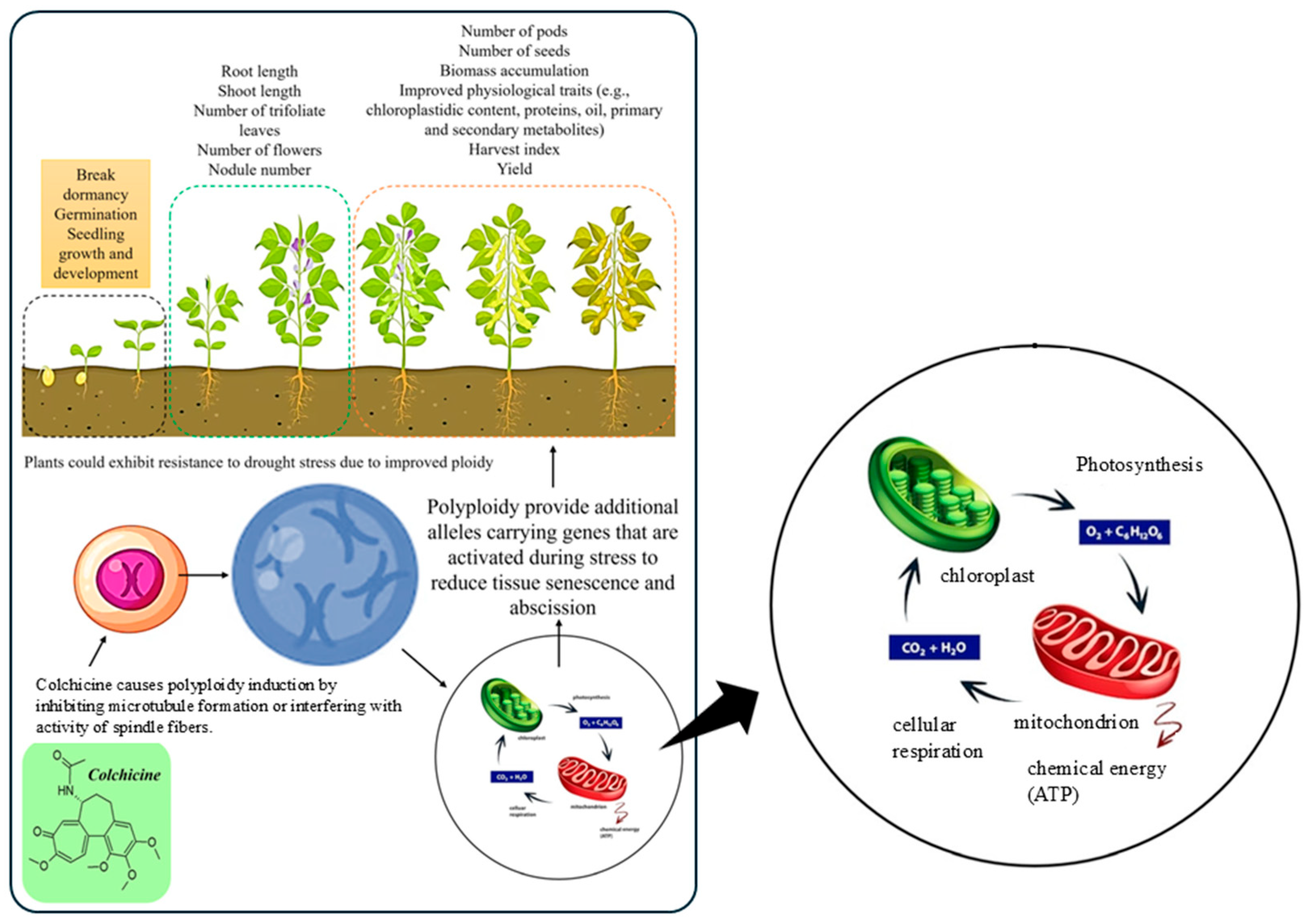
The Role of Colchicine in Plant Breeding
Abstract
1. Introduction
2. Comparison of Colchicine with Other Agents
2.1. Agents with Similar Mechanism to Colchicine
2.2. Agents with Different Mechanisms
3. Methods of Colchicine Application
3.1. In Vitro System
3.2. The Ex Vitro System
3.3. The In-Vivo System
4. Colchicine in Medicinal Plant Breeding
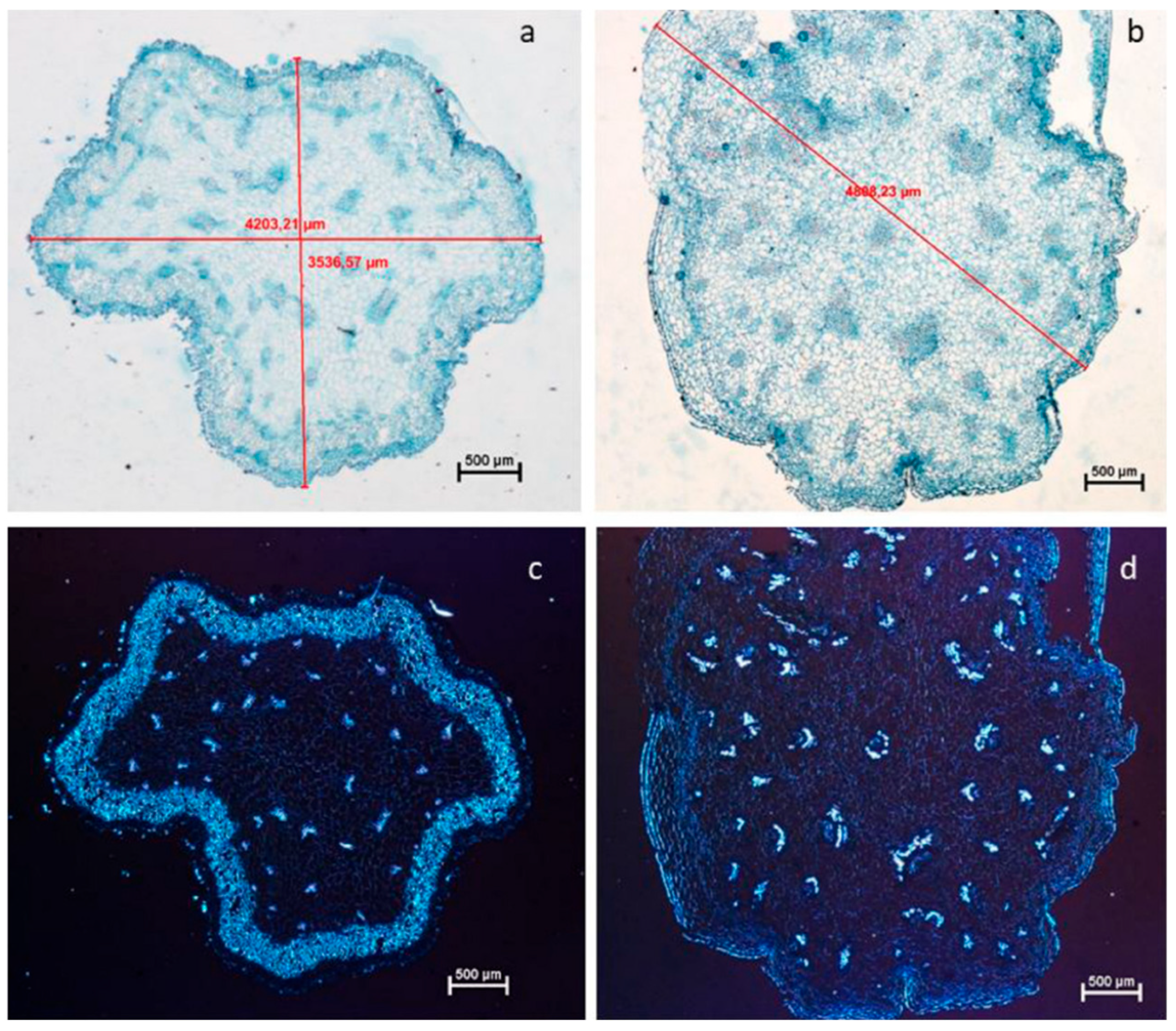
4.1. Applications in Chromosome Doubling
4.2. Impact on Secondary Metabolite Production
4.3. Genetic Stability in Polyploid Plants
4.4. Optimization of Dosage and Treatment Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, G.; Memon, A.G.; Pandey, B.; Khan, M.S.; Iqbal, M.S.; Srivastava, J.K. Colchicine Induced Mutation in Nigella sativa Plant for the Assessment of Morpho-Physiological and Biochemical Parameter Vis-A-Vis In Vitro Anti-Inflammatory Activity. Open Biotechnol. J. 2021, 15, 173–182. [Google Scholar] [CrossRef]
- Alam, Q.; Khah, M.A.; Azad, Z.R.A.A. Comparative Analysis of Different Chemical Mutagens in Inducing Chromosomal Aberrations in Meiotic Cells of Triticum aestivum L. Cytologia 2022, 87, 99–105. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Raouf Fard, F. Effect of different antimitotic agents on polyploid induction of anise hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Yousef, E.; Elsadek, M. A Comparative study of morphological and volatile oil composition characteristics in diploid and tetraploid garlic plants. Egypt. J. Hortic. 2020, 47, 295–308. [Google Scholar] [CrossRef]
- Amin, R.; Laskar, R.A.; Khursheed, S.; Raina, A.; Khan, S. Genetic sensitivity towards MMS mutagenesis assessed through in vitro growth and cytological test in Nigella sativa L. Life Sci. Int. Res. J. 2016, 3, 1–9. [Google Scholar]
- Zhang, R.; Rao, S.; Wang, Y.; Qin, Y.; Qin, K.; Chen, J. Chromosome Doubling Enhances Biomass and Carotenoid Content in Lycium chinense. Plants 2024, 13, 439. [Google Scholar] [CrossRef]
