HDAC Inhibitors Enhance the Chemosensitivity of Osteosarcoma Cells to Etoposide by Suppressing the Hippo/YAP Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. HDACis Enhanced the Sensitivity of Osteosarcoma Cells to VP16 to Inhibit Osteosarcoma Cell Proliferation
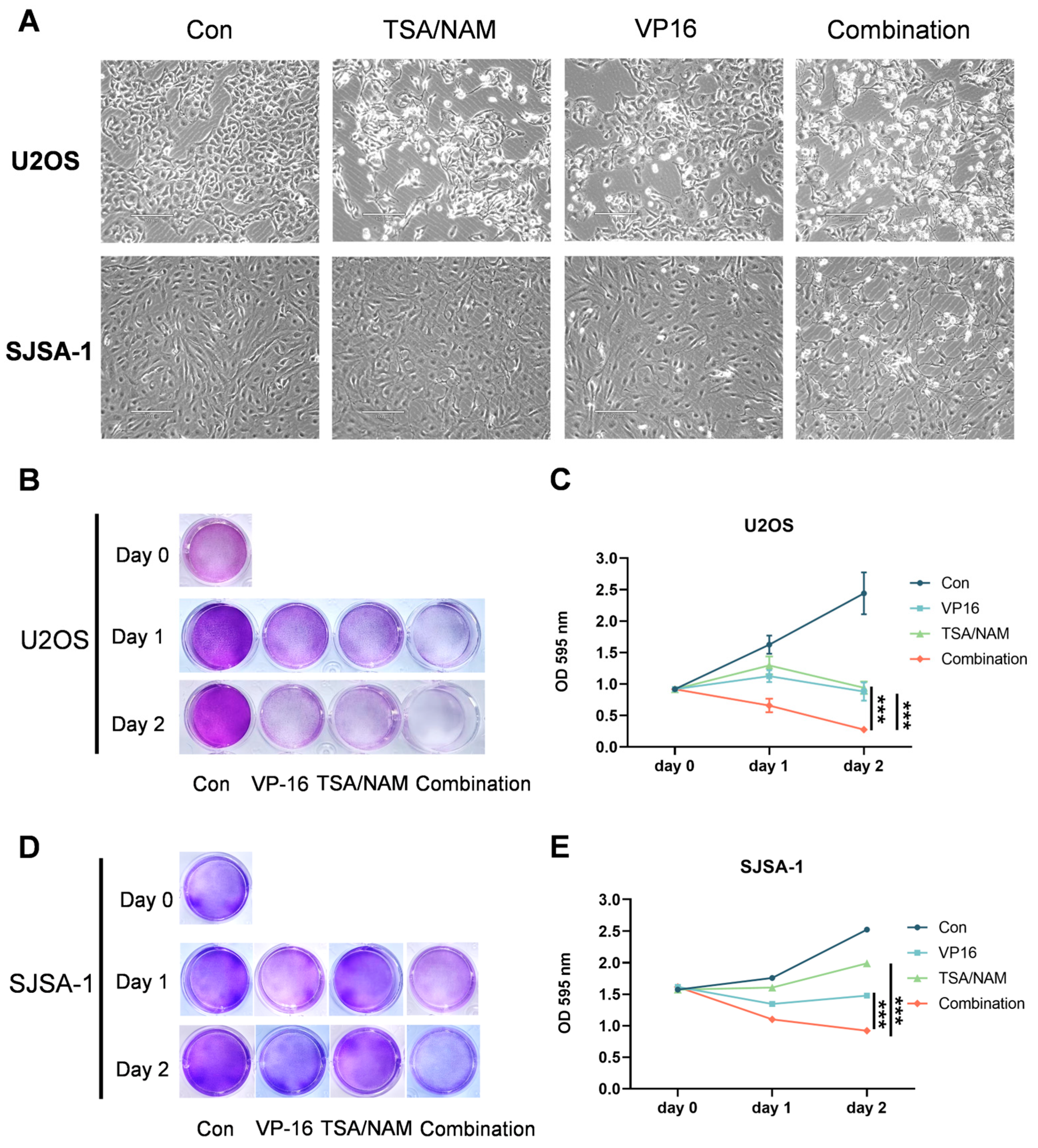
2.2. HDACis Promoted the Inhibitory of VP16 on the Cell Growth of Osteosarcoma Cells
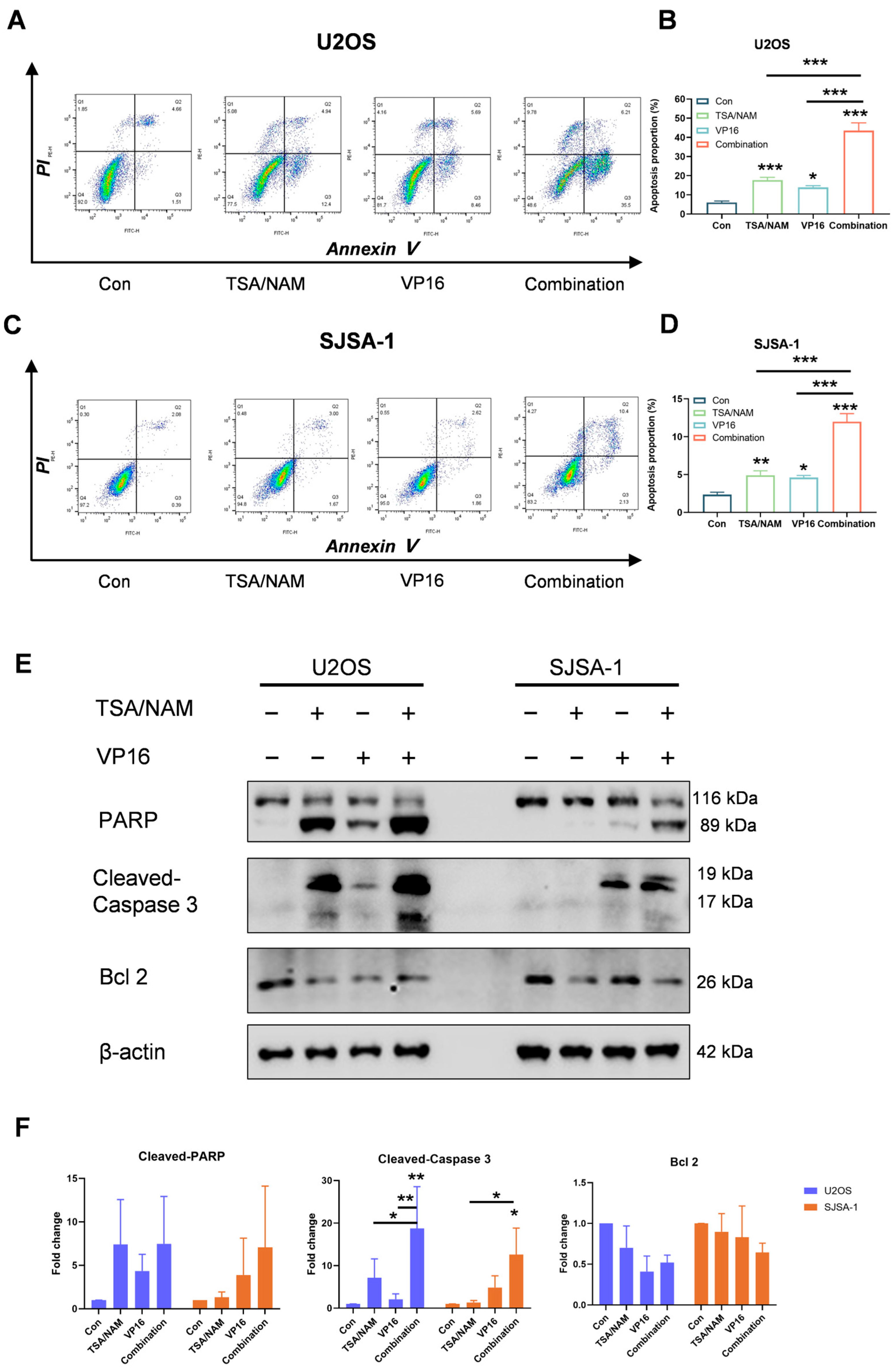
2.3. HDACis Enhanced the Chemosensitivity of VP16 by Inducing Apoptosis of Osteosarcoma Cells
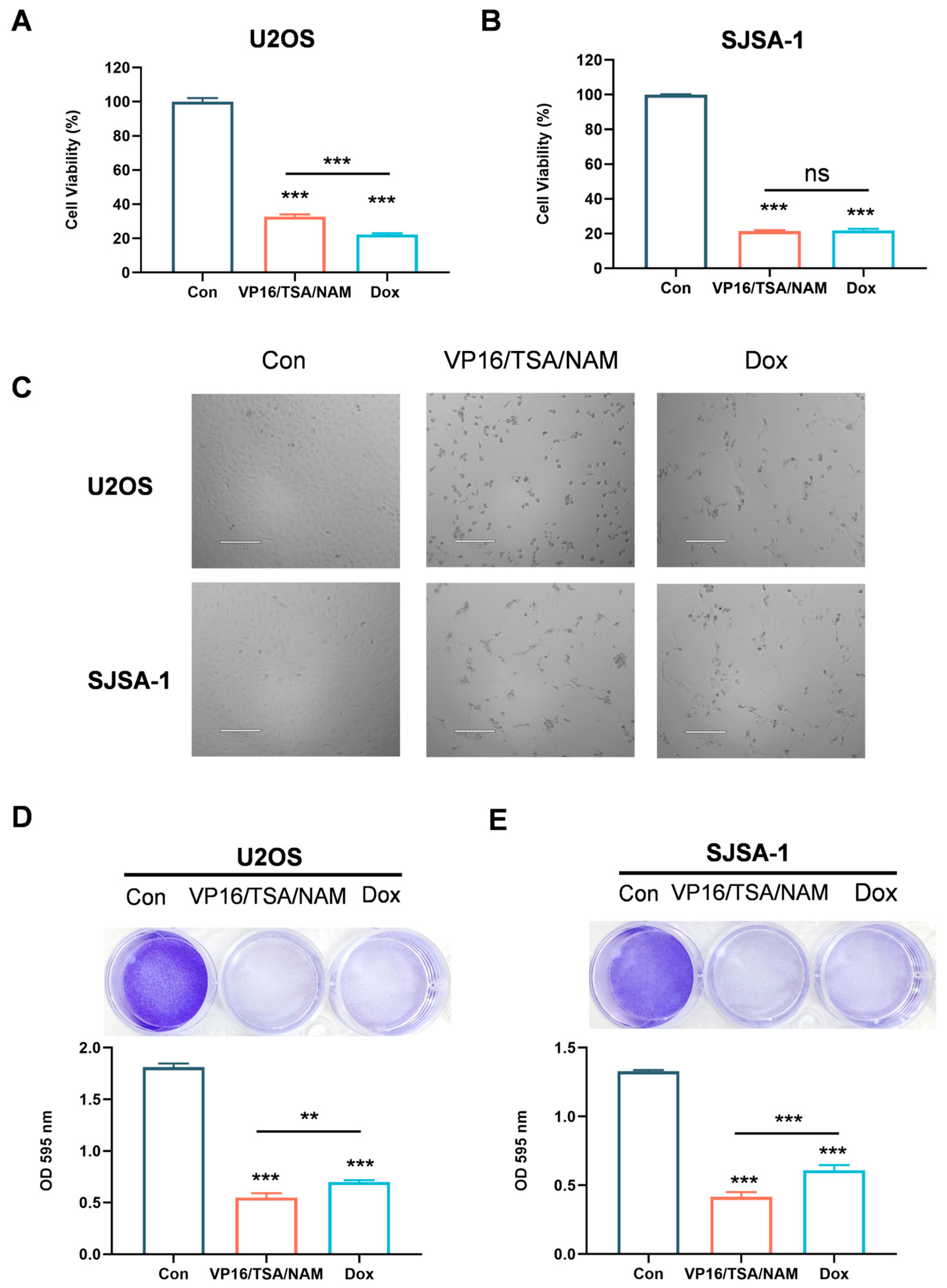
2.4. Combination of HDACis and VP16 Exerted Comparable Inhibitory Effect with Doxorubicin
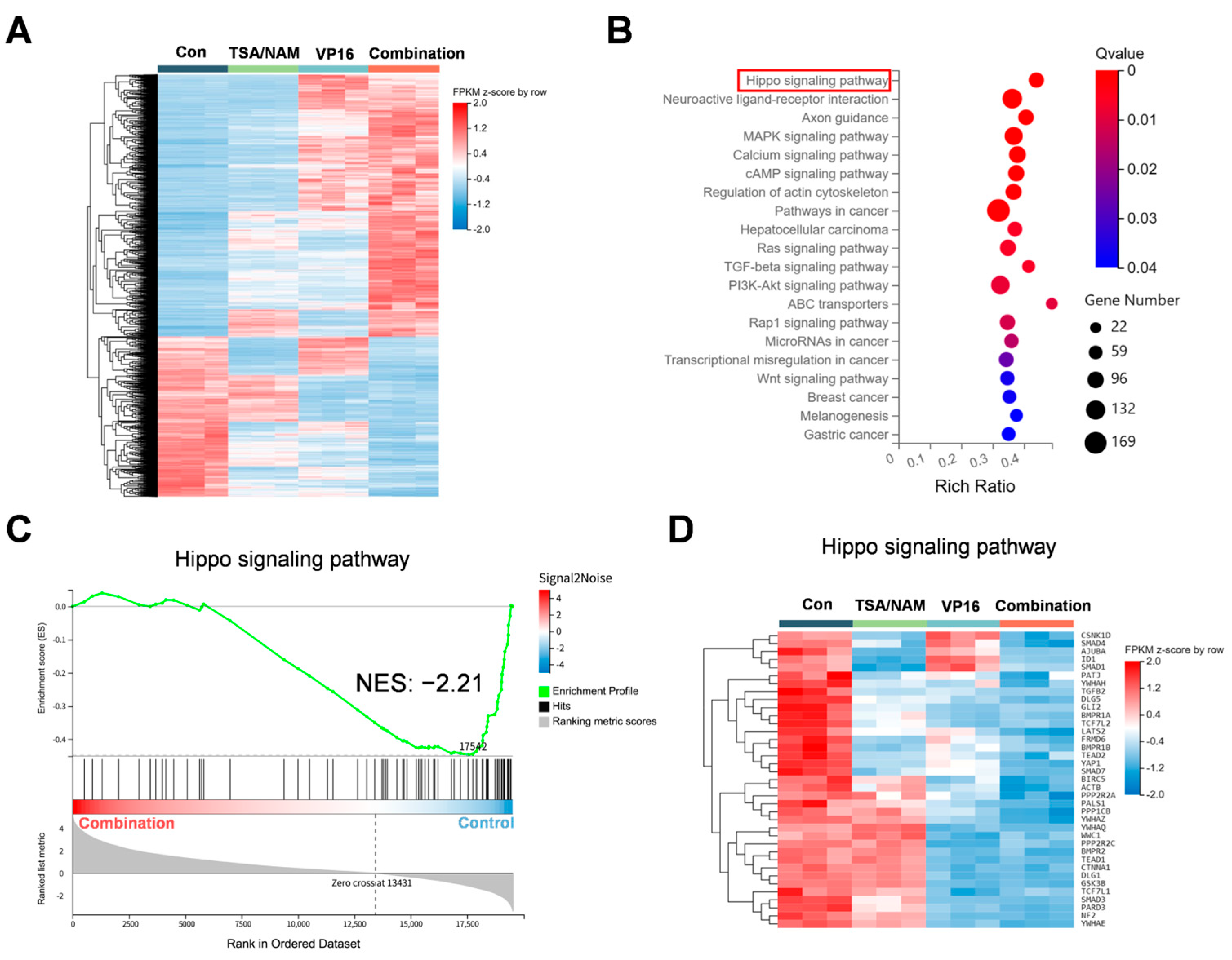
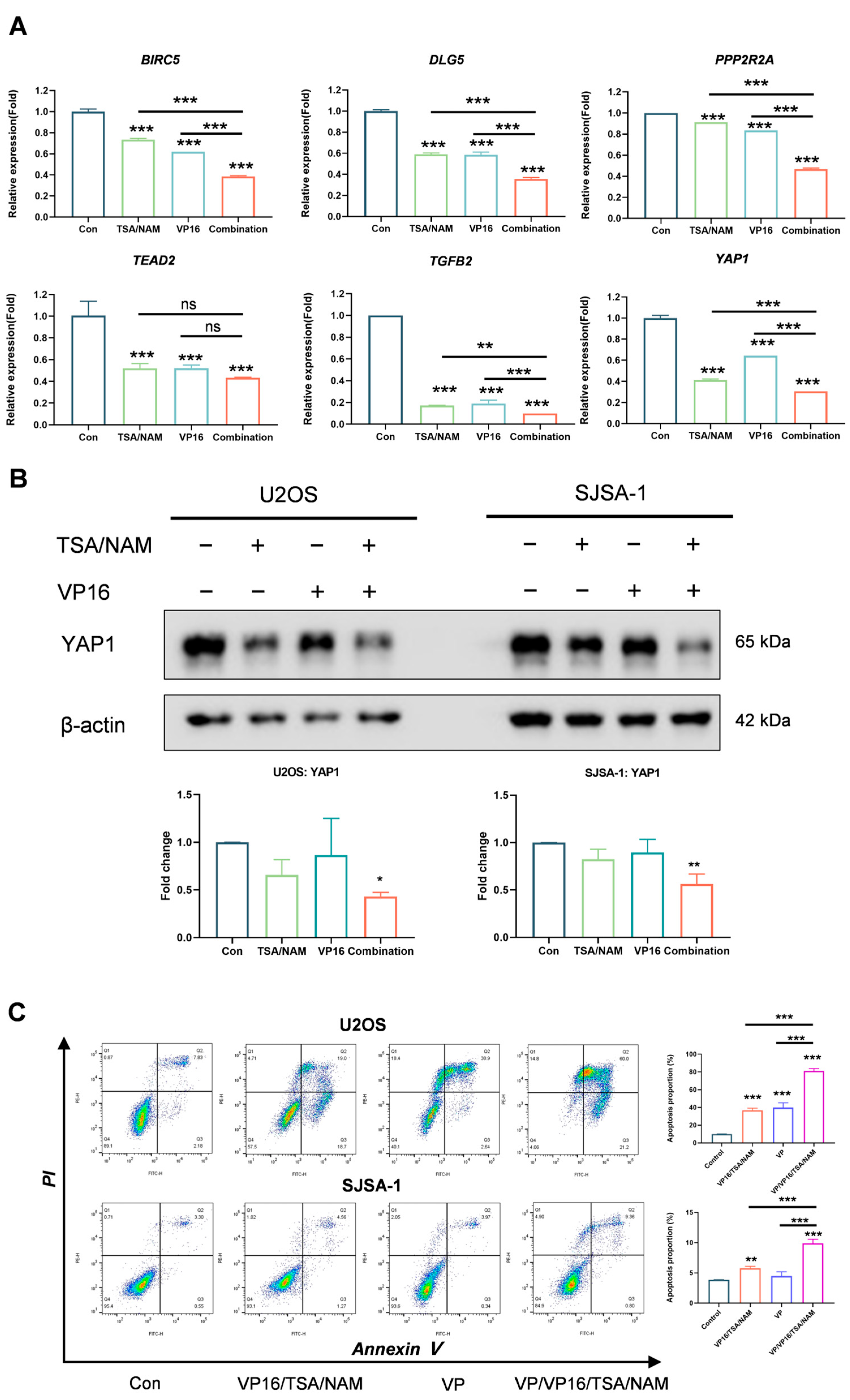
2.5. Hippo/YAP1 Pathway Involved in HDACi Augmented Sensitivity of Osteosarcoma Cells to VP16
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CCK-8 Assay
4.3. Cell Growth Assay
4.4. Apoptosis Assay
4.5. Compare Antitumor Effect of Combination and Doxorubicin
4.6. Western Blot
4.7. RT-qPCR
4.8. RNA-Sequence Analysis
4.9. YAP Inhibitor Combination Experiment
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| DEGs | Differential expression genes |
| DMEM | Dulbecco’s modified Eagle’s medium |
| Dox | Doxorubicin |
| FBS | Fetal bovine serum |
| GSEA | Gene Set Enrichment Analysis |
| HDACis | Histone deacetylases inhibitors |
| LATS1/2 | Large tumor suppressor 1/2 |
| MST1/2 | Mammalian ste20-like kinases 1/2 |
| NAM | Nicotinamide |
| O-ASCs | Omental adipose-derived stromal cells |
| PI | Prodium iodide |
| SAHA | Suberoylanilide hydroxamic acid |
| TAZ | Transcriptional coactivator with a PDZ-binding motif |
| TSA | Trichostatin A |
| VP16 | Etoposide |
| YAP1 | Yes-associated protein 1 |
