Blockade of Dopamine D3 Receptors in the Ventral Tegmental Area Mitigates Fear Memory Generalization
Abstract
1. Introduction
2. Results
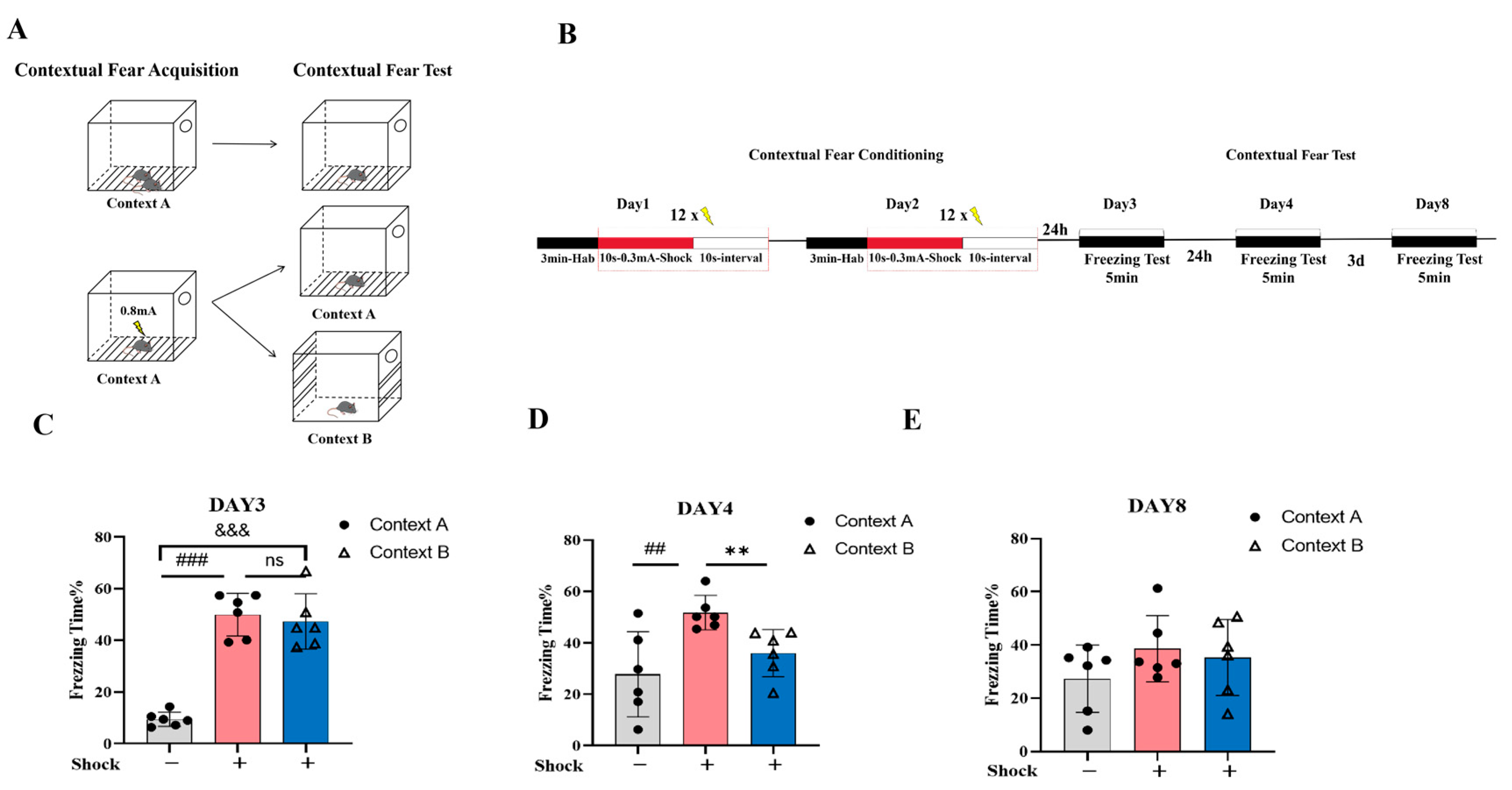
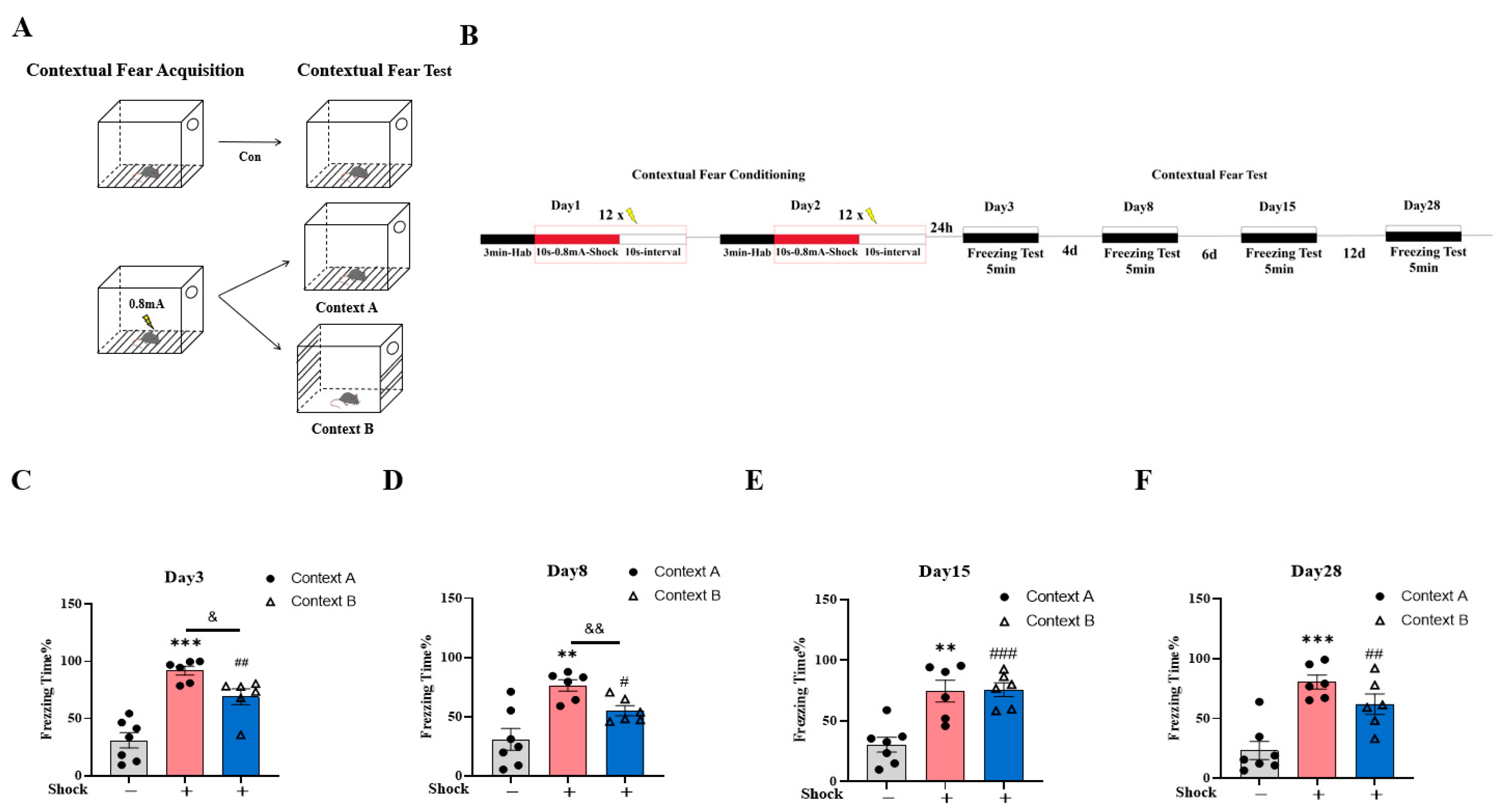
2.1. Validation of the Fear Generalization Model
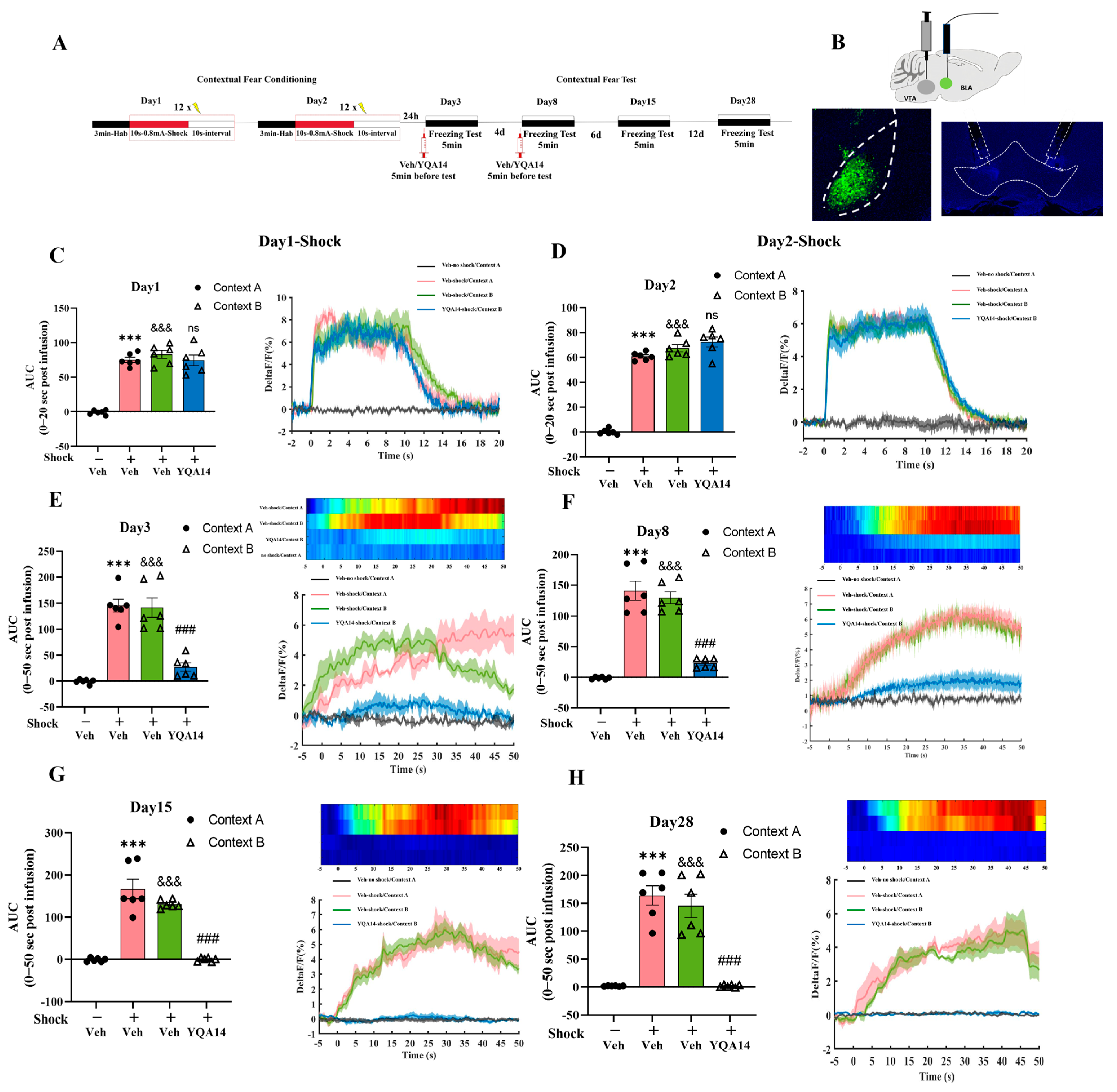
2.2. D3R Antagonism in VTA Attenuates Fear Generalization
2.3. D3R Antagonism in VTA Normalizes BLA Hyperactivity in the Fear Generalization Model
3. Discussion
3.1. Attenuation of Fear Generalization by Blockade of D3R in VTA
3.2. D3R Blockade Normalizes BLA Neuronal Hyperexcitability During Fear Generalization
3.3. Emotional Regulatory Role of VTA-to-BLA Dopaminergic Projections
3.4. Therapeutic Implications and Unresolved Mechanisms
3.5. Strengths and Limitations of the Present Study
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Administration
4.3. The Cannulae and Optic Fiber Implantation Surgeries
4.4. Behavioral Assays
4.4.1. Fear Generalization in Contextual Fear Conditioning with Electric Foot-Shock Models in Mice
4.4.2. Microinjection of D3R Antagonist-YQA14 into VTA Before Fear Generalization Test
4.4.3. Dynamic State in BLA Neurons During Fear Generalization Test
4.4.4. Fiber Photometry Imaging Data Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lesuis, S.L.; Park, S.; Hoorn, A.; Rashid, A.J.; Mocle, A.J.; Salter, E.W.; Vislavski, S.; Gray, M.T.; Torelli, A.M.; DeCristofaro, A.; et al. Stress disrupts engram ensembles in the lateral amygdala to generalize threat memory in mice. Cell 2024, 188, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, L.S.; Fadok, J.P.; Argilli, E.; Garelick, M.G.; Jones, G.L.; Dickerson, T.M.K.; Allen, J.M.; Mizumori, S.J.Y.; Bonci, A.; Palmiter, R.D. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat. Neurosci. 2011, 14, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Onat, S.; Büchel, C. The neuronal basis of fear generalization in humans. Nat. Neurosci. 2015, 18, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor, J.E.; Paz, R. Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. Biol. Psychiatry 2015, 78, 336–343. [Google Scholar] [CrossRef]
