Ameliorative Effects of Soybean Powder Fermented by Bacillus subtilis on Constipation Induced by Loperamide in Rats
Abstract
1. Introduction
2. Results
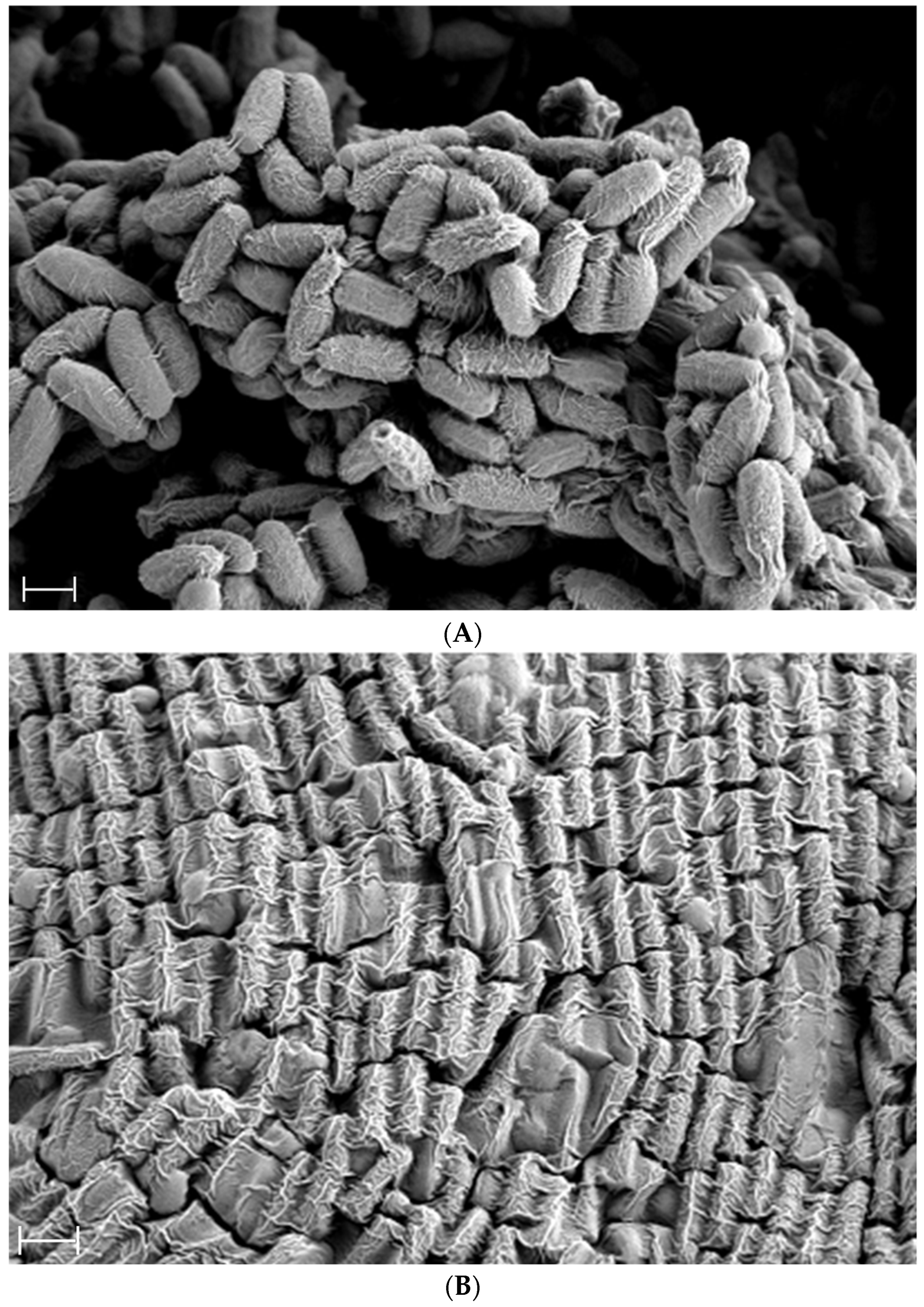
2.1. Morphological and Chemical Characteristics of Bacillus subtilis-Fermented Soybean Powder
2.2. Body Weight Changes in Loperamide-Induced Constipated Rats
2.3. Effects on Fecal Pellet Output
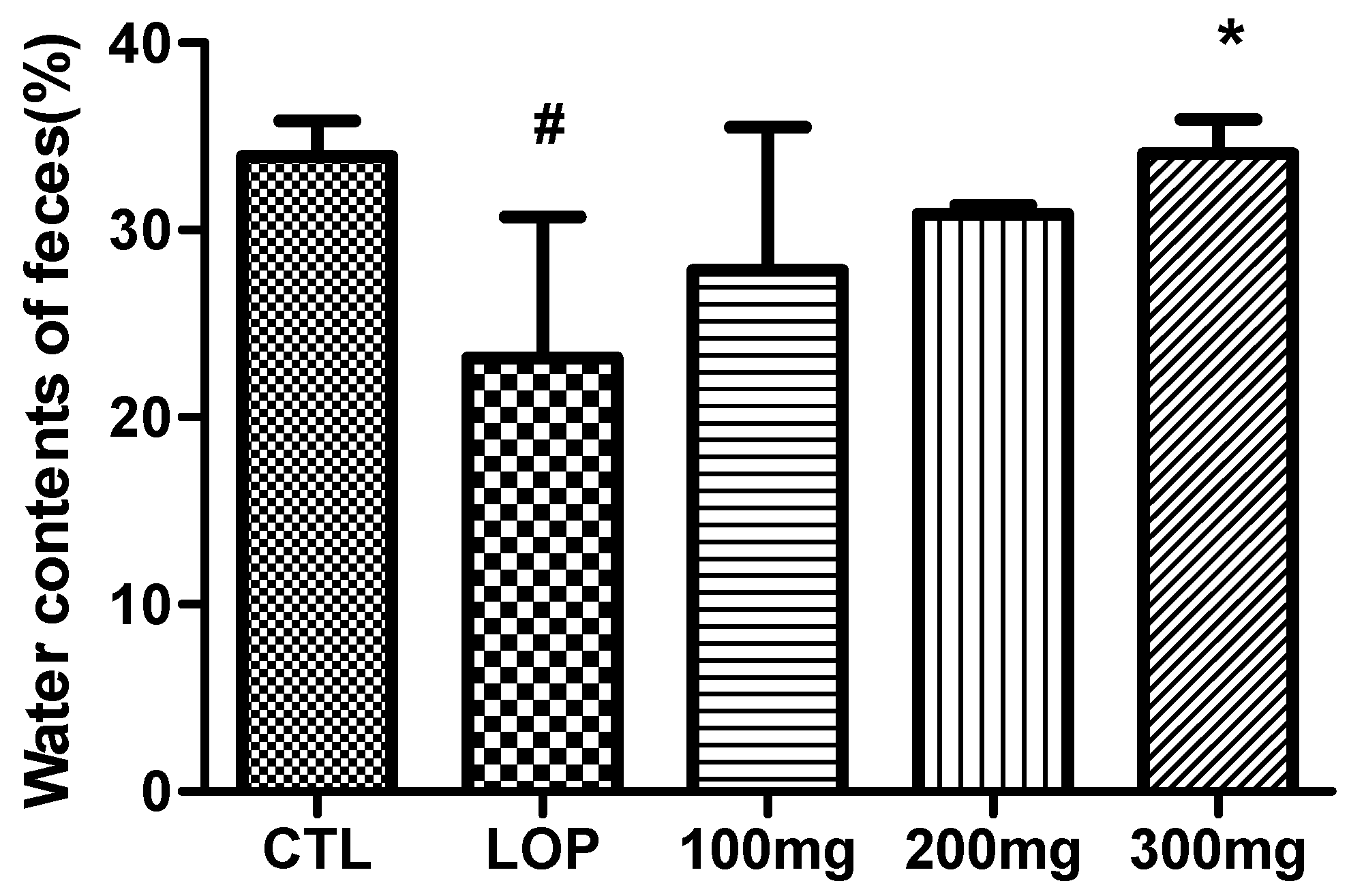
2.4. Effects on Fecal Water Content
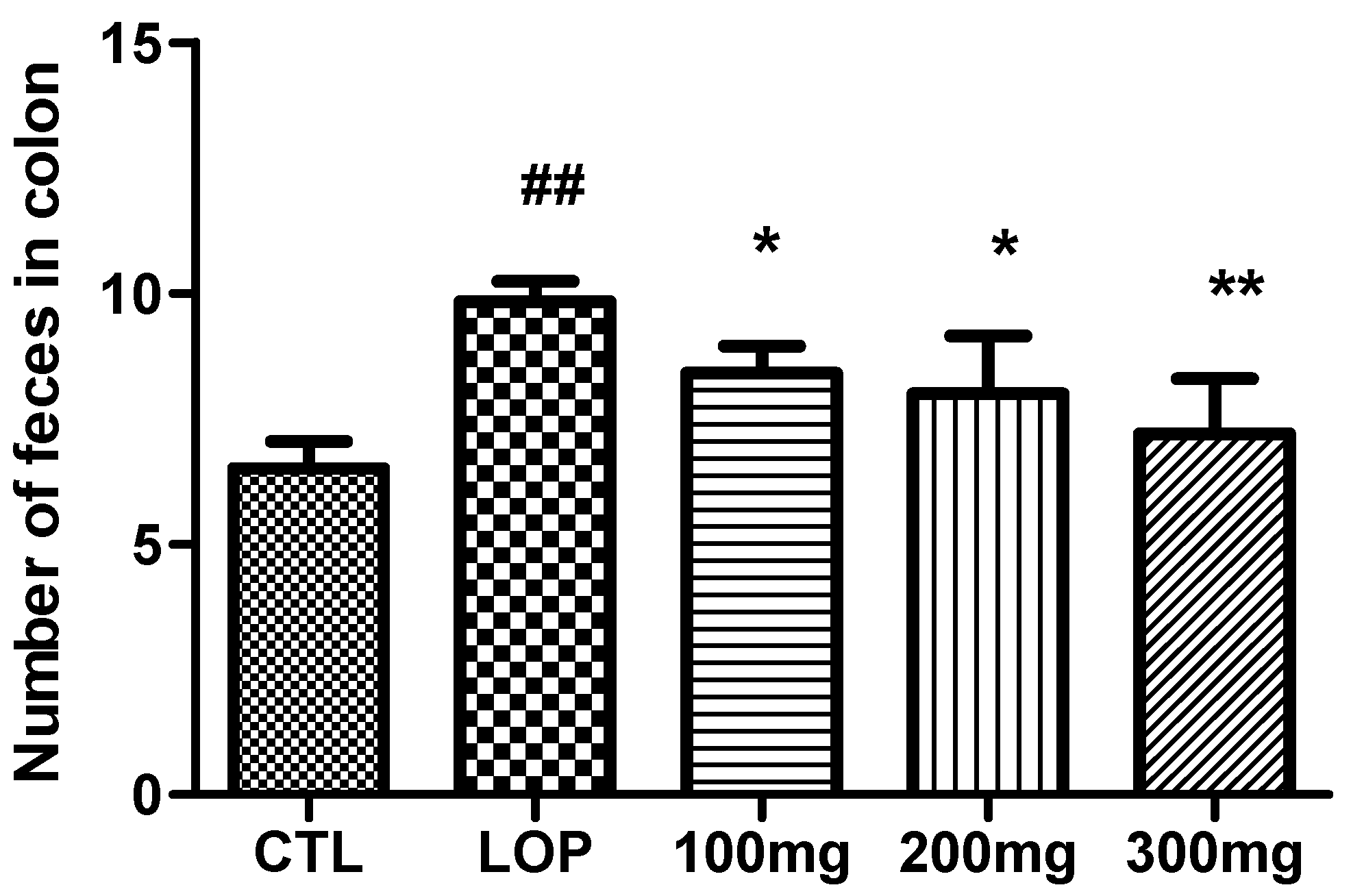
2.5. Colonic Fecal Retention
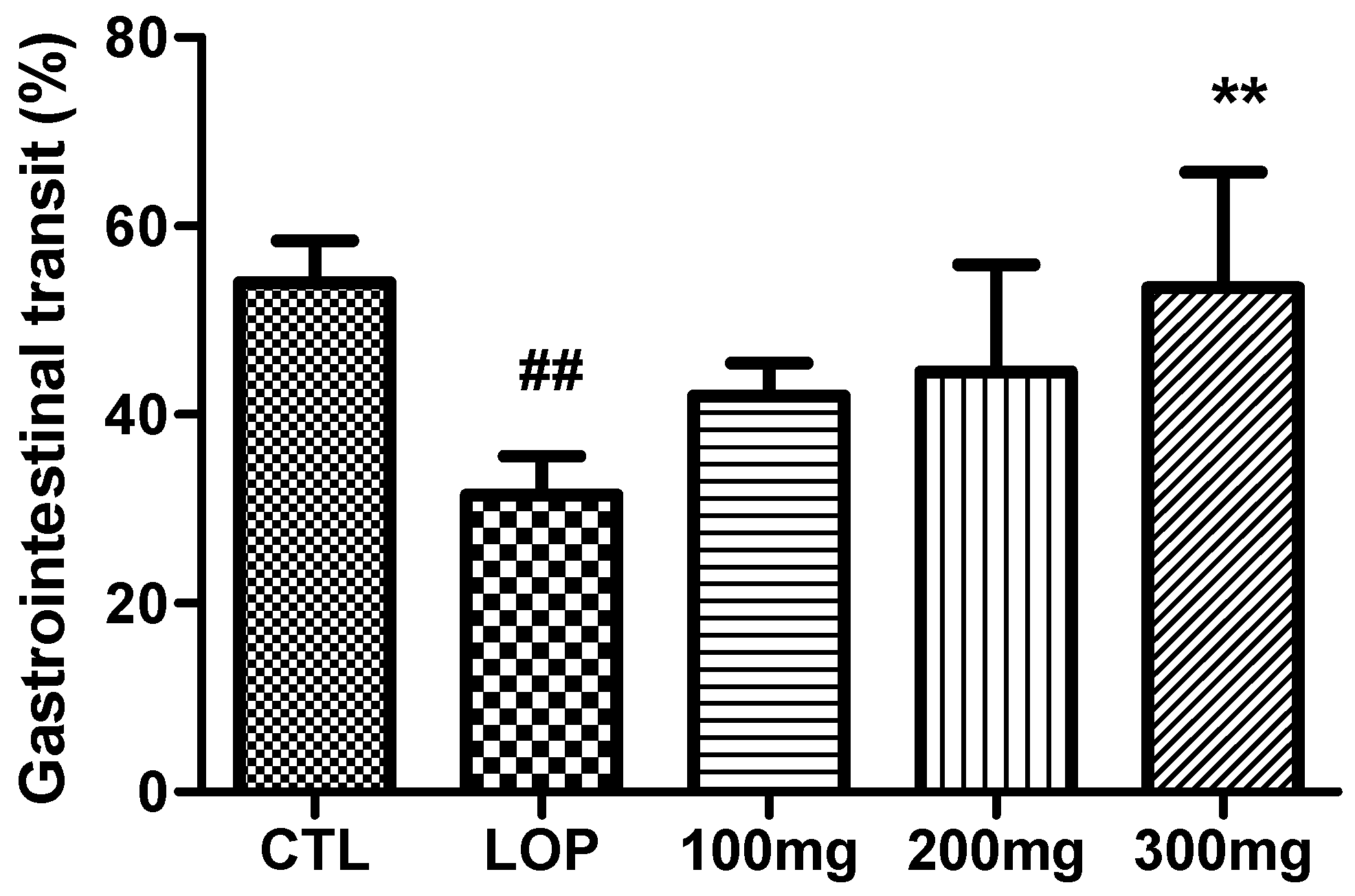
2.6. Improvement in Gastrointestinal Transit
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of Bacillus subtilis DKU_09
4.2. Preparation of Fermented Soybean and Morphological Analysis
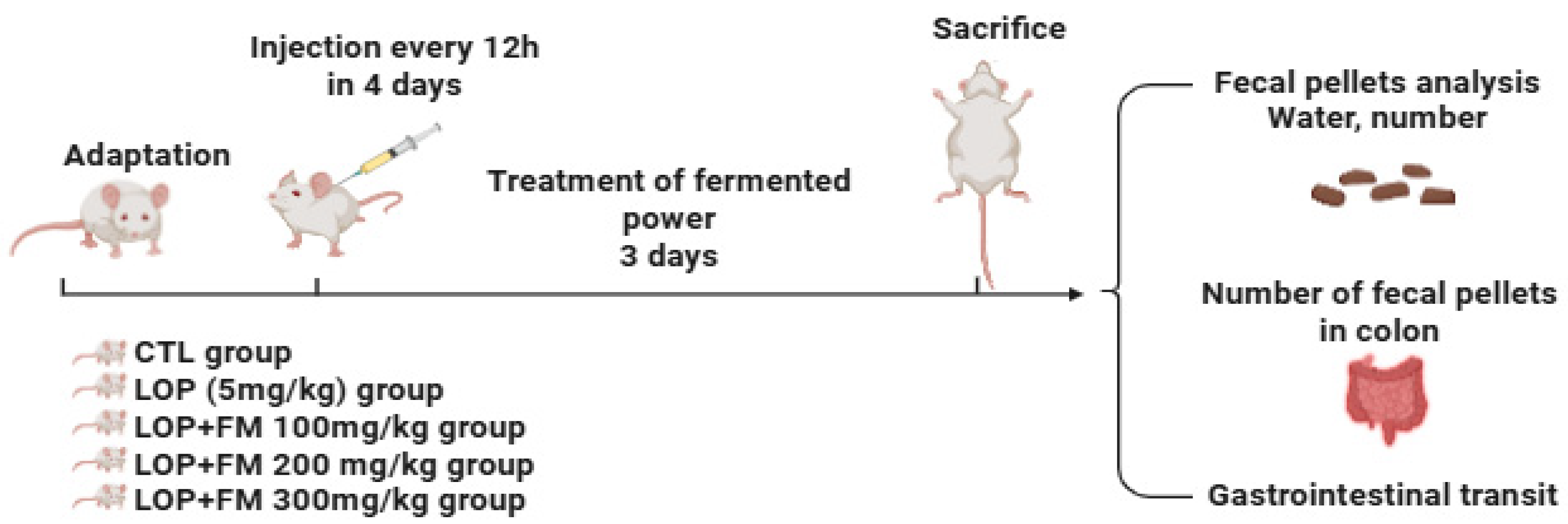
4.3. Animal Experiments Design
4.4. Total Number of Faecal Pellets, Water Contents, and Fecal Retention in the Colon
4.5. Gastrointestinal Transit Ratio
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McHugh, M.E.; Miller-Saultz, D. Assessment and management of gastrointestinal symptoms in advanced illness. Prim. Care 2011, 38, 225–246. [Google Scholar] [CrossRef]
- Portalatin, M.; Winstead, N. Medical management of constipation. Clin. Colon. Rectal. Surg. 2012, 25, 12–19. [Google Scholar] [CrossRef]