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Li, Y. Effects of microenvironment in osteosarcoma on chemoresistance and the promise of immunotherapy as an osteosarcoma therapeutic modality. Front. Immunol. 2022, 13, 871076. [Google Scholar] [CrossRef]
- Wen, Y.; Tang, F.; Tu, C.; Hornicek, F.; Duan, Z.; Min, L. Immune checkpoints in osteosarcoma: Recent advances and therapeutic potential. Cancer Lett. 2022, 547, 215887. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, V.; Villamayor, M.L.; Chamorro, J.; Rosero, D.I.; Pozas, J.; San Román, M.; Calvo, J.C.; Pérez de Aguado, P.; Moreno, J.; Guerrero, P.; et al. Receptor Tyrosine Kinase Inhibitors for the Treatment of Recurrent and Unresectable Bone Sarcomas. Int. J. Mol. Sci. 2022, 23, 13784. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Zhao, F.; Wei, F.; Shi, Y.T.; Wen, J.K.; Yang, C.; Zhang, H.S.; Li, C.C.; Liu, C.; Ye, W.C.; et al. Visible-Light Photocatalyzed Skeletal Rearrangement Enables the Synthesis of Highly Functionalized Xanthenes with Antitumor Activity. Angew. Chem. Int. Ed. Engl. 2024, 64, e202420671. [Google Scholar] [CrossRef]
- de Nigris, F.; Ruosi, C.; Napoli, C. Clinical efficiency of epigenetic drugs therapy in bone malignancies. Bone 2021, 143, 115605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yuan, M.; Zhang, M.; Lei, C.; Aras, O.; Zhang, X.; An, F. Combining histone deacetylase inhibitors (HDACis) with other therapies for cancer therapy. Eur. J. Med. Chem. 2021, 226, 113825. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, W.G. Targeting histone deacetylases for cancer therapy: From molecular mechanisms to clinical implications. Int. J. Biol. Sci. 2014, 10, 757–770. [Google Scholar] [CrossRef]
- McGuire, J.J.; Nerlakanti, N.; Lo, C.H.; Tauro, M.; Utset-Ward, T.J.; Reed, D.R.; Lynch, C.C. Histone deacetylase inhibition prevents the growth of primary and metastatic osteosarcoma. Int. J. Cancer 2020, 147, 2811–2823. [Google Scholar] [CrossRef]
