Femtosecond Laser-Engineered β-TCP Scaffolds: A Comparative Study of Green-Synthesized AgNPs vs. Ion Doping Against S. aureus for Bone Regeneration
Abstract
1. Introduction
2. Results and Discussion
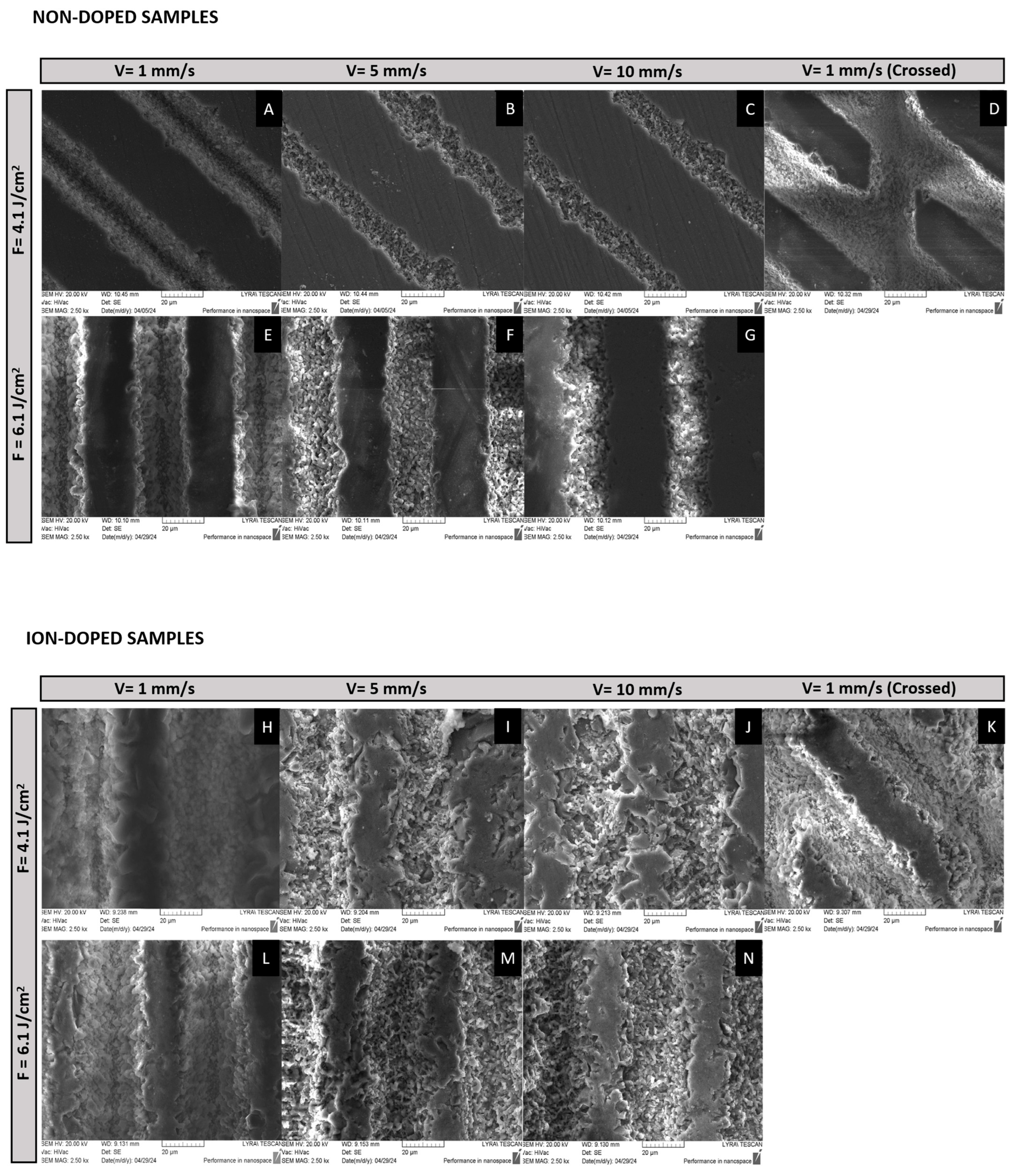
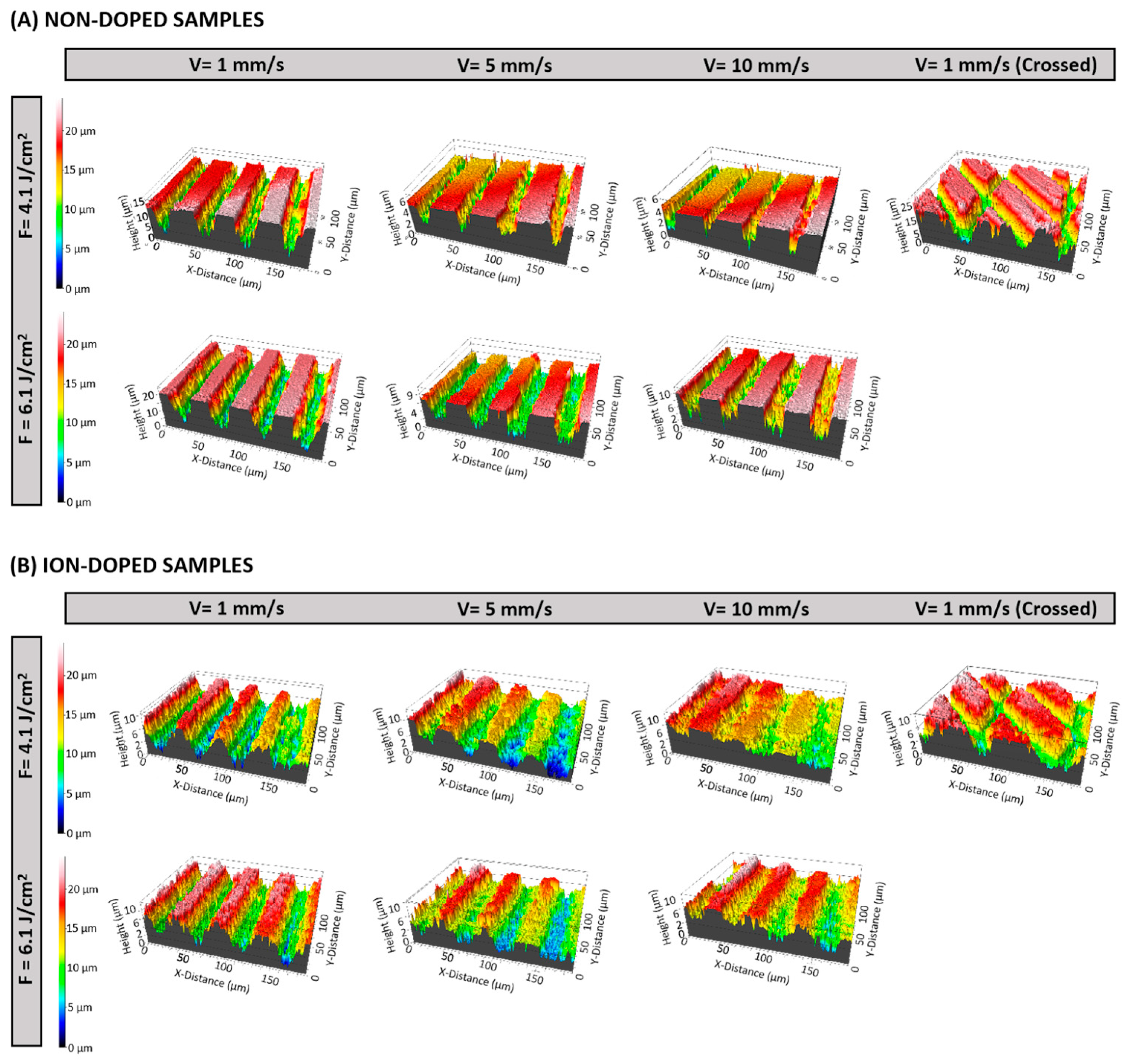
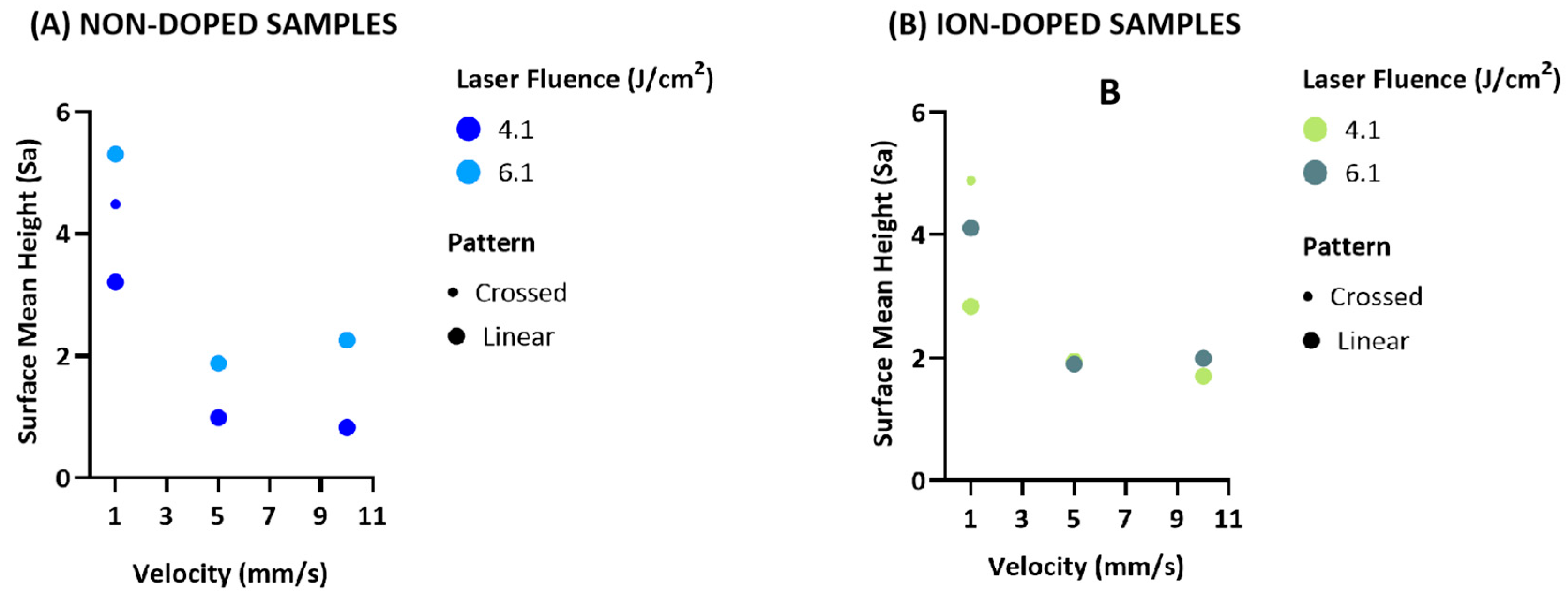
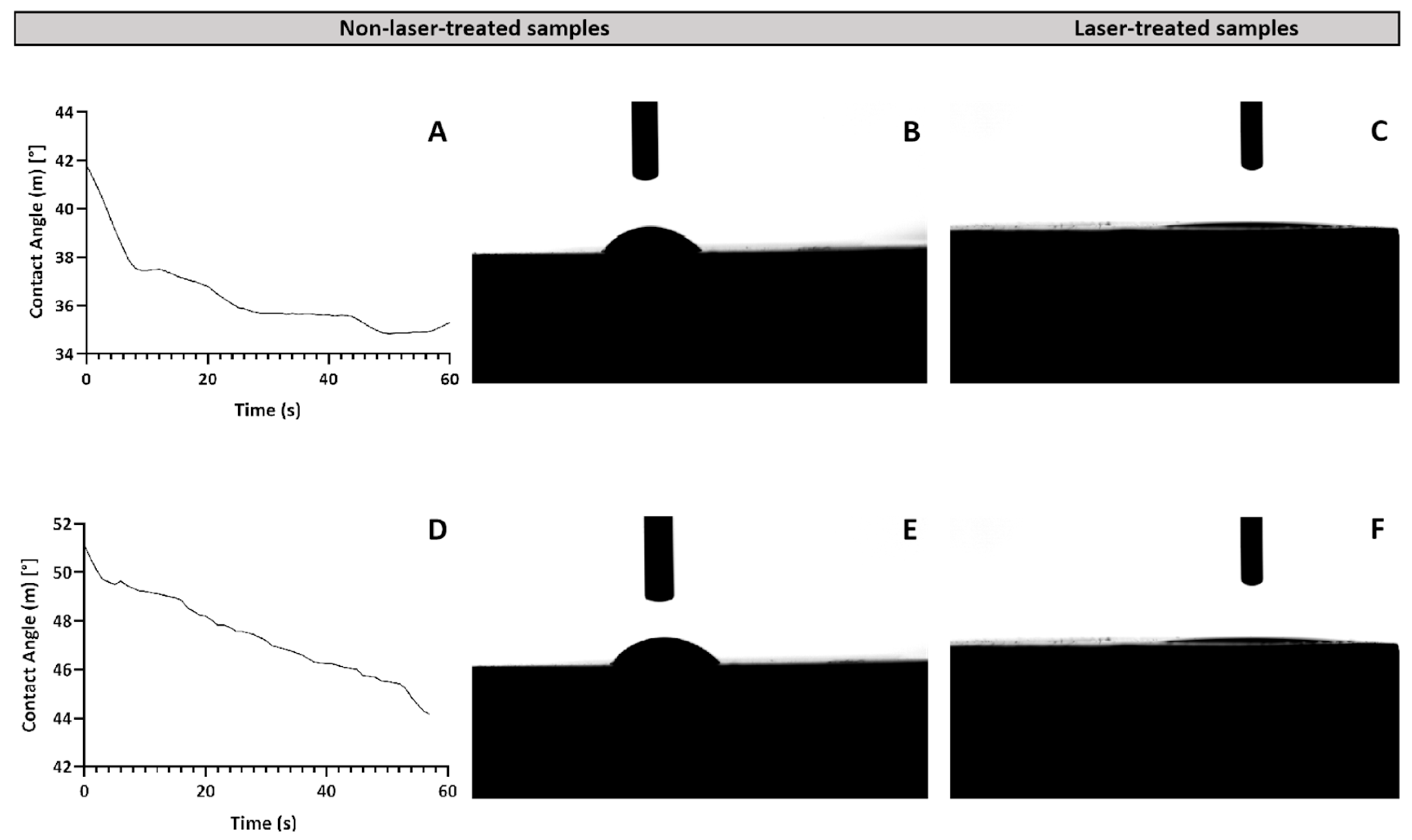
2.1. Morphological Characterization of the Laser-Treated β-TCP Scaffolds
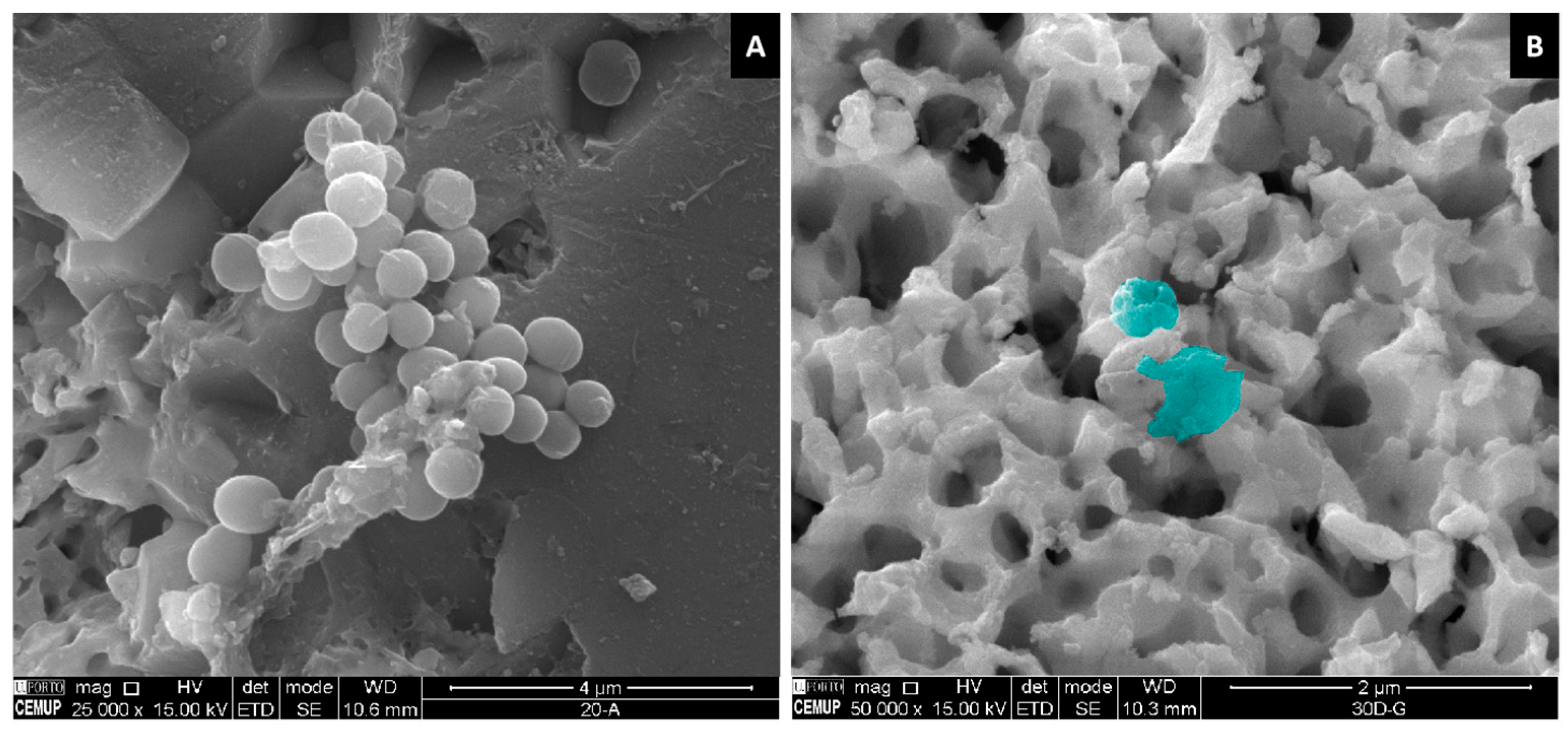
2.1.1. SEM
2.1.2. Three-Dimensional Optical Profilometry
2.1.3. Wettability Analysis
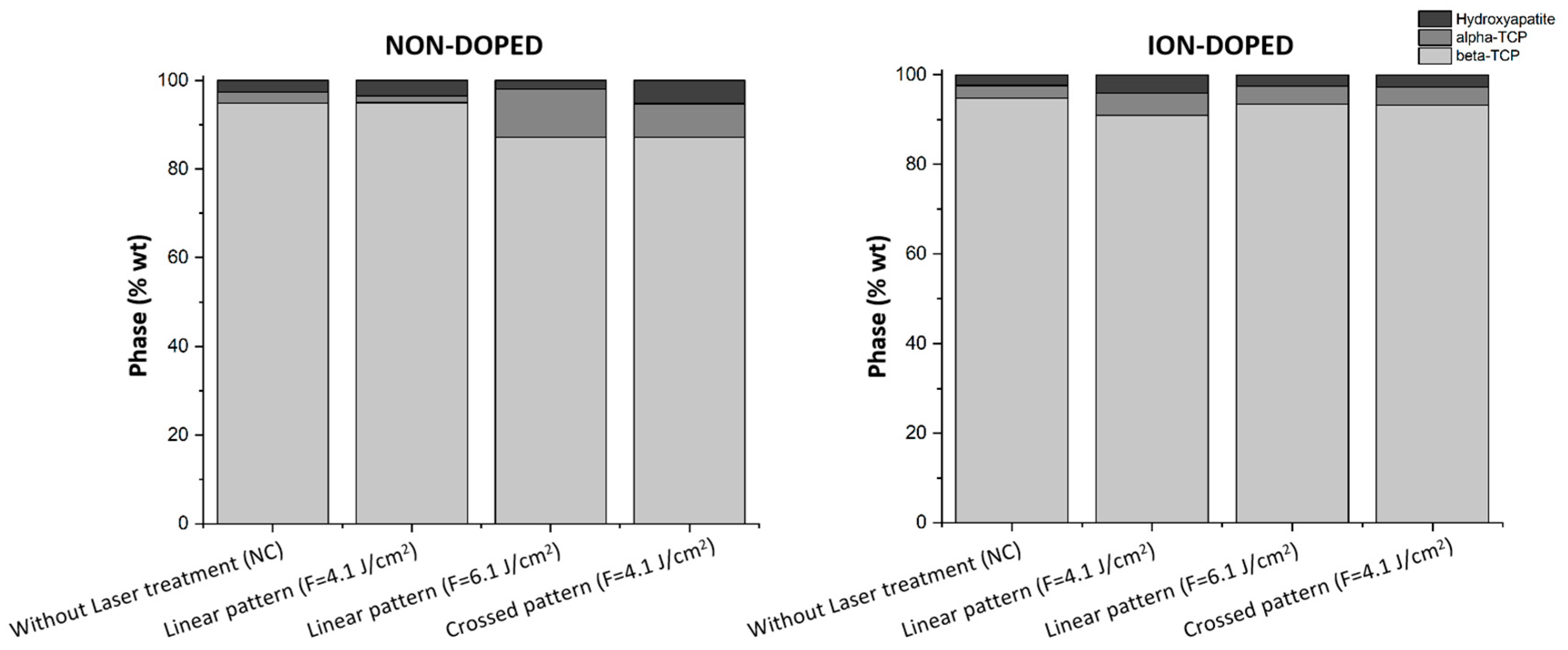
2.1.4. XRD Analysis
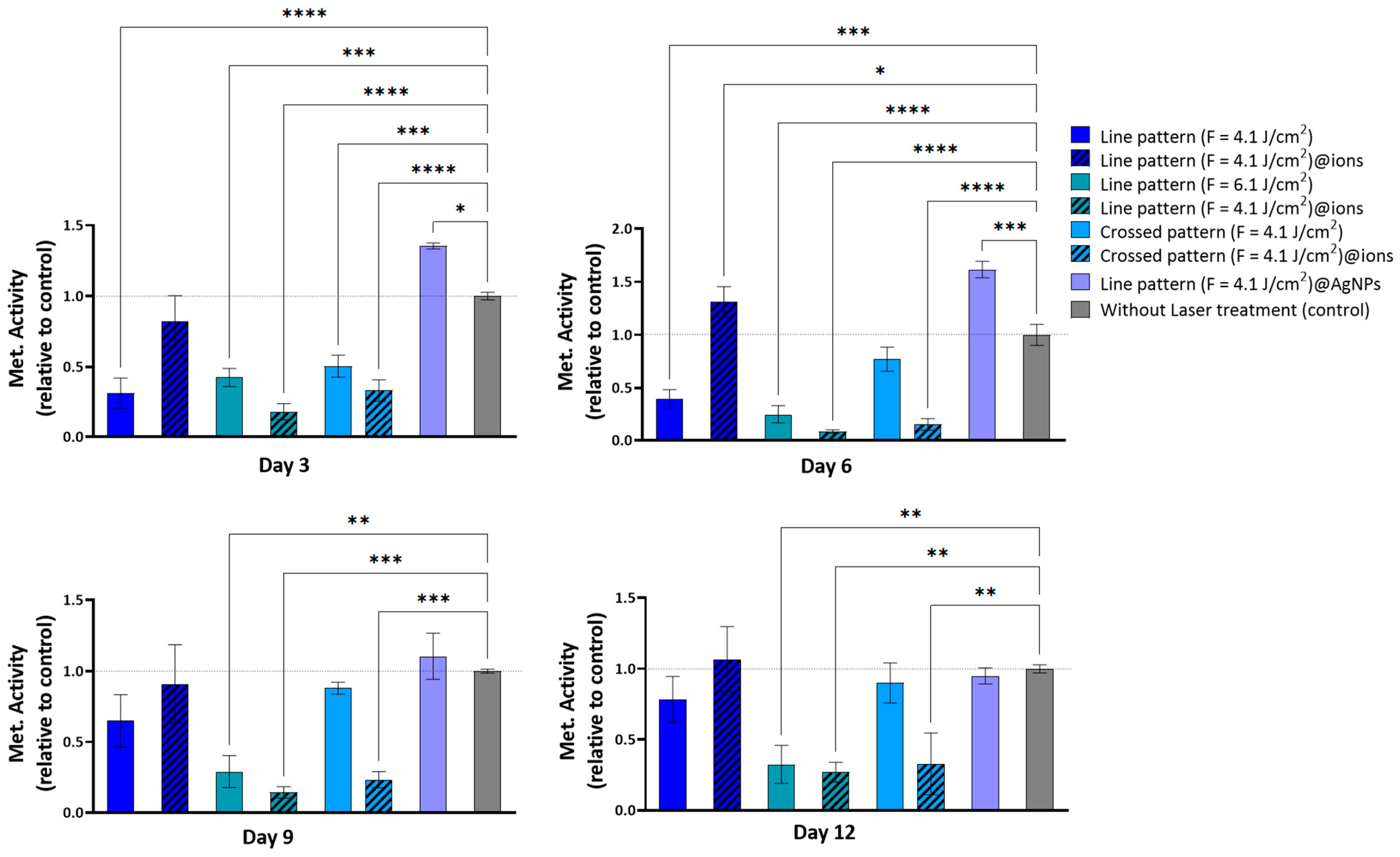
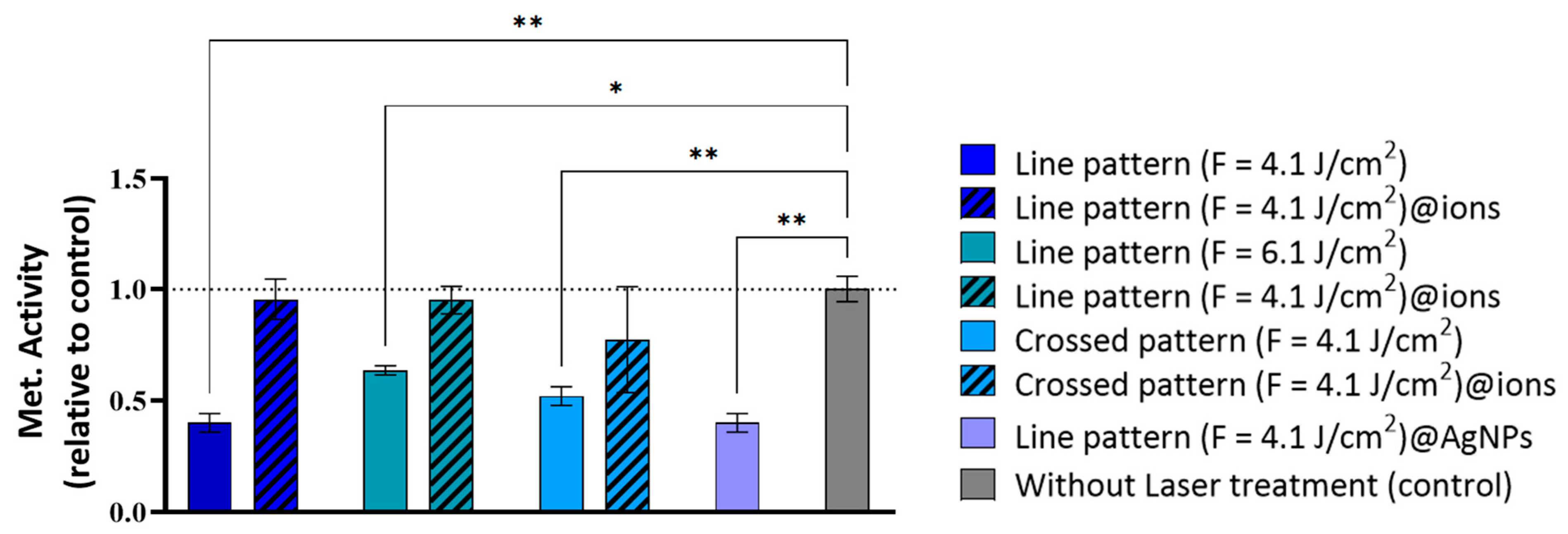
2.2. In Vitro Cytocompatibility
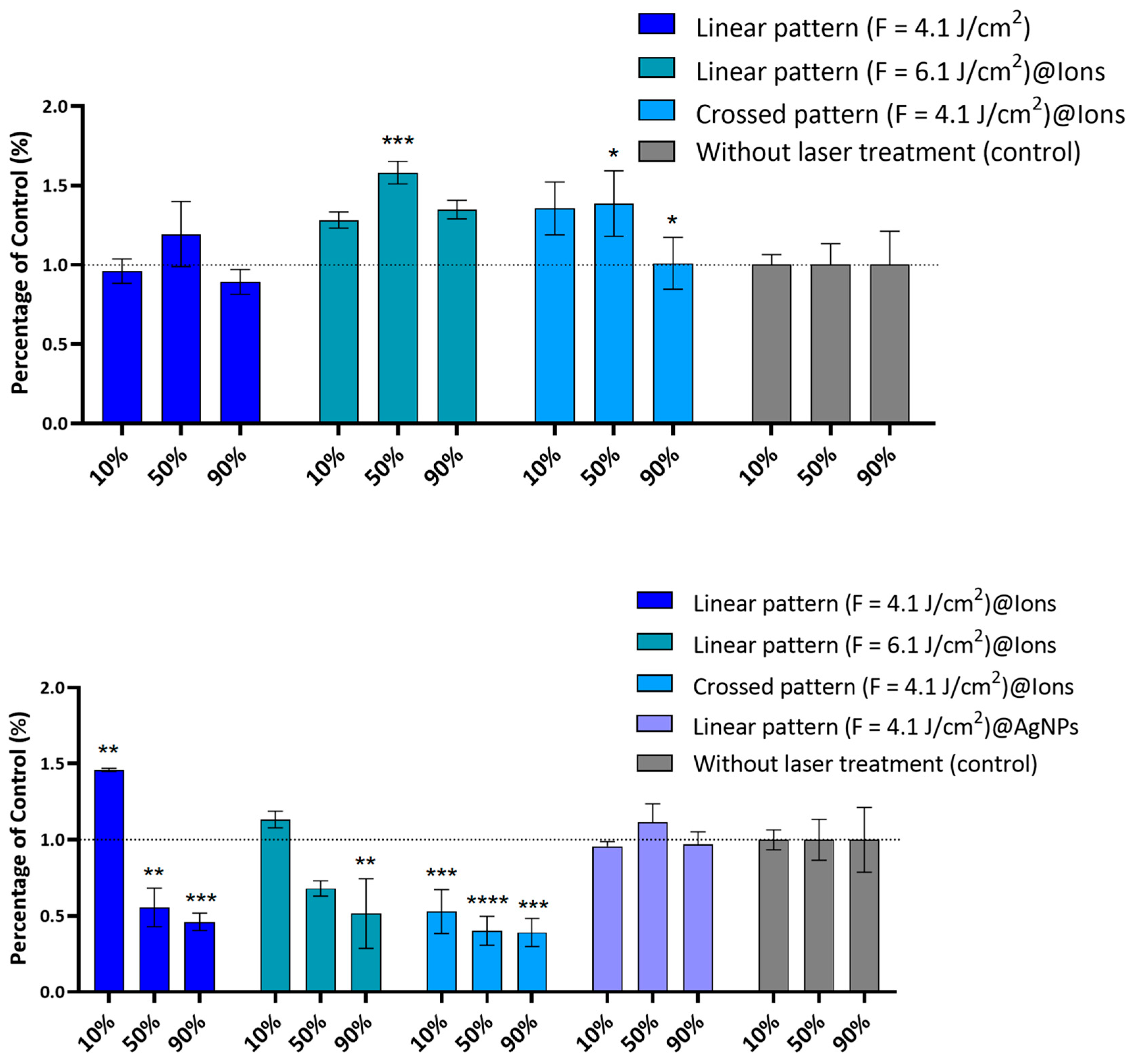
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Chemicals
3.2. Fabrication of β-TCP Scaffolds
3.3. Laser Processing of β-TCP Scaffolds
3.4. Morphological Characterization of the Laser-Treated β-TCP Scaffolds
3.4.1. SEM
3.4.2. Three-Dimensional Optical Profilometry
3.4.3. Wettability Analysis
3.4.4. XRD Analysis
3.5. Green Laser-Assisted Synthesis of AgNPs and Deposition in β-TCP Pellets
3.6. Biological Activity
3.6.1. In Vitro Cytocompatibility
Cell Culture Conditions
Direct Cytocompatibility Assay
3.7. Antibacterial Activity
3.7.1. Bacterial Culture Conditions
3.7.2. Antibacterial Direct Assay
3.7.3. Antibacterial Indirect Assay
3.8. SEM Characterization of β-TCP Scaffolds Post-Biological Testing
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Clauss, M.; Trampuž, A.; Borens, O.; Bohner, M.; Ilchmann, T. Biofilm Formation on Bone Grafts and Bone Graft Substitutes: Comparison of Different Materials by a Standard In Vitro Test and Microcalorimetry. Acta Biomater. 2010, 6, 3791–3797. [Google Scholar] [CrossRef]
- Hameed, S.; Sharif, S.; Ovais, M.; Xiong, H. Emerging Trends and Future Challenges of Advanced 2D Nanomaterials for Combating Bacterial Resistance. Bioact. Mater. 2024, 38, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Ketonis, C.; Parvizi, J.; Jones, L.C. Evolving Strategies to Prevent Implant-Associated Infections. J. Am. Acad. Orthop. Surg. 2012, 20, 478–480. [Google Scholar] [CrossRef]
