Abstract
Phosphate invert glasses (PIGs) have been attracting attention as materials for bone repair. PIGs have a high flexibility in chemical composition because they are composed of orthophosphate and pyrophosphate and can easily incorporate various ions in their glass networks. In our previous work, incorporation of niobium (Nb) into melt-quench-derived PIGs was effective in terms of controlling their ion release, and Nb ions promoted the activity of osteoblast-like cells. In the present work, a liquid-phase method was used for synthesizing Nb-containing PIGs, as this method allows us to prepare a glass precursor solution at room temperature, which can be attributed to improved glass-shape design. Nb-containing PIGs were successfully prepared, and their ion release behavior was controlled by changing the Nb content in the PIGs. The functions of Nb varied according to its content. For example, in the case of PIGs containing a larger amount of Nb, Nb acted as both the network modifier and former while also inducing the formation of chain-like structures. These glasses possessed a gradual ion release in a tris-HCl buffer solution. Cotton-wool-like structured scaffolds were fabricated using the synthesized Nb-containing glass using a wet-spinning method. Because the scaffolds possess excellent flexibility and controllable ion release, they are good candidates for new biomaterials.
1. Introduction
Glass has been extensively studied as a material for bone repair. The most common type of glass is silicate glass, since 45S5-type bioactive glass, which is a silicate system containing a minor amount of phosphate, has been developed as the first “bioactive” material [1,2,3,4]. On the other hand, phosphate glass also has been attracting attention as a new type of bioactive glass [5,6,7,8]. Phosphate glass is classified according to phosphorus content as ultraphosphate glass (P2O5 > 50 mol%), metaphosphate glass (P2O5 = 50 mol%), polyphosphate glass (P2O5 < 50 mol%) and phosphate invert glass (PIG, P2O5 ≤ 40 mol%) [9,10,11]. The PIGs are composed of ortho-phosphate (QP0) and pyro-phosphate (QP1) and a larger amounts of network modifiers than network formers. The modified ions in the glass-network structure are QP0- and QP1-bonded via non-bridged oxygen, indicating a highly ion-bonded state [12]. The glasses have a high flexibility in chemical composition because it is easy to incorporate several types of elements into the phosphate system [13,14,15,16,17].
Ions dissolved from glasses and ceramics have been reported to have a variety of effects on bone repair [18,19,20]. For example, calcium (Ca) ions improve osteoblast differentiation and calcification [21], while magnesium ions increase bone strength and promote osteoblast adhesion, proliferation and differentiation [22,23]. These inorganic ions have appropriate amount ranges for exhibiting such positive effects on bone formation. Therefore, it is important to control the chemical durability of the glass to provide an appropriate amount of inorganic ions to bone-forming cells. Because PIGs consist of pyrophosphate and orthophosphate groups and possess easier chemical durability control than the other phosphate glass systems, they are expected to be a good candidate for the material providing therapeutic ions [13,14,15,16,17]. For example, our group has reported that the introduction of intermediate oxides such as titanium (Ti) or niobium (Nb) into PIGs prepared by melt-quenching improves the chemical durability of the glass [17]. We also revealed that Nb ions promoted the differentiation and mineralization of mouse osteoblast-like cells [24]. Thus, Nb plays a role both in controlling the chemical durability of PIGs and in enhancing their bone-forming ability.
We have recently succeeded the preparation of Ti-containing PIGs by a liquid-phase method [25]. This method allows us to obtain amorphous phosphate materials by mixing phosphate solution and cation solution [26]. A glass precursor solution can be prepared at room temperature through the method, which is attributed to excellent flexibility in regard to glass-shape design. Although there have been several reports on glasses with a PIG composition synthesized by this method [26,27,28], we have shown for the first time that ion elution can be controlled by introducing intermediate oxides into glasses prepared by this method. The prepared Ti-containing PIGs exhibited a controlled ion release depending on the amount of the introduced Ti; this is because TiO2 is one of the intermediate oxides, and some of Ti in the glasses functions as a network former crosslinking pyrophosphate and/or orthophosphate group. As above-mentioned, because Nb ions have been found to promote some activities of osteoblast-like cells [24], we expected the introduction of Nb instead of Ti to be effective for not only controlling the chemical durability but also enhancing the bone-forming ability of the PIGs prepared by the liquid-phase method.
The shape of a material is an important factor in its use as a biomaterial. The cotton-wool-like structured materials possess good flexibility and excellent handling. These materials contain a large space (pore) for cell migration and nutrition penetration, which can enhance tissue regeneration by cells in the body [29,30]. Based on these reports, we prepared cotton-wool-like structured materials consisting of the Nb-containing PIGs using a wet-spinning method (WS). In the WS method, normally polymer is dissolved in a solvent that dissolves the polymer well (good solvent) and extruded into another solvent in which the polymer is less soluble (poor solvent) to fabricate fibers. In this study, a mixture of the glass precursor synthesized with the liquid-phase method and polyvinyl alcohol (PVA) was shaped into a fibrous structure with the WS method. PVA is a biodegradable polymer known for its hydrophilic and biocompatible properties and is widely used in the biomaterials field [31,32].
In the present work, Nb-containing PIGs were synthesized by a liquid-phase method. The influences of Nb introduction on the glass-network structure and ion-releasing behavior of the glass were evaluated. In addition, we fabricated fibrous materials with a cotton-wool-like structure using the Nb-containing PIGs. Finally, mouse osteoblast-like cells (MC3T3-E1) were cultured on the fabricated fibrous materials to assess their cell compatibility. This is the first report of the fabrication of PIG-containing fibers by the liquid-phase method combined with WS.
2. Results
2.1. Glass Structure of Prepared Samples
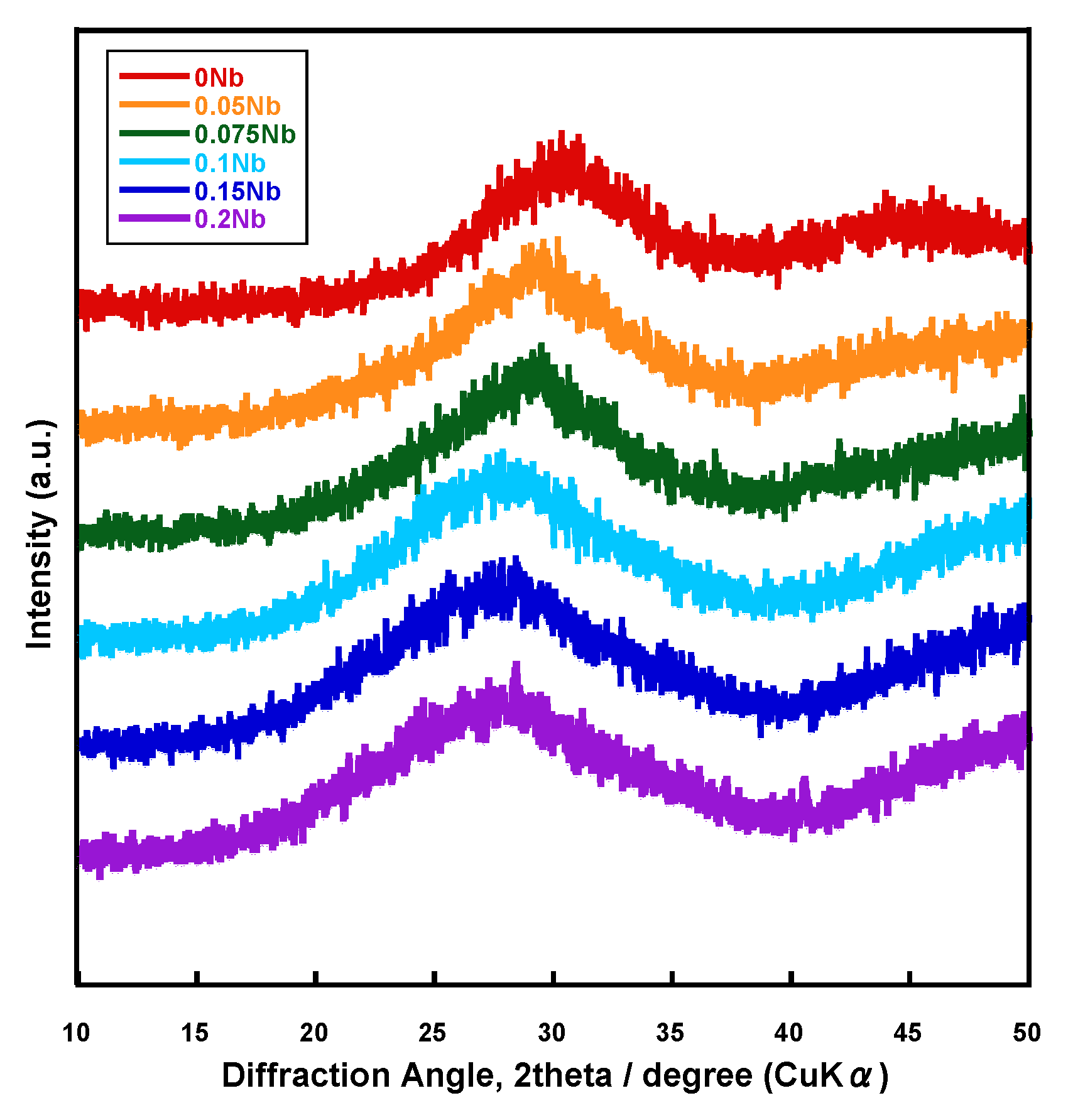
Figure 1 shows the X-ray diffractometry (XRD) patterns of the prepared samples named xNbs (x is the concentration of niobium chloride in the solution used for the synthesis, 0~0.2). Halo patterns originating from amorphous phases were observed for all of the samples.
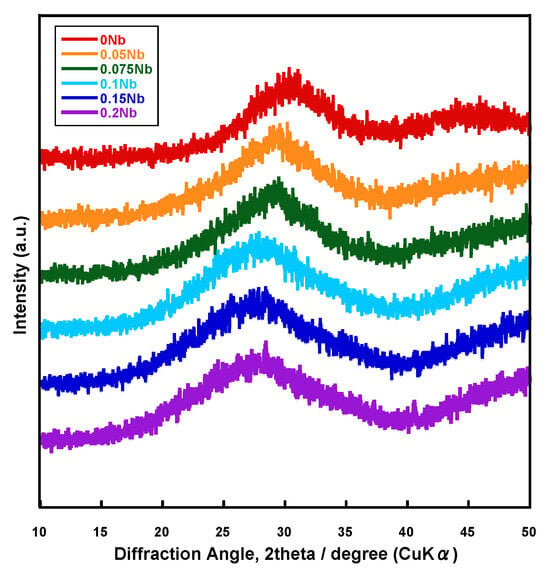
Figure 1.
XRD patterns of the samples.
Table 1 shows the composition of xNbs as determined by energy dispersive X-ray spectroscopy (EDS), and Figure S1 shows the EDS spectra of xNbs. The contents of P2O5 and K2O were almost the same among the samples and were 33~38 mol% and 4~10 mol%, respectively. On the other hand, the contents of CaO and Nb2O5 varied widely; the content of CaO decreased with increasing Nb2O5. In particular, the composition changed significantly between 0.075Nb and 0.1 Nb; the contents of Nb2O5 drastically increased and almost became even with that of CaO for 0.1 Nb.

