Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure–Activity Relationship: In Vivo, In Vitro, and In Silico Studies
Abstract
1. Introduction
2. Results
2.1. Cytotoxic Activity
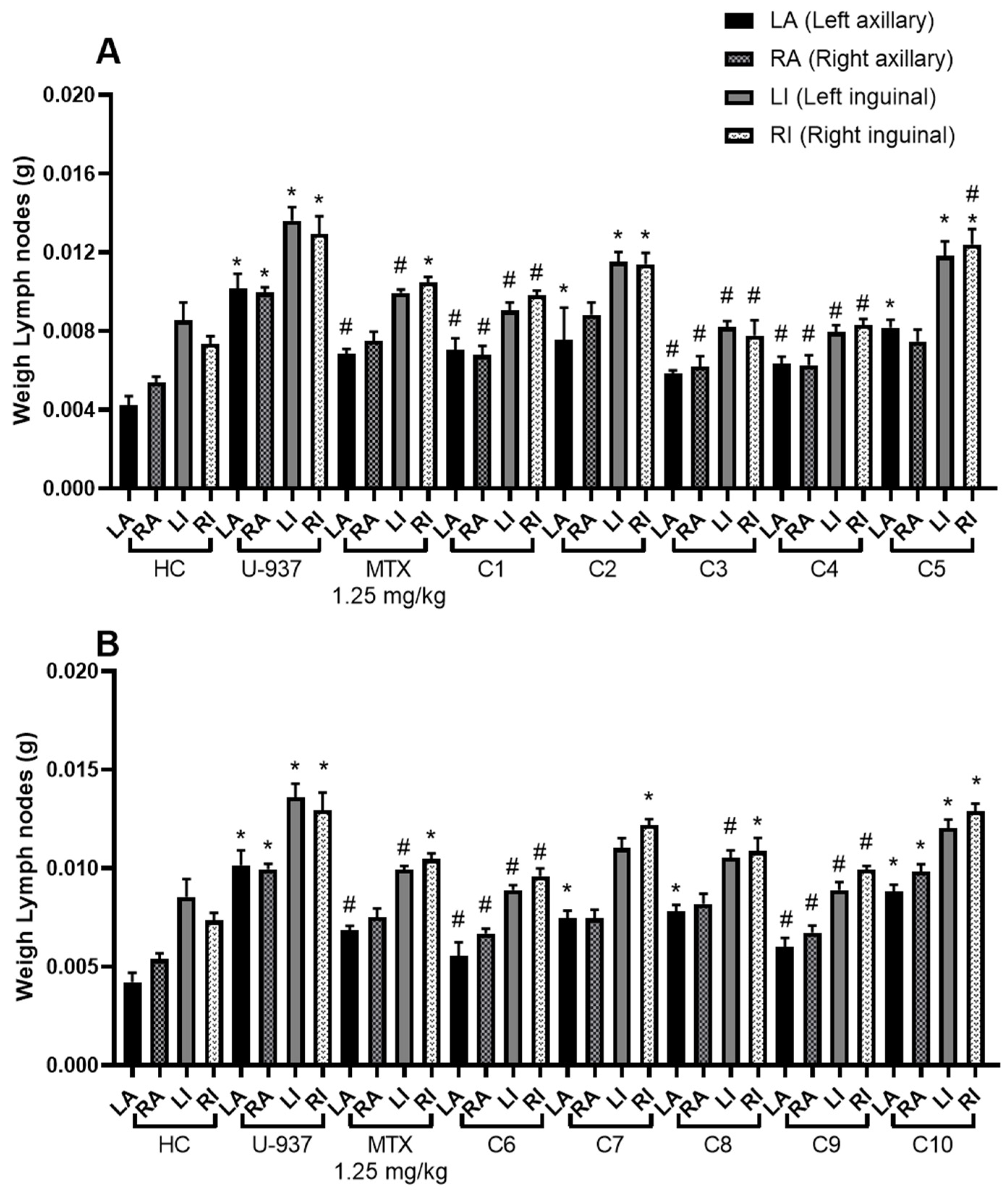
2.2. Anti-lymphoma Activity
2.3. Toxicoinformatic and Pharmaceutical Analysis of Acyclic Terpenoids
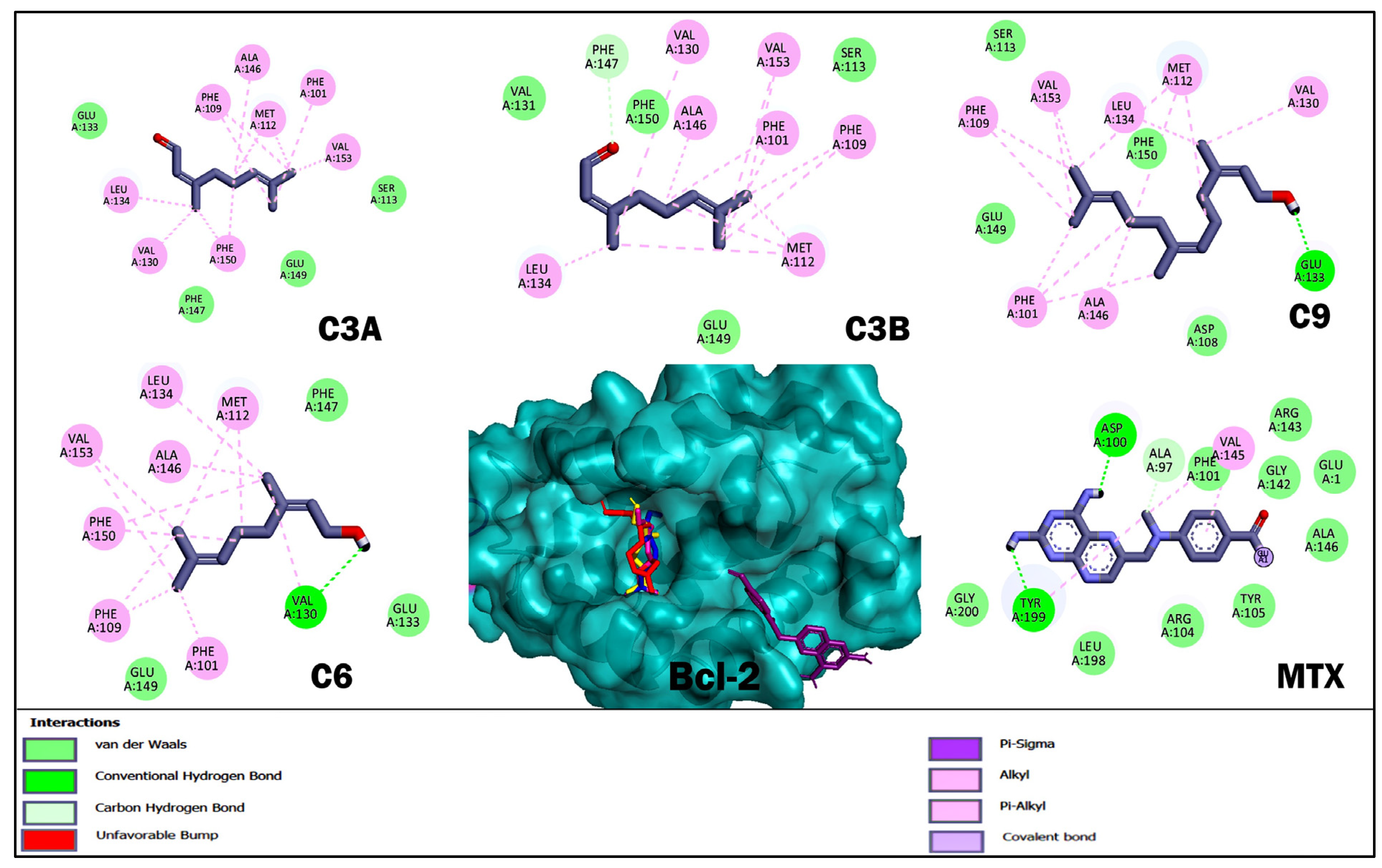
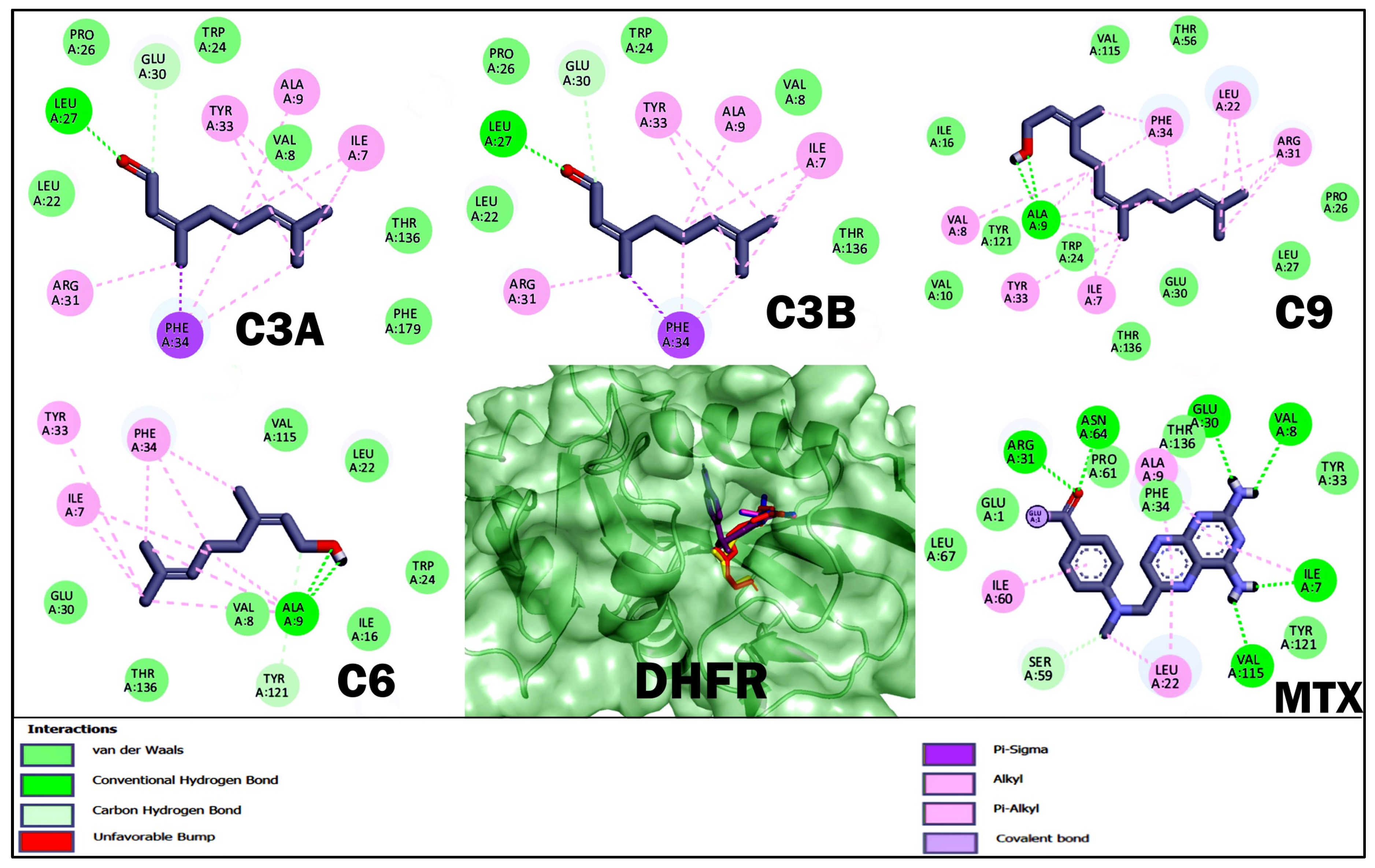
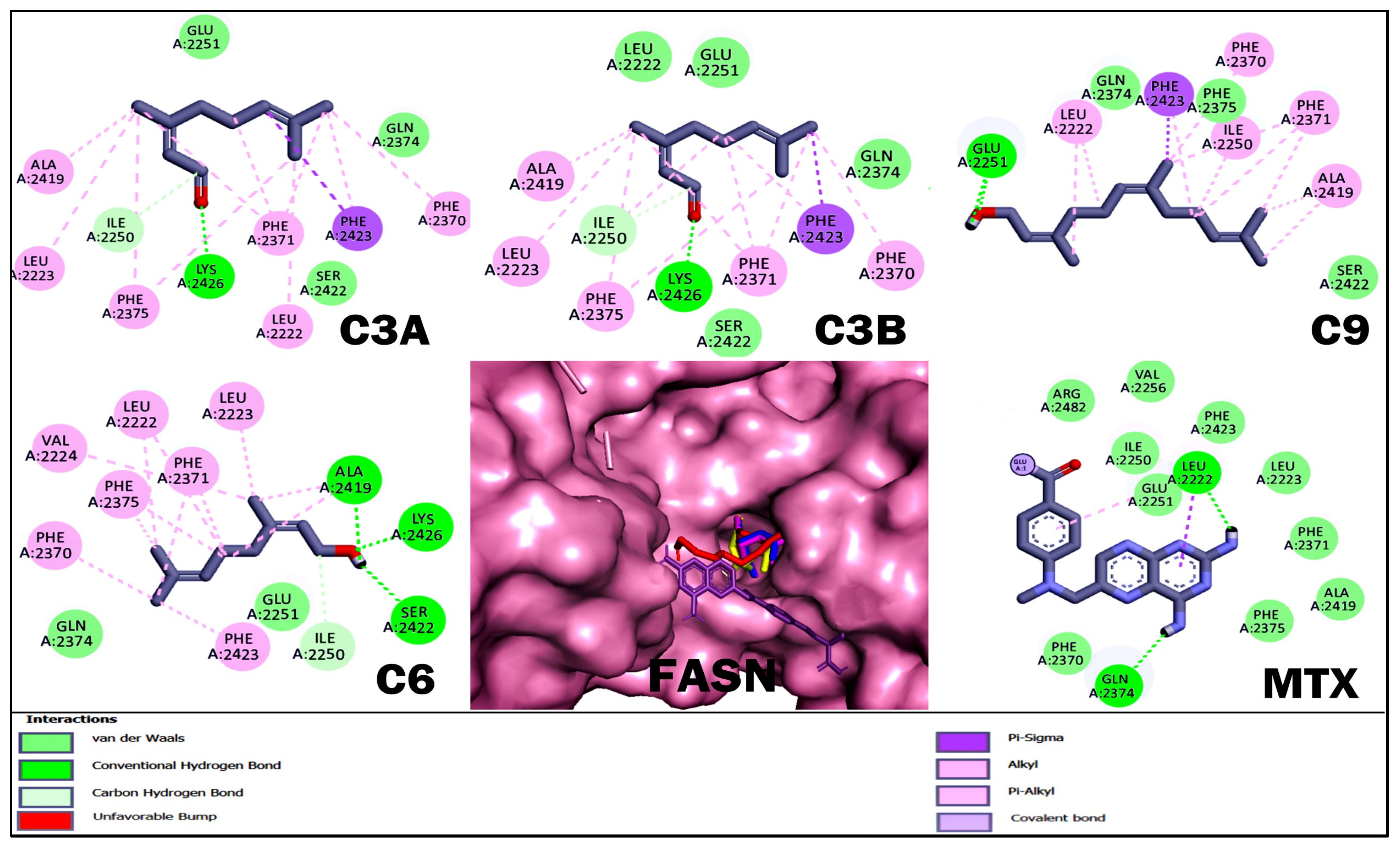
2.4. Molecular Docking Studies of Acyclic Terpenoids
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell-Based Assay
4.2.1. Cell Line
4.2.2. Cytotoxic Activity
4.3. Animals
4.4. Anti-lymphoma Activity
4.5. In Silico Toxicology and Pharmaceutical Properties
4.6. Studies of Molecular Docking of Acyclic Terpenoids
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Romancik, J.T. The Development and Application of Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma. J. Pers. Med. 2025, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Shaikh, H. Non-Hodgkin Lymphoma; StatPearls [Internet]; StatPearls Publishing: Miami, FL, USA, 2023. [Google Scholar]
- Luo, J.; Craver, A.; Bahl, K.; Stepniak, L.; Moore, K.; King, J.; Aschebrook-Kilfoy, B. Etiology of non-Hodgkin lymphoma: A review from epidemiologic studies. J. Natl. Cancer Cent. 2022, 2, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Salud Publica. Available online: https://www.gob.mx/salud/prensa/294-mexico-registra-al-ano-mas-de-195-mil-casos-de-cancer-secretaria-de-salud#:~:text=Del%20linfoma%20no%20Hodgkin%20hay,en%20mayores%20de%2060%20a%C3%B1os (accessed on 18 December 2024).