- Zhang, X.; Zu, H.; Zhao, D.; Yang, K.; Tian, S.; Yu, X.; Lu, F.; Liu, B.; Yu, X.; Wang, B.; et al. Ion Channel Functional Protein Kinase TRPM7 Regulates Mg Ions to Promote the Osteoinduction of Human Osteoblast via PI3K Pathway: In Vitro Simulation of the Bone-Repairing Effect of Mg-Based Alloy Implant. Acta Biomater. 2017, 63, 369–382. [Google Scholar] [CrossRef]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The Synergistic Effects of Sr and Si Bioactive Ions on Osteogenesis, Osteoclastogenesis and Angiogenesis for Osteoporotic Bone Regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of Strontium Ions with Potential Antibacterial Activity on In Vivo Bone Regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial Mechanism of Silver Nanoparticles in Pseudomonas aeruginosa: Proteomics Approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhou, Q.; Zhang, W.; Wang, J.; Liu, Y.; Yu, M.; Qiu, Y.; Ma, Z.; Liu, S. A Synergistic Antibacterial Study of Copper-Doped Polydopamine on Ti3C2Tx Nanosheets with Enhanced Photothermal and Fenton-like Activities. Materials 2023, 16, 7583. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Hu, L.; Yan, X.; Zhao, X.; Zhou, Q.; Cai, Y.; Jiang, G. Surface Ligand Controls Silver Ion Release of Nanosilver and Its Antibacterial Activity against Escherichia coli. Int. J. Nanomed. 2017, 12, 3193–3206. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Das, S.S.; Khatoon, A.; Ansari, M.; Afzal, M.; Hasnain, S.; Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Subha, V.; Ravindran, E.; Kumar, A.B.H.; Renganathan, S. Bactericidal Effect of Silver Nanoparticles from Aqueous Root Extracts of Catharanthus roseus. Int. J. Nanoparticles 2019, 11, 294. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef]
- Kang, J.; Dietz, M.J.; Hughes, K.; Xing, M.; Li, B. Silver Nanoparticles Present High Intracellular and Extracellular Killing against Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 1578–1585. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized over the Range 5–100 Nm Using the Same Protocol and Their Antibacterial Efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized Silver Nanoparticles Enhance Contact Killing and Show Highest Efficacy: Elucidation of the Mechanism of Bactericidal Action of Silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef]
