsid NTD and the CTD (Figure 8) [63,201,232]. It is likely that additional host factors also bind in this pocket. A highly resistant virus with five CA mutations (Q67H, K70R, H87P, T107N, and L111I) was discovered during the first characterization of PF74 [237]. H87P is distinct from the other residues due to its location in the CYPA-binding loop, which is distal from the inhibitor binding site. The substitution of H87P reduced the sensitivity of HIV-1 to another CA inhibitor, GSK878, by 3−4-fold, similar to LEN [233]. Together, these data suggest that CYPA binding to the core indirectly modulates the antiviral activity of these capsid inhibitors.
5. Conclusions and Perspectives
The research on HIV-1 evolution and invasion has uncovered a dizzying array of host factors that can interact with the CA and the viral core (Figure 9). The detailed mechanisms of TRIM5α interaction with HIV are unclear. Continued research on how CYPA regulates TRIM5 activity and other molecules will contribute to our understanding of complex host-retrovirus relationships, particularly those involving species-specific replication and zoonosis.
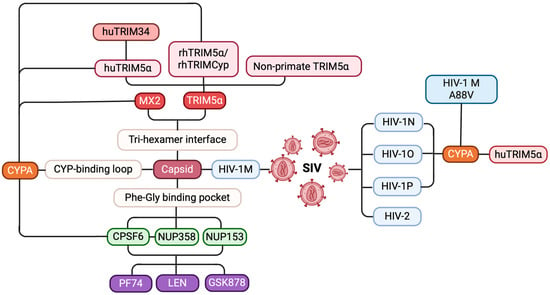
Figure 9.
A mind map of host–virus interactions affecting HIV evolution and replication. HIV evolution and cross-species transmission (blue): SIVs evolved through cross-species transmission, leading to the emergence of HIV-1 subtypes M, N, O, and P, as well as HIV-2, with HIV-1 M being the primary strain responsible for the global pandemic. Host restriction factors and their mechanisms (red): host restriction factors such as TRIM5α and MX2 effectively inhibit HIV-1 M infection by binding to the tri-hexamer interface of the capsid. Viral cofactors that facilitate replication (green): viral cofactors like CPSF6, NUP358, and NUP153 promote viral replication by interacting with the FG pocket of the capsid. CPSF6 promotes the nuclear entry of HIV-1 pre-integration complexes by cooperating with NUP358. CYPA’s role in HIV capsid interactions (orange): CYPA protects the virus from huTRIM5α restriction by binding to the CYPA-binding loop of the capsid and modulates the antiviral activity of huTRIM34, rhTRIM5α, and MX2. Additionally, the A88V mutation in HIV-1 M alters the trans-to-cis isomerization of the CYPA-binding loop, restoring the inhibitory effect of huTRIM5α. Key targets for small-molecule inhibitors (purple): small-molecule inhibitors, such as PF74, LEN, and GSK878, disrupt viral replication by interfering with the interactions between the capsid and CPSF6, NUP358, and NUP153.
In addition, the discovery that CYPA regulates TRIM5α to restrict poxviruses also provides new ideas for the development of antiviral drugs for diseases in different fields. CYPA plays important but ill-defined roles in various steps of the HIV-1 replication cycle, not only regulating a variety of host factors but also greatly influencing the sensitivity of capsid inhibitors, which form a complex network of relationships centered on CYPA. Therefore, disrupting CYPA-CA interactions appears to be a promising target in anti-HIV drug design.
Author Contributions
All authors have contributed to the text and figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported, in part, by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) 270650915 (Research Training Group GRK 2158) and the Heinz Ansmann Foundation for AIDS research, given to C.M. T.W. was supported by CSC (funding number: 202308620126). A.P.T. was supported by the German Academic Exchange Service (DAAD) (funding number: 91611196).
Acknowledgments
We are grateful for the computational support and infrastructure provided by the “Zentrum für Informations- und Medientechnologie” (ZIM) at the Heinrich Heine University Düsseldorf and the computing time provided by the John von Neumann Institute for Computing (NIC) on the supercomputer JUWELS at Jülich Supercomputing Centre (JSC) (user ID: VSK33).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed]
- Twizerimana, A.P.; Scheck, R.; Häussinger, D.; Münk, C. Post-Entry Restriction Factors of SIVcpz. Future Virol. 2018, 13, 727–745. [Google Scholar] [CrossRef]
- Gao, F.; Bailes, E.; Robertson, D.L.; Chen, Y.; Rodenburg, C.M.; Michael, S.F.; Cummins, L.B.; Arthur, L.O.; Peeters, M.; Shaw, G.M.; et al. Origin of HIV-1 in the Chimpanzee Pan Troglodytes Troglodytes. Nature 1999, 397, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Hahn, B.H. The Evolution of HIV-1 and the Origin of AIDS. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2487–2494. [Google Scholar] [CrossRef]
- Chen, Z.; Telfier, P.; Gettie, A.; Reed, P.; Zhang, L.; Ho, D.D.; Marx, P.A. Genetic Characterization of New West African Simian Immunodeficiency Virus SIVsm: Geographic Clustering of Household-Derived SIV Strains with Human Immunodeficiency Virus Type 2 Subtypes and Genetically Diverse Viruses from a Single Feral Sooty Mangabey Troop. J. Virol. 1996, 70, 3617–3627. [Google Scholar]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2019 Update of the Drug Resistance Mutations in HIV-1. Top. Antivir. Med. 2019, 27, 111–121. [Google Scholar]
- Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [CrossRef]
- Qu, K.; Ke, Z.; Zila, V.; Anders-Össwein, M.; Glass, B.; Mücksch, F.; Müller, R.; Schultz, C.; Müller, B.; Kräusslich, H.-G.; et al. Maturation of the Matrix and Viral Membrane of HIV-1. Science 2021, 373, 700–704. [Google Scholar] [CrossRef]
- Novikova, M.; Zhang, Y.; Freed, E.O.; Peng, K. Multiple Roles of HIV-1 Capsid during the Virus Replication Cycle. Virol. Sin. 2019, 34, 119–134. [Google Scholar] [CrossRef]
- Freed, E.O. HIV-1 Assembly, Release and Maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. [Google Scholar] [CrossRef]
- Samal, A.B.; Green, T.J.; Saad, J.S. Atomic View of the HIV-1 Matrix Lattice; Implications on Virus Assembly and Envelope Incorporation. Proc. Natl. Acad. Sci. USA 2022, 119, e2200794119. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Wilson, I.A. The HIV-1 Envelope Glycoprotein Structure: Nailing down a Moving Target. Immunol. Rev. 2017, 275, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Pornillos, O.; Ganser-Pornillos, B.K.; Yeager, M. Atomic Level Modeling of the HIV Capsid. Nature 2011, 469, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Welker, R.; Hohenberg, H.; Tessmer, U.; Huckhagel, C.; Kräusslich, H.-G. Biochemical and Structural Analysis of Isolated Mature Cores of Human Immunodeficiency Virus Type 1. J. Virol. 2000, 74, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Kotov, A.; Zhou, J.; Flicker, P.; Aiken, C. Association of Nef with the Human Immunodeficiency Virus Type 1 Core. J. Virol. 1999, 73, 8824–8830. [Google Scholar] [CrossRef]
- Accola, M.A.; Öhagen, Å.; Göttlinger, H.G. Isolation of Human Immunodeficiency Virus Type 1 Cores: Retention of Vpr in the Absence of P6gag. J. Virol. 2000, 74, 6198–6202. [Google Scholar] [CrossRef]
- Scoca, V.; Di Nunzio, F. The HIV-1 Capsid: From Structural Component to Key Factor for Host Nuclear Invasion. Viruses 2021, 13, 273. [Google Scholar] [CrossRef]
- Masenga, S.K.; Mweene, B.C.; Luwaya, E.; Muchaili, L.; Chona, M.; Kirabo, A. HIV–Host Cell Interactions. Cells 2023, 12, 1351. [Google Scholar] [CrossRef]
- Yamashita, M.; Engelman, A.N. Capsid-Dependent Host Factors in HIV-1 Infection. Trends Microbiol. 2017, 25, 741–755. [Google Scholar] [CrossRef]
- Zhuang, S.; Torbett, B.E. Interactions of HIV-1 Capsid with Host Factors and Their Implications for Developing Novel Therapeutics. Viruses 2021, 13, 417. [Google Scholar] [CrossRef]