Table 1.
Chemical compositions of the samples determined by EDS (mol%).
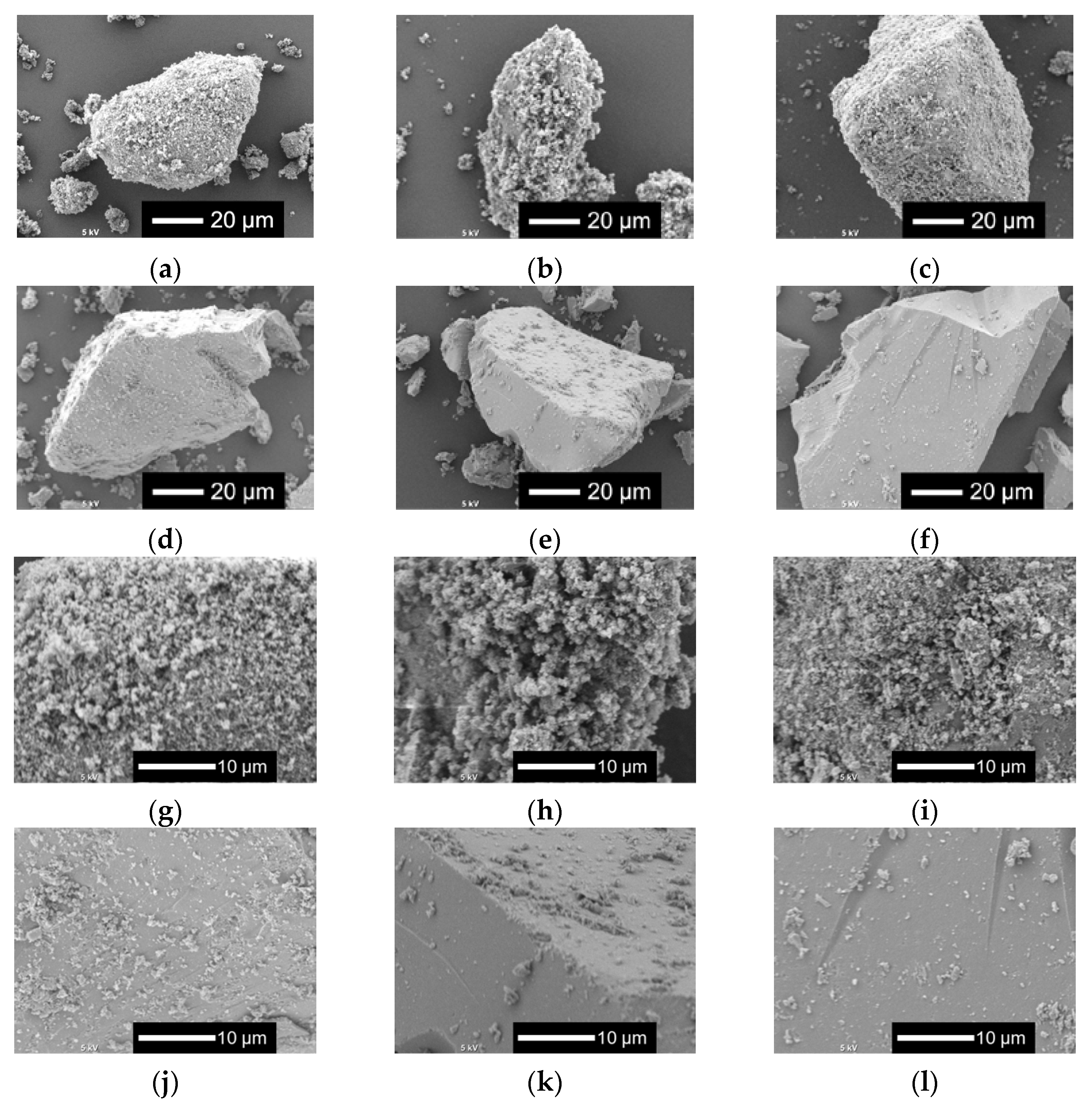
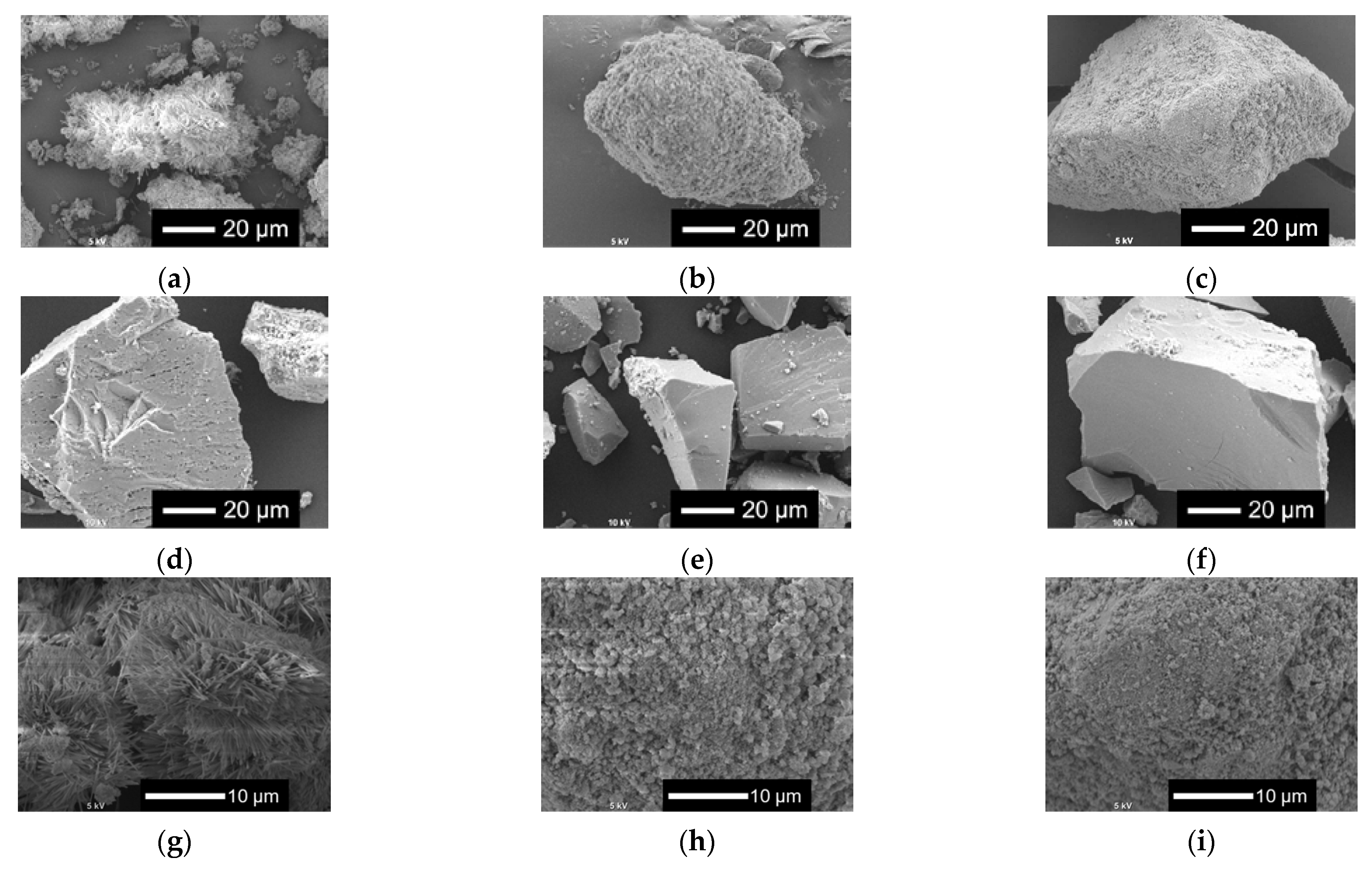
Figure 2 shows the scanning electron microscopy (SEM) images of xNbs. The surface roughness of the sample particles changed with the content of Nb in the samples. As the Nb content increased, the surface became smoother. For the samples with no or a smaller amount of Nb, tiny particles aggregated to the surfaces of the glass sample particles.
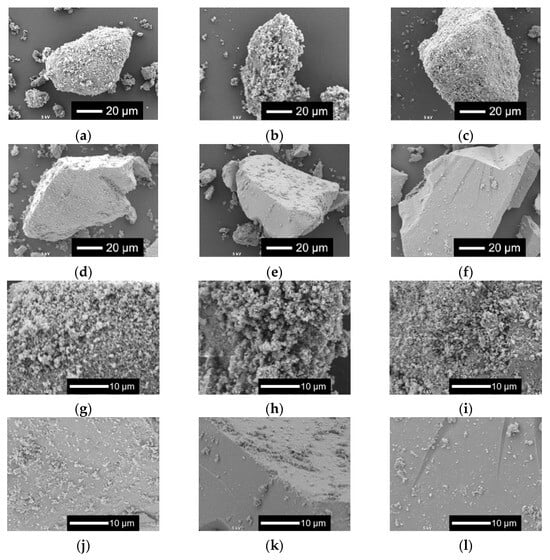
Figure 2.
SEM images of the samples. (a–f) Low-magnified images; (g–l) high-magnified images: (a,g) 0 Nb; (b,h) 0.05 Nb; (c,i) 0.075 Nb; (d,j) 0.1 Nb; (e,k) 0.15 Nb; (f,l) 0.2 Nb.
Figure S2 shows the thermogravimetry–differential thermal analysis (TG-DTA) curves of the samples, and Table 2 shows the glass transition temperature (Tg) and crystallization temperature (Tc) of the samples read from the TG-DTA curves. Two values were detected for Tg and Tc of 0.05 to 0.2 Nb.

Table 2.
Tg and Tc of the samples read from the TG-DTA curves (°C).
