Abstract
This review focuses on non-surgical treatment options for rotator cuff injuries and highlights the potential of mesenchymal stem cells (MSCs) as a potential regenerative approach. MSCs, sourced from various tissues like bone marrow and adipose tissue, exhibit promising mechanisms in vitro, influencing tendon-related gene expression and microenvironment modulation. Animal studies support this, showcasing MSCs’ ability to reduce inflammation, improve tissue remodeling, and enhance repaired tendon strength. Human trials, while varied and limited, suggest that MSCs might lower retear rates and enhance post-repair outcomes, but randomized controlled trials yield mixed results, emphasizing the necessity for standardized investigations. Ultimately, while cell-based therapies demonstrate an excellent safety profile, more rigorous clinical trials are necessary to determine their efficacy in improving patient outcomes and achieving lasting structural changes in rotator cuff injuries.
1. Introduction
Shoulder pain is the third-most-common musculoskeletal complaint (behind back and knee pain) in the United States [1]. The prevalence of shoulder pain ranges from 14 to 34% [2,3,4,5,6,7] each year; about 1% of the population who are 45 years and older present with shoulder pain to primary care settings [8]. In the United States, the direct healthcare expenses attributable to shoulder disorders was estimated to be USD 7 billion in 2000 [9], and rotator cuff tears are considered one of the most expensive diseases treated in American hospitals [1]. Rotator cuff disorders are the underlying problems in 65–70% of patients with shoulder pain [10,11]. Despite this enormous public health impact, there are no disease-modifying treatments for rotator cuff tears.
The major symptomatic manifestations of rotator cuff tears include chronic shoulder pain, impaired mobility, and functional impairments. These arise due to progressive pathological remodeling of the tendon, leading to increased fibroblast cellularity, neovascularity, thinning/loss of collagen matrix, and fatty infiltration [12]. Rotator cuff tears can be treated non-operatively and operatively. The current non-surgical standard-of-care therapies such as physical therapy address biomechanical and functional deficits but do not regenerate the underlying structural tendon tear. In addition, non-steroidal anti-inflammatory drugs (NSAIDs), modalities (acupuncture, iontophoresis, etc.), and glucocorticoids injections provide symptomatic relief but do not prevent the progression of disease. Moreover, the rotator cuff tear size, muscle atrophy, and fatty infiltration may progress over 5 to 10 years with non-operative treatments [13,14]. The current guidelines for pharmacological therapeutic strategies that have been adopted by many professional organizations are largely focused on symptom relief in partial-thickness rotator cuff tears and do not offer disease-modifying benefits [14]. Alternative injections such as hyaluronic acid have limited evidence to support their use, and platelet-rich plasma has limited evidence that does not support routine use for treatment of rotator cuff tears [14]. Surgical treatments such as rotator cuff repair are also aimed at either debriding the tendon or anchoring a torn tendon back to the humeral head and do not alter the underlying tendon biology. Moreover, incomplete or failed tendon healing occurs in 20–25% of patients [14]. Thus, current treatments for rotator cuff tears are sub-optimal, and there is a significant need for disease-modifying therapies (DMTs).
Given the overall frequency of shoulder pain and rotator cuff tears, further treatment modalities are needed to aid with healing. Emerging regenerative options are based upon repurposing mesenchymal stem cells (MSCs) to directly treat existing tears in muscle fibers or augment surgical treatment options in cases of full-thickness tears. The purpose of this review is to provide a brief overview of MSCs and an update of the current literature regarding their clinical applications in treating rotator cuff tears.
2. Methods
Given the overall paucity of human controlled trials regarding the use of MSCs for rotator cuff pathology, the decision was made to pursue a scoping review. The aim of this study was two-fold: (1) to synthesize the current basic science of MSCs, understand the different subtypes of MSCs, and the current in vivo research of the use of MSCs for rotator cuff tears (RCTs), and (2) to evaluate the current literature regarding the use of MSCs for RCTs in humans. A detailed literature search (September 2023 to December 2023) in seven databases (PubMed (NLM); CINAHL; Scopus (Elsevier); ClinicalTrials.gov; and Proquest Dissertation and Thesis) in order to evaluate the evidence base for MSCs for RCTs (see Figure 1). For the purpose of this review, MSCs were defined as nonhematopoietic multipotent cells, which are capable of differentiating into a variety of cells of mesenchymal lineage [15], which could include tissue derived from almost all organs, including bones, adipose tissue, etc. Randomized control trials, as well as cohort studies and case series, were considered for inclusion.
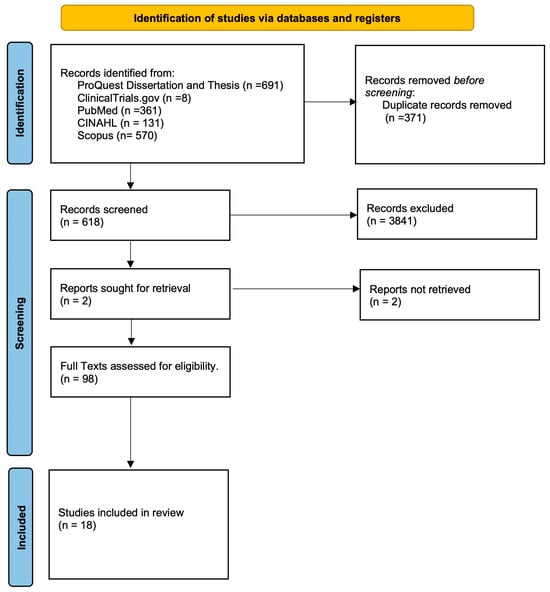
Figure 1.