- Temple, J.; Tripler, T.N.; Shen, Q.; Xiong, Y. A Snapshot of HIV-1 Capsid–Host Interactions. Curr. Res. Struct. Biol. 2020, 2, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Betancor, G.; Dicks, M.D.J.; Jimenez-Guardeño, J.M.; Ali, N.H.; Apolonia, L.; Malim, M.H. The GTPase Domain of MX2 Interacts with the HIV-1 Capsid, Enabling Its Short Isoform to Moderate Antiviral Restriction. Cell Rep. 2019, 29, 1923–1933.e3. [Google Scholar] [CrossRef] [PubMed]
- Grütter, M.G.; Luban, J. TRIM5 Structure, HIV-1 Capsid Recognition, and Innate Immune Signaling. Curr. Opin. Virol. 2012, 2, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Meuser, M.E.; Cunanan, C.J.; Cocklin, S. Structure, Function, and Interactions of the HIV-1 Capsid Protein. Life 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Achuthan, V.; Perreira, J.M.; Sowd, G.A.; Puray-Chavez, M.; McDougall, W.M.; Paulucci-Holthauzen, A.; Wu, X.; Fadel, H.J.; Poeschla, E.M.; Multani, A.S.; et al. Capsid-CPSF6 Interaction Licenses Nuclear HIV-1 Trafficking to Sites of Viral DNA Integration. Cell Host Microbe 2018, 24, 392–404.e8. [Google Scholar] [CrossRef]
- Schaller, T.; Ocwieja, K.E.; Rasaiyaah, J.; Price, A.J.; Brady, T.L.; Roth, S.L.; Hué, S.; Fletcher, A.J.; Lee, K.; KewalRamani, V.N.; et al. HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency. PLoS Pathog. 2011, 7, e1002439. [Google Scholar] [CrossRef]
- Stamnes, M.A.; Rutherford, S.L.; Zuker, C.S. Cyclophilins: A New Family of Proteins Involved in Intracellular Folding. Trends Cell Biol. 1992, 2, 272–276. [Google Scholar] [CrossRef]
- Schmid, F.X. Prolyl Isomerase: Enzymatic Catalysis of Slow Protein-Folding Reactions. Annu. Rev. Biophys. Biomol. Struct. 1993, 22, 123–143. [Google Scholar] [CrossRef]
- Braaten, D.; Wellington, S.; Warburton, D.; Luban, J. Assignment of Cyclophilin A (PPIA) to Human Chromosome Band 7p13 by in Situ Hybridization. Cytogenet. Genome Res. 1996, 74, 262. [Google Scholar] [CrossRef]
- Schiene-Fischer, C.; Yu, C. Receptor Accessory Folding Helper Enzymes: The Functional Role of Peptidyl Prolyl Cis/Trans Isomerases. FEBS Lett. 2001, 495, 1–6. [Google Scholar] [CrossRef]
- Lang, K.; Schmid, F.X.; Fischer, G. Catalysis of Protein Folding by Prolyl Isomerase. Nature 1987, 329, 268–270. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, J.; Madson, C.J.; Belshan, M. Cyclophilin B Enhances HIV-1 Infection. Virology 2016, 489, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Haendler, B.; Hofer, E. Characterization of the Human Cyclophilin Gene and of Related Processed Pseudogenes. Eur. J. Biochem. 1990, 190, 477–482. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Pham, U.; Posthuma, C.C.; Snijder, E.J. Cyclophilins and Cyclophilin Inhibitors in Nidovirus Replication. Virology 2018, 522, 46–55. [Google Scholar] [CrossRef]
- Maneix, L.; Iakova, P.; Lee, C.G.; Moree, S.E.; Lu, X.; Datar, G.K.; Hill, C.T.; Spooner, E.; King, J.C.K.; Sykes, D.B.; et al. Cyclophilin A Supports Translation of Intrinsically Disordered Proteins and Affects Haematopoietic Stem Cell Ageing. Nat. Cell Biol. 2024, 26, 593–603. [Google Scholar] [CrossRef]
- Liao, Y.; Luo, D.; Peng, K.; Zeng, Y. Cyclophilin A: A Key Player for Etiological Agent Infection. Appl. Microbiol. Biotechnol. 2021, 105, 1365–1377. [Google Scholar] [CrossRef]
- Bedir, M.; Outwin, E.; Colnaghi, R.; Bassett, L.; Abramowicz, I.; O’Driscoll, M. A Novel Role for the Peptidyl-Prolyl Cis-Trans Isomerase Cyclophilin A in DNA-Repair Following Replication Fork Stalling via the MRE11-RAD50-NBS1 Complex. EMBO Rep. 2024, 25, 3432–3455. [Google Scholar] [CrossRef]
- Zhong, Z.; Ning, J.; Boggs, E.A.; Jang, S.; Wallace, C.; Telmer, C.; Bruchez, M.P.; Ahn, J.; Engelman, A.N.; Zhang, P.; et al. Cytoplasmic CPSF6 Regulates HIV-1 Capsid Trafficking and Infection in a Cyclophilin A-Dependent Manner. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- De Iaco, A.; Luban, J. Cyclophilin A Promotes HIV-1 Reverse Transcription but Its Effect on Transduction Correlates Best with Its Effect on Nuclear Entry of Viral cDNA. Retrovirology 2014, 11, 11. [Google Scholar] [CrossRef]
- Padron, A.; Prakash, P.; Pandhare, J.; Luban, J.; Aiken, C.; Balasubramaniam, M.; Dash, C. Emerging Role of Cyclophilin A in HIV-1 Infection: From Producer Cell to the Target Cell Nucleus. J. Virol. 2023, 97, e00732-23. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Gao, G. SUN1 Regulates HIV-1 Nuclear Import in a Manner Dependent on the Interaction between the Viral Capsid and Cellular Cyclophilin A. J. Virol. 2018, 92, e00229-18. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, X.; Satoh, T.; Gentili, M.; Cerboni, S.; Silvin, A.; Conrad, C.; Ahmed-Belkacem, A.; Rodriguez, E.C.; Guichou, J.-F.; Bosquet, N.; et al. Nuclear Envelope Protein SUN2 Promotes Cyclophilin-A-Dependent Steps of HIV Replication. Cell Rep. 2016, 15, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Sokolskaja, E.; Berthoux, L.; Luban, J. Cyclophilin A and TRIM5α Independently Regulate Human Immunodeficiency Virus Type 1 Infectivity in Human Cells. J. Virol. 2006, 80, 2855–2862. [Google Scholar] [CrossRef]
- Kim, K.; Dauphin, A.; Komurlu, S.; McCauley, S.M.; Yurkovetskiy, L.; Carbone, C.; Diehl, W.E.; Strambio-De-Castillia, C.; Campbell, E.M.; Luban, J. Cyclophilin A Protects HIV-1 from Restriction by Human TRIM5α. Nat. Microbiol. 2019, 4, 2044–2051. [Google Scholar] [CrossRef]
- Selyutina, A.; Persaud, M.; Simons, L.M.; Bulnes-Ramos, A.; Buffone, C.; Martinez-Lopez, A.; Scoca, V.; Di Nunzio, F.; Hiatt, J.; Marson, A.; et al. Cyclophilin A Prevents HIV-1 Restriction in Lymphocytes by Blocking Human TRIM5α Binding to the Viral Core. Cell Rep. 2020, 30, 3766–3777.e6. [Google Scholar] [CrossRef]
- Burse, M.; Shi, J.; Aiken, C. Cyclophilin A Potentiates TRIM5α Inhibition of HIV-1 Nuclear Import without Promoting TRIM5α Binding to the Viral Capsid. PLoS ONE 2017, 12, e0182298. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, Q.; Liang, Z.; Qiao, W.; Cen, S.; Liang, C. The Highly Polymorphic Cyclophilin A-Binding Loop in HIV-1 Capsid Modulates Viral Resistance to MxB. Retrovirology 2015, 12, 1. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, Q.; Ding, S.; Qian, J.; Xu, F.; Zhou, J.; Cen, S.; Guo, F.; Liang, C. The Interferon-Inducible MxB Protein Inhibits HIV-1 Infection. Cell Host Microbe 2013, 14, 398–410. [Google Scholar] [CrossRef]
- Miles, R.J.; Kerridge, C.; Hilditch, L.; Monit, C.; Jacques, D.A.; Towers, G.J. MxB Sensitivity of HIV-1 Is Determined by a Highly Variable and Dynamic Capsid Surface. eLife 2020, 9, e56910. [Google Scholar] [CrossRef]
- Twizerimana, A.P.; Becker, D.; Zhu, S.; Luedde, T.; Gohlke, H.; Münk, C. The Cyclophilin A-Binding Loop of the Capsid Regulates the Human TRIM5α Sensitivity of Nonpandemic HIV-1. Proc. Natl. Acad. Sci. USA 2023, 120, e2306374120. [Google Scholar] [CrossRef]
- Berthoux, L.; Sebastian, S.; Sokolskaja, E.; Luban, J. Cyclophilin A Is Required for TRIM5α-Mediated Resistance to HIV-1 in Old World Monkey Cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14849–14853. [Google Scholar] [CrossRef] [PubMed]