- Talei, D.; Nekouei, M.K.; Mardi, M.; Kadkhodaei, S. Improving productivity of steviol glycosides in Stevia rebaudiana via induced polyploidy. J. Crop Sci. Biotechnol. 2020, 23, 301–309. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, F.; Fu, J.; Xiao, Y.; Wu, J. In vitro polyploid induction using colchicine for Zingiber Officinale Roscoe cv. ‘Fengtou’ ginger. Plant Cell Tissue Organ Cult. 2020, 142, 87–94. [Google Scholar] [CrossRef]
- Sanaei-Hoveida, Z.; Mortazavian, S.M.M.; Norouzi, M.; Sadat-Noori, S.A. Elevating morphology and essential oil in cumin genotypes through polyploidy induction. Sci. Hortic. 2024, 329, 113031. [Google Scholar] [CrossRef]
- Revathi, B.S.; Thomas, B. In vivo polyploidy induction in Dendrobium crumenatum through colchicine treatment. J. Appl. Hortic. 2022, 24, 317–321. [Google Scholar] [CrossRef]
- Moetamedipoor, S.A.; Jowkar, A.; Saharkhiz, M.J.; Hassani, H.S. Hexaploidy induction improves morphological, physiological and phytochemical characteristics of mojito mint (Mentha × villosa). Sci. Hortic. 2022, 295, 110810. [Google Scholar] [CrossRef]
- Bharati, R.; Fernández-Cusimamani, E.; Gupta, A.; Novy, P.; Moses, O.; Severová, L.; Svoboda, R.; Šrédl, K. Oryzalin induces polyploids with superior morphology and increased levels of essential oil production in Mentha spicata L. Ind. Crops Prod. 2023, 198, 116683. [Google Scholar] [CrossRef]
- Islam, M.A.; Tarannum, F.; Dina, A.H.; Ahmed, M.; Haque, M.A.; Ercişli, S.; Rasul, M.G.; Simsek, D.; Hasan, M. Phenotypic and Biochemical Trait Improvement in Husk Tomatoes (Physalis sp.) Through EMS-Induced Mutagenesis. Horticulturae 2024, 10, 913. [Google Scholar] [CrossRef]
- Hamarashid, S.H.; Khaledian, Y.; Soleimani, F. In vitro polyploidy-mediated enhancement of secondary metabolites content in Stachys byzantina L. Genet. Resour. Crop Evol. 2022, 69, 719–728. [Google Scholar] [CrossRef]
- Laskar, R.A.; Khan, S. Mutagenic effects of MH and MMS on induction of variability in broad bean (Vicia faba L.). Annu. Res. Rev. Biol. 2014, 4, 1129–1140. [Google Scholar] [CrossRef]
- Sadat Noori, S.A.; Norouzi, M.; Karimzadeh, G.; Shirkool, K.; Niazian, M. Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult. 2017, 130, 543–551. [Google Scholar] [CrossRef]