- Roh, M.S.; Kim, C.W.; Park, B.S.; Kim, G.C.; Jeong, J.H.; Kwon, H.C.; Suh, D.J.; Cho, K.H.; Yee, S.B.; Yoo, Y.H. Mechanism of histone deacetylase inhibitor Trichostatin A induced apoptosis in human osteosarcoma cells. Apoptosis 2004, 9, 583–589. [Google Scholar] [CrossRef]
- Magar, A.G.; Morya, V.K.; Koh, Y.-H.; Noh, K.-C. Synergistic HDAC4/8 Inhibition Sensitizes Osteosarcoma to Doxorubicin via pAKT/RUNX2 Pathway Modulation. Int. J. Mol. Sci. 2025, 26, 3574. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, X.; Jin, J.; Xu, H.; Gao, Q.; Wang, Y.; Zhao, J. Histone Deacetylase Inhibitor Trichostatin a Promotes the Apoptosis of Osteosarcoma Cells through p53 Signaling Pathway Activation. Int. J. Biol. Sci. 2016, 12, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.P.; Paschou, S.A.; Ryu, H.S. The Role of Nicotinamide in Cancer Chemoprevention and Therapy. Biomolecules 2020, 10, 477. [Google Scholar] [CrossRef]
- Zhang, J.G.; Zhao, G.; Qin, Q.; Wang, B.; Liu, L.; Liu, Y.; Deng, S.C.; Tian, K.; Wang, C.Y. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology 2013, 13, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Jafary, H.; Ahmadian, S.; Soleimani, M. Synergistic anticancer activity of valproate combined with nicotinamide enhances anti-proliferation response and apoptosis in MIAPaca2 cells. Mol. Biol. Rep. 2014, 41, 3801–3812. [Google Scholar] [CrossRef] [PubMed]
- Jafary, H.; Ahmadian, S.; Soleimani, M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumor Biol. 2014, 35, 2701–2710. [Google Scholar] [CrossRef]
- Martín-Bernabé, A.; Tarragó-Celada, J.; Cunin, V.; Michelland, S.; Cortés, R.; Poignant, J.; Boyault, C.; Rachidi, W.; Bourgoin-Voillard, S.; Cascante, M.; et al. Quantitative Proteomic Approach Reveals Altered Metabolic Pathways in Response to the Inhibition of Lysine Deacetylases in A549 Cells under Normoxia and Hypoxia. Int. J. Mol. Sci. 2021, 22, 3378. [Google Scholar] [CrossRef]
