Abstract
The primary objective of this paper is to delineate and elucidate the contemporary advancements, developments, and prevailing trajectories concerning intrastent restenosis (ISR). We aim to provide a thorough overview of the most recent developments in this area, covering various aspects such as pathophysiological insights, therapeutic approaches, and new strategies for tackling the complex challenges of ISR in modern clinical settings. The authors have undertaken a study to address a relatively new medical challenge, recognizing its significant impact on the morbidity and mortality of individuals with cardiovascular diseases. This effort is driven by the need to fully understand, analyze, and possibly improve the outcomes of this emerging medical issue within the cardiovascular disease field. We acknowledge its considerable clinical implications and the necessity for innovative methods to mitigate its effects on patient outcomes. Therefore, our emphasis was directed towards elucidating the principal facets of the condition’s prevalence, expounding upon the foundational mechanisms underscoring conspicuous restenosis, and delineating the risk factors relevant in shaping the contemporary landscape of diagnostic and therapeutic modalities. This thorough examination aims to provide a comprehensive understanding of the various dimensions of the condition, including epidemiological data, pathophysiological complexities, and clinical considerations critical for evaluating and enhancing current diagnostic and treatment approaches.
1. Introduction
Restenosis refers to the narrowing of a blood vessel’s diameter following an angioplasty procedure [1]. Intrastent restenosis (ISR) is a challenging medical problem [2]. A meta-analysis showed that percutaneous coronary intervention (PCI) for ISR is associated with a higher incidence of adverse cardiac events than PCI for de novo lesions. This happens especially because of a higher incidence of risk-adjusted major adverse cardiac events compared with PCI for de novo lesions at a median of ≈30 months [3].
Although it is a lesser encountered problem than before due to the use of drug-eluting stents, it continues to play a major role in modern medical practice because of extensive stents being implanted in current practice. The 2020 National Cardiovascular Data Registry, including 5,100,394 patients, shows that reinterventions for ISR were necessary for 542,112 patients, representing 10.6 percent of all the PCIs [4].
It is essential to know that ISR incidence depends on a series of variables such as the stent type, patient population, cardiovascular risk factors, medication used, and follow-up duration [5]. When it comes to the stents used, it was reported that the incidence of ISR ranged from 5 to 30 percent in patients with bare-metal stents (BMSs) [6]. Introducing drug-eluting stents (DESs) has significantly reduced the ISR rates to 2 to 10 percent [7]. Despite these results, due to millions of DESs used for treating cardiovascular problems, it can be said that ISR is a public health problem worldwide [8].
In this context, this review aims to underscore the primary challenges engendered by ISR, recognizing its consequential influence on individuals with a prior history of coronary angioplasty. The paramount objective is to traverse the pathophysiological intricacies inherent to restenosis, thereby seeking a comprehensive understanding of the underlying issues. In doing so, we aim to navigate through diverse diagnostic modalities and therapeutic interventions, propounding a nuanced exploration of approaches to mitigate the adverse impact of restenosis in individuals who have undergone coronary angioplasty procedures.
2. ISR—Definition, Incidence, and Pathophysiology
ISR is characterized by a progressive luminal constriction within the stent, predominantly manifesting within the timeframe of 3 to 12 months subsequent to stent angioplasty [9]. From a clinical perspective, this phenomenon manifests as recurrent angina or heart failure [10]. It is noteworthy that it may also manifest as acute myocardial infarction in approximately 10% of afflicted patients [7]. In contrast, intrastent thrombosis constitutes an acute thrombotic occlusion, representing a dire event with the potential for precipitating sudden cardiac death or protracted acute myocardial infarction [11]. Despite the early revascularization, the mortality at 6 months is very high in this case [9].
The incidence of ISR is contingent upon various factors, including the stent type, the intricacy of the stented lesions, and the presence of risk factors [5]. Significantly elevated restenosis rates are observed in instances characterized by lesion complexity, such as those involving small vessels, extended lesions, or bifurcation lesions [12]. Determining the incidence of ISR is challenging due to its dependence on a multifactorial and variable interplay of factors. During the period in which balloon angioplasties were used, restenosis rates ranged from 32% to 55% among all procedures, subsequently declining to a range of 17% to 41% [13,14,15,16] with the advent of bare-metal stents [17,18,19]. A pivotal achievement in addressing this complication was the adoption of DESs, leading to a reduction in restenosis rates to levels below 10% [20,21]. ISR seems to manifest more frequently in patients suffering from multivessel disease compared to those affected solely by single-vessel disease [22].
ISR refers to a condition characterized by a significant reduction, typically equal to or exceeding 50%, in the diameter of the coronary lumen [23].
The advent of coronary stents has substantively transformed the therapeutic landscape for individuals afflicted by both chronic coronary syndromes and acute coronary syndromes [24,25]. Nevertheless, the widespread adoption of percutaneous revascularization procedures has ushered in a novel pathological phenomenon: ISR. The pathophysiology of ISR is a complex process involving many cellular mechanisms and depends on a series of risk factors [26].
It is characterized by the re-narrowing of a coronary artery at the site of a previously implanted stent [27]. The predominant mechanism underlying ISR is characterized by tissue proliferation, commonly referred to as neointimal hyperplasia [28]. This process reflects an exaggerated homeostatic healing response triggered by the arterial wall injury incurred during stent implantation, as the literature delineates [29]. The causative factors implicated in this phenomenon encompass localized inflammation arising from mechanical injury to the intimal and medial layers. This subsequently drives an aggressive neointimal hyperplasia marked by the proliferation of smooth muscle cells and the deposition of extracellular matrix [30,31]. Moreover, hypersensitivity reactions to the metallic components and polymer constituents intrinsic to early-generation DESs have been established as recognized mechanisms contributing to neointimal hyperplasia [32].
Coronary atherosclerotic disease is most commonly managed through the intervention of angioplasty with stent placement, a therapeutic modality that, while lifesaving, has been associated with adverse events that curtail its enduring efficacy [33]. Technical inadequacies, exemplified by factors such as stent malposition or inadequate expansion, resulting in the establishment of laminar blood flow patterns, subsequently promote the development of neointimal hyperplasia [34]. After angioplasty with a stent, a prevailing concern in long-term progression is the occurrence of intrastent neo-atherosclerosis [35]. Notably, the utilization of old-generation bare-metal stents has been constrained in adherence to contemporary medical practice guidelines due to a notable incidence of ISR, which has historically ranged from 17% to 41% [10]. While the introduction of new-generation DESs has substantially reduced the rate of such complications [20], DES-related failures persist as a matter of concern, affecting up to 10% of all implanted stents [20,21].
