Abstract
Human papillomavirus (HPV) integration is recognized as a hallmark event in cervical carcinogenesis. However, it does not represent a routine phase of the viral life cycle but rather a stochastic occurrence, often constituting a dead-end pathway for the virus. High-risk human papillomavirus (hr-HPV) exhibits a greater propensity for integration. The progression from initial infection to genomic integration constitutes a dynamic multi-step oncogenic process in the development of cervical cancer (CC). This process involves viral entry, immune evasion, persistent infection, and ultimately integration. This article innovatively provides a comprehensive overview of this multi-stage mechanism: HPV, via the L1/L2 proteins, mediates internalization and establishes infection. Subsequently, under the influence of factors such as the host’s genetic background, vaginal microbiota imbalance, and immune evasion, the host’s DNA damage response (DDR) pathways are activated. Viral DNA integrates into host genome vulnerable sites (e.g., 3q28 and 8q24) through microhomology-mediated end joining (MMEJ) or other alternative pathways. Following integration, the expression of viral oncogenes persists, triggering host genomic rearrangements, aberrant epigenetic modifications, and immune microenvironment remodeling, all of which collectively drive cervical cancer progression. The study further reveals the clinical potential of HPV integration as a highly specific molecular biomarker, offering new perspectives for precision screening and targeted therapy. This dynamic model deepens our understanding of the HPV carcinogenic mechanism and provides a theoretical basis for intervention strategies.
1. Background
Globally, cervical cancer (CC) ranks as the fourth most common cancer in women, after breast, colorectal, and lung cancers. According to 2022 global cancer statistics, approximately 661,021 new cases and 348,189 deaths occur annually [1]. The incidence and mortality rates vary significantly due to disparities in socioeconomic levels and healthcare services, ranging from 2.2 (1.9–2.4) in Iraq to 84.6 (74.8–94.3) in Eswatini for incidence and from 1.0 (0.8–1.2) in Switzerland to 55.7 (47.7–63.7) in Eswatini for mortality [2]. The World Health Organization (WHO)’s global strategy to eliminate CC aims to reduce its incidence to less than 4 cases per 100,000 women annually within this century [3]. While advancements in prevention and screening technologies have substantially decreased CC rates in high-income countries, low- and middle-income countries (LMICs) still face incidence and mortality rates far exceeding the WHO’s elimination threshold [2]. In most nations, CC is still a considerable public health concern, particularly in sub-Saharan Africa, with advanced treatment posing significant challenges, making it a pressing global public health issue [4].
Nearly all CC cases are linked to persistent infections with high-risk human papillomavirus, such as HPV 16/18 [5]. HPV testing, the primary technique for screening CC, exhibits high sensitivity (>95%) for early infection detection but lower specificity (~80%) [6,7]. Traditional HPV testing relies on viral DNA detection but does not accurately distinguish which HPV infections will progress to CC and which are transient. Furthermore, a limited number of HPV-positive individuals will go on to develop CC—for instance, only 15–20% of infections with HPV16/18 will develop into high-grade squamous intraepithelial lesions (HSILs), but these types pose a much higher risk compared to other oncogenic HPV types [8,9]. Thus, a key challenge in CC prevention lies in effectively triaging HPV-positive individuals to identify high-risk populations for early identification and prevention of CC.
To address the issue, researchers have proposed HPV integration testing as a potential solution [10,11,12]. HPV integration involves inserting the HPV genome into the host’s genetic material, a phenomenon often tied to cervical carcinogenesis. The integration rate of HPV gradually increases from healthy tissue to precancerous abnormalities and eventually to invasive carcinoma [10]. Scientific studies have proven that HPV genome integration is not only related to changes in viral replication capacity but is also closely linked to cellular transformation and tumorigenesis [13]. Consequently, HPV integration testing is expected to serve as a more precise stratification tool, providing critical guidance for further diagnosis and management of high-risk human papillomavirus (hR-HPV)-positive individuals. This review will explore the dynamic process from HPV infection to integration, discuss the role of HPV integration in CC development, analyze its potential as a screening tool, and assess its clinical applicability, aiming to offer a foundational theory for optimizing CC screening strategies.
2. Prerequisites for HPV Integration
2.1. Viral Factors
HPV consists of a small, circular DNA structure that is double-stranded and non-enveloped with a diameter of approximately 50–60 nm and a genome length of around 8000 base pairs. It exhibits an icosahedral-shaped capsid symmetry. The genome of the virus is split into three sections: the early transcription region, the late transcription region, and the long control region (LCR) or upstream regulatory region (URR). The early region encodes proteins E1 to E8, playing key roles during viral replication, control of transcription, and cancerous transformation. The late region is responsible for encoding the L1 and L2 capsid proteins, involved in viral particle formation and structure. The LCR is the replication origin and contains the transcriptional promoter and enhancers [14].
To date, over 400 HPV genotypes (more than 200 affecting humans) have been identified and classified into five genera: Alpha, Beta, Gamma, Mu, and Nu [15]. Low-risk HPV types primarily cause benign lesions and rarely integrate into the host genome. In contrast, high-risk HPV infection is a major cause of HPV integration into host cells, increasing the risk of carcinogenesis through persistent immune responses and chronic inflammation. According to the WHO, HPVs considered high-risk are types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 [16]. HPV integration is detected in over 80% of cervical cancers [17], with HPV16 (clade α9) being the most prevalent oncogenic type, followed by HPV18 and HPV45 (clade α7) [18,19]. Compared to the α9 lineage, the α7 lineage shows a significantly higher integration frequency [20,21], as verified in a comprehensive genomic study of cervical cancer, which found integration across all HPV18 samples and 76% of HPV16 samples [17]. Furthermore, HPV16 is closely associated with squamous cell carcinoma, whereas HPV18 correlates with adenosquamous carcinoma [22], suggesting that different HPV subtypes exhibit different tropisms [17] and molecular differences [23].
Persistent high-copy HPV DNA increases the probability of replication errors, suggesting that viral load increase may enhance the chances of HPV integration [24,25]. In addition, studies show that, during their lifetime, nearly 80% of women will contract HPV, but less than 10% of infected women experience persistent infections [26].
2.2. Genetic Susceptibility to CC
Several genetic loci linked to CC risk have been identified through genome-wide association studies (GWASs), particularly inside the major histocompatibility complex (MHC) area. Three independent loci in the MHC were observed to be significantly correlated with CC risk: near MICA, between HLA-DQA1 and HLA-DRB1, and at HLA-DPB2 [27,28]. Furthermore, new susceptibility loci related to HPV infection have been identified through GWASs, including ARRDC3 at 5q14, EXOC1 at 4q12, and CLPTM1L at 17q12 [29,30,31]. These genetic variants may influence CC development by affecting host immune responses, DNA repair mechanisms, and cell cycle regulation, which in turn could impact HPV integration frequency and location [32]. Although candidate gene studies have historically reported numerous associations, many findings suffer from limited reproducibility or inconsistent effect sizes across populations. New understandings of CC pathogenesis have emerged from GWAS and post-GWAS studies, highlighting the complex interplay between genetic factors and HPV infection in disease development [33].
2.3. Vaginal Microbiota and Local Immune Environment
The vaginal microbiota in women is diverse, with symbiotic and antagonistic interactions influenced by various internal and external factors, forming a complex vaginal ecosystem. Imbalance in the vaginal microbiota, often termed dysbiosis, plays a critical role in promoting HPV infection and integration [34,35]. Lactobacillus species predominantly make up the normal vaginal microbiota, with a small number of other microbes coexisting; vaginal pH ranges from 3.8 to 4.5. When the vaginal ecosystem is disrupted, changes characterized by abnormal vaginal microbiota and pH occur, primarily due to a reduction in Lactobacillus, leading to pH increase and promoting the proliferation of pathogenic bacteria like Gardnerella vaginalis, associated with bacterial vaginosis [36,37]. This shift may trigger chronic inflammation, compromise the cervical epithelial barrier, and facilitate HPV infection [38,39,40]. Additionally, microbial metabolites like butyrate can regulate host gene expression by suppressing histone deacetylase (HDAC) activity, affecting the HPV life cycle [41].
2.4. Other Factors
Several factors are tightly associated with HPV infection and CC, including the following: First, nutritional status, as malnutrition can reduce immune function, increasing infection risk [42]. Second, individuals with weakened immune function are more susceptible to HPV, including those with HIV infection, organ transplant recipients, autoimmune disease patients, long-term immunosuppressive drug users, and other immunocompromised patients [43,44].
Additionally, lifestyle habits are significant risk factors, with long-term smoking being an important adjunct factor in CC development. Benzopyrene in tobacco induces APOBEC mutations, increasing the risk of HPV genomic fragmentation [45]. Nicotine suppresses the TLR9 pathway, weakening antiviral immunity [46]. Other factors include estrogen levels, long-term oral contraceptive use, early sexual activity, multiple sexual partners, and multiple pregnancies and births [47].
3. Molecular Mechanisms of HPV Entry, Persistent Infection, and Genomic Integration
Following successful nuclear entry, the HPV genome persists as a circular episome [48]. During this stage, viral DNA replicates using the host’s replication machinery while expressing only a limited set of genes, thereby minimizing detectable interference with host cell functions and evading immune surveillance. This phase generally lasts 6–24 months, and most infected individuals clear the virus through immune-mediated mechanisms [9].
A critical transition in HPV infection is the integration of viral DNA into the host genome [49]. This event is particularly common in high-risk HPV types and is a key step toward malignant transformation. Paradoxically, integration also represents a dead end for the viral life cycle. From a clinical perspective, integration marks the transition from infection to carcinogenesis. Cytological abnormalities such as low-grade squamous intraepithelial lesions (LSILs) or HSILs may become detectable at this stage. Integration often occurs during persistent infection (exceeding two years), yet the progression from integration to invasive cancer typically requires additional years or even decades [50], providing a crucial window for clinical intervention. HPV completes the process from infection to genomic integration through a series of precisely regulated molecular mechanisms. There are complex interactions between the virus and host factors in this process.
3.1. From HPV Viral Entry to Persistent Infection
3.1.1. Differentiation-Dependent Strategy of the HPV Life Cycle
HPV employs a highly evolved differentiation-dependent replication strategy, wherein its life cycle is intimately associated with the differentiation process of the host’s epithelial cells. Following microtrauma-mediated infection of undifferentiated basal keratinocytes, early viral genes maintain low-copy viral genome replication. As host cells differentiate toward the upper layers, HPV induces the ATM-mediated DNA damage response (DDR) and related pathways to promote viral genome amplification [51,52]. Mature viral particles are ultimately released in large quantities from terminally differentiated keratinocytes [53]. This strategy—localizing persistent infection to basal cells while restricting productive infection to differentiated layers—not only ensures long-term viral infection but also significantly enhances immune evasion.
3.1.2. HPV Infection
At the onset of infection, HPV binds to heparan sulfate proteoglycans (HSPGs) on the surface of host cells via the L1 major capsid protein (Figure 1A) [54]. This initial binding typically occurs on basal keratinocytes. HPV then interacts with laminin-332 (formerly laminin-5) secreted by migrating keratinocytes, serving as a “transient receptor,” facilitating interaction with secondary receptors (such as α6β4 integrins) (Figure 1C), or transferring to adjacent cells expressing secondary receptors (Figure 1D) [55,56]. This process not only enhances virus attachment but also results in structural changes in the capsid, exposing crucial domains of the L2 secondary capsid protein. These changes further facilitate furin protease-mediated cleavage of the L2 protein at the cell membrane, exposing the conserved, cross-neutralizing RG-1 epitope—a critical step for subsequent viral internalization (Figure 1B) [57,58].