- Squire, L.R.; Alvarez, P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr. Opin. Neurobiol. 1995, 5, 169–177. [Google Scholar] [CrossRef]
- Drew, M.R.; Huckleberry, K.A. Modulation of Aversive Memory by Adult Hippocampal Neurogenesis. Neurotherapeutics 2017, 14, 646–661. [Google Scholar] [CrossRef]
- Lopresto, D.; Schipper, P.; Homberg, J.R. Neural circuits and mechanisms involved in fear generalization: Implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2016, 60, 31–42. [Google Scholar] [CrossRef]
- Likhtik, E.; Paz, R. Amygdala–prefrontal interactions in (mal) adaptive learning. Trends Neurosci. 2015, 38, 158–166. [Google Scholar] [CrossRef]
- Jasnow, A.M.; Lynch, J.F., III; Gilman, T.L.; Riccio, D.C. Perspectives on fear generalization and its implications for emotional disorders. J. Neurosci. Res. 2017, 95, 821–835. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Peng, J.; Jiang, Y.; Sun, F.; Cui, M. Research progress on the involvement of dopaminergic neurons in the ventral tegmental area in the regulation of anxiety-like behavior. Chin. J. Nerv. Ment. Dis. 2023, 49, 565–569. [Google Scholar]
- Niibori, Y.; Yu, T.S.; Epp, J.R.; Akers, K.G.; Josselyn, S.A.; Frankland, P.W. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat. Commun. 2012, 3, 1253. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain Res. 1994, 61, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Moran, P.M.; Joseph, M.H. The role of dopamine in conditioning and latent inhibition: What, when, where and how? Neurosci. Biobehav. Rev. 2005, 29, 963–976. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hikosaka, O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 2009, 459, 837–841. [Google Scholar] [CrossRef]
- Milad, M.R.; Wright, C.I.; Orr, S.P.; Pitman, R.K.; Quirk, G.J.; Rauch, S.L. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol. Psychiatry 2007, 62, 446–454. [Google Scholar] [CrossRef]
