Prevalence and Molecular Characterization of Chronic and Occult Hepatitis B Virus Infection Among Pregnant Women in St. Petersburg, Russia
Abstract
1. Introduction
2. Results
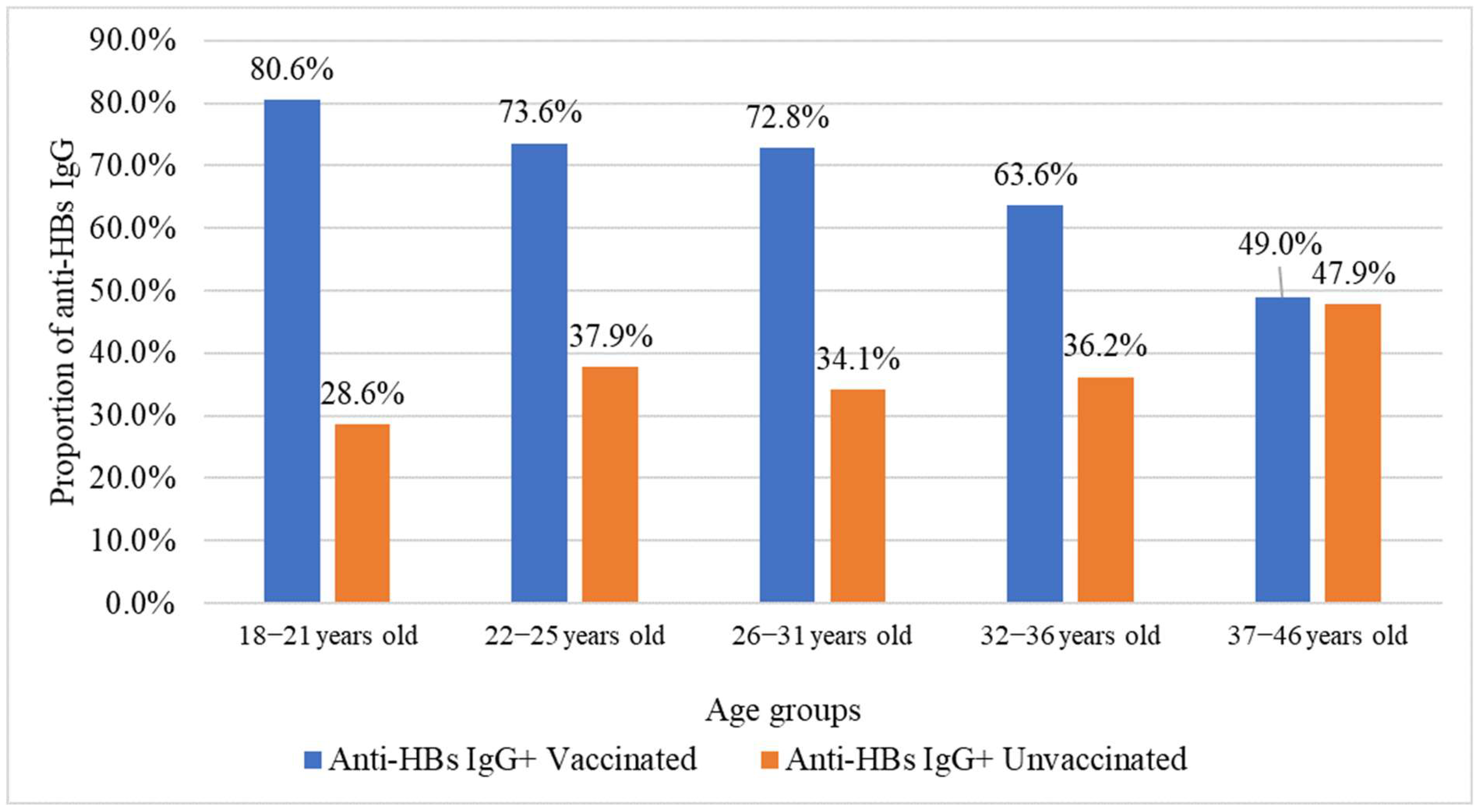
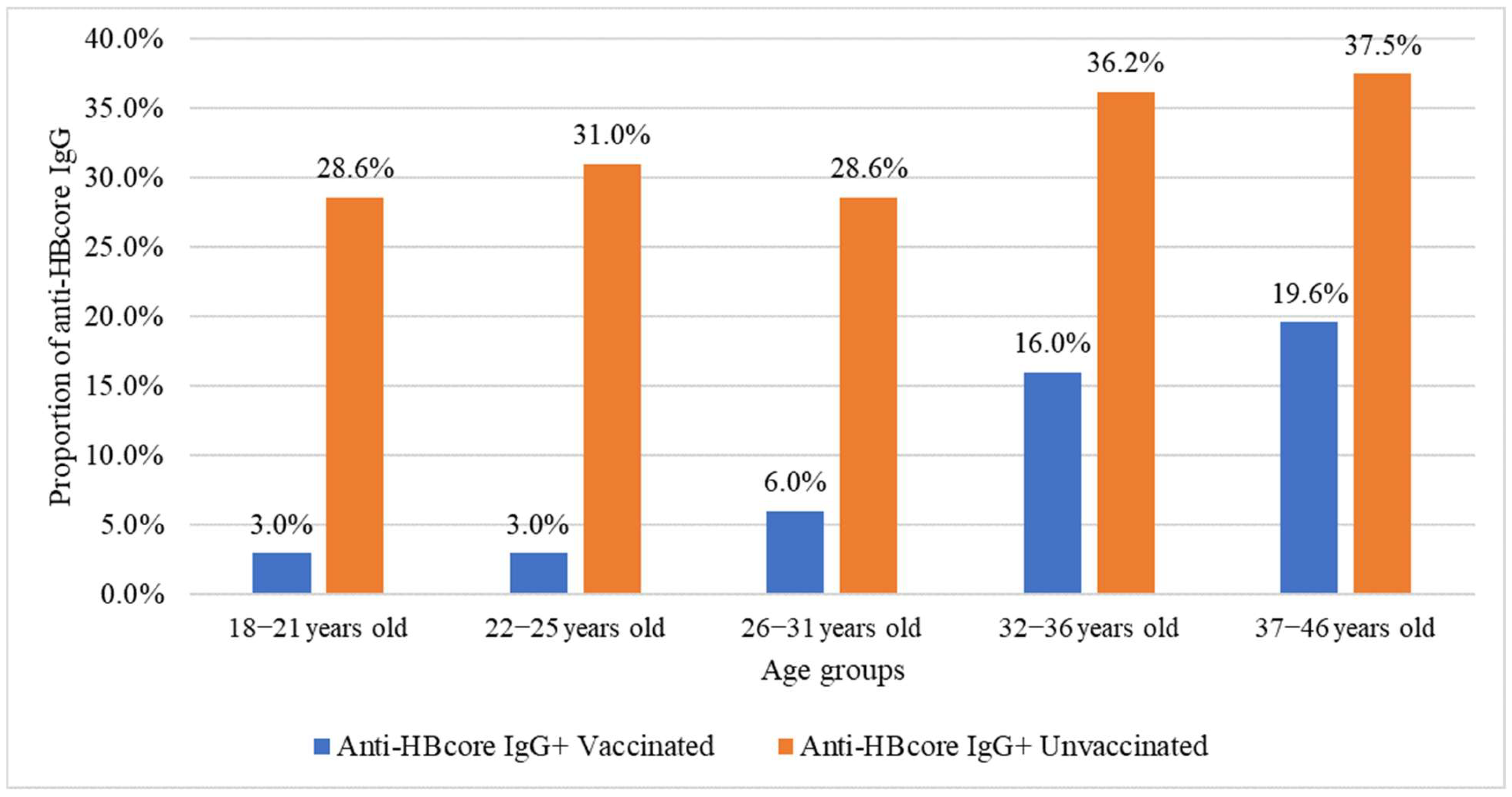
2.1. Hepatitis B Vaccination Status
2.2. Prevalence and Distribution of HBV Serological Markers in the Study Group
2.3. Prevalence of HBV DNA in the Study Group
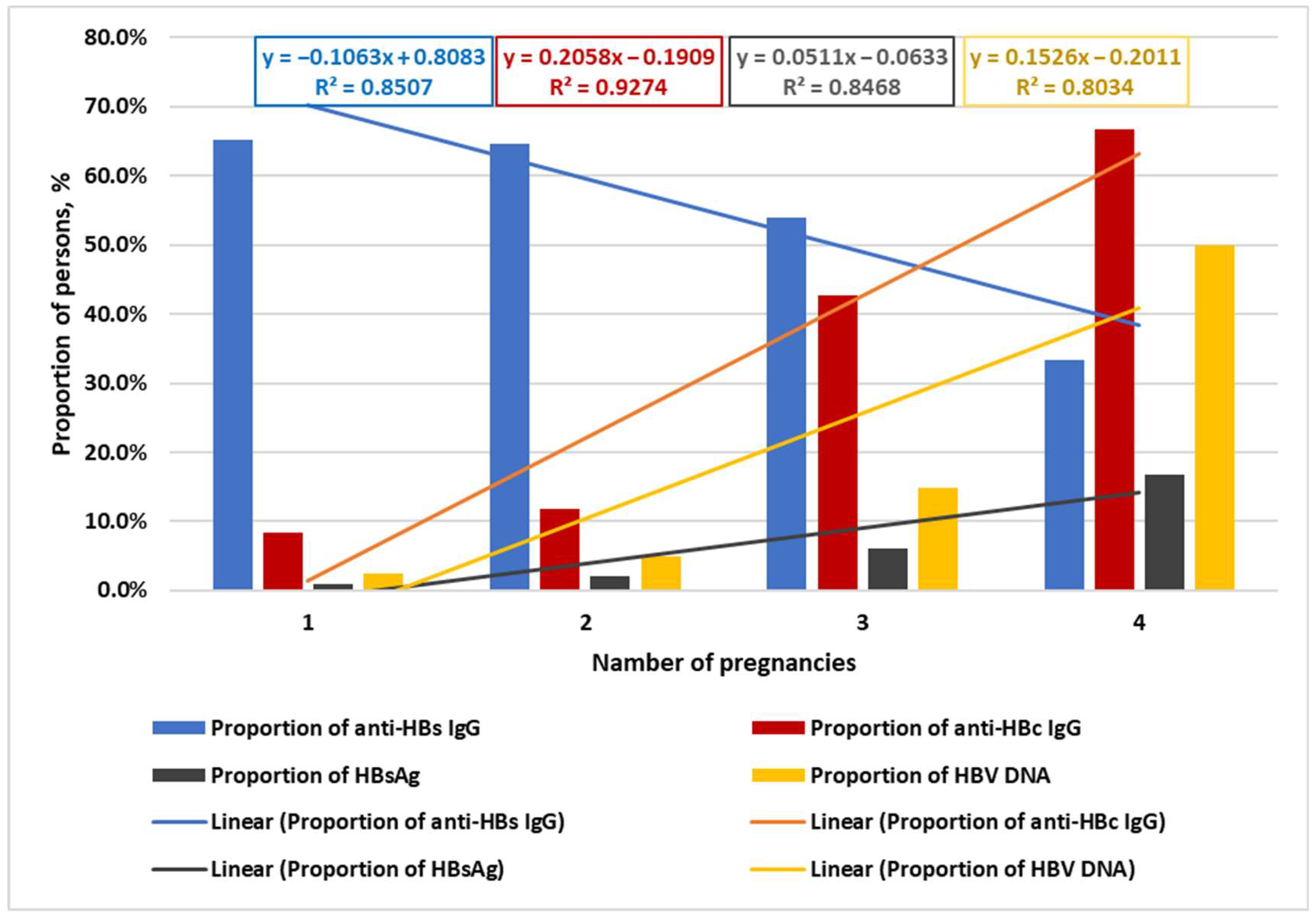
2.4. Association Between HBV Vaccination, Age, Number of Pregnancies, and Analyzed Markers
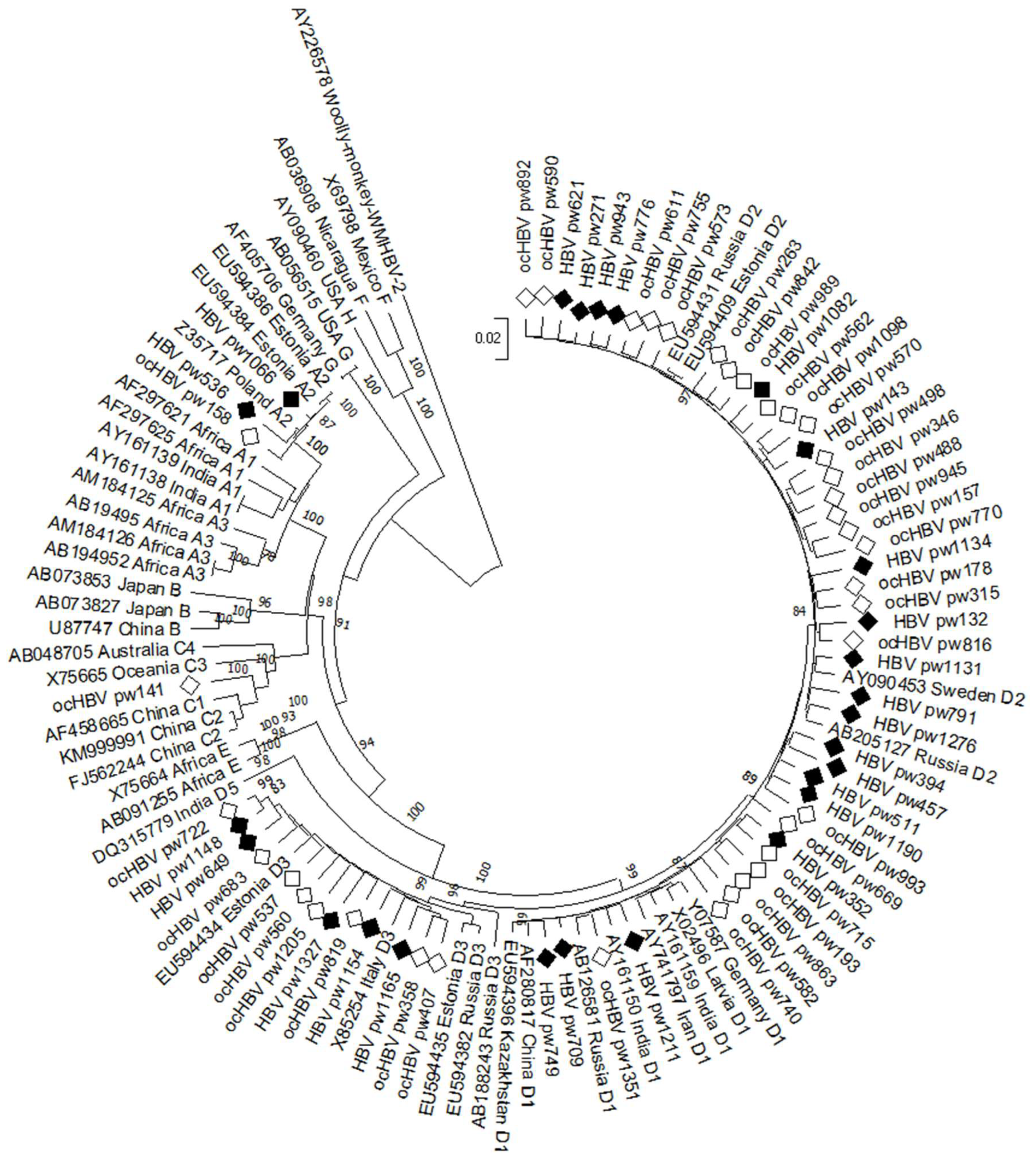
2.5. HBV Genotyping
2.6. Identification of Clinically Significant HBV Mutations
2.7. Analysis of RT Region Mutations
2.8. Analysis of Mutations in the MHR of the HBV Genome
2.9. Analysis of Mutations in the Precore/Core Region of the HBV Genome
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Sample Transportation and Storage
4.2.2. Enzyme-Linked Immunosorbent Assay
4.2.3. Nucleic Acid Extraction
4.2.4. Polymerase Chain Reaction and Sequencing
4.2.5. Genotype and Mutation Analysis
4.2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Regional Strategic Framework for Vaccine-Preventable Diseases and Immunization in the Western Pacific 2021–2030. 2022. Available online: https://www.who.int/publications/i/item/9789290619697 (accessed on 15 March 2025).