- Vilcherrez-Atoche, J.A.; Silva, J.C.; Clarindo, W.R.; Mondin, M.; Cardoso, J.C. In Vitro Polyploidization of Brassolaeliocattleya Hybrid Orchid. Plants 2023, 12, 281. [Google Scholar] [CrossRef]
- Nura, S.; Adamu, A.K.; Adelanwa, M.A.; Usman, I.S.; Shehu, K. Colchicine-induced mutagenesis for improved growth and yield of fonio (Digitaria exilis [Kippist] Stapf.). Bayero J. Pure Appl. Sci. 2017, 10, 126–133. [Google Scholar] [CrossRef]
- Karami, L.; Modarresi, M.; Kohanmoo, M.A.; Zahabi Ahmadi, F.; Irian, S. Polyploidy Induction in German Chamomile (Matricaria chamomilla L.) by Herbicide Trifluralin. Nova Biol. Reper. 2019, 6, 311–319. [Google Scholar] [CrossRef]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. Colchicine induces tetraploids in in vitro cultures of Digitalis lanata Ehrh.: Enhanced production of biomass and cardiac glycosides. Ind. Crops Prod. 2021, 174, 114167. [Google Scholar] [CrossRef]
- Taratima, W.; Rohmah, K.N.; Plaikhuntod, K.; Maneerattanarungroj, P.; Trunjaruen, A. Optimal protocol for in vitro polyploid induction of Cymbidium aloifolium (L.) Sw. BMC Plant Biol. 2023, 23, 295. [Google Scholar] [CrossRef]
- Arisha, M.H.; Shah, S.N.; Gong, Z.H.; Jing, H.; Li, C.; Zhang, H.X. Ethyl methane sulfonate induced mutations in M2 generation and physiological variations in M1 generation of peppers (Capsicum annuum L.). Front. Plant Sci. 2015, 6, 399. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, S.I.; Meiningrum, N.I.; Yudha, Y.S.; Nurcholis, W. Variability of agromorphological traits in Portulaca grandiflora through induced mutation using colchicine. Biodiversitas J. Biol. Divers. 2024, 25, 2484–2493. [Google Scholar] [CrossRef]
- Mangena, P.; Mushadu, P.N. Colchicine-induced polyploidy in leguminous crops enhances morpho-physiological characteristics for drought stress tolerance. Life 2023, 13, 1966. [Google Scholar] [CrossRef]
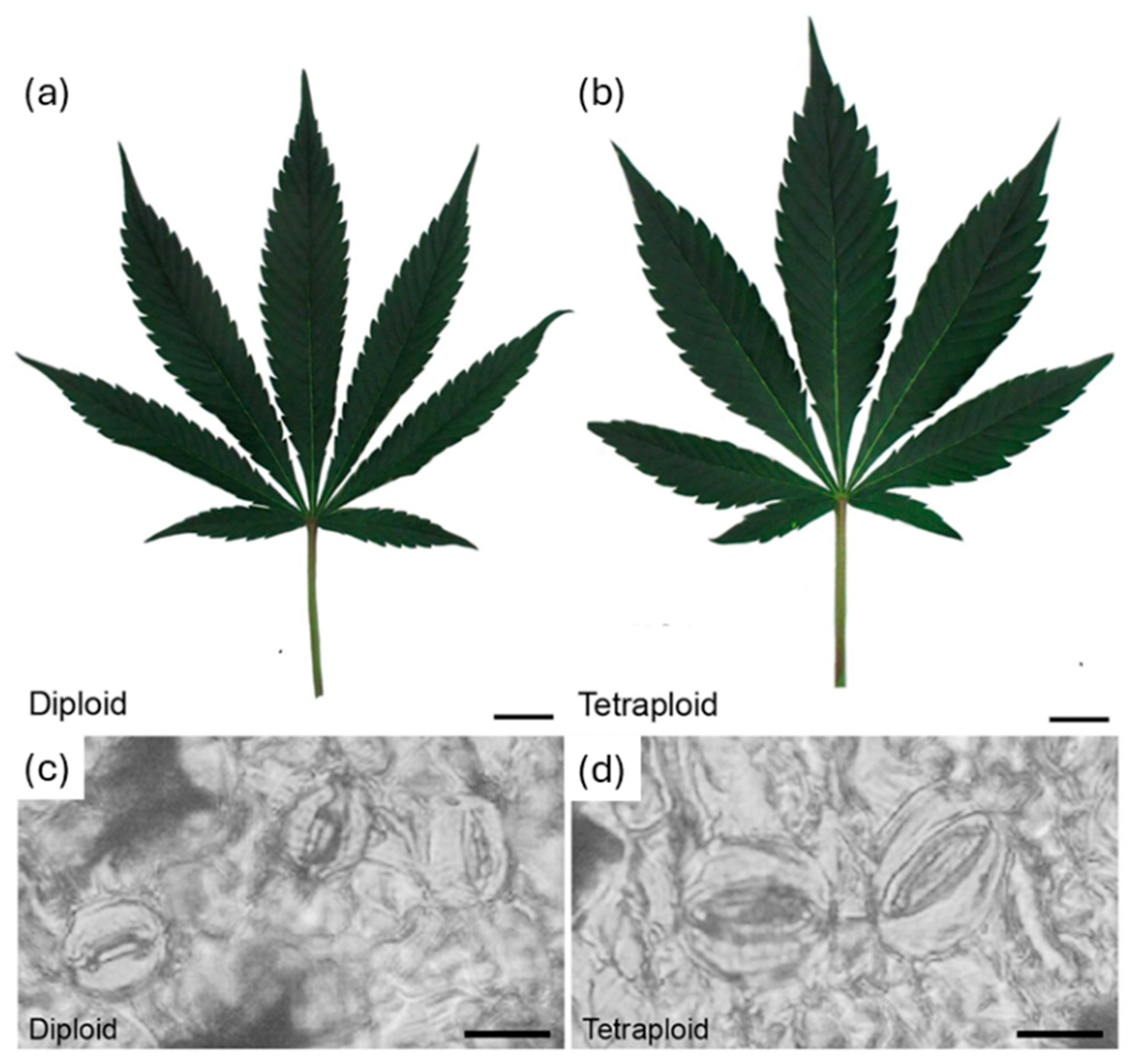
- Parsons, J.L.; Martin, S.L.; James, T.; Golenia, G.; Boudko, E.A.; Hepworth, S.R. Polyploidization for the Genetic Improvement of Cannabis sativa. Front. Plant Sci. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, B.; Švécarová, M.; Bednář, J.; Ondřej, V. In Vitro Polyploidization of Thymus vulgaris L. and Its Effect on Composition of Essential Oils. Agronomy 2021, 11, 596. [Google Scholar] [CrossRef]