- Tanner, M.K.; Hohorst, A.A.; Westerman, J.D.; Mendoza, C.S.; Han, R.; Moya, N.A.; Jaime, J.; Alvarez, L.M.; Dryden, M.Q.; Balolia, A.; et al. Pharmacological manipulations of the dorsomedial and dorsolateral striatum during fear extinction reveal opposing roles in fear renewal. Neurobiol. Learn. Mem. 2024, 212, 107937. [Google Scholar] [CrossRef]
- Correia, R.; Coimbra, B.; Domingues, A.V.; Wezik, M.; Vieitas-Gaspar, N.; Gaspar, R.; Sousa, N.; Pinto, L.; Rodrigues, A.J.; Soares-Cunha, C. Involvement of nucleus accumbens D2–medium spiny neurons projecting to the ventral pallidum in anxiety-like behaviour. J. Psychiatry Neurosci. 2023, 48, E267–E284. [Google Scholar] [CrossRef]
- Yang, M.-D.; Cun, X.-F.; Wu, N.; Li, J.; Song, R. Dopamine D3 receptor in the nucleus accumbens modulates opioid taking and seeking in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 139, 111389. [Google Scholar] [CrossRef]
- Sias, A.C.; Jafar, Y.; Goodpaster, C.M.; Armenta, K.R.; Wrenn, T.M.; Griffin, N.K.; Patel, K.; Lamparelli, A.C.; Sharpe, M.J.; Wassum, K.M. Dopamine projections to the basolateral amygdala drive the encoding of identity-specific reward memories. Nat. Neurosci. 2024, 27, 728–736. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R.; Sibley, D.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Yang, R.-F.; Wu, N.; Su, R.-B.; Li, J.; Peng, X.-Q.; Gardner, E.L. YQA14: A novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice: YQA14 inhibits cocaine self-administration. Addict. Biol. 2012, 17, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, G.D.; Mascio, M.D.; Matteo, V.D.; Esposito, E. Effects of acute and repeated administration of amisulpride, a dopamine D2/D3 receptor antagonist, on the electrical activity of midbrain dopaminergic neurons. Pharmacol. Exp. Ther. 1998, 287, 51–57. [Google Scholar] [CrossRef]
- Sokoloff, P.; Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 2017, 45, 2–19. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Volman, S.F.; Lammel, S.; Margolis, E.B.; Kim, Y.; Richard, J.M.; Roitman, M.F.; Lobo, M.K. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J. Neurosci. 2013, 33, 17569–17576. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Herry, C.; Ciocchi, S.; Senn, V.; Demmou, L.; Demmou, C.; Lüthi, A. Switching on and off fear by distinct neuronal circuits. Nature 2008, 454, 600–606. [Google Scholar] [CrossRef]