- Nelson, A.D.; Camilleri, M.; Chirapongsathorn, S.; Vijayvargiya, P.; Valentin, N.; Shin, A.; Erwin, P.J.; Wang, Z.; Murad, M.H. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: A systematic review and network meta-analysis. Gut 2017, 66, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligne, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W., 3rd. Health benefits of kimchi (korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Qiu, K.; Li, C.L.; Wang, J.; Qi, G.H.; Gao, J.; Zhang, H.J.; Wu, S.G. Effects of dietary supplementation with bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-cultures of lactobacillus acidophilus and bacillus subtilis enhance mucosal barrier by modulating gut microbiota-derived short-chain fatty acids. Nutrients 2022, 14, 4475. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Garvey, S.M.; Mah, E.; Blonquist, T.M.; Kaden, V.N.; Spears, J.L. The probiotic bacillus subtilis bs50 decreases gastrointestinal symptoms in healthy adults: A randomized, double-blind, placebo-controlled trial. Gut Microbes 2022, 14, 2122668. [Google Scholar] [CrossRef]
- Mazhar, S.; Khokhlova, E.; Colom, J.; Simon, A.; Deaton, J.; Rea, K. In vitro and in silico assessment of probiotic and functional properties of bacillus subtilis de111((r)). Front. Microbiol. 2022, 13, 1101144. [Google Scholar] [CrossRef]