- Leick, M.B.; Maus, M.V.; Frigault, M.J. Clinical perspective: Treatment of aggressive B cell lymphomas with FDA-approved CAR-T cell therapies. Mol. Ther. 2021, 29, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.J.; Luminari, S.; Barr, P.M.; Barta, S.K.; Danilov, A.V.; Hill, B.T.; Okosun, J. Follicular lymphoma: Recent and emerging therapies, treatment strategies, and remaining unmet needs. Oncologist 2019, 24, e1236–e1250. [Google Scholar] [CrossRef]
- Ansell, S.M. Hodgkin lymphoma: Diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 1574–1583. [Google Scholar] [CrossRef]
- Baena-Gómez, M.A.; Matilla, M.M.; Atienza, A.L.; Catalán, M.A.; Marqués, C.H.; López, L.M. Non-Hodgkin lymphoma: Excellent results at the expense of the high toxicity of the treatment. An. Pediatría 2015, 82, 381–387. [Google Scholar] [CrossRef]
- Al-Naeeb, A.B.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Jiang, Y.; Jin, B.; Wang, L. Functions of representative terpenoids and their biosynthesis mechanisms in medicinal plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenos: Sustancias útiles en la salud humana. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.; Alshawsh, M.A. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- NCT02255084. Vaginal Self-Sampling and Human Papillomavirus Testing in Unscreened Women. Available online: https://clinicaltrials.gov/show/NCT02255084 (accessed on 20 December 2024).
- Cortellini, A.; Verna, L.; Cannita, K.; Napoleoni, L.; Parisi, A.; Ficorella, C.; Porzio, G. Topical menthol for treatment of chemotherapy-induced peripheral neuropathy. Indian J. Palliat. Care 2017, 23, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Gao, S.; Mao, Y.; Xue, Q.; Yuan, Z.; Li, N.; Su, K.; Yang, K.; Lv, F.; Qiu, B.; et al. Open three-stage transthoracic oesophagectomy versus minimally invasive thoraco-laparoscopic oesophagectomy for oesophageal cancer: Protocol for a multicentre prospective, open and parallel, randomised controlled trial. BMJ Open 2015, 5, e008328. [Google Scholar] [CrossRef]
- Miller, J.A.; Thomson, P.; Hakim, I.A.; Lopez, A.M.; Vining, D.; Chew, W.M.; Chow, H.H.S. Abstract A79: Human breast tissue bioavailability of topically applied limonene. Can Prev Res. 2010, 3 (Suppl. S1), A79. [Google Scholar] [CrossRef]
- Passiglia, F.; Listì, A.; Castiglia, M.; Perez, A.; Rizzo, S.; Bazan, V.; Russo, A. EGFR inhibition in NSCLC: New findings and opened questions? Crit. Rev. Oncol. Hematol. 2017, 112, 126–135. [Google Scholar] [CrossRef]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Gerecitano, J.F. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Fowler, N.H.; Wang, M.L. Breakthrough therapies in B-cell non-Hodgkin lymphoma. Ann. Oncol. 2016, 27, 778–787. [Google Scholar] [CrossRef]
- Fowler-Shorten, D.J.; Hellmich, C.; Markham, M.; Bowles, K.M.; Rushworth, S.A. BCL-2 inhibition in haematological malignancies: Clinical application and complications. Blood Rev. 2024, 65, 101195. [Google Scholar] [CrossRef]
- Rana, R.M.; Rampogu, S.; Zeb, A.; Son, M.; Park, C.; Lee, G.; Lee, K.W. In silico study probes potential inhibitors of human dihydrofolate reductase for cancer therapeutics. J. Clin. Med. 2019, 8, 233. [Google Scholar] [CrossRef]
- Rao, A.S.; Tapale, S.R. A Study on dihydrofolate reductase and its inhibitors: A review. Int. J. Pharma Sci. Res. 2013, 4, 2535. [Google Scholar] [CrossRef]
- Peeters, R.; Cuenca-Escalona, J.; Zaal, E.A.; Hoekstra, A.T.; Balvert, A.C.; Vidal-Manrique, M.; Van Spriel, A.B. Fatty acid metabolism in aggressive B-cell lymphoma is inhibited by tetraspanin CD37. Nat. Commun. 2022, 13, 5371. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Pedarra, S.; Verdura, S.; Pardo, M.A.; Espin Garcia, R.; Serrano-Hervás, E.; Menendez, J.A. Fatty acid synthase (FASN) is a tumor-cell-intrinsic metabolic checkpoint restricting T-cell immunity. Cell Death Discov. 2024, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; Vander Steen, T.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Lupu, R. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Discov. 2021, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Juarez, D.; Fruman, D.A. Targeting the mevalonate pathway in cancer. Trends Cancer 2021, 7, 525–540. [Google Scholar] [CrossRef]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef]
- Yeganehjoo, H.; DeBose-Boyd, R.; McFarlin, B.; Mo, H. Synergistic impact of d-δ-tocotrienol and geranylgeraniol on the growth and HMG CoA reductase of human DU145 prostate carcinoma cells. Nutr. Cancer 2017, 69, 682–691. [Google Scholar] [CrossRef]
- Ramírez-Santos, J.; Calzada, F.; Ordoñez-Razo, R.M.; Mendieta-Wejebe, J.E.; Velázquez-Domínguez, J.A.; Argüello-García, R.; Barbosa, E. In vivo, in vitro and in silico anticancer activity of ilama leaves: An edible and medicinal plant in México. Molecules 2024, 29, 1956. [Google Scholar] [CrossRef]