As we mentioned before, using BMSs leads to a high rate of restenosis that can reach 40%. The principal etiology of this complication is commonly attributed to the incremental expansion of the extracellular fibrotic matrix, resulting in a gradual reduction in the luminal diameter [20]. In the contemporary era marked by the utilization of DESs, the pathogenesis of ISR appears to be contingent upon several interrelated factors, including the host’s heightened material sensitivity, the suppression of healing processes due to antiproliferative medications, and the organism’s reaction to stent implantation [36,37] (Figure 1). The pathophysiology of ISR (Figure 2) appears to involve a complex cascade of events, including the hyperplasia of smooth muscle cells, the migration of pro-inflammatory cells, the recruitment of marrow progenitors, including bone-marrow-derived progenitor cells (BMPCs), and the proliferation of the extracellular matrix, as suggested by the existing literature [36,37]. Hence, the interplay between the systemic pro-inflammatory response and the concurrent local pro-inflammatory response induced by the endothelial disruption during stent implantation collectively orchestrates the vascular healing process [38]. This dynamic interaction culminates in forming a neointimal zone characterized by the proliferation of unregulated smooth muscle fibers, ultimately contributing to the clinical manifestation of restenosis [39]. At the local level, the injury induced by stent deployment initiates a multifaceted cascade of processes, commencing with endothelial disruption and subsequent exposure of the intimal layer, which imparts a prothrombotic effect [38]. This local inflammatory milieu subsequently triggers the release of cytokines and growth factors, activates platelets, and fosters the proliferation and migration of smooth muscle fibers. These alterations can culminate in two potential outcomes: vascular healing or pathological progression. The latter pathological process, in particular, may eventually predispose individuals to ISR. Endothelial activation, prompted by cellular injury induced by the mechanical impact of the stent, initiates a cascade of events, including platelet activation. Notably, platelets are often the primary cells to respond to stent placement [40,41].
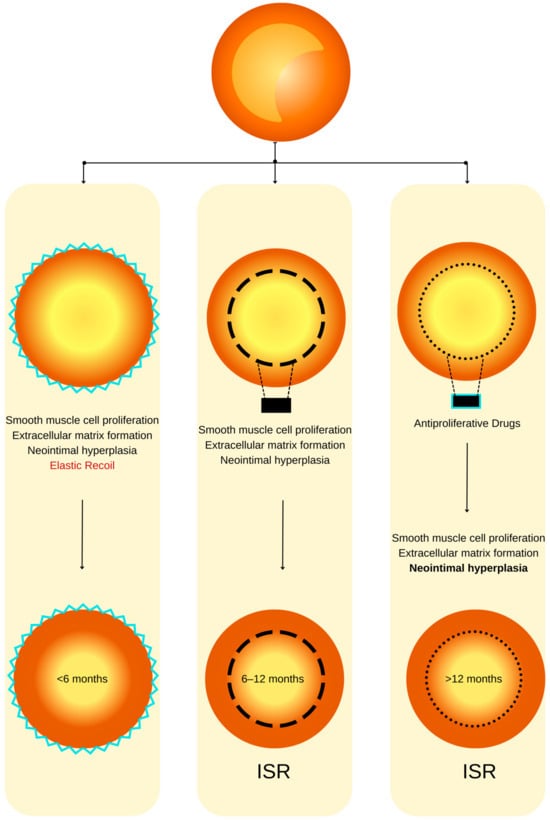
Figure 1.
ISR physiopathology (adapted from an open–access source [32]). (Left): Coronary restenosis after conventional balloon angioplasty primarily arises from the phenomenon of elastic recoil of the arterial vessel wall. Moreover, the trauma inflicted on the coronary artery triggers the initiation of smooth muscle cell proliferation, their migration, and the deposition of an extracellular matrix. This cascade of events culminates in the formation of neointimal hyperplasia, which ultimately contributes to the pathogenesis of restenosis. (Center): The deployment of a BMS is associated with a heightened level of vascular injury, thereby augmenting the magnitude of neointimal hyperplasia and elevating the risk of in-stent restenosis. (Right): DESs dispense antiproliferative agents, effectively mitigating the extent of neointimal hyperplasia and correspondingly diminishing the susceptibility to in-stent restenosis.
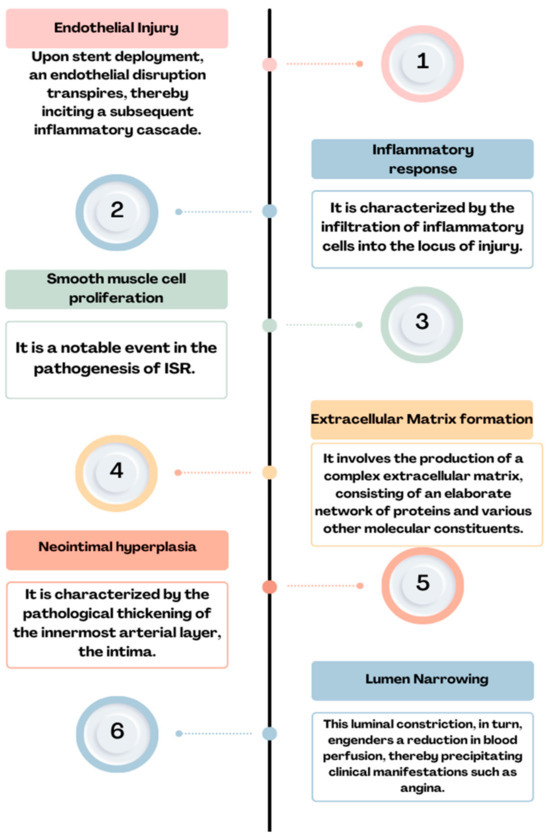
Figure 2.
Physiopathology of ISR: endothelial injury→ inflammatory response→ smooth muscle cell proliferation→ extracellular matrix formation→ neointimal hyperplasia→ lumen narrowing = ISR.
After arterial injury, platelets promptly adhere to the affected site and initiate the release of thromboxane A2. The glycoprotein (GP) IIb/IIIa complex engages with fibrinogen, facilitating platelet aggregation and activation [42]. Furthermore, activated platelets release many bioactive factors, among which the platelet-derived growth factor (PDGF) is prominent. PDGF exhibits both mitogenic and chemotactic properties, significantly influencing smooth muscle cells [43]. It contributes to the induction of oxidative stress, thereby facilitating the transition of smooth muscle cells from a contractile phenotype to a synthetic one [44]. The release of histamine from mast cells and platelets has been implicated in the pathogenesis of intimal hyperplasia [44]. This assertion supports studies involving porcine models of endothelial dysfunction, wherein a substantial 20- to 90-fold elevation in histamine concentration has been documented [45]. Interleukin-1, when released by platelets, triggers an upregulation in the production of interleukin-6 and interleukin-8, exerting pro-inflammatory effects. This cascade of events includes the stimulation of smooth muscle fiber migration, achieved through the activation of actin polymerization and the initiation of tyrosine phosphorylation at the cytoskeletal protein level, particularly in association with focal adhesion and smooth muscle fiber proliferation [38]. Extracellular vesicles emanating from platelets elicit a response in smooth muscle fibers, inducing the production of interleukin-6 while concurrently triggering the expression of αIIbβ3 and P-selectin. These cellular changes promote interactions between smooth muscle fibers and monocytes [46].
Furthermore, platelet-derived microvesicles have been demonstrated to induce endothelial protein C receptor (EPCR) proliferation characterized by the presence of von Willebrand factor (vWF+) and CD34+ markers. This effect is mediated through the release of transforming growth factor (TGF)-β1, as evidenced in a rat arterial injury model [47].
Within the vessel wall, the coexistence of an inflammatory response stemming from the presence of the foreign body, coupled with the anti-inflammatory effect exerted by the drug released from the stent, collectively engenders a deceleration in the process of reendothelialization. The exposure of adhesion molecules, such as P-selectin, serves as a stimulus for the recruitment of corresponding monocytes and the subsequent secretion of pro-inflammatory cytokines, namely interleukins 6 and 8. This cascade of events culminates in the infiltration of neutrophils, monocytes, and macrophages into the subendothelial space [38]. Elevated levels of monocytes and eosinophils at the three-month mark following PCI serve as predictive factors for late ISR after deploying a pharmacologically active stent [48]. While the precise involvement of mast cells in the pathophysiology of restenosis remains a subject of ongoing investigation, it is noteworthy that mast cells have been observed to release chymase [49], an enzyme implicated in generating angiotensin II and tumor growth factor-β. This consequential cascade of molecular events subsequently fosters fibroblast proliferation and is associated with the development of neointimal formation [50,51]. Experimental evidence in animal models has demonstrated the efficacy of chymase expression inhibitors in attenuating neointimal formation [52].