- Homaidan Shmeit, Y.; Fernandez, E.; Novy, P.; Kloucek, P.; Orosz, M.; Kokoska, L. Autopolyploidy effect on morphological variation and essential oil content in Thymus vulgaris L. Sci. Hortic. 2020, 263, 109095. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J. In vitro tetraploid induction from multigenotype protocorms and tetraploid regeneration in Dendrobium officinale. Plant Cell Tissue Organ Cult. 2020, 141, 289–298. [Google Scholar] [CrossRef]
- Lei, L.; Liu, G.; Yan, D.; Zhang, M.; Cui, Q.; Zhao, Q.; Chu, L.; Wen, L.; Wang, L.; Du, Q.; et al. Manipulation of ploidy for blueberry breeding: In vitro chromosome doubling of diploid Vaccinium duclouxii (Lévl.) Hand.-Mazz by trifluralin. Sci. Hortic. 2023, 317, 112056. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Zhou, X.J.; Khan, M.A.; Su, L.; Meng, H.W. In vitro induction of tetraploid garlic with trifluralin. Genet. Mol. Res. 2012, 11, 2620–2628. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Trzewik, A.; Marasek-Ciolakowska, A. In vitro polyploidisation of tulips (Tulipa gesneriana L.)—Phenotype assessment of tetraploids. Sci. Hortic. 2018, 242, 155–163. [Google Scholar] [CrossRef]
- Ebrahimzadeh, H.; Soltanloo, H.; Shariatpanahi, M.E.; Eskandari, A.; Ramezanpour, S.S. Improved chromosome doubling of parthenogenetic haploid plants of cucumber (Cucumis sativus L.) using colchicine, trifluralin, and oryzalin. Plant Cell Tissue Organ Cult. 2018, 135, 407–417. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Chen, J.-J.; Cao, Y.-M.; Duan, J.-X.; Cai, X.-D. Induction of tetraploids in ‘Red Flash’ caladium using colchicine and oryzalin: Morphological, cytological, photosynthetic and chilling tolerance analysis. Sci. Hortic. 2020, 272, 109524. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Li, Y.; Xu, Y.; Wei, Q.; Wang, J.; Lin, F.; Gong, D.; Liu, F.; Wang, Y.; et al. A Novel Banana Mutant “RF 1” (Musa spp. ABB, Pisang Awak Subgroup) for Improved Agronomic Traits and Enhanced Cold Tolerance and Disease Resistance. Front. Plant Sci. 2021, 12, 730718. [Google Scholar]