- Grewe, B.F.; Gründemann, J.; Kitch, L.J.; Lecoq, J.A.; Parker, J.G.; Marshall, J.D.; Larkin, M.C.; Jercog, P.E.; Grenier, F.; Li, J.Z.; et al. Neural ensemble dynamics underlying a long-term associative memory. Nature 2017, 543, 670–675. [Google Scholar] [CrossRef]
- Bissière, S.; Plachta, N.; Hoyer, D.; McAllister, K.H.; Olpe, H.-R.; Grace, A.A.; Cryan, J.F. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol. Psychiatry 2008, 63, 821–831. [Google Scholar] [CrossRef]
- de Oliveira, A.R.; Reimer, A.E.; de Macedo, C.E.A.; de Carvalho, M.C.; Silva, M.A.d.S.; Brandão, M.L. Conditioned fear is modulated by D2 receptor pathway connecting the ventral tegmental area and basolateral amygdala. Neurobiol. Learn. Mem. 2011, 95, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, J.A.; Grace, A.A. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature 2002, 417, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Albrechet-Souza, L.; Carvalho, M.C.; Brandão, M.L. D(1)-like receptors in the nucleus accumbens shell regulate the expression of contextual fear conditioning and activity of the anterior cingulate cortex in rats. Int. J. Neuropsychopharmacol. 2013, 16, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Mayberg, H.S. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat. Neurosci. 2007, 10, 1116–1124. [Google Scholar] [CrossRef]
- Leggio, G.M.; Torrisi, S.A.; Castorina, A.; Platania, C.B.M.; Impellizzeri, A.A.R.; Fidilio, A.; Caraci, F.; Bucolo, C.; Drago, F.; Salomone, S. Dopamine D3 receptor-dependent changes in alpha6 GABAA subunit expression in striatum modulate anxiety-like behaviour: Responsiveness and tolerance to diazepam. Eur. Neuropsychopharmacol. 2015, 25, 1427–1436. [Google Scholar] [CrossRef]