- Frew, A.J.; Johnstone, R.W.; Bolden, J.E. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009, 280, 125–133. [Google Scholar] [CrossRef]
- Tang, F.; Choy, E.; Tu, C.; Hornicek, F.; Duan, Z. Therapeutic applications of histone deacetylase inhibitors in sarcoma. Cancer Treat. Rev. 2017, 59, 33–45. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.; Im, E.; Kim, N.D. Etoposide as a Key Therapeutic Agent in Lung Cancer: Mechanisms, Efficacy, and Emerging Strategies. Int. J. Mol. Sci. 2025, 26, 796. [Google Scholar] [CrossRef]
- Unland, R.; Clemens, D.; Heinicke, U.; Potratz, J.C.; Hotfilder, M.; Fulda, S.; Wardelmann, E.; Frühwald, M.C.; Dirksen, U. Suberoylanilide hydroxamic acid synergistically enhances the antitumor activity of etoposide in Ewing sarcoma cell lines. Anticancer Drugs 2015, 26, 843–851. [Google Scholar] [CrossRef]
- Hajji, N.; Wallenborg, K.; Vlachos, P.; Nyman, U.; Hermanson, O.; Joseph, B. Combinatorial action of the HDAC inhibitor trichostatin A and etoposide induces caspase-mediated AIF-dependent apoptotic cell death in non-small cell lung carcinoma cells. Oncogene 2008, 27, 3134–3144. [Google Scholar] [CrossRef]
- Schmidt, O.; Nehls, N.; Prexler, C.; von Heyking, K.; Groll, T.; Pardon, K.; Garcia, H.D.; Hensel, T.; Gürgen, D.; Henssen, A.G.; et al. Class I histone deacetylases (HDAC) critically contribute to Ewing sarcoma pathogenesis. J. Exp. Clin. Cancer Res. 2021, 40, 322. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, F.-F.; Li, J.; Dong, J.-B.; He, H.-Y.; Li, X.-F.; Lu, Q.; Zhang, W.-X.; Shao, C.-M.; Yao, Z.-N.; et al. Investigating the synergy of Shikonin and Valproic acid in inducing apoptosis of osteosarcoma cells via ROS-mediated EGR1 expression. Phytomedicine 2024, 126, 155459. [Google Scholar] [CrossRef]