- Paynich, M.L.; Jones-Burrage, S.E.; Knight, K.L. Exopolysaccharide from bacillus subtilis induces anti-inflammatory m2 macrophages that prevent t cell-mediated disease. J. Immunol. 2017, 198, 2689–2698. [Google Scholar] [CrossRef]
- Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Paradee, N.; Prommaban, A.; Ounjaijean, S.; Rerkasem, K.; Parklak, W.; Prakit, K.; Saengsitthisak, B.; et al. Antioxidant potential and cytotoxic effect of isoflavones extract from thai fermented soybean (thua-nao). Molecules 2021, 26, 7432. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.S.; Son, D.; Chung, Y.S.; Kwon, Y.H. Antioxidant activity and anti-inflammatory activity of ethanol extract and fractions of doenjang in lps-stimulated raw 264.7 macrophages. Nutr. Res. Pract. 2015, 9, 569–578. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, S.H.; Park, K.Y.; Yu, B.P.; Kim, N.D.; Jung, J.H.; Chung, H.Y. The anti-inflammatory action of fermented soybean products in kidney of high-fat-fed rats. J. Med. Food 2011, 14, 232–239. [Google Scholar] [CrossRef]
- Jang, S.E.; Kim, K.A.; Han, M.J.; Kim, D.H. Doenjang, a fermented korean soybean paste, inhibits lipopolysaccharide production of gut microbiota in mice. J. Med. Food 2014, 17, 67–75. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, I.S.; Park, S.B.; Yang, H.J.; Jeong, D.Y.; Kim, S.Y. Comparison of laxative effects of fermented soybeans (cheonggukjang) containing toxins and biogenic amines against loperamide-induced constipation mouse model. Nutr. Res. Pract. 2022, 16, 435–449. [Google Scholar] [CrossRef]