- Shimakawa, Y.; Lemoine, M.; Njai, H.F.; Bottomley, C.; Ndow, G.; Goldin, R.D.; Jatta, A.; Jeng-Barry, A.; Wegmuller, R.; Moore, S.E.; et al. NAatural history of chronic HBV infection in West Africa: A longitudinal population-based study from the Gambia. Gut 2016, 65, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Cheng, P.N.; Kao, J.H. Systematic review: Chronic viral hepatitis and metabolic derangement. Aliment. Pharmacol. Ther. 2020, 51, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Keane, E.; Funk, A.L.; Shimakawa, Y. Systematic review with meta-analysis: The risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Aliment. Pharmacol. Ther. 2016, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Fu, S.; Wu, Y.; Tian, Z.; Feng, Y.; Li, J.; Luo, X.; Yang, Y.; Ji, F.; Chen, Y.; et al. Incidence of mother-to-child transmission of hepatitis B in relation to maternal peripartum antiviral prophylaxis: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2022, 101, 1197–1206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMahon, B.J.; Alward, W.L.; Hall, D.B.; Heyward, W.L.; Bender, T.R.; Francis, D.P.; Maynard, J.E. Acute hepatitis B virus infection: Relation of age to the clinical expression of disease and subsequent development of the carrier state. J. Infect. Dis. 1985, 151, 599–603. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Hepatitis B Key Facts. Available online: http://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 20 April 2025).