- Ganser, B.K.; Li, S.; Klishko, V.Y.; Finch, J.T.; Sundquist, W.I. Assembly and Analysis of Conical Models for the HIV-1 Core. Science 1999, 283, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Gitti, R.K.; Lee, B.M.; Walker, J.; Summers, M.F.; Yoo, S.; Sundquist, W.I. Structure of the Amino-Terminal Core Domain of the HIV-1 Capsid Protein. Science 1996, 273, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Gamble, T.R.; Yoo, S.; Vajdos, F.F.; Von Schwedler, U.K.; Worthylake, D.K.; Wang, H.; McCutcheon, J.P.; Sundquist, W.I.; Hill, C.P. Structure of the Carboxyl-Terminal Dimerization Domain of the HIV-1 Capsid Protein. Science 1997, 278, 849–853. [Google Scholar] [CrossRef]
- Gamble, T.R.; Vajdos, F.F.; Yoo, S.; Worthylake, D.K.; Houseweart, M.; Sundquist, W.I.; Hill, C.P. Crystal Structure of Human Cyclophilin A Bound to the Amino-Terminal Domain of HIV-1 Capsid. Cell 1996, 87, 1285–1294. [Google Scholar] [CrossRef]
- Kovaleski, B.J.; Kennedy, R.; Khorchid, A.; Kleiman, L.; Matsuo, H.; Musier-Forsyth, K. Critical Role of Helix 4 of HIV-1 Capsid C-Terminal Domain in Interactions with Human Lysyl-tRNA Synthetase. J. Biol. Chem. 2007, 282, 32274–32279. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Kelly, B.N.; Hua, Y.; Whitby, F.G.; Stout, C.D.; Sundquist, W.I.; Hill, C.P.; Yeager, M. X-Ray Structures of the Hexameric Building Block of the HIV Capsid. Cell 2009, 137, 1282–1292. [Google Scholar] [CrossRef]
- Byeon, I.-J.L.; Meng, X.; Jung, J.; Zhao, G.; Yang, R.; Ahn, J.; Shi, J.; Concel, J.; Aiken, C.; Zhang, P.; et al. Structural Convergence between CryoEM and NMR Reveals Novel Intersubunit Interactions Critical for HIV-1 Capsid Function. Cell 2009, 139, 780–790. [Google Scholar] [CrossRef]
- Bayro, M.J.; Tycko, R. Structure of the Dimerization Interface in the Mature HIV-1 Capsid Protein Lattice from Solid State NMR of Tubular Assemblies. J. Am. Chem. Soc. 2016, 138, 8538–8546. [Google Scholar] [CrossRef]
- Zhao, G.; Perilla, J.R.; Yufenyuy, E.L.; Meng, X.; Chen, B.; Ning, J.; Ahn, J.; Gronenborn, A.M.; Schulten, K.; Aiken, C.; et al. Mature HIV-1 Capsid Structure by Cryo-Electron Microscopy and All-Atom Molecular Dynamics. Nature 2013, 497, 643–646. [Google Scholar] [CrossRef]
- Matreyek, K.A.; Yücel, S.S.; Li, X.; Engelman, A. Nucleoporin NUP153 Phenylalanine-Glycine Motifs Engage a Common Binding Pocket within the HIV-1 Capsid Protein to Mediate Lentiviral Infectivity. PLoS Pathog. 2013, 9, e1003693. [Google Scholar] [CrossRef] [PubMed]
- Twizerimana, A.P.; Scheck, R.; Becker, D.; Zhang, Z.; Wammers, M.; Avelar, L.; Pflieger, M.; Häussinger, D.; Kurz, T.; Gohlke, H.; et al. Cell Type-Dependent Escape of Capsid Inhibitors by Simian Immunodeficiency Virus SIVcpz. J. Virol. 2020, 94, e01338-20. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Fletcher, A.J.; Schaller, T.; Elliott, T.; Lee, K.; KewalRamani, V.N.; Chin, J.W.; Towers, G.J.; James, L.C. CPSF6 Defines a Conserved Capsid Interface That Modulates HIV-1 Replication. PLoS Pathog. 2012, 8, e1002896. [Google Scholar] [CrossRef]
- Rebensburg, S.V.; Wei, G.; Larue, R.C.; Lindenberger, J.; Francis, A.C.; Annamalai, A.S.; Morrison, J.; Shkriabai, N.; Huang, S.-W.; KewalRamani, V.; et al. Sec24C Is an HIV-1 Host Dependency Factor Crucial for Virus Replication. Nat. Microbiol. 2021, 6, 435–444. [Google Scholar] [CrossRef]
- Yin, L.; Braaten, D.; Luban, J. Human Immunodeficiency Virus Type 1 Replication Is Modulated by Host Cyclophilin A Expression Levels. J. Virol. 1998, 72, 6430–6436. [Google Scholar] [CrossRef]
- Braaten, D.; Franke, E.K.; Luban, J. Cyclophilin A Is Required for an Early Step in the Life Cycle of Human Immunodeficiency Virus Type 1 before the Initiation of Reverse Transcription. J. Virol. 1996, 70, 3551–3560. [Google Scholar] [CrossRef]
- Sokolskaja, E.; Sayah, D.M.; Luban, J. Target Cell Cyclophilin A Modulates Human Immunodeficiency Virus Type 1 Infectivity. J. Virol. 2004, 78, 12800–12808. [Google Scholar] [CrossRef]
- Wilbourne, M.; Zhang, P. Visualizing HIV-1 Capsid and Its Interactions with Antivirals and Host Factors. Viruses 2021, 13, 246. [Google Scholar] [CrossRef]
- Braaten, D.; Luban, J. Cyclophilin A Regulates HIV-1 Infectivity, as Demonstrated by Gene Targeting in Human T Cells. EMBO J. 2001, 20, 1300–1309. [Google Scholar] [CrossRef]
- Aiken, C. Mechanistic Independence of Nef and Cyclophilin A Enhancement of Human Immunodeficiency Virus Type 1 Infectivity. Virology 1998, 248, 139–147. [Google Scholar] [CrossRef][Green Version]
- Ni, T.; Gerard, S.; Zhao, G.; Dent, K.; Ning, J.; Zhou, J.; Shi, J.; Anderson-Daniels, J.; Li, W.; Jang, S.; et al. Intrinsic Curvature of the HIV-1 CA Hexamer Underlies Capsid Topology and Interaction with Cyclophilin A. Nat. Struct. Mol. Biol. 2020, 27, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Chiramel, A.I.; Meyerson, N.R.; McNally, K.L.; Broeckel, R.M.; Montoya, V.R.; Méndez-Solís, O.; Robertson, S.J.; Sturdevant, G.L.; Lubick, K.J.; Nair, V.; et al. TRIM5α Restricts Flavivirus Replication by Targeting the Viral Protease for Proteasomal Degradation. Cell Rep. 2019, 27, 3269–3283.e6. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, B.; Wittmann, S.; Lagisquet, J.; Deutschmann, J.; Eissmann, K.; Ross, J.J.; Biesinger, B.; Gramberg, T. Human TRIM5α Senses and Restricts LINE-1 Elements. Proc. Natl. Acad. Sci. USA 2020, 117, 17965–17976. [Google Scholar] [CrossRef]
- Liu, C.; Perilla, J.R.; Ning, J.; Lu, M.; Hou, G.; Ramalho, R.; Himes, B.A.; Zhao, G.; Bedwell, G.J.; Byeon, I.-J.; et al. Cyclophilin A Stabilizes the HIV-1 Capsid through a Novel Non-Canonical Binding Site. Nat. Commun. 2016, 7, 10714. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Y.; Richardson, S.; Sreekumar, M.; Albarnaz, J.D.; Smith, G.L. TRIM5α Restricts Poxviruses and Is Antagonized by CypA and the Viral Protein C6. Nature 2023, 620, 873–880. [Google Scholar] [CrossRef]
- Peng, W.; Shi, J.; Márquez, C.L.; Lau, D.; Walsh, J.; Faysal, K.M.R.; Byeon, C.H.; Byeon, I.-J.L.; Aiken, C.; Böcking, T. Functional Analysis of the Secondary HIV-1 Capsid Binding Site in the Host Protein Cyclophilin A. Retrovirology 2019, 16, 10. [Google Scholar] [CrossRef]
- Steinkasserer, A.; Harrison, R.; Billich, A.; Hammerschmid, F.; Werner, G.; Wolff, B.; Peichl, P.; Palfi, G.; Schnitzel, W.; Mlynar, E. Mode of Action of SDZ NIM 811, a Nonimmunosuppressive Cyclosporin A Analog with Activity against Human Immunodeficiency Virus Type 1 (HIV-1): Interference with Early and Late Events in HIV-1 Replication. J. Virol. 1995, 69, 814–824. [Google Scholar] [CrossRef]
- Bukovsky, A.A.; Weimann, A.; Accola, M.A.; Göttlinger, H.G. Transfer of the HIV-1 Cyclophilin-Binding Site to Simian Immunodeficiency Virus from Macaca Mulatta Can Confer Both Cyclosporin Sensitivity and Cyclosporin Dependence. Proc. Natl. Acad. Sci. USA 1997, 94, 10943–10948. [Google Scholar] [CrossRef]
- Braaten, D.; Aberham, C.; Franke, E.K.; Yin, L.; Phares, W.; Luban, J. Cyclosporine A-Resistant Human Immunodeficiency Virus Type 1 Mutants Demonstrate That Gag Encodes the Functional Target of Cyclophilin A. J. Virol. 1996, 70, 5170–5176. [Google Scholar] [CrossRef]
- Bosco, D.A.; Eisenmesser, E.Z.; Pochapsky, S.; Sundquist, W.I.; Kern, D. Catalysis of Cis/Trans Isomerization in Native HIV-1 Capsid by Human Cyclophilin A. Proc. Natl. Acad. Sci. USA 2002, 99, 5247–5252. [Google Scholar] [CrossRef]
- Johnson, W.E.; Sawyer, S.L. Molecular Evolution of the Antiretroviral TRIM5 Gene. Immunogenetics 2009, 61, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Chandrasekaran, V.; Carter, S.D.; Woodward, C.L.; Christensen, D.E.; Dryden, K.A.; Pornillos, O.; Yeager, M.; Ganser-Pornillos, B.K.; Jensen, G.J.; et al. Primate TRIM5 Proteins Form Hexagonal Nets on HIV-1 Capsids. eLife 2016, 5, e16269. [Google Scholar] [CrossRef] [PubMed]