Neo-atherosclerosis represents a mechanistic phenomenon observed in the context of DES utilization, and its incidence is on the rise. This process is typified by the accretion of lipid-laden foamy macrophages, occasionally accompanied by a necrotic core predominantly localized within the stent deployment region [35].
To offer an at-a-glance perspective, Table 1 schematically shows the cells involved in ISR, as well as the mechanism by which they contribute.

Table 1.
Cell types and their role in ISR.
3. Risk Factors for ISR
3.1. Patient-Related Factors
The clinical prognosticators associated with in-stent restenosis (ISR) encompass a spectrum of factors, comprising, but not limited to, chronic kidney disease, elderly age, male gender, diabetes mellitus, and elevated body mass index, among various others [53]. Furthermore, established cardiovascular risk factors associated with atherosclerosis, such as hypertension, smoking, and dyslipidemia, have been evidenced to exert a pathogenetic influence on the development of neointimal hyperplasia [54].
3.2. Clinical Factors
ISR is a multifactorial phenomenon, subject to variability contingent upon individual patient characteristics, angiographic predictors, and the intricacies associated with the angioplasty procedure [55]. Notably, in the context of diabetic patients, it is imperative to underscore that the second generation of DESs exhibit a notable susceptibility to ISR, manifesting with an incidence rate of 8.7% [56]. In contrast, non-diabetic patients present a relatively diminished propensity for ISR, as evidenced by a prevalence of 5.7% [35].
Concerning gender-based prevalence, a study published in 2023 employed intracoronary imaging, specifically, optical coherence tomography (OCT), to demonstrate that the incidence of ISR exhibits a greater frequency among male patients [57]. Hence, the imaging data reveal a notable discrepancy, whereby men exhibit a significantly elevated risk in contrast to women, characterized by a higher incidence of thin-cap fibrous atherosclerosis (TCFA) (37.4% [n = 77] vs. 9.3% [n = 4], p < 0.001), as well as in-stent neo-atherosclerosis (ISNA) (82.0% [n = 169] vs. 62.8% [n = 27], p = 0.005).
Another study, conducted by Bajdechi et al. and published in 2023, explored a distinct patient cohort, specifically, individuals afflicted with Human Immunodeficiency Virus (HIV), revealing their heightened susceptibility to acute coronary events. This elevated risk can be attributed, in part, to the influence of antiviral medications and the concurrent discordant inflammatory response [58]. While patients afflicted with HIV exhibit an elevated risk of recurrent acute coronary syndrome, it is noteworthy that the stage of the disease does not exert a discernible influence on the prevalence of ISR [58].
Arterial hypertension, identified as an independent cardiovascular risk factor [59], was shown for the first time to be responsible for intrastent restenosis in a retrospective study involving 796 patients who underwent angiography due to angina recurrence or reversible myocardial ischemia [60]. The study revealed that effective blood pressure control during the initial PCI procedure correlated with a 24% lower risk of ISR. Additionally, factors such as total cholesterol levels, the use of beta-blockers or antiplatelet agents, and the site of stent implantation were linked to further reductions in ISR, particularly among patients with BMSs [60].
Moreover, it is well established that the cessation of antiplatelet medications ranks among the primary risk factors associated with intrastent restenosis. Consequently, non-adherence to pharmacological treatment regimens represents an additional significant risk factor for the development of ISR [61]. Additionally, individuals presenting with hypertension and a diagnosis of heart failure manifest an elevated susceptibility to ISR [62].
Furthermore, in a retrospective observational study, the incidence of restenosis in the coronary stent group was higher compared to the non-stent group when considering factors such as a family history of CHD, a history of type 2 diabetes, hypertension, smoking, drinking, withdrawal of aspirin, use of conventional doses of statins, calcified lesions, having ≥3 implanted stents, stent length ≥30 mm, and stent diameter <3 mm [63].
3.3. Angiographic Factors
As evidenced by the existing literature, the deployment of a stent in a vessel elicits a localized inflammatory response. This inflammatory cascade constitutes a pivotal component in the genesis of a pathological phenomenon recognized as ISR [64]. Furthermore, the intricacies of this multifaceted phenomenon extend beyond the mere presence of a stent, encompassing an array of stent-dependent, intra-stent, and extra-stent risk factors. The schematic delineation portrayed in Figure 3 illustrates the angiographic patterns under discussion. It is imperative to underscore that this classification holds pivotal significance in prognosis and expeditious patient triage for both clinical and investigative objectives [65].
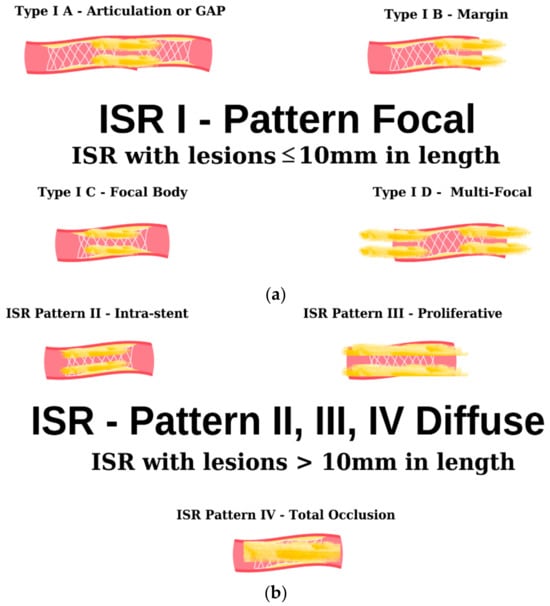
Figure 3.
Angiographic classification of intrastent restenosis (adapted from an open-access source [65]). (a) Type I: there are four types of focal ISR described in the image above. (b) Type II: the observed lesions are confined strictly to the confines of the stent and do not exhibit any extension beyond its proximal or distal extremities. Type III: Diffuse, proliferative ISR. The identified lesions manifest a length exceeding 10 mm, exhibiting an extension that surpasses the boundaries of the stent at both its proximal and distal margins. Type IV: total occlusion of the stent resulting in no coronary perfusion.
In a retrospective study [66], the utilization of BMSs was associated with an ISR rate of 30%. Subsequently, with the advent of second-generation DESs, this proportion exhibited a notable reduction, declining to 12%. Consequently, the use of BMSs has been identified as a risk factor for ISR, thereby underscoring the rationale behind its diminishing prevalence in contemporary clinical practice in favor of the more favored DESs.
4. Clinical Presentation and Diagnosis
In individuals with a prior history of angioplasty involving stent placement, the reappearance of anginal symptoms can stem from several potential factors, including incomplete revascularization (signifying the presence of unresolved lesions), the advancement of underlying atherosclerotic disease, or the occurrence of ISR [67]. Diagnosis is typically confirmed through coronary angiography, and treatment often entails concurrent intervention to address the identified lesions during the same procedure. Although coronary angiography remains the gold standard for diagnosing intrastent restenosis, new data suggest the useful application of coronary CT angiography for diagnosing this pathology. A study published in 2024, which included 102 patients with symptoms raising suspicion of intrastent restenosis, demonstrated that in a large proportion of cases (86.3% of the patients), the CT results, when compared with coronary angiography, were able to exclude ISR (false-negative result) [68]. Considering this finding, we anticipate that in the future, CT scans will additionally assist in the early diagnosis of ISR, facilitating prompt treatment according to current standards.
