The Association Between Chemerin Levels and Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Protocol and Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Definition of the Outcome of Interest
2.5. Assessment of Risk of Bias
2.6. Data Extraction
2.7. Statistical Analysis
3. Results
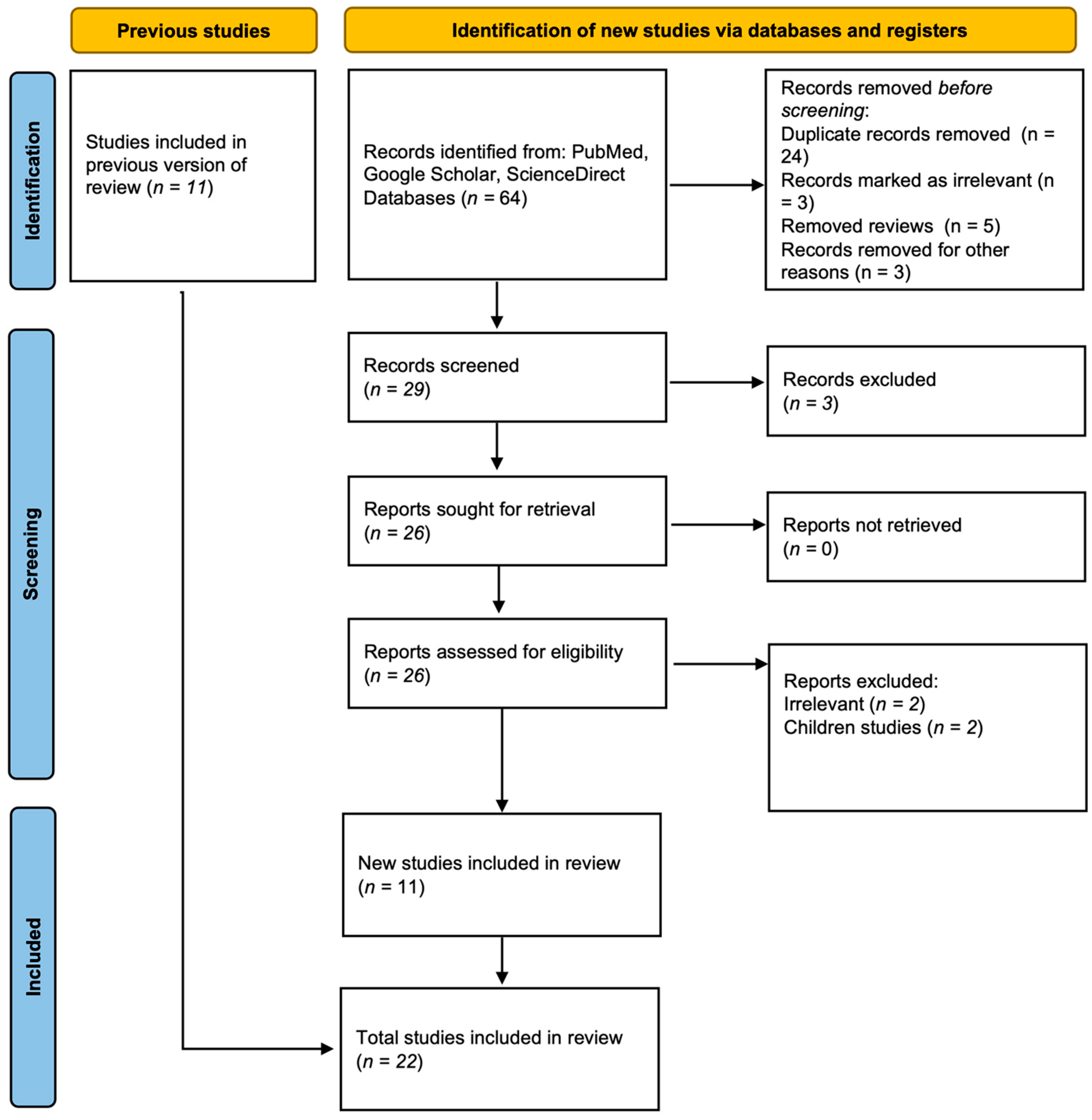
3.1. Study Selection
3.2. Features of Selected Studies
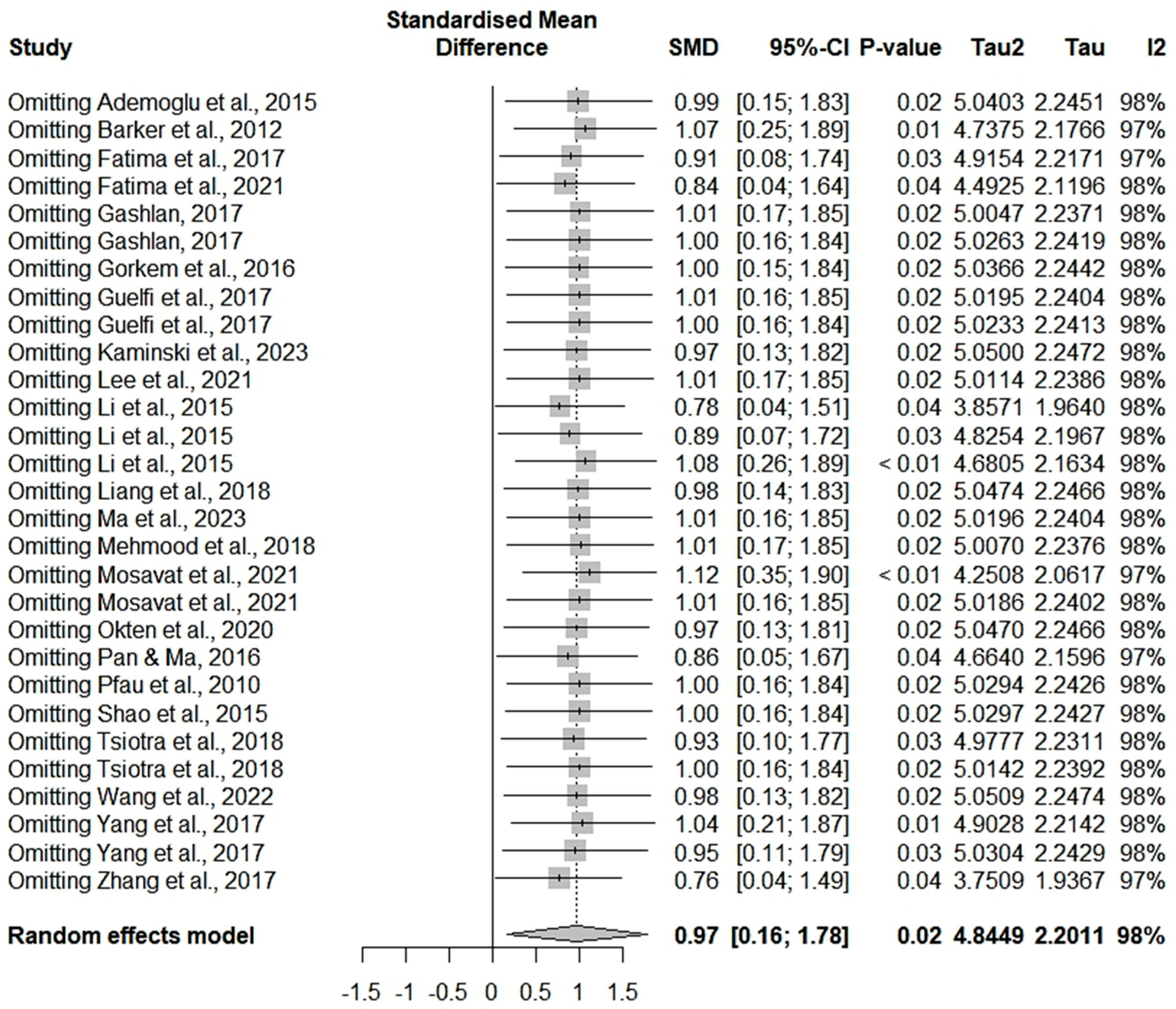
3.3. Meta-Analysis Results
3.4. Subgroup Analysis and Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Standards of Medical Care in Diabetes-2014. Diabetes Care 2014, 37 (Suppl. 1), 14–80. [CrossRef]
- Jaffe, A.; Giveon, S.; Rubin, C.; Novikov, I.; Ziv, A.; Kalter-Leibovici, O. Gestational Diabetes Risk in a Multi-Ethnic Population. Acta Diabetol. 2020, 57, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M.; Stumvoll, M. Adipokines in Gestational Diabetes. Lancet Diabetes Endocrinol. 2014, 2, 488–499. [Google Scholar] [CrossRef]
- Hamdan, H.Z.; Hassan, A.A.; Adam, I. Minerals in Pregnancy and Newborns. In Molecular Nutrition: Mother and Infant; Academic Press: Cambridge, MA, USA, 2021; pp. 155–177. [Google Scholar] [CrossRef]
- Hamdan, H.Z.; Hamdan, S.Z.; Adam, I. Detection of Novel Hub-Methylated Differentially Expressed Genes in Pregnant Women with Gestational Diabetes Mellitus via WGCNA of Epigenome-Wide and Transcriptome-Wide Profiling. Int. J. Health Sci. 2025, 19, 3941. [Google Scholar] [CrossRef]
- Singh, A.; Jaiswar, S.P.; Priyadarshini, A.; Deo, S. Linking of Oxidative Stress and Mitochondrial DNA Damage to the Pathophysiology of Idiopathic Intrauterine Growth Restriction. Int. J. Health Sci. 2023, 17, 15–22. [Google Scholar]
- Kralisch, S.; Bluher, M.; Paschke, R.; Stumvoll, M.; Fasshauer, M. Adipokines and Adipocyte Targets in the Future Management of Obesity and the Metabolic Syndrome. Mini Rev. Med. Chem. 2007, 7, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.; Lim, R.; Rice, G.E.; Lappas, M. Increased Chemerin Concentrations in Fetuses of Obese Mothers and Correlation with Maternal Insulin Sensitivity. J. Matern. Fetal Neonatal Med. 2012, 25, 2274–2280. [Google Scholar] [CrossRef]
- Görkem, Ü.; Küçükler, F.K.; Toğrul, C.; Güngör, T. Are Adipokines Associated with Gestational Diabetes Mellitus? J. Turk. Ger Gynecol. Assoc. 2016, 17, 186. [Google Scholar] [CrossRef]
- Ademoglu, E.; Berberoglu, Z.; Dellal, F.; Keskin, A.M.; Kose, A.; Candan, Z.; Bekdemir, H.; Erdamar, H.; Culha, C.; Aral, Y. Higher Levels of Circulating Chemerin in Obese Women with Gestational Diabetes Mellitus. Acta Endocrinol. Buchar. 2015, 11, 32–38. [Google Scholar] [CrossRef]
