The G311E Mutant Gene of MATE Family Protein DTX6 Confers Diquat and Paraquat Resistance in Rice Without Yield or Nutritional Penalties
Abstract
1. Introduction
2. Results
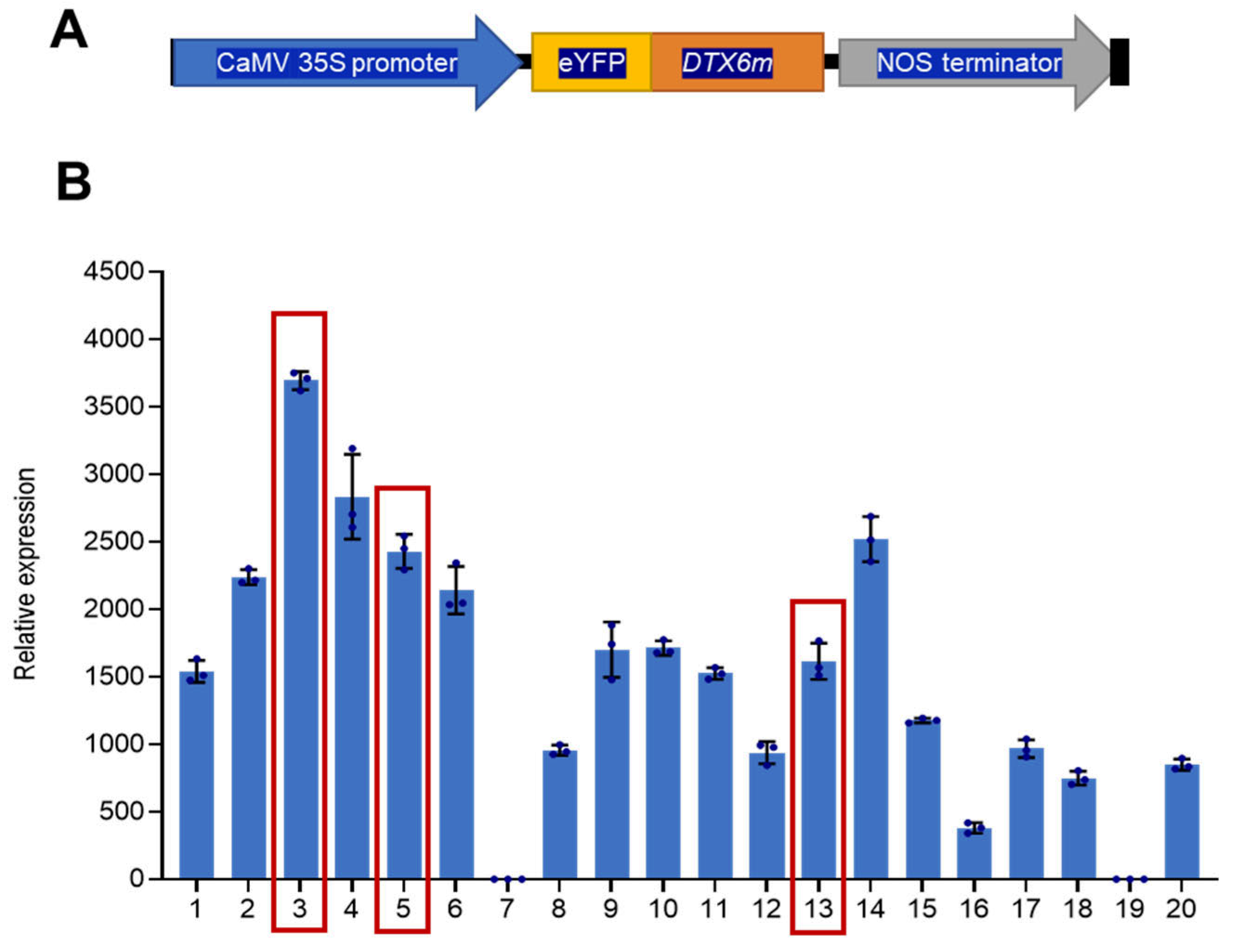
2.1. Generation of DTX6m-Expressing Rice Lines
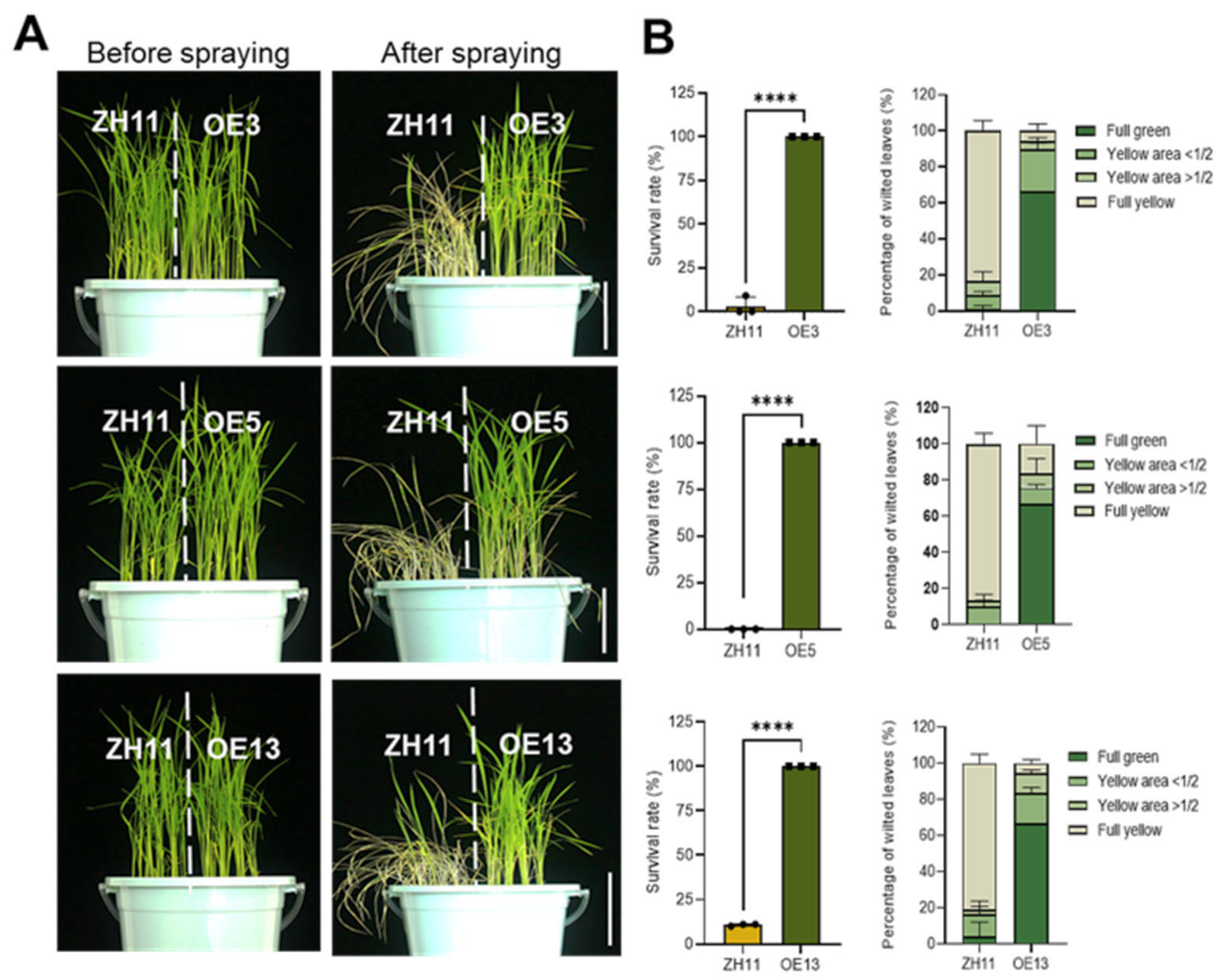
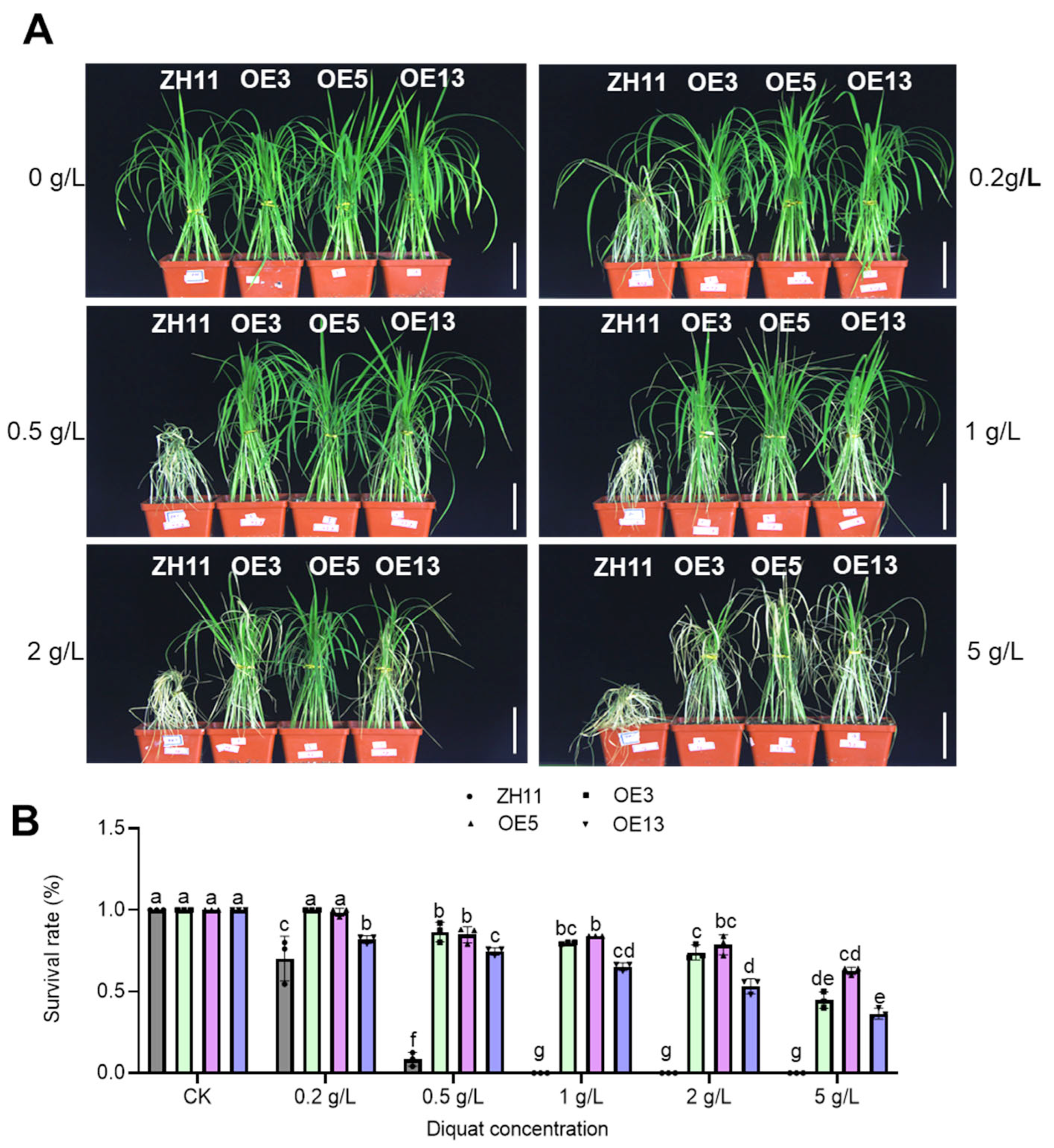
2.2. DTX6m-Overexpression Lines Exhibit Strong Paraquat and Diquat Resistance
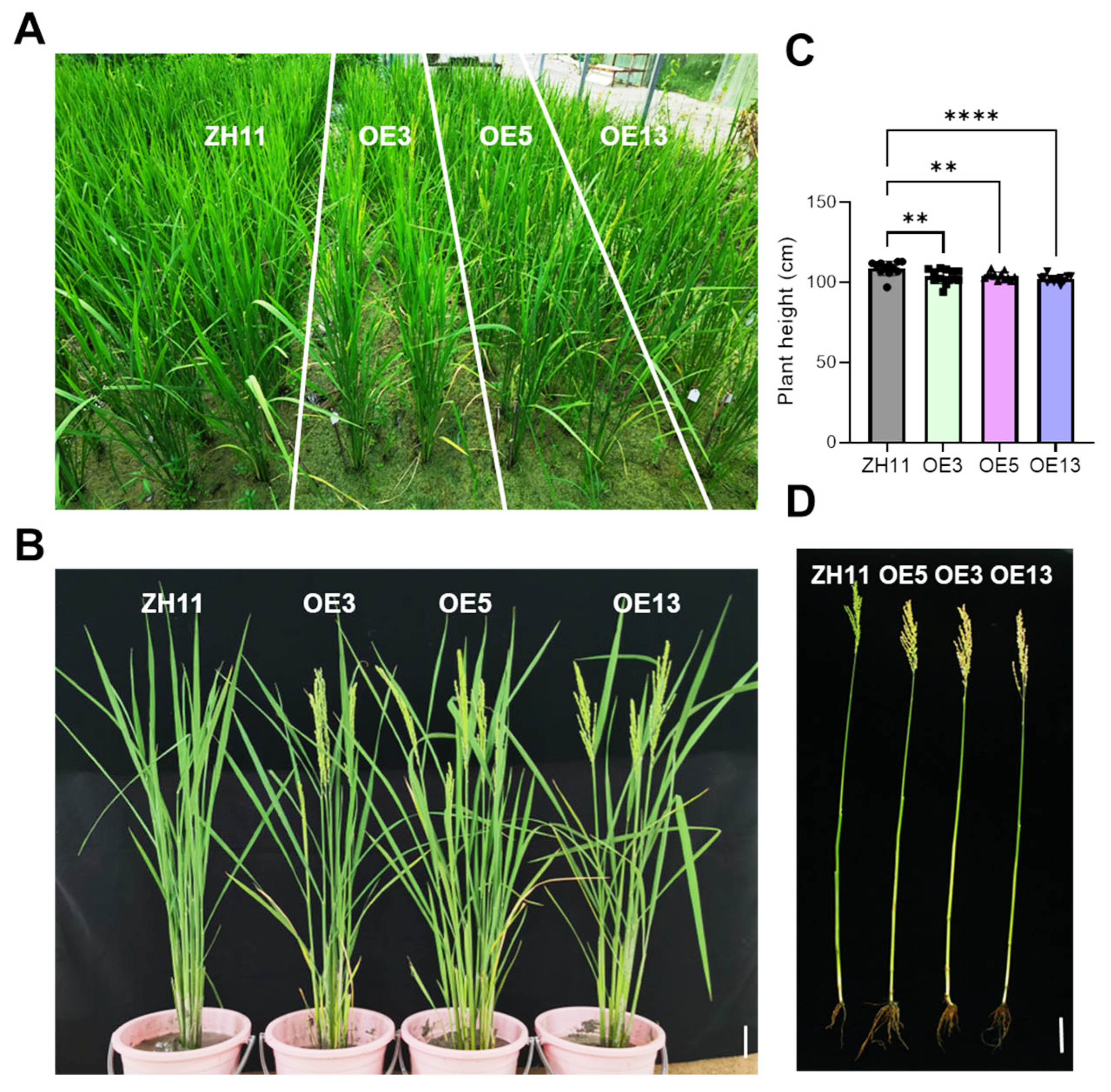
2.3. DTX6m-Overexpression Lines Had Early Flowering and Semi-Dwarf Growth Phenotypes in the Field
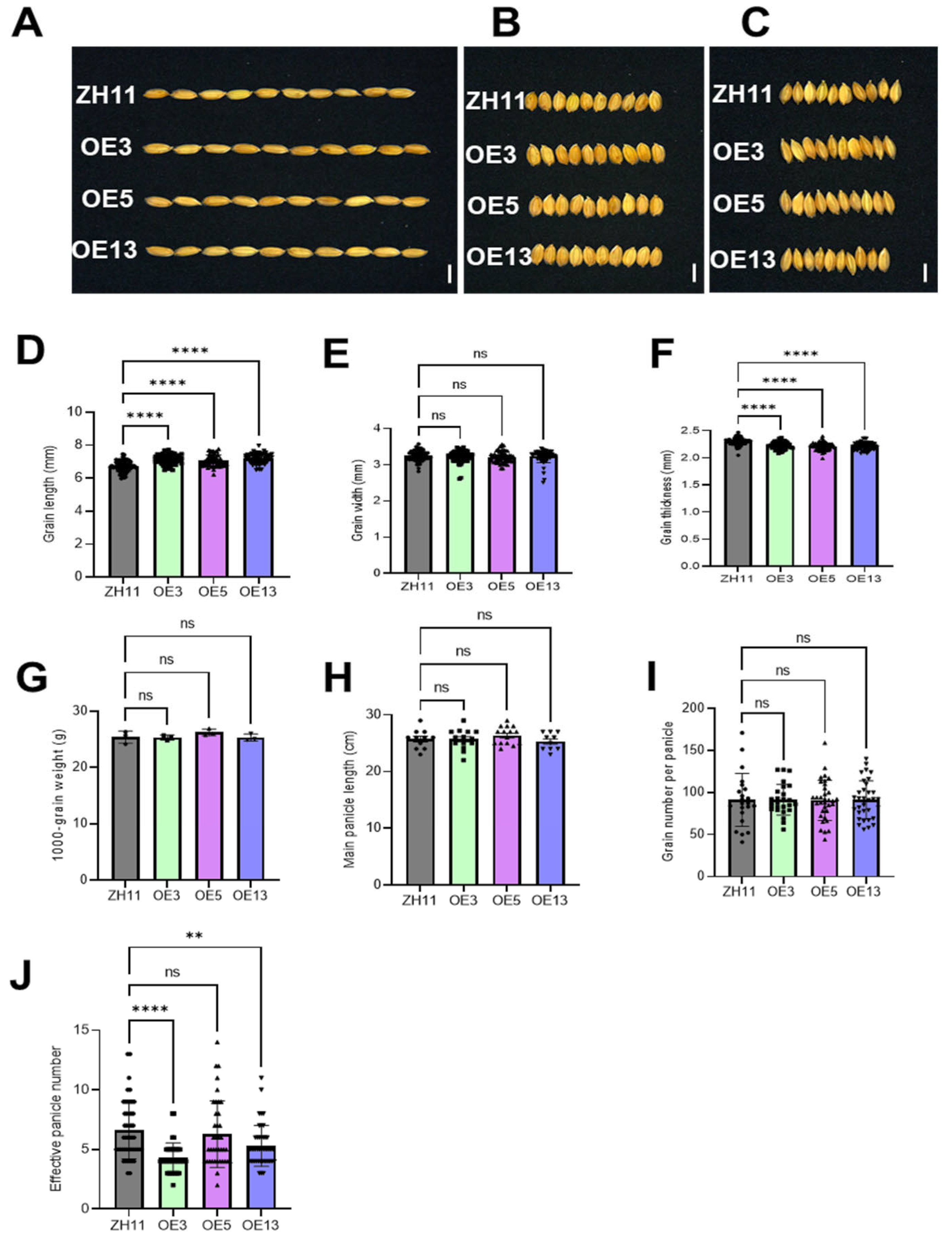
2.4. Analysis of the Field Agronomic Traits of DTX6m-Transgenic Lines
2.5. Seed Storage Reserve Accumulation Is Not Consistently Affected in the Transgenic Lines, Except the Free Amino Acid Level
3. Discussion
3.1. Evolutionary Analysis of Arabidopsis and Rice MATE Family Genes
3.2. DTX6m Largely Increased the Resistance of Rice to Paraquat and Diquat
3.3. The Transgenic Rice Had Slightly Early Flowering and a Dwarf Phenotype Without Compromised Yield and Nutritional Value
4. Materials and Methods
4.1. Rice Variety and Growth Conditions
4.2. Generation of Transgenic Lines
4.3. RNA Extraction and RT-qPCR
4.4. Herbicide Spraying Assay of Soil-Grown Seedlings
4.5. Agronomic Trait Analysis of Transgenic Rice Plants
4.6. Nutritional Components of Rice Seeds
4.6.1. Free Fatty Acids
4.6.2. Total Sugar
4.6.3. Soluble Sugar
4.6.4. Starch Content
4.6.5. Protein Levels