- Skorupka, K.A.; Roganowicz, M.D.; Christensen, D.E.; Wan, Y.; Pornillos, O.; Ganser-Pornillos, B.K. Hierarchical Assembly Governs TRIM5α Recognition of HIV-1 and Retroviral Capsids. Sci. Adv. 2019, 5, eaaw3631. [Google Scholar] [CrossRef] [PubMed]
- Ganser-Pornillos, B.K.; Pornillos, O. Restriction of HIV-1 and Retroviruses by TRIM5. Nat. Rev. Microbiol. 2019, 17, 546–556. [Google Scholar] [CrossRef]
- Stremlau, M.; Perron, M.; Lee, M.; Li, Y.; Song, B.; Javanbakht, H.; Diaz-Griffero, F.; Anderson, D.J.; Sundquist, W.I.; Sodroski, J. Specific Recognition and Accelerated Uncoating of Retroviral Capsids by the TRIM5α Restriction Factor. Proc. Natl. Acad. Sci. USA 2006, 103, 5514–5519. [Google Scholar] [CrossRef]
- Imam, S.; Kömürlü, S.; Mattick, J.; Selyutina, A.; Talley, S.; Eddins, A.; Diaz-Griffero, F.; Campbell, E.M. K63-Linked Ubiquitin Is Required for Restriction of HIV-1 Reverse Transcription and Capsid Destabilization by Rhesus TRIM5α. J. Virol. 2019, 93, e00558-19. [Google Scholar] [CrossRef]
- Keown, J.R.; Black, M.M.; Ferron, A.; Yap, M.; Barnett, M.J.; Pearce, F.G.; Stoye, J.P.; Goldstone, D.C. A Helical LC3-Interacting Region Mediates the Interaction between the Retroviral Restriction Factor Trim5α and Mammalian Autophagy-Related ATG8 Proteins. J. Biol. Chem. 2018, 293, 18378–18386. [Google Scholar] [CrossRef]
- Saha, B.; Chisholm, D.; Kell, A.M.; Mandell, M.A. A Non-Canonical Role for the Autophagy Machinery in Anti-Retroviral Signaling Mediated by TRIM5α. PLoS Pathog. 2020, 16, e1009017. [Google Scholar] [CrossRef]
- Mandell, M.A.; Jain, A.; Arko-Mensah, J.; Chauhan, S.; Kimura, T.; Dinkins, C.; Silvestri, G.; Münch, J.; Kirchhoff, F.; Simonsen, A.; et al. TRIM Proteins Regulate Autophagy and Can Target Autophagic Substrates by Direct Recognition. Dev. Cell 2014, 30, 394–409. [Google Scholar] [CrossRef]
- Merindol, N.; El-Far, M.; Sylla, M.; Masroori, N.; Dufour, C.; Li, J.; Cherry, P.; Plourde, M.B.; Tremblay, C.; Berthoux, L. HIV-1 Capsids from B27/B57+ Elite Controllers Escape Mx2 but Are Targeted by TRIM5α, Leading to the Induction of an Antiviral State. PLoS Pathog. 2018, 14, e1007398. [Google Scholar] [CrossRef]
- Pertel, T.; Hausmann, S.; Morger, D.; Züger, S.; Guerra, J.; Lascano, J.; Reinhard, C.; Santoni, F.; Uchil, P.D.; Chatel, L.; et al. TRIM5 Is an Innate Immune Sensor for the Retrovirus Capsid Lattice. Nature 2011, 472, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Mael, A.A.; Ikeda, Y. Alpha Interferon Enhances TRIM5α-Mediated Antiviral Activities in Human and Rhesus Monkey Cells. J. Virol. 2007, 81, 10201–10206. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, E.E.; Shioda, T. Impact of TRIM5α in Vivo. AIDS 2015, 29, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- OhAinle, M.; Helms, L.; Vermeire, J.; Roesch, F.; Humes, D.; Basom, R.; Delrow, J.J.; Overbaugh, J.; Emerman, M. A Virus-Packageable CRISPR Screen Identifies Host Factors Mediating Interferon Inhibition of HIV. eLife 2018, 7, e39823. [Google Scholar] [CrossRef]
- Carthagena, L.; Parise, M.C.; Ringeard, M.; Chelbi-Alix, M.K.; Hazan, U.; Nisole, S. Implication of TRIMalpha and TRIMCyp in Interferon-Induced Anti-Retroviral Restriction Activities. Retrovirology 2008, 5, 59. [Google Scholar] [CrossRef]
- Nisole, S.; Stoye, J.P.; Saïb, A. TRIM Family Proteins: Retroviral Restriction and Antiviral Defence. Nat. Rev. Microbiol. 2005, 3, 799–808. [Google Scholar] [CrossRef]
- Meroni, G.; Diez-Roux, G. TRIM/RBCC, a Novel Class of ‘Single Protein RING Finger’ E3 Ubiquitin Ligases. BioEssays 2005, 27, 1147–1157. [Google Scholar] [CrossRef]
- Sanchez, J.G.; Okreglicka, K.; Chandrasekaran, V.; Welker, J.M.; Sundquist, W.I.; Pornillos, O. The Tripartite Motif Coiled-Coil Is an Elongated Antiparallel Hairpin Dimer. Proc. Natl. Acad. Sci. USA 2014, 111, 2494–2499. [Google Scholar] [CrossRef]
- Goldstone, D.C.; Walker, P.A.; Calder, L.J.; Coombs, P.J.; Kirkpatrick, J.; Ball, N.J.; Hilditch, L.; Yap, M.W.; Rosenthal, P.B.; Stoye, J.P.; et al. Structural Studies of Postentry Restriction Factors Reveal Antiparallel Dimers That Enable Avid Binding to the HIV-1 Capsid Lattice. Proc. Natl. Acad. Sci. USA 2014, 111, 9609–9614. [Google Scholar] [CrossRef]
- Ganser-Pornillos, B.K.; Chandrasekaran, V.; Pornillos, O.; Sodroski, J.G.; Sundquist, W.I.; Yeager, M. Hexagonal Assembly of a Restricting TRIM5α Protein. Proc. Natl. Acad. Sci. USA 2011, 108, 534–539. [Google Scholar] [CrossRef]
- Javanbakht, H.; Diaz-Griffero, F.; Stremlau, M.; Si, Z.; Sodroski, J. The Contribution of RING and B-Box 2 Domains to Retroviral Restriction Mediated by Monkey TRIM5α. J. Biol. Chem. 2005, 280, 26933–26940. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Griffero, F.; Qin, X.; Hayashi, F.; Kigawa, T.; Finzi, A.; Sarnak, Z.; Lienlaf, M.; Yokoyama, S.; Sodroski, J. A B-Box 2 Surface Patch Important for TRIM5α Self-Association, Capsid Binding Avidity, and Retrovirus Restriction. J. Virol. 2009, 83, 10737–10751. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sodroski, J. The TRIM5α B-Box 2 Domain Promotes Cooperative Binding to the Retroviral Capsid by Mediating Higher-Order Self-Association. J. Virol. 2008, 82, 11495–11502. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yeung, D.F.; Fiegen, A.M.; Sodroski, J. Determinants of the Higher Order Association of the Restriction Factor TRIM5α and Other Tripartite Motif (TRIM) Proteins. J. Biol. Chem. 2011, 286, 27959–27970. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Christensen, D.E.; Nelson, C.; Tan, C.P.; Schaller, T.; Lehner, P.J.; Sundquist, W.I.; Towers, G.J. TRIM5α Requires Ube2W to Anchor Lys63-linked Ubiquitin Chains and Restrict Reverse Transcription. EMBO J. 2015, 34, 2078–2095. [Google Scholar] [CrossRef]
- Herkules, F.; Yu, C.H.; Taylor, A.B.; Dougherty, V.; Weintraub, S.T.; Ivanov, D.N. Structural and Functional Asymmetry of RING Trimerization Controls Priming and Extension Events in TRIM5α Autoubiquitylation. Nat. Commun. 2022, 13, 7104. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wada, K.; Tanji, K.; Tanaka, M.; Kamitani, T. Ubiquitination of E3 Ubiquitin Ligase TRIM5 Alpha and Its Potential Role. FEBS J. 2008, 275, 1540–1555. [Google Scholar] [CrossRef]
- Lienlaf, M.; Hayashi, F.; Di Nunzio, F.; Tochio, N.; Kigawa, T.; Yokoyama, S.; Diaz-Griffero, F. Contribution of E3-Ubiquitin Ligase Activity to HIV-1 Restriction by TRIM5αrh: Structure of the RING Domain of TRIM5α. J. Virol. 2011, 85, 8725–8737. [Google Scholar] [CrossRef]
- Tozawa, T.; Matsunaga, K.; Izumi, T.; Shigehisa, N.; Uekita, T.; Taoka, M.; Ichimura, T. Ubiquitination-Coupled Liquid Phase Separation Regulates the Accumulation of the TRIM Family of Ubiquitin Ligases into Cytoplasmic Bodies. PLoS ONE 2022, 17, e0272700. [Google Scholar] [CrossRef]
- Wu, X.; Anderson, J.L.; Campbell, E.M.; Joseph, A.M.; Hope, T.J. Proteasome Inhibitors Uncouple Rhesus TRIM5α Restriction of HIV-1 Reverse Transcription and Infection. Proc. Natl. Acad. Sci. USA 2006, 103, 7465–7470. [Google Scholar] [CrossRef]
