The Extracellular Matrix Influences the miRNA Landscape of Human Mesenchymal Stromal/Stem Cells
Abstract
1. Introduction
2. Results
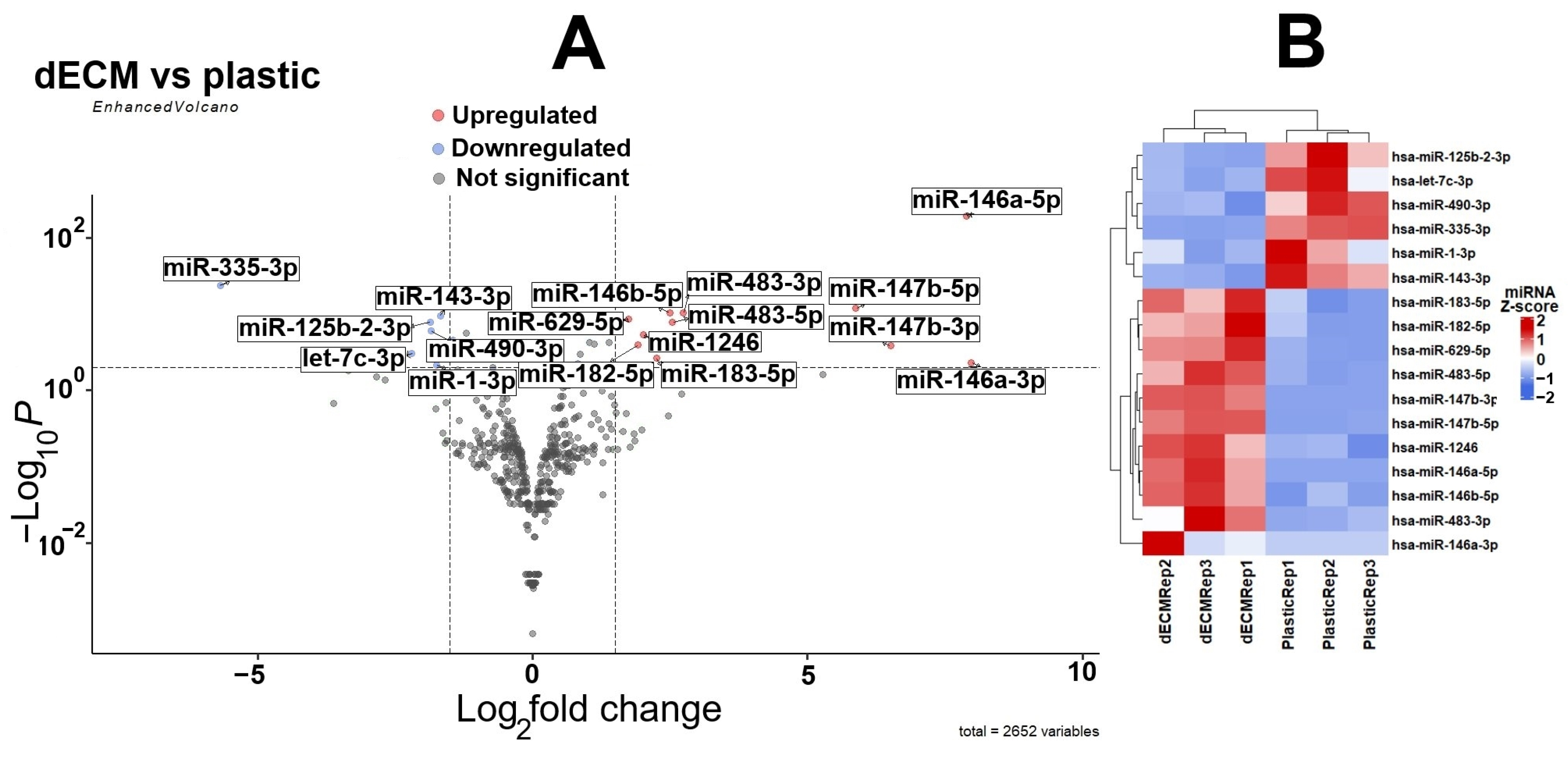
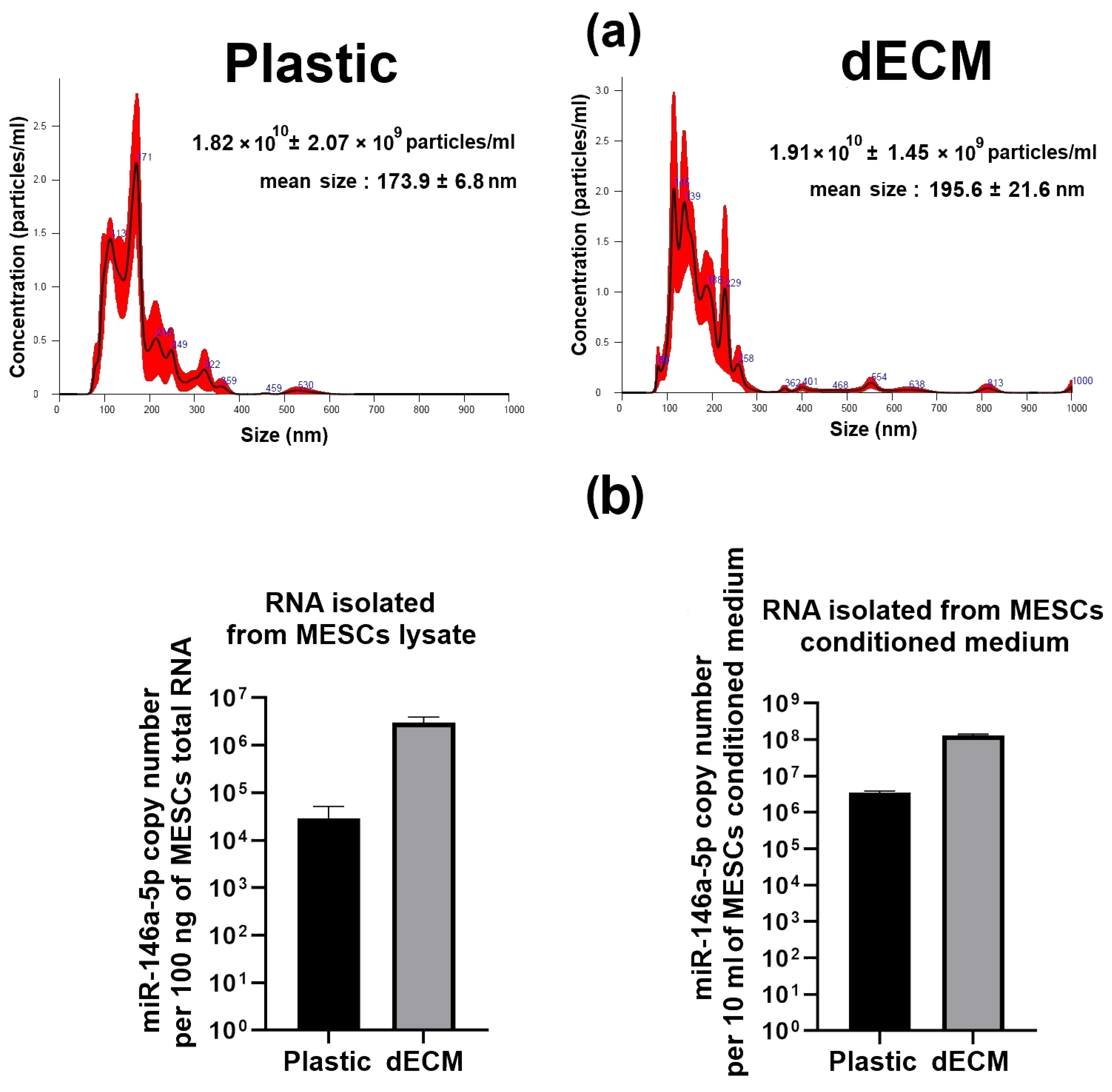
2.1. Differential Expression of miRNAs in MESCs Cultured on dECM
2.2. Differential miR-146a-5p Expression in MSCs Derived from Different Sources
2.3. miR-146a-5p Mimic Promotes Proliferation of C2C12 Myoblasts
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of dECM
4.3. Conditioned Medium Preparation
4.4. miRNA-seq
4.5. miRNA RT-qPCR
4.6. C2C12 Transfection with miRNA Oligonucleotides
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM-dECM | Conditioned medium derived from MESCs cultured on decellularized cell-derived extracellular matrix |
| dECM | decellularized cell-derived extracellular matrix |
| DMEM | Dulbecco’s modified Eagle medium |
| DP-MSCs | Mesenchymal stromal/stem cells isolated from dental pulp |
| ECM | Extracellular matrix |
| FC | Fold change |
| Fet-MSCs | Mesenchymal stromal/stem cells derived from fetal bone marrow |
| MSCs | Mesenchymal stromal/stem cells |
| MESCs | Mesenchymal stromal/stem cells derived from desquamated endometrium of menstrual blood |
| NC | Negative control |
| RT-qPCR | Reverse transcription–quantitative polymerase chain reaction |
| SD | Standard deviation |
| TEM | Transmission electron microscopy |
| WJ-MSCs | Mesenchymal stromal/stem cells isolated from Wharton’s jelly |
References
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-Art Review. Sultan Qaboos Univ. Med. J. 2018, 18, e264. [Google Scholar] [CrossRef]
- Fernández-Francos, S.; Eiro, N.; Costa, L.A.; Escudero-Cernuda, S.; Fernández-Sánchez, M.L.; Vizoso, F.J. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Cha, M.-J.; Hwang, K.-C. Cell Adhesion and Long-Term Survival of Transplanted Mesenchymal Stem Cells: A Prerequisite for Cell Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, M.A.; Shevchenko, E.K.; Molokotina, Y.D.; Makarevich, P.I.; Beloglazova, I.B.; Zubkova, E.S.; Dergilev, K.V.; Tsokolaeva, Z.I.; Penkov, D.; Hsu, M.-N.; et al. Transplantation of Adipose Stromal Cell Sheet Producing Hepatocyte Growth Factor Induces Pleiotropic Effect in Ischemic Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 3088. [Google Scholar] [CrossRef] [PubMed]