- Wang, T.; Cui, H.; Ma, N.; Jiang, Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncol. Lett. 2013, 6, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.H.; Trovillion, E.M.; Sholler, C.; Gandra, D.; McKinney, K.Q.; Mulama, D.; Dykema, K.J.; Nagulapally, A.B.; Oesterheld, J.; Saulnier Sholler, G.L. Panobinostat Synergizes with Chemotherapeutic Agents and Improves Efficacy of Standard-of-Care Chemotherapy Combinations in Ewing Sarcoma Cells. Cancers 2024, 16, 3565. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, F.; Wang, H.; Cai, W.; Gao, J.; Li, Y.; Cai, S. MiR-34a-5p promotes the multi-drug resistance of osteosarcoma by targeting the CD117 gene. Oncotarget 2016, 7, 28420–28434. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Shahar, N.; Larisch, S. Inhibiting the inhibitors: Targeting anti-apoptotic proteins in cancer and therapy resistance. Drug Resist. Updates 2020, 52, 100712. [Google Scholar] [CrossRef]
- Bressenot, A.; Marchal, S.; Bezdetnaya, L.; Garrier, J.; Guillemin, F.; Plénat, F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J. Histochem. Cytochem. 2009, 57, 289–300. [Google Scholar] [CrossRef]
- Bernard, A.; Chevrier, S.; Beltjens, F.; Dosset, M.; Viltard, E.; Lagrange, A.; Derangère, V.; Oudot, A.; Ghiringhelli, F.; Collin, B.; et al. Cleaved Caspase-3 Transcriptionally Regulates Angiogenesis-Promoting Chemotherapy Resistance. Cancer Res. 2019, 79, 5958–5970. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Dong, J. The Hippo Signaling Pathway in Drug Resistance in Cancer. Cancers 2021, 13, 318. [Google Scholar] [CrossRef]
- Kumar, R.; Hong, W. Hippo Signaling at the Hallmarks of Cancer and Drug Resistance. Cells 2024, 13, 564. [Google Scholar] [CrossRef]