- Fatima, S.S.; Alam, F.; Chaudhry, B.; Khan, T.A. Elevated Levels of Chemerin, Leptin, and Interleukin-18 in Gestational Diabetes Mellitus. J. Matern. Fetal Neonatal Med. 2017, 30, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Quan, X.; Lan, Y.; Ye, J.; Wei, Q.; Yin, X.; Fan, F.; Xing, H. Serum Chemerin Level during the First Trimester of Pregnancy and the Risk of Gestational Diabetes Mellitus. Gynecol. Endocrinol. 2017, 33, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Ji, H.; Li, C.J.; Wang, P.H.; Yu, P.; Yu, D.M. Chemerin Expression in Chinese Pregnant Women with and without Gestational Diabetes Mellitus. Ann. d’Endocrinologie 2015, 76, 19–24. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Pope, C.F.; Crawford, L.A.; Vasavada, R.C.; Jagasia, S.M.; Gannon, M. Gestational Diabetes Mellitus Resulting from Impaired Beta-Cell Compensation in the Absence of FoxM1, a Novel Downstream Effector of Placental Lactogen. Diabetes 2010, 59, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhou, M.; Xu, X.K.; Qu, F.; Chen, D. Is Chemerin Associated with Gestational Diabetes Mellitus? An Evidence-Based Clinical Research from Chinese Women. J. Obstet. Gynaecol. 2018, 38, 482–487. [Google Scholar] [CrossRef]
- Pan, B.-L.; Ma, R.-M. Correlation of Serum Omentin-1 and Chemerin with Gestational Diabetes Mellitus. J. South. Med. Univ. 2016, 36, 1231–1236. [Google Scholar]
- Bozaoglu, K.; Segal, D.; Shields, K.A.; Cummings, N.; Curran, J.E.; Comuzzie, A.G.; Mahaney, M.C.; Rainwater, D.L.; Vandeberg, J.L.; MacCluer, J.W.; et al. Chemerin Is Associated with Metabolic Syndrome Phenotypes in a Mexican-American Population. J. Clin. Endocrinol. Metab. 2009, 94, 3085–3088. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin Is a Novel Adipokine Associated with Obesity and Metabolic Syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Garces, M.F.; Sanchez, E.; Ruíz-Parra, A.I.; Rubio-Romero, J.A.; Angel-Müller, E.; Suarez, M.A.; Bohórquez, L.F.; Bravo, S.B.; Nogueiras, R.; Diéguez, C.; et al. Serum Chemerin Levels during Normal Human Pregnancy. Peptides 2013, 42, 138–143. [Google Scholar] [CrossRef]
- Fatima, S.S.; Rehman, R.; Muhammad, J.S.; Martins, R.; Mohammed, N.; Khan, U. Association of Chemerin Gene Promoter Methylation in Maternal Blood and Breast Milk during Gestational Diabetes. J. Dev. Orig. Health Dis. 2022, 13, 108–114. [Google Scholar] [CrossRef]
- Mosavat, M.; Mirsanjari, M.; Lwaleed, B.A.; Kamarudin, M.; Omar, S.Z. Adipocyte-Specific Fatty Acid-Binding Protein (AFABP) and Chemerin in Association with Gestational Diabetes: A Case-Control Study. J. Diabetes Res. 2021, 2021, 5533802. [Google Scholar] [CrossRef] [PubMed]