- Kumari, M.; Shukla, S.; Pandey, S.; Giri, V.P.; Bhatia, A.; Tripathi, T.; Kakkar, P.; Nautiyal, C.S.; Mishra, A. Enhanced Cellular Internalization: A Bactericidal Mechanism More Relative to Biogenic Nanoparticles than Chemical Counterparts. ACS Appl. Mater. Interfaces 2017, 9, 4519–4533. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef]
- Oliver, S.; Wagh, H.; Liang, Y.; Yang, S.; Boyer, C. Enhancing the Antimicrobial and Antibiofilm Effectiveness of Silver Nanoparticles Prepared by Green Synthesis. J. Mater. Chem. B 2018, 6, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Sousa, A.; Sá, S.; Soares, S.; Pereira, A.C.; Rocha, A.C.; Pais, P.; Ferreira, D.; Almeida, C.; Luís, C.; et al. Harvesting the Power of Green Synthesis: Gold Nanoparticles Tailored for Prostate Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 2277. [Google Scholar] [CrossRef]
- Palencia, M.S.; Berrio, M.E.; Palencia, S.L. Effect of Capping Agent and Diffusivity of Different Silver Nanoparticles on Their Antibacterial Properties. J. Nanosci. Nanotechnol. 2017, 17, 5197–5204. [Google Scholar] [CrossRef]
- Khansarizadeh, M.; Mokhtarzadeh, A.; Rashedinia, M.; Taghdisi, S.M.; Lari, P.; Abnous, K.H.; Ramezani, M. Identification of Possible Cytotoxicity Mechanism of Polyethylenimine by Proteomics Analysis. Hum. Exp. Toxicol. 2016, 35, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.C.; Lan, W.H.; Chan, C.P.; Hsieh, C.C.; Chang, M.C.; Jeng, J.H. The Effects of Extracellular Citric Acid Acidosis on the Viability, Cellular Adhesion Capacity and Protein Synthesis of Cultured Human Gingival Fibroblasts. Aust. Dent. J. 1999, 44, 123–130. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Chyu, J.Y.; Yang, R.-S.; Chen, C.-H.; Shen, C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface Potential and Charges Impact on Cell Responses on Biomaterials Interfaces for Medical Applications. Mater. Sci. Eng. C 2019, 104, 109883. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Filipov, E.; Angelova, L.; Vig, S.; Fernandes, M.H.; Moreau, G.; Lasgorceix, M.; Buchvarov, I.; Daskalova, A. Investigating Potential Effects of Ultra-Short Laser-Textured Porous Poly-ε-Caprolactone Scaffolds on Bacterial Adhesion and Bone Cell Metabolism. Polymers 2022, 14, 2382. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yao, S.; Zhang, H.; Cai, M.; Liu, W.; Pan, R.; Chen, C.; Wang, X.; Wang, L.; Zhong, M. Biocompatible Nano-Ripples Structured Surfaces Induced by Femtosecond Laser to Rebel Bacterial Colonization and Biofilm Formation. Opt. Laser Technol. 2020, 124, 105973. [Google Scholar] [CrossRef]
- Gnilitskyi, I.; Rymar, S.; Iungin, O.; Vyshnevskyy, O.; Parisse, P.; Potters, G.; Zayats, A.V.; Moshynets, O. Femtosecond Laser Modified Metal Surfaces Alter Biofilm Architecture and Reduce Bacterial Biofilm Formation. Nanoscale Adv. 2023, 5, 6659–6669. [Google Scholar] [CrossRef]
- Siddiquie, R.Y.; Gaddam, A.; Agrawal, A.; Dimov, S.S.; Joshi, S.S. Anti-Biofouling Properties of Femtosecond Laser-Induced Submicron Topographies on Elastomeric Surfaces. Langmuir 2020, 36, 5349–5358. [Google Scholar] [CrossRef]
- Carvalho, A.; Grenho, L.; Fernandes, M.H.; Daskalova, A.; Trifonov, A.; Buchvarov, I.; Monteiro, F.J. Femtosecond Laser Microstructuring of Alumina Toughened Zirconia for Surface Functionalization of Dental Implants. Ceram. Int. 2020, 46, 1383–1389. [Google Scholar] [CrossRef]
- Wu, R.; Li, Y.; Shen, M.; Yang, X.; Zhang, L.; Ke, X.; Yang, G.; Gao, C.; Gou, Z.; Xu, S. Bone Tissue Regeneration: The Role of Finely Tuned Pore Architecture of Bioactive Scaffolds before Clinical Translation. Bioact. Mater. 2021, 6, 1242–1254. [Google Scholar] [CrossRef]
- Xu, S.; Dou, H.; Sun, K.; Ye, Y.; Li, Z.; Wang, H.; Liao, W.; Liu, H.; Miao, X.; Yuan, X.; et al. Scan Speed and Fluence Effects in Femtosecond Laser Induced Micro/Nano-Structures on the Surface of Fused Silica. J. Non-Cryst. Solids 2018, 492, 56–62. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Zhang, X.; Chu, Z.; Ren, B.; Yue, C.; Jiang, B.; Liu, X. Influence of Femtosecond Laser Pulse Sequence on the Morphology and Roughness of Titanium Surface Micro-Patterns. J. Manuf. Process. 2023, 97, 248–259. [Google Scholar] [CrossRef]