Flow chart of included studies.
3. Basic Science of Mesenchymal Stem Cells
MSCs are defined as nonhematopoietic multipotent cells, which are capable of differentiating into a variety of cells of mesenchymal lineage [15]. It is believed that they can be derived from the connective tissue of almost all organs, including bones, adipose tissue, dental pulp, as well as isolated from the human placenta, umbilical cord, and various fetal tissues [16]. The minimal criteria for identifying MSCs, as defined by the International Society for Cellular Therapy (ISCT), require the following: (1) must be plastic-adherent when maintained in standard culture conditions, (2) must express CD73, CD90, and CD105, and lack expression of CD11b, CD14, CD19, CD34, CD45, CD79a and HLA-DR surface molecules, and (3) be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro [17].
Although initial studies theorized that MSCs repaired tissues through the differentiation and engraftment into injuries’ tissues, more recent research has shown that MSCs are able to mediate tissue repair, but they have only transient engraftment into the injured issues [16,18]. Recent studies suggest that MSCs’ therapeutic effects are mediated through the release of paracrine factors, mitochondrial transfer, and extracellular vesicle secretion [16,18,19]. MSCs produce an abundance of paracrine factors, including cytokines, chemokines, growth factors, and microRNA. Caplan and colleagues proposed that within local injury, MSCs actively participate in the suppression of local immune reactions within local tissues, as well as wound repair, tissue regeneration, and angiogenesis [20,21]. Furthermore, research has shown that MSCs can mediate the stimulation of the recruitment, proliferation, and differentiation of tissue-specific cells [21,22,23] and attenuate the oxidative stress response [21,24].
Although the ISCT definition states that MSCs must be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro, some studies have shown that under appropriate conditions, MSCs can differentiate into other tissues, like tendon, skeletal muscle, myocardium, and smooth muscle [21,25,26]. Although MSCs can be harvested from a variety of tissues, many of these cells share similar characteristics. Research has shown some differences between these cells, which may, however, potentially lead to differences in differentiation propensity. For example, the global miRNA expression profile of MSCs varies according to the tissue of origin, which may affect cellular properties, such as proliferation, differentiation, and paracrine activities [27]. As such, it is important to understand the different MSCs’ harvesting sites/subtypes.
3.1. Mesenchymal Stem Cell Subtypes
MSCs can be harvested from multiple tissue sources of mesenchymal origin, including the placenta, umbilical cords, adipose tissue, bone marrow, as well as other tissues. Although multiple potential sources exist, the most commonly utilized adult sources are bone marrow and adipose tissue in orthopedics [28]. This is in part due to the ease at which these tissues are obtained, but also due to the success that these tissues have shown in producing a large number of MSCs and paracrine effects [28,29,30]. For the purpose of this review, we will further explore bone-marrow-derived MSCs, adipose-derived MSCs, umbilical-cord-derived MSCs, muscle, and peripheral blood.
3.1.1. Bone-Marrow-Derived MSCs
Bone-marrow-derived MSCs (BM-MSCs) were the first MSCs identified and, thus, have been the most extensively studied, both in vitro and for their therapeutic properties [31,32]. BM-MSCs have been shown to comprise 0.001% to 0.01% of total marrow mononuclear cells [21,33,34]. As with all MSCs, BM-MSCs are thought to exert therapeutic effects through their ability to regulate cell proliferation/differentiation, ability to secrete trophic factors, and immunomodulatory activity. However, research has shown specific differences in BM-MSCs compared to other subtypes. Immunologically, BM-MSCs have been shown to strongly express CD49f, PODXL, CD 106, and cytochrome p450 and not express or minimally express CD54 and CD34, as compared to other MSCs [21,35,36]. In addition, some studies have demonstrated that BM-MSCs are more prone to osteogenic differentiation than other MSCs [21,35,37]. Additionally, some in vitro studies have found that some MSCs have decreased chondrogenic differentiation potential when compared to BM-MSCs [21,36,38].
As previously stated, this was the first stem cell discovered, and as such, it has been extensively investigated as a potential therapy for a wide variety of conditions, including, but not limited to, cardiovascular, neurological, orthopedic, oncologic, rheumatologic, and gastrological diseases.
3.1.2. Adipose Tissue MSCs
Adipose tissue MSCs (AT-MSCs) have been also extensively studied due to their advantageous ability to be conveniently sourced as subcutaneous AT, which is abundantly found throughout the body. Unlike BM-MSCs, it is estimated that approximately 98–100% of cells obtained through AT are viable [39,40]. Thus, when compared to BM MASCs, AT-MSCs contain a 500-fold greater number of MSCs when isolated from an equivalent amount of adipose tissue [21,41]. MSCs can be harvest by either enzymatically digesting adipose tissue to yield a stromal vascular fraction (SVF) or through mechanical breakdown to yield micro-fragmented adipose tissue (MFAT). Studies have shown that MFAT contains higher concentrations of AT-MSCs when compared to SVF making it an ideal choice in clinical applications, especially given its comparative ease of accessibility [42,43]. However, one limitation of AT-MSCs is that certain donor characteristics, like age, can affect the ability of AT-MSCs to expand and differentiate, notably in the chondrogenic and osteogenic lineages [21,40,44,45]. However, these effects have not been clinically verified.