- Bulut, A.; Akca, G.; Keskin Aktan, A.; Akbulut, K.G.; Babül, A. The Significance of Blood and Salivary Oxidative Stress Markers and Chemerin in Gestational Diabetes Mellitus. Taiwan. J. Obstet. Gynecol. 2021, 60, 695–699. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Ju, H.; Sun, M. Circulating Chemerin Levels and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2018, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, J.; Zuo, C.; Deng, D.; Pan, F.; Chen, R.; Zhu, J.; Chen, C.; Ye, S. Circulating Apelin, Chemerin and Omentin Levels in Patients with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2020, 19, 26. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training, 2nd ed.; John Wiley & Sons: Chichester, UK, 2024. [Google Scholar]
- Pfau, D.; Stepan, H.; Kratzsch, J.; Verlohren, M.; Verlohren, H.J.; Drynda, K.; Lössner, U.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Circulating Levels of the Adipokine Chemerin in Gestational Diabetes Mellitus. Horm. Res. Paediatr. 2010, 74, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liu, M.; Li, F.; Xu, X. The Expression of Chemerin in Maternal Peripheral, Umbilical Cord Blood and Placenta in Gestational Diabetic Patients. J. Biomater. Tissue Eng. 2015, 5, 833–837. [Google Scholar] [CrossRef]
- Gashlan, H.M. Relationship between Levels of Retinol Binding Protein 4, Vaspin and Chemerin and Insulin Resistance in Gestational Diabetes Mellitus. Int. J. Pharm. Res. Allied Sci. 2017, 6, 236–250. [Google Scholar]
- Guelfi, K.J.; Ong, M.J.; Li, S.; Wallman, K.E.; Doherty, D.A.; Fournier, P.A.; Newnham, J.P.; Keelan, J.A. Maternal Circulating Adipokine Profile and Insulin Resistance in Women at High Risk of Developing Gestational Diabetes Mellitus. Metabolism 2017, 75, 54–60. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, H.; Xiao, H.; Tian, X.; Wang, Y.; Yun, X.; Xu, Y. Interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNF-α) Single Nucleotide Polymorphisms (SNPs), Inflammation and Metabolism in Gestational Diabetes Mellitus in Inner Mongolia. Med. Sci. Monit. 2017, 23, 4149–4157. [Google Scholar] [CrossRef]
- Tsiotra, P.C.; Halvatsiotis, P.; Patsouras, K.; Maratou, E.; Salamalekis, G.; Raptis, S.A.; Dimitriadis, G.; Boutati, E. Circulating Adipokines and MRNA Expression in Adipose Tissue and the Placenta in Women with Gestational Diabetes Mellitus. Peptides 2018, 101, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Ye, C.; Connelly, P.W.; Hanley, A.J.; Zinman, B.; Retnakaran, R. Rising Plasminogen Activator Inhibitor-1 and Hypoadiponectinemia Characterize the Cardiometabolic Biomarker Profile of Women with Recent Gestational Diabetes. Cardiovasc. Diabetol. 2018, 17, 133. [Google Scholar] [CrossRef]