- Qiu, C.; Panwisawas, C.; Ward, M.; Basoalto, H.C.; Brooks, J.W.; Attallah, M.M. On the Role of Melt Flow into the Surface Structure and Porosity Development during Selective Laser Melting. Acta Mater. 2015, 96, 72–79. [Google Scholar] [CrossRef]
- Sasidharan Pillai, R.; Sglavo, V.M. Effect of MgO Addition on Solid State Synthesis and Thermal Behavior of Beta-Tricalcium Phosphate. Ceram. Int. 2014, 41, 2512–2518. [Google Scholar] [CrossRef]
- Kliuev, M.; Wiessner, M.; Büttner, H.; Maradia, U.; Wegener, K. Super-Hydrophobic and Super-Hydrophilic Effect by Means of EDM Surface Structuring of γ-TiAl. Procedia CIRP 2020, 95, 393–398. [Google Scholar] [CrossRef]
- Arahira, T.; Maruta, M.; Matsuya, S. Characterization and In Vitro Evaluation of Biphasic α-Tricalcium Phosphate/β-Tricalcium Phosphate Cement. Mater. Sci. Eng. C 2017, 74, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, J.; Xu, W.; Wang, Y.; Hou, G.; Sun, T.; Luo, L. Effects of Sr2+/Zn2+ Co-Substitution on Crystal Structure and Properties of Nano-β-Tricalcium Phosphate. Ceram. Int. 2017, 44, 6096–6103. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, F.; Groth, T.; Vecchio, K.S. Preparation, Characterization and Mechanical Performance of Dense β-TCP Ceramics With/without Magnesium Substitution. J. Mater. Sci. Mater. Med. 2008, 19, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, M.; Sglavo, V.M. Effect of Mg2+ Doping on Beta–Alpha Phase Transition in Tricalcium Phosphate (TCP) Bioceramics. Acta Biomater. 2016, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Understanding the Influence of MgO and SrO Binary Doping on the Mechanical and Biological Properties of β-TCP Ceramics. Acta Biomater. 2010, 6, 4167–4174. [Google Scholar] [CrossRef]
- Shuai, C.; Zhuang, J.; Peng, S.; Wen, X. Inhibition of Phase Transformation from β- to α-Tricalcium Phosphate with Addition of Poly (L-Lactic Acid) in Selective Laser Sintering. Rapid Prototyp. J. 2014, 20, 369–376. [Google Scholar] [CrossRef]