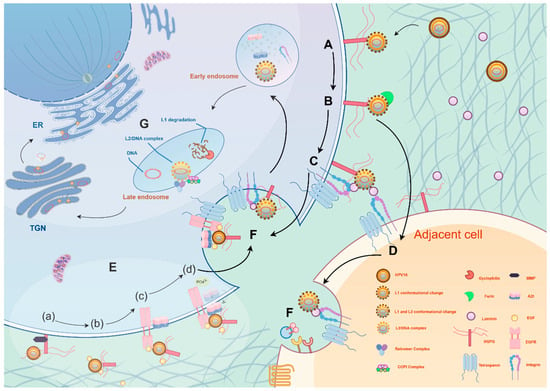
Figure 1.
HPV invades host cells through a multi-step, precisely regulated mechanism. (A) First, the virus L1 protein binds to heparan sulfate proteoglycan (HSPG) on the cell membrane, triggering a conformational change. (B) The L2 protein is cleaved by furin protease, exposing the RG-1 epitope, after which the virus enters the cell through two pathways: (C) One pathway involves binding to laminin-5 after adsorption, followed by interaction with integrin α6β4, (D) or the virus transfers to adjacent cells. (E) The second pathway involves the formation of a virus–HSPG–EGF complex, activating the EGFR/Src pathway. (a) The HSPG bound to the virus is cleaved and released by Matrix Metalloproteinase (MMP). (b) The virus, HSPG, and EGF form a high-molecular weight complex. (c) This complex binds to EGFR on the plasma membrane, inducing Src kinase activation. (d) Active Src phosphorylates Annexin A2 (AnxA2), causing AnxA2’s extracellular translocation and binding with the virus. (F) The virus is internalized after binding to a four-transmembrane protein. (G) The virus is sequentially transported from early endosomes to late endosomes, then to the Trans-Golgi Network (TGN). During this process, L1 is degraded, and L2 cooperates with the Retromer/COPI complex for retrograde transport. Ultimately, the viral genome enters the cell nucleus via mitosis, completing the infection. The figure was made by Figdraw (www.figdraw.com).
The HPV internalization pathway relies on an atypical endocytosis mechanism dependent on actin remodeling and tyrosine kinase signaling (Figure 1E,F) [59,60]. During this process, the C-terminal sequence of L2 can penetrate the endosomal membrane and directly interact with the host cell’s COPI complex, relocating the virus from the endosome to the Golgi apparatus or endoplasmic reticulum [61]. During host cell division, the nuclear membrane temporarily disassembles, and the viral genome, along with L2 protein complexes, enters the cell nucleus (Figure 1G) [62]. Within the nucleus, the HPV genome frequently localizes near nuclear domain 10 (ND10) bodies [63]. These bodies play critical roles in intrinsic immune defense, yet paradoxically provide a favorable niche for viral replication and transcription programs [64]. HPV often reorganizes ND10 components—for instance, the viral L2 protein displaces Sp100 and recruits Daxx, thereby establishing a localized microenvironment conducive to viral transcription and replication initiation [65].
3.1.3. Transcriptional and Replicational Regulation of HPV
The initial phase of HPV infection is characterized via the expression of E1 and E2 proteins, which cooperatively initiate viral DNA replication [66]. E2, the master transcriptional regulator, modulates early promoter activity via conserved E2 binding sites (E2BSs) [67] and recruits the E1 helicase to the replication origin, forming a sequence-specific complex that triggers a cascade of replication events [68,69]. E1, an ATP-dependent helicase, unwinds viral DNA [69] and recruits host replication factors while simultaneously inducing cellular DNA damage responses to promote viral replication [70,71]. Notably, the E2-bromodomain-containing protein 4 (E2-BRD4) complex is known to tether the viral genome to host chromatin for persistent replication in some HPV types; this mechanism exhibits type-specific variation [72]. For instance, HPV16 primarily relies on TopBP1 for chromatin attachment during mitosis, as demonstrated by its essential role in plasmid segregation and retention [73]. Additionally, the viral E8^E2 protein engages with cellular transcriptional repressor factors, suppressing transcription and replication to maintain low-level infection [74,75]. In the higher differentiated layers, viral DNA amplifies to high copy numbers, and L1/L2 capsid proteins autonomously form complete virions. The natural shedding of surface keratinocytes completes the viral dissemination cycle.
3.1.4. Immune Evasion Strategy
The life cycle of HPV, which depends on differentiation, is central to its immune evasion strategy: viral replication remains confined to epithelial cells, avoiding inflammation triggered by cell lysis; high viral loads are restricted to terminally differentiated layers, which are immunologically privileged and destined for desquamation. HPV evades innate immune surveillance by trafficking within endosomal vesicles [76], while the L2 protein counteracts the antiviral effects of PML nuclear bodies [77,78]. Furthermore, viral proteins disrupt the body’s innate immune signaling pathways and postpone adaptive immune reactions, thereby sustaining persistent infection [79]. Notably, the E5 oncoprotein of hr-HPV mediates immune evasion through multiple mechanisms to sustain persistent infection [80]: it primarily sequesters MHC-I/II and CD1d molecules within the endoplasmic reticulum and Golgi apparatus, disrupting antigen presentation and impairing T and NK cell responses. Concurrently, E5 activates the EGF-R signaling axis, upregulating COX-2/PGE2 and NF-κB pathways to foster an immunosuppressive microenvironment [81]. This process is accompanied by inactivation of the TGF-β/SMAD tumor-suppressive pathway, further suppressing NK cell cytotoxicity and interferon-mediated antiviral activity [80].
3.2. HPV Integration Process
HPV integration is recognized as a hallmark event in cervical carcinogenesis. Integration results in the loss of the virus’s normal replication ability, figuratively described as a “dead end” for replication [82]. Despite the loss of normal replication, this event induces sustained upregulation of E6/E7 oncogenes by disrupting the E2 gene, thereby indirectly potentiating the oncogenic potential.
3.2.1. Disruption of Viral Genome and Activation of Viral Oncogenes
Viral genome integration is a pivotal event in HPV-associated malignant transformation, often accompanied by disruption of E2/E1 genes [83,84]. The hinge region of E2 is a frequent breakpoint [83]. Loss of E2 function derepresses the E6/E7 oncogenes, as E2 normally suppresses their transcription by regulating the early promoter and polyadenylation signal (PAS). Concurrently, truncation of the E1 C-terminal domain impairs its synergy with E2, further exacerbating E6/E7 overexpression [85,86]. Notably, some studies indicate that the E5 segment is often deleted in integrated HPV clinical samples. This may stem from its genomic location at replication fork convergence points [87]. Following E5 loss, a cascade of events—from single-cell signaling dysregulation to population-level integration—is exponentially accelerated. Specifically, E5 deletion activates the TGF-β/IFN-κ axis, leading to increased secretion of IFN-κ. As a secretory cytokine, IFN-κ diffuses into the microenvironment, promoting clearance of cells harboring episomal viral genomes. Although integrated cells maintain high IFN-κ expression, the viral genome—sheltered within host chromosomes—evades immune eradication, thereby facilitating survival and clonal expansion of integrated clones. This mechanism suggests that E5 loss is not stochastic but rather a necessity under immune micro-pressure, sacrificing local immunosuppressive function (via E5 deletion) to secure long-term advantages conferred by genomic integration. These findings provide a novel paradigm for understanding the evolutionary trajectory of HPV-associated cancers.
These events culminate in the overexpression of the E6 and E7 oncogenes. The E6 and E7 proteins drive tumorigenesis through multiple pathways: E6 mediates p53 ubiquitination and degradation via E6AP, inhibiting apoptosis and DNA repair while activating telomerase and disrupting cell polarity [88,89,90]; E7 binds Rb to release E2F, inducing cell cycle dysregulation and generating replicative stress, double-strand breaks (DSBs), and other severe genomic lesions [91,92,93]. Moreover, E6/E7 promotes chronic inflammation via the COX-2/PGE2 axis [94], and the E5 protein amplifies inflammatory responses through the EGFR/NF-κB pathway [81]. The resulting reactive oxygen and nitrogen species (ROS/RNS) induce DNA damage, facilitating viral integration into the host genome [95].
3.2.2. HPV Integration Mechanisms
Integrating the HPV genome into host DNA is a complex, multi-mechanism process centered on the repair-mediated fusion of viral DNA with breakpoints in the host genome. The process initiates with the formation of host genome DSBs, which may be induced by viral proteins [96] or cellular stress responses [97]. Subsequent steps are driven by the DDR, primarily involving four molecular mechanisms: microhomology-mediated end joining (MMEJ), non-homologous end joining (NHEJ), fork stalling and template switching (FoSTeS), and microhomology-mediated break-induced replication (MMBIR).
Early studies proposed NHEJ as the dominant pathway for HPV integration [98], given its reliance on the DNA-protein kinase catalytic subunit (DNA-PKcs)/Ku70 complex for erroneous ligation of DNA ends, which can lead to random viral insertion into critical host genes or regulatory regions, triggering genomic instability and oncogenic risk [99] (Figure 2A). However, emerging evidence reveals widespread enrichment of microhomologous sequences at HPV integration sites, suggesting that MMEJ may instead be the predominant mechanism [100].
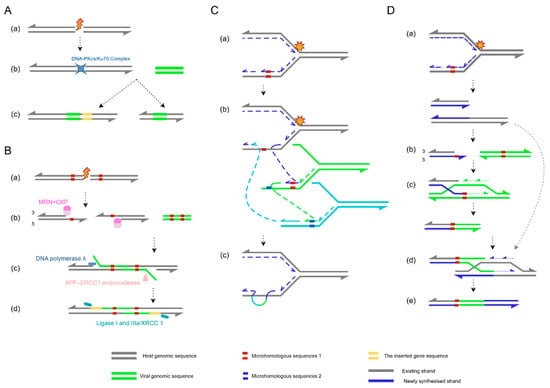
Figure 2.
Schematic overview of HPV integration mechanisms. (A) Non-homologous end joining (NHEJ): (a) A double-strand break (DSB) occurs in the host genome; (b) the DNA-protein kinase catalytic subunit (DNA-PKcs)/Ku70 complex directly binds broken ends without requiring homologous sequences; (c) ligation often introduces insertions or deletions at the junction site. (B) Microhomology-mediated end joining (MMEJ): (a) Host genomic damage induces a DSB; (b) the Mre11-Rad50-Nbs1(MRN) complex and retinoblastoma binding protein 8(CtIP) cooperatively resect 5′→3′ ends, exposing 3′ microhomology regions; (c) microhomologous sequences in HPV DNA pair with host microhomology regions; the excision repair cross-complementing rodent repair deficiency, complementation group 4–excision repair cross-complementing rodent repair deficiency, complementation group 1 (XPF-ERCC1) endonuclease trims non-complementary overhangs, followed by gap filling by DNA polymerase λ; (d) DNA ligase I and IIIa/ X-ray complementing defective repair in Chinese hamster cells 1 (XRCC1) ligate the DNA backbone, completing viral integration. This process frequently causes host sequence deletions or rearrangements. (C) Fork stalling and template switching (FoSTeS): (a) During host DNA replication, the fork stalls at damaged sites; (b) the stalled fork switches templates to nearby homologous sequences (e.g., HPV DNA, green); (c) HPV DNA is copied into the host genome via template switching, generating complex rearrangements or repeats. (D) Microhomology-mediated break-induced replication (MMBIR): (a) Host DNA breakage or fork collapse produces a single-ended DSB; (b) 5′→3′ resection exposes microhomology regions (red) and creates a 3′ single-stranded overhang; (c) the 3′ overhang invades HPV microhomology regions, priming DNA synthesis; (d) after that, the strand disengages and reanneals to the original template; (e) host–HPV chimeric molecules form, driving genomic rearrangements or HPV integration. The figure was made by Figdraw (www.figdraw.com).