3.1.3. Umbilical Cord Blood MSCs
Umbilical cord blood MSCs (UCB-MSCs) are considered an abundant source of mesenchymal stem cells. Since the MSCs derived from UCB are typically discarded at birth, some consider this a less expensive and the least invasive method of collecting MSCs compared to their adult source counterparts [46,47]. Another potential advantage of UCB is that, due to their immaturity, UCB-MSCs have been shown to be less immunogenic. In addition, they have been found to have a similar doubling time when compared to BM-MSCs [39,46,47,48]. Lastly, research has shown that they may have the highest expansion potential among all subtypes of MSCs [47].
3.1.4. Muscle-Derived MSCs
As with other MSCs, muscle-derived MSCs (M-MSCs) are able to differentiate into multiple mesenchymal tissues, like myogenic, chondrogenic, and osteogenic linages. Of note, M-MSCs are committed to a myogenic lineage, while satellite cells are capable of multi-lineage differentiation [49]. Satellite cells are mononuclear cells that surround each muscle fiber and the plasma membrane of the fiber. These are thought to be the main cell type responsible for skeletal muscle regeneration. Studies have shown multiple potential applications for M-MSCs, including augmenting muscle healing following injury, both skeletal and cardiac, the promotion of peripheral nerve regeneration, and the promotion of vascular regeneration [39,50,51].
3.1.5. Peripheral Blood MSCs
Peripheral blood progenitor cells have been shown to be mobilized through the use of filgrastim, a granulocyte-CSF [48,52]. An advantage of using mobilized peripheral blood MSCs (PB-MSCs) is the ease at which they can be accessed and obtained. They share the same ability to differentiate into mesenchymal lineages as other subtypes of MSCs; however, studies have shown that the doubling time of PB-MSCs is almost 95 h, which is longer than most other sources [48,53]. Lastly, another disadvantage of this subtype is that their capacity to differentiate into bone and chondral lineages has been shown to be lower than BM-MSCs.
4. MSCs for Rotator Cuff Injury
4.1. In Vitro Data
Tendon disruption seen in rotator cuff tears (RCTs) leads to decreased muscle fibers and mass, which subsequently leads to increased fat content [54,55]. That being said, RCTs are thought to induce fatty infiltration, and classification systems have been developed to quantify the severity of rotator cuff tears based on the degree of fatty infiltration [56]. It has been shown that surgical rotator cuff repairs have lower surgical success rates in patients with a more advanced Goutallier stage [55,57]. It is believed that the use of MSCs for tendinopathy reduces the inflammatory environment, shifting to a more reparative environment [58,59].
In vitro culture studies have attempted to better understand the mechanisms by which MSCs can aid in the repair of tendinopathies like RCTs. One study found that crosstalk between tendon cells and MSCs led to an upregulation of tendon-related genes, like scelraxis and tenomodulin, as well as tendon ECM markers, like type 1 collagen and decorin [59,60,61]. Another theory is that paracrine factors play a role in MSCs, supporting tendon cells. Sevivas and colleagues found that pre-conditioning tendon cells in vitro with the BM-MSCs’ secretome results in improved biomechanical performance when transferred to a rat model of rotator cuff tears [62]. Another potential mechanism by which MSCs treat RTC is through the generation of tendons like tissue. In one in vitro study, researchers were able to culture BMMSCs in fibrin gels and spontaneously generate collagen fibrils similar to embryonic tendons [59,63]. The rationale by which this occurs in vitro is due to TGF-B3 signaling.
Further studies have examined the potential cellular mechanisms by which AT-MSCs are able to improve tendon healing. AT-MSCs may also use a cellular crosstalk mechanisms in order in upregulate tendon-related genes [64,65]. Furthermore, in co-cultures with AT-MSCs and tendon explants, it was found that the collagenolytic activity of matrix metalloproteinase (MMPs) was increased. In addition to fastened extracellular matrix remodeling, the same study by Costa-Almeida and colleagues found an accelerated deposition of type 1 college and increased ratio of type 1 to type 3 collagen [66]. Thus, MSCs may play a role in shifting the microenvironment to induce repair and reduce fibrotic healing. Altogether, in vitro studies support the potential therapeutic effect for MSCs though multiple different mechanisms that lead to modulation of the microenvironment.
4.2. Clinical Applications of Mesenchymal Stem Cells in Rotator Cuff Disease
4.2.1. In Vivo Studies
RCTs can manifest in varying severity and with varying levels of fibrosis and fatty infiltration. In a study conducted by Mora et al. in 2014, a rat model of acute supraspinatus tear followed by repair was used to investigate the effects of AT-MSCs [67]. Their findings a revealed notable reduction in acute inflammation, edema, and a decreased presence of neutrophils in histology. Similarly, Chen et al. (2015) conducted a study using human-adipose-derived MSCs in a rat model of RCT [68]. They observed improved fiber arrangement and tendon organization, as well as reduced inflammation. These findings suggest that AT-MSCs can help mitigate the initial inflammatory response following RCT. They also show promise in chronic disease, as seen in a study by Gunmucio et al. (2016), who investigated stromal vascular stem cell treatment in conjunction with surgical repair in a rat model of chronic RCT [69]. Their results showed a significant reduction in muscle fibrosis, up to 40% when compared to repair alone.