A notable proportion of patients with bare-metal stents may manifest acute coronary syndrome, with prevalence rates varying from 3% to 20% [69,70]. Furthermore, in two relatively limited-scale investigations, individuals with ISR following the deployment of DESs exhibited clinical symptoms, including unstable angina, in a range between 27% and 50%, and a subset of patients, approximately 5% to 11%, experienced acute myocardial infarction [4,71,72].
Intravascular Imaging
ISR manifests through a complex interplay of diverse mechanical and biological elements, encompassing drug resistance and hypersensitivity reactions to drug polymers [10]. This challenge’s intricate nature necessitates identifying and modifying underlying causative factors. Employing intravascular imaging methods, such as optical coherence tomography (OCT) (Figure 4), or intravascular ultrasound (IVUS), offers a promising avenue for delineating mechanistic intricacies and potentially mitigating the condition [73]. These imaging modalities have notably contributed to elucidating the correlation between specific lesion characteristics and the predisposition to ISR [74]. Nonetheless, the current body of evidence remains insufficient to substantiate the efficacy of intravascular imaging in improving clinical outcomes or forestalling recurrent episodes of ISR [73].
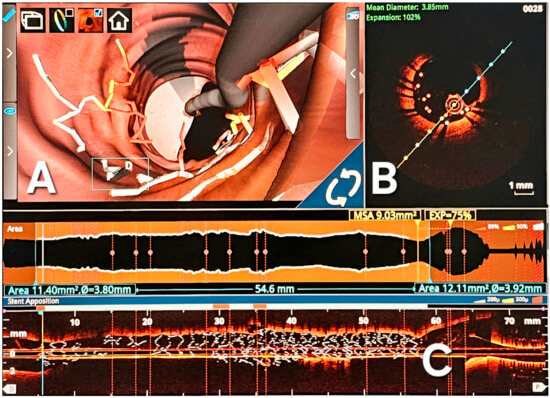
Figure 4.
OCT images used for optimal stent optimization. (A) Comprehensive three-dimensional intravascular reconstruction, vividly displaying instances of strut malposition; (B) Transversal section delineating the specifics of strut malposition, quantifying it at a length of 0.6 mm. This sectional view augments the understanding of the spatial irregularities within the stent deployment. (C) Longitudinal 3D reconstruction highlighting the stent along its entire length; significant malposition is observed at the distal end of the stent; D-Longitudinal reconstruction of the stent.
5. Clinical Outcomes of ISR
Technological progress, specifically, the advancements in percutaneous coronary interventions, has substantially improved clinical outcomes for acute and chronic coronary syndromes [75]. Presently, the use of new-generation DESs should be considered the gold standard in the treatment of these patient types, regardless of clinical presentation, lesion type, or patient comorbidities [76].
However, the limitations of DESs are related to local inflammatory reactions and late thrombosis [77]. Consequently, alternatives to stent usage have been explored. This has led to the development of Drug-Eluting Balloons (DEBs), which are pharmacologically active and serve both to restore the luminal diameter of the vessel and to deliver drugs without implanting a metal layer in the endothelium [78].
DEBs have emerged as a treatment option for small vessels [79], bifurcations [80], and, recently, ISR [81]. Extensive clinical research has been conducted to demonstrate the utility of DEBs, especially in cases of ISR (Table 2).

Table 2.
Clinical trials focused on DEBs.
When it comes to the chemotherapy used, the coating of balloons with Sirolimus [82] and Paclitaxel [85,86] has been of interest; these have also been compared in clinical studies, with both demonstrating effectiveness [83].
Data suggest the utility of using Paclitaxel-coated balloons in reducing the incidence of restenosis. These findings indicate that local chemotherapy treatment does not necessitate the additional implantation of DESs. The treatment of ISR with Paclitaxel is safe and lowers the rate of needing re-intervention for ISR [87].
Although treating ISR with pharmacologically active balloons has shown satisfactory results in preliminary studies, a recent study highlighted that the treatment of ISR with DEBs had a higher risk of recurrence and major adverse cardiac events (MACEs), compared to patients treated with DESs [88].
Although the treatment of ISR with DEBs appears to have higher rates of MACE compared to DESs, after adjusting for variables, the outcome is similar regarding both strategies [88]. Therefore, using DEBs for ISR can be a supported choice, although further studies are needed to validate the types of lesions for which DEBs are best suited.
6. Treatment
Fundamentally, the therapeutic approach to ISR aligns closely with managing stenosis in native coronary arteries. However, it is crucial to emphasize that the presence of an additional stent layer requires increased vigilance and careful consideration during the treatment process [89].
In response to the significance of this issue, a compendium of therapeutic modalities has been contemplated and devised. In comprehensive meta-analyses comparing various treatment modalities, two specific approaches have consistently emerged as superior: the utilization of DESs and drug-coated balloons (DCBs) [90,91]. These two therapeutic options align with the prevailing recommendations outlined in contemporary clinical guidelines [92].
Given the discernible reduction in restenosis rates attributed to the implementation of DESs, these stent types are notably favored in contemporary clinical practice for managing de novo coronary lesions [93]. Furthermore, many meta-analyses consistently position DESs as the primary choice for managing ISR [4,70] contrasting them with alternative interventions such as Paclitaxel-coated balloons [94], intravascular brachytherapy [95,96,97], and conventional balloon angioplasty [98,99,100].
The principal drawback associated with DESs pertains to the enduring presence of a non-absorbable stent scaffold within the vessel. This characteristic can give rise to notable therapeutic complexities, particularly when confronted with a recurrence of ISR. Pharmacologically active balloons are constructed by affixing an active agent onto the surface of a conventional angioplasty balloon [101]. The composition of the balloon coating typically comprises two key constituents: an active lipophilic drug and an excipient, the latter serving to enhance drug solubility and promote its transfer from the balloon surface to the vessel wall [97].
Pharmacologically active balloons offer antiproliferative advantages without necessitating the addition of an extra stent layer, rendering them an appealing alternative for comparison with DESs. Their utility is particularly pertinent in circumstances where the incorporation of an extra stent layer is undesirable, such as in the context of bifurcation lesions or cases involving the presence of multiple stent layers. Moreover, patients who have undergone angioplasty with a pharmacologically active balloon exhibit a differential need for antiplatelet regimens, making them a favored choice, especially in patient populations characterized by an elevated risk of bleeding [81].
Numerous investigations have undertaken comparative assessments between DESs and Paclitaxel-coated drug-coated balloons in treating ISR [102,103,104,105,106,107,108]. Despite the conceptual merits attributed to DCBs, a comprehensive meta-analysis has indicated that the recurrent deployment of DESs for ISR management proves to be more efficacious in reducing target lesion revascularization (TLR) rates at the three-year mark as compared to DCBs [109]. Furthermore, the application of DESs yields superior outcomes concerning the angiographic assessment of residual stenosis. In most comparisons between drug-coated balloons and DESs, these advantages in terms of the angiographic results persist through extended long-term follow-up periods. In individuals experiencing restenosis with bare-metal stents, the available evidence suggests a substantial degree of comparability in both the clinical efficacy and safety profiles between the utilization of DESs and drug-coated balloons [94]. In light of this consideration, the preferred approach in such circumstances involves the utilization of drug-coated balloons, primarily due to the absence of an additional strut layer.