- Okten, S.B.; Bildacı, T.B. Salivary Leptin and Chemerin; a Novel Way of Gestational Diabetes Screening. Gynecol. Endocrinol. 2020, 36, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, J.A.; Kim, J.H.; Kwak, S.H.; Choi, S.H.; Jang, H.C. Longitudinal Changes of High Molecular Weight Adiponectin Are Associated with Postpartum Development of Type 2 Diabetes Mellitus in Patients with Gestational Diabetes Mellitus. Endocrinol. Metab. 2021, 36, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Li, M.; Zhao, R. Chemerin Levels and Its Genetic Variants Are Associated with Gestational Diabetes Mellitus: A Hospital-Based Study in a Chinese Cohort. Gene 2022, 807, 145888. [Google Scholar] [CrossRef]
- Kamiński, M.; Mierzyński, R.; Poniedziałek-Czajkowska, E.; Sadowska, A.; Sotowski, M.; Leszczyńska-Gorzelak, B. Comparative Evaluation of Adipokine Metrics for the Diagnosis of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2023, 25, 175. [Google Scholar] [CrossRef]
- Ma, Z.; Chu, L.; Zhang, Y.; Lu, F.; Zhu, Y.; Wu, F.; Zhang, Z. Is Chemerin Associated with Gestational Diabetes Mellitus? A Case-Control Study. Diabetes Metab. Syndr. Obes. 2023, 16, 2271–2281. [Google Scholar] [CrossRef]
- Coustan, D.; Carpenter, M. The Diagnosis of Gestational Diabetes. Diabetes Care 1998, 21 (Suppl. 2), B5–B8. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 December 2021).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Sedgwick, P. Meta-Analyses: Heterogeneity and Subgroup Analysis. BMJ 2013, 346, f4040. [Google Scholar] [CrossRef]
- Savva, S.C.; Lamnisos, D.; Kafatos, A.G. Predicting Cardiometabolic Risk: Waist-to-Height Ratio or BMI. A Meta-Analysis. Diabetes Metab. Syndr. Obes. 2013, 6, 403–419. [Google Scholar] [CrossRef]
- Cheng, C.H.; Ho, C.C.; Yang, C.F.; Huang, Y.C.; Lai, C.H.; Liaw, Y.P. Waist-to-Hip Ratio Is a Better Anthropometric Index than Body Mass Index for Predicting the Risk of Type 2 Diabetes in Taiwanese Population. Nutr. Res. 2010, 30, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.J.; Bonde, L.; Svare, J.A.; Randeva, H.S.; Asmar, M.; Larsen, S.; Vilsbøll, T.; Knop, F.K. Decreased Plasma Chemerin Levels in Women with Gestational Diabetes Mellitus. Diabet. Med. 2014, 31, 936–940. [Google Scholar] [CrossRef]
