Abstract
This review explores recent advancements in gene therapy as a potential treatment for neurodegenerative diseases, focusing on intervention mechanisms, administration routes, and associated limitations. Following the PRISMA procedure guidelines, we systematically analyzed studies published since 2020 using the PICO framework to derive reliable conclusions. The efficacy of various gene therapies was evaluated for Parkinson’s disease (n = 12), spinal muscular atrophy (n = 8), Huntington’s disease (n = 3), Alzheimer’s disease (n = 3), and amyotrophic lateral sclerosis (n = 6). For each condition, we assessed the therapeutic approach, curative or disease-modifying potential, delivery methods, advantages, drawbacks, and side effects. Results indicate that gene therapies targeting specific genes are particularly effective in monogenic disorders, with promising clinical outcomes expected in the near future. In contrast, in polygenic diseases, therapies primarily aim to promote cell survival. A major challenge remains: the translation of animal model success to human clinical application. Additionally, while intracerebral delivery methods enhance therapeutic efficacy, they are highly invasive. Despite these hurdles, gene therapy represents a promising frontier in the treatment of neurodegenerative diseases, underscoring the need for continued research to refine and personalize treatments for each condition.
1. Introduction
Neurodegenerative diseases (NDDs) are a group of disorders affecting the nervous system, marked by their high prevalence and significant impact on patient health [1]. These diseases typically lack curative treatment and have a poor prognosis, leading to progressive disability and reduced life expectancy [2]. NDDs with a genetic basis can be broadly classified into two categories [3], described below.
1.1. Monogenic Diseases
Monogenic neurodegenerative diseases are caused by mutations in a single gene, leading to a clear, predictable inheritance pattern and providing a more straightforward target for gene therapy interventions. In contrast to polygenic diseases, where multiple genetic and environmental factors contribute to disease onset, monogenic disorders offer unique opportunities for therapeutic strategies that directly address the underlying genetic defect. Among the most studied monogenic neurodegenerative diseases are Huntington’s disease (HD) and spinal muscular atrophy (SMA), both of which have well-characterized genetic causes. These disorders were chosen as focal points for this review due to their devastating clinical impact, the availability of established animal models, and recent advances in gene therapies [4,5,6,7,8,9,10,11,12,13,14,15].
- -
- Huntington’s disease (HD) is a hereditary degenerative disorder characterized by the expansion of CAG triplet repeats (>36) in the first exon of the HTT gene located on chromosome 4q. The repetition of amino acid glutamine causes the production of an abnormal huntingtin (HTT) protein [6]. This protein aggregates causing the extensive degeneration of the cortex and basal ganglia [4,16], with the severity of symptoms correlating with the number of glutamine repeats [17].
- -
- Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease caused by a biallelic mutation of the neuronal survival gene SMN1 on chromosome 5q. It is the main cause of childhood mortality of genetic origin, due to a protein deficiency that results in the progressive degeneration of motor neurons, leading to significant weakness and muscle atrophy [1,18]. The severity of SMA depends on the number of functional SMN2 gene copies, which modulate the harmful effects of SMN1 deficiency, affecting the age of onset and prognosis [19,20,21].
1.2. Polygenic Diseases
Polygenic neurodegenerative diseases are driven by the complex interplay of multiple genes and environmental factors, making their underlying mechanisms more difficult to target with therapeutic interventions. Unlike monogenic disorders, polygenic diseases do not follow a simple inheritance pattern, and their genetic basis often involves variations in several genes, each contributing modestly to disease susceptibility. Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) are prime examples of polygenic neurodegenerative diseases, each with a multifactorial etiology involving both genetic predispositions and environmental influences. These disorders were chosen in this review for their high prevalence, significant societal impact, and the progress made in understanding their genetic contributions, despite the challenges posed by their polygenic nature. Recent gene therapy approaches in these diseases aim to modify broader biological processes, such as enhancing cell survival and reducing toxic protein accumulations, rather than targeting a single causative gene [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
- -
- Alzheimer’s disease (AD) is the leading cause of dementia [24]. Although the pathogenesis of the sporadic form is unknown, familial AD (<5% of cases) shows mutations in genes coding for amyloid-beta precursor protein (APP), presenilin 1 and 2 (PSEN1-2), and microtubule-associated protein Tau (MAPT). The accumulation of β-amyloid plaques and tangles of hyperphosphorylated tau is associated with memory loss and cognitive decline, with death typically occurring 5–12 years after diagnosis [43].