4.6.6. Free Amino Acid Levels
4.7. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, S.; Kaur, R.; Chauhan, B.S. Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop Prot. 2018, 103, 65–72. [Google Scholar] [CrossRef]
- Kuang, Y.J.; Yu, H.Y.; Qi, F.Y.; Zhou, X.P.; Li, X.J.; Zhou, H.B. Developing herbicide-resistant crops through genome editing technologies: A review. Crop Prot. 2024, 183, 106745. [Google Scholar] [CrossRef]
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F.J.; Li, Y.P.; Cheng, J.Q.; Wu, C.L.; Cao, J.L.; Jin, S.X. Herbicide Resistance: Another Hot Agronomic Trait for Plant Genome Editing. Plants 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Siddique, K.H.M.; Rehman, H.; Aziz, T.; Lee, D.J.; Wahid, A. Rice direct seeding: Experiences, challenges and opportunities. Soil Till. Res. 2011, 111, 87–98. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S. Weed control in dry direct-seeded rice using tank mixtures of herbicides in South Asia. Crop Prot. 2015, 72, 90–96. [Google Scholar] [CrossRef]
- Kumar, V.; Bellinder, R.R.; Gupta, R.K.; Malik, R.K.; Brainard, D.C. Role of herbicide-resistant rice in promoting resource conservation technologies in rice-wheat cropping systems of India: A review. Crop Prot. 2008, 27, 290–301. [Google Scholar] [CrossRef]
- Beckie, H.J.; Busi, R.; Bagavathiannan, M.V.; Martin, S.L. Herbicide resistance gene flow in weeds: Under-estimated and under-appreciated. Agric. Ecosyst. Environ. 2019, 283, 106566. [Google Scholar] [CrossRef]