- Hong, H.A.; Huang, J.M.; Khaneja, R.; Hiep, L.V.; Urdaci, M.C.; Cutting, S.M. The safety of bacillus subtilis and bacillus indicus as food probiotics. J. Appl. Microbiol. 2008, 105, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Sorokulova, I.B.; Pinchuk, I.V.; Denayrolles, M.; Osipova, I.G.; Huang, J.M.; Cutting, S.M.; Urdaci, M.C. The safety of two bacillus probiotic strains for human use. Dig. Dis. Sci. 2008, 53, 954–963. [Google Scholar] [CrossRef]
- Aichbichler, B.W.; Wenzl, H.H.; Santa Ana, C.A.; Porter, J.L.; Schiller, L.R.; Fordtran, J.S. A comparison of stool characteristics from normal and constipated people. Dig. Dis. Sci. 1998, 43, 2353–2362. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.W.; Cho, H.R.; Kim, K.Y.; Lee, J.K.; Sohn, J.H.; Ku, S.K. Laxative effects of fermented rice extract in rats with loperamide-induced constipation. Exp. Ther. Med. 2014, 8, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Opioid receptors in the gastrointestinal tract. Regul. Pept. 2009, 155, 11–17. [Google Scholar] [CrossRef]
- Holzer, P. Treatment of opioid-induced gut dysfunction. Expert Opin. Investig. Drugs 2007, 16, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hong, J.-H.; Jeong, Y.S.; Jung, H.H. Evaluation of two bacillus subtilis strains isolated from korean fermented food as probiotics against loperamide-induced constipation in mice. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 797–806. [Google Scholar] [CrossRef]