- Likhtik, E.; Stujenske, J.M.; Topiwala, M.A.; Harris, A.Z.; Gordon, J.A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 2014, 17, 106–113. [Google Scholar] [CrossRef]
- Sun, L.; You, J.; Sun, F.; Cui, M.; Wang, J.; Wang, W.; Wang, D.; Liu, D.; Xu, Z.; Qiu, C.; et al. Reactivating a positive feedback loop VTA-BLA-NAc circuit associated with positive experience ameliorates the attenuated reward sensitivity induced by chronic stress. Neurobiol. Stress. 2021, 15, 100370. [Google Scholar] [CrossRef]
- Zhang, X.; Flick, K.; Rizzo, M.; Pignatelli, M.; Tonegawa, S. Dopamine induces fear extinction by activating the reward-responding amygdala neurons. Proc. Natl. Acad. Sci. USA 2025, 122, e2501331122. [Google Scholar] [CrossRef]
- Li, C.; Rainnie, D.G. Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the D1-like family of dopamine receptors and group II metabotropic glutamate receptors. J. Physiol. 2014, 592, 4329–4351. [Google Scholar] [CrossRef]
- Marcellino, D.; Ferré, S.; Casadó, V.; Cortés, A.; Foll, L.B.; Mazzola, C.; Drago, F.; Saur, O.; Stark, H.; Soriano, A.; et al. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008, 283, 26016–26025. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narváez, M.; Wydra, K.; Tarakanov, A.O.; Li, X.; Millón, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the Role of GPCR Heteroreceptor Complexes in Modulating the Brain Networks in Health and Disease. Front. Cell Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.; Diaz, J.; Carroll, P.; Griffon, N.; Schwartz, J.-C.; Sokoloff, P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 2001, 411, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Weera, M.M.; Agoglia, A.E.; Douglass, E.; Jiang, Z.; Rajamanickam, S.; Shackett, R.S.; A Herman, M.; Justice, N.J.; Gilpin, N.W. Generation of a CRF1-Cre transgenic rat and the role of central amygdala CRF1 cells in nociception and anxiety-like behavior. eLife 2022, 11, e67822. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Cabal, L.; Liu, J.; Pollandt, S.; Schmidt, K.; Shinnick-Gallagher, P.; Gallagher, J.P. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: Functional switch after chronic cocaine administration. J. Neurosci. 2008, 28, 529–542. [Google Scholar] [CrossRef]