- Candotti, D.; Laperche, S. Hepatitis B Virus Blood Screening: Need for Reappraisal of Blood Safety Measures? Front. Med. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Saitta, C.; Pollicino, T.; Raimondo, G. Occult Hepatitis B Virus Infection: An Update. Viruses 2022, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostankova, Y.V.; Shchemelev, A.N.; Boumbaly, S.; Balde, T.A.L.; Zueva, E.B.; Valutite, D.E.; Serikova, E.N.; Davydenko, V.S.; Skvoroda, V.V.; Vasileva, D.A.; et al. Prevalence of HIV and Viral Hepatitis Markers among Healthcare Workers in the Republic of Guinea. Diagnostics 2023, 13, 378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boumbaly, S.; Balde, T.A.L.; Semenov, A.V.; Ostankova, Y.V.; Serikova, E.N.; Naidenova, E.V.; Valutite, D.E.; Shchemelev, A.N.; Zueva, E.B.; Esaulenko, E.V.; et al. Prevalence of viral hepatitis B markers among blood donors in the Republic of Guinea. Vopr. Virusol. 2022, 67, 59–68. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Ji, D.Z.; Pang, X.Y.; Shen, D.T.; Liu, S.N.; Goyal, H.; Xu, H.G. Global prevalence of occult hepatitis B: A systematic review and meta-analysis. J. Viral Hepat. 2022, 29, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Franzè, M.S.; Pollicino, T.; Raimondo, G.; Squadrito, G. Occult hepatitis B virus infection in hepatitis C virus negative chronic liver diseases. Liver Int. 2022, 42, 963–972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, Y.; Wu, Y.H.; Wu, W.; Zhang, W.J.; Yang, J.; Chen, Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: A meta-analysis. Liver Int. 2012, 32, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Haidich, A.B.; Apostolidou-Kiouti, F.; Chalevas, P.; Papatheodoridis, G.V. Hepatitis B virus reactivation in HBsAg-negative, anti-HBc-positive patients receiving immunosuppressive therapy: A systematic review. Ann. Gastroenterol. 2018, 31, 480–490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seto, W.K.; Chan, T.S.; Hwang, Y.Y.; Wong, D.K.; Fung, J.; Liu, K.S.; Gill, H.; Lam, Y.F.; Lau, E.H.Y.; Cheung, K.S.; et al. Hepatitis B reactivation in occult viral carriers undergoing hematopoietic stem cell transplantation: A prospective study. Hepatology 2017, 65, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Kramvis, A. Molecular characteristics and clinical relevance of African genotypes and subgenotypes of hepatitis B virus. S. Afr. Med. J. 2018, 108, 17–21. Available online: https://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S0256-95742018000900008 (accessed on 1 September 2025). [PubMed]
- Stasi, C.; Silvestri, C.; Voller, F. Emerging Trends in Epidemiology of Hepatitis B Virus Infection. J. Clin. Transl. Hepatol. 2017, 5, 272–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Ostankova, Y.V.; Semenov, A.V.; Zueva, E.B.; Totolian, A.A. The first cases of hepatitis B virus subgenotype D4 detection in patients with chronic, acute, and occult hepatitis B in the Russian Federation. Mol. Genet. Microbiol. Virol. 2020, 35, 221–228. [Google Scholar] [CrossRef]
- Mokaya, J.; McNaughton, A.L.; Hadley, M.J.; Beloukas, A.; Geretti, A.M.; Goedhals, D.; Matthews, P.C. A systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa: A call for urgent action. PLoS Neglected Trop. Dis. 2018, 12, e0006629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shoraka, S.; Hosseinian, S.M.; Hasibi, A.; Ghaemi, A.; Mohebbi, S.R. The role of hepatitis B virus genome variations in HBV-related HCC: Effects on host signaling pathways. Front. Microbiol. 2023, 14, 1213145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phan, N.M.H.; Faddy, H.M.; Flower, R.L.; Dimech, W.J.; Spann, K.M.; Roulis, E.V. Low Genetic Diversity of Hepatitis B Virus Surface Gene amongst Australian Blood Donors. Viruses 2021, 13, 1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.H.; Wu, C.C.; Chen, X.W.; Li, X.; Li, J.; Lu, M.J. Genetic variation of hepatitis B virus and its significance for pathogenesis. World J. Gastroenterol. 2016, 22, 126–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hossain, M.G.; Ueda, K. Investigation of a Novel Hepatitis B Virus Surface Antigen (HBsAg) Escape Mutant Affecting Immunogenicity. PLoS ONE 2017, 12, e0167871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yll, M.; Cortese, M.F.; Guerrero-Murillo, M.; Orriols, G.; Gregori, J.; Casillas, R.; González, C.; Sopena, S.; Godoy, C.; Vila, M.; et al. Conservation and variability of hepatitis B core at different chronic hepatitis stages. World J. Gastroenterol. 2020, 26, 2584–2598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quarleri, J. Core promoter: A critical region where the hepatitis B virus makes decisions. World J. Gastroenterol. 2014, 20, 425–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.L.; Ren, J.P.; Wang, X.Q.; Wang, X.H.; Yang, S.F.; Xiong, Y. Mutations in pre-core and basic core promoter regions of hepatitis B virus in chronic hepatitis B patients. World J. Gastroenterol. 2016, 22, 3268–3274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Y.; Zhang, L.; Dai, Y.; Hu, Y.; Xu, B.; Zhou, Y.H. Conservative Evolution of Hepatitis B Virus Precore and Core Gene During Immune Tolerant Phase in Intrafamilial Transmission. Virol. Sin. 2020, 35, 388–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mixson-Hayden, T.; Lee, D.; Ganova-Raeva, L.; Drobeniuc, J.; Stauffer, W.M.; Teshale, E.; Kamili, S. Hepatitis B virus and hepatitis C virus infections in United States-bound refugees from Asia and Africa. Am. J. Trop. Med. Hyg. 2014, 90, 1014–1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akinbodewa, A.A.; Gbadegesin, B.A.; Adejumo, O.A.; Ahmed, S.D.; Uwameiye, O.; Dada, S.A.; Okunola, O.; Osho, P.O. A Multicentre Study of Awareness and Practice of Vaccination Against Infectious Diseases Among Haemo-Dialysis Subjects in Nigeria. W. Afr. J. Med. 2019, 36, 239–245. [Google Scholar] [PubMed]
- Boucheron, P.; Lu, Y.; Yoshida, K.; Zhao, T.; Funk, A.L.; Lunel-Fabiani, F.; Guingané, A.; Tuaillon, E.; van Holten, J.; Chou, R.; et al. Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bian, Q.; Zhu, Y.; Pang, Q.; Chang, L.; Li, R.; Tiongson, B.C.; Zhang, H.; Pan, C.Q. Real-world study of tenofovir disoproxil fumarate to prevent hepatitis B transmission in mothers with high viral load. Aliment. Pharmacol. Ther. 2019, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.P.; Chalid, M.T.; Rasyak, M.R.; El Khobar, K.E.; Turyadi; Sjahril, R.; Wahyuni, R.; Setiady, Y.; Muljono, D.H. Characteristics of hepatitis B virus surface protein and occult hepatitis B infection in infants with immunoprophylaxis failure from Indonesia. Vaccine 2025, 56, 127130. [Google Scholar] [CrossRef] [PubMed]
- Delghandi, S.; Raoufinia, R.; Shahtahmasbi, S.; Meshkat, Z.; Gouklani, H.; Gholoobi, A. An overview of occult hepatitis B infection (OBI) with emphasis on HBV vaccination. Heliyon 2024, 10, e37097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rospotrebnadzor. On the State of Sanitary and Epidemiological Well-Being of the Population in the Russian Federation in 2023: State Report; Federal Service for Consumer Rights Protection and Human Welfare: Moscow, Russia, 2024; 364p. [Google Scholar]
- Order of the Ministry of Health of the Russian Federation No. 1122n of December 6, 2021 “On the Approval of the National Calendar of Preventive Vaccinations, the Calendar of Preventive Vaccinations for Epidemic Indications, and the Procedure for Conducting Preventive Vaccinations”. Available online: https://ivo.garant.ru/#/document/403258640/paragraph/1:0 (accessed on 1 September 2025).