- Gao, Y.; Qu, G.; Huang, S.; Liu, Z.; Zhang, M.; Fu, W.; Ren, J.; Feng, H. Comparison Between Germinated Seed and Isolated Microspore EMS Mutagenesis in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Horticulturae 2022, 8, 232. [Google Scholar] [CrossRef]
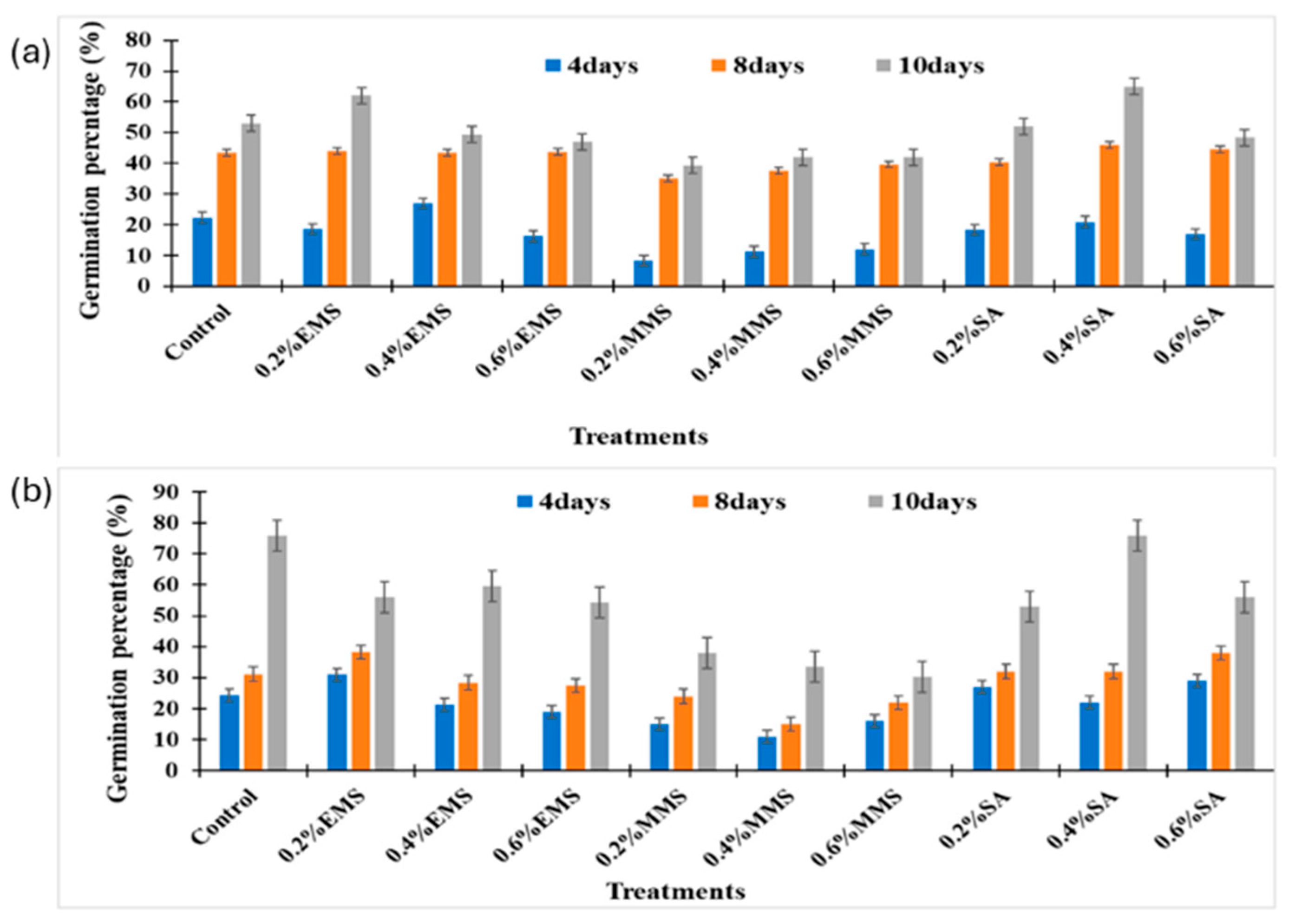
- Opoku Gyamfi, M.; Eleblu, J.S.Y.; Sarfoa, L.G.; Asante, I.K.; Opoku-Agyemang, F.; Danquah, E.Y. Induced variations of ethyl methane sulfonate mutagenized cowpea (Vigna unguiculata L. walp) plants. Front. Plant Sci. 2022, 13, 952247. [Google Scholar] [CrossRef]
- Din, A.; Qadri, Z.A.; Wani, M.A.; Banday, N.; Iqbal, S.; Nazki, I.T.; Wani, F.J. Enhancing Flower Color Diversity in Chrysanthemum cv. “Candid” Through Ethyl Methane Sulfonate Mutagenesis: A Promising Approach for Ornamental Crop Improvement. ACS Agric. Sci. Technol. 2023, 3, 1004–1013. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhry, S.; Laskar, R.A. Studies on qualitative and quantitative characters of mutagenised chili populations induced through MMS and EMS. Vegetos 2020, 33, 793–799. [Google Scholar] [CrossRef]
- Naaz, N.; Ansari, S.B.; Khan, S.; Choudhary, S.; Jahan, R. Physio-morphological variations induced by methyl methane sulphonate in Trigonella foenum-graecum L. Int. J. Bot. Stud. 2020, 5, 37–42. [Google Scholar]
- Samadi, N.; Naghavi, M.R.; Moratalla-Lopez, N.; Alonso, G.L.; Shokrpour, M. Morphological, molecular and phytochemical variations induced by colchicine and EMS chemical mutagens in Crocus sativus L. Food Chem. 2022, 4, 100086. [Google Scholar] [CrossRef]
- Hamisu, A.; Koul, B.; Arukha, A.P.; Al Nadhari, S.; Rabbee, M.F. Evaluation of the Impact of Chemical Mutagens on the Phenological and Biochemical Characteristics of Two Varieties of Soybean (Glycine max L.). Life 2024, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Cabahug, R.A.M.; Ha, M.K.T.T.; Lim, K.-B.; Hwang, Y.-J. LD50 determination and phenotypic evaluation of three Echeveria varieties induced by chemical mutagens. Toxicol. Environ. Health Sci. 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Shariat, A.; Sefidkon, F. Tetraploid induction in savory (Satureja khuzistanica): Cytological, morphological, phytochemical and physiological changes. Plant Cell Tissue Organ Cult. 2021, 146, 137–148. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Kong, B.; Zhou, Q.; Sang, Y.; Zhang, P. In vitro octaploid induction of Populus hopeiensis with colchicine. BMC Plant Biol. 2022, 22, 176. [Google Scholar] [CrossRef]