- Jin, M.; Chen, L.; Deng, X.W.; Tang, X.Y. Development of herbicide resistance genes and their application in rice. Crop J. 2022, 10, 26–35. [Google Scholar] [CrossRef]
- Shah, D.M.; Horsch, R.B.; Klee, H.J.; Kishore, G.M.; Winter, J.A.; Tumer, N.E.; Hironaka, C.M.; Sanders, P.R.; Gasser, C.S.; Aykent, S.; et al. Engineering Herbicide Tolerance in Transgenic Plants. Science 1986, 233, 478–481. [Google Scholar] [CrossRef]
- Das, T.K.; Behera, B.; Nath, C.P.; Ghosh, S.; Sen, S.; Raj, R.; Ghosh, S.; Sharma, A.R.; Yaduraju, N.T.; Nalia, A.; et al. Herbicides use in crop production: An analysis of cost-benefit, non-target toxicities and environmental risks. Crop Prot. 2024, 181, 106691. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.G.; McClelland, M.R. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Farrington, J.A.; Ebert, M.; Land, E.J.; Fletcher, K. Bipyridylium Quaternary-Salts and Related Compounds 5. Pulse-Radiolysis Studies of Reaction of Paraquat Radical with Oxygen—Implications for Mode of Action of Bipyridyl Herbicides. Biochim. Biophys. Acta (BBA)-Bioenerg. 1973, 314, 372–381. [Google Scholar] [CrossRef]
- Fussell, K.C.; Udasin, R.G.; Gray, J.P.; Mishin, V.; Smith, P.J.S.; Heck, D.E.; Laskin, J.D. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic. Biol. Med. 2011, 50, 874–882. [Google Scholar] [CrossRef]