- Hasanvand, Z.; Sadeghi, A.; Rezvanfar, M.R.; Goodarzi, M.T.; Rahmannezhad, G.; Mashayekhi, F.J. Association between Chemerin Rs17173608 and Rs4721 Gene Polymorphisms and Gestational Diabetes Mellitus in Iranian Pregnant Women. Gene 2018, 649, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Müssig, K.; Staiger, H.; Machicao, F.; Thamer, C.; Machann, J.; Schick, F.; Claussen, C.D.; Stefan, N.; Fritsche, A.; Häring, H.U. RARRES2, Encoding the Novel Adipokine Chemerin, Is a Genetic Determinant of Disproportionate Regional Body Fat Distribution: A Comparative Magnetic Resonance Imaging Study. Metabolism 2009, 58, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, H.Z.; Hamdan, S.Z.; Adam, I. Association of Selenium Levels with Preeclampsia: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2023, 201, 2105–2122. [Google Scholar] [CrossRef]
- Lloyd, J.W.; Zerfass, K.M.; Heckstall, E.M.; Evans, K.A. Diet-Induced Increases in Chemerin Are Attenuated by Exercise and Mediate the Effect of Diet on Insulin and HOMA-IR. Ther. Adv. Endocrinol. Metab. 2015, 6, 189–198. [Google Scholar] [CrossRef]
- Human Chemerin ELISA—Quantikine DCHM00: R&D Systems. Available online: https://www.rndsystems.com/products/human-chemerin-quantikine-elisa_dchm00#product-details (accessed on 4 July 2025).
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A Myth Rather than an Exercise-Inducible Myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Huang, C.; Ge, L.; Xue, L.; Xiao, Z.; Xiao, T.; Zhao, H.; Ren, P.; Zhang, J.V. Chemerin: A Functional Adipokine in Reproductive Health and Diseases. Biomedicines 2022, 10, 1910. [Google Scholar] [CrossRef]
- Hamdan, H.Z.; Ali, T.; Adam, I. Association between Retinol-Binding Protein 4 Levels and Preeclampsia: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5201. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin Regulates β-Cell Function in Mice. Sci. Rep. 2011, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin Is a Novel Adipocyte-Derived Factor Inducing Insulin Resistance in Primary Human Skeletal Muscle Cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef]
- Su, X.; Cheng, Y.; Zhang, G.; Wang, B. Chemerin in Inflammatory Diseases. Clin. Chim. Acta 2021, 517, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Moreli, J.B.; Corrêa-Silva, S.; Damasceno, D.C.; Sinzato, Y.K.; Lorenzon-Ojea, A.R.; Borbely, A.U.; Rudge, M.V.C.; Bevilacqua, E.; Calderon, I.M.P. Changes in the TNF-Alpha/IL-10 Ratio in Hyperglycemia-Associated Pregnancies. Diabetes Res. Clin. Pract. 2015, 107, 362–369. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Wang, D.; Zhu, H.; Kang, L.; Jiang, J. Expression and Correlation of Chemerin and FABP4 in Peripheral Blood of Gestational Diabetes Mellitus Patients. Exp. Ther. Med. 2019, 19, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Mazaki-Tovi, S.; Kasher-Meron, M.; Hemi, R.; Haas, J.; Gat, I.; Lantsberg, D.; Hendler, I.; Kanety, H. Chemerin Is Present in Human Cord Blood and Is Positively Correlated with Birthweight. Am. J. Obstet. Gynecol. 2012, 207, 412.e1–412.e10. [Google Scholar] [CrossRef]