Furthermore, MSCs have shown promise in enhancing functional outcomes following RCT repair. In a rabbit model of chronic RCT, researchers demonstrated that adipose-derived MSC exosomes, when injected after surgical repair, led to a significantly higher tendon load to failure, increased muscular stiffness, and improved tendon stress tolerance compared to surgical repair alone [70]. This suggests that MSCs can play a pivotal role in augmenting the mechanical integrity and functional performance of repaired tendons. Shin (2020) utilized adipose-derived MSC cell sheets in a rat model to improve tensile strength, particularly at the enthesis following rotator cuff repair [71]. The nearly two-fold increase in tensile strength highlights the potential of MSC-based therapies to enhance the structural integrity of the repaired tendon, which is crucial for functional recovery. Finally, multiple studies have employed scaffold matrices populated with MSCs in rat models of RCT, resulting in increased tendon tensile strength [72,73].
While the precise mechanisms underlying these improvements remain unclear, it is believed that MSCs may modulate this process through a paracrine activity of suppressing pro-inflammatory cytokines, which can hinder tendon healing after injury, and by simultaneously promoting angiogenesis to improve cellular healing [74]. Nevertheless, these in vivo trials provided compelling evidence for the therapeutic potential of mesenchymal stem cells in addressing both the structural and functional aspects of tendon healing in RCTs. These studies suggest that MSCs can reduce inflammation, enhance tissue remodeling, and improve the mechanical properties of repaired tendons, which has prompted human clinical trials.
4.2.2. Human Trials
Overall, our review found 18 case reports/series, RCTs, and case control series examining the effects of MSCs of rotator cuff tears (see Table 1) [75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92]. The most notable features of each study are summarized in Table 1. Of note, one of the included studies was a 2-year follow-up from the initial study. While human trials for MSC use in musculoskeletal applications have been on the rise in recent years, there continues to be a paucity of well-designed studies, especially randomized controlled trials, that examine the effects of MSCs of RCT. There is a lot of variance in target populations, MSC type and application, and follow-up time frames. We aim to synthesize the available evidence below.

Table 1.
Current published studies examining MSCs for rotator cuff tears. Included are the study name, study design, pathology, number of total participants, harvesting site, outcomes, and results.
Of the 18 studies reviewed, 12 were either case series, case reports, or case-controlled studies. Five of the included non-randomized controlled studies examined BMAC, while the other seven examined AT-MSCs/MFAT. In an earlier human-based study involving MSCs, Hernigou and colleagues showed, in their 2014 case-controlled study, that using bone-marrow-derived MSCs as an adjunct to surgical rotator cuff repair could help prevent retears in the future (as seen at 10 yr follow-up) and improve the quality of the repair [82]. Interestingly, they also found that the number of MSCs that were transplanted positively correlated with a patient’s tendon integrity. Similarly, a cohort study conducted by Kim et al. (2017) showed that using adipose-derived MSCs to augment surgical rotator cuff repair significantly decreased retear rates, as seen on MRI [87]. However, they did not note any clinical differences in patients on follow-up. On the other hand, 10 of the 12 included studies found improvements in pain and/or functional outcome scores. In fact, Jo et al. (2018) found improvements in pain of up to 80%, with arthroscopic evaluation demonstrating near-full healing of the tear defect [85,86]. The positive results from these types of studies helped open the door to future randomized control trials. Currently, there are nine registered trials on clinical trials.gov classified as “recruiting, unknown status, active, or completed” (see Table 2).

Table 2.
This table represents currently registered studies on clinicaltrails.gov when searching for “Rotator Cuff Tear” and “Stem Cells”. Only studies classified as recruiting, unknown status, active, or completed were included in the table. Any study classified as withdrawn or suspended was not included. The quality of these studies was not evaluated as they are currently ongoing.
4.2.3. Human Randomized Control Trials
Of the 18 studies, our review found that only 5 were randomized control trials. Two of these RCTs examined BMAC, while the other three studies examined AT-MSCs/MFAT. One of the most recently published trials was by Cole et al. (2023) in 2023, where they compared arthroscopic rotator cuff repair alone versus repair augmented with concentrated bone marrow aspirate in 91 patients [79]. They found that patient-reported pain and function outcomes were not statistically different between groups. However, they noted significantly lower retear rates (18% vs. 57%; p < 0.001) in the augmented repair group based on Sugaya classification on one-year MRI scans. In contrast, Randelli et al. (2022) conducted a similar trial but, this time, using adipose-derived MSCs in the form of microfragmented adipose tissue to augment arthroscopic repair [92]. They followed patients for a total of 24 months and found that at 6 months, the augmented repair group had statistically significant improvements over the control group in patient-reported pain and function. Interestingly, these differences were not seen at any other follow-up point. These studies suggest that MSCs can certainly help augment the healing process when used in conjunction with arthroscopic repair, but their clinical significance for patient-reported metrics needs to be further investigated.