- Fathurrahman, F.; Mardaleni, M.; Krisianto, A. Effect of colchicine mutagen on phenotype and genotype of Vigna unguiculata var. sesquipedalis the 7th generation. Biodiversitas J. Biol. Divers. 2023, 24, 1408–1416. [Google Scholar] [CrossRef]
- Azizan, N.I.; Shamsiah, A.; Hasan, N.A.; Hussein, S. Morphological characterization of Colchicine-induced Mutants in Stevia rebaudiana. IOP Conf. Ser. Earth Environ. Sci. 2021, 757, 012006. [Google Scholar] [CrossRef]
- Ren, J.; Lu, X.; Duan, Y.-Y.; Xiao, G.-A.; Xie, K.-D.; Wu, X.-M.; Guo, W.-W. In vivo tetraploid induction of mono-embryonic citrus genotypes by colchicine treatment. Sci. Hortic. 2024, 338, 113701. [Google Scholar] [CrossRef]
- Kara, Z.; Yazar, K. Induction of polyploidy in grapevine (Vitis vinifera L.) seedlings by in vivo colchicineapplications. Turk. J. Agric. For. 2022, 46, 152–159. [Google Scholar] [CrossRef]
- McLeod, A.; Contreras, R.; Halstead, M.; Vining, K. In Vivo and In Vitro Chromosome Doubling of ‘I3’ Hemp. HortScience 2023, 58, 1018–1022. [Google Scholar] [CrossRef]
- Abdoli, M.; Moieni, A.; Naghdi Badi, H. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol. Plant. 2013, 35, 2075–2083. [Google Scholar] [CrossRef]
- Majdi, M.; Karimzadeh, G.; Malboobi, M.A.; Omidbaigi, R.; Mirzaghaderi, G. Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): Morphological, physiological, cytological, and phytochemical changes. HortScience 2010, 45, 16–21. [Google Scholar] [CrossRef]
- Estaji, A.; Hosseini, B.; Ghotbi Ravandi, E.; Dehghan, E.; Sefidkon, F. The effects of colchicine-induced autotetraploidy on selected characteristics of nuruozak (Salvia leriifolia). Cytol. Genet. 2017, 51, 74–81. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.; Li, X.; Jin, Z.; Li, J. In Vitro Inducted Tetraploid Elsholtzia splendens Nakai ex F. Maek. Alters Polyphenol Species and Synthesis. Plants 2024, 13, 3374. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, G.; Thirugnanasampandan, R.; Gogulramnath, M. Effect of colchicine induced tetraploidy on morphology, cytology, essential oil composition, gene expression and antioxidant activity of Citrus limon (L.) Osbeck. Physiol. Mol. Biol. Plants 2020, 26, 271–279. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, B.; Zou, J.; Luo, Z.; Yang, H.; Zhou, P.; Chen, X.; Zhou, W. Induction of tetraploids in Paper Mulberry (Broussonetia papyrifera (L.) L’Her. ex Vent.) by colchicine. BMC Plant Biol. 2023, 23, 574. [Google Scholar] [CrossRef]
- Kasmiyati, S.; Kristiani, E.B.E.; Herawati, M.M. Effect of Induced Polyploidy on Plant Growth, Chlorophyll and Flavonoid Content of Artemisia cina. Biosaintifika J. Biol. Biol. Educ. 2020, 12, 90–96. [Google Scholar] [CrossRef]
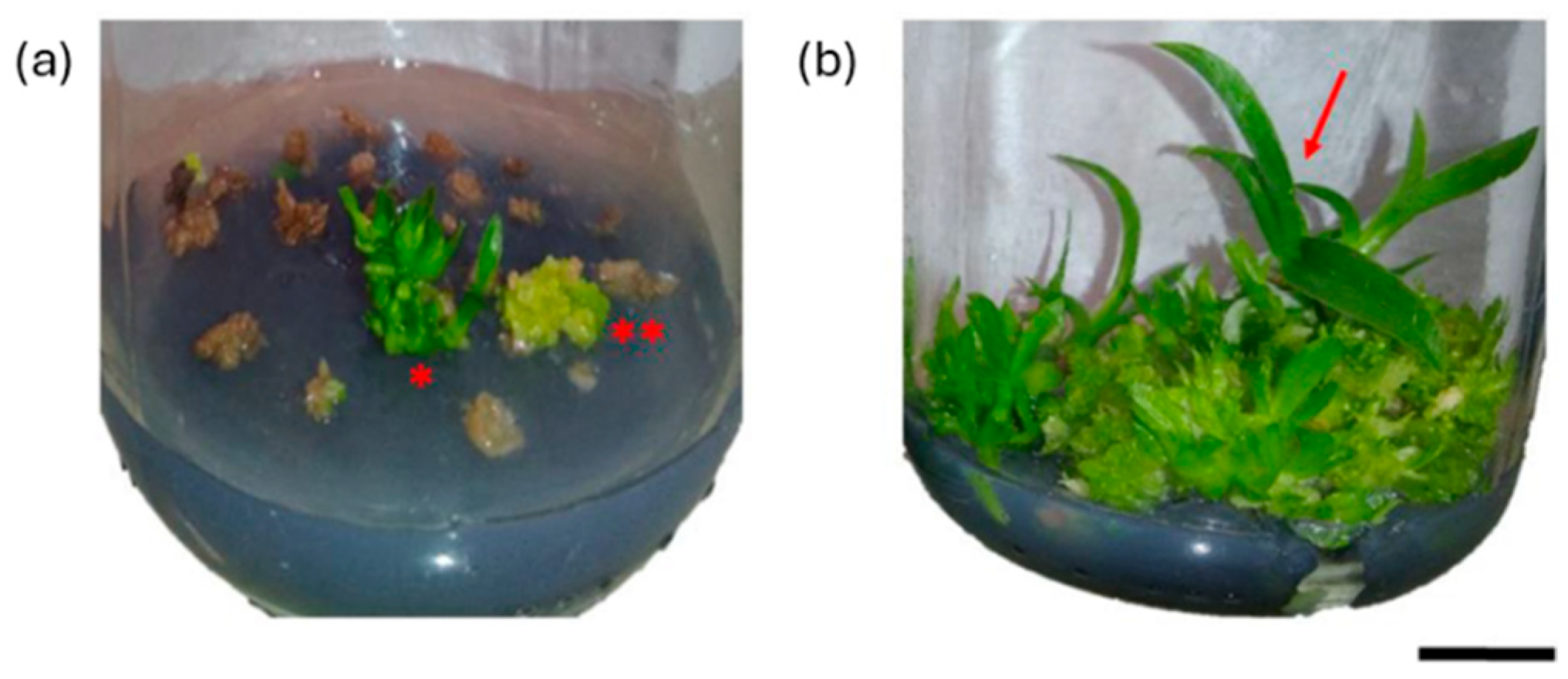
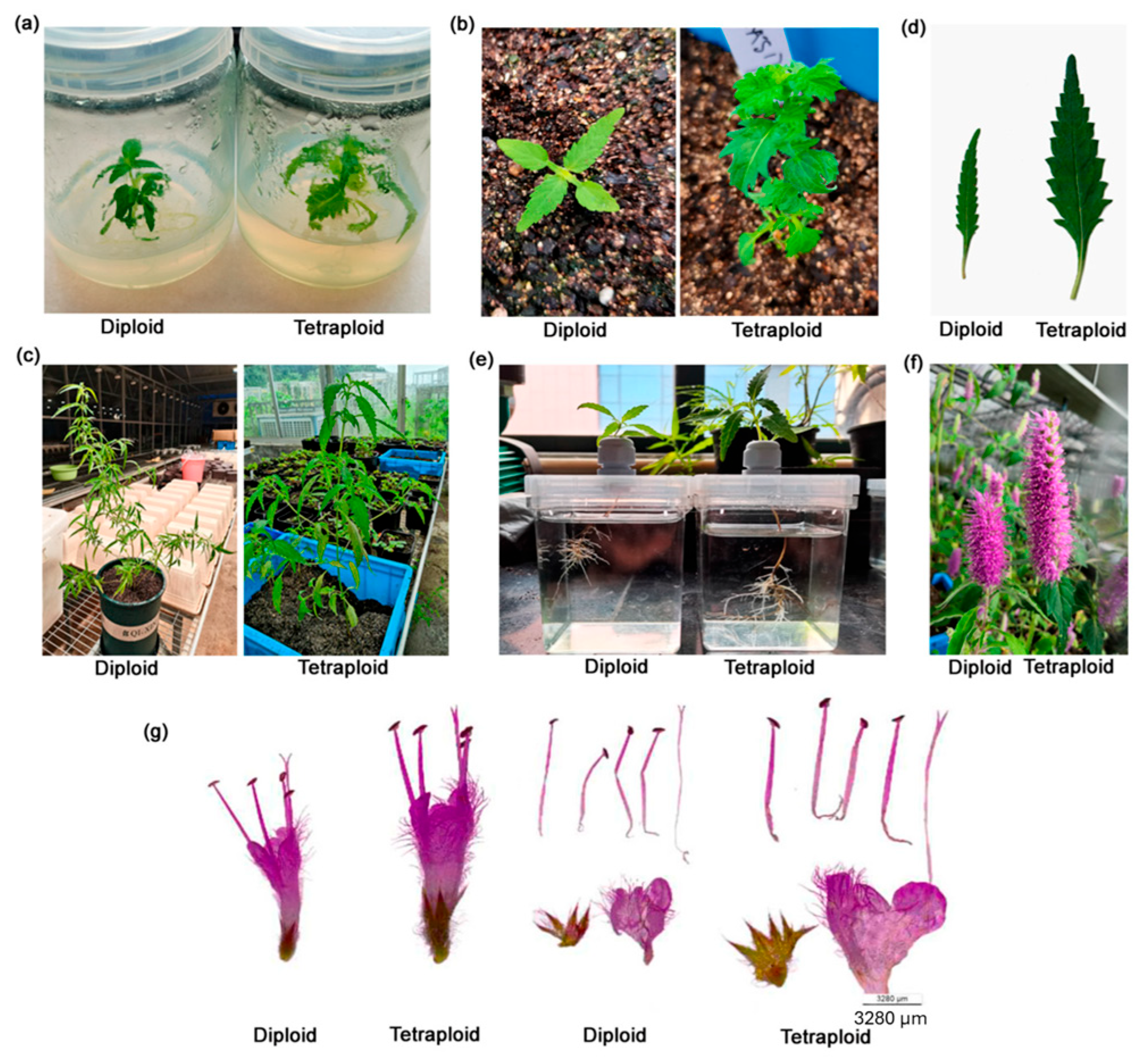
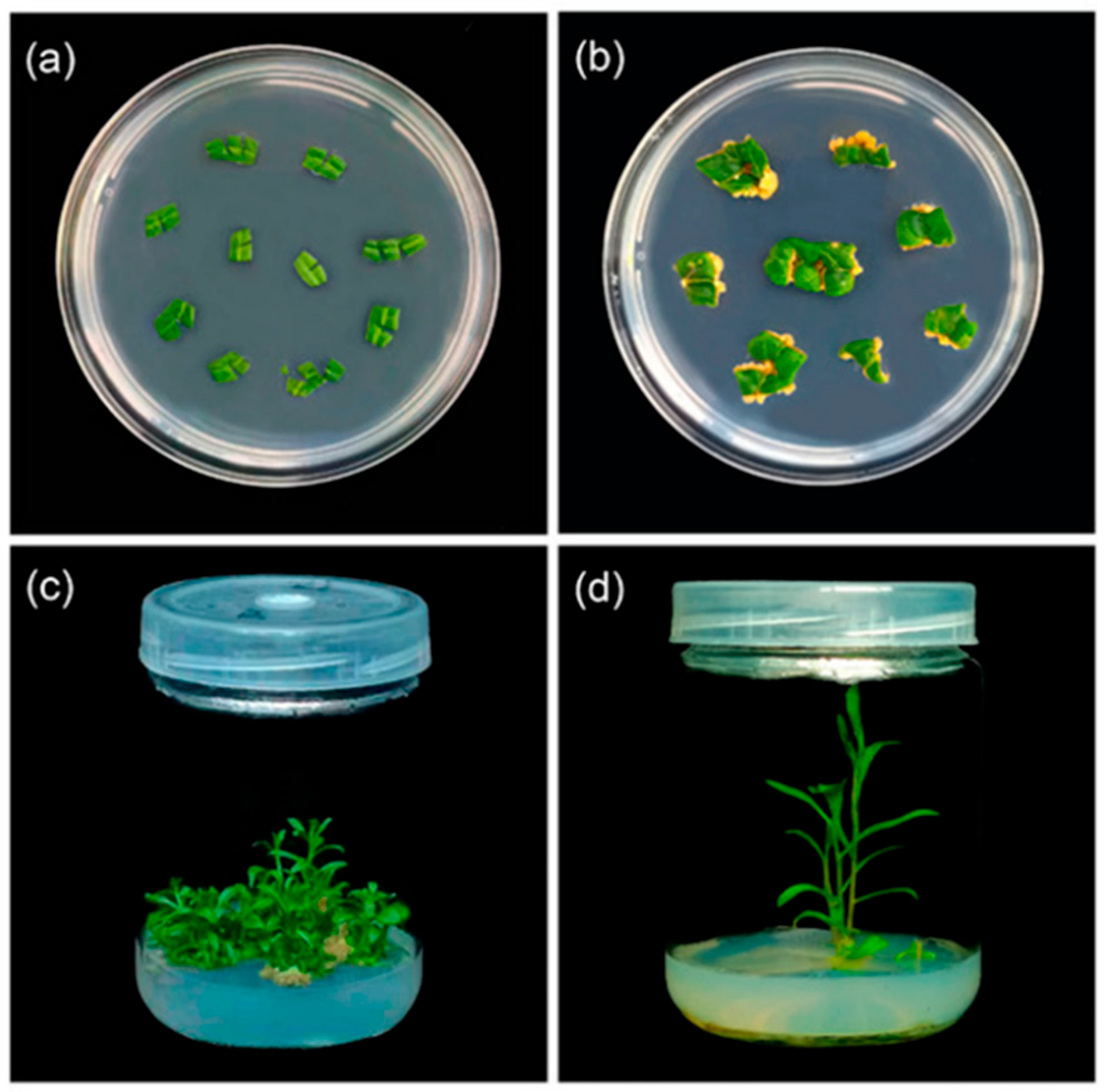
| No. | Agent Type | Example(s) | Mode of Action | Key Application Outcomes | References |
|---|---|---|---|---|---|
| 1 | Antimitotic | Colchicine | Disrupts microtubule formation (polyploidy) | Induces polyploidy; alters morphology and secondary metabolite production | [3,20,21,43,44] |
| 2 | Dinitroanilines | Oryzalin, Trifluralin | Binds plant tubulin (polyploidy) | Safer polyploidy induction in Mentha, Cannabis, Thymus, Blueberry | [12,25,26,27,28,29,31,32,33] |
| 3 | Alkylating Agent | EMS (ethyl methanesulfonate) | Alkylates guanine (point mutations) | Trait improvement in Banana, Tomato, Cowpea, Pepper | [13,22,34,35,36,37] |
| 4 | Alkylating Agent | MMS (methyl methanesulfonate) | Induces chromosomal aberrations | Morphological and physiological variation in Nigella, Chili, Fenugreek | [5,15,38,39] |
| 5 | Sulfonic Acid Deriv. | SA (sodium azide) | Produces point mutations via base alteration | Enhances protein, lipid, and fiber traits in soybean | [41] |
| No. | Plant Species | Colchicine Conc. and Time | Application Method | Observed Effects | References |
|---|---|---|---|---|---|
| 1 | Nigella sativa | 0.025%, 8 h | Seed soaking | ↑ shoot/root length, flavonoids, antioxidants, chlorophyll, phenolics | [1] |