- Ramírez-Santos, J.; Calzada, F.; Mendieta-Wejebe, J.E.; Ordoñez-Razo, R.M.; Martinez-Casares, R.M.; Valdes, M. Understanding the anti-lymphoma activity of Annona macroprophyllata Donn and its acyclic terpenoids: In vivo, in vitro, and in silico studies. Molecules 2022, 27, 7123. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Siddiqi, T. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. The Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Zubair, A.C.; Ali, S.A.; Rees, R.C.; Goepel, J.R.; Winfield, D.A.; Goyns, M.H. Analysis of the colonization of unirradiated and irradiated SCID mice by human lymphoma and non-malignant lymphoid cells. Leuk. Lymphoma 1996, 22, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G.J. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON 2018, 23, 346–352. [Google Scholar] [PubMed]
- Saini, D.; Chaudhary, P.K.; Chaudhary, J.K.; Kaur, H.; Verma, G.K.; Pramanik, S.D.; Prasad, R. Molecular mechanisms of antiproliferative and pro-apoptotic effects of essential oil active constituents in MCF7 and T24 cancer cell lines: In vitro insights and in silico modelling of proapoptotic gene product-compound interactions. Apoptosis 2024, 30, 805–825. [Google Scholar] [CrossRef]
- Silva, G.D.S.E.; Marques, J.N.J.; Linhares, E.P.M.; Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.J.; Qin, H.W.; Hu, W.J.; Liu, Y.J.; Xiao, G.S.; Yang, D.L. Study on mechanism of inhibiting proliferation of head and neck cancer cells by citral, active ingredient of lemon essential oil. J. Chin. Mater. Med. 2024, 49, 2364–2375. [Google Scholar] [CrossRef]
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways. Int. J. Oncol. 2016, 48, 1772–1782. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential anti-inflammatory and anti-cancer properties of farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Joo, J.H.; Ueda, E.; Bortner, C.D.; Yang, X.P.; Liao, G.; Jetten, A.M. Farnesol activates the intrinsic apoptosis pathway and the ATF4-ATF3-CHOP cascade of ER stress in human T-lymphoblastic leukemia Molt4 cells. Biochem. Pharmacol. 2015, 97, 256–268. [Google Scholar] [CrossRef]
- Pollastri, M.P. Overview on the Rule of Five. Curr. Protoc. Pharmacol. 2010, 49, 9–12. [Google Scholar] [CrossRef]
- Špičáková, A.; Szotáková, B.; Dimunová, D.; Myslivečková, Z.; Kubíček, V.; Ambrož, M.; Skálová, L. Nerolidol and farnesol inhibit some cytochrome P450 activities but did not affect other xenobiotic-metabolizing enzymes in rat and human hepatic subcellular fractions. Molecules 2017, 22, 509. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, M.; Hou, S.; Yuan, J.; Chang, X.; Gao, S.; Li, J. Therapeutic Potential of Terpenoids in Cancer Treatment: Targeting Mitochondrial Pathways. Cancer Rep. 2024, 7, e70006. [Google Scholar] [CrossRef] [PubMed]
- Moradipoodeh, B.; Jamalan, M.; Zeinali, M.; Fereidoonnezhad, M.; Mohammadzadeh, G. Actividad anticancerígena in vitro e in silico de la amigdalina en la línea celular de cáncer de mama humano SK-BR-3. Mol. Biol. Rep. 2019, 46, 6361–6370. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Elmore, S.W. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Rana, R.M.; Rampogu, S.; Abid, N.B.; Zeb, A.; Parate, S.; Lee, G.; Lee, K.W. In silico study identified methotrexate analog as potential inhibitor of drug resistant human dihydrofolate reductase for cancer therapeutics. Molecules 2020, 25, 3510. [Google Scholar] [CrossRef]
- Pemble, C.W., 4th; Johnson, L.C.; Kridel, S.J.; Lowther, W.T. Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by Orlistat. Nat. Struct. Mol. Biol. 2007, 14, 704–709. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- Mo, H.; Jeter, R.; Bachmann, A.; Yount, S.T.; Shen, C.L.; Yeganehjoo, H. The potential of isoprenoids in adjuvant cancer therapy to reduce adverse effects of statins. Front. Pharmacol 2019, 9, 1515. [Google Scholar] [CrossRef]
- Galle, M.; Crespo, R.; Rodenak Kladniew, B.; Montero Villegas, S.; Polo, M.; de Bravo, M.G. Suppression by geraniol of the growth of A549 human lung adenocarcinoma cells and inhibition of the mevalonate pathway in culture and in vivo: Potential use in cancer chemotherapy. Nutr. Cancer. 2014, 66, 888–895. [Google Scholar] [CrossRef]
- Joo, K.M.; Kim, S.; Koo, Y.J.; Lee, M.; Lee, S.H.; Choi, D.; Lim, K.M. Development and validation of UPLC method for WST-1 cell viability assay and its application to MCTT HCE™ eye irritation test for colorful substances. Toxicol. Vitro 2019, 60, 412–419. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. NOM-062-ZOO-1999: Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. 1999. Available online: https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 5 February 2024).