- Rospotrebnadzor. On the State of Sanitary and Epidemiological Well-Being of the Population in the Russian Federation in 2024: State Report; Federal Service for Consumer Rights Protection and Human Welfare: Moscow, Russia, 2025; 424p. [Google Scholar]
- Shulakova, N.I. Epidemiological and Immunological Effectiveness of Mass Vaccination of the Population of Russia Against Hepatitis B: Abstract of the Dissertation for the Degree of Senior Doctor of Medical Sciences. Ph.D. Thesis, Central Research Institute of Epidemiology (CRIE), Moscow, Russia, 2017; 48p. [Google Scholar]
- Semenenko, T.A.; Akimkin, V.G. Seroepidemiology in the surveillance of vaccine-preventable diseases. J. Microbiol. Epidemiol. Immunobiol. 2018, 95, 87–94. [Google Scholar] [CrossRef]
- Ostankova, Y.V.; Serikova, E.N.; Shirshova, N.Y.; Kusevitskaya, M.B.; Gorskaya, O.A.; Basina, V.V.; Mashkov, I.A.; Zueva, E.B.; Schemelev, A.N.; Reingardt, D.E.; et al. Prevalence of occult hepatitis B infection among blood donors in Saint Petersburg. Russ. J. Infect. Immun. 2023, 13, 1129–1140. [Google Scholar] [CrossRef]
- Anufrieva, E.V.; Ostankova, Y.V.; Serikova, E.N.; Shchemelev, A.N.; Davydenko, V.S.; Reingardt, D.E.; Zueva, E.B.; Totolian, A.A. Prevalence of Serological and Molecular-Biological HIV-Infection, HBV and HCV Markers among Medical Workers. Probl. Part. Danger. Infect. 2024, 54–62. (In Russian) [Google Scholar] [CrossRef]
- Anufrieva, E.V.; Serikova, E.N.; Ostankova, Y.V.; Shchemelev, A.N.; Davydenko, V.S.; Reingardt, D.E.; Zueva, E.B.; Totolian, A.A. The structure of some blood-borne infections distribution among persons from penitentiary institutions the markers. HIV Infect. Immunosuppr. Disord. 2023, 15, 95–104. (In Russian) [Google Scholar] [CrossRef]
- Akimkina, V.G.; Totoliana, A.A. (Eds.) Viral Hepatitis in the Russian Federation; Saint-Petersburg Pasteur Institute: Saint Petersburg, Russia, 2024; 196p. [Google Scholar]
- Komarova, A.L.; Smirnova, N.I. Prevalence of HBsAg among pregnant women in a region of moderate endemicity. Infect. Dis. 2021, 2, 42–47. [Google Scholar]
- Ganina, A.A.; Kyuregyan, K.K.; Mikhaylov, M.I. Detection rate of hepatitis among pregnant women screened for TORCH-infections. Meditsinskiy Alfavit 2016, 3, 41. [Google Scholar]
- Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Kartashov, M.Y.; Svirin, K.A.; Krivosheina, E.I.; Chub, E.; Ternovoi, V.A.; Kochneva, G.V. Evaluation of the intensity of post-vaccination immunity to HBV among HIVinfected and conditionally healthy persons in Western Siberia. Epidemiol. Vaccinal Prev. 2023, 22, 139–147. (In Russian) [Google Scholar] [CrossRef]
- Tursunbaeva, M.S. HBV infection and pregnancy: Current aspects of epidemiology, clinical presentation, testing, and prevention of vertical transmission. Med. Educ. Bull. 2025, 1, 129–140. Available online: https://www.elibrary.ru/item.asp?id=82366319 (accessed on 11 September 2025).
- Shilova, I.V.; Ostankova, Y.V.; Goryacheva, L.G.; Semenov, A.V. HBV—Infection in children with perinatal infection. Clinical report of familial hepatitis B. J. Infectol. 2021, 13, 120–124. (In Russian) [Google Scholar] [CrossRef]
- Bai, G.Q.; Li, S.H.; Yue, Y.F.; Shi, L. The study on role of peripheral blood mononuclear cell in HBV intrauterine infection. Arch. Gynecol. Obstet. 2011, 283, 317–321. [Google Scholar] [CrossRef]
- CDC. Hepatitis B Questions and Answers for Health Professionals//Updated July 2023. Available online: https://www.cdc.gov/hepatitis-b/hcp/clinical-overview (accessed on 1 May 2025).
- Tian, Q.; Jia, J. Hepatitis B virus genotypes: Epidemiological and clinical relevance in Asia. Hepatol. Int. 2016, 10, 854–860. [Google Scholar] [CrossRef]
- Tallo, T.; Tefanova, V.; Priimägi, L.; Schmidt, J.; Katargina, O.; Michailov, M.; Mukomolov, S.; Magnius, L.; Norder, H. D2: Major subgenotype of hepatitis B virus in Russia and the Baltic region. J. Gen. Virol. 2008, 89 Pt 8, 1829–1839. [Google Scholar] [CrossRef]
- Ostankova, Y.V.; Semenov, A.V.; Zueva, E.B.; Gabdrakhmanov, I.A.; Kozlov, K.V.; Zhdanov, K.V.; Totolian, A.A. Variety of the hepatitis B virus genovariants in the military. J. Infectology 2019, 11, 46–53. (In Russian) [Google Scholar] [CrossRef]
- Semenov, A.V.; Ostankova, Y.V.; Serikova, E.N.; Zueva, E.B.; Totolian, A.A. Optimization of the algorithm diagnosis chronic hepatitis B markers in patients with newly diagnosed HIV infection. Klin. Lab. Diagn. 2020, 65, 574–579. (In English) [Google Scholar] [CrossRef]
- Serikova, E.N.; Ostankova, Y.V.; Anufrieva, E.V.; Reingardt, D.E.; Schemelev, A.N.; Zueva, E.B.; Ivanova, A.R.; Semenov, A.V.; Totolian, A.A. Prevalence of parenteral viral hepatitis B and C markers among migrants in the North-West Federal District. HIV Infect. Immunosuppr. Disord. 2024, 16, 94–106. (In Russian) [Google Scholar] [CrossRef]
- Bottecchia, M.; Madejón, A.; Sheldon, J.; García-Samaniego, J.; Barreiro, P.; Soriano, V. Hepatitis B virus genotype A2 harbours an L217R polymorphism which may account for a lower response to adefovir. J. Antimicrob. Chemother. 2008, 62, 626–627. [Google Scholar] [CrossRef]
- Baxter, C.; Ngcapu, S.; Blackard, J.T.; Powell, E.A.; Penton, P.K.; Abdool Karim, S.S. Frequency of Hepatitis B Virus Resistance Mutations in Women Using Tenofovir Gel as Pre-Exposure Prophylaxis. Viruses 2019, 11, 569. [Google Scholar] [CrossRef]
- Chung, H.J.; Chen, X.; Yu, Y.; Lee, H.K.; Song, C.H.; Choe, H.; Lee, S.; Kim, H.J.; Hong, S.T. A critical role of hepatitis B virus polymerase in cirrhosis, hepatocellular carcinoma, and steatosis. FEBS openbio 2017, 8, 130–145. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, L.; Yan, Y.; Wang, L. Paradoxical HBsAg and anti-HBs coexistence among Chronic HBV Infections: Causes and Consequences. Int. J. Biol. Sci. 2021, 17, 1125–1137. [Google Scholar] [CrossRef]