- Boutsikou, T.; Briana, D.D.; Boutsikou, M.; Kafalidis, G.; Stamati, L.; Baka, S.; Hassiakos, D.; Gourgiotis, D.; Malamitsi-Puchner, A. Cord Blood Chemerin and Obestatin Levels in Large for Gestational Age Infants. J. Matern.-Fetal Neonatal Med. 2013, 26, 123–126. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, Y.; Wang, Z.; Wei, L.; Zhang, H.; Fang, C.; Zhu, S.; Du, Y.; Su, R.; Li, W.; et al. Chemerin Alleviates the Placental Oxidative Stress and Improves Fetal Overgrowth of Gestational Diabetes Mellitus Mice Induced by High Fat Diet. Mol. Med. 2024, 30, 239. [Google Scholar] [CrossRef]
- Liang, Z.; Han, L.; Sun, D.; Chen, Y.; Wu, Q.; Zhang, L.; Zhou, M.; Chen, D. Chemerin-Induced Macrophages Pyroptosis in Fetal Brain Tissue Leads to Cognitive Disorder in Offspring of Diabetic Dams. J. Neuroinflammat. 2019, 16, 226. [Google Scholar] [CrossRef]
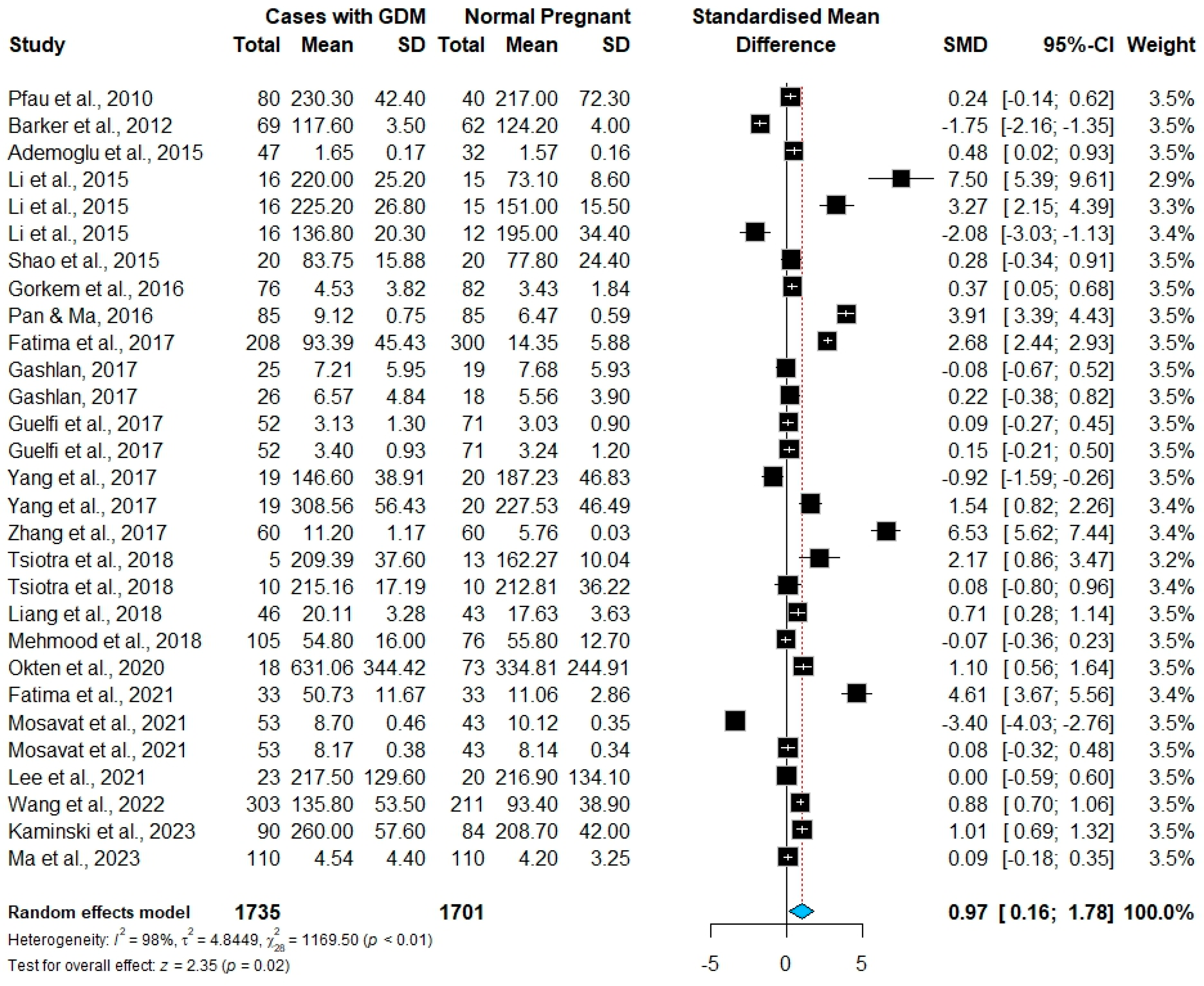

| Study | Country | Continent | Study Design | Sampling Trimester | No. Cases/No. Controls | Chemerin Mean (SD) in GDM Cases, ng/mL | Chemerin Mean (SD) in Controls, ng/mL | GDM Cases Mean Age, Years | GDM Cases Mean BMI, kg/m2 | Commercial ELISA Kit | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfau et al., 2010 [28] | Germany | Europe | Case–control | Third | 80/40 | 230.3 (42.4) | 217 (72.3) | 33 | 24.9 | BioVendor (Karasek, Czech Republic) | 8 |
| Barker et al., 2012 [9] | Australia | Australia | Case–control | Third | 69/62 | 117.6 (3.5) | 124.2 (4) | 35.2 | 31.2 | R & D systems (Minneapolis, MN, USA) | 7 |
| Ademoglu et al., 2015 [11] | Turkey | Europe | Case–control | Second | 47/32 | 1.65 (0.17) | 1.57 (0.16) | 30.2 | 31.3 | BioVendor (Karasek, Czech Republic) | 6 |
| Li et al., 2015 [14] | China | Asia | Case–control | Third | 16/15 | 220 (25.2) | 73.1 (8.6) | 29.3 | 23 | HCB(Vancouver, BC, Canada) | 7 |
| Li et al., 2015 [14] | China | Asia | Case–control | Third | 16/15 | 225.2 (26.8) | 151 (15.5) | 29.3 | 27.1 | 7 | |
| Li et al., 2015 [14] | China | Asia | Case–control | Third | 16/12 | 136.8 (20.3) | 195 (34.4) | 29.14 | 33 | 7 | |
| Shao et al., 2015 [29] | China | Asia | Case–control | Third | 20/20 | 83.75 (15.88) | 77.8 (24.4) | 30 | 28.73 | NA | 6 |
| Görkem et al., 2016 [10] | Turkey | Europe | Cross-sectional | Second | 76/82 | 4.53 (3.82) | 3.43 (1.84) | 27.59 | 36.25 | BioVendor (Karasek, Czech Republic) | 6 |
| Pan & Ma, 2016 [17] | China | Asia | Case–control | Third | 85/85 | 9.12 (0.75) | 6.47 (0.59) | 28.1 | 23.6 | NA | 7 |
| Fatima et al., 2017 [12] | Pakistan | Asia | Case–control | Second | 208/300 | 93.39 (45.43) | 14.35 (5.88) | 27.3 | 24.83 | Glory Bioscience (Shanghai, China) | 8 |