- Calzada, F.; Solares-Pascasio, J.I.; Valdes, M.; Garcia-Hernandez, N.; Velazquez, C.; Ordoñez-Razo, R.M.; Barbosa, E. Anti-lymphoma potential of the ethanol extract and rutin obtained of the leaves from Schinus molle Linn. Pharmacogn. Res. 2018, 10, 119–123. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. 2017 Calculation of Molecular Properties and Bioactivity Score. Available online: http://www.molinspiration.com/services/logp.html (accessed on 5 January 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Cao, D. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, gkae303. [Google Scholar] [CrossRef]
- Hanwell, M.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.; Lindstrom, W.; Sanner, M.; Belew, R.; Goodshell, D.; Olson, A. Autodock4 and AutodockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
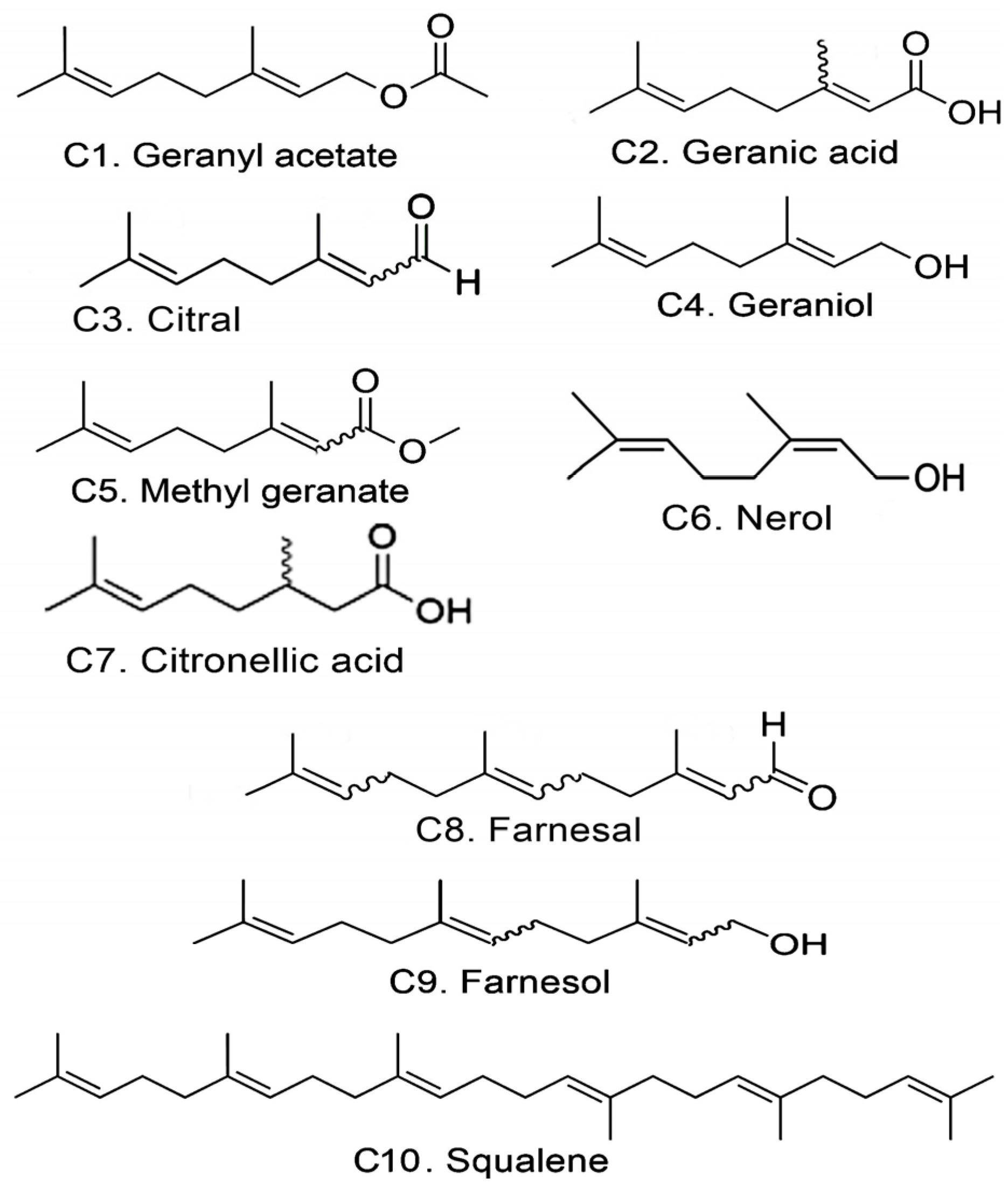
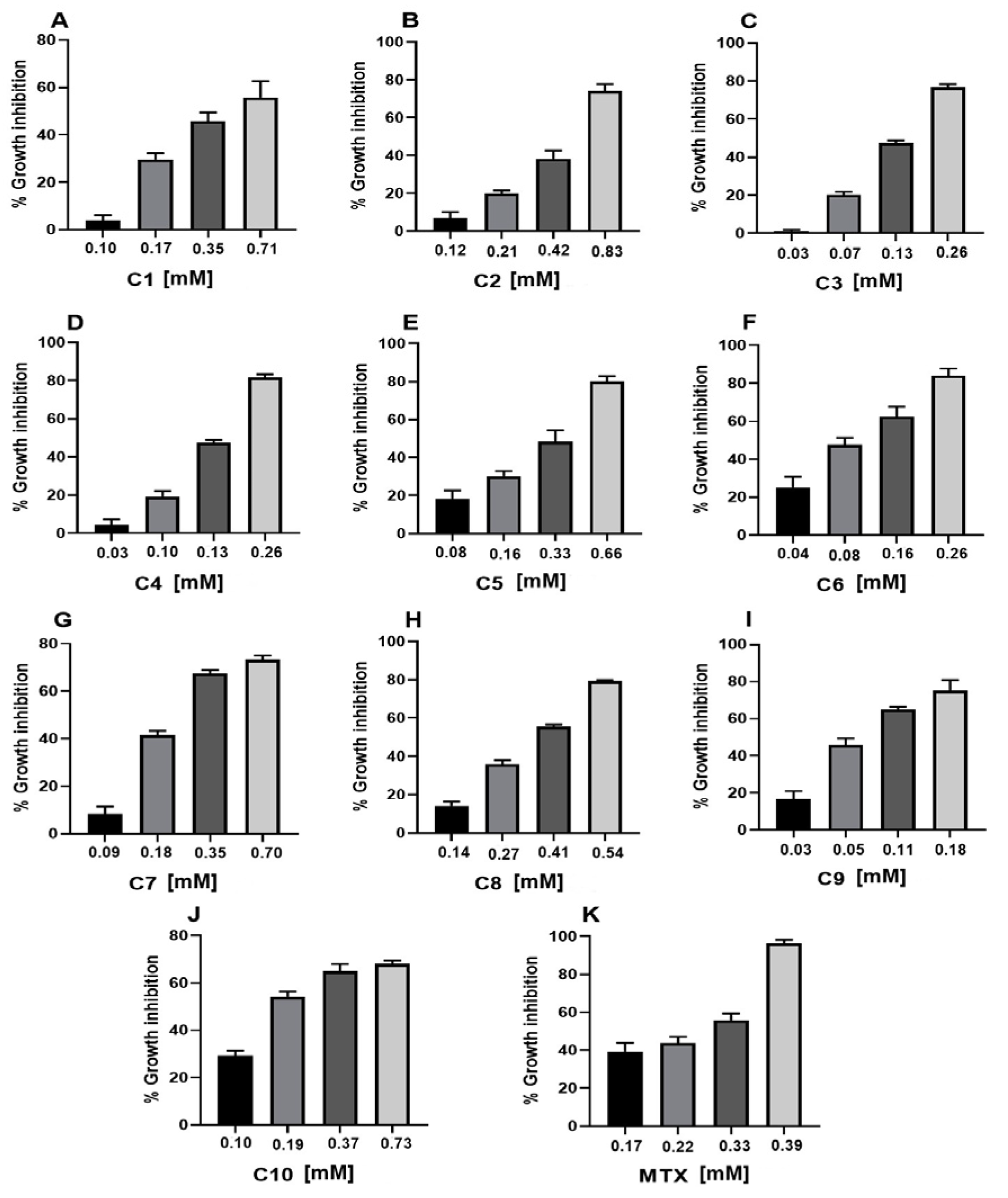

| Sample | CC50 (mM) a |
|---|---|
| Geranyl acetate (C1) | 0.55 ± 0.01 * |
| Geranic acid (C2) | 0.33 ± 0.01 * |
| Citral (C3) | 0.16 ± 0.01 * |
| Geraniol (C4) | 0.17 ± 0.01 * |
| Methyl geranate (C5) | 0.38 ± 0.01 * |
| Nerol (C6) | 0.11 ± 0.01 * |
| Citronellic acid (C7) | 0.34 ± 0.01 * |
| Farnesal (C8) | 0.36 ± 0.01 * |
| Farnesol (C9) | 0.09 ± 0.01 * |
| Squalene (C10) | 0.26 ± 0.01 |
| MTX | 0.25 ± 0.01 |
| Treatment | Inhibition of Lymph Nodes Growth (%) |
|---|---|
| Geranyl acetate (C1) | 65.93 ± 5.27 |
| Geranic acid (C2) | 34.68 ± 5.51 * |
| Citral (C3) | 88.06 ± 3.44 * |
| Geraniol (C4) | 84.28 ± 2.27 * |
| Methyl geranate (C5) | 32.15 ± 4.23 * |
| Nerol (C6) | 75.20 ± 4.33 * |
| Citronellic acid (C7) | 40.16 ± 2.58 |
| Farnesal (C8) | 43.82 ± 3.75 |
| Farnesol (C9) | 71.37 ± 3.86 * |
| Squalene (C10) | 14.42 ± 1.96 * |
| MTX | 56.17 ± 3.11 |
| Acyclic Terpenoid | C3A | C3B | C9 | C6 | C4 |
|---|---|---|---|---|---|
| Physicochemical | |||||
| TPSA | 17.07 | 17.07 | 20.23 | 20.23 | 20.23 |
| Lipophilicity (logP) | 3.28 | 3.24 | 4.70 | 2.51 | 2.95 |
| Water solubility (logS) | −2.96 | −3 | −4.41 | −2.62 | −3.03 |
| Rotatable bonds | 4 | 4 | 7 | 4 | 4 |
| Number of H-bond donors | 0 | 0 | 1 | 1 | 1 |
| Number of H-bond acceptors | 1 | 1 | 1 | 1 | 1 |
| Druglikeness | |||||
| Lipinski | Yes | Yes | Yes | Yes | Yes |
| Ghose | No | No | Yes | No | No |
| Veber | Yes | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes | Yes |
| Pharmacokinetic | |||||
| Human intestinal absorption | High | High | High | High | High |
| BBBp | Yes | Yes | Yes | Yes | Yes |
| Volume of distribution | 0.54 | 0.64 | 2.20 | 2.29 | 1.98 |