Conversely, in the more complex scenario of ISR in patients with DESs, the deployment of DESs for treatment demonstrates a modestly greater efficacy when compared to the use of DCBs, particularly concerning the requirement for target lesion revascularization [106]. Nevertheless, this increased efficacy necessitates a thorough evaluation of the potential implications of implanting an additional stent layer [89].
Hence, the deployment of either pharmacologically active stents or pharmacologically active balloons warrants consideration in the context of ISR. The selection between these two techniques necessitates careful evaluation based on patient-specific characteristics, the nature of the lesion, underlying risk factors, and the particular stent type involved in the stenosis.
Importantly, to reduce the incidence of ISR, it is advisable to implement a series of strategies. These include appropriate lesion preparation and the utilization of intracoronary imaging to ensure accurate stent apposition to the vessel walls, particularly in the case of complex lesions [110].
In addition to the interventional treatment of ISR, managing risk factors plays a crucial role [63]. Consequently, patients with a history of ISR or previous PCI for coronary artery disease require careful monitoring by their current healthcare provider. Pharmacological treatment should be meticulously guided according to the current medical guidelines [111]. Furthermore, lifestyle modifications and addressing modifiable risk factors are essential for the long-term outcome of these patients [111].
7. Conclusions
Despite concerted endeavors and notable advancements, ISR persists as a formidable challenge within the medical domain. The elucidation of novel therapeutic modalities mandates a comprehensive understanding of the intricacies inherent to restenosis, particularly the underlying pathophysiological mechanisms. The imperative lies in consolidating and coordinating the concerted efforts and specialized knowledge dispersed across the global medical community. Such systematic organization is requisite to facilitate the discernment and subsequent formulation of innovative solutions to ameliorate the persistence of restenosis.
Given the significant clinical implications marked by the escalated rates of morbidity and mortality among patients with a prior history of coronary angioplasty featuring stent implantation, research endeavors have primarily pivoted towards the prophylaxis of intrastent restenosis (ISR). Consequently, the advent of successive generations of stents heralded a notable reduction in ISR incidences, albeit its persistent manifestation. Hence, a pragmatic stance necessitates addressing this enduring challenge, propelling prospects toward adopting bioresorbable stents as a promising avenue for mitigation and potentially circumventing ISR’s deleterious consequences.
Author Contributions
I.-T.B., A.-G.N., A.S.-U. and E.A. participated in reviewing, writing, and revision. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the EEA Grants 2014-2021, under Project contract no. 33/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wassif, H.; Welt, F.G.P. Restenosis of Stented Coronary Arteries; SCAI Interventional Cardiology Board Review: Second Edition; StatPearls: Treasure Island, FL, USA, 2023; pp. 10–15. [Google Scholar]
- Alraies, M.C.; Darmoch, F.; Tummala, R.; Waksman, R. Diagnosis and management challenges of in-stent restenosis in coronary arteries. World J. Cardiol. 2017, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, A.; Dang, A.T.; Mahana, I.; Elzeneini, M.; Alonso, F.; Banerjee, S.; Kumbhani, D.J.; Elgendy, I.Y.; Mintz, G.S. Outcomes of Percutaneous Coronary Intervention for In-Stent Restenosis Versus De Novo Lesions: A Meta-Analysis. J. Am. Heart Assoc. 2023, 12, 29300. [Google Scholar] [CrossRef] [PubMed]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis after Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef]
- Alexandrescu, D.-M.; Mitu, O.; Costache, I.I.; Macovei, L.; Mitu, I.; Alexandrescu, A.; Arsenescu Georgescu, C. Risk factors associated with intra-stent restenosis after percutaneous coronary intervention. Exp. Ther. Med. 2021, 22, 1141. [Google Scholar] [CrossRef]
- Alfonso, F.; Jose Pérez-Vizcayno, M.; Cárdenas, A.; García del Blanco, B.; Seidelberger, B.; Iñiguez, A.; Gómez-Recio, M.; Masotti, M.; Teresa Velázquez, M.; Sanchís, J.; et al. A Randomized Comparison of Drug-Eluting Balloon Versus Everolimus-Eluting Stent in Patients with Bare-Metal Stent-In-Stent Restenosis The RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: Paclitaxel-eluting Balloon vs. Everolimus-eluting Stent). J. Am. Coll. Cardiol. 2014, 63, 1378–1386. [Google Scholar]
- Ullrich, H.; Olschewski, M.; Münzel, T.; Gori, T. Coronary In-Stent Restenosis: Predictors and Treatment. Dtsch. Arztebl. Int. 2021, 118, 637. [Google Scholar] [CrossRef]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef] [PubMed]
- Coronary Artery Stent Thrombosis: Clinical Presentation and Management—UpToDate. Available online: https://www.uptodate.com/contents/coronary-artery-stent-thrombosis-clinical-presentation-and-management/print (accessed on 25 November 2023).
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment. J. Thorac. Dis. 2016, 8, E1150. [Google Scholar] [CrossRef]
- Modi, K.; Soos, M.P.; Mahajan, K. Stent Thrombosis; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Li, M.; Hou, J.; Gu, X.; Weng, R.; Zhong, Z.; Liu, S. Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China. Eur. J. Med. Res. 2022, 27, 12. [Google Scholar] [CrossRef]
- Fishman, R.F.; Kuntz, R.E.; Carrozza, J.P.; Miller, M.J.; Senerchia, C.C.; Schnitt, S.J.; Diver, D.J.; Safian, R.D.; Baim, D.S. Long-term results of directional coronary atherectomy: Predictors of restenosis. J. Am. Coll. Cardiol. 1992, 20, 1101–1110. [Google Scholar] [CrossRef]
- Le Feuvre, C.; Bonan, R.; Lespérance, J.; Gosselin, G.; Joyal, M.; Crépeau, J. Predictive factors of restenosis after multivessel percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1994, 73, 840–844. [Google Scholar] [CrossRef]
- Bakhai, A.; Booth, J.; Delahunty, N.; Nugara, F.; Clayton, T.; McNeill, J.; Davies, S.W.; Cumberland, D.C.; Stables, R.H.; SV Stent Investigators. The SV stent study: A prospective, multicentre, angiographic evaluation of the BiodivYsio phosphorylcholine coated small vessel stent in small coronary vessels. Int. J. Cardiol. 2005, 102, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Valgimigli, M.; Biondi-Zoccai, G.G.L.; Abbate, A.; Garcia Garcia, H.M.; Anselmi, M.; Turri, M.; McFadden, E.P.; Vassanelli, C.; Serruys, P.W.; et al. Clinical effectiveness of bare-metal stenting compared with balloon angioplasty in total coronary occlusions: Insights from a systematic overview of randomized trials in light of the drug-eluting stent era. Am. Heart J. 2006, 151, 682–689. [Google Scholar] [CrossRef]