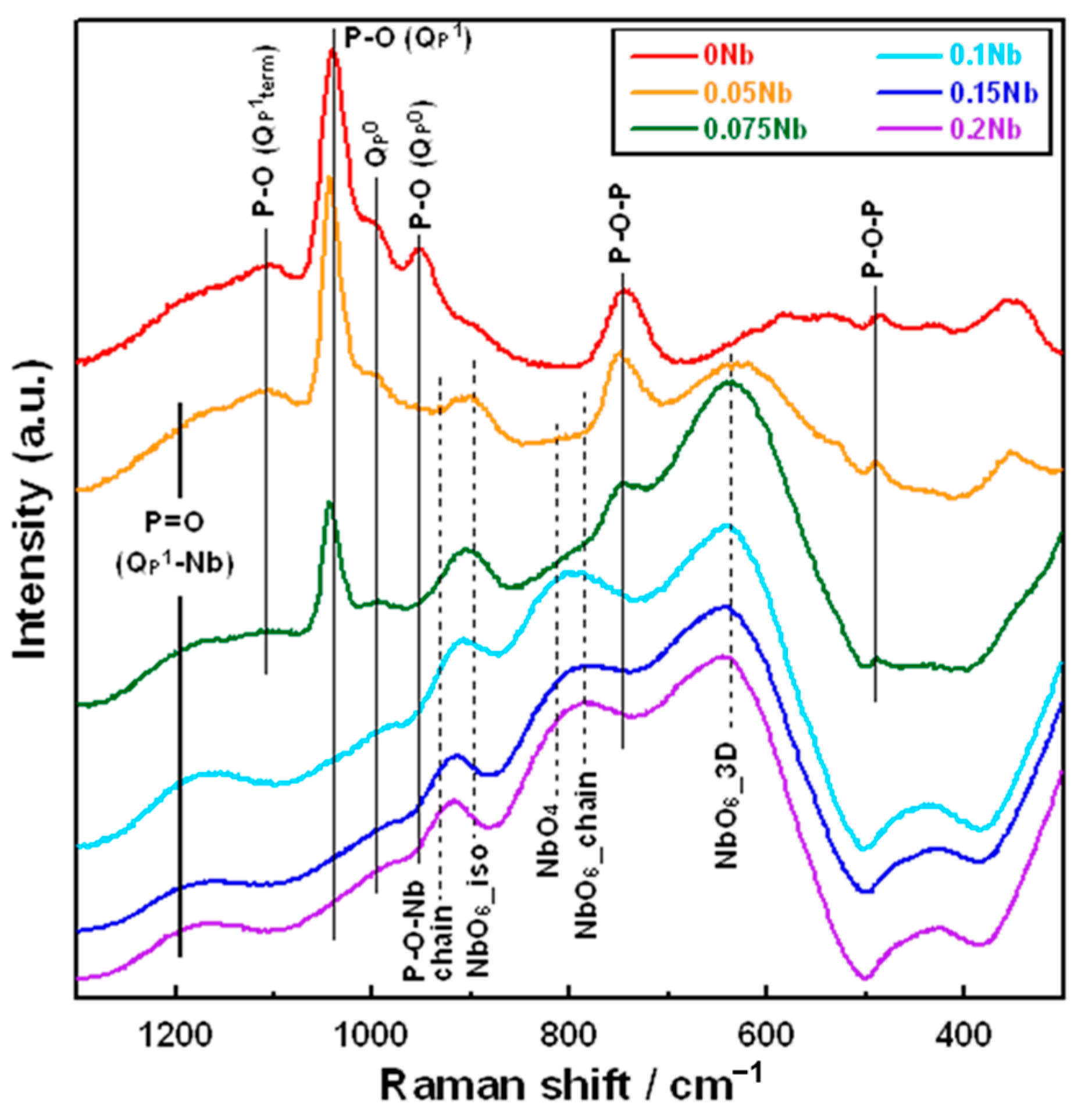
Figure 3 shows the laser Raman spectra of xNb. There are obvious differences in the shape of the spectrum between the xNb (x ≤ 0.075) and the xNb (0.1 ≤ x) group. For 0 Nb, we observed strong peaks at 740 and 1040 cm−1, which originated from the Qp1 structure, and weak peaks at 950 and 990 cm−1, which originated from the Qp0 structure [33]. For 0.05 Nb and 0.075 Nb, in addition to these peaks mentioned above, a peak at 630 cm−1 derived from the NbO6_3D structure and a peak at 900 cm−1 derived from the NbO6_iso structure appeared [34].
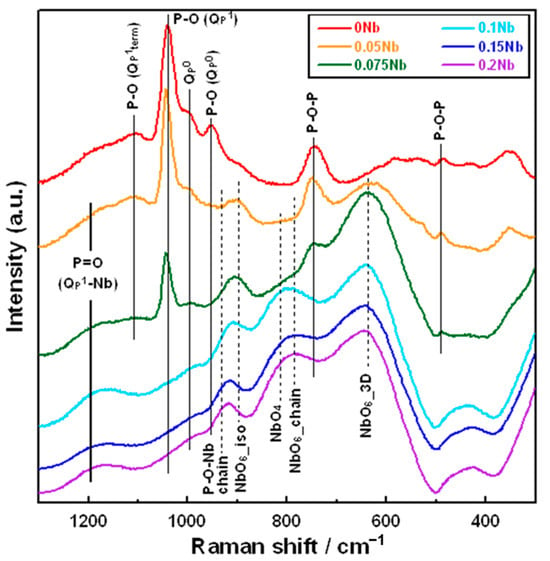
Figure 3.
Raman spectra of the samples.
In the xNb (0.1 ≤ x) group, peaks related to Nb with the other structures were observed. For 0.1 Nb, peaks derived from the NbO6_chain structure at 780 cm−1, the NbO4 structure at 810 cm−1 [34] and the P-O-Nb chain structure at 930 cm−1 were also observed [35]. For 0.15 Nb and 0.2 Nb, the peaks derived from the NbO6_chain and NbO4 structures possessed a slightly stronger intensity, while the peak derived from the NbO6_3D structure was weaker compared to that for 0.1 Nb.
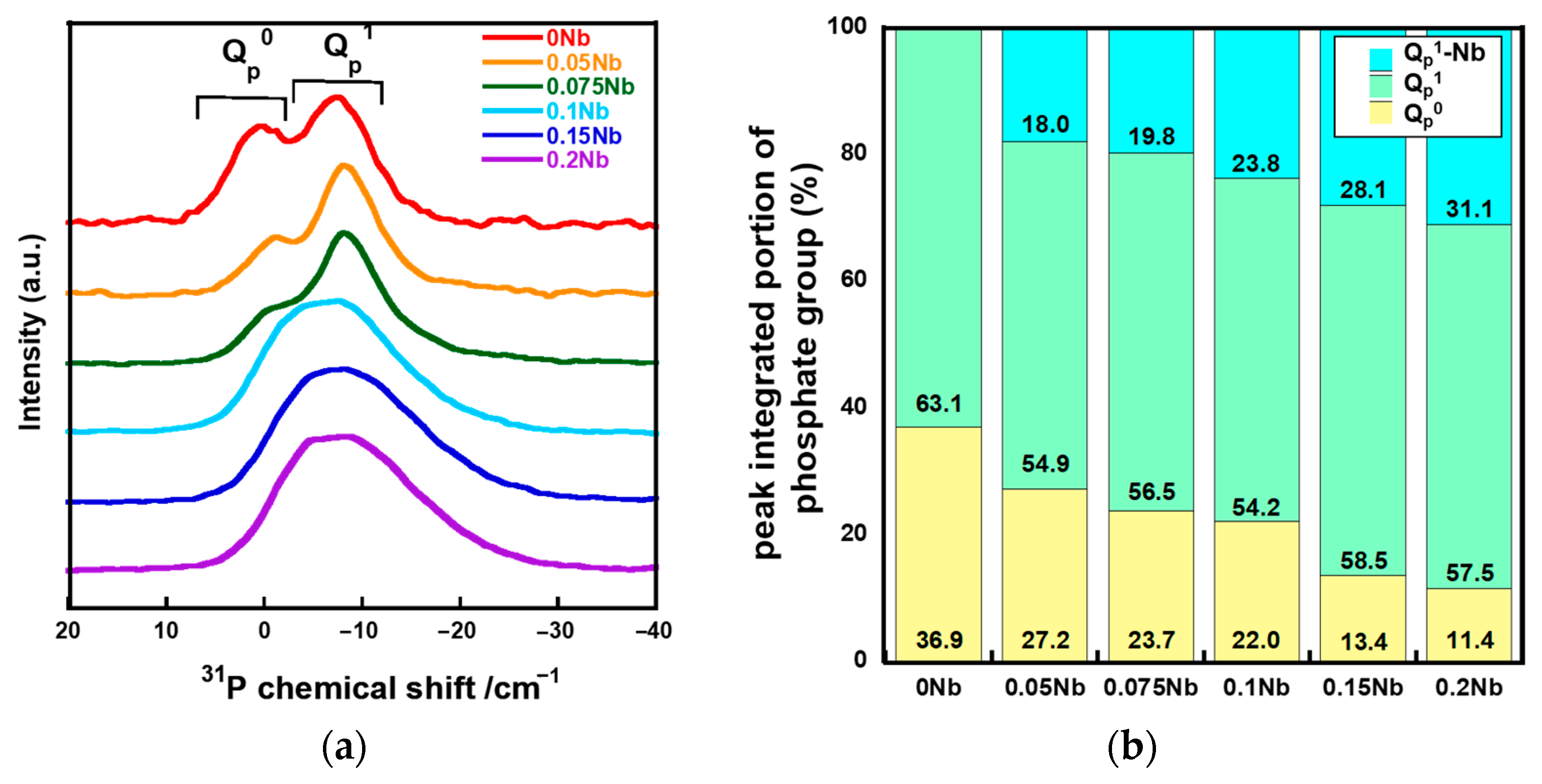
Figure 4a shows the solid-state 31P magic angle spinning nuclear magnetic resonance (31P MAS-NMR) spectra of xNbs. The spectra of 0 Nb, 0.05 Nb and 0.075 Nb exhibit peaks corresponding to Qp0 and Qp1 at around 2 ppm and -6 ppm, respectively [36,37]. For 0.1 Nb, 0.15 Nb and 0.2 Nb, a broad peak was extended to -22 ppm which corresponds to the Qp2 region. The peak in the spectrum for 0.15 Nb and 0.2 Nb exhibits an expansion that is slightly larger than 0.1 Nb. In our previous work, such expanding of the peak was observed in the 31P MAS-NMR spectra of the Ti-containing PIGs prepared by the liquid-phase method due to Ti-bonding to QP1 [25]. Therefore, the 31P MAS-NMR spectra of xNbs were deconvoluted into peaks of QP0, QP1, and QP1-Nb (Figure S3). Figure 4b shows the integrated area ratios of QP0, QP1, and QP1-Nb obtained from the deconvolution. The QP0 peak areas decreased with the increase in the Nb content, whereas those of QP1-Nb increased.
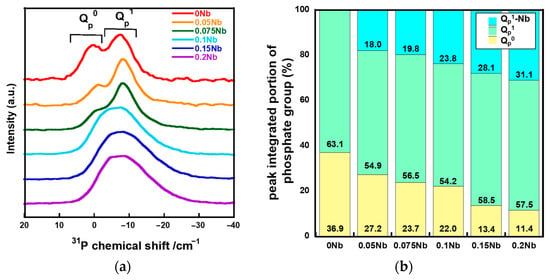
Figure 4.
31P MAS-NMR results of the samples. (a) spectra; (b) fractured peak integrated portion of QP0, QP1, and QP1-Nb.