- -
- Parkinson’s disease (PD) is characterized by resting tremor, bradykinesia, and postural instability, along with cognitive impairment [33,44]. Motor dysfunction is produced by the degradation of dopaminergic neurons in the substantia nigra pars compacta [36,45,46]. PD can be sporadic (10–15%) or familial (85–90%). The average age of onset for PD is estimated to be around 60 years, though some patients experience significantly earlier onset. PD is linked to increased mortality and a reduced life expectancy compared to the general population, particularly when diagnosed before the age of 70 [47,48]. Alterations have been identified in the membrane-interacting gene α-synuclein (SNCA), [49,50]; in mitochondrial quality control genes, such as phosphatase and tensin homolog (PTEN) and leucine-rich repeat kinase 2 (LRRK2) [49,51]; in oxidative damage control genes, such as the gene that encodes the parkinsonism associated deglycase (PARK7); and the gene that encodes the lysosomal enzyme glucosylceramidase Beta 1 (GBA1) [52].
- -
- Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder affecting motor neurons, with both familial (20%) and sporadic (80%) forms [53]. In both, protein aggregation leads to neuronal degeneration, which leads to progressive paralysis and, often, death within 3–5 years [54]. ALS is frequently associated with the mutation of the superoxide dismutase 1 (SOD1) gene, causing the accumulation of abnormal SOD1 protein [54], and with the mutation of the TAR DNA-binding protein 43 (TDP-43) gene. The TDP-43 protein plays a crucial role in the regulation of RNA splicing, which is essential for the proper expression of genes. Additionally, the dysregulation of TDP-43 has been linked to alterations in neurotransmitter systems, particularly affecting gamma-aminobutyric acid (GABA), an important inhibitory neurotransmitter in the brain. These changes can disrupt neural signaling and may contribute to various NDDs [42,55].
Gene therapy is defined as the process of modifying the structure or expression of genetic material for the treatment of genetically based diseases caused by the mutation of one or a small number of genes [3]. This approach involves delivering therapeutic genetic material through various carriers, such as viral vectors [56], liposomes [57], nanoparticles [58], or exosomes [59]. These systems are designed to transfer genes or oligonucleotides efficiently, while aiming for minimal toxicity.
Ideally, gene therapy should be delivered in a single dose through a safe, minimally invasive route [14,60]. Effective gene therapy for NDDs requires a thorough understanding of disease-related metabolic pathways, the bioavailability of the therapeutic molecules [7,31,46], and the specific properties of neuronal circuits involved [43,50,51].
Gene therapy can be administered through two primary approaches: (1) ex vivo, where genetically modified cells are reintroduced into the patient, and (2) in vivo, where genes or oligonucleotides are delivered directly through naked DNA sequences, nonviral particles, or plasmid or viral vectors [61]. Ex vivo techniques typically involve the use of human pluripotent cells (PSCs), mesenchymal stem cells (MSCs), or hematopoietic stem cells (HSCs) [62]. In vivo techniques include gene addition strategies and genome editing methods, such us CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats associated protein 9), as well as mRNA modifications using antisense oligonucleotides (ASOs) or small-interfering ribonucleic acid (siRNA) for gene silencing. However, these RNA-based therapies have shown limited effectiveness in treating neurological disorders due to depot effects, where over 99% of therapeutic RNA becomes trapped in endosomes. This entrapment significantly hinders the delivery of RNA to target sites. Efforts are underway to develop slow-release RNA therapies capable of sustaining long-term responses, such as those required for PD. These advancements aim to enhance endosomal escape by freeing up 50% or more of the RNA therapeutics, enabling a more rapid and efficient delivery to target cells [63].