- Lazarevic, I.; Banko, A.; Miljanovic, D.; Cupic, M. Immune-Escape Hepatitis B Virus Mutations Associated with Viral Reactivation upon Immunosuppression. Viruses 2019, 11, 778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Mokhtar, M.A.; Hetta, H.F.; Mekky, M.A.; Abd El-Kareem, D.M.; Ramadan, M.; Salah, M.; Mohamed, N.A.; El-Masry, E.A.; Adel, S.; Sayed, I.M. Characterization of Antigen Escape Mutations in Chronic HBV-Infected Patients in Upper Egypt. Infect. Drug Resist. 2021, 14, 2419–2427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piermatteo, L.; D’Anna, S.; Bertoli, A.; Bellocchi, M.; Carioti, L.; Fabeni, L.; Alkhatib, M.; Frazia, S.; Lichtner, M.; Mastroianni, C.; et al. Unexpected rise in the circulation of complex HBV variants enriched of HBsAg vaccine-escape mutations in HBV genotype-D: Potential impact on HBsAg detection/quantification and vaccination strategies. Emerg. Microbes Infect. 2023, 12, 2219347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Chaar, M.; Candotti, D.; Crowther, R.A.; Allain, J.P. Impact of Hepatitis B Virus Surface Protein Mutations on the Diagnosis of Occult Hepatitis B Virus Infection. Hepatology 2010, 52, 1600–1610. [Google Scholar] [CrossRef]
- Colagrossi, L.; Hermans, L.E.; Salpini, R.; Di Carlo, D.; Pas, S.D.; Alvarez, M.; Ben-Ari, Z.; Boland, G.; Bruzzone, B.; Coppola, N.; et al. Immune-escape mutations and stop-codons in HBsAg develop in a large proportion of patients with chronic HBV infection exposed to anti-HBV drugs in Europe. BMC Infect. Dis. 2018, 18, 251. [Google Scholar] [CrossRef]
- Gencay, M.; Hübner, K.; Gohl, P.; Seffner, A.; Weizenegger, M.; Neofytos, D.; Batrla, R.; Woeste, A.; Kim, H.S.; Westergaard, G.; et al. Ultra-deep sequencing reveals high prevalence and broad structural diversity of hepatitis B surface antigen mutations in a global population. PLoS ONE 2017, 12, e0172101. [Google Scholar] [CrossRef]
- Candotti, D.; Assennato, S.M.; Laperche, S.; Allain, J.P.; Levicnik-Stezinar, S. Multiple HBV transfusion transmissions from undetected occult infections: Revising the minimal infectious dose. Gut 2019, 68, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mabunda, N.; Zicai, A.F.; Ismael, N.; Vubil, A.; Mello, F.; Blackard, J.T.; Lago, B.; Duarte, V.; Moraes, M.; Lewis, L.; et al. Molecular and serological characterization of occult hepatitis B among blood donors in Maputo, Mozambique. Memórias Inst. Oswaldo Cruz 2020, 115, e200006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayashi, S.; Khan, A.; Simons, B.C.; Homan, C.; Matsui, T.; Ogawa, K.; Kawashima, K.; Murakami, S.; Takahashi, S.; Isogawa, M.; et al. An association between core mutations in hepatitis B virus genotype F1b and hepatocellular carcinoma in Alaskan native people. Hepatology 2019, 69, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Al-Anazi, M.R.; Nazir, N.; Abdo, A.A.; Sanai, F.M.; Al-Hamoudi, W.K.; Alswat, K.A.; Al-Ashgar, H.I.; Khan, M.Q.; Albenmousa, A.; et al. The correlation between hepatitis B virus precore/core mutations and the progression of severe liver disease. Front. Cell. Infect. Microbiol. 2018, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Review on hepatitis B virus precore/core promoter mutations and their correlation with genotypes and liver disease severity. World J. Hepatol. 2022, 14, 708–718. [Google Scholar] [CrossRef]
- Liu, C.J.; Chen, B.F.; Chen, P.J.; Lai, M.Y.; Huang, W.L.; Kao, J.H.; Chen, D.S. Role of hepatitis B viral load and basal core promoter mutation in hepatocellular carcinoma in hepatitis B carriers. J. Infect. Dis. 2006, 193, 1258–1265. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Gu, C.; Yin, J.; He, Y.; Xie, J.; Cao, G. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: A meta-analysis. J. Natl. Cancer Inst. 2009, 101, 066–1082. [Google Scholar] [CrossRef]
- Cheng, H.; Su, H.; Wang, S.; Shao, Z.; Men, K.; Li, M.; Li, S.; Zhang, J.; Xu, J.; Zhang, H.; et al. Association between genomic heterogeneity of hepatitis B virus and intrauterine infection. Virology 2009, 387, 168–175. [Google Scholar] [CrossRef]
- Reuter, T.; Gomes-Gouvea, M.S.; Chuffi, S.; Duque, U.H.; Perini, W.; Azevedo, R.S.; Pinho, J.R.R. Core Promoter and Pre-Core Variants of the Hepatitis B Virus (HBV) Are Frequent in Chronic Hepatitis B HBeAg-Negative Patients Infected by Genotypes A and D. Viruses 2023, 15, 2339. [Google Scholar] [CrossRef]
- Sanaei, N.; Hashemi, S.M.A.; Dehno, S.Z.S.; Asl, M.M.; Moini, M.; Malek-Hosseini, S.A.; Hosseini, S.Y.; Sarvari, J. Precore/core mutations of hepatitis B virus genotype D arising in different states of infection. Clin. Exp. Hepatol. 2022, 8, 21–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendenhall, M.A.; Hong, X.; Hu, J. Hepatitis B Virus Capsid: The Core in Productive Entry and Covalently Closed Circular DNA Formation. Viruses 2023, 15, 642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, S.S.; Gao, S.; Castillo, E.; Coffin, C.S. Presence of precore (C)/C promoter mutants in peripheral blood mononuclear cells of chronic hepatitis B (CHB) carriers during pregnancy does not correlate with increased risk of liver disease in 4 years of follow-up. Dig. Dis. Sci. 2019, 65, 204–214. [Google Scholar] [CrossRef]
- Ostankova, Y.V.; Semenov, A.V.; Totolian, A.A. Hepatitis B virus identification in a blood plasma at a low viral load. Klin. Lab. Diagn. 2019, 64, 635–640. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Ostankova, Y.V.; Serikova, E.N.; Semenov, A.V.; Totolian, A.A. Method for hepatitis B virus DNA detecting in biological material at low viral load based on nested PCR with detection on three viral targets in real-time mode. Klin. Lab. Diagn. 2022, 67, 530–537. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gohar, M.; Rehman, I.U.; Ullah, A.; Khan, M.A.; Yasmin, H.; Ahmad, J.; Butt, S.; Ahmad, A. Phylogenetic Analysis and Emerging Drug Resistance against Different Nucleoside Analogues in Hepatitis B Virus Positive Patients. Microorganisms 2023, 11, 2622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanford University HBVseq. Available online: https://hivdb.stanford.edu/HBV/HBVseq/development/HBVseq.html (accessed on 14 December 2024).
- HIV-GRADE, HBV. Available online: https://www.hiv-grade.de/cms/grade/results/hbv (accessed on 16 December 2024).
- Genafor Geno2pheno HBV. Available online: https://hbv.geno2pheno.org/ (accessed on 24 December 2024).
- GraphPad Software Inc. Available online: https://www.graphpad.com/support/prism-5-updates/ (accessed on 11 August 2024).