Mixed results were noted in studies that investigated non-surgical MSC injections as well. Centeno et al. (2020) compared bone marrow concentrate plus PRP injection to exercise therapy in partial-thickness supraspinatus tear and found significant improvements in pain and function outcomes at 12 months [76]. Similarly, Hurd et al. (2020) compared injections of adipose-derived MSCs versus steroids in partial-thickness rotator cuff tears who had failed treatment with physical therapy [84]. They found statistically significant improvements in pain and function outcomes at 12 months [84]. However, this was in a small sample size of 16 patients, as their published results were pilot data, and the study is still ongoing. On the other hand, Chun et al. (2022) compared injections with adipose-derived MSC plus fibrin glue, fibrin glue only, and saline only for the treatment of partial-thickness supraspinatus tears and found no significant differences in pain or functional patient-reported outcomes [78].
While some studies [76,84,92] suggest there may be short-term clinical benefit for patients, larger, more rigorous trials will be required to fully elucidate the extent of the clinical benefit that can be expected from this orthobiologic treatment. For instance, future studies can include comparisons between different formulations of MSCs (bone marrow vs. adipose, etc.), a comparison of treatment efficacy in full-thickness vs. partial-thickness tears, and long-term follow-ups.
5. Future Directions with MFAT
A major challenge in developing drug-modifying therapy is the necessity to modulate several dysregulated pathways that impact pain, intra- and peri-tendinous inflammation, and structural tendon loss. One approach to achieve such disease modification is through orthobiologic agents, such as MSCs, that are formulated with specific tendon stem/progenitor cells that can potentially reduce tendon inflammation and pain, enhance overall function, and repair tendon tear loss [93].
Overall, there are limited RCTs examining the effect of MSCs on rotator cuff tears. To date, there is only one double-blinded randomized control trial that shows the beneficial effects of MSCs at 6 months [80]; however, this is in conjunction with arthroscopic repair of large rotator cuff tears compared to arthroscopic repair alone and does not include a non-operative injection arm of MSCs alone. In addition, only a few unblinded prospective trials evaluating MSC-based therapies for rotator cuff tears exist and demonstrate an excellent safety profile; however, translating the results of these trials into clinical practice is challenging due to key limitations, including the following: (1) heterogeneity of MSC formulations, (2) lack of standardization for dosing and/or administration frequency, (3) lack of trials utilizing endpoints that assess disease-modifying properties [85,86,87]. Moreover, no trial has comprehensively defined a formulation that is reproducible with specific biological properties in patients with partial-thickness rotator cuff tears. Given this, it is important that future clinical trials focus on standardizing formulations and developing standardized administration frequencies in order to properly assess the outcomes of MSCs when used for rotator cuff tears.
6. Conclusions
Cell-based therapy has certainly been shown to be safe in human use when derived from both bone marrow and adipose tissues. This review has clearly shown that a fair number of studies have been conducted to demonstrate safety; however, more well-designed robust clinical trials need to be carried out to assess its efficacy in patient outcomes and determine mechanistically if structural modification is a resulting long-term outcome.
Author Contributions
All authors contributed equally to the conceptualization, writing—review and editing. Investigation and analysis of resources N.H., A.M. and N.B.J. P.J. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Colvin, A.C.; Egorova, N.; Harrison, A.K.; Moskowitz, A.; Flatow, E.L. National trends in rotator cuff repair. J. Bone Jt. Surg. Am. 2012, 94, 227–233. [Google Scholar] [CrossRef]
- Urwin, M.; Symmons, D.; Allison, T.; Brammah, T.; Busby, H.; Roxby, M.; Simmons, A.; Williams, G. Estimating the burden of musculoskeletal disorders in the community: The comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann. Rheum. Dis. 1998, 57, 649–655. [Google Scholar] [CrossRef]
- Bergenudd, H.; Lindgärde, F.; Nilsson, B.; Petersson, C.J. Shoulder pain in middle age. A study of prevalence and relation to occupational work load and psychosocial factors. Clin. Orthop. Relat. Res. 1988, 231, 234–238. [Google Scholar] [CrossRef]
- Andersson, H.I.; Ejlertsson, G.; Leden, I.; Rosenberg, C. Chronic pain in a geographically defined general population: Studies of differences in age, gender, social class, and pain localization. Clin. J. Pain 1993, 9, 174–182. [Google Scholar] [CrossRef]