Initially regarded as a backup repair pathway—sometimes termed alternative NHEJ (alt-NHEJ)—MMEJ can be upregulated upon NHEJ impairment. Yet growing evidence indicates that it may serve as a preferred repair mechanism, particularly in contexts of homologous recombination (HR) deficiency [101]. Recent HPV integration site capture studies confirm that microhomology-mediated repair is the primary mechanism for viral integration [102]. This pathway begins with 5′-to-3′ DNA end resection by the Mre11-Rad50-Nbs1 (MRN) complex in coordination with retinoblastoma binding protein 8 (CtIP), exposing microhomologous sequences at 3′ overhangs [103,104]. After annealing of these microhomology regions, non-complementary flaps are trimmed by the excision repair cross-complementing rodent repair deficiency, complementation group 4–excision repair cross-complementing rodent repair deficiency, complementation group 1(XPF-ERCC1) endonuclease complex [105], gaps are filled by DNA polymerase λ [106], and ligation is completed by DNA ligase I or III [107] (Figure 2B).
Beyond classical repair pathways, HPV integration can also occur via replication-dependent mechanisms like FoSTeS and MMBIR, which generate complex genomic rearrangements [108]. During DNA replication, fork stalling caused by damage or topological stress may trigger template switching, often resulting in interchromosomal rearrangements (Figure 2C). Conversely, replication fork collapse can activate MMBIR—a variant of break-induced replication (BIR) that relies on microhomology rather than long homologous sequences. Unlike canonical Rad51-dependent BIR, MMBIR employs Rad52 to catalyze annealing of short microhomologies [109,110]. Notably, stress conditions such as hypoxia reduce Rad51 levels, promoting MMBIR as an alternative repair mechanism [111]. MMBIR involves 5′ strand resection at DSBs to generate 3′ single-stranded overhangs, which invade microhomology regions of alternative DNA templates to form D-loops and restart synthesis (Figure 2D).
Recent studies propose that microhomology-mediated repair (MMR) generates looping viral–host DNA intermediates, which are amplified through aberrant activation of viral replication origins, ultimately producing head-to-tail concatemers of viral and host DNA [112]. The intricate rearrangement patterns formed by these collaborative mechanisms are a hallmark of HPV-associated cancer genomes.
4. Distribution Characteristics of HPV Integration Sites
Several studies have investigated the distribution of HPV integration sites in cell lines and clinical samples. Earlier research suggested that HPV integration sites were randomly distributed on chromosomes [113,114]. However, deeper studies proposed different views, indicating that HPV integration events are not entirely random but show some bias. The concept of “productive integration” has been introduced to explain this phenomenon. Productive integration refers to the virus retaining key oncogenes (E6/E7) and continuously expressing them, directly promoting tumorigenesis. Some studies believe that integration sites are randomly distributed initially, with most being silenced, while productive integration is non-random and closely associated with immune evasion and tumor progression [115].
Different HPV types exhibit different integration tendencies on chromosomal bands, such as HPV16 being prone to forming integration clusters on chromosomes 3 (3q28) and 13 (13q22.1), while HPV18 is more likely to integrate on chromosome 8 (8q24.21) [116]. These regions are rich in cancer-associated genes such as MYC, FHIT, KLF5 and MACROD2, considered HPV integration “hotspots” [117,118,119]. Different histological types of CC exhibit heterogeneity in HPV integration sites, such as adenocarcinoma commonly showing integration at 17q12, while squamous carcinoma exhibits more integration events at 21p11.2 [120]. Additionally, new integration hotspots, such as 14q32.2, 10p15, 2q37, 2q22.3, 3p14.2, 8q24.22, 14q24.1, 17p11.1, 17q23.1, and 17q23.2, have been discovered in recent genome-wide studies, providing new clues for understanding the molecular mechanisms of CC [85,121].
Regarding the distribution of genomic elements, HPV often integrates at common fragile sites (CFSs), transcriptionally active regions, CpG regions, and enhancer regions [49,113,117,122,123]. CFSs are regions on chromosomes susceptible to replication stress-induced breaks. Studies have shown that integration sites are often enriched in FANCD2-associated fragile sites and enhancer-rich regions, with FANCD2 being a protein involved in DNA repair. This enrichment may contribute to genomic instability, further promoting cancer development [49].
5. Trigger Effects of HPV Integration
HPV integration types are typically classified into single-copy integration, multi-copy integration, and mixed types. A recent study discovered a new type of integration lacking E6/E7 genes, suggesting that although E6/E7 oncogenes are key contributors to the development of cancer, they are not the only mechanism, highlighting the diversity and complexity of cancer progression mechanisms [124]. The HPV genome’s integration acts like a “trigger” for CC development, initiating a series of complex chain reactions. This process not only alters the virus’s own genome structure but also causes a chain of transformations in the host genome, including the induction of host genome instability and transformations in host gene expression regulation. This virus–host genome interaction plays a crucial role in the occurrence and progression of CC, further promoting cancer development.
Notably, although viral genomic integration is a key event in carcinogenesis, it is not an absolute requirement for malignant transformation. Approximately 15% of cervical carcinomas have been found to retain HPV in a purely episomal form, demonstrating that integration is not an obligatory step in oncogenesis [125]. Specifically, episomal HPV achieves carcinogenesis through dynamic epigenetic mechanisms—such as increased acetylation of histone H4 within the upstream regulatory region (URR), particularly at late promoter regions. This not only drives early transcriptional activation of the E6/E7 oncogenes but also compensates for later transcriptional downregulation by maintaining high viral copy numbers, thereby ensuring sustained oncoprotein expression. Remarkably, this process exhibits fundamental similarities to the integration pathway in in vitro models: both depend on E6/E7-mediated degradation of p53 and pRb to promote uncontrolled proliferation and share immune evasion strategies (e.g., downregulation of interferon-stimulated genes). Furthermore, episomal forms of HPV16 can form polymers and mutants through abnormal replication and rearrangement, ultimately leading to cancerous transformation [126]. These findings provide a mechanistic basis for the episome as an independent oncogenic pathway.
Recent studies have established that oropharyngeal cancer has now surpassed cervical cancer as the most common HPV-associated malignancy. In the United States and other developed countries, approximately 70% of oropharyngeal cancer cases are HPV-positive [127]. Dynamic epigenetic regulation may explain the enhanced sensitivity of these tumors to radiotherapy and chemotherapy (e.g., reversible histone modifications) [128], offering a rationale for HDAC-targeted therapeutic strategies.
Furthermore, the coexistence of episomal and integrated HPV copies acts as an amplifier during carcinogenesis—a phenomenon elucidated in studies such as that by Kadaja et al. [129]. When both forms are present in a cell, E1 and E2 proteins expressed from episomal HPV activate replication origins at integrated sites, triggering “onion-skin replication”. This process generates aberrant replication intermediates, including linear DNA, branched structures, and circular plasmids, directly causing double-strand breaks (DSBs). Moreover, these lesions recruit cellular repair machinery, which often fails due to overloaded capacity, subsequently activating the ATM-Chk2 pathway and ultimately inducing irreversible chromosomal abnormalities such as translocations [129].
5.1. HPV Integration Leads to Host Genome Instability
Studies show that HPV integration directly promotes genomic instability [112], leading to focal structural alterations, generating virus–host fusion transcripts, forming super-enhancers, and other alterations in the host genome. These genomic changes, in turn, exacerbate genomic instability, forming a vicious cycle.
5.1.1. Chromosomal Structural Variations or Chromosomal Rearrangements
In a study of HPV-CCDC106 integration sites, HPV integration significantly altered the local chromosomal three-dimensional structure [130]. Whole-genome sequencing (WGS), transcriptome sequencing, chromatin immunoprecipitation (ChIP) sequencing, and high-throughput chromosome conformation capture (Hi-C) analysis of fresh tumor tissues revealed significant genomic variations and differential expression features on chromosome 19 [130]. HPV integration split a topologically associating domain (TAD) into two smaller TADs and hijacked an enhancer element from the PEG3 gene to the CCDC106 site, leading to PEG3 repression and CCDC106 upregulation [130].
Further research has shown that HPV integration can trigger structural and numerical variations in chromosomes [131]. Studies on HPV16 integration have observed that chromosomal translocation events occur simultaneously with HPV integration, suggesting that this process may lead to chromosomal instability [132]. Virus integration disrupts telomere stability or interferes with centromere function, leading to mitotic abnormalities [133]. Additionally, HPV-16 E6 and E7 proteins cooperate to disrupt centrosome duplication, promoting the formation of multipolar spindles and further exacerbating aneuploidy [134,135].
The breakage–fusion–bridge (BFB) cycle, a key genomic rearrangement mechanism in cell lines, can lead to complex inversions and gene amplification events on chromosomes. For example, on chromosome 11, genes such as YAP1, BIRC2, and BIRC3 frequently undergo amplification through the BFB cycle, and these genetic alterations are significantly associated with early CC diagnosis and higher invasiveness [136]. Furthermore, recent studies reveal that, following integration into the host genome, HPV forms virus–host heterocateny—complex concatemeric structures exhibiting high instability. These complexes mediate the capture, amplification, and rearrangement of host genomic segments through dynamic excision and reintegration processes [137]. This mechanism underlies the diverse and interconnected genomic rearrangement patterns observed within individual tumors, substantially promoting intratumoral heterogeneity and clonal evolution.
5.1.2. Virus–Host Fusion Transcripts
Studies of HPV16- and HPV18-related CCs and precancerous lesions have found that HPV integration can induce the production of virus–host fusion transcripts, which is reflected in genomic analyses of CCs: 83% of HPV-positive tumors exhibited virus–host fusion transcripts [17]. These transcripts may use the host’s PAS, affecting the expression of viral oncogenes [138]. Research shows that despite multiple integrated copies of viral DNA, the virus–host fusion transcripts typically originate from a single integrated HPV DNA sequence, and host genome elements are crucial for the effective expression of viral oncogenes. This aberrant expression may lead to abnormal proliferation and carcinogenesis [139]. Furthermore, virus–host fusion transcripts are more stable than those produced by free viruses, possibly because integrated HPV lacks the unstable core motif “AUUUA” in its 3’ UTR [140].
5.1.3. Virus–Cell Super-Enhancers
HPV integration sites frequently appear at transcriptional regulatory hubs, typically associated with cell-specific enhancers. Studies have shown that HPV16 integration can hijack and polymerize cell enhancers to generate virus–cell super-enhancers (SEs). In studies of CC cell lines, approximately 26 copies of HPV16 integrated into the intergenic region of chromosome 2p23.2, interspersed with amplified flanking cell DNA, with this region being rich in super-enhancer markers such as H3K27ac and Brd4. The hybrid elements formed by this integration can significantly upregulate the expression of E6/E7 oncogenes [141].
Notably, HPV-derived SEs can also function through extrachromosomal DNA (ecDNA). By conducting a multi-omics analysis of six HPV-positive and three HPV-negative cell lines, seven high-activity HPV breakpoint-induced cellular super-enhancers (BP-cSEs) were identified. These elements are located on HPV–human hybrid ecDNA and mediate transcriptional activation through long-distance intra- and interchromosomal interactions. This process regulates the expression of multiple target genes, and pathway analysis confirms their enrichment in carcinogenic networks [142]. These findings reveal a new mechanism by which HPV integration forms SEs through chromosomal and extrachromosomal mechanisms, providing new insights into viral carcinogenesis.
5.2. Downstream Effects of HPV Integration-Induced Oncoprotein Dysregulation
5.2.1. Epigenetic Modifications
HPV genome integration can lead to widespread changes in both viral and host epigenetic features [143], promoting immune evasion and tumorigenesis. Allele-specific differentially methylated regions (DMRs) near integration sites can span megabases, independently of transcriptional status, suggesting widespread epigenetic dysregulation as a direct consequence of HPV integration—a finding corroborated by recent nanopore sequencing data [144]. Some studies have found that high methylation of immune-stimulating CpG sites in the E6/E7 region of integrated HPV16 weakens TLR9 recognition [145]; meanwhile, methylation of URR and E2BSs disrupts the transcriptional repressive function of E2 on viral oncogenes [146,147,148,149]. Furthermore, methylation of the L1/L2 regions further promotes immune evasion and persistent infection [150]. A multi-omics analysis of 50 CC cases found that HPV integration near host genes such as MIR205HG and PROS1 alters their methylation and expression patterns. Specifically, integration at the MIR205HG enhancer region upregulates its expression, while high methylation in the PROS1 promoter region silences its expression [150].