- Somers, N.; Jean, F.; Lasgorceix, M.; Curto, H.; Urruth, G.; Thuault, A.; Petit, F.; Leriche, A. Influence of Dopants on Thermal Stability and Densification of β-Tricalcium Phosphate Powders. Open Ceram. 2021, 7, 100168. [Google Scholar] [CrossRef]
- Shaikh, S.; Kedia, S.; Singh, A.K.; Sharma, K.; Sinha, S. Surface Treatment of 45S5 Bio-Glass Using Femtosecond Laser to Achieve Superior Growth of Hydroxyapatite. arXiv 2016, arXiv:1610.05510. [Google Scholar] [CrossRef]
- Dautova, Y.; Kozlova, D.; Skepper, J.N.; Epple, M.; Bootman, M.D.; Proudfoot, D. Fetuin-A and Albumin Alter Cytotoxic Effects of Calcium Phosphate Nanoparticles on Human Vascular Smooth Muscle Cells. PLoS ONE 2014, 9, e97565. [Google Scholar] [CrossRef]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.G.; Porter, A.E.; Skepper, J.N. Hydroxyapatite Nano and Microparticles: Correlation of Particle Properties with Cytotoxicity and Biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Verberckmoes, S.C.; De Broe, M.E.; D’Haese, P.C. Dose-Dependent Effects of Strontium on Osteoblast Function and Mineralization. Kidney Int. 2003, 64, 534–543. [Google Scholar] [CrossRef]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High Magnesium Inhibits Human Osteoblast Differentiation In Vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, C.; Li, J.; Zhu, Y.; Zhang, X. High Extracellular Magnesium Inhibits Mineralized Matrix Deposition and Modulates Intracellular Calcium Signaling in Human Bone Marrow-Derived Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2014, 450, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Rest, J.R. The Histological Effects of Copper and Zinc on Chick Embryo Skeletal Tissues in Organ Culture. Br. J. Nutr. 1976, 36, 243–254. [Google Scholar] [CrossRef]
- Murni, N.S.; Dambatta, M.S.; Yeap, S.K.; Froemming, G.R.A.; Hermawan, H. Cytotoxicity Evaluation of Biodegradable Zn–3Mg Alloy toward Normal Human Osteoblast Cells. Mater. Sci. Eng. C 2015, 49, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of Phenolic Compounds towards Free Radicals under In Vitro Conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Goldbeter, A. The Balance between Cell Cycle Arrest and Cell Proliferation: Control by the Extracellular Matrix and by Contact Inhibition. Interface Focus 2014, 4, 20130075. [Google Scholar] [CrossRef]