- Mitchell, C.; Adebajo, A.; Hay, E.; Carr, A. Shoulder pain: Diagnosis and management in primary care. BMJ 2005, 331, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Pope, D.P.; Croft, P.R.; Pritchard, C.M.; Macfarlane, G.J.; Silman, A.J. The frequency of restricted range of movement in individuals with self-reported shoulder pain: Results from a population-based survey. Br. J. Rheumatol. 1996, 35, 1137–1141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Speed, C. Shoulder pain. Clin. Evid. 2005, 14, 1543–1560. [Google Scholar]
- Chen, A.L.; Shapiro, J.A.; Ahn, A.K.; Zuckerman, J.D.; Cuomo, F. Rotator cuff repair in patients with type I diabetes mellitus. J. Shoulder Elb. Surg. 2003, 12, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Crossley, K.L.; O’Neil, M.; Al-Zakwani, I. Estimates of Direct Healthcare Expenditures among Individuals with Shoulder Dysfunction in the United States; American Society of Shoulder and Elbow Therapists: Warrenville, IL, USA, 2004. [Google Scholar]
- John, M.; Pap, G.; Angst, F.; Flury, M.P.; Lieske, S.; Schwyzer, H.K.; Simmen, B.R. Short-term results after reversed shoulder arthroplasty (Delta III) in patients with rheumatoid arthritis and irreparable rotator cuff tear. Int. Orthop. 2010, 34, 71–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ostör, A.J.K.; Richards, C.A.; Prevost, A.T.; Speed, C.A.; Hazleman, B.L. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology 2005, 44, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Moosmayer, S.; Lund, G.; Seljom, U.S.; Haldorsen, B.; Svege, I.C.; Hennig, T.; Pripp, A.H.; Smith, H.J. At a 10-Year Follow-up, Tendon Repair Is Superior to Physiotherapy in the Treatment of Small and Medium-Sized Rotator Cuff Tears. J. Bone Jt. Surg. Am. 2019, 101, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Chahal, J. Management of Rotator Cuff Injuries. J. Am. Acad. Orthop. Surg. 2020, 28, e193–e201. [Google Scholar] [CrossRef] [PubMed]
- Shenaq, D.S.; Rastegar, F.; Petkovic, D.; Zhang, B.Q.; He, B.C.; Chen, L.; Zuo, G.W.; Luo, Q.; Shi, Q.; Wagner, E.R.; et al. Mesenchymal Progenitor Cells and Their Orthopedic Applications: Forging a Path towards Clinical Trials. Stem Cells Int. 2010, 2010, 519028. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.I.; Ide, C.; Nabeshima, Y.I. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef]
- Shim, W.S.; Jiang, S.; Wong, P.; Tan, J.; Chua, Y.L.; Tan, Y.S.; Sin, Y.K.; Lim, C.H.; Chua, T.; Teh, M.; et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem. Biophys. Res. Commun. 2004, 324, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Gartner, S.; Kaplan, H.S. Long-term culture of human bone marrow cells. Proc. Natl. Acad. Sci. USA 1980, 77, 4756–4759. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.P.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Friedenstein, A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976, 47, 327–359. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Locatelli, F.; Fibbe, W.E. Mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2009, 1176, 101–117. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Ghetu, N.; Wallace, C.G.; Wei, F.C.; Liao, S.K. The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. J. Stem Cell Res. Ther. 2014, 4, 237. [Google Scholar] [CrossRef]
- Noël, D.; Caton, D.; Roche, S.; Bony, C.; Lehmann, S.; Casteilla, L.; Jorgensen, C.; Cousin, B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp. Cell Res. 2008, 314, 1575–1584. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bochev, I.; Elmadjian, G.; Kyurkchiev, D.; Tzvetanov, L.; Altankova, I.; Tivchev, P.; Kyurkchiev, S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol. Int. 2008, 32, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, V.; Clausen, P.; Matuska, A.M. Micro-fragmented adipose tissue cellular composition varies by processing device and analytical method. Sci. Rep. 2022, 12, 16107. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and Properties of Mesenchymal Stem Cells Derived From Microfragmented Adipose Tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef]
- Zhu, M.; Kohan, E.; Bradley, J.; Hedrick, M.; Benhaim, P.; Zuk, P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2009, 3, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011, 7, 269–291. [Google Scholar] [CrossRef]
- Gang, E.J.; Hong, S.H.; Jeong, J.A.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2004, 321, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.S.; Roshin, G.E.; Divya, T.S.; Rasheed, V.A.; Santhoshkumar, T.R.; Elizabeth, K.E.; James, J.; Pillai, R.M. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res. Ther. 2012, 3, 57. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Gómez-García, R.; Granados-Montiel, J.; Berebichez-Fastlicht, E.; Olivos-Meza, A.; Granados, J.; Velasquillo, C.; Ibarra, C. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells-Their Current Uses and Potential Applications. Stem Cells Int. 2017, 2017, 2638305. [Google Scholar] [CrossRef]