- Saha, B.; Olsvik, H.; Williams, G.L.; Oh, S.; Evjen, G.; Sjøttem, E.; Mandell, M.A. TBK1 Is Ubiquitinated by TRIM5α to Assemble Mitophagy Machinery. Cell Rep. 2024, 43, 114294. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.L.; Wu, L.I.; Emerman, M.; Malik, H.S. Positive Selection of Primate TRIM5α Identifies a Critical Species-Specific Retroviral Restriction Domain. Proc. Natl. Acad. Sci. USA 2005, 102, 2832–2837. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.A.; Menezes, A.N.; Schrago, C.G.; Moreira, M.A.M.; Bonvicino, C.R.; Soares, M.A.; Seuánez, H.N. Evolution of TRIM5alpha B30.2 (SPRY) Domain in New World Primates. Infect. Genet. Evol. 2010, 10, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; OhAinle, M.; Esteves, P.J. Patterns of Evolution of TRIM Genes Highlight the Evolutionary Plasticity of Antiviral Effectors in Mammals. Genome Biol. Evol. 2023, 15, evad209. [Google Scholar] [CrossRef] [PubMed]
- Águeda-Pinto, A.; Lemos de Matos, A.; Pinheiro, A.; Neves, F.; de Sousa-Pereira, P.; Esteves, P.J. Not so Unique to Primates: The Independent Adaptive Evolution of TRIM5 in Lagomorpha Lineage. PLoS ONE 2019, 14, e0226202. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Águeda-Pinto, A.; Pinheiro, A.; Rebelo, H.; Esteves, P.J. Evolution of TRIM5 and TRIM22 in Bats Reveals a Complex Duplication Process. Viruses 2022, 14, 345. [Google Scholar] [CrossRef]
- Tareen, S.U.; Sawyer, S.L.; Malik, H.S.; Emerman, M. An Expanded Clade of Rodent Trim5 Genes. Virology 2009, 385, 473–483. [Google Scholar] [CrossRef]
- Song, B.; Gold, B.; O’hUigin, C.; Javanbakht, H.; Li, X.; Stremlau, M.; Winkler, C.; Dean, M.; Sodroski, J. The B30.2(SPRY) Domain of the Retroviral Restriction Factor TRIM5α Exhibits Lineage-Specific Length and Sequence Variation in Primates. J. Virol. 2005, 79, 6111–6121. [Google Scholar] [CrossRef]
- Biris, N.; Tomashevski, A.; Bhattacharya, A.; Diaz-Griffero, F.; Ivanov, D.N. Rhesus Monkey TRIM5α SPRY Domain Recognizes Multiple Epitopes That Span Several Capsid Monomers on the Surface of the HIV-1 Mature Viral Core. J. Mol. Biol. 2013, 425, 5032–5044. [Google Scholar] [CrossRef]
- Roganowicz, M.D.; Komurlu, S.; Mukherjee, S.; Plewka, J.; Alam, S.L.; Skorupka, K.A.; Wan, Y.; Dawidowski, D.; Cafiso, D.S.; Ganser-Pornillos, B.K.; et al. TRIM5α SPRY/Coiled-Coil Interactions Optimize Avid Retroviral Capsid Recognition. PLoS Pathog. 2017, 13, e1006686. [Google Scholar] [CrossRef]
- Yang, H.; Ji, X.; Zhao, G.; Ning, J.; Zhao, Q.; Aiken, C.; Gronenborn, A.M.; Zhang, P.; Xiong, Y. Structural Insight into HIV-1 Capsid Recognition by Rhesus TRIM5α. Proc. Natl. Acad. Sci. USA 2012, 109, 18372–18377. [Google Scholar] [CrossRef] [PubMed]
- Biris, N.; Yang, Y.; Taylor, A.B.; Tomashevski, A.; Guo, M.; Hart, P.J.; Diaz-Griffero, F.; Ivanov, D.N. Structure of the Rhesus Monkey TRIM5α PRYSPRY Domain, the HIV Capsid Recognition Module. Proc. Natl. Acad. Sci. USA 2012, 109, 13278–13283. [Google Scholar] [CrossRef] [PubMed]
- Kovalskyy, D.B.; Ivanov, D.N. Recognition of the HIV Capsid by the TRIM5α Restriction Factor Is Mediated by a Subset of Pre-Existing Conformations of the TRIM5α SPRY Domain. Biochemistry 2014, 53, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Morger, D.; Zosel, F.; Bühlmann, M.; Züger, S.; Mittelviefhaus, M.; Schuler, B.; Luban, J.; Grütter, M.G. The Three-Fold Axis of the HIV-1 Capsid Lattice Is the Species-Specific Binding Interface for TRIM5α. J. Virol. 2018, 92, e01541-17. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Skorupka, K.A.; Pak, A.J.; Ganser-Pornillos, B.K.; Pornillos, O.; Voth, G.A. TRIM5α Self-Assembly and Compartmentalization of the HIV-1 Viral Capsid. Nat. Commun. 2020, 11, 1307. [Google Scholar] [CrossRef]
- Anderson, J.L.; Campbell, E.M.; Wu, X.; Vandegraaff, N.; Engelman, A.; Hope, T.J. Proteasome Inhibition Reveals That a Functional Preintegration Complex Intermediate Can Be Generated during Restriction by Diverse TRIM5 Proteins. J. Virol. 2006, 80, 9754–9760. [Google Scholar] [CrossRef]
- O’Connor, C.; Pertel, T.; Gray, S.; Robia, S.L.; Bakowska, J.C.; Luban, J.; Campbell, E.M. P62/Sequestosome-1 Associates with and Sustains the Expression of Retroviral Restriction Factor TRIM5α. J. Virol. 2010, 84, 5997–6006. [Google Scholar] [CrossRef]
- Imam, S.; Talley, S.; Nelson, R.S.; Dharan, A.; O’Connor, C.; Hope, T.J.; Campbell, E.M. TRIM5α Degradation via Autophagy Is Not Required for Retroviral Restriction. J. Virol. 2016, 90, 3400–3410. [Google Scholar] [CrossRef]
- Perez-Caballero, D.; Hatziioannou, T.; Zhang, F.; Cowan, S.; Bieniasz, P.D. Restriction of Human Immunodeficiency Virus Type 1 by TRIM-CypA Occurs with Rapid Kinetics and Independently of Cytoplasmic Bodies, Ubiquitin, and Proteasome Activity. J. Virol. 2005, 79, 15567–15572. [Google Scholar] [CrossRef]
- Saha, B.; Salemi, M.; Williams, G.L.; Oh, S.; Paffett, M.L.; Phinney, B.; Mandell, M.A. Interactomic Analysis Reveals a Homeostatic Role for the HIV Restriction Factor TRIM5α in Mitophagy. Cell Rep. 2022, 39, 110797. [Google Scholar] [CrossRef]
- Oh, S.; Mandell, M.A. Regulation of Mitochondria-Derived Immune Activation by ‘Antiviral’ TRIM Proteins. Viruses 2024, 16, 1161. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Mandell, M.A. The Retroviral Restriction Factor TRIM5/TRIM5α Regulates Mitochondrial Quality Control. Autophagy 2023, 19, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.; Schubert, D.; LaBonte, J.; Munson, L.; Gibson, S.; Scammell, J.; Ferrigno, P.; Sodroski, J. Species-Specific, Postentry Barriers to Primate Immunodeficiency Virus Infection. J. Virol. 1999, 73, 10020–10028. [Google Scholar] [CrossRef]
- Nisole, S.; Lynch, C.; Stoye, J.P.; Yap, M.W. A Trim5-Cyclophilin A Fusion Protein Found in Owl Monkey Kidney Cells Can Restrict HIV-1. Proc. Natl. Acad. Sci. USA 2004, 101, 13324–13328. [Google Scholar] [CrossRef]
- Sayah, D.M.; Sokolskaja, E.; Berthoux, L.; Luban, J. Cyclophilin A Retrotransposition into TRIM5 Explains Owl Monkey Resistance to HIV-1. Nature 2004, 430, 569–573. [Google Scholar] [CrossRef]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The Cytoplasmic Body Component TRIM5α Restricts HIV-1 Infection in Old World Monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Sawyer, S.L.; Emerman, M.; Malik, H.S. Discordant Evolution of the Adjacent Antiretroviral Genes TRIM22 and TRIM5 in Mammals. PLoS Pathog. 2007, 3, e197. [Google Scholar] [CrossRef]
- Hatziioannou, T.; Cowan, S.; Goff, S.P.; Bieniasz, P.D.; Towers, G.J. Restriction of Multiple Divergent Retroviruses by Lv1 and Ref1. EMBO J. 2003, 22, 385–394. [Google Scholar] [CrossRef]
- Hatziioannou, T.; Perez-Caballero, D.; Yang, A.; Cowan, S.; Bieniasz, P.D. Retrovirus Resistance Factors Ref1 and Lv1 Are Species-Specific Variants of TRIM5α. Proc. Natl. Acad. Sci. USA 2004, 101, 10774–10779. [Google Scholar] [CrossRef]
- Schaller, T.; Hué, S.; Towers, G.J. An Active TRIM5 Protein in Rabbits Indicates a Common Antiviral Ancestor for Mammalian TRIM5 Proteins. J. Virol. 2007, 81, 11713–11721. [Google Scholar] [CrossRef]
- Si, Z.; Vandegraaff, N.; O’hUigin, C.; Song, B.; Yuan, W.; Xu, C.; Perron, M.; Li, X.; Marasco, W.A.; Engelman, A.; et al. Evolution of a Cytoplasmic Tripartite Motif (TRIM) Protein in Cows That Restricts Retroviral Infection. Proc. Natl. Acad. Sci. USA 2006, 103, 7454–7459. [Google Scholar] [CrossRef] [PubMed]
- Boso, G.; Shaffer, E.; Liu, Q.; Cavanna, K.; Buckler-White, A.; Kozak, C.A. Evolution of the Rodent Trim5 Cluster Is Marked by Divergent Paralogous Expansions and Independent Acquisitions of TrimCyp Fusions. Sci. Rep. 2019, 9, 11263. [Google Scholar] [CrossRef] [PubMed]
- McEwan, W.A.; Schaller, T.; Ylinen, L.M.; Hosie, M.J.; Towers, G.J.; Willett, B.J. Truncation of TRIM5 in the Feliformia Explains the Absence of Retroviral Restriction in Cells of the Domestic Cat. J. Virol. 2009, 83, 8270–8275. [Google Scholar] [CrossRef]