- Qi, S.; Zhu, Y.; Liu, X.; Li, P.; Wang, Y.; Zeng, Y.; Yu, A.; Wang, Y.; Sha, Z.; Zhong, Z.; et al. WWC proteins mediate LATS1/2 activation by Hippo kinases and imply a tumor suppression strategy. Mol. Cell 2022, 82, 1850–1864.e1857. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhou, X. Targeting Hippo signaling in cancer: Novel perspectives and therapeutic potential. MedComm 2023, 4, e375. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Wu, Y.N.; Huang, J.Q.; Wang, W.; Xu, M.; Jia, J.P.; Han, G.; Mao, B.B.; Bi, W.Z. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin. J. Cancer 2016, 35, 47. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Y.L.; Yu, C.; Chang, T.; Fan, H.Y. YAP/TEAD co-activator regulated pluripotency and chemoresistance in ovarian cancer initiated cells. PLoS ONE 2014, 9, e109575. [Google Scholar] [CrossRef]
- Song, S.; Honjo, S.; Jin, J.; Chang, S.S.; Scott, A.W.; Chen, Q.; Kalhor, N.; Correa, A.M.; Hofstetter, W.L.; Albarracin, C.T.; et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clin. Cancer Res. 2015, 21, 2580–2590. [Google Scholar] [CrossRef]
- Ferraiuolo, M.; Pulito, C.; Finch-Edmondson, M.; Korita, E.; Maidecchi, A.; Donzelli, S.; Muti, P.; Serra, M.; Sudol, M.; Strano, S.; et al. Agave negatively regulates YAP and TAZ transcriptionally and post-translationally in osteosarcoma cell lines. Cancer Lett. 2018, 433, 18–32. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhai, Y.; Yuan, Y.; Wang, J.; Jin, Y.; Dang, L.; Song, L.; Chen, C.; Wang, Y. Cinobufacini injection suppresses the proliferation of human osteosarcoma cells by inhibiting PIN1-YAP/TAZ signaling pathway. Front. Pharmacol. 2023, 14, 1081363. [Google Scholar] [CrossRef] [PubMed]