| Plasma protein binding | 92.5% | 93.3% | 97.06% | 91.09% | 90.82% |
| CYP1A2 inhibitor | No | No | Yes | No | No |
| CYP2C19 inhibitor | Yes | Yes | No | No | No |
| CYP2C19 substrate | Yes | Yes | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No |
| CYP2D6 substrate | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No |
| Clearance | 7.51 | 7.51 | 8.36 | 9.94 | 9.77 |
| T1/2 | 1.69 | 1.78 | 0.670 | 0.903 | 0.82 |
| Toxicity | |||||
| Mutagenic | No | No | No | No | No |
| Carcinogenic | No | No | No | No | No |
| Neurotoxicity | No | - | No | No | No |
| Rat Oral Acute Toxicity | No | Low | No | No | No |
| H-HT | Low | Low | Low | Low | Low |
| Predicted Toxicity Class b | 5 | 4 | 5 | 5 | 5 |
| Compound | Bcl-2 | ||
|---|---|---|---|
| ΔG (kcal/mol) | H-BR | NPI | |
| C3A | −5.07 | Ser 113, Glu 133, Phe 147, Glu 149 | Phe 101, Phe 109, Met 112, Val 130, Leu 134, Ala 146, Phe 150, Val 153 |
| C3B | −4.83 | Ser 113, Val 131, Phe 147, Glu 149, Phe 150 | Phe 101, Phe 109, Met 112, Val 130, Leu 134, Ala 146, Val 153 |
| C9 | −5.76 | Asp 108, Ser 113, Glu 133, Glu 149, Phe 150 | Phe 101, Phe 109, Met 112, Val 130, Leu 134, Ala 146, Val 153 |
| C6 | −5.17 | Val 130, Glu 133, Phe 147, Glu 149 | Phe 101, Phe 109, Met 112, Leu 134, Ala 146, Phe 150, Val 153 |
| MTX | −5.8 | Ala 97, Asp 100, Phe 101, Arg 104, Tyr 105, Gly 142, Arg 143, Ala 146, Leu 198, Tyr 199, Gly 200 | Val 145 |
| Compound | DHFR | ||
| C3A | −4.51 | Val 8, Leu 22, Trp 24, Pro 26, Leu 27, Glu 30, Phe 134, Thr 136, Phe 179 | Ile 7, Ala 9, Arg 31, Tyr 33, Phe 34 |
| C3B | −4.48 | Val 8, Leu 22, Trp 24, Pro 26, Leu 27, Glu 30, Thr 136, Phe 179 | Ile 7, Ala 9, Arg 31, Tyr 33, Phe 34 |
| C9 | −5.61 | Ala 9, Val 10, Ile 16, Trp 24, Pro 26, Leu 27, Glu 30, Thr 56, Val 115, Trp 121, Thr 136 | Ile 7, Val 8, Leu 22, Arg 31, Tyr 33, Phe 34 |
| C6 | −4.82 | Val 8, Ala 9, Val 10, Ile 16, Leu 22, Trp 24, Glu 30, Thr 56, Val 115, Tyr 121, Thr 136 | Ile 7, Tyr 33, Phe 34 |
| MTX | −8.58 | Ile 7, Val 8, Glu 30, Arg 31, Tyr 33, Phe 34, Thr 56, Ser 59, Pro 61, Asn 64, Leu 67, Val 115, Tyr 121, Thr 136, Phe 179 | Ala 9, Leu 22, Ile 60 |
| Compound | HMG-CoA reductase | ||
| C3A | −5.52 | Gly 656, Met 657, Gly 765, Asp 767, Glu 801, Ile 802, Gly 803, Val 805, Gly 806, Gly 807, Thr 809 | Ala 654, Met 655 |
| C3B | −5.5 | Gly 656, Met 657, Gly 765, Gln 766, Asp 767, Glu 801, Ile 802, Gly 803, Val 805, Gly 806, Gly 807, Gly 808, Thr 809 | Ala 654 |