Importantly, E6/E7 can exacerbate genomic instability and promote malignant transformation by regulating epigenetic modification factors such as HDAC, DNMT3B [151], and RNA-binding proteins such as RNASEH2A [152]. These findings confirm the critical importance of HPV integration in the course of epigenetic reprogramming of CC and supply important evidence for the development of novel biomarkers and therapeutic targets.
5.2.2. Somatic Mutations
HPV integration can induce mutations in the host genome through various mechanisms. Typical mechanisms include the following: (1) Direct induction of mutations by disrupting host genome integrity. For instance, HPV integration commonly targets the regulatory regions of tumor-suppressor genes (such as TP53 and SCAI) or proto-oncogenes (such as PIK3CA and ERBB2) [132,153], promoting carcinogenesis through insertion mutations or promoter activation. (2) Disrupting DNA repair systems (such as RAD51B and FBXW7 mutations), exacerbating genomic instability and leading to mutation accumulation [153]. These mutations are often enriched near HPV integration sites and are linked to the advancement of tumors. Studies show that mutations in genes like LRP1B in HPV16-integrated CCs are significantly associated with poor prognosis [153,154], indicating the key role of integration-associated mutations in disease progression.
5.2.3. Copy Number Variations
HPV integration can induce large-scale copy number variations (CNVs) in the host genome [133,155]. The integration process is accompanied by the formation of DSBs, which activate NHEJ or MMR [132], leading to the amplification or deletion of local genes. For example, HPV16 integration into the 3q28 chromosomal region containing the PIK3CA gene can trigger amplification of this region, activating the PI3K/Akt/mTOR signaling pathway [156]. Studies show that the upregulation of MYC and HMGA2 oncogenes is closely associated with HPV integration in their flanking regions [100]. Additionally, the amplification of genes such as CD274 (PD-L1) and PDCD1LG2 (PD-L2) suggests a link between CNVs and immune therapy response [17]. Comparative genomic hybridization (CGH) analysis reveals that the amplification of 1q, 3q, and 20q, along with the deletion of 11q and 13q, is considerably elevated in HPV-positive cervical tumors compared to HPV-negative ones, revealing the existence of integration-specific CNV patterns [157,158].
5.3. Virus-Mediated Immune Microenvironment Remodeling
Comprehensive analysis of single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics (ST) has uncovered a dynamic “homeostasis–imbalance–malignancy” transition in the immune microenvironment during CC development [159]. In early stages, immune cells effectively eliminate tumor cells to maintain homeostasis. However, as the disease progresses, the accumulation of immunosuppressive cells (e.g., Tregs and M2-type tumor-associated macrophages [TAMs]) drives immune evasion, facilitating tumor infection and metastasis [160,161]. HPV oncoproteins play a pivotal role in this process. They impair antigen presentation by suppressing dendritic cell function, downregulate immune surveillance molecules such as TLR9 [162,163], disrupt interferon synthesis, and promote the secretion of immunosuppressive factors, thereby polarizing the immune microenvironment and fostering immune escape. Viral genome integration further exacerbates immune evasion through PD-L1-mediated T cell exhaustion and HLA loss, creating conditions for persistent viral infection and malignant transformation [164,165]. In advanced stages, the immune microenvironment deteriorates further, a process characterized by heightened immunosuppression and functional exhaustion of immune cells. During this phase, tumor cells engage in complex crosstalk with immune cells to promote infection and metastasis [166]. For instance, PCLAF+ tumor-associated epithelial cells (TAEpis) suppress CD8+ T cell function, supporting tumor growth [161].
6. Clinical Translation: From Triage to Targeted Therapy
HPV integration holds potential application value in CC triage screening. Compared to cytological examination triage, HPV integration shows similar sensitivity and negative predictive value for detecting CIN3+ lesions, but with higher specificity, and a significantly lower referral rate for colposcopy [10,167]. Therefore, combining HPV integration detection may optimize triage strategies for high-risk patients, enabling more effective screening of high-risk individuals and reducing unnecessary diagnostic and therapeutic procedures.
Additionally, targeted therapeutic strategies for HPV integration mainly include inhibiting DNA repair mechanisms and viral oncogene expression. HPV integration induces genomic instability via MMEJ, and inhibiting MMEJ-related repair enzymes such as poly(ADP-ribose) polymerase (PARP) may effectively block the integration process [168]. Another strategy is to target the expression of the E6 and E7 genes, which interfere with the cell cycle [91]. For example, using small interfering RNA (siRNA) technology can effectively reduce the expression levels of E6 and E7, thereby enhancing the effectiveness of chemotherapy drugs [169]. Furthermore, gene editing technologies like CRISPR/Cas9 could precisely repair the genomic abnormalities caused by HPV integration, offering a potential therapeutic approach [170]. Meanwhile, inhibiting ecDNA excision and recombination—key steps in heterocateny—may disrupt clonal evolution [137], presenting a promising strategy for HPV-associated cancers. While these strategies are still in the exploratory stage, future research is expected to provide new options for the treatment of CC.
7. Conclusions
HPV integration represents a pivotal event in carcinogenesis, yet its accidental nature and the existence of episome-driven oncogenic pathways must be acknowledged. The process from HPV infection to integration is a dynamic multi-stage process involving complex interactions between the virus and host cells. In the initial stage, HPV enters the host cell via L1/L2 proteins, establishing initial infection. Later, under the combined influence of host genetic background and ecological imbalance, the viral genome integrates into the human chromosome via mechanisms such as MMEJ and NHEJ. This process plays a pivotal role in CC development, triggering a cascade of events. HPV integration not only directly induces localized genomic disruption—including DNA breakage, cellular super-enhancer formation, and virus–host fusion transcripts—but also indirectly amplifies genomic instability through sustained E6/E7 expression, which promotes the accumulation of somatic mutations, CNVs, epigenetic modifications, and activation of immune escape mechanisms. High-throughput technologies have revolutionized HPV integration research. Techniques like WGS [133,171], scRNA-seq [139], long-read sequencing [124], and the Hi-C technique [102] allow researchers to capture specific integration sites, frequencies, and their genomic impacts with high precision and efficiency.
Future research directions should focus on elucidating the integration mechanisms of different HPV subtypes and their specific impacts on the host genome, in particular how distinct integration patterns influence the clinical manifestations and prognosis of CC. Additionally, investigations into the distribution and functional consequences of HPV integration across different tissue types—especially the differences in integration patterns between adenocarcinoma and squamous cell carcinoma—are warranted. A deeper understanding of the molecular mechanisms underlying HPV integration, as well as potential alternative pathways, remains crucial. Concurrently, the mechanisms driving carcinogenesis in HPV-negative patients deserve further attention.
Collectively, the dynamic process from HPV infection to integration not only reveals the complex interplay between the virus and host cells but also provides a theoretical foundation for early diagnosis and targeted therapy of CC. Moving forward, targeted interventions against HPV integration will emerge as pivotal strategies in cancer screening and treatment, offering novel clinical approaches. Supported by high-throughput technologies, research in this field will accelerate our understanding of HPV-mediated oncogenesis and enhance the precision of early diagnosis and therapeutic interventions.
Author Contributions
S.L. first suggested writing a review paper on the role of HPV integration in CCs. J.L. searched for the published studies. S.L. and J.L. discussed the topic and wrote the manuscript together. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001410-7. [Google Scholar]
- Bray, F.; Parkin, D.M.; Gnangnon, F.; Tshisimogo, G.; Peko, J.-F.; Adoubi, I.; Assefa, M.; Bojang, L.; Awuah, B.; Koulibaly, M.; et al. Cancer in Sub-Saharan Africa in 2020: A Review of Current Estimates of the National Burden, Data Gaps, and Future Needs. Lancet Oncol. 2022, 23, 719–728. [Google Scholar] [CrossRef]
- Cervical Cancer. Available online: https://www.who.int/health-topics/cervical-cancer (accessed on 7 September 2025).