- Morice, S.; Mullard, M.; Brion, R.; Dupuy, M.; Renault, S.; Tesfaye, R.; Brounais-Le Royer, B.; Ory, B.; Redini, F.; Verrecchia, F. The YAP/TEAD Axis as a New Therapeutic Target in Osteosarcoma: Effect of Verteporfin and CA3 on Primary Tumor Growth. Cancers 2020, 12, 3847. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Zhao, L.; Zhou, J.; Cheng, Y.; Xu, B.; Wang, J.; Wei, L. Spontaneous formation of tumorigenic hybrids between human omental adipose-derived stromal cells and endometrial cancer cells increased motility and heterogeneity of cancer cells. Cell Cycle 2019, 18, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.P.; Pacheco, A.R.; Coutinho, L.; Oliveira, H.; Pinho, S.; Almeida, L.; Fernandes, E.; Santos, C. Combination of etoposide and fisetin results in anti-cancer efficiency against osteosarcoma cell models. Arch. Toxicol. 2017, 92, 1205–1214. [Google Scholar] [CrossRef]
- Yuan, X.-W.; Zhu, X.-F.; Huang, X.-F.; Sheng, P.-Y.; He, A.-S.; Yang, Z.-B.; Deng, R.; Feng, G.-K.; Liao, W.-M. Interferon-α enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin by p53-dependent apoptosis. Acta Pharmacol. Sin. 2007, 28, 1835–1841. [Google Scholar] [CrossRef][Green Version]
- Cao, Z.; Kon, N.; Liu, Y.; Xu, W.; Wen, J.; Yao, H.; Zhang, M.; Wu, Z.; Yan, X.; Zhu, W.G.; et al. An unexpected role for p53 in regulating cancer cell-intrinsic PD-1 by acetylation. Sci. Adv. 2021, 7, eabf4148. [Google Scholar] [CrossRef]
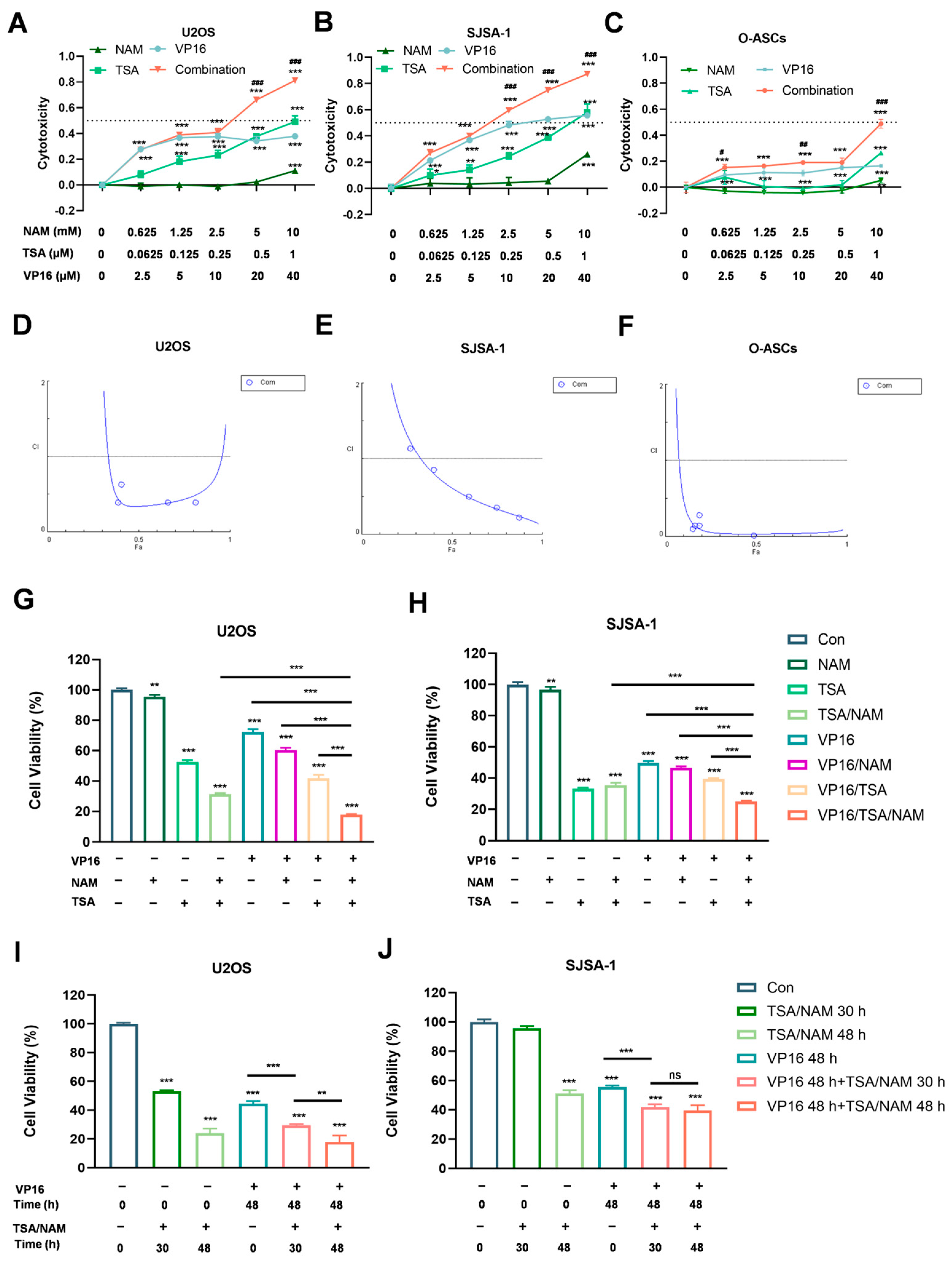
| VP16 (µM) | TSA (µM) | NAM (mM) | U2OS | SJSA-1 | O-ASCs | |||
|---|---|---|---|---|---|---|---|---|
| FA | CI | FA | CI | FA | CI | |||
| 2.5 | 0.0625 | 0.625 | 0.273 | 5.009 | 0.271 | 1.132 | 0.152 | 0.100 |
| 5 | 0.125 | 1.25 | 0.389 | 0.388 | 0.401 | 0.853 | 0.164 | 0.143 |
| 10 | 0.25 | 2.5 | 0.408 | 0.629 | 0.595 | 0.495 | 0.190 | 0.141 |
| 20 | 0.5 | 5 | 0.661 | 0.386 | 0.749 | 0.349 | 0.190 | 0.281 |