- Alexandrushkina, N.; Nimiritsky, P.; Eremichev, R.; Popov, V.; Arbatskiy, M.; Danilova, N.; Malkov, P.; Akopyan, Z.; Tkachuk, V.; Makarevich, P. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int. J. Mol. Sci. 2020, 21, 5567. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wan, F.; Morimatsu, M.; Xu, Q.; Feng, T.; Yang, H.; Gong, Y.; Ma, S.; Chang, Y.; Zhang, S.; et al. Cell Sheet Formation Enhances the Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cells on Myocardial Infarction as a Bioactive Material. Bioact. Mater. 2021, 6, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Bou-Ghannam, S.; Kim, K.; Grainger, D.W.; Okano, T. 3D Cell Sheet Structure Augments Mesenchymal Stem Cell Cytokine Production. Sci. Rep. 2021, 11, 8170. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, R.E.; Burova, E.B. Conditioned Medium of Human Mesenchymal Stromal/Stem Cells Cultured on Decellularized Extracellular Matrix Promotes Murine Skeletal Muscle Repair after Acute Injury. Biochem. Biophys. Res. Commun. 2024, 736, 150511. [Google Scholar] [CrossRef]
- Ushakov, R.; Ratushnyy, A.; Buravkova, L.; Tolkunova, E.; Burova, E. The Decellularized Cell-Derived Extracellular Matrix Enhances the Paracrine Function of Human Mesenchymal Stromal/Stem Cells. Int. J. Mol. Sci. 2024, 25, 2419. [Google Scholar] [CrossRef]
- Sen, C.K.; Ghatak, S. MiRNA Control of Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2629–2640. [Google Scholar] [CrossRef]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal MicroRNAs Derived from Mesenchymal Stem Cells: Cell-to-Cell Messages. Cell Commun. Signal. 2020, 18, 149. [Google Scholar] [CrossRef]
- Powsner, E.H.; Kronstadt, S.M.; Nikolov, K.; Aranda, A.; Jay, S.M. Mesenchymal Stem Cell Extracellular Vesicle Vascularization Bioactivity and Production Yield Are Responsive to Cell Culture Substrate Stiffness. Bioeng. Transl. Med. 2025, 10, e10743. [Google Scholar] [CrossRef]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef]
- Waldemer-Streyer, R.J.; Kim, D.; Chen, J. Muscle Cell-Derived Cytokines in Skeletal Muscle Regeneration. FEBS J. 2022, 289, 6463–6483. [Google Scholar] [CrossRef]