- Zielonka, J.; Münk, C. Cellular Restriction Factors of Feline Immunodeficiency Virus. Viruses 2011, 3, 1986–2005. [Google Scholar] [CrossRef]
- Ylinen, L.M.J.; Keckesova, Z.; Webb, B.L.J.; Gifford, R.J.M.; Smith, T.P.L.; Towers, G.J. Isolation of an Active Lv1 Gene from Cattle Indicates That Tripartite Motif Protein-Mediated Innate Immunity to Retroviral Infection Is Widespread among Mammals. J. Virol. 2006, 80, 7332–7338. [Google Scholar] [CrossRef]
- Yap, M.W.; Stoye, J.P. Apparent Effect of Rabbit Endogenous Lentivirus Type K Acquisition on Retrovirus Restriction by Lagomorph Trim5αs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120498. [Google Scholar] [CrossRef][Green Version]
- Wei, Y.; Zou, C.; Zeng, S.; Xue, C.; Cao, Y. Characterization of Porcine Tripartite Motif Genes as Host Restriction Factors against PRRSV and PEDV Infection. Virus Res. 2019, 270, 197647. [Google Scholar] [CrossRef]
- Wood, A.; Webb, B.L.J.; Bartosch, B.; Schaller, T.; Takeuchi, Y.; Towers, G.J. Porcine Endogenous Retroviruses PERV A and A/C Recombinant Are Insensitive to a Range of Divergent Mammalian TRIM5α Proteins Including Human TRIM5α. J. Gen. Virol. 2009, 90, 702–709. [Google Scholar] [CrossRef]
- Pacheco, B.; Menéndez-Arias, L.; Sodroski, J. Characterization of Two Distinct Early Post-Entry Blocks to HIV-1 in Common Marmoset Lymphocytes. Sci. Rep. 2016, 6, 37489. [Google Scholar] [CrossRef]
- Song, B.; Javanbakht, H.; Perron, M.; Park, D.H.; Stremlau, M.; Sodroski, J. Retrovirus Restriction by TRIM5α Variants from Old World and New World Primates. J. Virol. 2005, 79, 3930–3937. [Google Scholar] [CrossRef]
- Virgen, C.A.; Kratovac, Z.; Bieniasz, P.D.; Hatziioannou, T. Independent Genesis of Chimeric TRIM5-Cyclophilin Proteins in Two Primate Species. Proc. Natl. Acad. Sci. USA 2008, 105, 3563–3568. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Webb, B.L.J.; Ylinen, L.M.J.; Verschoor, E.; Heeney, J.L.; Towers, G.J. Independent Evolution of an Antiviral TRIMCyp in Rhesus Macaques. Proc. Natl. Acad. Sci. USA 2008, 105, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.; Kozyrev, Y.; Hu, S.-L. TRIMCyp Expression in Old World Primates Macaca Nemestrina and Macaca Fascicularis. Proc. Natl. Acad. Sci. USA 2008, 105, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Kuang, Y.-Q.; Liu, H.-L.; Zheng, Y.-T.; Su, B. A Novel Fusion Gene, TRIM5-Cyclophilin A in the Pig-Tailed Macaque Determines Its Susceptibility to HIV-1 Infection. AIDS 2007, 21 (Suppl. S8), S19–S26. [Google Scholar] [CrossRef]
- Yoo, S.; Myszka, D.G.; Yeh, C.; McMurray, M.; Hill, C.P.; Sundquist, W.I. Molecular Recognition in the HIV-1 Capsid/Cyclophilin A Complex. J. Mol. Biol. 1997, 269, 780–795. [Google Scholar] [CrossRef]
- Price, A.J.; Marzetta, F.; Lammers, M.; Ylinen, L.M.J.; Schaller, T.; Wilson, S.J.; Towers, G.J.; James, L.C. Active Site Remodeling Switches HIV Specificity of Antiretroviral TRIMCyp. Nat. Struct. Mol. Biol. 2009, 16, 1036–1042. [Google Scholar] [CrossRef]
- Wagner, J.M.; Christensen, D.E.; Bhattacharya, A.; Dawidziak, D.M.; Roganowicz, M.D.; Wan, Y.; Pumroy, R.A.; Demeler, B.; Ivanov, D.N.; Ganser-Pornillos, B.K.; et al. General Model for Retroviral Capsid Pattern Recognition by TRIM5 Proteins. J. Virol. 2018, 92, e01563-17. [Google Scholar] [CrossRef]
- Diaz-Griffero, F.; Kar, A.; Perron, M.; Xiang, S.-H.; Javanbakht, H.; Li, X.; Sodroski, J. Modulation of Retroviral Restriction and Proteasome Inhibitor-Resistant Turnover by Changes in the TRIM5α B-Box 2 Domain. J. Virol. 2007, 81, 10362–10378. [Google Scholar] [CrossRef]
- Diaz-Griffero, F.; Vandegraaff, N.; Li, Y.; McGee-Estrada, K.; Stremlau, M.; Welikala, S.; Si, Z.; Engelman, A.; Sodroski, J. Requirements for Capsid-Binding and an Effector Function in TRIMCyp-Mediated Restriction of HIV-1. Virology 2006, 351, 404–419. [Google Scholar] [CrossRef]
- Zuliani-Alvarez, L.; Govasli, M.L.; Rasaiyaah, J.; Monit, C.; Perry, S.O.; Sumner, R.P.; McAlpine-Scott, S.; Dickson, C.; Rifat Faysal, K.M.; Hilditch, L.; et al. Evasion of cGAS and TRIM5 Defines Pandemic HIV. Nat. Microbiol. 2022, 7, 1762–1776. [Google Scholar] [CrossRef]
- Torimiro, J.N.; Javanbakht, H.; Diaz-Griffero, F.; Kim, J.; Carr, J.K.; Carrington, M.; Sawitzke, J.; Burke, D.S.; Wolfe, N.D.; Dean, M.; et al. A Rare Null Allele Potentially Encoding a Dominant-Negative TRIM5α Protein in Baka Pygmies. Virology 2009, 391, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kondo, M.; Chen, J.; Miyoshi, H.; Suzuki, H.; Ohashi, T.; Shida, H. Inhibitory Effect of Human TRIM5α on HIV-1 Production. Microbes Infect. 2010, 12, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.W.; Guo, L.; Xin, F.; Yang, X.; Riley, J.L. Stabilized Human TRIM5α Protects Human T Cells From HIV-1 Infection. Mol. Ther. 2014, 22, 1084–1095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Li, X.; Stremlau, M.; Lee, M.; Sodroski, J. Removal of Arginine 332 Allows Human TRIM5α To Bind Human Immunodeficiency Virus Capsids and To Restrict Infection. J. Virol. 2006, 80, 6738–6744. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.T.; Bouchard, A.; Grütter, M.G.; Berthoux, L. Generation of Human TRIM5α Mutants with High HIV-1 Restriction Activity. Gene Ther. 2010, 17, 859–871. [Google Scholar] [CrossRef]
- Jimenez-Guardeño, J.M.; Apolonia, L.; Betancor, G.; Malim, M.H. Immunoproteasome Activation Enables Human TRIM5α Restriction of HIV-1. Nat. Microbiol. 2019, 4, 933–940. [Google Scholar] [CrossRef]
- Ribeiro, C.M.S.; Sarrami-Forooshani, R.; Setiawan, L.C.; Zijlstra-Willems, E.M.; van Hamme, J.L.; Tigchelaar, W.; van der Wel, N.N.; Kootstra, N.A.; Gringhuis, S.I.; Geijtenbeek, T.B.H. Receptor Usage Dictates HIV-1 Restriction by Human TRIM5α in Dendritic Cell Subsets. Nature 2016, 540, 448–452. [Google Scholar] [CrossRef]
- Towers, G.; Bock, M.; Martin, S.; Takeuchi, Y.; Stoye, J.P.; Danos, O. A Conserved Mechanism of Retrovirus Restriction in Mammals. Proc. Natl. Acad. Sci. USA 2000, 97, 12295–12299. [Google Scholar] [CrossRef]
- Cornejo-Latorre, C.; Cortés-Calva, P.; Álvarez-Castañeda, S.T. The Evolutionary History of the Subgenus Haplomylomys (Cricetidae: Peromyscus). J. Mammal. 2017, 98, 1627–1640. [Google Scholar] [CrossRef]
- Dietrich, I.; Macintyre, A.; McMonagle, E.; Price, A.J.; James, L.C.; McEwan, W.A.; Hosie, M.J.; Willett, B.J. Potent Lentiviral Restriction by a Synthetic Feline TRIM5 Cyclophilin A Fusion. J. Virol. 2010, 84, 8980–8985. [Google Scholar] [CrossRef]
- Dietrich, I.; McEwan, W.A.; Hosie, M.J.; Willett, B.J. Restriction of the Felid Lentiviruses by a Synthetic Feline TRIM5-CypA Fusion. Vet. Immunol. Immunopathol. 2011, 143, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wongsrikeao, P.; Saenz, D.; Rinkoski, T.; Otoi, T.; Poeschla, E. Antiviral Restriction Factor Transgenesis in the Domestic Cat. Nat. Methods 2011, 8, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Keckesova, Z.; Ylinen, L.M.J.; Towers, G.J. Cyclophilin A Renders Human Immunodeficiency Virus Type 1 Sensitive to Old World Monkey but Not Human TRIM5α Antiviral Activity. J. Virol. 2006, 80, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, L.M.J.; Keckesova, Z.; Wilson, S.J.; Ranasinghe, S.; Towers, G.J. Differential Restriction of Human Immunodeficiency Virus Type 2 and Simian Immunodeficiency Virus SIVmac by TRIM5α Alleles. J. Virol. 2005, 79, 11580–11587. [Google Scholar] [CrossRef]
- Goldschmidt, V.; Ciuffi, A.; Ortiz, M.; Brawand, D.; Muñoz, M.; Kaessmann, H.; Telenti, A. Antiretroviral Activity of Ancestral TRIM5α. J. Virol. 2008, 82, 2089–2096. [Google Scholar] [CrossRef]