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.L.M.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence Regarding Human Papillomavirus Testing in Secondary Prevention of Cervical Cancer. Vaccine 2012, 30 (Suppl. S5), F88–F99, Erratum in Vaccine 2013, 31, 6266. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Lewkowitz, A.K.; Chen, F.; Lin, M.J.; Hu, S.-Y.; Zhang, X.; Pan, Q.-J.; Ma, J.-F.; Niyazi, M.; Li, C.-Q.; et al. Pooled Analysis of a Self-Sampling HPV DNA Test as a Cervical Cancer Primary Screening Method. J. Natl. Cancer Inst. 2012, 104, 178–188. [Google Scholar] [CrossRef]
- Khan, M.J.; Castle, P.E.; Lorincz, A.T.; Wacholder, S.; Sherman, M.; Scott, D.R.; Rush, B.B.; Glass, A.G.; Schiffman, M. The Elevated 10-Year Risk of Cervical Precancer and Cancer in Women with Human Papillomavirus (HPV) Type 16 or 18 and the Possible Utility of Type-Specific HPV Testing in Clinical Practice. J. Natl. Cancer Inst. 2005, 97, 1072–1079. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The Natural History of Human Papillomavirus Infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Hu, T.; Li, K.; He, L.; Huang, F.; Yang, F.; Chen, S.; Wang, H.; Ma, D.; Huang, X.; Wu, P. Testing for Viral DNA Integration among HPV-Positive Women to Detect Cervical Precancer: An Observational Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2024, 131, 309–318. [Google Scholar] [CrossRef]
- Izadi, N.; Strmiskova, J.; Anton, M.; Hausnerova, J.; Bartosik, M. LAMP-Based Electrochemical Platform for Monitoring HPV Genome Integration at the mRNA Level Associated with Higher Risk of Cervical Cancer Progression. J. Med. Virol. 2024, 96, e70008. [Google Scholar] [CrossRef] [PubMed]
- Bouchilloux, S.; Fer, F.; Lemée, F.; Barradeau, S.; Dvorak, V.; Kubickova, S.; Ventruba, P.; Tachezy, R.; Trnková, M.; Janda, P.; et al. Correlation between Integration of High-Risk HPV Genome into Human DNA Detected by Molecular Combing and the Severity of Cervical Lesions: First Results of the EXPL-HPV-002 Study. Ceska Gynekol. 2019, 84, 84–92. [Google Scholar]
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV Integration and Its Progression to Cervical Cancer. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 61, 134–144. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Oncogenic Human Papillomaviruses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160273. [Google Scholar] [CrossRef]
- McBride, A.A. Human Papillomaviruses: Diversity, Infection and Host Interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Special Programme of Research, Development, and Research Training in Human Reproduction (World Health Organization). In WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003082-4. [Google Scholar]
- Albert Einstein College of Medicine; Analytical Biological Services; Barretos Cancer Hospital; Baylor College of Medicine; Beckman Research Institute of City of Hope; Buck Institute for Research on Aging; Canada’s Michael Smith Genome Sciences Centre; Harvard Medical School; Helen F. Graham Cancer Center & Research Institute at Christiana Care Health Services; HudsonAlpha Institute for Biotechnology; et al. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Salavatiha, Z.; Farahmand, M.; Shoja, Z.; Jalilvand, S. A Meta-Analysis of Human Papillomavirus Prevalence and Types among Iranian Women with Normal Cervical Cytology, Premalignant Lesions, and Cervical Cancer. J. Med. Virol. 2021, 93, 4647–4658. [Google Scholar] [CrossRef]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human Papillomavirus Types in 115,789 HPV-Positive Women: A Meta-Analysis from Cervical Infection to Cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Gagliardi, A.; Porter, V.L.; Zong, Z.; Bowlby, R.; Titmuss, E.; Namirembe, C.; Griner, N.B.; Petrello, H.; Bowen, J.; Chan, S.K.; et al. Analysis of Ugandan Cervical Carcinomas Identifies Human Papillomavirus Clade-Specific Epigenome and Transcriptome Landscapes. Nat. Genet. 2020, 52, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Løvestad, A.H.; Repesa, A.; Costanzi, J.-M.; Lagström, S.; Christiansen, I.K.; Rounge, T.B.; Ambur, O.H. Differences in Integration Frequencies and APOBEC3 Profiles of Five High-Risk HPV Types Adheres to Phylogeny. Tumour Virus Res. 2022, 14, 200247. [Google Scholar] [CrossRef]
- Mohammed, F.A.; Tune, K.K.; Jett, M.; Muhie, S. Cervical Cancer Stages, Human Papillomavirus Integration, and Malignant Genetic Mutations: Integrative Analysis of Datasets from Four Different Cohorts. Cancers 2023, 15, 5595. [Google Scholar] [CrossRef]
- Berti, F.C.B.; Mathias, C.; Garcia, L.E.; Gradia, D.F.; de Araújo-Souza, P.S.; Cipolla, G.A.; de Oliveira, J.C.; Malheiros, D. Comprehensive Analysis of ceRNA Networks in HPV16- and HPV18-Mediated Cervical Cancers Reveals XIST as a Pivotal Competing Endogenous RNA. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166172. [Google Scholar] [CrossRef]
- Mir, B.A.; Ahmad, A.; Farooq, N.; Priya, M.V.; Siddiqui, A.H.; Asif, M.; Manzoor, R.; Ishqi, H.M.; Alomar, S.Y.; Rahaman, P.F.; et al. Increased Expression of HPV-E7 Oncoprotein Correlates with a Reduced Level of pRb Proteins via High Viral Load in Cervical Cancer. Sci. Rep. 2023, 13, 15075. [Google Scholar] [CrossRef]
- Ibragimova, M.K.; Tsyganov, M.M.; Karabut, I.V.; Churuksaeva, O.N.; Shpileva, O.N.; Bychkov, V.A.; Kolomiets, L.A.; Litviakov, N.V. Integrative and Episomal Forms of Genotype 16 of Human Papillomavirus in Patients with Cervical Intraepithelial Neoplasia and Cervical Cancer. Vopr. Virusol. 2016, 61, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Burchell, A.N.; Winer, R.L.; de Sanjosé, S.; Franco, E.L. Chapter 6: Epidemiology and Transmission Dynamics of Genital HPV Infection. Vaccine 2006, 24 (Suppl. S3), S52–S61. [Google Scholar] [CrossRef]
- Chen, D.; Juko-Pecirep, I.; Hammer, J.; Ivansson, E.; Enroth, S.; Gustavsson, I.; Feuk, L.; Magnusson, P.K.E.; McKay, J.D.; Wilander, E.; et al. Genome-Wide Association Study of Susceptibility Loci for Cervical Cancer. J. Natl. Cancer Inst. 2013, 105, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, D.; Schürmann, P.; Mao, Q.; Wang, Y.; Bretschneider, L.-M.; Speith, L.-M.; Hülse, F.; Enßen, J.; Bousset, K.; Jentschke, M.; et al. Association of Genomic Variants at the Human Leukocyte Antigen Locus with Cervical Cancer Risk, HPV Status and Gene Expression Levels. Int. J. Cancer 2020, 147, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Kukimoto, I.; Li, Z.; Li, S.; Li, N.; Hu, Z.; Takahashi, A.; Inoue, S.; Yokoi, S.; Chen, J.; et al. Genome-Wide Association Study of Cervical Cancer Suggests a Role for ARRDC3 Gene in Human Papillomavirus Infection. Hum. Mol. Genet. 2019, 28, 341–348. [Google Scholar] [CrossRef]
- Bowden, S.J.; Bodinier, B.; Kalliala, I.; Zuber, V.; Vuckovic, D.; Doulgeraki, T.; Whitaker, M.D.; Wielscher, M.; Cartwright, R.; Tsilidis, K.K.; et al. Genetic Variation in Cervical Preinvasive and Invasive Disease: A Genome-Wide Association Study. Lancet Oncol. 2021, 22, 548–557. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Hu, Z.; Li, S.; Wang, S.; Liu, J.; Wu, C.; He, L.; Zhou, J.; Li, Z.; et al. A Genome-Wide Association Study Identifies Two New Cervical Cancer Susceptibility Loci at 4q12 and 17q12. Nat. Genet. 2013, 45, 918–922. [Google Scholar] [CrossRef]
- Joo, J.; Omae, Y.; Hitomi, Y.; Park, B.; Shin, H.-J.; Yoon, K.-A.; Sawai, H.; Tsuiji, M.; Hayashi, T.; Kong, S.-Y.; et al. The Association of Integration Patterns of Human Papilloma Virus and Single Nucleotide Polymorphisms on Immune- or DNA Repair-Related Genes in Cervical Cancer Patients. Sci. Rep. 2019, 9, 13132. [Google Scholar] [CrossRef]
- Chen, D.; Gyllensten, U. Lessons and Implications from Association Studies and Post-GWAS Analyses of Cervical Cancer. Trends Genet. TIG 2015, 31, 41–54. [Google Scholar] [CrossRef]
- Yu, T.; Gao, S.; Jin, F.; Yan, B.; Wang, W.; Wang, Z. Characteristics of the Vaginal Microbiota and Vaginal Metabolites in Women with Cervical Dysplasia. Front. Cell. Infect. Microbiol. 2024, 14, 1457216, Erratum in Front. Cell. Infect. Microbiol. 2024, 14, 1527287. [Google Scholar] [CrossRef] [PubMed]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Danielsson, D.; Teigen, P.K.; Moi, H. The Genital Econiche: Focus on Microbiota and Bacterial Vaginosis. Ann. N. Y. Acad. Sci. 2011, 1230, 48–58. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Wei, X.; Zhu, J.; Wang, X.; Xie, X.; Lu, W. The Alterations of Vaginal Microbiome in HPV16 Infection as Identified by Shotgun Metagenomic Sequencing. Front. Cell. Infect. Microbiol. 2020, 10, 286. [Google Scholar] [CrossRef]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; van de Wijgert, J.H.H.M. Cervicovaginal Microbiome Dysbiosis Is Associated with Proteome Changes Related to Alterations of the Cervicovaginal Mucosal Barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef]
- Doerflinger, S.Y.; Throop, A.L.; Herbst-Kralovetz, M.M. Bacteria in the Vaginal Microbiome Alter the Innate Immune Response and Barrier Properties of the Human Vaginal Epithelia in a Species-Specific Manner. J. Infect. Dis. 2014, 209, 1989–1999. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota Metabolite Butyrate Constrains Neutrophil Functions and Ameliorates Mucosal Inflammation in Inflammatory Bowel Disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Letafati, A.; Sakhavarz, T.; Khosravinia, M.M.; Ardekani, O.S.; Sadeghifar, S.; Norouzi, M.; Naseri, M.; Ghaziasadi, A.; Jazayeri, S.M. Exploring the Correlation between Progression of Human Papillomavirus Infection towards Carcinogenesis and Nutrition. Microb. Pathog. 2023, 183, 106302. [Google Scholar] [CrossRef] [PubMed]
- Varada, S.; Posnick, M.; Alessa, D.; Ramírez-Fort, M.K. Management of Cutaneous Human Papillomavirus Infection in Immunocompromised Patients. Curr. Probl. Dermatol. 2014, 45, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.C.; Burnett, A.F.; Willet, G.D.; Young, M.A.; Doniger, J. High Frequency of Latent and Clinical Human Papillomavirus Cervical Infections in Immunocompromised Human Immunodeficiency Virus-Infected Women. Obs. Gynecol. 1992, 79, 321–327. [Google Scholar] [CrossRef]
- Revathidevi, S.; Murugan, A.K.; Nakaoka, H.; Inoue, I.; Munirajan, A.K. APOBEC: A molecular driver in cervical cancer pathogenesis. Cancer Lett. 2021, 496, 104–116. [Google Scholar] [CrossRef]