- Liao, Z.; Zheng, R.; Shao, G. Mechanisms and Application Strategies of MiRNA-146a Regulating Inflammation and Fibrosis at Molecular and Cellular Levels (Review). Int. J. Mol. Med. 2023, 51, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, C.; Jiang, L.; Zhu, X.; Zhou, F.; Xia, M.; Chen, Y. The Bone Mesenchymal Stem Cell-Derived Exosomal MiR-146a-5p Promotes Diabetic Wound Healing in Mice via Macrophage M1/M2 Polarization. Mol. Cell. Endocrinol. 2024, 579, 112089. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Tan, J.; Duan, Y.; Duan, J.; Wang, W.; Jin, F.; Jin, Z.; Yuan, X.; Liu, Y. Cyclic Stretch Induced MiR-146a Upregulation Delays C2C12 Myogenic Differentiation through Inhibition of Numb. Biochem. Biophys. Res. Commun. 2009, 378, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Xing, L.; Wu, J.; Wen, S.; Luo, J.; Chen, T.; Fan, Y.; Zhu, J.; Yang, L.; Liu, J.; et al. Skeletal Muscle-Derived Exosomal MiR-146a-5p Inhibits Adipogenesis by Mediating Muscle-Fat Axis and Targeting GDF5-PPARγ Signaling. Int. J. Mol. Sci. 2023, 24, 4561. [Google Scholar] [CrossRef]
- Eremichev, R.; Kulebyakina, M.; Alexandrushkina, N.; Nimiritsky, P.; Basalova, N.; Grigorieva, O.; Egiazaryan, M.; Dyikanov, D.; Tkachuk, V.; Makarevich, P. Scar-Free Healing of Endometrium: Tissue-Specific Program of Stromal Cells and Its Induction by Soluble Factors Produced After Damage. Front. Cell Dev. Biol. 2021, 9, 616893. [Google Scholar] [CrossRef]
- Huang, Y.; Crawford, M.; Higuita-Castro, N.; Nana-Sinkam, P.; Ghadiali, S.N. MiR-146a Regulates Mechanotransduction and Pressure-Induced Inflammation in Small Airway Epithelium. FASEB J. 2012, 26, 3351–3364. [Google Scholar] [CrossRef]
- Gronau, L.; Duecker, R.P.; Jerkic, S.-P.; Eickmeier, O.; Trischler, J.; Chiocchetti, A.G.; Blumchen, K.; Zielen, S.; Schubert, R. Dual Role of MicroRNA-146a in Experimental Inflammation in Human Pulmonary Epithelial and Immune Cells and Expression in Inflammatory Lung Diseases. Int. J. Mol. Sci. 2024, 25, 7686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Liu, Q.; Wang, Y.; Wang, H.; He, L.; Jin, X.; Li, N. MicroRNA MiR-147b Promotes Tumor Growth via Targeting UBE2N in Hepatocellular Carcinoma. Oncotarget 2017, 8, 114072–114080. [Google Scholar] [CrossRef] [PubMed]