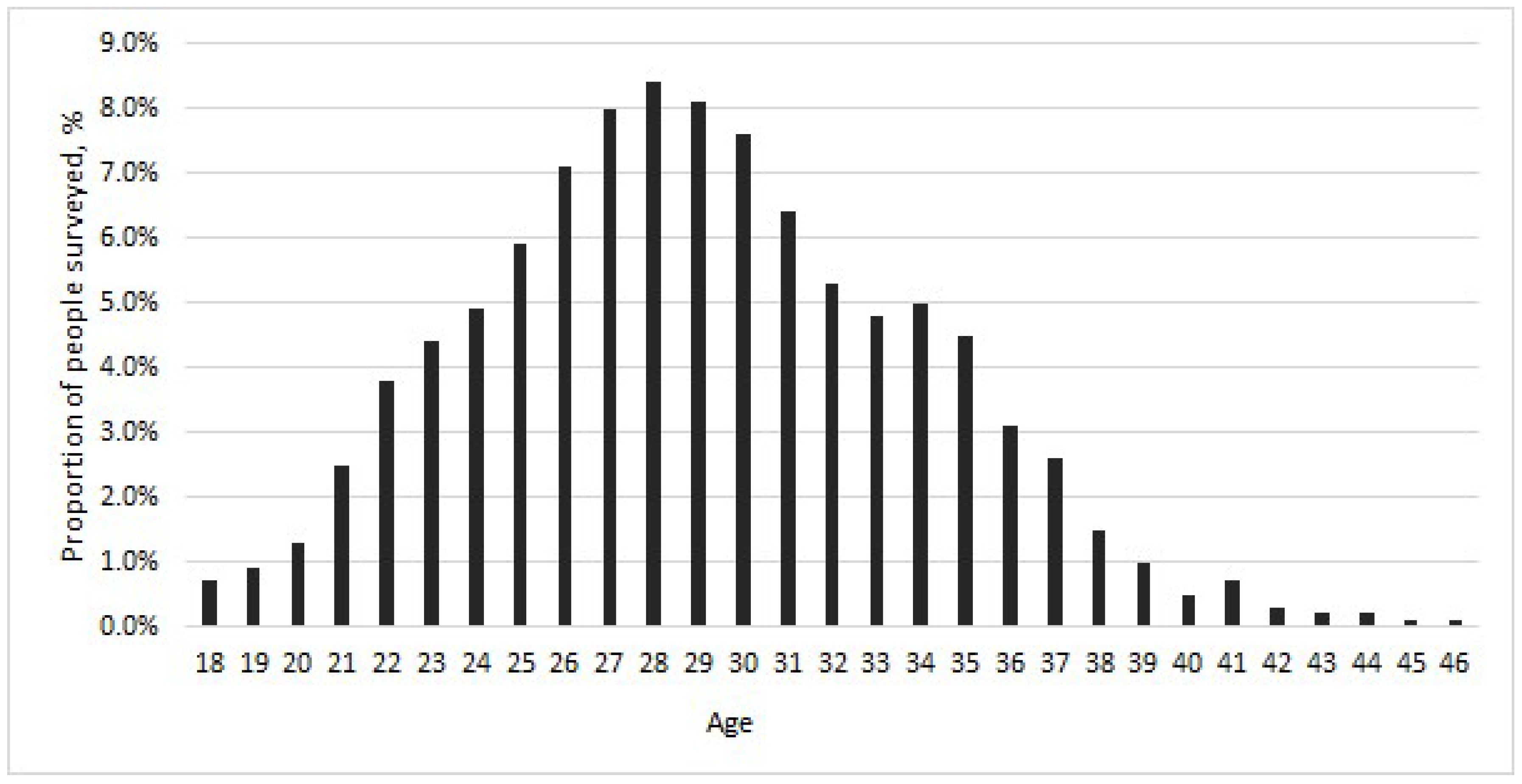
| Age Group, Years | Number of Volunteers | Vaccinated Against HBV | |
|---|---|---|---|
| N | abs, (n) | %, 95% CI | |
| 18–21 years | 74 | 67 | 90.5% (81.5–96.1%) |
| 22–25 years | 260 | 231 | 88.9% (84.4–92.4%) |
| 26–31 years | 624 | 533 | 85.4% (82.4–88.1%) |
| 32–36 years | 311 | 206 | 66.2% (60.7–71.5%) |
| 37–46 years | 99 | 51 | 51.5% (41.3–61.7%) |
| Total: | 1368 | 1088 | 79.5% (77.3–81.6%) |
| Age Group, Years | Number of Volunteers | Anti-HBs IgG | Anti-HBcore IgG | HBsAg | Anti-HBs IgG + Anti-HBcore IgG | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | |
| 18–21 years | 74 | 56 | 75.7% (64.3–84.9%) | 4 | 5.4% (1.5–13.3%) | 0 | 0% (0–4.9%) | 2 | 0.15% (0.02–0.53%) |
| 22–25 years | 260 | 181 | 69.6% (63.6–75.2%) | 16 | 6.2% (3.6–9.8%) | 2 | 0.8% (0.1–2.8%) | 6 | 0.44% (0.16–0.95%) |
| 26–31 years | 624 | 419 | 67.2% (63.3–70.8%) | 58 | 9.3% (7.1–11.9%) | 11 | 1.8% (0.9–3.1%) | 18 | 1.32% (0.78–2.07%) |
| 32–36 years | 311 | 169 | 54.3% (48.6–60.0%) | 71 | 22.8% (18.3–27.9%) | 10 | 3.2% (1.6–5.8%) | 18 | 1.32% (0.78–2.07%) |
| 37–46 years | 99 | 48 | 48.5% (38.3–58.8%) | 28 | 28.3% (19.7–38.2%) | 3 | 3.0% (0.6–8.6%) | 10 | 0.73% (0.35–1.34%) |
| Total: | 1368 | 873 | 63.8% (61.2–66.4%) | 177 | 12.9% (11.2–14.8%) | 26 | 1.9% (1.3–2.8%) | 54 | 3.95% (2.98–5.12%) |
| Pregnancy Number | Number of Volunteers | Anti-HBs IgG | Anti-HBcore IgG | HBsAg | |||
|---|---|---|---|---|---|---|---|
| N | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | |
| 5th pregnancy | 1 | 0 | 0.0% (0.0–97.5%) | 1 | 100.0% (2.5–100%) | 0 | 0.0% (0.0–97.5%) |
| 4th pregnancy | 6 | 2 | 33.3% (4.3–77.7%) | 4 | 66.7% (22.3–95.7%) | 1 | 16.7% (0.4–64.1%) |
| 3rd pregnancy | 115 | 62 | 53.9% (44.4–63.3%) | 49 | 42.6% (33.4–52.2%) | 7 | 6.1% (2.5–12.1%) |
| 2nd pregnancy | 553 | 357 | 64.6% (60.4–68.6%) | 65 | 11.8% (9.2–14.8%) | 11 | 2.0% (1.0–3.5%) |
| 1st pregnancy | 693 | 452 | 65.2% (61.6–68.8%) | 58 | 8.4% (6.4–10.7%) | 7 | 1.0% (0.4–2.1%) |
| Hepatitis B Serological Markers | Prevalence | |
|---|---|---|
| abs, (n), N = 1368 | %, 95% CI | |
| HBsAg | 9 | 0.7% (0.3–1.3%) |
| Anti-HBs IgG | 818 | 59.8% (57.1–62.4%) |
| Anti-HBc IgG | 115 | 8.4% (7.0–10.0%) |
| HBsAg, anti-HBs IgG | 1 | 0.1% (0.0–0.4%) |
| HBsAg, anti-HBc IgG | 8 | 0.6% (0.3–1.2%) |
| HBsAg, anti-HBs IgG, anti-HBc IgG | 8 | 0.6% (0.3–1.2%) |
| Anti-HBs IgG, anti-HBc IgG | 46 | 3.4% (2.5–4.5%) |
| Hepatitis B Serological Markers | Number of Volunteers | 1st Pregnancy | 2nd Pregnancy | 3rd Pregnancy | 4th Pregnancy | 5th Pregnancy | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | |
| HBsAg | 9 | 2 | 22.2% (2.8–60.0%) | 4 | 44.4% (13.7–78.8%) | 2 | 22.2% (2.8–60.0%) | 1 | 11.1% (0.3–48.3%) | 0 | 0.0% (0.0–33.6%) |
| Anti-HBs IgG | 818 | 433 | 52.9% (49.5–56.4%) | 336 | 41.1% (37.7–44.5%) | 48 | 5.9% (4.4–7.7%) | 1 | 0.1% (0.0–0.7%) | 0 | 0.0% (0.0–0.5%) |
| Anti-HBc IgG | 115 | 39 | 33.9% (25.4–43.3%) | 41 | 35.7% (26.9–45.1%) | 31 | 26.9% (19.1–36.0%) | 3 | 2.6% (0.5–7.4%) | 1 | 0.9% (0.0–4.8%) |
| HBsAg, anti-HBs IgG | 1 | 1 | 100.0% (2.5–100%) | 0 | 0.0% (0–97.5%) | 0 | 0.0% (0.0–97.5%) | 0 | 0.0% (0.0–97.5%) | 0 | 0.0% (0.0–97.5%) |
| HBsAg, anti-HBc IgG | 8 | 1 | 12.5% (0.3–52.7%) | 3 | 37.5% (8.5–75.5%) | 4 | 50.0% (15.7–84.3%) | 0 | 0.0% (0.0–36.9%) | 0 | 0.0% (0.0–36.9%) |
| HBsAg, anti-HBs IgG, anti-HBc IgG | 8 | 3 | 37.5% (8.5–75.5%) | 4 | 50.0% (15.7–84.3%) | 1 | 12.5% (0.3–52.7%) | 0 | 0.0% (0.0–36.9%) | 0 | 0.0% (0.0–36.9%) |
| Anti-HBs IgG, anti-HBc IgG | 46 | 15 | 32.6% (19.5–48.0%) | 17 | 36.9% (23.2–52.5%) | 13 | 28.3% (16.0–43.5%) | 1 | 2.2% (0.1–11.5%) | 0 | 0.0% (0.0–7.7%) |
| Age Group, Years | Number of Volunteers | HBV DNA | |
|---|---|---|---|
| N | abs, (n) | %, 95% CI | |
| 18–21 years | 74 | 1 | 1.35% (0.0–7.3%) |
| 22–25 years | 260 | 7 | 2.7% (1.1–5.5%) |
| 26–31 years | 624 | 30 | 4.8% (3.3–6.8%) |
| 32–36 years | 311 | 21 | 6.8% (4.2–10.1%) |
| 37–46 years | 99 | 5 | 5.1% (1.7–11.4%) |
| Total: | 1368 | 64 | 4.7% (3.6–5.9%) |
| Pregnancy Number | Number of Volunteers | HBV DNA | |
|---|---|---|---|
| N | abs, (n) | %, 95% CI | |
| 5th pregnancy | 1 | 0 | 0.0% (0.0–97.5%) |
| 4th pregnancy | 6 | 3 | 50.0% (11.8–88.2%) |
| 3rd pregnancy | 115 | 17 | 14.8% (8.9–22.6%) |
| 2nd pregnancy | 553 | 27 | 4.9% (3.2–7.0%) |
| 1st pregnancy | 693 | 17 | 2.5% (1.4–3.9%) |
| Age Group, Years | Number of Volunteers | HBV DNA+, HBsAg+ | HBV DNA+, HbsAg− | ||
|---|---|---|---|---|---|
| N | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | |
| 18–21 years | 74 | 0 | 0.0% (0.0–4.9%) | 1 | 1.35% (0.0–7.3%) |
| 22–25 years | 260 | 2 | 0.8% (0.1–2.8%) | 5 | 1.9% (0.6–4.4%) |