- E Yarur, H.; Zegers, J.; Vega-Quiroga, I.; Novoa, J.; Ciruela, F.; E Andres, M.; Gysling, K. Functional Interplay of Type-2 Corticotrophin Releasing Factor and Dopamine Receptors in the Basolateral Amygdala-Medial Prefrontal Cortex Circuitry. Int. J. Neuropsychopharmacol. 2021, 24, 221–228. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Wu, J.; Wang, Y.; Johnson, N.L.; Bhattarai, J.P.; Li, G.; Wang, W.; Guevara, C.; Shoenhard, H.; Fuccillo, M.V.; et al. Ventral striatal islands of Calleja neurons bidirectionally mediate depression-like behaviors in mice. Nat. Commun. 2023, 14, 6887. [Google Scholar] [CrossRef]
- Kiss, B.; Laszlovszky, I.; Krámos, B.; Visegrády, A.; Bobok, A.; Lévay, G.; Lendvai, B.; Román, V. Neuronal Dopamine D3 Receptors: Translational Implications for Preclinical Research and CNS Disorders. Biomolecules 2021, 11, 104. [Google Scholar] [CrossRef]
- Bontempi, L.; Savoia, P.; Bono, F.; Fiorentini, C.; Missale, C. Dopamine D3 and acetylcholine nicotinic receptor heteromerization in midbrain dopamine neurons: Relevance for neuroplasticity. Eur. Neuropsychopharmacol. 2017, 27, 313–324. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, M.; Escartín-Pérez, R.E.; Leyva-Gómez, G.; Avalos-Fuentes, J.A.; Paz-Bermúdez, F.J.; Loya-López, S.I.; Aceves, J.; Erlij, D.; Cortés, H.; Florán, B. Blockade of Intranigral and Systemic D3 Receptors Stimulates Motor Activity in the Rat Promoting a Reciprocal Interaction among Glutamate, Dopamine, and GABA. Biomolecules 2019, 9, 511. [Google Scholar] [CrossRef]
- Villalobos-Escobedo, F.S.; Jijón-Lorenzo, R.; Avalos-Fuentes, J.A.; Paz-Bermúdez, F.; Recillas-Morales, S.; Rojas, I.C.; Leyva-Gómez, G.; Cortés, H.; Florán, B. Dopamine D3 receptor modulates D2 receptor effects on cAMP and GABA release at striatopallidal terminals-Modulation by the Ca2+-Calmodulin-CaMKII system. Eur. J. Neurosci. 2024, 59, 1441–1459. [Google Scholar] [CrossRef]