- Kratovac, Z.; Virgen, C.A.; Bibollet-Ruche, F.; Hahn, B.H.; Bieniasz, P.D.; Hatziioannou, T. Primate Lentivirus Capsid Sensitivity to TRIM5 Proteins. J. Virol. 2008, 82, 6772–6777. [Google Scholar] [CrossRef]
- Saito, A.; Henning, M.S.; Serrao, E.; Dubose, B.N.; Teng, S.; Huang, J.; Li, X.; Saito, N.; Roy, S.P.; Siddiqui, M.A.; et al. Capsid-CPSF6 Interaction Is Dispensable for HIV-1 Replication in Primary Cells but Is Selected during Virus Passage In Vivo. J. Virol. 2016, 90, 6918–6935. [Google Scholar] [CrossRef]
- Berthoux, L.; Sebastian, S.; Sokolskaja, E.; Luban, J. Lv1 Inhibition of Human Immunodeficiency Virus Type 1 Is Counteracted by Factors That Stimulate Synthesis or Nuclear Translocation of Viral cDNA. J. Virol. 2004, 78, 11739–11750. [Google Scholar] [CrossRef]
- 1 Towers, G.J.; Hatziioannou, T.; Cowan, S.; Goff, S.P.; Luban, J.; Bieniasz, P.D. Cyclophilin A Modulates the Sensitivity of HIV-1 to Host Restriction Factors. Nat. Med. 2003, 9, 1138–1143. [Google Scholar] [CrossRef]
- Stremlau, M.; Song, B.; Javanbakht, H.; Perron, M.; Sodroski, J. Cyclophilin A: An Auxiliary but Not Necessary Cofactor for TRIM5α Restriction of HIV-1. Virology 2006, 351, 112–120. [Google Scholar] [CrossRef]
- Pacheco, B.; Finzi, A.; Stremlau, M.; Sodroski, J. Adaptation of HIV-1 to Cells Expressing Rhesus Monkey TRIM5α. Virology 2010, 408, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Emerman, M. Cyclophilin A Interacts with Diverse Lentiviral Capsids. Retrovirology 2006, 3, 70. [Google Scholar] [CrossRef]
- Newman, R.M.; Johnson, W.E. A Brief History of TRIM5alpha. AIDS Rev. 2007, 9, 114–125. [Google Scholar]
- Lin, T.-Y.; Emerman, M. Determinants of Cyclophilin A-Dependent TRIM5α Restriction against HIV-1. Virology 2008, 379, 335–341. [Google Scholar] [CrossRef]
- Bosco, D.A.; Eisenmesser, E.Z.; Clarkson, M.W.; Wolf-Watz, M.; Labeikovsky, W.; Millet, O.; Kern, D. Dissecting the Microscopic Steps of the Cyclophilin A Enzymatic Cycle on the Biological HIV-1 Capsid Substrate by NMR. J. Mol. Biol. 2010, 403, 723–738. [Google Scholar] [CrossRef]
- Lu, M.; Hou, G.; Zhang, H.; Suiter, C.L.; Ahn, J.; Byeon, I.-J.L.; Perilla, J.R.; Langmead, C.J.; Hung, I.; Gor’kov, P.L.; et al. Dynamic Allostery Governs Cyclophilin A–HIV Capsid Interplay. Proc. Natl. Acad. Sci. USA 2015, 112, 14617–14622. [Google Scholar] [CrossRef]
- Mamede, J.I.; Damond, F.; de Bernardo, A.; Matheron, S.; Descamps, D.; Battini, J.-L.; Sitbon, M.; Courgnaud, V. Cyclophilins and Nucleoporins Are Required for Infection Mediated by Capsids from Circulating HIV-2 Primary Isolates. Sci. Rep. 2017, 7, 45214. [Google Scholar] [CrossRef]
- Stevens, A.; Bock, M.; Ellis, S.; LeTissier, P.; Bishop, K.N.; Yap, M.W.; Taylor, W.; Stoye, J.P. Retroviral Capsid Determinants of Fv1 NB and NR Tropism. J. Virol. 2004, 78, 9592–9598. [Google Scholar] [CrossRef]
- Yap, M.W.; Nisole, S.; Lynch, C.; Stoye, J.P. Trim5α Protein Restricts Both HIV-1 and Murine Leukemia Virus. Proc. Natl. Acad. Sci. USA 2004, 101, 10786–10791. [Google Scholar] [CrossRef]
- Olsen, J.C. Gene Transfer Vectors Derived from Equine Infectious Anemia Virus. Gene Ther. 1998, 5, 1481–1487. [Google Scholar] [CrossRef]
- Keckesova, Z.; Ylinen, L.M.J.; Towers, G.J. The Human and African Green Monkey TRIM5α Genes Encode Ref1 and Lv1 Retroviral Restriction Factor Activities. Proc. Natl. Acad. Sci. USA 2004, 101, 10780–10785. [Google Scholar] [CrossRef] [PubMed]
- Braaten, D.; Franke, E.K.; Luban, J. Cyclophilin A Is Required for the Replication of Group M Human Immunodeficiency Virus Type 1 (HIV-1) and Simian Immunodeficiency Virus SIV(CPZ)GAB but Not Group O HIV-1 or Other Primate Immunodeficiency Viruses. J. Virol. 1996, 70, 4220–4227. [Google Scholar] [CrossRef] [PubMed]
- Saenz, D.T.; Teo, W.; Olsen, J.C.; Poeschla, E.M. Restriction of Feline Immunodeficiency Virus by Ref1, Lv1, and Primate TRIM5α Proteins. J. Virol. 2005, 79, 15175–15188. [Google Scholar] [CrossRef] [PubMed]
- Dettwiler, S.; Aringhieri, C.; Cardinale, S.; Keller, W.; Barabino, S.M.L. Distinct Sequence Motifs within the 68-kDa Subunit of Cleavage Factor Im Mediate RNA Binding, Protein-Protein Interactions, and Subcellular Localization. J. Biol. Chem. 2004, 279, 35788–35797. [Google Scholar] [CrossRef]
- Maertens, G.N.; Cook, N.J.; Wang, W.; Hare, S.; Gupta, S.S.; Öztop, I.; Lee, K.; Pye, V.E.; Cosnefroy, O.; Snijders, A.P.; et al. Structural Basis for Nuclear Import of Splicing Factors by Human Transportin 3. Proc. Natl. Acad. Sci. USA 2014, 111, 2728–2733. [Google Scholar] [CrossRef]
- Jang, S.; Cook, N.J.; Pye, V.E.; Bedwell, G.J.; Dudek, A.M.; Singh, P.K.; Cherepanov, P.; Engelman, A.N. Differential Role for Phosphorylation in Alternative Polyadenylation Function versus Nuclear Import of SR-like Protein CPSF6. Nucleic Acids Res. 2019, 47, 4663–4683. [Google Scholar] [CrossRef]
- Lee, K.; Mulky, A.; Yuen, W.; Martin, T.D.; Meyerson, N.R.; Choi, L.; Yu, H.; Sawyer, S.L.; KewalRamani, V.N. HIV-1 Capsid-Targeting Domain of Cleavage and Polyadenylation Specificity Factor 6. J. Virol. 2012, 86, 3851–3860. [Google Scholar] [CrossRef]
- Yang, Q.; Coseno, M.; Gilmartin, G.M.; Doublié, S. Crystal Structure of a Human Cleavage Factor CFIm25/CFIm68/RNA Complex Provides an Insight into Poly(A) Site Recognition and RNA Looping. Structure 2011, 19, 368–377. [Google Scholar] [CrossRef]
- Henning, M.S.; Dubose, B.N.; Burse, M.J.; Aiken, C.; Yamashita, M. In Vivo Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects. PLoS Pathog. 2014, 10, e1003868. [Google Scholar] [CrossRef]
- Lee, K.; Ambrose, Z.; Martin, T.D.; Oztop, I.; Mulky, A.; Julias, J.G.; Vandegraaff, N.; Baumann, J.G.; Wang, R.; Yuen, W.; et al. Flexible Use of Nuclear Import Pathways by HIV-1. Cell Host Microbe 2010, 7, 221–233. [Google Scholar] [CrossRef]
- Price, A.J.; Jacques, D.A.; McEwan, W.A.; Fletcher, A.J.; Essig, S.; Chin, J.W.; Halambage, U.D.; Aiken, C.; James, L.C. Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly. PLoS Pathog. 2014, 10, e1004459. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.; Valle-Casuso, J.C.; White, T.E.; Brandariz-Nuñez, A.; Bosche, W.J.; Reszka, N.; Gorelick, R.; Diaz-Griffero, F. The Ability of TNPO3-Depleted Cells to Inhibit HIV-1 Infection Requires CPSF6. Retrovirology 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, A.; Santoni, F.; Vannier, A.; Guipponi, M.; Antonarakis, S.; Luban, J. TNPO3 Protects HIV-1 Replication from CPSF6-Mediated Capsid Stabilization in the Host Cell Cytoplasm. Retrovirology 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Sowd, G.A.; Serrao, E.; Wang, H.; Wang, W.; Fadel, H.J.; Poeschla, E.M.; Engelman, A.N. A Critical Role for Alternative Polyadenylation Factor CPSF6 in Targeting HIV-1 Integration to Transcriptionally Active Chromatin. Proc. Natl. Acad. Sci. USA 2016, 113, E1054–E1063. [Google Scholar] [CrossRef]
- Xue, G.; Yu, H.J.; Buffone, C.; Huang, S.-W.; Lee, K.; Goh, S.L.; Gres, A.T.; Guney, M.H.; Sarafianos, S.G.; Luban, J.; et al. The HIV-1 Capsid Core Is an Opportunistic Nuclear Import Receptor. Nat. Commun. 2023, 14, 3782. [Google Scholar] [CrossRef]
- Selyutina, A.; Simons, L.M.; Kirby, K.A.; Bulnes-Ramos, A.; Hu, P.; Sarafianos, S.G.; Hultquist, J.F.; Diaz-Griffero, F. TRIM5α Restriction of HIV-1-N74D Viruses in Lymphocytes Is Caused by a Loss of Cyclophilin A Protection. Viruses 2022, 14, 363. [Google Scholar] [CrossRef]