- Stettler, C.; Wandel, S.; Allemann, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.E.; Stone, G.W.; Leon, M.B.; de Lezo, J.S.; et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet 2007, 370, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Mehilli, J.; Pache, J.; Kaiser, C.; Valgimigli, M.; Kelbæk, H.; Menichelli, M.; Sabaté, M.; Suttorp, M.J.; Baumgart, D.; et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 2007, 356, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- García del Blanco, B.; Rumoroso Cuevas, J.R.; Hernández Hernández, F.; Trillo Nouche, R. Spanish cardiac catheterization and coronary intervention registry. 22nd official report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990–2012). Rev. Esp. Cardiol. (Engl. Ed.) 2013, 66, 894–904. [Google Scholar] [CrossRef]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Zhao, L.P.; Xu, W.T.; Wang, L.; Li, H.; Shao, C.L.; Gu, H.B.; Chan, S.P.; Xu, H.F.; Yang, X.J. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron. Artery Dis. 2015, 26, 5–10. [Google Scholar] [CrossRef]
- Chiastra, C.; Migliavacca, F. Modeling of Blood Flow in Stented Coronary Arteries. In Heat Transfer and Fluid Flow in Biological Processes; Academic Press: Cambridge, MA, USA, 2015; pp. 335–370. [Google Scholar]
- Bertolone, D.T.; Gallinoro, E.; Esposito, G.; Paolisso, P.; Bermpeis, K.; De Colle, C.; Fabbricatore, D.; Mileva, N.; Valeriano, C.; Munhoz, D.; et al. Contemporary Management of Stable Coronary Artery Disease. High Blood Press. Cardiovasc. Prev. 2022, 29, 207. [Google Scholar] [CrossRef]
- Pham, V.; Moroni, A.; Gall, E.; Benedetti, A.; Zivelonghi, C.; Picard, F. Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review. J. Clin. Med. 2023, 12, 2833. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of Drug-Eluting Stents A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review Circulation: Cardiovascular Interventions. Circ. Cardiovasc. Interv. 2019, 12, 7023. [Google Scholar] [CrossRef]
- Lee, M.S.; Banka, G. In-stent Restenosis. Interv. Cardiol. Clin. 2016, 5, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.A.; Jamil, R.T.; Siddiqui, W.J. Neointimal Hyperplasia; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 2010, 56, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.R.; Lally, C. An investigation of damage mechanisms in mechanobiological models of in-stent restenosis. J. Comput. Sci. 2018, 24, 132–142. [Google Scholar] [CrossRef]
- He, R.; Zhao, L.; Silberschmidt, V.V.; Liu, Y. Mechanistic evaluation of long-term in-stent restenosis based on models of tissue damage and growth. Biomech. Model Mechanobiol. 2020, 19, 1425–1446. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Buckley, L.; Durán, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzmán, L.A. Restenosis, Stent Thrombosis, and Bleeding Complications: Navigating between Scylla and Charybdis. J. Am. Coll. Cardiol. 2018, 71, 1676–1695. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–577. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.K.; Lian, S.S.; Zhong, L.; Collet, C.; Foin, N.; Ang, H.Y. Stent malapposition generates stent thrombosis: Insights from a thrombosis model. Int. J. Cardiol. 2022, 353, 43–45. [Google Scholar] [CrossRef]
- Nusca, A.; Viscusi, M.M.; Piccirillo, F.; De Filippis, A.; Nenna, A.; Spadaccio, C.; Nappi, F.; Chello, C.; Mangiacapra, F.; Grigioni, F.; et al. In Stent Neo-Atherosclerosis: Pathophysiology, Clinical Implications, Prevention, and Therapeutic Approaches. Life 2022, 12, 393. [Google Scholar] [CrossRef]
- Virmani, R.; Farb, A. Pathology of in-stent restenosis. Curr. Opin. Lipidol. 1999, 10, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Yutani, C.; Ishibashi-Ueda, H.; Suzuki, T.; Kojima, A. Histologic evidence of foreign body granulation tissue and de novo lesions in patients with coronary stent restenosis. Cardiology 1999, 92, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Clare, J.; Ganly, J.; Bursill, C.A.; Sumer, H.; Kingshott, P.; de Haan, J.B. The Mechanisms of Restenosis and Relevance to Next Generation Stent Design. Biomolecules 2022, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular Smooth Muscle Cell Proliferation in Restenosis. Circ. Cardiovasc. Interv. 2011, 4, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Sexton, T.; Smyth, S.S. Translational Implications of Platelets as Vascular First Responders. Circ. Res. 2018, 122, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Hytönen, J.; Leppänen, O.; Braesen, J.H.; Schunck, W.H.; Mueller, D.; Jung, F.; Mrowietz, C.; Jastroch, M.; Von Bergwelt-Baildon, M.; Kappert , K.; et al. Activation of Peroxisome Proliferator-Activated Receptor-δ as Novel Therapeutic Strategy to Prevent In-Stent Restenosis and Stent Thrombosis. Arter. Thromb. Vasc. Biol. 2016, 36, 1534–1548. [Google Scholar] [CrossRef]
- Reinthaler, M.; Jung, F.; Landmesser, U.; Lendlein, A. Trend to move from permanent metals to degradable, multifunctional polymer or metallic implants in the example of coronary stents. Expert. Rev. Med. Devices 2016, 13, 1001–1003. [Google Scholar] [CrossRef]
- Factors Inducing In-Stent Restenosis: An In-Vitro Model|Request PDF. Available online: https://www.researchgate.net/publication/8246560_Factors_inducing_in-stent_restenosis_An_in-vitro_model (accessed on 25 November 2023).
- Jung, F.; Raghunath, M.; Blocki, A.; Gori, T. Restenosis after Coronary Stent Implantation: Cellular Mechanisms and Potential of Endothelial Progenitor Cells (A Short Guide for the Interventional Cardiologist). Cells 2022, 11, 2094. [Google Scholar]
- Fang, Y.I.; Namiki, H.; Tsunoda, E.; Shioda, S.; Shibata, M.; Nakatani, M.; Katagiri, T.; Takeyama, Y.; Ohata, H.; Honda, K.; et al. Marked increase in the histamine content of neointima after stent implantation of pig coronary artery and growth-promoting effects of histamine in cultured smooth muscle cells. Life Sci. 2005, 77, 241–251. [Google Scholar] [CrossRef]
- Vajen, T.; Benedikter, B.J.; Heinzmann, A.C.A.; Vasina, E.M.; Henskens, Y.; Parsons, M.; Maguire, P.B.; Stassen, F.R.; Heemskerk, J.W.M.; Schurgers, L.J.; et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell. Vesicles 2017, 6, 1322454. [Google Scholar] [CrossRef]
- Yan, J.; Bao, H.; Fan, Y.J.; Jiang, Z.L.; Qi, Y.X.; Han, Y. Platelet-derived microvesicles promote endothelial progenitor cell proliferation in intimal injury by delivering TGF-β1. FEBS J. 2020, 287, 5196–5217. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, H.; Lin, Z.; Fan, L.; Guo, Y.; Zhang, Y.; Chen, L. The Early Predictive Value of Circulating Monocytes and Eosinophils in Coronary DES Restenosis. Front. Cardiovasc. Med. 2022, 9, 764622. [Google Scholar] [CrossRef]
- Kennedy, S.; Wu, J.; Wadsworth, R.M.; Lawrence, C.E.; Maffia, P. Mast cells and vascular diseases. Pharmacol. Ther. 2013, 138, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Montone, R.A.; Sabato, V.; Crea, F. Role of Allergic Inflammatory Cells in Coronary Artery Disease. Circulation 2018, 138, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Miyazaki, M. Development and Application of Chymase Inhibitors: Effect of Chymase Inhibitor on Vascular Proliferation. Jpn. J. Pharmacol. 2002, 90, 223–227. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, B.; Wang, H.; Tao, Q.; Ge, S.; Zhai, Z. Tumor necrosis factor alpha-stimulated gene-6 (TSG-6) inhibits the inflammatory response by inhibiting the activation of P38 and JNK signaling pathway and decreases the restenosis of vein grafts in rats. Heart Vessel. 2017, 32, 1536–1545. [Google Scholar] [CrossRef]