| 26–31 years | 624 | 11 | 1.8% (0.9–3.1%) | 19 | 3.0% (1.8–4.7%) |
| 32–36 years | 311 | 10 | 3.2% (1.6–5.8%) | 11 | 3.5% (1.8–6.2%) |
| 37–46 years | 99 | 3 | 3.0% (0.6–8.6%) | 2 | 2.0% (0.3–7.1%) |
| Total: | 1368 | 26 | 1.9% (1.3–2.8%) | 38 | 2.8% (2.0–3.8%) |
| Hepatitis B Serological Markers | HBV DNA Positive (N = 64) | HBV DNA+, HBsAg+ (N = 26) | HBV DNA+, HbsAg− (N = 38) | |||
|---|---|---|---|---|---|---|
| abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | |
| HBsAg | 9 | 14.1% (6.6–25.0%) | 9 | 34.6% (17.2–55.7%) | 0 | 0.0% (0.0–9.3%) |
| Anti-HBs IgG | 1 | 1.6% (0.0–8.4%) | 0 | 0.0% (0.0–13.2%) | 1 | 2.6% (0.1–13.8%) |
| Anti-HBc IgG | 25 | 39.1% (27.1–52.1%) | 0 | 0.0% (0.0–13.2%) | 25 | 65.8% (48.7–80.4%) |
| HBsAg, anti-HBs IgG | 1 | 1.6% (0.0–8.4%) | 1 | 3.9% (0.1–19.6%) | 0 | 0.0% (0.0–9.3%) |
| HBsAg, anti-HBc IgG | 8 | 12.5% (5.6–23.2%) | 8 | 30.8% (14.3–51.8%) | 0 | 0.0% (0.0–9.3%) |
| HBsAg, anti-HBs IgG, anti-HBc IgG | 8 | 12.5% (5.6–23.2%) | 8 | 30.8% (14.3–51.8%) | 0 | 0.0% (0.0–9.3%) |
| Anti-HBs IgG, anti-HBc IgG | 10 | 15.6% (7.8–26.9%) | 0 | 0.0% (0.0–13.2%) | 10 | 26.3% (13.4–43.1%) |
| Seronegative | 2 | 3.1% (0.4–10.8%) | 0 | 0.0% (0.0–13.2%) | 2 | 5.3% (0.6–17.8%) |
| Vaccination Status | HBV DNA+, HBsAg+ (N = 26) | HBV DNA+, HbsAg− (N = 38) | ||
|---|---|---|---|---|
| abs, (n) | %, 95% CI | abs, (n) | %, 95% CI | |
| vaccinated | 1 | 3.85% (0.1–19.6%) | 26 | 68.4% (51.4–82.5%) |
| unvaccinated | 25 | 96.15% (80.4–99.9%) | 12 | 31.6% (17.5–48.7%) |
| Mutation | Frequency of Occurrence in the Group (N = 64) | |||
|---|---|---|---|---|
| abs, n | % | 95% CI − | 95% CI + | |
| Y100SY/C/F | 4 | 6.3% | 1.7% | 15.2% |
| Q101R/H | 2 | 3.1% | 0.4% | 10.8% |
| L109R | 2 | 3.1% | 0.4% | 10.8% |
| I110S/L | 4 | 6.3% | 1.7% | 15.2% |
| P111Q | 2 | 3.1% | 0.4% | 10.8% |
| T113S/P | 63 | 98.4% | 91.6% | 100.0% |
| T114S | 62 | 96.9% | 89.2% | 99.6% |
| T118L/V/A/G/M | 30 | 46.9% | 34.3% | 59.8% |
| G119E | 1 | 1.6% | 0.0% | 8.4% |
| P120T/L/S/Q | 5 | 7.8% | 2.6% | 17.3% |
| R122K/I/Q | 6 | 9.4% | 3.5% | 19.3% |
| T123A | 1 | 1.6% | 0.0% | 8.4% |
| C124R/W/G | 3 | 4.7% | 1.0% | 13.1% |
| T125M | 4 | 6.3% | 1.7% | 15.2% |
| T126S/N | 2 | 3.1% | 0.4% | 10.8% |
| T127P | 25 | 39.1% | 27.1% | 52.1% |
| A128V/A/G/F | 25 | 39.1% | 27.1% | 52.1% |
| Q129R/H | 4 | 6.3% | 1.7% | 15.2% |
| G130R/A | 2 | 3.1% | 0.4% | 10.8% |
| N131T | 61 | 95.3% | 86.9% | 99.0% |
| S132SY | 1 | 1.6% | 0.0% | 8.4% |
| M133I | 1 | 1.6% | 0.0% | 8.4% |
| Y134H/N/F/D | 6 | 9.4% | 3.5% | 19.3% |
| C137Y/W | 3 | 4.7% | 1.0% | 13.1% |
| C139W/F | 2 | 3.1% | 0.4% | 10.8% |
| T140ST | 1 | 1.6% | 0.0% | 8.4% |
| K141R | 1 | 1.6% | 0.0% | 8.4% |
| P142L | 1 | 1.6% | 0.0% | 8.4% |
| S143P/L/T | 8 | 12.5% | 5.6% | 23.2% |
| D144G/E/H | 5 | 7.8% | 2.6% | 17.3% |
| G145A/R/E | 6 | 9.4% | 3.5% | 19.3% |
| N146S | 1 | 1.6% | 0.0% | 8.4% |
| G159A/G/E | 6 | 9.4% | 3.5% | 19.3% |
| E164G/K | 5 | 7.8% | 2.6% | 17.3% |
| A166V | 1 | 1.6% | 0.0% | 8.4% |
| S167L/P | 3 | 4.7% | 1.0% | 13.1% |
| Mutation | Frequency of Occurrence in the CHB Group (N = 26) | Frequency of Occurrence in the OBI Group (N = 38) | ||
|---|---|---|---|---|
| abs, n | %, 95% CI | abs, n | %, 95% CI | |
| P120T/L/S/Q | 0 | 0% | 5 | 13.2% (4.4–28.1%) |
| R122K/I/Q | 1 | 3.85% (0.1–19.6%) | 5 | 13.2% (4.4–28.1%) |
| C124R/W/G | 0 | 0% | 3 | 7.9% (1.7–21.4%) |
| T126S/N | 0 | 0% | 2 | 5.3% (0.6–17.7%) |
| Q129R/H | 0 | 0% | 4 | 10.5% (2.9–24.8%) |
| M133I | 0 | 0% | 1 | 2.6% (0.1–13.8%) |
| C137Y/W | 0 | 0% | 3 | 7.9% (1.7–21.4%) |
| Y134H/N/F/D | 1 | 3.85% (0.1–19.6%) | 3 | 7.9% (1.7–21.4%) |
| K141R | 0 | 0% | 1 | 2.6% (0.1–13.8%) |
| P142L | 0 | 0% | 1 | 2.6% (0.1–13.8%) |
| S143P/L/T | 2 | 7.69% (0.9–25.1%) | 6 | 15.8% (6.0–31.3%) |
| D144G/E/H | 0 | 0% | 4 | 10.5% (2.9–24.8%) |
| G145A/R/E | 0 | 0% | 5 | 13.2% (4.4–28.1%) |
| Sample | Vaccinated Against HBV | Anti-HBs IgG | Mutation |
|---|---|---|---|
| ocHBV_pw315 | Yes | Yes | T113P, T114A, T118V, K122R, P127T, A128V, N131T, F134Y, G145R, A159G, Y161F, V168A |
| ocHBV_pw560 | Yes | Yes | T113P, T114A, K122R, T126N, T127P, N131T, F134Y, D144E, A159G, Y161F, V168A |
| ocHBV_pw570 | Yes | Yes | Y100C, I110L, T113P, T114A, T118G, K122R, P127T, A128V, N131T, F134Y, A159G, K160R, Y161F, W163S, E164K, W165C, V168A, V177A |
| ocHBV_pw770 | Yes | Yes | D99A, L109R, T113S, T114S, T118V, K122R, P127T, A128V, N131T, F134Y, A159G, Y161F, E164G, S167L, V168A |
| ocHBV_pw863 | Yes | Yes | T113S, T114S, K122R, T127P, N131T, F134Y, G145A, A159G, Y161F, V168A |
| Mutation | Frequency of Occurrence in the Group (N = 64) | |
|---|---|---|
| abs, n | %, 95% CI | |
| L3R | 1 | 1.56% (0.04–8.40%) |
| H5D/V | 2 | 3.13% (0.38–10.84%) |
| C12F | 5 | 7.81% (2.59–17.30%) |