- Turkowski, K.; Herzberg, F.; Günther, S.; Weigert, A.; Haselbauer, T.; Fink, L.; Brunn, D.; Grimminger, F.; Seeger, W.; Sültmann, H.; et al. MiR-147b Mediated Suppression of DUSP8 Promotes Lung Cancer Progression. Oncogene 2024, 43, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zemelko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artzibasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikolsky, N.N. Multipotent Mesenchymal Stem Cells of Desquamated Endometrium: Isolation, Characterization, and Application as a Feeder Layer for Maintenance of Human Embryonic Stem Cells. Cell Tissue Biol. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Krylova, T.A.; Koltsova, A.M.; Zenin, V.V.; Musorina, A.S.; Yakovleva, T.K.; Poljanskaya, G.G. Comparative Characteristics of New Lines of Mesenchymal Stem Cells Derived from Human Embryonic Stem Cells, Bone Marrow, and Foreskin. Cell Tissue Biol. 2012, 6, 95–107. [Google Scholar] [CrossRef]
- Koltsova, A.M.; Krylova, T.A.; Musorina, A.S.; Zenin, V.V.; Turilova, V.I.; Yakovleva, T.K.; Poljanskaya, G.G. The Dynamics of Cell Properties during Long-Term Cultivation of Two Lines of Mesenchymal Stem Cells Derived from Wharton’s Jelly of Human Umbilical Cord. Cell Tissue Biol. 2018, 12, 7–19. [Google Scholar] [CrossRef]
- Koltsova, A.M.; Zenin, V.V.; Turilova, V.I.; Yakovleva, T.K.; Poljanskaya, G.G. The Derivation and Characterization of Mesenchymal Stem Cell Line, Isolated from Human Pulp of a Deciduous Tooth. Tsitologiya 2018, 60, 955–968. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushakov, R.; Burova, E. The Extracellular Matrix Influences the miRNA Landscape of Human Mesenchymal Stromal/Stem Cells. Int. J. Mol. Sci. 2025, 26, 8830. https://doi.org/10.3390/ijms26188830
Ushakov R, Burova E. The Extracellular Matrix Influences the miRNA Landscape of Human Mesenchymal Stromal/Stem Cells. International Journal of Molecular Sciences. 2025; 26(18):8830. https://doi.org/10.3390/ijms26188830
Chicago/Turabian StyleUshakov, Roman, and Elena Burova. 2025. "The Extracellular Matrix Influences the miRNA Landscape of Human Mesenchymal Stromal/Stem Cells" International Journal of Molecular Sciences 26, no. 18: 8830. https://doi.org/10.3390/ijms26188830
APA StyleUshakov, R., & Burova, E. (2025). The Extracellular Matrix Influences the miRNA Landscape of Human Mesenchymal Stromal/Stem Cells. International Journal of Molecular Sciences, 26(18), 8830. https://doi.org/10.3390/ijms26188830