2.2. Ion Release from Samples in Tris-HCl Buffer Solution
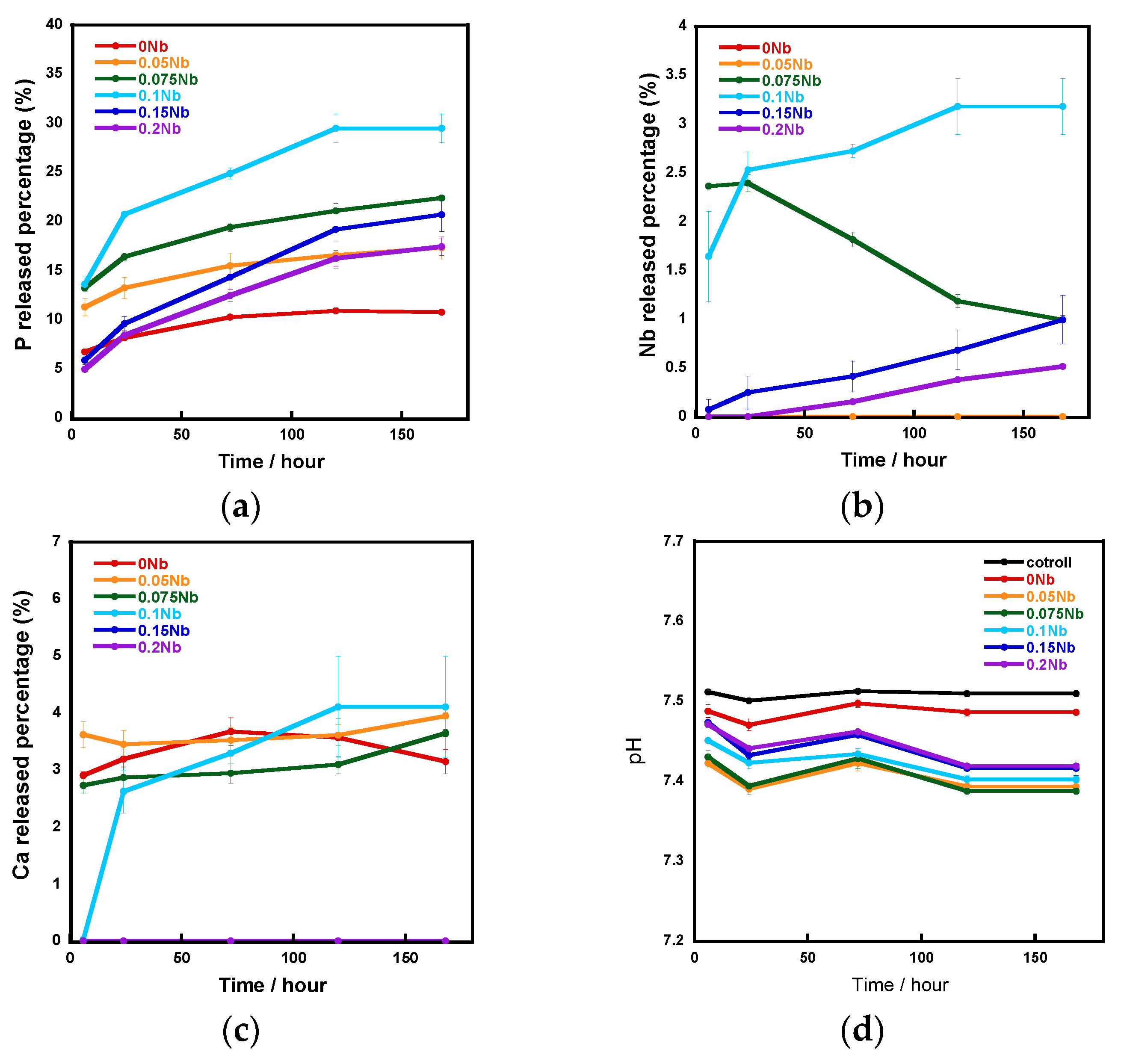
Figure 5 shows the ion release amounts in a tris-HCl buffer solution measured using an inductively coupled plasma atomic emission spectrometer (ICP-AES) and the pH of the aforementioned tris-HCl buffer solution. The vertical axis shows the percentage of released P, Nb and Ca element amounts relative to the total amount of each element contained in the samples, which were calculated using the results of the EDS composition analysis. The release percentage of P and Nb increased with the increase in the Nb content from 0 to 0.1 and decreased with increase in that from 0.1 to 0.2. In the case of Ca release, although the percentages for 0 Nb to 0.1 Nb were similar, no release was observed for 0.15 Nb and 0.2 Nb. During the 7-day soaking, the pH of the solution was 7.4~7.5.
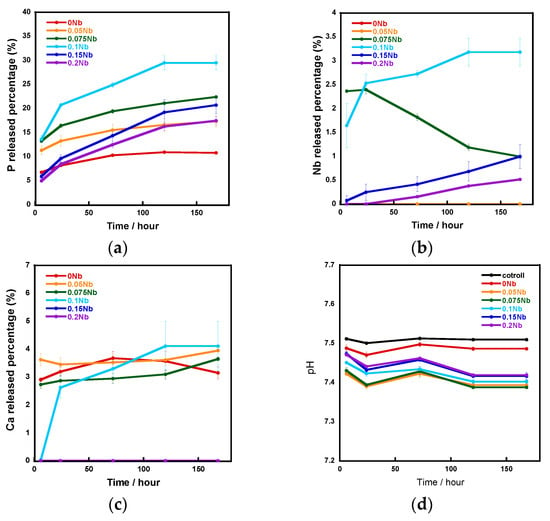
Figure 5.
The ratio of released ions to total amount in samples during immersion in a tris-HCl buffer solution for 7 days: (a) P; (b) Nb; (c) Ca; (d) pH of the tris-HCl buffer solution.
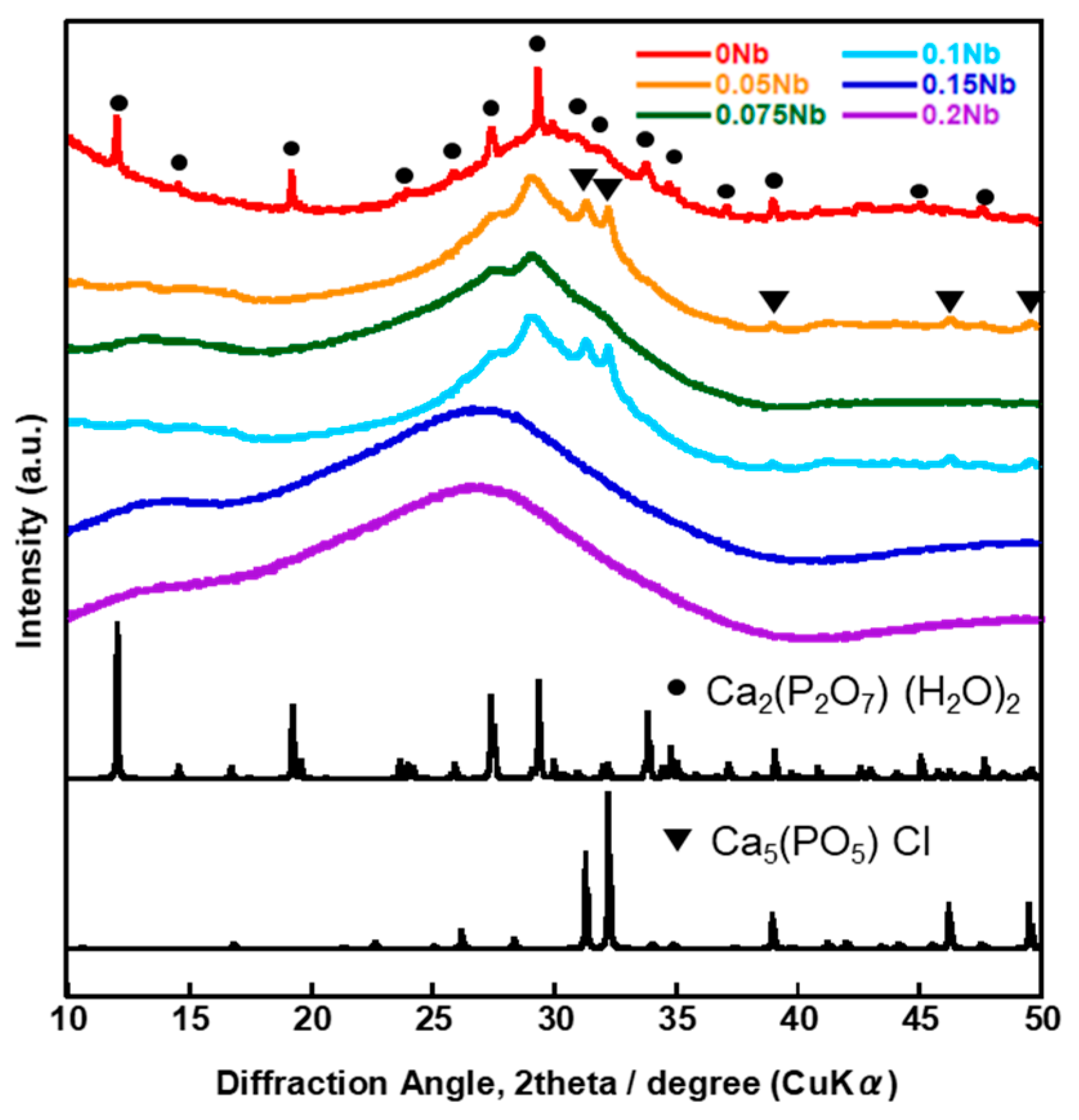
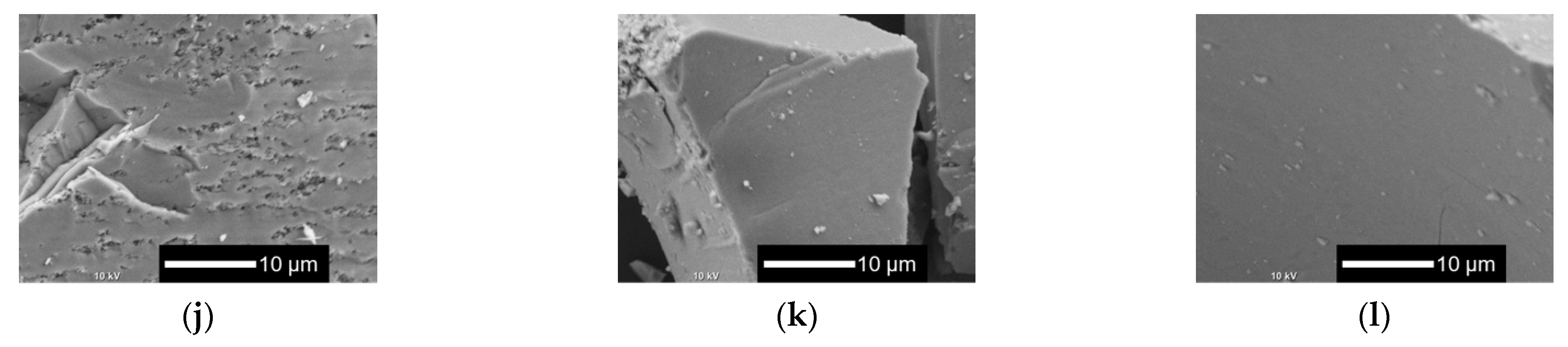
Figure 6 shows the XRD patterns and Figure 7 shows the SEM images of xNbs after immersion in tris-HCl buffer solution for 7 days. For the XRD patterns, peaks derived from calcium-phosphate-based crystalline phases were observed for 0 to 0.1 Nb. However, in the case of 0.05 to 0.1 Nb, because their patterns contain only a few sharp peaks and predominantly consist of a broad peak, the amount of the precipitates was expected to be small. No sharp peaks were found for 0.15 and 0.2 Nb. These results were supported by the SEM observation. Although needle-shaped precipitates were observed on 0 Nb, no precipitates were observed for the other samples.