- Guo, H.H.; Li, L.; Gao, L. Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples. Molecules 2023, 28, 684. [Google Scholar] [CrossRef]
- Kelemen, A.; Garda, T.; Kónya, Z.; Erdödi, F.; Ujlaky-Nagy, L.; Juhász, G.P.; Freytag, C.; M-Hamvas, M.; Máthé, C. Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems. Plants 2024, 13, 1896. [Google Scholar] [CrossRef]
- Schulteis, B.M.; Moisinho, I.S.; Butler-Jones, A.; Besançon, T.E.; Brunharo, C.; Sosnoskie, L.M. Response of Horseweed from New York Vineyards and Orchards to Paraquat and Diquat. HortScience 2025, 60, 554–562. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, M.M.; Wang, W.J.; Wang, Q.; Huang, M.Q.; Li, C.Q.; Lian, Q.C.; Xia, J.Q.; Qi, J.; Xiang, C.B.; et al. Changing Gly311 to an acidic amino acid in the MATE family protein DTX6 enhances resistance to the dihydropyridine herbicides. Mol. Plant 2021, 14, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Q.; Nazish, T.; Javaid, A.; Ali, M.; Liu, Q.Q.; Wang, L.; Zhang, Z.Y.; Zhang, Z.S.; Huang, Y.J.; Wu, J.; et al. A gain-of-function mutation of the MATE family transporter DTX6 confers paraquat resistance in Arabidopsis. Mol. Plant 2021, 14, 2126–2133. [Google Scholar] [CrossRef]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2006, 27, 587–593. [Google Scholar] [CrossRef]
- Li, L.G.; He, Z.Y.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef]