- Abou-Khalil, R.; Yang, F.; Lieu, S.; Julien, A.; Perry, J.; Pereira, C.; Relaix, F.; Miclau, T.; Marcucio, R.; Colnot, C. Role of muscle stem cells during skeletal regeneration. Stem Cells 2015, 33, 1501–1511. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert. Opin. Biol. Ther. 2010, 10, 505–517. [Google Scholar] [CrossRef]
- Peng, H.; Huard, J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl. Immunol. 2004, 12, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.J.; Schriber, J.R.; Grimes, K.; Long, G.D.; Negrin, R.S.; Raimondi, C.M.; Horning, S.J.; Brown, S.L.; Miller, L.; Blume, K.G. Granulocyte colony-stimulating factor “mobilized” peripheral blood progenitor cells accelerate granulocyte and platelet recovery after high-dose chemotherapy. Blood 1993, 81, 2031–2035. [Google Scholar] [CrossRef]
- Tondreau, T.; Meuleman, N.; Delforge, A.; Dejeneffe, M.; Leroy, R.; Massy, M.; Mortier, C.; Bron, D.; Lagneaux, L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: Proliferation, expression, and plasticity. Stem Cells 2005, 23, 1105–1112. [Google Scholar] [CrossRef]
- Kang, J.R.; Gupta, R. Mechanisms of fatty degeneration in massive rotator cuff tears. J. Shoulder Elb. Surg. 2012, 21, 175–180. [Google Scholar] [CrossRef]
- Gupta, R.; Rao, R.; Johnston, T.R.; Uong, J.; Yang, D.S.; Lee, T.Q. Muscle stem cells and rotator cuff injury. JSES Rev. Rep. Tech. 2021, 1, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Somerson, J.S.; Hsu, J.E.; Gorbaty, J.D.; Gee, A.O. Classifications in Brief: Goutallier Classification of Fatty Infiltration of the Rotator Cuff Musculature. Clin. Orthop. Relat. Res. 2016, 474, 1328–1332. [Google Scholar] [CrossRef]
- Gerber, C.; Wirth, S.H.; Farshad, M. Treatment options for massive rotator cuff tears. J. Shoulder Elb. Surg. 2011, 20, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef]
- Luo, Q.; Song, G.; Song, Y.; Xu, B.; Qin, J.; Shi, Y. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology 2009, 61, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Wang, B.; Sun, Y.; Xu, J.; Yuk-Wai, L.W.; Xu, L.; Zhang, J.; Li, G. The Use of Cocultured Mesenchymal Stem Cells with Tendon-Derived Stem Cells as a Better Cell Source for Tendon Repair. Tissue Eng. Part A 2016, 22, 1229–1240. [Google Scholar] [CrossRef]
- Sevivas, N.; Teixeira, F.G.; Portugal, R.; Direito-Santos, B.; Espregueira-Mendes, J.; Oliveira, F.J.; Silva, R.F.; Sousa, N.; Sow, W.T.; Nguyen, L.T.; et al. Mesenchymal Stem Cell Secretome Improves Tendon Cell Viability In Vitro and Tendon-Bone Healing In Vivo When a Tissue Engineering Strategy Is Used in a Rat Model of Chronic Massive Rotator Cuff Tear. Am. J. Sports Med. 2018, 46, 449–459. [Google Scholar] [CrossRef]
- Kapacee, Z.; Yeung, C.Y.; Lu, Y.; Crabtree, D.; Holmes, D.F.; Kadler, K.E. Synthesis of embryonic tendon-like tissue by human marrow stromal/mesenchymal stem cells requires a three-dimensional environment and transforming growth factor β3. Matrix Biol. 2010, 29, 668–677. [Google Scholar] [CrossRef]
- Veronesi, F.; Torricelli, P.; Della Bella, E.; Pagani, S.; Fini, M. In vitro mutual interaction between tenocytes and adipose-derived mesenchymal stromal cells. Cytotherapy 2015, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Calejo, I.; Reis, R.L.; Gomes, M.E. Crosstalk between adipose stem cells and tendon cells reveals a temporal regulation of tenogenesis by matrix deposition and remodeling. J. Cell Physiol. 2018, 233, 5383–5395. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Berdecka, D.; Rodrigues, M.; Reis, R.; Gomes, M. Tendon explant cultures to study the communication between adipose stem cells and native tendon niche. J. Cell. Biochem. 2018, 119, 3653–3662. [Google Scholar] [CrossRef]
- Mora, M.V.; Antuña, S.A.; Arranz, M.G.; Carrascal, M.T.; Barco, R. Application of Adipose tissue-derived Stem Cells in a Rat Rotator Cuff Repair Model. Injury 2014, 45, S22–S27. [Google Scholar] [CrossRef]
- Chen, H.S.; Su, Y.T.; Chan, T.M.; Su, Y.J.; Syu, W.S.; Harn, H.J.; Lin, S.Z.; Chiu, S.C. Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplant. 2015, 24, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Flood, M.D.; Roche, S.M.; Sugg, K.B.; Momoh, A.O.; Kosnik, P.E.; Bedi, A.; Mendias, C.L. Stromal vascular stem cell treatment decreases muscle fibrosis following chronic rotator cuff tear. Int. Orthop. 2016, 40, 759–764. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Song, W.; Yu, W.; He, Y. Adipose Stem Cell-Derived Exosomes Decrease Fatty Infiltration and Enhance Rotator Cuff Healing in a Rabbit Model of Chronic Tears. Am. J. Sports Med. 2020, 48, 1456–1464. [Google Scholar] [CrossRef]
- Shin, M.J.; Shim, I.K.; Kim, D.M.; Choi, J.H.; Lee, Y.N.; Jeon, I.H.; Kim, H.; Park, D.; Kholinne, E.; Yang, H.S.; et al. Engineered Cell Sheets for the Effective Delivery of Adipose-Derived Stem Cells for Tendon-to-Bone Healing. Am. J. Sports Med. 2020, 48, 3347–3358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Y.; Reisdorf, R.L.; Qi, J.; Lu, C.K.; Berglund, L.J.; Amadio, P.C.; Moran, S.L.; Steinmann, S.P.; An, K.N.; et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials 2019, 192, 189–198. [Google Scholar] [CrossRef]