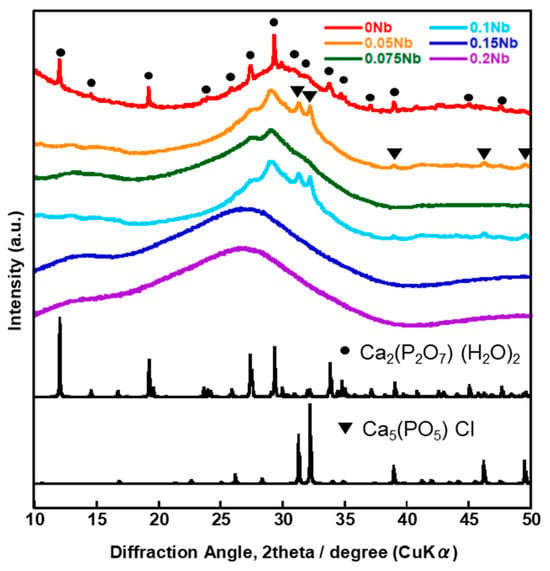
Figure 6.
XRD patterns of the samples after immersion in tris-HCl buffer solution for 7 days.
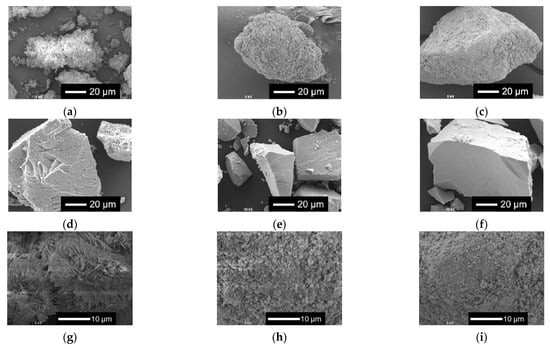

Figure 7.
SEM images of the samples after immersion in tris-HCl buffer solution for 7 days. (a–f) Low-magnified images; (g–l) high-magnified images: (a,g) 0 Nb; (b,h) 0.05 Nb; (c,i) 0.075 Nb; (d,j) 0.1 Nb; (e,k) 0.15 Nb; (f,l) 0.2 Nb.
2.3. Structure and Ion Release Behavior of Fibrous Scaffolds
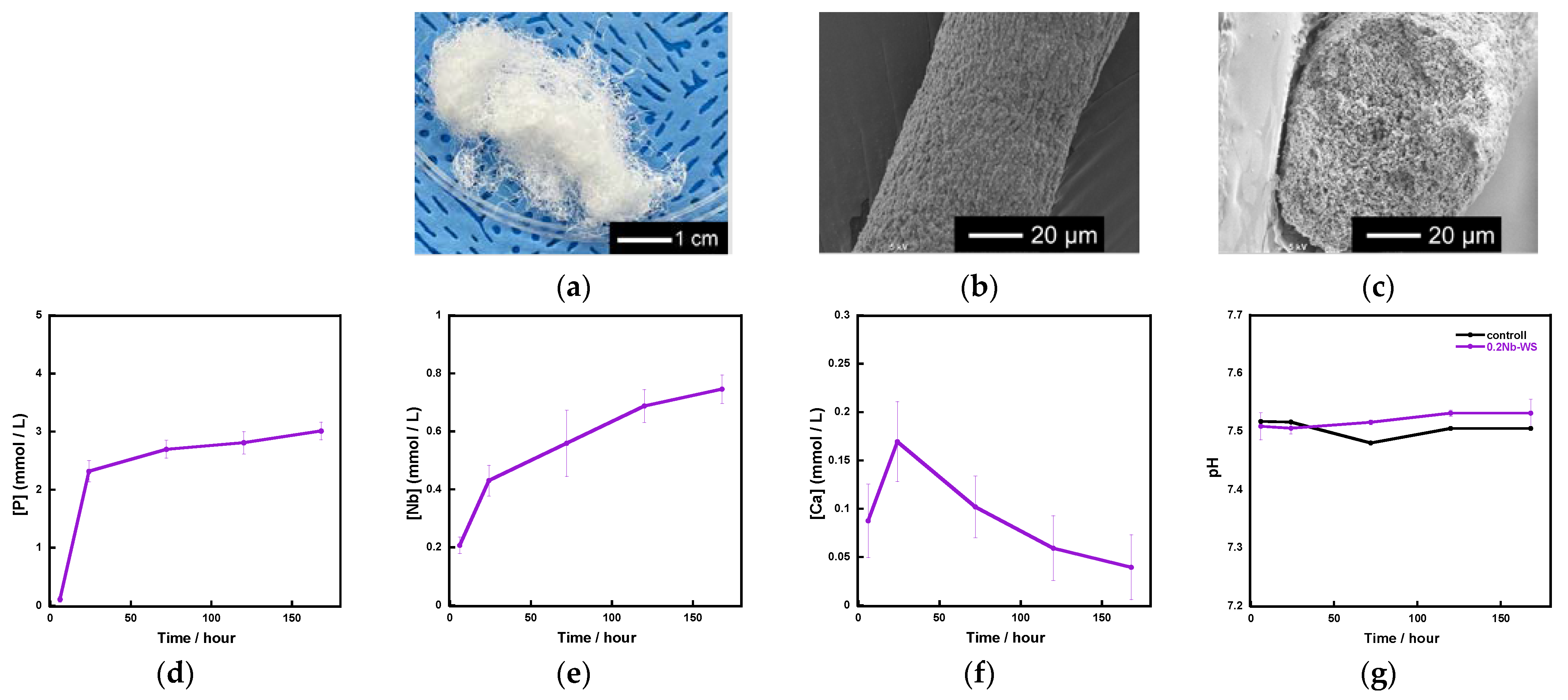
Based on the above results, we used 0.2 Nb to fabricate fibrous scaffolds because 0.2 Nb contained the largest amount of Nb among the synthesized samples and possessed a gradual ion release. Figure 8a shows the appearance of the fibrous scaffold consisting of 0.2 Nb and a polyvinyl alcohol (PVA) composite prepared by a wet-spinning method. They possessed a cotton-wool-like structure and excellent flexibility. The fiber diameter was found to be 40~120 μm from the SEM observation for 20 parts, which were randomly picked, of the samples. There were no micrometer-sized pores on the surface or inside of a single fiber (Figure 8b,c). From the results of the immersion test using tris-HCl buffer solution (Figure 8d–f), the fibers exhibited a gradual ion release of P and Nb. However, in the case of Ca, its concentration increased until day 1 and then gradually decreased. During the 7 days of immersion, the pH of the solution was about 7.5 (Figure 8g) and only changed by a small amount.
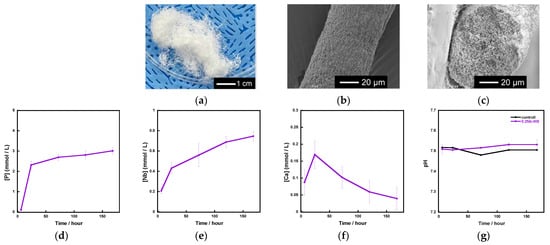
Figure 8.
Fibrous scaffolds consisting of a 0.2 Nb-PVA composite: (a) appearance; (b) SEM image of a single fiber; (c) SEM image of a cross section of the fiber; (d–f) concentration of the ions released from the scaffold samples in the tris-HCl buffer solution; (d) P; (e)Nb; (f) Ca; (g) pH of tris-HCl buffer solution.
2.4. Cell Proliferation on the Fibrous Scaffolds
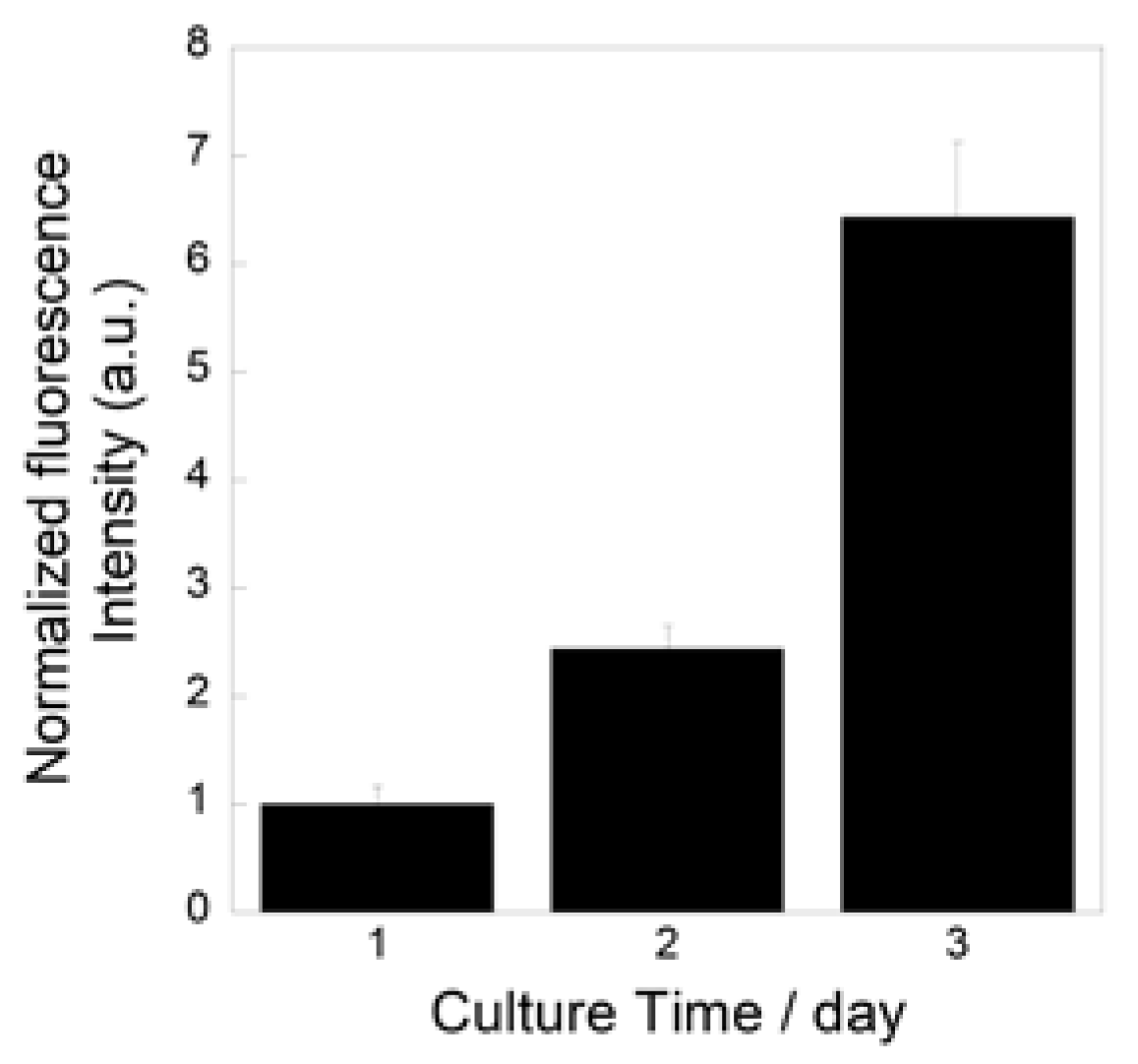
Figure 9 show the metabolic activity levels of the MC3T3-E1 cells cultured on the prepared fibrous samples. The levels increased during the culture, which indicates that the cells successfully proliferated on the samples.