- Ribatti, D. A Revisited Concept: Contact Inhibition of Growth. From Cell Biology to Malignancy. Exp. Cell Res. 2017, 359, 17–19. [Google Scholar] [CrossRef]
- Bispo, D.S.C.; Jesus, C.S.H.; Correia, M.; Ferreira, F.; Bonifazio, G.; Goodfellow, B.J.; Oliveira, M.B.; Mano, J.F.; Gil, A.M. NMR Metabolomics Assessment of Osteogenic Differentiation of Adipose-Tissue-Derived Mesenchymal Stem Cells. J. Proteome Res. 2022, 21, 654–670. [Google Scholar] [CrossRef]
- Matta, C.; Szűcs-Somogyi, C.; Kon, E.; Robinson, D.; Neufeld, T.; Altschuler, N.; Berta, A.; Hangody, L.; Veréb, Z.; Zákány, R. Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells Is Enhanced by an Aragonite Scaffold. Differentiation 2019, 107, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, P.; Yu, Z.; Zhang, X.; Shen, L.; Shi, H.; Yan, H.; Wang, L.; Tian, Y. Effects of Periodic Surface Structures Induced by Femtosecond Laser Irradiation on the Antibacterial Properties of Zr-Based Amorphous Material. Optik 2022, 268, 169760. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Liao, S.; Jiang, C.; Wang, L.; Tang, Y.; Wu, G.; Dai, G.; Chen, L. Quantitative Proteomics Reveals the Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2020, 19, 3109–3122. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial Effect of Silver Nanoparticles on Staphylococcus aureus. BioMetals 2010, 24, 135–141. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Schober, Y.; Römpp, A.; Ghassempour, A.; Spengler, B. Proteomics Study of Silver Nanoparticles Toxicity on Bacillus thuringiensis. Ecotoxicol. Environ. Saf. 2014, 100, 122–130. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Tarafder, S.; Vahabzadeh, S.; Bose, S. Effects of MgO, ZnO, SrO, and SiO2 in Tricalcium Phosphate Scaffolds on In Vitro Gene Expression and In Vivo Osteogenesis. Mater. Sci. Eng. C 2019, 96, 10–19. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1997.