| Gashlan 2017 [30] | KSA | Asia | Cross-sectional | Second | 25/19 | 7.21 (5.95) | 7.68 (5.93) | 32.4 | 33.4 | Elabscience Company | 7 |
| Gashlan 2017 [30] | KSA | Asia | Cross-sectional | Third | 26/18 | 6.57 (4.84) | 5.56 (3.9) | 33.4 | 34.1 | 6 | |
| Guelfi et al., 2017 [31] | Australia | Australia | RCT | First | 52/71 | 3.13 (1.3) | 3.03 (0.9) | 34.4 | 26.9 | R & D systems (Minneapolis, MN, USA) | 6 |
| Guelfi et al., 2017 [31] | Australia | Australia | RCT | Second | 52/71 | 3.4 (0.93) | 3.24 (1.2) | 34.4 | 26.9 | 7 | |
| Yang et al., 2017 [13] | China | Asia | Case–control | First | 19/20 | 146.6 (38.91) | 187.23 (46.83) | 26.84 | 22.74 | R & D systems (Minneapolis, MN, USA) | 7 |
| Yang et al., 2017 [13] | China | Asia | Case–control | Third | 19/20 | 308.56 (56.43) | 227.53 (46.49) | 26.84 | 25.67 | 7 | |
| Zhang et al., 2017 [32] | China | Asia | Case–control | First | 60/60 | 11.2 (1.17) | 5.76 (0.03) | 29.13 | 38.68 | TaKaRa Biotechnology (Kusatsu, Japan) | 7 |
| Tsiotra et al., 2018 [33] | Greece | Europe | Case–control | Third | 5/13 | 209.39 (37.6) | 162.27 (10.04) | 29.8 | 26.9 | Millipore (Burlington, MA, USA) | 7 |
| Tsiotra et al., 2018 [33] | Greece | Europe | Case–control | Third | 10/10 | 215.16 (17.19) | 212.81 (36.22) | 27.7 | 36 | 7 | |
| Liang et al., 2018 [16] | China | Asia | Case–control | Third | 46/43 | 20.11 (3.28) | 17.63 (3.63) | 31.89 | 21.52 | NA | 8 |
| Mehmood et al., 2018 [34] | Canada | America | Cohort | Second | 105/76 | 54.8 (16) | 55.8 (12.7) | 35 | 25.78 | Millipore (Burlington, MA, USA) | 8 |
| Okten et al., 2020 [35] | Turkey | Europe | Case–control | Second | 18/73 | 631.06 (344.42) | 334.81 (244.91) | 32.05 | 27.06 | Shanghai Korain Biotech | 8 |
| Fatima et al., 2022 [21] | Pakistan | Asia | Case–control | Second | 33/33 | 50.73 (11.67) | 11.06 (2.86) | 24.23 | 25.22 | Glory Bioscience (Shanghai, China) | 7 |
| Mosavat et al., 2021 [22] | Malaysia | Asia | Case–control | Second | 53/43 | 8.7 (0.46) | 10.12 (0.35) | 33.2 | 27 | R & D systems (Minneapolis, MN, USA) | 6 |
| Mosavat et al., 2021 [22] | Malaysia | Asia | Case–control | Third | 53/43 | 8.17 (0.38) | 8.14 (0.34) | 33.2 | 27 | 6 | |
| Lee et al., 2021 [36] | South Korea | Asia | Cohort | Second | 23/20 | 217.5 (129.6) | 216.9 (134.1) | 31.8 | 23.1 | BioVendor (Karasek, Czech Republic) | 8 |
| Wang et al., 2022 [37] | China | Asia | Case–control | Second | 303/211 | 135.8 (53.5) | 93.4 (38.9) | 32.7 | 31.9 | R & D systems (Minneapolis, MN, USA) | 7 |
| Kamiński et al., 2023 [38] | Poland | Europe | Case–control | Second | 90/84 | 260 (57.6) | 208.7 (42) | 26.6 | 26.7 | BioVendor (Karasek, Czech Republic) | 7 |