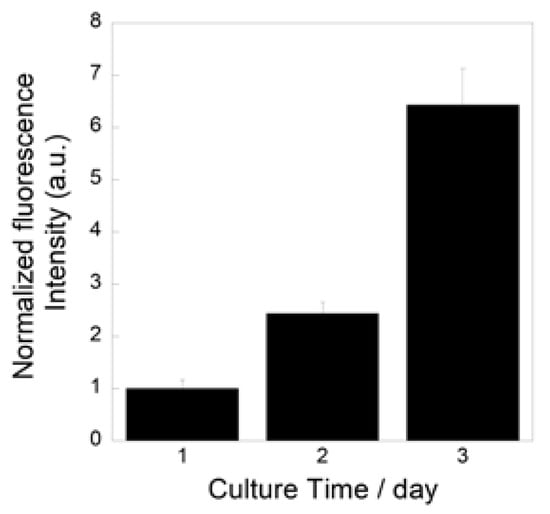
Figure 9.
Metabolic activity levels of MC3T3-E1 cells cultured on prepared fibrous samples.
3. Discussion
Since all of the prepared samples were found to consist of an amorphous phase from their XRD patterns, as well as to exhibit Tg from their DTA results, we confirmed that Nb-containing glasses were successfully prepared by the liquid-phase method. The spectra of laser Raman and 31P MAS-NMR (Figure 3 and Figure 4) suggest that these glasses were composed of Qp0 and Qp1, and their glass network was varied depending on the amount of Nb introduced. The Nb content in the resulting samples (Table 1) and the glass-network structures (Figure 3 and Figure 4) changed drastically when the amount of Nb introduced during synthesis (x value) increased from 0.075 to 0.1.
For the xNb group (x ≤ 0.075), Raman spectra revealed that Nb formed a six coordination unit that functions as a network modifier in glass networks. The 31P MAS NMR spectra demonstrated that Qp0 peak shifts to the higher field side after introducing Nb. It has been reported that the chemical shift in the 31P MAS NMR spectrum is sensitive to the electronegativity, the charge and the radius of next-nearest-neighbor cations [38]. Nb has higher field strength than calcium. Thus, the observed peak shift must indicate the increase in the electron density surrounding the phosphorus in the Qp0 group due to the substitution of Ca, which coordinates to the Qp0 group, with Nb.
Raman spectra revealed that the xNb (0.1 ≤ x) group contains a P-O-Nb chain structure and six and four coordination units of Nb. This indicates that Nb can act as both a network former and a network modifier. Although Nb was reported to predominantly form a six coordination unit [39], the glasses with (50-y)P2O5–yNb2O5–50SrO (y = 15 and 20) and 40P2O5–20Nb2O5–40SrO systems were found to contain Nb with six and four coordination units from their Raman spectra, and the content of the four coordination unit increased with the increase in the y value from 15 to 20 [34]. Therefore, Nb with a four coordination unit is expected to form and act as a network former in the xNb, and its content must increase with the increase in the amount of Nb incorporated. In our previous study on melt-quench-derived glasses with a CaO–P2O5–TiO2/Nb2O5 system, the amount of Nb with a four coordination unit in the glasses increased with increase in the amount of CaO replaced with P2O5, and this phenomenon was also observed for Ti in the TiO2-sereis glasses. This suggests that the role of Nb in the glasses was similar to that of Ti [17]. We also revealed that, in the case of Ti-containing PIGs synthesized by the liquid-phase method, the amount of Ti with a four coordination unit increased with the increase in the content of Ti in the glasses [25]. Based on these reports, we expect that the Nb in the xNb plays the same role as Ti in the Ti-containing PIGs and that some of the Nb forms a four coordination unit and acts as a network former. On the other hand, because Nb5+ exhibits a high field strength (1.26 Å−2) which is close to that of network-forming cations (>1.3 Å−2) [40], all Nb in the glass might act as a network former regardless of its coordination unit. Nevertheless, in the case of the xNb (0.1 ≤ x) group, a large amount of Nb was incorporated into the glasses and acted as a network former.
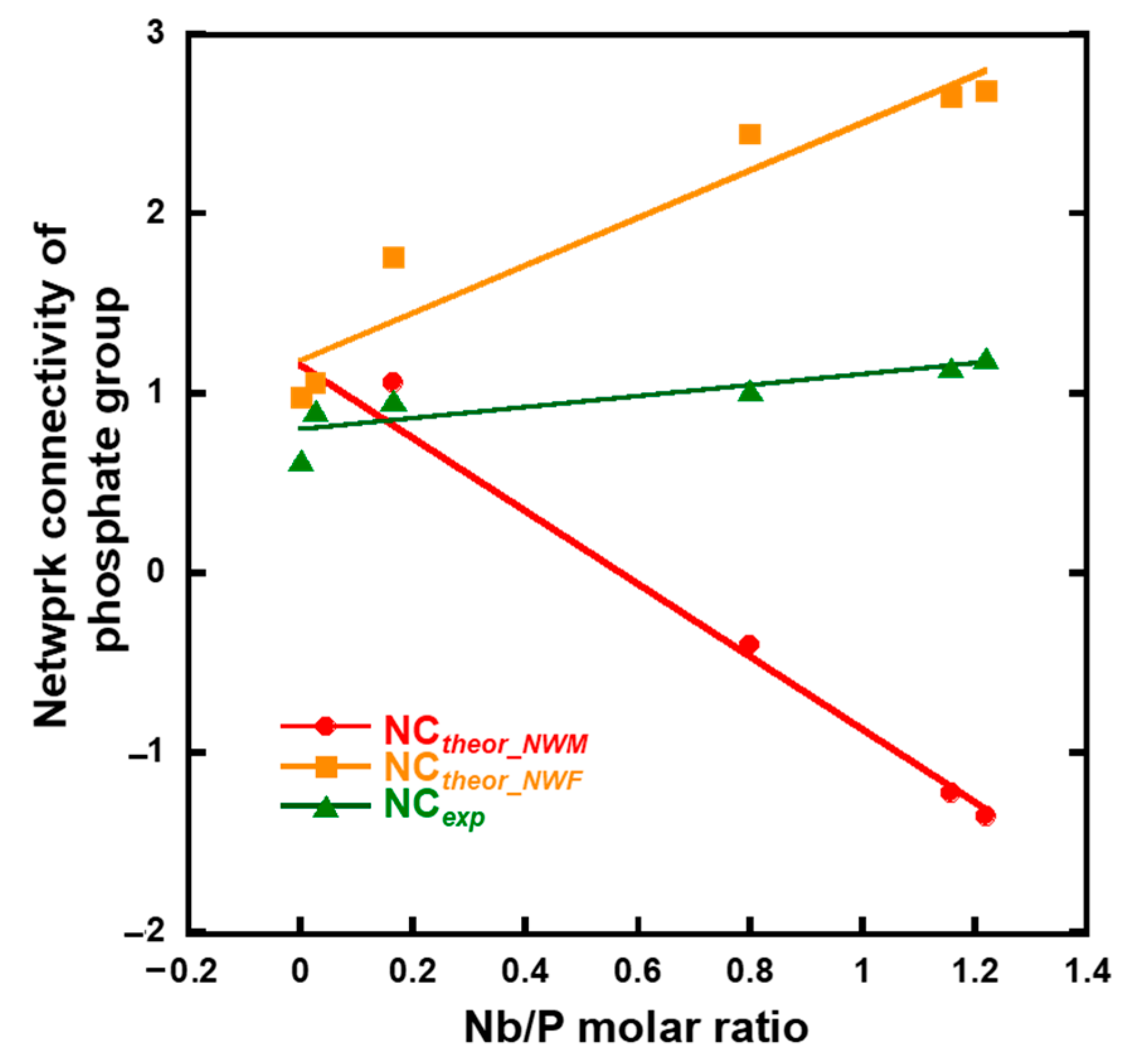
The network connectivity of xNb was calculated using Equations (1)–(3) (Figure 10). The experimental network connectivity (NCexp) was calculated using the deconvoluted 31PMAS-NMR spectra (Figure S5 and Figure 4b), assuming the presence of QPb units (b: number of bridging oxygen atoms of P). The NCexp was calculated using Equation (1), where the number of bridging oxygen atoms (b value) of QP1-Nb is two.
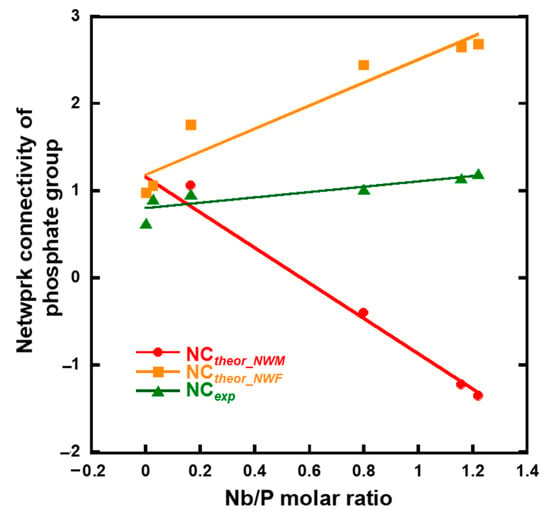
Figure 10.
Calculated network connectivity of the samples.
The theoretical network connectivity is regarded as the number of bridging oxygen atoms per network-forming element [41]. When we assume that all Nb2O5 in xNb act as either a network modifier or network former, the network connectivity of xNb can be calculated in two different ways, with Nb2O5 acting as an network modifier (NCtheor_NWM) or network former (NCtheor_NWF), using Equations (2) and (3) [15]:
where [P2O5], [CaO], [K2O] and [Nb2O5] are the molar fractions of phosphate, calcium oxide, potassium oxide, and niobium oxide, respectively. The NCexp value of 0 Nb and 0.05 Nb was approximately the same as NCtheor_NWM and NCtheor_NWF. Although the NCexp value of 0.075 Nb was still close to that of NCtheor_NWM, the values of xNb (0.1 ≤ x) gradually approached NCtheor_NWF, and the differences in the values between NCexp and NCtheor_NWM drastically increased with an increase in the Nb content (Figure 10). This indicates that a large number of the Nb in xNb acted as a network former, especially in xNb (0.1 ≤ x).