| C9 | −6.72 | Gly 656, Asn 658, Lys 691, Gly 765, Gln 766, Asp 767, Gln 770, Glu 801, Ile 802, Gly 803, Val 805, Gly 806, Gly 807, Thr 809 | Ala 654, Met 655, Met 657, Met 659 |
| C6 | −5.87 | Gly 656, Met 657, Gly 765, Asp 767, Gln 770, Glu 801, Ile 802, Gly 803, Val 805, Gly 806, Gly 807, Gly 808, Thr 809 | Ala 654 |
| MTX | −5.26 | Tyr 517, Val 522, Cys 526, Tyr 533, Met 534, Pro 535, Ile 536, Pro 537, Val 538, Thr 557, Thr 558, Tyr 761, Ile 762, Ala 763, Cys 764, Gly 765 Gln 766, Ala 768, Gly 808, Leu 811, Pro 813 | Pro 535 |
| Compound | FASN | ||
| C3A | −4.86 | Ile 2250, Glu 2251, Gln 2374, Ser 2422, Lys 2426 | Leu 2222, Leu 2223, Phe 2370, Phe 2371, Phe 2375. Ala 2419, Phe 2423 |
| C3B | −4.8 | Leu 2222, Ile 2250, Glu 2251, Gln 2374, Ser 2422, Lys 2426 | Leu 2223, Phe 2370, Phe 2371, Phe 2375, Ala 2419, Phe 2423 |
| C9 | −5.79 | Leu 2223, Pro 2249, Glu 2251, Gln 2374, Phe 2375, Ser 2422, Lys 2426 | Ala 2419, Leu 2222, Ile 2250, Phe 2370, Phe 2371, Phe 2423 |
| C6 | −5.09 | Ile 2250, Glu 2251, Gln 2374, Ala 2419, Ser 2422, Lys 2426 | Leu 2222, Leu 2223, Val 2224, Phe 2370, Phe 2371, Phe 2375, Phe 2423 |
| MTX | −4.93 | Leu 2222, Leu 2223, Ile 2250, Glu 2251, Val 2256, Phe 2370, Phe 2371, Gln 2374, Phe 2375, Ala 2419, Phe 2423, Arg 2482 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzada, F.; Ramírez-Santos, J.; Ordoñez-Razo, R.M.; Valdes, M.; Velázquez, C.; Barbosa, E. Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure–Activity Relationship: In Vivo, In Vitro, and In Silico Studies. Int. J. Mol. Sci. 2025, 26, 5683. https://doi.org/10.3390/ijms26125683
Calzada F, Ramírez-Santos J, Ordoñez-Razo RM, Valdes M, Velázquez C, Barbosa E. Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure–Activity Relationship: In Vivo, In Vitro, and In Silico Studies. International Journal of Molecular Sciences. 2025; 26(12):5683. https://doi.org/10.3390/ijms26125683
Chicago/Turabian StyleCalzada, Fernando, Jesica Ramírez-Santos, Rosa María Ordoñez-Razo, Miguel Valdes, Claudia Velázquez, and Elizabeth Barbosa. 2025. "Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure–Activity Relationship: In Vivo, In Vitro, and In Silico Studies" International Journal of Molecular Sciences 26, no. 12: 5683. https://doi.org/10.3390/ijms26125683
APA StyleCalzada, F., Ramírez-Santos, J., Ordoñez-Razo, R. M., Valdes, M., Velázquez, C., & Barbosa, E. (2025). Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure–Activity Relationship: In Vivo, In Vitro, and In Silico Studies. International Journal of Molecular Sciences, 26(12), 5683. https://doi.org/10.3390/ijms26125683