- Rogers, E.E.; Guerinot, M.L. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 2002, 14, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Peeters, A.J.M.; Léon-Kloosterziel, K.M.; Koornneef, M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.M.; Debeaujon, I.; Klein, M. The MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhao, F.G.; Tang, R.J.; Yu, Y.X.; Song, J.L.; Wang, Y.; Li, L.G.; Luan, S. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2036–E2045. [Google Scholar] [CrossRef]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.Z.; Sumner, L.W.; Tang, Y.H.; Dixon, R.A. MATE2 Mediates Vacuolar Sequestration of Flavonoid Glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, J.; Park, M.J.; Kim, Y.S.; Kim, S.G.; Jung, J.H.; Park, C.M. A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochem. J. 2012, 442, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Liu, X.Y.; Xue, H.; Wu, Y.; Shi, L.; Wang, R.; Chen, Y.; Xu, N.; Zhao, J.; Shao, J.X.; et al. Noncanonical ATG8-ABS3 interaction controls senescence in plants. Nat. Plants 2019, 5, 212–224. [Google Scholar] [CrossRef]
- Hoekenga, O.A.; Maron, L.G.; Piñeros, M.A.; Cançado, G.M.A.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef]
- Zhou, G.F.; Delhaize, E.; Zhou, M.X.; Ryan, P.R. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Ann. Bot. 2013, 112, 603–612. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.E.; Inzé, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.M.; Moriyama, Y.; Yazaki, K. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Zhu, H.F.; Pan, Y.J.; Yu, Y.X.; Luan, S.; Li, L.G. A DTX/MATE-Type Transporter Facilitates Abscisic Acid Efflux and Modulates ABA Sensitivity and Drought Tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Zhang, G.H.; Hu, B.H.; Wu, J.; Chen, W.L.; Ren, Z.J.; Liu, Y.L.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, eabc8873. [Google Scholar] [CrossRef]
- Nawrath, C.; Heck, S.; Parinthawong, N.; Métraux, J.P. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 2002, 14, 275–286. [Google Scholar] [CrossRef]
- Serrano, M.; Wang, B.J.; Aryal, B.; Garcion, C.; Abou-Mansour, E.; Heck, S.; Geisler, M.; Mauch, F.; Nawrath, C.; Métraux, J.P. Export of Salicylic Acid from the Chloroplast Requires the Multidrug and Toxin Extrusion-Like Transporter EDS5. Plant Physiol. 2013, 162, 1815–1821. [Google Scholar] [CrossRef]
- Sun, X.L.; Gilroy, E.M.; Chini, A.; Nurmberg, P.L.; Hein, I.; Lacomme, C.; Birch, P.R.J.; Hussain, A.; Yun, B.W.; Loake, G.J. encodes a MATE-transporter that negatively regulates plant disease resistance. New Phytol. 2011, 192, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci. Rep. 2014, 4, 3964. [Google Scholar] [CrossRef]
- Dobritzsch, M.; Lübken, T.; Eschen-Lippold, L.; Gorzolka, K.; Blum, E.; Matern, A.; Marillonnet, S.; Böttcher, C.; Dräger, B.; Rosahl, S. MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides. Plant Cell 2016, 28, 583–596. [Google Scholar] [CrossRef]