This is supported by the results of 31P MAS-NMR; a peak broadens to −22 ppm, which is considered to the region for the Qp2 structure. The deconvolution results demonstrated that the content of the Qp2-like structure (denoted by QP1-Nb in Figure S3 increased with an increase in the Nb content. This suggests that Qp1 in the glasses is cross-linked by Nb and forms a Qp2-like structure because their Raman spectra do not exhibit peaks corresponding to the Qp2 chain structure around 700 and 1170 cm−1. It has been reported that 31P MAS-NMR peaks of P(OH)2(OSi) and H4P2O7 were observed in almost the same region, making them almost impossible to assign [42,43]. This means that the peak corresponding to Qp1 is found in the same region regardless of the P-O-Si or P-O-P bond in Qp1. On the basis of these things, the xNb (0.1 ≤ x) group is expected to contain more long-chain structures, such as (-O-P-O-P-O-Nb-O-). This is supported by the results of the measurement of Tg and Tc by TG-DTA; Tg and Tc were observed at two different temperatures for xNb. Because long-chain structures require more energy for migration and crystallization than short structures, the appearance of additional Tg and Tc is due to the formation of the above-mentioned long-chain structure. In addition, Nb2O5 might form three-dimensional cluster structures, because the NbO6_3D structure was contained in the Nb-containing samples, as shown in the Raman spectra (Figure 3). Such structures were reported to form in metaphosphate glasses prepared by the melt-quenching method [44,45]. The cluster formation also may contribute to the appearance of two different Tg and Tc. Further investigation will be needed to clarify more details about the Nb state.
The results of the immersion tests demonstrated that, in the case of xNb (x ≤ 0.1) group, the percentage of P and Nb release increased with the increasing Nb content in the glasses (Figure 5). The formation of Ca2(P2O7) (H2O)2 or Ca5(PO5) Cl crystals was found for 0 to 0.1 Nb after immersion from the XRD results (Figure 6), and the needle-shaped precipitate was observed for 0 Nb from the SEM observation (Figure 7). These results indicate that 0 Nb possessed the lowest chemical durability among the prepared samples and released a large amount of ions, which induced the crystal precipitation. Because Nb exhibits a higher field strength compared to Ca, the substitution of Ca with Nb can contribute to enhancement of the chemical durability of glasses [46]. As shown in Table 1, the content of CaO in the prepared glasses decreased with the increase in that of Nb2O5. Thus, the ion release of the glasses was suppressed by Nb substitution, which contributed to the delay of the crystal precipitation with the dependence on the Nb content.
In the case of the group xNb (0.1 ≤ x), the percentage of P and Nb release decreased with the increasing Nb content (Figure 5). Furthermore, no crystal peaks or precipitates were observed for 0.15 Nb and 0.2 Nb even after immersion in a tris-HCl buffer solution for 7 days, according to the results of XRD and SEM (Figure 6 and Figure 7). On the basis of the results of characterization on their glass structure, these samples were expected to have a long-chain structure, such as (-O-P-O-P-O-Nb-O-). It has been reported that the formation of P-O-Nb bonds increased the chemical durability of phosphate glasses prepared by the melt-quenching method [17]. Thus, with the increasing Nb content, the formation of the long-chain structure containing P-O-Nb bonds contributed to the improved chemical durability and the sustained ion-providing ability.
We successfully fabricated the cotton-wool-like scaffolds using Nb-containing glass. They possessed good flexibility and porous structures which can induce cell migration. Because the 0.2 Nb and PVA were mixed in the liquid phase before the wet-spinning process, the surfaces and insides of the obtained fibers possessed dense and homogeneous mixed structures (Figure 8b,c). They gradually released P and Nb ions in the buffer solution, and the release was sustained during the immersion (Figure 8d,e). The release of Ca ions was stopped after 1 day of immersion, and the ion concentration decreased after that (Figure 8f). On the basis of the results of the immersion test using the glass powders, the decrease in the Ca concentration is expected to be due to the precipitation of calcium phosphate-based crystals on the fiber surfaces. Another possible reason is the formation of a Nb2O5 gel on the fiber surface. The gel formation might suppress the ion release from the glass. Further investigation will be needed to clarify the state of the fiber surfaces. Because the osteoblast-like cells successfully proliferated on the fibrous scaffolds, as shown in Figure 9, the scaffolds are expected to possess a good cell compatibility. Thus, the prepared fibrous scaffolds are expected to act as good candidates for the materials used for bone regeneration due to their ability to provide therapeutic ions over a long period while having no effect on the pH of the surrounding solution.
4. Materials and Methods
4.1. Preparation of Samples
A quantity of 50 mL of an aqueous solution containing K4P2O7 (0.2 M, Sigma-Aldrich) (Solution A) and 50 mL of an aqueous solution containing CaCl2 (1 M, Wako Pure Chemical) and NbCl5 (0~0.2 M, Wako Pure Chemical) (Solution B) were prepared separately. Solution B was added dropwise to Solution A at a rate of 2 mL/min. The resulting suspension was filtered by suction, washed with ultrapure water and ethanol and dried at 200 °C for 1 d. The samples were indicated by xNb (x: concentration of niobium chloride concentration in Solution B, 0~0.2 M).
4.2. Characterization of Glass Structure
The glass nature was confirmed by XRD (Philips, X’ Pert Pro α1) with a CuKα X-ray source at 3.3 °/min with a scan step of 0.26 °. The morphology of the samples was observed using SEM (JEOL, Tokyo, Japan, JSM-6000). The glass composition was determined by EDS (JEOL, Tokyo, Japan, JED-2300) (n = 3). The glass transition temperature (Tg) and crystallization temperature (Tc) were determined by TG-DTA (Rigaku, Tokyo, Japan, Thermoplus, TG8120) with a heating rate: 5 °C/min using 20 mg of glass powders (n = 1). The glass structure was evaluated by laser Raman spectroscopy on samples in the Raman shift region between 100 and 1800 cm−1 (HORIBA, Kyoto, Japan, LabRam HR800). The samples were excited by the 514 nm line of a neodymium:yttrium aluminum garnet solid-state laser with a power of 50 mW. The exposure time was 20 s and the cumulative number was 16. The 31P MAS-NMR (JEOL, Tokyo, Japan, JNM-ECA600II) was also used to clarify the phosphate structures in the glasses at 242.955 MHz in a 3.2 mm rotor spinning at 15 kHz. A single-pulse experiment with a 2.77 μs pulse width, 5 s recycle delay and cumulated number of 128 was performed for each sample. The chemical shift was referenced to the signal of NH4H2PO4 as 1.0 ppm.
4.3. Measurement of Ion Release from Samples in Tris-HCl Buffer Solution
Tris-HCl buffer solution was prepared by dissolving 6.118 g of tris (hydroxymethyl) aminomethane (Kishida Chemical, 99.0%) in 1 L of distilled water and adjusting the pH to 7.40 using 1 mol/L of HCl. The samples were sieved to a particle size of 32~125 μm. Seventy-five mg of the sample was immersed in 50 mL of buffer solution and maintained at 37.0 °C at 120 rpm (n = 3). At each time point (6, 24, 48, 72 h), 0.5 mL of solution was removed from each sample and 0.5 mL of fresh buffer solution was added. The collected solution was diluted 20 times, and the concentrations of P, Ca, and Nb in the resulting solution were determined using ICP-AES (Shimadzu, Kyoto, Japan, ICPS-7510). Their dissolution rates were calculated using the following equation [17]:
where x (ppm) is the ion amount dissolved in the tris-HCl buffer solution after soaking, measured by ICP-AES. WAtom is the atomic weight of the ion, and [AtomRate] is the atomic rate of the ion calculated from the nominal glass composition. Wsample (g), Mglass (g/mol), and Vsolution (L) are the weight of the sample soaked, the molecular weight of the glass, and the volume of solution used for soaking, respectively. The pH of the buffer solutions were measured at each time point. The control sample was the buffer solution without any samples. The samples after soaking in the buffer solution for 7 d were washed with ethanol and dried at 60 °C for 1 day, followed by the SEM observation and the examination using XRD at 0.1 °/min with a scan step of 0.05°.
4.4. Fabrication of Fibrous Scaffolds and Measurement of Their Ion Release
A glass precursor solution of 0.2 Nb was prepared with the above-mentioned method. After finishing adding Solution B to Solution A, the resulting solution was centrifuged at 2100 rpm (720× g) for 10 min. The supernatant was collected, mixed with ultrapure water, stirred using a vortex, and centrifuged with the same condition. After repeating this process twice, the supernatant was collected and mixed with a solution of 7 wt% PVA (Mw = 85,000–124,000, Sigma–Aldrich, Tokyo, Japan) with a weight ratio of 1: 1, followed by stirring for 3 h. The PVA solution was prepared by adding PVA to water heated at 60 °C and then cooled to room temperature before being mixed with the glass precursor solution. For the wet-spinning process, the mixture solution containing PVA and the glass precursor of 0.2 Nb was placed in a syringe attached with a 30 G needle and then extruded into an acetone bath with an extrusion speed of 50 µL/min, resulting in the formation of fibers. The fibers were collected from the acetone bath and dried at room temperature. For measurement of their ion release, 20 mg of the prepared fibers were immersed in 20 mL of tris-HCl buffer solution and maintained at 37.0 °C at 120 rpm (n = 3). The ion concentration of the buffer solution was measured by ICP-AES with the above-mentioned method. The pH amounts of the buffer solutions were measured at each time point. The control sample was the buffer solution without any samples.
4.5. Cell Culture Tests for Fibrous Scaffolds
The prepared fibrous samples were sterilized with UV irradiation for one day. Ten mg of the samples was placed in a 96-well plate. A suspension of mouse osteoblast-like cells (MC3T3-E1 cells, Riken PRC, Ibaraki, Japan) were prepared using MEMα (Wako Pure Chemical, Osaka, Japan) containing 10 vol.% fetal bovine serum (FBS, Cosmo Bio, Tokyo, Japan) and 1 vol.% of Penicillin–Streptomycin (Gibco). The cells were seeded onto the samples at a density of 5.0 × 103 cells/well (n = 4) and incubated in a CO2 incubator at 37 °C (humidified atmosphere of 95% air and 5% CO2). The culture medium was replaced with a fresh one every day.
The proliferation level of the cells after being cultured on the sample was measured using the alamarBlue cell viability reagent (Thermo Fisher Scientific, Tokyo, Japan), following instructions from the kit. Briefly, the alamarBlue reagent was diluted with the culture medium with a ratio of 1: 10 in volume; then, 110 μL of the prepared solution was added in each well and incubated in the CO2 incubator for 4 h. To measure the metabolic activity of the cells, the fluorescence of the medium taken from each well was measured with an excitation wavelength of 540 nm and emission wavelength of 590 nm using a multimode plate reader (PerkinElmer, Shelton, WA, USA, EnSpire).