- Chen, P.; Cui, L.; Fu, S.C.; Shen, L.; Zhang, W.; You, T.; Ong, T.Y.; Liu, Y.; Yung, S.H.; Jiang, C. The 3D-Printed PLGA Scaffolds Loaded with Bone Marrow-Derived Mesenchymal Stem Cells Augment the Healing of Rotator Cuff Repair in the Rabbits. Cell Transplant. 2020, 29, 963689720973647. [Google Scholar] [CrossRef]
- Huang, Y.; He, B.; Wang, L.; Yuan, B.; Shu, H.; Zhang, F.; Sun, L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res. Ther. 2020, 11, 496. [Google Scholar] [CrossRef]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J. Pain. Res. 2015, 8, 269–276. [Google Scholar] [CrossRef]
- Centeno, C.; Fausel, Z.; Stemper, I.; Azuike, U.; Dodson, E. A Randomized Controlled Trial of the Treatment of Rotator Cuff Tears with Bone Marrow Concentrate and Platelet Products Compared to Exercise Therapy: A Midterm Analysis. Stem Cells Int. 2020, 2020, 5962354. [Google Scholar] [CrossRef]
- Cherian, C.; Malanga, G.A.; Hogaboom, N.; Pollack, M.A.; Dyson-Hudson, T.A. Autologous, micro-fragmented adipose tissue as a treatment for chronic shoulder pain in a wheelchair using individual with spinal cord injury: A case report. Spinal Cord. Ser. Cases 2019, 5, 46. [Google Scholar] [CrossRef]
- Chun, S.W.; Kim, W.; Lee, S.Y.; Lim, C.Y.; Kim, K.; Kim, J.G.; Park, C.H.; Hong, S.H.; Yoo, H.J.; Chung, S.G. A randomized controlled trial of stem cell injection for tendon tear. Sci. Rep. 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.J.; Kaiser, J.T.; Wagner, K.R.; Sivasundaram, L.; Otte, R.S.; Tauro, T.M.; White, G.M.; Ralls, M.L.; Yanke, A.B.; Forsythe, B.; et al. Prospective Randomized Trial of Biologic Augmentation with Bone Marrow Aspirate Concentrate in Patients Undergoing Arthroscopic Rotator Cuff Repair. Am. J. Sports Med. 2023, 51, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Ellera Gomes, J.L.; da Silva, R.C.; Silla, L.M.; Abreu, M.R.; Pellanda, R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.L.; Dodson, A.; Martin, J. Microfragmented adipose tissue in the treatment of a full-thickness supraspinatus tear: A case report. Regen. Med. 2023, 18, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Flouzat Lachaniette, C.H.; Delambre, J.; Zilber, S.; Duffiet, P.; Chevallier, N.; Rouard, H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int. Orthop. 2014, 38, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Hogaboom, N.; Malanga, G.; Cherian, C.; Dyson-Hudson, T. A pilot study to evaluate micro-fragmented adipose tissue injection under ultrasound guidance for the treatment of refractory rotator cuff disease in wheelchair users with spinal cord injury. J. Spinal Cord. Med. 2021, 44, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Hurd, J.L.; Facile, T.R.; Weiss, J.; Hayes, M.; Hayes, M.; Furia, J.P.; Maffulli, N.; Winnier, G.E.; Alt, C.; Schmitz, C.; et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: A prospective, randomized, controlled first-in-human pilot study. J. Orthop. Surg. Res. 2020, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Kim, P.S.; Yoon, J.Y.; Yoon, K.S. Intratendinous Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: A First-In-Human Trial. Stem Cells 2018, 36, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Yoon, K.S. Intratendinous Injection of Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: A 2-Year Follow-Up Study. Arthroscopy 2020, 36, 971–980. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, D.H.; Park, J.W.; Park, S.; Kim, S.J. Effect of Bone Marrow Aspirate Concentrate-Platelet-Rich Plasma on Tendon-Derived Stem Cells and Rotator Cuff Tendon Tear. Cell Transplant. 2017, 26, 867–878. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, E.K.; Kim, S.J.; Song, D.H. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J. Orthop. Surg. Res. 2018, 13, 1. [Google Scholar] [CrossRef]
- Marathe, A.; Song, B.; Jayaram, P. Microfragmented Adipose Tissue With Adjuvant Platelet-Rich Plasma Combination Therapy for Partial-Thickness Supraspinatus Tear. Cureus 2021, 13, e15583. [Google Scholar] [CrossRef] [PubMed]
- Striano, R.D.; Malanga, G.A.; Bilbool, N.; Azatullah, K. Refractory Shoulder Pain with Osteoarthritis, and Rotator Cuff Tear, Treated with Micro-Fragmented Adipose Tissue. Orthop. Spine Sports Med. 2018, 2, 014. [Google Scholar]
- Randelli, P.S.; Cucchi, D.; Fossati, C.; Boerci, L.; Nocerino, E.; Ambrogi, F.; Menon, A. Arthroscopic Rotator Cuff Repair Augmentation With Autologous Microfragmented Lipoaspirate Tissue Is Safe and Effectively Improves Short-term Clinical and Functional Results: A Prospective Randomized Controlled Trial With 24-Month Follow-up. Am. J. Sports Med. 2022, 50, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Lugano, G.; Perucca Orfei, C.; Menon, A.; Ragni, E.; Colombini, A.; De Luca, P.; Randelli, P.; de Girolamo, L. Autologous microfragmented adipose tissue reduces inflammatory and catabolic markers in supraspinatus tendon cells derived from patients affected by rotator cuff tears. Int. Orthop. 2021, 45, 419–426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).