- Wang, L.; Bei, X.; Gao, J.; Li, Y.; Yan, Y.; Hu, Y. The similar and different evolutionary trends of MATE family occurred between rice and Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 207. [Google Scholar] [CrossRef]
- Du, Z.X.; Su, Q.T.; Wu, Z.; Huang, Z.; Bao, J.Z.; Li, J.B.; Tu, H.; Zeng, C.H.; Fu, J.R.; He, H.H. Genome-wide characterization of MATE gene family and expression profiles in response to abiotic stresses in rice. BMC Ecol. Evol. 2021, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.W.; Wang, Y.; Wang, F.P.; Zhao, S.; Song, J.L.; Feng, F.; Zhao, J.R.; Yang, J.X. Expanding base editing scope to near-PAMless with engineered CRISPR/Cas9 variants in plants. Mol. Plant 2021, 14, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 2008, 3, 824–834. [Google Scholar] [CrossRef]
- Laurell, S.; Tibbling, G. Colorimetric Micro-Determination of Free Fatty Acids in Plasma. Clin. Chim. Acta 1967, 16, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hron, W.T.; Menahan, L.A. A Sensitive Method for the Determination of Free Fatty-Acids in Plasma. J. Lipid Res. 1981, 22, 377–381. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Hansen, J.; Moller, I. Percolation of Starch and Soluble Carbohydrates from Plant-Tissue for Quantitative-Determination with Anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Yu, J.; Li, S.M.; Xu, Q.Y.; Zheng, X.; Shao, Y.F.; Zhu, D.W. Extraction and determination of rice protein components. Qual. Saf. Agro Prod. 2023, 2, 10–14. [Google Scholar]