- Pelliccia, F.; Zimarino, M.; Niccoli, G.; Morrone, D.; De Luca, G.; Miraldi, F.; De Caterina, R. In-stent restenosis after percutaneous coronary intervention: Emerging knowledge on biological pathways. Eur. Heart J. Open 2023, 3, oead083. [Google Scholar] [CrossRef]
- Klein, L.W.; Nathan, S.; Maehara, A.; Messenger, J.; Mintz, G.S.; Ali, Z.A.; Rymer, J.; Sandoval, Y.; Al-Azizi, K.; Mehran, R.; et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100971. [Google Scholar] [CrossRef]
- Ninno, F.; Tsui, J.; Balabani, S.; Díaz-Zuccarini, V. A systematic review of clinical and biomechanical engineering perspectives on the prediction of restenosis in coronary and peripheral arteries. JVS Vasc. Sci. 2023, 4, 100128. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Serruys, P.W.; Silber, S.; Kelbaek, H.; Richardt, G.; Morel, M.A.; Negoita, M.; Buszman, P.E.; Windecker, S. Comparison of zotarolimus- and everolimus-eluting coronary stents: Final 5-year report of the RESOLUTE all-comers trial. Circ. Cardiovasc. Interv. 2015, 8, e002230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Jiang, M.; Feng, H.; Han, Y.; Zhang, X.; Chen, Y.; Gao, L. The effect of sex differences on neointimal characteristics of in-stent restenosis in drug-eluting stents: An optical coherence tomography study. Heliyon 2023, 9, e19073. [Google Scholar] [CrossRef]
- Bajdechi, M.; Gurghean, A.; Bataila, V.; Scafa-Udriște, A.; Bajdechi, G.E.; Radoi, R.; Oprea, A.C.; Chioncel, V.; Mateescu, I.; Zekra, L.; et al. Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome. Diagnostics 2023, 13, 2682. [Google Scholar] [CrossRef]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109. [Google Scholar] [CrossRef]
- Tocci, G.; Barbato, E.; Coluccia, R.; Modestino, A.; Pagliaro, B.; Mastromarino, V.; Giovannelli, F.; Berni, A.; Volpe, M. Blood Pressure Levels at the Time of Percutaneous Coronary Revascularization and Risk of Coronary In-Stent Restenosis. Am. J. Hypertens. 2016, 29, 509. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, D.; Crisan, A.; Mitu, O.; Macovei, L.; Costache, I.I.; Ivona, M.I.; Frasinariu, O.; Alexandrescu, A.; Georgescu, C.A. Antiplatelet Therapy and Inflammatory Status Associated with Intra Stent Restenosis after Percutaneous Coronary Intervention. Med.-Surg. J. 2021, 125, 335–342. Available online: https://www.revmedchir.ro/index.php/revmedchir/article/view/2446 (accessed on 25 November 2023). [CrossRef]
- Singh, M.; Gersh, B.J.; McClelland, R.L.; Ho, K.K.L.; Willerson, J.T.; Penny, W.F.; Holmes, D.R. Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: Insights from the Prevention of Restenosis with Tranilast and Its Outcomes (PRESTO) trial. Circulation 2004, 109, 2727–2731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Zhao, K.; Bian, Y.J.; Liu, Y.; Xue, Y.T. Risk factors for in-stent restenosis after coronary stent implantation in patients with coronary artery disease: A retrospective observational study. Medicine 2022, 101, E31707. [Google Scholar] [CrossRef]
- Ochijewicz, D.; Tomaniak, M.; Opolski, G.; Kochman, J. Inflammation as a determinant of healing response after coronary stent implantation. Int. J. Cardiovasc. Imaging 2021, 37, 791. [Google Scholar] [CrossRef]
- Mehran, R.; Dangas, G.; Abizaid, A.S.; Mintz, G.S.; Lansky, A.J.; Satler, L.F.; Pichard, A.D.; Kent, K.M.; Stone, G.W.; Leon, M.B. Angiographic Patterns of In-Stent Restenosis. Circulation 1999, 100, 1872–1878. [Google Scholar] [CrossRef]
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.L.; Kastrati, A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014, 100, 153–159. [Google Scholar] [CrossRef]
- De Luca, L.; Rosano, G.M.C.; Spoletini, I. Post-percutaneous coronary intervention angina: From physiopathological mechanisms to individualized treatment. Cardiol. J. 2022, 29, 850. [Google Scholar] [CrossRef] [PubMed]
- Elwany, M.N.; Abskharoun, M.; Dawood, M.; Al-Tahan, S.M.; Sanhoury, M. The utility and effectiveness of the newer generation high-resolution coronary computed tomography angiography in the evaluation of coronary in-stent restenosis. Curr. Probl. Cardiol. 2024, 49, 102212. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; John, J.M.; Chew, D.P.; Lee, D.S.; Ellis, S.G.; Bhatt, D.L. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 2006, 151, 1260–1264. [Google Scholar] [CrossRef]
- Walters, D.L.; Harding, S.A.; Walsh, C.R.; Wong, P.; Pomerantsev, E.; Jang, I.K. Acute coronary syndrome is a common clinical presentation of in-stent restenosis. Am. J. Cardiol. 2002, 89, 491–494. [Google Scholar] [CrossRef]
- Mishkel, G.J.; Moore, A.L.; Markwell, S.; Shelton, M.C.; Shelton, M.E. Long-term outcomes after management of restenosis or thrombosis of drug-eluting stents. J. Am. Coll. Cardiol. 2007, 49, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, M.; Gori, T.; Pierli, C.; Casini, S.; Sinicropi, G.; Buti, A.; Iadanza, A.; Bravi, A. Symptomatic failure after sirolimus-eluting stent implantation: A rare but challenging condition. Can. J. Cardiol. 2007, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Abouelnour, A.; Gori, T. Intravascular imaging in coronary stent restenosis: Prevention, characterization, and management. Front. Cardiovasc. Med. 2022, 9, 843734. [Google Scholar] [CrossRef]
- Perera, D.; Postema, P.; Rashid, R.; Patel, S.; Blows, L.; Marber, M.; Redwood, S. Does a well developed collateral circulation predispose to restenosis after percutaneous coronary intervention? An intravascular ultrasound study. Heart 2006, 92, 763. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Mach, F.; Räber, L. Lipid-lowering therapy and percutaneous coronary interventions. EuroIntervention 2021, 16, 1389–1403. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Byrne, R.A.; Colleran, R.; Kastrati, A. Strengths and limitations of real world data in patients treated with coronary stents. Circ. Cardiovasc. Interv. 2018, 11, 7239. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Q.; Yang, H.; Jing, Z.; Ming, C. Clinical outcomes of drug-eluting balloon for treatment of small coronary artery in patients with acute myocardial infarction. Intern. Emerg. Med. 2021, 16, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Hildick-Smith, D.; Arunothayaraj, S.; Stankovic, G.; Chen, S.L. Percutaneous coronary intervention of bifurcation lesions. EuroIntervention 2022, 18, E273–E291. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Coughlan, J.J.; Giacoppo, D.; Kastrati, A.; Byrne, R.A. Management of in-stent restenosis. EuroIntervention 2022, 18, E103–E123. [Google Scholar] [CrossRef] [PubMed]
- Study Details|SELUTION SLRTM 014 In-Stent Restenosis|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04280029 (accessed on 20 January 2024).