- Chen, W.; Chung, H.H.; Cheng, J.T. Opiate-induced constipation related to activation of small intestine opioid mu2-receptors. World J. Gastroenterol. 2012, 18, 1391–1396. [Google Scholar] [CrossRef]
- Makizaki, Y.; Uemoto, T.; Yokota, H.; Yamamoto, M.; Tanaka, Y.; Ohno, H. Improvement of loperamide-induced slow transit constipation by bifidobacterium bifidum g9-1 is mediated by the correction of butyrate production and neurotransmitter profile due to improvement in dysbiosis. PLoS ONE 2021, 16, e0248584, Erratum in: PLoS ONE 2022, 17, e0267927. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin b complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Fermented soy products: A review of bioactives for health from fermentation to functionality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70080. [Google Scholar] [CrossRef]
- Nakashima, Y.; Onuki, K.; Hibi, T.; Ohno, R.I.; Sugawa, H.; Tominaga, Y.; Yasuda, S.; Kinoshita, H. Soymilk yogurt fermented using pediococcus pentosaceus tokai 759 m improves mice gut microbiota and reduces pro-inflammatory cytokine production. Biosci. Biotechnol. Biochem. 2024, 88, 1349–1361. [Google Scholar] [CrossRef]
- Zhang, X.; Al-Dossary, A.; Hussain, M.; Setlow, P.; Li, J. Applications of bacillus subtilis spores in biotechnology and advanced materials. Appl. Environ. Microbiol. 2020, 86, e01096-20. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Kim, J.-H.; Jeung, H.-W.; Lee, C.-U.; Kim, D.-S.; Li, B.; Lee, G.-H.; Sung, M.-S.; Ha, K.-C.; Back, H.-I.J.F.; et al. Effects of ficus carica paste on loperamide-induced constipation in rats. Food Chem. Toxicol. 2012, 50, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Wintola, O.A.; Sunmonu, T.O.; Afolayan, A.J. The effect of aloe ferox mill. In the treatment of loperamide-induced constipation in wistar rats. BMC Gastroenterol. 2010, 10, 95. [Google Scholar] [CrossRef] [PubMed]



| Compound/Metric | Control | 1 Day | 2 Days | 3 Days | 4 Days |
|---|---|---|---|---|---|
| Daidzein | 46.06 | 108.1 | 237.43 | 429.96 | 615.42 |
| Daidzin | 394.22 | 227.76 | 109.18 | 47.46 | 71.11 |
| Genistein | 12.52 | 93.9 | 215.37 | 429.17 | 594.96 |
| Genistin | 282.38 | 176.98 | 92.55 | 46.66 | 61.67 |
| Total Aglycones | 58.58 | 202 | 452.8 | 859.13 | 1210.39 |
| Total Glycosides | 676.6 | 404.74 | 201.73 | 94.12 | 132.78 |
| Total Isoflavones | 735.18 | 606.74 | 654.54 | 953.26 | 1343.17 |
| Aglycone Ratio (%) | 8% | 33% | 69% | 90% | 90% |
| Sample | Putative Species | Related GenBank Sequence | Query Cover (%) | Identity (%) |
|---|---|---|---|---|