| Ma et al., 2023 [39] | China | Asia | Case–control | Second | 110/110 | 4.54 (4.4) | 4.2 (3.25) | 32.24 | 28.99 | Meilian ( Shanghai, China) | 6 |
| Subgroup | Number of Studies | Number of GDM | Number of Controls | SMD (95% CI) | I2-Index |
|---|---|---|---|---|---|
| Trimester | |||||
| First/second | 16 | 1274 | 1305 | 0.83 (−0.25; 1.91) | 98.0% |
| Third | 13 | 461 | 396 | 1.16 (−13; 2.45) | 98.0% |
| Continent | |||||
| Asia | 18 | 1131 | 1087 | 1.39 (0.12; 2.66) * | 98.0% |
| Others | 11 | 604 | 614 | 0.29 (−0.23; 81) | 93.0% |
| Study Design | |||||
| Case–control | 22 | 1376 | 1344 | 1.27 (0.20; 2.33) * | 98.0% |
| Others | 7 | 359 | 357 | 0.11 (−0.04; 0.27) | 0.0% |
| Patients’ age | |||||
| ≥30 years | 16 | 1082 | 952 | −0.05 (−0.57; 0.47) | 95.0% |
| <30 years | 13 | 653 | 749 | 2.31 (0.82; 3.79) * | 98.0% |
| Patients’ BMI | |||||
| ≥28 kg/m2 | 11 | 762 | 636 | 0.45 (−0.83; 1.73) | 97.0% |
| <28 kg/m2 | 18 | 973 | 1065 | 1.30 (0.24; 2.35) * | 98.0% |
| Covariate | Estimation Coefficient | Standard Error | p-Value | 95% CI |
|---|---|---|---|---|
| NOS score | 0.64 | 0.679 | 0.340 | (−0.68; 1.97) |
| Year of publication | −0.02 | 0.196 | 0.928 | (−0.40; 0.36) |
| Sample size | 0.01 | 0.004 | 0.689 | (−0.01; 0.02) |
| Trimester | ||||
| First/second | Reference | Reference | ||
| Third | 0.56 | 1.253 | 0.654 | (−1.89; 3.01) |
| Continent | ||||
| Asia | Reference | Reference | ||
| Others | −1.17 | 1.004 | 0.241 | (−3.14; 0.79) |
| Study Design | ||||
| Case–control | Reference | Reference | ||
| Others | 0.75 | 1.085 | 0.487 | (−1.37; 2.87) |
| BMI | ||||
| ≥28 kg/m2 | −0.48 | 0.972 | ||
| <28 kg/m2 | Reference | Reference | 0.618 | (−2.38; 1.42) |
| Age | ||||
| ≥30 years | −2.13 | 0.877 | ||
| <30 years | Reference | Reference | 0.015 | (−3.82; −0.411) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldharee, H.; Makki, Y.R.; Hamdan, H.Z. The Association Between Chemerin Levels and Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 6622. https://doi.org/10.3390/ijms26146622
Aldharee H, Makki YR, Hamdan HZ. The Association Between Chemerin Levels and Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(14):6622. https://doi.org/10.3390/ijms26146622
Chicago/Turabian StyleAldharee, Hitham, Yousif R. Makki, and Hamdan Z. Hamdan. 2025. "The Association Between Chemerin Levels and Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 14: 6622. https://doi.org/10.3390/ijms26146622
APA StyleAldharee, H., Makki, Y. R., & Hamdan, H. Z. (2025). The Association Between Chemerin Levels and Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(14), 6622. https://doi.org/10.3390/ijms26146622