- Study Details|Treatment of In-Stent Restenosis 2 Study|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03667313 (accessed on 20 January 2024).
- Study Details|Treatment of Coronary In-Stent Restenosis (ISR) by a Sirolimus Coated or a Paclitaxel Coated Balloon|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03242096 (accessed on 20 January 2024).
- Study Details|Treatment of In-Stent Restenosis by Paclitaxel Coated PTCA Balloons (PACCOCATH-ISR I)|ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/study/NCT00106587 (accessed on 28 January 2024).
- Study Details|Treatment of in-Stent Restenosis by Paclitaxel Coated PTCA Balloons (PACCOCATH-ISR II)|ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/study/NCT00409981 (accessed on 28 January 2024).
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; Böhm, M.; Speck, U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Kheifets, M.; Rahat, O.; Bental, T.; Levi, A.; Vaknin-Assa, H.; Greenberg, G.; Codner, P.; Witberg, G.; Kornowski, R.; Perl, L. Outcomes of Drug Eluting Balloons for In-Stent Restenosis: Large Cohort Analysis and Single Center Clinical Experience. Can. J. Cardiol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Byrne, R.A.; Rivero, F.; Kastrati, A. Current Treatment of In-Stent Restenosis. J. Am. Coll. Cardiol. 2014, 63, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, D.; Gargiulo, G.; Aruta, P.; Capranzano, P.; Tamburino, C.; Capodanno, D. Treatment strategies for coronary in-stent restenosis: Systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ 2015, 351, h5392. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Stefanini, G.G.; Mavridis, D.; Siontis, K.C.; Alfonso, F.; Pérez-Vizcayno, M.J.; Byrne, R.A.; Kastrati, A.; Meier, B.; Salanti, G.; et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: A network meta-analysis. Lancet 2015, 386, 655–664. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardiothoracic Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef]
- Cassese, S.; Byrne, R.A.; Schulz, S.; Hoppman, P.; Kreutzer, J.; Feuchtenberger, A.; Ibrahim, T.; Ott, I.; Fusaro, M.; Schunkert, H.; et al. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur. Heart J. 2015, 36, 94–99. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.P.M.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.Y.; Degenhardt, R.; Pleva, L.; et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: A comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur. Heart J. 2020, 41, 3715–3728. [Google Scholar] [CrossRef]
- Holmes, D.R.; Teirstein, P.; Satler, L.; Sketch, M.; O’Malley, J.; Popma, J.J.; Kuntz, R.E.; Fitzgerald, P.J.; Wang, H.; Caramanica, E.; et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: The SISR randomized trial. JAMA 2006, 295, 1264–1273. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; O’Shaughnessy, C.D.; Martin, S.L.; Satler, L.; McGarry, T.; Turco, M.A.; Kereiakes, D.J.; Kelley, L.; Popma, J.J.; et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: The TAXUS V ISR randomized trial. JAMA 2006, 295, 1253–1263. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, S.W.; Koo, B.K.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Hong, M.K.; Kim, J.J.; Mori, K.; et al. Treatment of diffuse IN-stent restenosis with Drug-Eluting stents vs. intracoronary bEta-raDiation therapy: INDEED Study. Int. J. Cardiol. 2008, 131, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Mehilli, J.; Von Beckerath, N.; Dibra, A.; Hausleiter, J.; Pache, J.; Schühlen, H.; Schmitt, C.; Dirschinger, J.; Schömig, A. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: A randomized controlled trial. JAMA 2005, 293, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Pérez-Vizcayno, M.J.; Hernandez, R.; Bethencourt, A.; Martí, V.; López-Mínguez, J.R.; Angel, J.; Mantilla, R.; Morís, C.; Cequier, A.; et al. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: Results of the Restenosis Intrastent: Balloon Angioplasty versus Elective Sirolimus-Eluting Stenting (RIBS-II) trial. J. Am. Coll. Cardiol. 2006, 47, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, B.; Moulichon, R.; Teiger, E.; Brunel, P.; Metzger, J.P.; Pansieri, M.; Carrie, D.; Stoll, H.P.; Wittebols, K.; Spaulding, C.; et al. One-year results of the CRISTAL Trial, a randomized comparison of cypher sirolimus-eluting coronary stents versus balloon angioplasty for restenosis of drug-eluting stents. J. Interv. Cardiol. 2012, 25, 586–595. [Google Scholar] [CrossRef]
- Byrne, R.A.; Joner, M.; Alfonso, F.; Kastrati, A. Drug-coated balloon therapy in coronary and peripheral artery disease. Nat. Rev. Cardiol. 2014, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.W.; Dörr, O.; Wöhrle, J.; Krackhardt, F.; Ince, H.; Zeus, T.; Berland, J.; Piot, C.; Roubille, F.; Schult, I.; et al. A multicentre, randomised controlled clinical study of drug-coated balloons for the treatment of coronary in-stent restenosis. EuroIntervention 2020, 16, E328–E334. [Google Scholar] [CrossRef]
- Rittger, H.; Brachmann, J.; Sinha, A.M.; Waliszewski, M.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Breithardt, O.A.; et al. A Randomized, Multicenter, Single-Blinded Trial Comparing Paclitaxel-Coated Balloon Angioplasty With Plain Balloon Angioplasty in Drug-Eluting Stent Restenosis: The PEPCAD-DES Study. J. Am. Coll. Cardiol. 2012, 59, 1377–1382. [Google Scholar] [CrossRef]
- Habara, S.; Iwabuchi, M.; Inoue, N.; Nakamura, S.; Asano, R.; Nanto, S.; Hayashi, Y.; Shiode, N.; Saito, S.; Ikari, Y.; et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am. Heart J. 2013, 166, 527–533.e2. [Google Scholar] [CrossRef]
- Freisinger, E.; Koeppe, J.; Gerss, J.; Goerlich, D.; Malyar, N.M.; Marschall, U.; Faldum, A.; Reinecke, H. Mortality after use of paclitaxel-based devices in peripheral arteries: A real-world safety analysis. Eur. Heart J. 2020, 41, 3732–3739. [Google Scholar] [CrossRef]
- Verheye, S.; Vrolix, M.; Kumsars, I.; Erglis, A.; Sondore, D.; Agostoni, P.; Cornelis, K.; Janssens, L.; Maeng, M.; Slagboom, T.; et al. The SABRE Trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): Angiographic Results and 1-Year Clinical Outcomes. JACC Cardiovasc. Interv. 2017, 10, 2029–2037. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; di Palma, G.; Latini, R.A.; Elwany, M.; Orrego, P.S.; Seregni, R.G. Immediate and short-term performance of a novel sirolimus-coated balloon during complex percutaneous coronary interventions. The FAtebenefratelli SIrolimus COated-balloon (FASICO) registry. Cardiovasc. Revasc Med. 2017, 18, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Palop, R.; Pinar, E.; Lozano, Í.; Saura, D.; Picó, F.; Valdés, M. Utility of the fractional flow reserve in the evaluation of angiographically moderate in-stent restenosis. Eur. Heart J. 2004, 25, 2040–2047. [Google Scholar] [CrossRef]
- Waksman, R.; Chitturi, K.R. Myths and Truths in the Management of Drug-Eluting Stent In-Stent Restenosis. JACC Cardiovasc. Interv. 2024, 17, 14–16. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).