- Hasan, U.A.; Zannetti, C.; Parroche, P.; Goutagny, N.; Malfroy, M.; Roblot, G.; Carreira, C.; Hussain, I.; Müller, M.; Taylor-Papadimitriou, J.; et al. The Human Papillomavirus Type 16 E7 Oncoprotein Induces a Transcriptional Repressor Complex on the Toll-like Receptor 9 Promoter. J. Exp. Med. 2013, 210, 1369–1387. [Google Scholar] [CrossRef]
- Gómez, D.T.; Santos, J.L. Human Papillomavirus Infection and Cervical Cancer: Pathogenesis and Epidemiology. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 680–688. [Google Scholar]
- Liu, Y.; Ai, H. Comprehensive insights into human papillomavirus and cervical cancer: Pathophysiology, screening, and vaccination strategies. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189192. [Google Scholar] [CrossRef]
- Warburton, A.; Markowitz, T.E.; Katz, J.P.; Pipas, J.M.; McBride, A.A. Recurrent Integration of Human Papillomavirus Genomes at Transcriptional Regulatory Hubs. npj Genom. Med. 2021, 6, 101. [Google Scholar] [CrossRef]
- Wallace, N.A.; Khanal, S.; Robinson, K.L.; Wendel, S.O.; Messer, J.J.; Galloway, D.A. High-Risk Alphapapillomavirus Oncogenes Impair the Homologous Recombination Pathway. J. Virol. 2017, 91, e01084-17. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human Papillomaviruses Activate the ATM DNA Damage Pathway for Viral Genome Amplification upon Differentiation. PLoS Pathog. 2009, 5, e1000605. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A. Impact of Replication Stress in Human Papillomavirus Pathogenesis. J. Virol. 2019, 93, e01012-17. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Replication and Partitioning of Papillomavirus Genomes. Adv. Virus Res. 2008, 72, 155–205. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; van Bodegraven, D.; Kádek, A.; L B Munguira, I.; Soria-Martinez, L.; Nentwich, S.; Saha, S.; Chardon, F.; Kavan, D.; Uetrecht, C.; et al. Glycan-Induced Structural Activation Softens the Human Papillomavirus Capsid for Entry through Reduction of Intercapsomere Flexibility. Nat. Commun. 2024, 15, 10076. [Google Scholar] [CrossRef]
- Nguyen, B.P.; Ryan, M.C.; Gil, S.G.; Carter, W.G. Deposition of Laminin 5 in Epidermal Wounds Regulates Integrin Signaling and Adhesion. Curr. Opin. Cell Biol. 2000, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Culp, T.D.; Budgeon, L.R.; Marinkovich, M.P.; Meneguzzi, G.; Christensen, N.D. Keratinocyte-Secreted Laminin 5 Can Function as a Transient Receptor for Human Papillomaviruses by Binding Virions and Transferring Them to Adjacent Cells. J. Virol. 2006, 80, 8940–8950. [Google Scholar] [CrossRef]
- Richards, R.M.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Cleavage of the Papillomavirus Minor Capsid Protein, L2, at a Furin Consensus Site Is Necessary for Infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Gambhira, R.; Roden, R.B.S.; Lowy, D.R.; Schiller, J.T. Mechanisms of Human Papillomavirus Type 16 Neutralization by L2 Cross-Neutralizing and L1 Type-Specific Antibodies. J. Virol. 2008, 82, 4638–4646. [Google Scholar] [CrossRef]
- Schelhaas, M.; Shah, B.; Holzer, M.; Blattmann, P.; Kühling, L.; Day, P.M.; Schiller, J.T.; Helenius, A. Entry of Human Papillomavirus Type 16 by Actin-Dependent, Clathrin- and Lipid Raft-Independent Endocytosis. PLoS Pathog. 2012, 8, e1002657. [Google Scholar] [CrossRef] [PubMed]
- Spoden, G.; Kühling, L.; Cordes, N.; Frenzel, B.; Sapp, M.; Boller, K.; Florin, L.; Schelhaas, M. Human Papillomavirus Types 16, 18, and 31 Share Similar Endocytic Requirements for Entry. J. Virol. 2013, 87, 7765–7773. [Google Scholar] [CrossRef]
- Harwood, M.C.; Woo, T.-T.; Takeo, Y.; DiMaio, D.; Tsai, B. HPV Is a Cargo for the COPI Sorting Complex during Virus Entry. Sci. Adv. 2023, 9, eadc9830. [Google Scholar] [CrossRef]
- Aydin, I.; Weber, S.; Snijder, B.; Samperio Ventayol, P.; Kühbacher, A.; Becker, M.; Day, P.M.; Schiller, J.T.; Kann, M.; Pelkmans, L.; et al. Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses. PLoS Pathog. 2014, 10, e1004162. [Google Scholar] [CrossRef]
- Day, P.M.; Baker, C.C.; Lowy, D.R.; Schiller, J.T. Establishment of Papillomavirus Infection Is Enhanced by Promyelocytic Leukemia Protein (PML) Expression. Proc. Natl. Acad. Sci. USA 2004, 101, 14252–14257. [Google Scholar] [CrossRef]
- Porter, S.S.; Stepp, W.H.; Stamos, J.D.; McBride, A.A. Host Cell Restriction Factors That Limit Transcription and Replication of Human Papillomavirus. Virus Res. 2017, 231, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Stepp, W.H.; Meyers, J.M.; McBride, A.A. Sp100 Provides Intrinsic Immunity against Human Papillomavirus Infection. Mbio 2013, 4, e00845-813. [Google Scholar] [CrossRef]
- Ozbun, M.A. Human Papillomavirus Type 31b Infection of Human Keratinocytes and the Onset of Early Transcription. J. Virol. 2002, 76, 11291–11300. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. The Papillomavirus E2 Proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yilmaz, G.; Biswas-Fiss, E.E.; Biswas, S. Mechanisms of Viral DNA Replication of Human Papillomavirus: E2 Protein-Dependent Recruitment of E1 DNA Helicase to the Origin of DNA Replication. Int. J. Mol. Sci. 2025, 26, 4333. [Google Scholar] [CrossRef]
- Rana, A.; Evande, R.; Biswas-Fiss, E.; Biswas, S. Abstract 1637 Molecular Mechanism of HPV DNA Replication Initiation, Mediated by E1 and E2 Proteins. J. Biol. Chem. 2024, 300, S681. [Google Scholar] [CrossRef]
- Baedyananda, F.; Chaiwongkot, A.; Varadarajan, S.; Bhattarakosol, P. HPV16 E1 Dysregulated Cellular Genes Involved in Cell Proliferation and Host DNA Damage: A Possible Role in Cervical Carcinogenesis. PLoS ONE 2021, 16, e0260841. [Google Scholar] [CrossRef]
- Sakakibara, N.; Mitra, R.; McBride, A.A. The Papillomavirus E1 Helicase Activates a Cellular DNA Damage Response in Viral Replication Foci. J. Virol. 2011, 85, 8981–8995. [Google Scholar] [CrossRef]
- Jang, M.K.; Shen, K.; McBride, A.A. Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome. PLoS Pathog. 2014, 10, e1004117. [Google Scholar] [CrossRef]
- Prabhakar, A.T.; James, C.D.; Das, D.; Fontan, C.T.; Otoa, R.; Wang, X.; Bristol, M.L.; Morgan, I.M. Interaction with TopBP1 Is Required for Human Papillomavirus 16 E2 Plasmid Segregation/Retention Function during Mitosis. J. Virol. 2022, 96, e0083022. [Google Scholar] [CrossRef]
- Dreer, M.; Fertey, J.; van de Poel, S.; Straub, E.; Madlung, J.; Macek, B.; Iftner, T.; Stubenrauch, F. Interaction of NCOR/SMRT Repressor Complexes with Papillomavirus E8^E2C Proteins Inhibits Viral Replication. PLoS Pathog. 2016, 12, e1005556. [Google Scholar] [CrossRef] [PubMed]
- Dreer, M.; van de Poel, S.; Stubenrauch, F. Control of Viral Replication and Transcription by the Papillomavirus E8^E2 Protein. Virus Res. 2017, 231, 96–102. [Google Scholar] [CrossRef]
- Uhlorn, B.L.; Jackson, R.; Li, S.; Bratton, S.M.; Van Doorslaer, K.; Campos, S.K. Vesicular Trafficking Permits Evasion of cGAS/STING Surveillance during Initial Human Papillomavirus Infection. PLoS Pathog. 2020, 16, e1009028. [Google Scholar] [CrossRef] [PubMed]
- Guion, L.; Bienkowska-Haba, M.; DiGiuseppe, S.; Florin, L.; Sapp, M. PML Nuclear Body-Residing Proteins Sequentially Associate with HPV Genome after Infectious Nuclear Delivery. PLoS Pathog. 2019, 15, e1007590. [Google Scholar] [CrossRef] [PubMed]
- Guion, L.G.; Sapp, M. The Role of Promyelocytic Leukemia Nuclear Bodies during HPV Infection. Front. Cell. Infect. Microbiol. 2020, 10, 35. [Google Scholar] [CrossRef]
- Hong, S.; Laimins, L.A. Manipulation of the Innate Immune Response by Human Papillomaviruses. Virus Res. 2017, 231, 34–40. [Google Scholar] [CrossRef]
- de Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R.; Venuti, A. hrHPV E5 Oncoprotein: Immune Evasion and Related Immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71. [Google Scholar] [CrossRef]
- Kim, S.-H.; Oh, J.-M.; No, J.-H.; Bang, Y.-J.; Juhnn, Y.-S.; Song, Y.-S. Involvement of NF-κB and AP-1 in COX-2 Upregulation by Human Papillomavirus 16 E5 Oncoprotein. Carcinogenesis 2009, 30, 753–757. [Google Scholar] [CrossRef]
- Molina, M.A.; Steenbergen, R.D.M.; Pumpe, A.; Kenyon, A.N.; Melchers, W.J.G. HPV Integration and Cervical Cancer: A Failed Evolutionary Viral Trait. Trends Mol. Med. 2024, 30, 890–902. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Gortsilas, P.; Kyriakopoulou, Z.; Ruether, I.G.A.; Dimitriou, T.G.; Orfanoudakis, G.; Markoulatos, P. Sites of Disruption within E1 and E2 Genes of HPV16 and Association with Cervical Dysplasia. J. Med. Virol. 2015, 87, 1973–1980. [Google Scholar] [CrossRef]
- Amaro-Filho, S.M.; Pereira Chaves, C.B.; Felix, S.P.; Basto, D.L.; de Almeida, L.M.; Moreira, M.A.M. HPV DNA Methylation at the Early Promoter and E1/E2 Integrity: A Comparison between HPV16, HPV18 and HPV45 in Cervical Cancer. Papillomavirus Res. 2018, 5, 172–179. [Google Scholar] [CrossRef]
- Xu, X.-S.; Ma, Y.-S.; Dai, R.-H.; Zhang, H.-L.; Yang, Q.-X.; Fan, Q.-Y.; Liu, X.-Y.; Liu, J.-B.; Feng, W.-W.; Meng, H.; et al. Identification of Novel Genomic Hotspots and Tumor-Relevant Genes via Comprehensive Analysis of HPV Integration in Chinese Patients of Cervical Cancer. Am. J. Cancer Res. 2024, 14, 4665–4682. [Google Scholar] [CrossRef]
- Titolo, S.; Pelletier, A.; Sauvé, F.; Brault, K.; Wardrop, E.; White, P.W.; Amin, A.; Cordingley, M.G.; Archambault, J. Role of the ATP-Binding Domain of the Human Papillomavirus Type 11 E1 Helicase in E2-Dependent Binding to the Origin. J. Virol. 1999, 73, 5282–5293. [Google Scholar] [CrossRef]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K.; Bodily, J.M. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 Oncoprotein Encoded by Human Papillomavirus Types 16 and 18 Promotes the Degradation of P53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Klingelhutz, A.J.; Foster, S.A.; McDougall, J.K. Telomerase Activation by the E6 Gene Product of Human Papillomavirus Type 16. Nature 1996, 380, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Banks, L. Molecular Mechanisms Underlying Human Papillomavirus E6 and E7 Oncoprotein-Induced Cell Transformation. Mutat. Res./Rev. Mutat. Res. 2017, 772, 23–35. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Warburton, A. The Role of Integration in Oncogenic Progression of HPV-Associated Cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Dannenberg, A.J. Cyclooxygenase-2 Transcription Is Regulated by Human Papillomavirus 16 E6 and E7 Oncoproteins: Evidence of a Corepressor/Coactivator Exchange. Cancer Res. 2007, 67, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Gonzaléz-García, M.C.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Valverde, M.; Rojas, E.; Rodríguez-Sastre, M.A.; García-Cuellar, C.M.; Lizano, M. Human Papillomavirus Types 16 and 18 Early-Expressed Proteins Differentially Modulate the Cellular Redox State and DNA Damage. Int. J. Biol. Sci. 2018, 14, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kessis, T.D.; Connolly, D.C.; Hedrick, L.; Cho, K.R. Expression of HPV16 E6 or E7 Increases Integration of Foreign DNA. Oncogene 1996, 13, 427–431. [Google Scholar]