| 2 | Cuminum cyminum (cumin) | 0.05% | In vitro or seed-based | ↑ essential oil yield (30–100%), ↑ cuminaldehyde, stem/root/seed size | [9] |
| 3 | Mentha spicata | 40 µM, 48 h | In vitro (oryzalin) | Tetraploids showed increased leaf area, bushiness, and carvone/limonene content | [12] |
| 4 | Stachys byzantina | 0.2%, 12 h | In vitro | ↑ linalool, chlorophyll, leaf size, ↓ stomatal density; 18% polyploidy rate | [14] |
| 5 | Portulaca grandiflora | 0.0–0.2% (drops), 3 days shading | Shoot tip (ex vitro) | ↑ stem diameter, branch number, leaf morphology; genotype-specific variation | [23] |
| 6 | Satureja khuzistanica | 0.05%, 4 days | In vitro leaf explants | ↑ chlorophyll, sugar, phenols; ↓ stomatal density; tetraploid confirmed by flow cytometry | [43] |
| 7 | Stevia rebaudiana | 1–2.5%, 48 h | Shoot tip (ex vitro) | Larger leaves, altered margins, ↑ stevioside, ↑ stomatal size/density | [46] |
| 8 | Citrus spp. | 0.025%, 2 h | Decapitated epicotyls | ↑ leaf thickness, ↓ oil gland/stomatal density, 20% tetraploid induction | [47] |
| 9 | Vitis vinifera | 1–6 g/L, 3 days | Cotyledon shoot tip | ↑ stomatal size, ↓ density; tetraploids confirmed; viability dose-dependent | [48] |
| 10 | Echinacea purpurea | 0.25% | Seedlings | ↑ chicoric/chlorogenic acid content, larger leaves, tetraploidy achieved | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.; Yun, S.; Gil, Y.; Park, M.-H. The Role of Colchicine in Plant Breeding. Int. J. Mol. Sci. 2025, 26, 6743. https://doi.org/10.3390/ijms26146743
Singh B, Yun S, Gil Y, Park M-H. The Role of Colchicine in Plant Breeding. International Journal of Molecular Sciences. 2025; 26(14):6743. https://doi.org/10.3390/ijms26146743
Chicago/Turabian StyleSingh, Baljinder, Sunyoung Yun, Yeji Gil, and Myoung-Hwan Park. 2025. "The Role of Colchicine in Plant Breeding" International Journal of Molecular Sciences 26, no. 14: 6743. https://doi.org/10.3390/ijms26146743
APA StyleSingh, B., Yun, S., Gil, Y., & Park, M.-H. (2025). The Role of Colchicine in Plant Breeding. International Journal of Molecular Sciences, 26(14), 6743. https://doi.org/10.3390/ijms26146743