5. Conclusions
Nb-containing phosphate glasses were prepared using the liquid-phase method, and their structure and ion release behavior were studied. The role of Nb in the glass network was changed depending on the amount of Nb introduced. In the case of the group xNb (x ≤ 0.075) group, Nb acted mainly as a network modifier and was introduced into the glass network through the substitution of Ca. Although the chemical durability of the glasses was enhanced by the substitution, the glasses still released ions, which induced the precipitation of calcium phosphate-based crystals during the 7 days of immersion in tris-HCl buffer solution. In the case of the xNb (0.1 ≤ x) group, Nb mainly acted as network former and formed long-chain structures such as (-O-P-O-P-O-Nb-O-). The chemical durability of the glasses was further improved by the formation of such chain structures. By using a combination method of the liquid-phase method and the wet-spinning method, we successfully fabricated cotton-wool-like structured materials with gradual ion-release properties. They can be good candidates for new biomaterials with the controllable ability of providing therapeutic ions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26010161/s1.
Author Contributions
Conceptualization, A.O. and S.L.; investigation, M.T. and S.S.; writing—original draft preparation, M.T.; writing—review and editing, A.O. and S.L.; funding acquisition, M.T, A.O. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Shiraishi Foundation of Science Development, Toyoaki Scholarship Foundation, a JSPS KAKENHI grant number 22K18201, 23KK0272, 24K22400, Nippon Sheet Glass Foundation for Materials Science and Engineering, and Glass Research Promotion Program (GIC, NGF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Neel, E.A.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef]
- Pandayil, J.T.; Boetti, N.G.; Janner, D. Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices-A Review. J. Func. Biomater. 2024, 15, 79. [Google Scholar] [CrossRef]
- Sharmin, N.; Rudd, C.D. Structure, thermal properties, dissolution behaviour and biomedical applications of phosphate glasses and fibres: A review. J. Mater. Sci. 2017, 52, 8733–8760. [Google Scholar] [CrossRef]
- Lepry, W.C.; Nazhat, S.N. A review of phosphate and borate sol–gel glasses for biomedical applications. Adv. NanoBiomed Res. 2020, 1, 2000055. [Google Scholar] [CrossRef]
- Walter, G.; Vogel, J.; Hoppe, U.; Hartmann, P. The structure of CaO–Na2O–MgO–P2O5 invert glass. J. Non-Cryst. Solids 2001, 296, 212–223. [Google Scholar] [CrossRef]
- Brauer, D.S.; Wilson, R.M.; Kasuga, T. Multicomponent phosphate invert glasses with improved processing. J. Non-Cryst. Solids 2012, 358, 1720–1723. [Google Scholar] [CrossRef]
- Patel, U.; Moss, R.M.; Hossain, K.M.Z.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; Hannon, A.C. Structural and physico-chemical analysis of calcium/strontium substituted, near-invert phosphate based glasses for biomedical applications. Acta Biomater. 2017, 60, 109–127. [Google Scholar] [CrossRef]
- Kasuga, T.; Abe, Y. Calcium phosphate invert glasses with soda and titania. J. Non-Cryst. Solids 1999, 243, 70–74. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structures and dissolution behaviors of MgO–CaO–P2O5–Nb2O5 glasses. J. Non-Cryst. Solids 2016, 438, 18–25. [Google Scholar] [CrossRef]
- Lee, S.; Obata, A.; Brauer, D.S.; Kasuga, T. Dissolution behavior and cell compatibility of alkali-free MgO-CaO-SrO-TiO2-P2O5 glasses for biomedical applications. Biomed. Glasses 2015, 1, 151–158. [Google Scholar] [CrossRef]
- Lee, S.; Shiraki, S.; Nagata, F.; Kato, K.; Kasuga, T. Structure and dissolution behavior of boron-containing calcium phosphate invert glasses. J. Non-Cryst. Solids 2022, 590, 121690. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structure and dissolution behavior of MgO-P2O5-TiO2/ Nb2O5 (Mg/P ≥1) invert glasses. J. Ceram. Soc. Jpn. 2015, 123, 942–948. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structures and dissolution behaviors of CaO–P2O5–TiO2/Nb2O5 (Ca/P ≥ 1) invert glasses. J. Non-Cryst. Solids 2015, 426, 35–42. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.H.; Kim, H.W.; Salih, V.; Wall, I.B.; Knowles, J.C. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef]
- Hinoi, E.; Takarada, T.; Yoneda, Y. Glutamate signaling system in bone. J. Pharmacol. Sci. 2004, 94, 215–220. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Wolf, F.I.A. Cittadini, Magnesium in cell proliferation and differentiation. Front. Biosci. 1999, 4, D607–D617. [Google Scholar] [CrossRef]
- Obata, A.; Takahashi, Y.; Miyajima, T.; Ueda, K.; Narushima, T.; Kasuga, T. Effects of Niobium Ions Released from Calcium Phosphate Invert Glasses Containing Nb2O5 on Osteoblast-Like Cell Functions. ACS Appl. Mater. Interfaces 2012, 4, 5684–5690. [Google Scholar] [CrossRef]
- Lee, S.; Shiraki, S.; Takahashi, M.; Obata, A.; Sakurai, M.; Nagata, F. Preparation and structure of titanium-containing pyrophosphate glasses prepared using the liquid-phase method. J. Am. Ceram. Soc. 2024, 108, e20144. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Foroutan, F.; McGuire, J.; Gupta, P.; Nikolaou, A.; Kyffin, B.; Kelly, N.; Hanna, J.; Gutierrez-Merino, J.; Knowels, J.C.; Baek, S.; et al. Antibacterial Copper Doped Calcium Phosphate Glasses for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 6054–6062. [Google Scholar] [CrossRef]
- Desdord, M.; Soulié, J.; Rey, C.; Combes, C. Tunable Behavior in Solution of 68 Amorphous Calcium Ortho/Pyrophosphate Materials: An Acellular In Vitro Study. ACS Biomater. Sci. Eng. 2022, 8, 2363–2374. [Google Scholar] [CrossRef]
- Obata, A.; Ozasa, H.; Kasuga, T.; Jones, J.R. Cotton wool-like poly (lactic acid)/vaterite composite scaffolds releasing soluble silica for bone tissue engineering. J. Mater. Sci.-Mater. Med. 2013, 24, 1649–1658. [Google Scholar] [CrossRef]
- Kasuga, T.; Obata, A.; Maeda, H.; Ota, Y.; Yao, X.; Oribe, K. Siloxane-poly (lactic acid)-vaterite composites with 3D cotton-like structure. J. Mater. Sci.-Mater. Med. 2012, 23, 2349–2357. [Google Scholar] [CrossRef]
- Ye, M.; Mohanty, P.; Ghosh, G. Morphology and properties of poly vinyl alcohol (PVA) scaffolds: Impact of process variables. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 42, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mahnama, H.; Dadbin, S.; Frounchi, M.; Rajabi, S. Preparation of biodegradable gelatin/PVA porous scaffolds for skin regeneration. Artif. Cells Nanomed. Biotechnol. 2017, 45, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Karakassides, M.A.; Sarannnti, A.; Koutselas, I. Preparation and structural study of binary phosphate glasses with high calcium and/or magnesium content. J. Non-Cryst. Solids 2004, 347, 69–79. [Google Scholar] [CrossRef]
- Hsu, S.M.; Wu, J.J.; Yung, S.W.; Chin, T.S.; Zhang, T.; Lee, Y.M.; Chu, C.M.; Ding, J.Y. Evaluation of chemical durability, thermal properties and structure characteristics of Nb–Sr-phosphate glasses by Raman and NMR spectroscopy. J. Non-Cryst. Solids 2012, 358, 14–19. [Google Scholar] [CrossRef]
- Donato, M.G.; Gagliardi, M.; Sirleto, L.; Messina, G.; Lipovskii, A.A.; Tagantsev, D.K.; Righini, G.C. Raman optical amplification properties of sodium–niobium–phosphate glasses. Appl. Phys. Lett. 2010, 97, 231111. [Google Scholar] [CrossRef]
- Gras, P.; Baker, A.; Combes, C.; Rey, C.; Sarda, S.; Wright, A.J.; Smith, M.E.; Hanna, J.V.; Gervais, C.; Laurencin, D.; et al. From crystalline to amorphous calcium pyrophosphates: A solid state Nuclear Magnetic Resonance perspective. Acta Biomater. 2016, 31, 348–357. [Google Scholar] [CrossRef]
- Slater, C.; Laurencin, D.; Burnell, V.; Smith, M.E.; Grover, L.M.; Hriljaca, J.A.; Wright, A.J. Enhanced stability and local structure in biologically relevant amorphous materials containing pyrophosphate. J. Mater. Chem. 2011, 21, 18783–18791. [Google Scholar] [CrossRef]
- Turner, G.L.; Smith, K.A.; Kirkpatrick, R.J.; Oldfieldt, E. Structure and Cation Effects on Phosphorus-31 NMR Chemical Shifts and Chemical-Shift Anisotropies of Orthophosphates. J. Magn. Reson. 1969, 70, 408–415. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. Determination of niobium-oxygen bond distances and bond orders by Raman spectroscopy. Solid. State Ion. 1991, 45, 201–213. [Google Scholar] [CrossRef]
- Komatsu, T.; Honma, T.; Tasheva, T.; Dimitrov, V. Structural role of Nb2O5 in glass-forming ability, electronic polarizability and nanocrystallization in glasses: A review. J. Non-Cryst. Solids 2022, 581, 121414. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; Law, R.V.; Hill, R.G. Effect of TiO2 addition on structure, solubility and crystallisation of phosphate invert glasses for biomedical applications. J. Non-Cryst. Solids. 2010, 356, 2626–2633. [Google Scholar] [CrossRef]
- Jain, S.K.; Tabassum, T.; Li, L.; Ren, L.; Fan, W.; Tsapatsis, M.; Caratzoulas, S.; Han, S.; Scott, S.L. P-Site Structural Diversity and Evolution in a Zeosil Catalyst. J. Am. Chem. Soc. 2021, 143, 1968–1983. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Azaïs, T.; Bonhomme-Coury, L.; Laurent, G.; Bonhomme, C. Efficiency of the Refocused 31P−29Si MAS-J-INEPT NMR Experiment for the Characterization of Silicophosphate Crystalline Phases and Amorphous Gels. Inorg. Chem. 2007, 46, 1379–1387. [Google Scholar] [CrossRef]
- Flambard, A.; Videau, J.J.; Delevoye, L.; Cardinal, T.; Labrugère, C.; Rivero, C.A.; Couzi, M.; Montagne, L. Structure and nonlinear optical properties of sodium–niobium phosphate glasses. J. Non-Cryst. Solids. 2008, 354, 3540–3547. [Google Scholar] [CrossRef]
- Flambard, A.; Montagne, L.; Delevoye, L.; Palavit, G.; Amoureux, J.P.; Videau, J.J. Solid-state NMR study of mixed network sodium–niobium phosphate glasses. J. Non-Cryst. Solids 2004, 345–346, 75–79. [Google Scholar] [CrossRef]
- Block, S.; Levins, E.M. Structural Interpretation of Immiscibility in Oxide Systems:11, Coordination Principles Applied to Immiscibility. J. Am. Chem. Soc. 1957, 40, 79–142. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).