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green Synthesis of Silver Nanoparticles Using Tea Leaf Extract and Evaluation of Their Stability and Antibacterial Activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Oliveira, M.; Angelova, L.; Grenho, L.; Fernandes, M.H.; Daskalova, A. Dual-Function Femtosecond Laser: β-TCP Structuring and AgNP Synthesis via Photoreduction with Azorean Green Tea for Enhanced Osteointegration and Antibacterial Properties. Materials 2024, 17, 5057. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- Staphylococcus aureus, ATCC® 25923TM; American Type Culture Collection: Manassas, VA, USA, 2012.



| Scanning Velocity (mm/s) | Fluence (J/cm2) | Pattern | Doping |
|---|---|---|---|
| 1 | 4.1 | Linear | NA |
| 1 | 4.1 | Linear | AgNPs |
| 1 | 4.1 | Linear | Ions |
| 1 | 6.1 | Linear | NA |
| 1 | 6.1 | Linear | Ions |
| 1 | 4.1 | Crossed | NA |
| 1 | 4.1 | Crossed | Ions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.; Angelova, L.; Avdeev, G.; Grenho, L.; Fernandes, M.H.; Daskalova, A. Femtosecond Laser-Engineered β-TCP Scaffolds: A Comparative Study of Green-Synthesized AgNPs vs. Ion Doping Against S. aureus for Bone Regeneration. Int. J. Mol. Sci. 2025, 26, 4888. https://doi.org/10.3390/ijms26104888
Oliveira M, Angelova L, Avdeev G, Grenho L, Fernandes MH, Daskalova A. Femtosecond Laser-Engineered β-TCP Scaffolds: A Comparative Study of Green-Synthesized AgNPs vs. Ion Doping Against S. aureus for Bone Regeneration. International Journal of Molecular Sciences. 2025; 26(10):4888. https://doi.org/10.3390/ijms26104888
Chicago/Turabian StyleOliveira, Marco, Liliya Angelova, Georgi Avdeev, Liliana Grenho, Maria Helena Fernandes, and Albena Daskalova. 2025. "Femtosecond Laser-Engineered β-TCP Scaffolds: A Comparative Study of Green-Synthesized AgNPs vs. Ion Doping Against S. aureus for Bone Regeneration" International Journal of Molecular Sciences 26, no. 10: 4888. https://doi.org/10.3390/ijms26104888
APA StyleOliveira, M., Angelova, L., Avdeev, G., Grenho, L., Fernandes, M. H., & Daskalova, A. (2025). Femtosecond Laser-Engineered β-TCP Scaffolds: A Comparative Study of Green-Synthesized AgNPs vs. Ion Doping Against S. aureus for Bone Regeneration. International Journal of Molecular Sciences, 26(10), 4888. https://doi.org/10.3390/ijms26104888