- Doi, E.; Shibata, D.; Matoba, T. Modified Colorimetric Ninhydrin Methods for Peptidase Assay. Anal. Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Han, J.; Sun, Z.; Zhao, M.; Zhang, Z.; An, S.; Shi, M.; Yang, J.; Ge, X. The G311E Mutant Gene of MATE Family Protein DTX6 Confers Diquat and Paraquat Resistance in Rice Without Yield or Nutritional Penalties. Int. J. Mol. Sci. 2025, 26, 6204. https://doi.org/10.3390/ijms26136204
Chen G, Han J, Sun Z, Zhao M, Zhang Z, An S, Shi M, Yang J, Ge X. The G311E Mutant Gene of MATE Family Protein DTX6 Confers Diquat and Paraquat Resistance in Rice Without Yield or Nutritional Penalties. International Journal of Molecular Sciences. 2025; 26(13):6204. https://doi.org/10.3390/ijms26136204
Chicago/Turabian StyleChen, Gaoan, Jiaying Han, Ziyan Sun, Mingming Zhao, Zihan Zhang, Shuo An, Muyu Shi, Jinxiao Yang, and Xiaochun Ge. 2025. "The G311E Mutant Gene of MATE Family Protein DTX6 Confers Diquat and Paraquat Resistance in Rice Without Yield or Nutritional Penalties" International Journal of Molecular Sciences 26, no. 13: 6204. https://doi.org/10.3390/ijms26136204
APA StyleChen, G., Han, J., Sun, Z., Zhao, M., Zhang, Z., An, S., Shi, M., Yang, J., & Ge, X. (2025). The G311E Mutant Gene of MATE Family Protein DTX6 Confers Diquat and Paraquat Resistance in Rice Without Yield or Nutritional Penalties. International Journal of Molecular Sciences, 26(13), 6204. https://doi.org/10.3390/ijms26136204