| DKU_09 | Bacillus subtilis subsp. subtilis | NR_102783.2 | 100 | 99.91 |
| Bacillus subtilis | NR_116183.1 | 100 | 99.91 | |
| Bacillus subtilis | NR_113265.1 | 100 | 99.91 | |
| Bacillus subtilis | NR_112629.1 | 100 | 99.91 | |
| Bacillus subtilis | NR_027552.1 | 100 | 99.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.S.; Kim, S.K.; Ban, J.Y.; Oh, C.-H. Ameliorative Effects of Soybean Powder Fermented by Bacillus subtilis on Constipation Induced by Loperamide in Rats. Int. J. Mol. Sci. 2025, 26, 7615. https://doi.org/10.3390/ijms26157615
Lee GS, Kim SK, Ban JY, Oh C-H. Ameliorative Effects of Soybean Powder Fermented by Bacillus subtilis on Constipation Induced by Loperamide in Rats. International Journal of Molecular Sciences. 2025; 26(15):7615. https://doi.org/10.3390/ijms26157615
Chicago/Turabian StyleLee, Gi Soo, Su Kang Kim, Ju Yeon Ban, and Chung-Hun Oh. 2025. "Ameliorative Effects of Soybean Powder Fermented by Bacillus subtilis on Constipation Induced by Loperamide in Rats" International Journal of Molecular Sciences 26, no. 15: 7615. https://doi.org/10.3390/ijms26157615
APA StyleLee, G. S., Kim, S. K., Ban, J. Y., & Oh, C.-H. (2025). Ameliorative Effects of Soybean Powder Fermented by Bacillus subtilis on Constipation Induced by Loperamide in Rats. International Journal of Molecular Sciences, 26(15), 7615. https://doi.org/10.3390/ijms26157615