- Song, D.; Ge, Y.; Chen, Z.; Shang, C.; Guo, Y.; Zhao, T.; Li, Y.; Wu, N.; Song, R.; Li, J. Role of dopamine D3 receptor in alleviating behavioural deficits in animal models of post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Lu, J.; Dou, H.; Lei, Y. Generalization gradients for fear and disgust in human associative learning. Sci. Rep. 2021, 11, 14210. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, M.; Wu, N.; Li, J.; Song, R. Blockade of dopamine D3 receptor in ventral tegmental area attenuating contextual fear memory. Biomed. Pharmacother. 2023, 158, 114179. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Li, Z.; Li, J.; Zhuang, X.; Zhang, Z. P-Glycoprotein (ABCB1) Limits the Brain Distribution of YQA-14, a Novel Dopamine D3 Receptor Antagonist. Chem. Pharm. Bull. 2015, 63, 512–518. [Google Scholar] [CrossRef]
- Chen, M.; Ma, S.; Liu, H.; Dong, Y.; Tang, J.; Ni, Z.; Tan, Y.; Duan, C.; Li, H.; Huang, H.; et al. Brain region-specific action of ketamine as a rapid antidepressant. Science 2024, 385, eado7010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Ding, X.; Wu, N.; Li, J.; Song, R. Blockade of Dopamine D3 Receptors in the Ventral Tegmental Area Mitigates Fear Memory Generalization. Int. J. Mol. Sci. 2025, 26, 6520. https://doi.org/10.3390/ijms26136520
Fang X, Ding X, Wu N, Li J, Song R. Blockade of Dopamine D3 Receptors in the Ventral Tegmental Area Mitigates Fear Memory Generalization. International Journal of Molecular Sciences. 2025; 26(13):6520. https://doi.org/10.3390/ijms26136520
Chicago/Turabian StyleFang, Xiangjun, Xiaoyan Ding, Ning Wu, Jin Li, and Rui Song. 2025. "Blockade of Dopamine D3 Receptors in the Ventral Tegmental Area Mitigates Fear Memory Generalization" International Journal of Molecular Sciences 26, no. 13: 6520. https://doi.org/10.3390/ijms26136520
APA StyleFang, X., Ding, X., Wu, N., Li, J., & Song, R. (2025). Blockade of Dopamine D3 Receptors in the Ventral Tegmental Area Mitigates Fear Memory Generalization. International Journal of Molecular Sciences, 26(13), 6520. https://doi.org/10.3390/ijms26136520