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA Integration and Carcinogenesis: Putative Roles for Inflammation and Oxidative Stress. Future Virol. 2011, 6, 45–57. [Google Scholar] [CrossRef]
- Ziegert, C.; Wentzensen, N.; Vinokurova, S.; Kisseljov, F.; Einenkel, J.; Hoeckel, M.; von Knebel Doeberitz, M. A Comprehensive Analysis of HPV Integration Loci in Anogenital Lesions Combining Transcript and Genome-Based Amplification Techniques. Oncogene 2003, 22, 3977–3984. [Google Scholar] [CrossRef]
- Lieber, M.R. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End-Joining Pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, D.; Wang, W.; Li, W.; Jia, W.; Zeng, X.; Ding, W.; Yu, L.; Wang, X.; Wang, L.; et al. Genome-Wide Profiling of HPV Integration in Cervical Cancer Identifies Clustered Genomic Hot Spots and a Potential Microhomology-Mediated Integration Mechanism. Nat. Genet. 2015, 47, 158–163. [Google Scholar] [CrossRef]
- Jiang, Y. Contribution of Microhomology to Genome Instability: Connection between DNA Repair and Replication Stress. Int. J. Mol. Sci. 2022, 23, 12937. [Google Scholar] [CrossRef]
- Groves, I.J.; Drane, E.L.A.; Michalski, M.; Monahan, J.M.; Scarpini, C.G.; Smith, S.P.; Bussotti, G.; Várnai, C.; Schoenfelder, S.; Fraser, P.; et al. Short- and Long-Range Cis Interactions between Integrated HPV Genomes and Cellular Chromatin Dysregulate Host Gene Expression in Early Cervical Carcinogenesis. PLoS Pathog. 2021, 17, e1009875. [Google Scholar] [CrossRef]
- Sartori, A.A.; Lukas, C.; Coates, J.; Mistrik, M.; Fu, S.; Bartek, J.; Baer, R.; Lukas, J.; Jackson, S.P. Human CtIP Promotes DNA End Resection. Nature 2007, 450, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.J.; Lees-Miller, S.P.; Tainer, J.A. Mre11-Rad50-Nbs1 Conformations and the Control of Sensing, Signaling, and Effector Responses at DNA Double-Strand Breaks. DNA Repair 2010, 9, 1299–1306. [Google Scholar] [CrossRef]
- Ahmad, A.; Robinson, A.R.; Duensing, A.; van Drunen, E.; Beverloo, H.B.; Weisberg, D.B.; Hasty, P.; Hoeijmakers, J.H.J.; Niedernhofer, L.J. ERCC1-XPF Endonuclease Facilitates DNA Double-Strand Break Repair. Mol. Cell. Biol. 2008, 28, 5082–5092. [Google Scholar] [CrossRef]
- Crespan, E.; Czabany, T.; Maga, G.; Hübscher, U. Microhomology-Mediated DNA Strand Annealing and Elongation by Human DNA Polymerases λ and β on Normal and Repetitive DNA Sequences. Nucleic Acids Res. 2012, 40, 5577–5590. [Google Scholar] [CrossRef]
- Liang, L.; Deng, L.; Nguyen, S.C.; Zhao, X.; Maulion, C.D.; Shao, C.; Tischfield, J.A. Human DNA Ligases I and III, but Not Ligase IV, Are Required for Microhomology-Mediated End Joining of DNA Double-Strand Breaks. Nucleic Acids Res. 2008, 36, 3297–3310. [Google Scholar] [CrossRef]
- Zhang, F.; Khajavi, M.; Connolly, A.M.; Towne, C.F.; Batish, S.D.; Lupski, J.R. The DNA Replication FoSTeS/MMBIR Mechanism Can Generate Genomic, Genic and Exonic Complex Rearrangements in Humans. Nat. Genet. 2009, 41, 849–853. [Google Scholar] [CrossRef]
- Hastings, P.J.; Lupski, J.R.; Rosenberg, S.M.; Ira, G. Mechanisms of Change in Gene Copy Number. Nat. Rev. Genet. 2009, 10, 551–564. [Google Scholar] [CrossRef]
- Hastings, P.J.; Ira, G.; Lupski, J.R. A Microhomology-Mediated Break-Induced Replication Model for the Origin of Human Copy Number Variation. PLoS Genet. 2009, 5, e1000327. [Google Scholar] [CrossRef]
- Bindra, R.S.; Glazer, P.M. Repression of RAD51 Gene Expression by E2F4/P130 Complexes in Hypoxia. Oncogene 2007, 26, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-Wide Analysis of HPV Integration in Human Cancers Reveals Recurrent, Focal Genomic Instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Vinokurova, S.; von Knebel Doeberitz, M. Systematic Review of Genomic Integration Sites of Human Papillomavirus Genomes in Epithelial Dysplasia and Invasive Cancer of the Female Lower Genital Tract. Cancer Res. 2004, 64, 3878–3884. [Google Scholar] [CrossRef] [PubMed]
- Vinokurova, S.; Wentzensen, N.; Kraus, I.; Klaes, R.; Driesch, C.; Melsheimer, P.; Kisseljov, F.; Dürst, M.; Schneider, A.; von Knebel Doeberitz, M. Type-Dependent Integration Frequency of Human Papillomavirus Genomes in Cervical Lesions. Cancer Res. 2008, 68, 307–313. [Google Scholar] [CrossRef]
- Fan, J.; Fu, Y.; Peng, W.; Li, X.; Shen, Y.; Guo, E.; Lu, F.; Zhou, S.; Liu, S.; Yang, B.; et al. Multi-Omics Characterization of Silent and Productive HPV Integration in Cervical Cancer. Cell Genom. 2023, 3, 100211. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.-K.; Liu, C.-Y.; Liu, H.-W.; Li, J.-T.; Li, F.; Mehryar, M.M.; Wang, Y.-J.; Zhan, S.-B.; Zhou, Y.-b.; Zhong, R.-G.; et al. Integrated HPV Genomes Tend to Integrate in Gene Desert Areas in the CaSki, HeLa, and SiHa Cervical Cancer Cell Lines. Life Sci. 2015, 127, 46–52. [Google Scholar] [CrossRef]
- Ferber, M.J.; Thorland, E.C.; Brink, A.A.T.P.; Rapp, A.K.; Phillips, L.A.; McGovern, R.; Gostout, B.S.; Cheung, T.H.; Chung, T.K.H.; Fu, W.Y.; et al. Preferential Integration of Human Papillomavirus Type 18 near the C-Myc Locus in Cervical Carcinoma. Oncogene 2003, 22, 7233–7242. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, Y.; Cui, X.; Huo, Q.; Zhu, L.; Zhang, A.; Tan, M.; Hong, Q.; Yang, Y.; Zhang, H.; et al. Characteristic of HPV Integration in the Genome and Transcriptome of Cervical Cancer Tissues. BioMed Res. Int. 2018, 2018, 6242173. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Lameiras, S.; Deloger, M.; Morel, A.; Vacher, S.; Lecerf, C.; Dupain, C.; Jeannot, E.; Girard, E.; Baulande, S.; et al. Human Papilloma Virus (HPV) Integration Signature in Cervical Cancer: Identification of MACROD2 Gene as HPV Hot Spot Integration Site. Br. J. Cancer 2021, 124, 777–785, Erratum in Br. J. Cancer 2023, 128, 1790. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, J.; Zeng, L.; Chen, G.; Cai, H.; Cao, H.; Ma, Q.; Wu, X. Characteristics of HPV Integration in Cervical Adenocarcinoma and Squamous Carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 17973–17986. [Google Scholar] [CrossRef]
- Bodelon, C.; Untereiner, M.E.; Machiela, M.J.; Vinokurova, S.; Wentzensen, N. Genomic Characterization of Viral Integration Sites in HPV-Related Cancers. Int. J. Cancer 2016, 139, 2001–2011. [Google Scholar] [CrossRef]
- Thorland, E.C.; Myers, S.L.; Persing, D.H.; Sarkar, G.; McGovern, R.M.; Gostout, B.S.; Smith, D.I. Human Papillomavirus Type 16 Integrations in Cervical Tumors Frequently Occur in Common Fragile Sites. Cancer Res. 2000, 60, 5916–5921. [Google Scholar]
- Ji, F.; Liao, H.; Pan, S.; Ouyang, L.; Jia, F.; Fu, Z.; Zhang, F.; Geng, X.; Wang, X.; Li, T.; et al. Genome-Wide High-Resolution Mapping of Mitotic DNA Synthesis Sites and Common Fragile Sites by Direct Sequencing. Cell Res. 2020, 30, 1009–1023. [Google Scholar] [CrossRef]
- Zhou, L.; Qiu, Q.; Zhou, Q.; Li, J.; Yu, M.; Li, K.; Xu, L.; Ke, X.; Xu, H.; Lu, B.; et al. Long-Read Sequencing Unveils High-Resolution HPV Integration and Its Oncogenic Progression in Cervical Cancer. Nat. Commun. 2022, 13, 2563. [Google Scholar] [CrossRef]
- Gray, E.; Pett, M.R.; Ward, D.; Winder, D.M.; Stanley, M.A.; Roberts, I.; Scarpini, C.G.; Coleman, N. In Vitro Progression of HPV16 Episome-Associated Cervical Neoplasia Displays Fundamental Similarities to Integrant-Associated Carcinogenesis. Cancer Res. 2010, 70, 4081–4091. [Google Scholar] [CrossRef]
- Rossi, N.M.; Dai, J.; Xie, Y.; Wangsa, D.; Heselmeyer-Haddad, K.; Lou, H.; Boland, J.F.; Yeager, M.; Orozco, R.; Freites, E.A.; et al. Extrachromosomal Amplification of Human Papillomavirus Episomes Is a Mechanism of Cervical Carcinogenesis. Cancer Res. 2023, 83, 1768–1781. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M. Epidemiological Trends in the Characterization of of Human Papillomavirus Virus-Related Oropharyngeal Cancer: A Global Perspective. Curr. Oncol. Rep. 2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M. HPV-Mediated Radiosensitivity in Oropharyngeal Squamous Cell Carcinoma: Molecular Mechanisms and Cellular Pathways. Curr. Oncol. Rep. 2025, 27, 634–641. [Google Scholar] [CrossRef]
- Kadaja, M.; Isok-Paas, H.; Laos, T.; Ustav, E.; Ustav, M. Mechanism of Genomic Instability in Cells Infected with the High-Risk Human Papillomaviruses. PLoS Pathog. 2009, 5, e1000397. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Hong, P.; Huang, X.; Lin, D.; Cao, G.; Wang, L.; Feng, B.; Wu, P.; Shen, H.; Xu, Q.; et al. HPV-CCDC106 Integration Alters Local Chromosome Architecture and Hijacks an Enhancer by Three-Dimensional Genome Structure Remodeling in Cervical Cancer. J. Genet. Genom. 2020, 47, 437–450. [Google Scholar] [CrossRef]
- Duensing, S.; Münger, K. The Human Papillomavirus Type 16 E6 and E7 Oncoproteins Independently Induce Numerical and Structural Chromosome Instability. Cancer Res. 2002, 62, 7075–7082. [Google Scholar] [PubMed]
- Zhao, J.-W.; Fang, F.; Guo, Y.; Zhu, T.-L.; Yu, Y.-Y.; Kong, F.-F.; Han, L.-F.; Chen, D.-S.; Li, F. HPV16 Integration Probably Contributes to Cervical Oncogenesis through Interrupting Tumor Suppressor Genes and Inducing Chromosome Instability. J. Exp. Clin. Cancer Res. 2016, 35, 180. [Google Scholar] [CrossRef]
- Zapatka, M.; Borozan, I.; Brewer, D.S.; Iskar, M.; Grundhoff, A.; Alawi, M.; Desai, N.; Sültmann, H.; Moch, H.; PCAWG Pathogens; et al. The Landscape of Viral Associations in Human Cancers. Nat. Genet. 2020, 52, 320–330, Erratum in Nat. Genet. 2023, 55, 1077. [Google Scholar] [CrossRef]
- Duensing, S.; Lee, L.Y.; Duensing, A.; Basile, J.; Piboonniyom, S.; Gonzalez, S.; Crum, C.P.; Munger, K. The Human Papillomavirus Type 16 E6 and E7 Oncoproteins Cooperate to Induce Mitotic Defects and Genomic Instability by Uncoupling Centrosome Duplication from the Cell Division Cycle. Proc. Natl. Acad. Sci. USA 2000, 97, 10002–10007. [Google Scholar] [CrossRef]