- Bester, S.M.; Adu-Ampratwum, D.; Annamalai, A.S.; Wei, G.; Briganti, L.; Murphy, B.C.; Haney, R.; Fuchs, J.R.; Kvaratskhelia, M. Structural and Mechanistic Bases of Viral Resistance to HIV-1 Capsid Inhibitor Lenacapavir. mBio 2022, 13, e01804-22. [Google Scholar] [CrossRef]
- Ohainle, M.; Kim, K.; Komurlu Keceli, S.; Felton, A.; Campbell, E.; Luban, J.; Emerman, M. TRIM34 Restricts HIV-1 and SIV Capsids in a TRIM5α-Dependent Manner. PLoS Pathog. 2020, 16, e1008507. [Google Scholar] [CrossRef]
- Twentyman, J.; Khalifeh, A.; Felton, A.L.; Emerman, M.; Ohainle, M. Primate TRIM34 Is a Broadly-Acting, TRIM5-Dependent Lentiviral Restriction Factor. Retrovirology 2023, 20, 15. [Google Scholar] [CrossRef]
- Kong, J.; Xu, B.; Wei, W.; Wang, X.; Xie, W.; Yu, X.-F. Characterization of the Amino-Terminal Domain of Mx2/MxB-Dependent Interaction with the HIV-1 Capsid. Protein Cell 2014, 5, 954–957. [Google Scholar] [CrossRef]
- Fribourgh, J.L.; Nguyen, H.C.; Matreyek, K.A.; Alvarez, F.J.D.; Summers, B.J.; Dewdney, T.G.; Aiken, C.; Zhang, P.; Engelman, A.; Xiong, Y. Structural Insight into HIV-1 Restriction by MxB. Cell Host Microbe 2014, 16, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Woodward, C.L.; Chow, S.A. The Nuclear Pore Complex. Nucleus 2010, 1, 18–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meier, K.; Jaguva Vasudevan, A.A.; Zhang, Z.; Bähr, A.; Kochs, G.; Häussinger, D.; Münk, C. Equine MX2 Is a Restriction Factor of Equine Infectious Anemia Virus (EIAV). Virology 2018, 523, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, L.; Zhong, C.; Yu, T.; Ju, Z.; Wang, M.; Xiong, H.; Zeng, Y.; Wang, J.; Hu, H.; et al. MxB Impedes the NUP358-Mediated HIV-1 Pre-Integration Complex Nuclear Import and Viral Replication Cooperatively with CPSF6. Retrovirology 2020, 17, 16. [Google Scholar] [CrossRef]
- Kane, M.; Rebensburg, S.V.; Takata, M.A.; Zang, T.M.; Yamashita, M.; Kvaratskhelia, M.; Bieniasz, P.D. Nuclear Pore Heterogeneity Influences HIV-1 Infection and the Antiviral Activity of MX2. eLife 2018, 7, e35738. [Google Scholar] [CrossRef]
- Goujon, C.; Moncorgé, O.; Bauby, H.; Doyle, T.; Ward, C.C.; Schaller, T.; Hué, S.; Barclay, W.S.; Schulz, R.; Malim, M.H. Human MX2 Is an Interferon-Induced Post-Entry Inhibitor of HIV-1 Infection. Nature 2013, 502, 559–562. [Google Scholar] [CrossRef]
- Smaga, S.S.; Xu, C.; Summers, B.J.; Digianantonio, K.M.; Perilla, J.R.; Xiong, Y. MxB Restricts HIV-1 by Targeting the Tri-Hexamer Interface of the Viral Capsid. Structure 2019, 27, 1234–1245.e5. [Google Scholar] [CrossRef]
- Dicks, M.D.J.; Betancor, G.; Jimenez-Guardeño, J.M.; Pessel-Vivares, L.; Apolonia, L.; Goujon, C.; Malim, M.H. Multiple Components of the Nuclear Pore Complex Interact with the Amino-Terminus of MX2 to Facilitate HIV-1 Restriction. PLoS Pathog. 2018, 14, e1007408. [Google Scholar] [CrossRef]
- Moschonas, G.D.; Delhaye, L.; Cooreman, R.; Hüsers, F.; Bhat, A.; Stylianidou, Z.; Bousser, E.D.; Pryck, L.D.; Grzesik, H.; Sutter, D.D.; et al. MX2 Forms Nucleoporin-Comprising Cytoplasmic Biomolecular Condensates That Lure Viral Capsids. Cell Host Microbe 2024, 32, 1705–1724.e14. [Google Scholar] [CrossRef]
- Layish, B.; Goli, R.; Flick, H.; Huang, S.-W.; Zhang, R.Z.; Kvaratskhelia, M.; Kane, M. Virus Specificity and Nucleoporin Requirements for MX2 Activity Are Affected by GTPase Function and Capsid-CypA Interactions. PLoS Pathog. 2024, 20, e1011830. [Google Scholar] [CrossRef]
- Lamorte, L.; Titolo, S.; Lemke, C.T.; Goudreau, N.; Mercier, J.-F.; Wardrop, E.; Shah, V.B.; von Schwedler, U.K.; Langelier, C.; Banik, S.S.R.; et al. Discovery of Novel Small-Molecule HIV-1 Replication Inhibitors That Stabilize Capsid Complexes. Antimicrob. Agents Chemother. 2013, 57, 4622–4631. [Google Scholar] [CrossRef] [PubMed]
- Kortagere, S.; Madani, N.; Mankowski, M.K.; Schön, A.; Zentner, I.; Swaminathan, G.; Princiotto, A.; Anthony, K.; Oza, A.; Sierra, L.-J.; et al. Inhibiting Early-Stage Events in HIV-1 Replication by Small-Molecule Targeting of the HIV-1 Capsid. J. Virol. 2012, 86, 8472–8481. [Google Scholar] [CrossRef] [PubMed]
- Thenin-Houssier, S.; Valente, S.T. HIV-1 Capsid Inhibitors as Antiretroviral Agents. Curr. HIV Res. 2016, 14, 270–282. [Google Scholar] [CrossRef]
- Bocanegra, R.; Rodríguez-Huete, A.; Fuertes, M.Á.; Del Álamo, M.; Mateu, M.G. Molecular Recognition in the Human Immunodeficiency Virus Capsid and Antiviral Design. Virus Res. 2012, 169, 388–410. [Google Scholar] [CrossRef]
- Ternois, F.; Sticht, J.; Duquerroy, S.; Kräusslich, H.-G.; Rey, F.A. The HIV-1 Capsid Protein C-Terminal Domain in Complex with a Virus Assembly Inhibitor. Nat. Struct. Mol. Biol. 2005, 12, 678–682. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Xu, S.; Huang, T.; Song, S.; Cherukupalli, S.; Zhan, P.; Liu, X. An Insight on Medicinal Aspects of Novel HIV-1 Capsid Protein Inhibitors. Eur. J. Med. Chem. 2021, 217, 113380. [Google Scholar] [CrossRef]
- Paik, J. Lenacapavir: First Approval. Drugs 2022, 82, 1499–1504. [Google Scholar] [CrossRef]
- Subramanian, R.; Tang, J.; Zheng, J.; Lu, B.; Wang, K.; Yant, S.R.; Stepan, G.J.; Mulato, A.; Yu, H.; Schroeder, S.; et al. Lenacapavir: A Novel, Potent, and Selective First-in-Class Inhibitor of HIV-1 Capsid Function Exhibits Optimal Pharmacokinetic Properties for a Long-Acting Injectable Antiretroviral Agent. Mol. Pharm. 2023, 20, 6213–6225. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical Targeting of HIV Capsid Protein with a Long-Acting Small Molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef]
- Kelly, B.N.; Kyere, S.; Kinde, I.; Tang, C.; Howard, B.R.; Robinson, H.; Sundquist, W.I.; Summers, M.F.; Hill, C.P. Structure of the Antiviral Assembly Inhibitor CAP-1 Bound to the HIV-1 CA Protein. J. Mol. Biol. 2007, 373, 355–366. [Google Scholar] [CrossRef]
- Zhou, J.; Price, A.J.; Halambage, U.D.; James, L.C.; Aiken, C. HIV-1 Resistance to the Capsid-Targeting Inhibitor PF74 Results in Altered Dependence on Host Factors Required for Virus Nuclear Entry. J. Virol. 2015, 89, 9068–9079. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gallazzi, F.; Hill, K.J.; Burke, D.H.; Lange, M.J.; Quinn, T.P.; Neogi, U.; Sönnerborg, A. GS-CA Compounds: First-In-Class HIV-1 Capsid Inhibitors Covering Multiple Grounds. Front. Microbiol. 2019, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, H.; Mallon, K.; Valera, L.; Parcella, K.; Cockett, M.I.; Kadow, J.F.; Gillis, E.P.; Krystal, M.; Fridell, R.A. Antiviral Properties of HIV-1 Capsid Inhibitor GSK878. Antimicrob. Agents Chemother. 2023, 67, e01694-22. [Google Scholar] [CrossRef]
- Wei, G.; Iqbal, N.; Courouble, V.V.; Francis, A.C.; Singh, P.K.; Hudait, A.; Annamalai, A.S.; Bester, S.; Huang, S.-W.; Shkriabai, N.; et al. Prion-like Low Complexity Regions Enable Avid Virus-Host Interactions during HIV-1 Infection. Nat. Commun. 2022, 13, 5879. [Google Scholar] [CrossRef]
- Saito, A.; Ferhadian, D.; Sowd, G.A.; Serrao, E.; Shi, J.; Halambage, U.D.; Teng, S.; Soto, J.; Siddiqui, M.A.; Engelman, A.N.; et al. Roles of Capsid-Interacting Host Factors in Multimodal Inhibition of HIV-1 by PF74. J. Virol. 2016, 90, 5808. [Google Scholar] [CrossRef]
- Cohen, J. The Long Shot. Science 2024, 386, 1208–1209. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, J.; Halambage, U.D.; Shah, V.B.; Burse, M.J.; Wu, H.; Blair, W.S.; Butler, S.L.; Aiken, C. Compensatory Substitutions in the HIV-1 Capsid Reduce the Fitness Cost Associated with Resistance to a Capsid-Targeting Small-Molecule Inhibitor. J. Virol. 2014, 89, 208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).