| 40 | 1 | 10 | 0.813 | 0.390 | 0.873 | 0.224 | 0.488 | 0.013 |
| Gene Name | Nucleotide Sequence (5′-3′) |
|---|---|
| BIRC5 | AGGACCACCGCATCTCTACAT |
| AAGTCTGGCTCGTTCTCAGTG | |
| DLG5 | TGAGGCGATCCACCATGAG |
| CCTCCCTGTATTTCTCCGACT | |
| PPP2R2A | CATACCAGGTGCATGAATACCTC |
| GGGTTATGTCTCGCTTTGTGTTT | |
| TEAD2 | CTTCGTGGAACCGCCAGAT |
| GGAGGCCACCCTTTTTCTCA | |
| TGFB2 | CCATCCCGCCCACTTTCTAC |
| AGCTCAATCCGTTGTTCAGGC | |
| YAP1 | TAGCCCTGCGTAGCCAGTTA |
| TCATGCTTAGTCCACTGTCTGT | |
| GAPDH | GACACCCACTCCTCCACCTTT |
| TTGCTGTAGCCAAATTCGTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Chen, Y.; Chen, M.; Fan, Q.; Sun, H.; Jin, D.; Liu, Y.; Xiong, Y.; Wang, D. HDAC Inhibitors Enhance the Chemosensitivity of Osteosarcoma Cells to Etoposide by Suppressing the Hippo/YAP Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 8935. https://doi.org/10.3390/ijms26188935
Cao Z, Chen Y, Chen M, Fan Q, Sun H, Jin D, Liu Y, Xiong Y, Wang D. HDAC Inhibitors Enhance the Chemosensitivity of Osteosarcoma Cells to Etoposide by Suppressing the Hippo/YAP Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(18):8935. https://doi.org/10.3390/ijms26188935
Chicago/Turabian StyleCao, Zhijie, Yulu Chen, Mengshan Chen, Qianjin Fan, Hui Sun, Dong Jin, Yajing Liu, Yanwen Xiong, and Donglai Wang. 2025. "HDAC Inhibitors Enhance the Chemosensitivity of Osteosarcoma Cells to Etoposide by Suppressing the Hippo/YAP Signaling Pathway" International Journal of Molecular Sciences 26, no. 18: 8935. https://doi.org/10.3390/ijms26188935
APA StyleCao, Z., Chen, Y., Chen, M., Fan, Q., Sun, H., Jin, D., Liu, Y., Xiong, Y., & Wang, D. (2025). HDAC Inhibitors Enhance the Chemosensitivity of Osteosarcoma Cells to Etoposide by Suppressing the Hippo/YAP Signaling Pathway. International Journal of Molecular Sciences, 26(18), 8935. https://doi.org/10.3390/ijms26188935