- Duensing, S.; Münger, K. Human Papillomaviruses and Centrosome Duplication Errors: Modeling the Origins of Genomic Instability. Oncogene 2002, 21, 6241–6248. [Google Scholar] [CrossRef]
- Rodriguez, I.; Rossi, N.M.; Keskus, A.; Xie, Y.; Ahmad, T.; Bryant, A.; Lou, H.; Paredes, J.G.; Milano, R.; Rao, N.; et al. Insights into the Mechanisms and Structure of Breakage-Fusion-Bridge Cycles in Cervical Cancer Using Long-Read Sequencing. Am. J. Hum. Genet. 2024, 111, 544–561. [Google Scholar] [CrossRef]
- Akagi, K.; Symer, D.E.; Mahmoud, M.; Jiang, B.; Goodwin, S.; Wangsa, D.; Li, Z.; Xiao, W.; Dunn, J.D.; Ried, T.; et al. Intratumoral Heterogeneity and Clonal Evolution Induced by HPV Integration. Cancer Discov. 2023, 13, 910–927. [Google Scholar] [CrossRef]
- Yu, L.; Lobanov, A.; Majerciak, V.; Mirza, S.; Band, V.; Liu, H.; Cam, M.; Zheng, Z.-M. Expression of HPV Oncogenes from a Single Integration Site in Cervical Cancer Cell Lines. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yu, L.; Majerciak, V.; Lobanov, A.; Mirza, S.; Band, V.; Liu, H.; Cam, M.; Hughes, S.H.; Lowy, D.R.; Zheng, Z.-M. HPV Oncogenes Expressed from Only One of Multiple Integrated HPV DNA Copies Drive Clonal Cell Expansion in Cervical Cancer. mBio 2024, 15, e00729-24. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, F.; Häfner, N.; Driesch, C.; Kraus Christiansen, I.; Beer, K.; Schmitz, M.; Runnebaum, I.B.; Dürst, M. Differences in Stability of Viral and Viral-Cellular Fusion Transcripts in HPV-Induced Cervical Cancers. Int. J. Mol. Sci. 2019, 21, 112. [Google Scholar] [CrossRef]
- Warburton, A.; Redmond, C.J.; Dooley, K.E.; Fu, H.; Gillison, M.L.; Akagi, K.; Symer, D.E.; Aladjem, M.I.; McBride, A.A. HPV Integration Hijacks and Multimerizes a Cellular Enhancer to Generate a Viral-Cellular Super-Enhancer That Drives High Viral Oncogene Expression. PLoS Genet. 2018, 14, e1007179. [Google Scholar] [CrossRef]
- Tian, R.; Huang, Z.; Li, L.; Yuan, J.; Zhang, Q.; Meng, L.; Lang, B.; Hong, Y.; Zhong, C.; Tian, X.; et al. HPV Integration Generates a Cellular Super-Enhancer Which Functions as ecDNA to Regulate Genome-Wide Transcription. Nucleic Acids Res. 2023, 51, 4237–4251. [Google Scholar] [CrossRef] [PubMed]
- Yanatatsaneejit, P.; Mutirangura, A.; Kitkumthorn, N. Human Papillomavirus’s Physical State and Cyclin A1 Promoter Methylation in Cervical Cancer. Int. J. Gynecol. Cancer 2011, 21, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Porter, V.L.; Ng, M.; O’Neill, K.; MacLennan, S.; Corbett, R.D.; Culibrk, L.; Hamadeh, Z.; Iden, M.; Schmidt, R.; Tsaih, S.-W.; et al. Rearrangements of Viral and Human Genomes at Human Papillomavirus Integration Events and Their Allele-Specific Impacts on Cancer Genome Regulation. Genome Res. 2025, 35, 653–670. [Google Scholar] [CrossRef]
- Sen, S.; Mandal, P.; Bhattacharya, A.; Kundu, S.; Roy Chowdhury, R.; Mondal, N.R.; Chatterjee, T.; Chakravarty, B.; Roy, S.; Sengupta, S. Impact of Viral and Host DNA Methylations on HPV16-Related Cervical Cancer Pathogenesis. Tumor Biol. 2017, 39, 101042831769979. [Google Scholar] [CrossRef]
- Chaiwongkot, A.; Vinokurova, S.; Pientong, C.; Ekalaksananan, T.; Kongyingyoes, B.; Kleebkaow, P.; Chumworathayi, B.; Patarapadungkit, N.; Reuschenbach, M.; von Knebel Doeberitz, M. Differential Methylation of E2 Binding Sites in Episomal and Integrated HPV 16 Genomes in Preinvasive and Invasive Cervical Lesions. Int. J. Cancer 2013, 132, 2087–2094. [Google Scholar] [CrossRef]
- Leung, T.-W.; Liu, S.S.; Leung, R.C.Y.; Chu, M.M.Y.; Cheung, A.N.Y.; Ngan, H.Y.S. HPV 16 E2 Binding Sites 1 and 2 Become More Methylated than E2 Binding Site 4 during Cervical Carcinogenesis. J. Med. Virol. 2015, 87, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, E.; Baraquin, A.; Ramanah, R.; Carcopino, X.; Morel, A.; Valmary-Degano, S.; Bravo, I.G.; de Sanjosé, S.; Riethmuller, D.; Mougin, C.; et al. Methylation of Human Papillomavirus Type 16 CpG Sites at E2-Binding Site 1 (E2BS1), E2BS2, and the Sp1-Binding Site in Cervical Cancer Samples as Determined by High-Resolution Melting Analysis-PCR. J. Clin. Microbiol. 2013, 51, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhi, Y.; Li, Y.; Fan, T.; Li, H.; Du, P.; Cheng, G.; Li, X. Study on the Relationship between Methylation Status of HPV 16 E2 Binding Sites and Cervical Lesions. Clin. Chim. Acta 2019, 493, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, Y.; Liu, B.; Rao, X.; Cao, C.; Peng, F.; Zhi, W.; Wu, P.; Peng, T.; Wei, Y.; et al. Multi-Omics Data Reveals Novel Impacts of Human Papillomavirus Integration on the Epigenomic and Transcriptomic Signatures of Cervical Tumorigenesis. J. Med. Virol. 2023, 95, e28789. [Google Scholar] [CrossRef]
- Sen, P.; Ganguly, P.; Ganguly, N. Modulation of DNA Methylation by Human Papillomavirus E6 and E7 Oncoproteins in Cervical Cancer. Oncol. Lett. 2018, 15, 11–22. [Google Scholar] [CrossRef]
- Xu, J.; Liu, H.; Yang, Y.; Wang, X.; Liu, P.; Li, Y.; Meyers, C.; Banerjee, N.S.; Wang, H.-K.; Cam, M.; et al. Genome-Wide Profiling of Cervical RNA-Binding Proteins Identifies Human Papillomavirus Regulation of RNASEH2A Expression by Viral E7 and E2F1. Mbio 2019, 10, e02687-18. [Google Scholar] [CrossRef]
- Rusan, M.; Li, Y.Y.; Hammerman, P.S. Genomic Landscape of Human Papillomavirus-Associated Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 2009–2019. [Google Scholar] [CrossRef]
- Cao, C.-H.; Liu, R.; Lin, X.-R.; Luo, J.-Q.; Cao, L.-J.; Zhang, Q.-J.; Lin, S.-R.; Geng, L.; Sun, Z.-Y.; Ye, S.-K.; et al. LRP1B Mutation Is Associated with Tumor HPV Status and Promotes Poor Disease Outcomes with a Higher Mutation Count in HPV-Related Cervical Carcinoma and Head & Neck Squamous Cell Carcinoma. Int. J. Biol. Sci. 2021, 17, 1744–1756. [Google Scholar] [CrossRef]
- Bodelon, C.; Vinokurova, S.; Sampson, J.N.; den Boon, J.A.; Walker, J.L.; Horswill, M.A.; Korthauer, K.; Schiffman, M.; Sherman, M.E.; Zuna, R.E.; et al. Chromosomal Copy Number Alterations and HPV Integration in Cervical Precancer and Invasive Cancer. Carcinogenesis 2016, 37, 188–196. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The Role of the PI3K/Akt/mTOR Signalling Pathway in Human Cancers Induced by Infection with Human Papillomaviruses. Mol. Cancer 2015, 14, 87. [Google Scholar] [CrossRef]
- Wilting, S.M.; Snijders, P.J.F.; Meijer, G.A.; Ylstra, B.; van den Ijssel, P.R.L.A.; Snijders, A.M.; Albertson, D.G.; Coffa, J.; Schouten, J.P.; van de Wiel, M.A.; et al. Increased Gene Copy Numbers at Chromosome 20q Are Frequent in Both Squamous Cell Carcinomas and Adenocarcinomas of the Cervix. J. Pathol. 2006, 209, 220–230. [Google Scholar] [CrossRef]
- Thomas, L.K.; Bermejo, J.L.; Vinokurova, S.; Jensen, K.; Bierkens, M.; Steenbergen, R.; Bergmann, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Chromosomal Gains and Losses in Human Papillomavirus-Associated Neoplasia of the Lower Genital Tract —A Systematic Review and Meta-Analysis. Eur. J. Cancer 2014, 50, 85–98. [Google Scholar] [CrossRef]
- Guo, C.; Qu, X.; Tang, X.; Song, Y.; Wang, J.; Hua, K.; Qiu, J. Spatiotemporally Deciphering the Mysterious Mechanism of Persistent HPV-Induced Malignant Transition and Immune Remodelling from HPV-Infected Normal Cervix, Precancer to Cervical Cancer: Integrating Single-Cell RNA-Sequencing and Spatial Transcriptome. Clin. Transl. Med. 2023, 13, e1219. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, B. The Role of Tumor-Associated Macrophages in HPV Induced Cervical Cancer. Front. Immunol. 2025, 16, 1586806. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Cheng, Y.; Yan, J.; Ren, F.; Jia, Y.; Li, J.; Wang, B.; Liu, J.; Wang, C.; et al. Single-Cell Transcriptomic Analyses Reveal Heterogeneity and Key Subsets Associated with Survival and Response to PD-1 Blockade in Cervical Squamous Cell Carcinoma. Cancer Cell Int. 2025, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Beglin, M.; Melar-New, M.; Laimins, L. Human Papillomaviruses and the Interferon Response. J. Interferon Cytokine Res. 2009, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.; Caux, C.; Hasan, U.; Bendriss-Vermare, N.; Olive, D. Impaired Toll-like Receptor 7 and 9 Signaling: From Chronic Viral Infections to Cancer. Trends Immunol. 2010, 31, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Smola, S.; Trimble, C.; Stern, P.L. Human Papillomavirus-Driven Immune Deviation: Challenge and Novel Opportunity for Immunotherapy. Ther. Adv. Vaccines 2017, 5, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, G.; Leventakou, D.; Saltiel, D.-R.; Zervoudi, E.; Logotheti, E.; Pettas, S.; Karagianni, K.; Daiou, A.; Hatzistergos, K.E.; Dafou, D.; et al. HPV16 E6 Oncogene Contributes to Cancer Immune Evasion by Regulating PD-L1 Expression through a miR-143/HIF-1a Pathway. Viruses 2024, 16, 113. [Google Scholar] [CrossRef]
- Sheng, B.; Pan, S.; Ye, M.; Liu, H.; Zhang, J.; Zhao, B.; Ji, H.; Zhu, X. Single-Cell RNA Sequencing of Cervical Exfoliated Cells Reveals Potential Biomarkers and Cellular Pathogenesis in Cervical Carcinogenesis. Cell Death Dis. 2024, 15, 130. [Google Scholar] [CrossRef]
- Tian, X.; Weng, D.; Chen, Y.; Wang, Y.; Li, X.; Wang, X.; Cao, C.; Gong, D.; Zeng, Z.; Wu, Q.; et al. Risk Assessment and Triage Strategy of Cervical Cancer Primary Screening on HPV Integration Status: 5-Year Follow-up of a Prospective Cohort Study. J. Natl. Cancer Cent. 2024, 4, 311. [Google Scholar] [CrossRef]
- Kaur, S.D.; Chellappan, D.K.; Aljabali, A.A.; Tambuwala, M.; Dua, K.; Kapoor, D.N. Recent Advances in Cancer Therapy Using PARP Inhibitors. Med. Oncol. 2022, 39, 241. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, N.; Jung, H.S.; Bae, S.H.; Chelakkot, C.; Hong, S.; Choi, J.-S.; Yim, D.-S.; Oh, Y.-K.; Choi, Y.-L.; Shin, Y.K. Effect of HPV E6/E7 siRNA with Chemotherapeutic Agents on the Regulation of TP53/E2F Dynamic Behavior for Cell Fate Decisions. Neoplasia 2017, 19, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, Z.; Ma, X. Description of CRISPR-Cas9 Development and Its Prospects in Human Papillomavirus-Driven Cancer Treatment. Front. Immunol. 2022, 13, 1037124. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Wang, M.; Liu, J.; Zhou, X.; Liang, Z.; Zhang, Q.; Long, S.; Quzhen, S.; Li, X.; et al. Human Papillomavirus Integration Perspective in Small Cell Cervical Carcinoma. Nat. Commun. 2022, 13, 5968. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).