| C14G/S | 7 | 10.94% (4.51–21.25%) |
| P15A | 2 | 3.13% (0.38–10.84%) |
| A19G | 5 | 7.81% (2.59–17.30%) |
| L22M | 2 | 3.13% (0.38–10.84%) |
| W28*/*W/*Y | 10 | 15.63% (7.76–26.86%) |
| G29D | 11 | 17.19% (8.90–28.68%) |
| Mutation | Frequency of Occurrence in the Group (N = 64) | |||
|---|---|---|---|---|
| abs, n | % | 95% CI − | 95% CI + | |
| T12S/N | 18 | 28.1% | 17.6% | 40.8% |
| E14Q | 4 | 6.3% | 1.7% | 15.2% |
| L16I | 2 | 3.1% | 0.4% | 10.8% |
| L19F | 1 | 1.6% | 0.0% | 8.4% |
| S21A | 4 | 6.3% | 1.7% | 15.2% |
| F24Y | 1 | 1.6% | 0.0% | 8.4% |
| S26N/T | 3 | 4.7% | 1.0% | 13.1% |
| V27I | 1 | 1.6% | 0.0% | 8.4% |
| S35A/T | 3 | 4.7% | 1.0% | 13.1% |
| Y38F | 4 | 6.3% | 1.7% | 15.2% |
| E40D | 11 | 17.2% | 8.9% | 28.7% |
| A41AP | 1 | 1.6% | 0.0% | 8.4% |
| E43K | 1 | 1.6% | 0.0% | 8.4% |
| E46D | 2 | 3.1% | 0.4% | 10.8% |
| C48V | 3 | 4.7% | 1.0% | 13.1% |
| S49T | 4 | 6.3% | 1.7% | 15.2% |
| P50H/A | 3 | 4.7% | 1.0% | 13.1% |
| L55I | 4 | 6.3% | 1.7% | 15.2% |
| I59V/M/F/S | 5 | 7.8% | 2.6% | 17.3% |
| G63A | 1 | 1.6% | 0.0% | 8.4% |
| E64DE | 5 | 7.8% | 2.6% | 17.3% |
| L65T | 1 | 1.6% | 0.0% | 8.4% |
| T67N/S/A | 10 | 15.6% | 7.8% | 26.9% |
| N74S/G/V/A/T/I | 61 | 95.3% | 86.9% | 99.0% |
| E77D/Q | 12 | 18.8% | 10.1% | 30.5% |
| D78H | 1 | 1.6% | 0.0% | 8.4% |
| P79Q | 11 | 17.2% | 8.9% | 28.7% |
| A80I/T/V/Q/G | 25 | 39.1% | 27.1% | 52.1% |
| S81AS | 1 | 1.6% | 0.0% | 8.4% |
| D83E/G | 3 | 4.7% | 1.0% | 13.1% |
| L84Q | 2 | 3.1% | 0.4% | 10.8% |
| V86IV | 1 | 1.6% | 0.0% | 8.4% |
| N87S/T/G | 61 | 95.3% | 86.9% | 99.0% |
| V89D | 1 | 1.6% | 0.0% | 8.4% |
| N90HN | 1 | 1.6% | 0.0% | 8.4% |
| T91V/N/S/D | 6 | 9.4% | 3.5% | 19.3% |
| N92H | 4 | 6.3% | 1.7% | 15.2% |
| M93V | 3 | 4.7% | 1.0% | 13.1% |
| L95I | 1 | 1.6% | 0.0% | 8.4% |
| I97F | 60 | 93.8% | 84.8% | 98.3% |
| W102RW | 1 | 1.6% | 0.0% | 8.4% |
| I105L/V | 3 | 4.7% | 1.0% | 13.1% |
| T109M | 2 | 3.1% | 0.4% | 10.8% |
| F110FL | 1 | 1.6% | 0.0% | 8.4% |
| E113D/A/Q | 9 | 14.1% | 6.6% | 25.0% |
| T114V/P/I | 4 | 6.3% | 1.7% | 15.2% |
| L116I/M | 50 | 78.1% | 66.0% | 87.5% |
| Y118DY | 1 | 1.6% | 0.0% | 8.4% |
| I126M | 1 | 1.6% | 0.0% | 8.4% |
| P130S/Q | 5 | 7.8% | 2.6% | 17.3% |
| A131P | 3 | 4.7% | 1.0% | 13.1% |
| R133KR | 1 | 1.6% | 0.0% | 8.4% |
| P135V/S/T | 3 | 4.7% | 1.0% | 13.1% |
| T142I/M | 2 | 3.1% | 0.4% | 10.8% |
| L143LR | 1 | 1.6% | 0.0% | 8.4% |
| T146C | 1 | 1.6% | 0.0% | 8.4% |
| T147A/S/C | 4 | 6.3% | 1.7% | 15.2% |
| V149I | 5 | 7.8% | 2.6% | 17.3% |
| R151C/A/G | 4 | 6.3% | 1.7% | 15.2% |
| G155C/V | 2 | 3.1% | 2.6% | 4.2% |
| R156G | 1 | 1.6% | 1.1% | 2.6% |
| S157T/A | 3 | 4.7% | 4.2% | 5.7% |
| R168P | 1 | 1.6% | 1.1% | 2.6% |
| Q171K | 1 | 1.6% | 1.1% | 2.6% |
| R174HR | 1 | 1.6% | 1.1% | 2.6% |
| S178T | 2 | 3.1% | 2.6% | 4.2% |
| Q179K/P | 5 | 7.8% | 7.3% | 8.8% |
| E182D/G | 3 | 4.7% | 4.2% | 5.7% |
| S183P | 4 | 6.3% | 5.7% | 7.3% |
| Q184K | 1 | 1.6% | 1.1% | 2.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostankova, Y.V.; Shchemelev, A.N.; Serikova, E.N.; Kusevitskaya, M.B.; Sannikov, M.V.; Gorskaya, O.A.; Basina, V.V.; Shirshova, N.Y.; Mashkov, I.A.; Zueva, E.B.; et al. Prevalence and Molecular Characterization of Chronic and Occult Hepatitis B Virus Infection Among Pregnant Women in St. Petersburg, Russia. Int. J. Mol. Sci. 2025, 26, 9079. https://doi.org/10.3390/ijms26189079
Ostankova YV, Shchemelev AN, Serikova EN, Kusevitskaya MB, Sannikov MV, Gorskaya OA, Basina VV, Shirshova NY, Mashkov IA, Zueva EB, et al. Prevalence and Molecular Characterization of Chronic and Occult Hepatitis B Virus Infection Among Pregnant Women in St. Petersburg, Russia. International Journal of Molecular Sciences. 2025; 26(18):9079. https://doi.org/10.3390/ijms26189079
Chicago/Turabian StyleOstankova, Yulia V., Alexander N. Shchemelev, Elena N. Serikova, Marina B. Kusevitskaya, Maksim V. Sannikov, Olga A. Gorskaya, Valentina V. Basina, Natalia Yu. Shirshova, Ilya A. Mashkov, Elena B. Zueva, and et al. 2025. "Prevalence and Molecular Characterization of Chronic and Occult Hepatitis B Virus Infection Among Pregnant Women in St. Petersburg, Russia" International Journal of Molecular Sciences 26, no. 18: 9079. https://doi.org/10.3390/ijms26189079
APA StyleOstankova, Y. V., Shchemelev, A. N., Serikova, E. N., Kusevitskaya, M. B., Sannikov, M. V., Gorskaya, O. A., Basina, V. V., Shirshova, N. Y., Mashkov, I. A., Zueva, E. B., Reingardt, D. E., & Totolian, A. A. (2025). Prevalence and Molecular Characterization of Chronic and Occult Hepatitis B Virus Infection Among Pregnant Women in St. Petersburg, Russia. International Journal of Molecular Sciences, 26(18), 9079. https://doi.org/10.3390/ijms26189079