Vectors can be packaged into viral and nonviral vehicles. Lentiviruses (LVs) are RNA viruses that have a reverse transcriptase and have been used in preclinical research [34] and clinical trials for NDDs [64]. LVs efficiently target a wide range of tissues and cell types, including the central nervous system. Their ability to stably integrate transgenes into the host genome supports sustained transgene expression. LVs are particularly advantageous for ex vivo gene correction, as these viruses have evolved to preferentially transduce human cells, providing some protection from the immune system. However, the risk of insertional mutagenesis during clinical applications remains a significant concern, and in vivo gene transfer with LVs faces challenges due to immune-mediated rejection [65,66]. In contrast, AAVs and their engineered capsid variants are extensively utilized in clinical settings [8,9,10,12,13,14,67]. AAVs, which belong to the Parvoviridae family, exhibit low oncogenicity and immunogenicity [1,68]. They offer high and stable transduction efficiencies, particularly in fully differentiated neural cells with minimal turnover, and generally present manageable side effects. Among AAVs, AAV9 is particularly favored for NDDs due to its ability to cross the blood–brain barrier (BBB) [16,69]. Nonviral vectors, typically nanoparticles, are used to deliver small genetic sequences and, more rarely, DNA plasmids, which are employed less frequently due to size constraints. These nanoparticles can have organic (e.g., lipid-based, liposomal, or polymeric) or inorganic structures, offering a high degree of design flexibility for targeted tissue delivery [70]. In the context of NDDs, nanoparticles are often administered intracranially, as they generally cannot cross the BBB. However, innovative approaches are emerging that may allow these vectors to bypass this barrier. Despite these advances, the lack of extensive clinical trials leaves unresolved questions regarding their safety and potential side effects [71].
The route administration for gene therapy is determined by several factors, including the desired systemic or localized effect, the specific target cells, the accessibility of these cells (e.g., overcoming barriers, such as the BBB or blood–nerve barrier), the titer and yield of the therapeutic product, and the potential side effects associated with high doses, such as inflammation caused by the administration method. This is crucial for determining the effectiveness and safety of the treatment. The advantages and disadvantages of each route of administration have been discussed in many publications [15,34,60]. Direct intracerebral delivery is preferred for localized effects [32,35,45]. Although it is highly invasive, it is used for the administration of vectors that do not cross the BBB or when high concentrations are needed. On the other hand, intravenous, intra-arterial, or intrathecal routes are less invasive but present challenges related to vector delivery into the central nervous system, as they must be able to cross the BBB [20]. The drawback of those routes of administration is the presence of immune reactions and toxicity [10,13,72]. Currently, the intravenous route has replaced the intra-arterial route. The intrathecal route consists of administering treatment in the subarachnoid space along the neuraxial axis and does not require crossing the BBB. Intracerebroventricular administration consists of direct drug delivery into the ventricles of the brain. This route seems to show the best concentration/invasiveness ratio, achieving a higher effect with lower doses; although, it can cause neurotoxicity [14]. Emerging delivery methods, such as the intranasal [73] and the intrauterine route [15], are under development to improve accessibility and reduce invasiveness.
Current treatments for NDDs primarily focus on symptom management and slowing disease progression [73], but recent advancements in gene therapy have shown promising potential in addressing the underlying genetic causes of these disorders, both in preclinical and early clinical trials [20]. However, the success of gene therapies is contingent upon several key factors, including the safety and efficacy of delivery vectors, the ability to achieve targeted gene expression, and the successful translation of preclinical results into human applications. This review aims to provide a comprehensive analysis of the current state of gene therapy techniques for the most prominent NDDs, including AD, ALS, HD, PD, and SMA. It evaluates their efficacy, safety profiles, and emerging research directions. The outcomes of this review will help identify the current challenges facing gene therapy, particularly the translation from animal models to clinical practice, and highlight future directions.
2. Methods
A systematic review was conducted to identify studies investigating gene therapy applications in NDDs, adhering to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [74]. The initial search was conducted on 15 June 2023, with an updated search on 15 December 2023, across multiple databases, including PubMed, Scopus, and Web of Science. The search targeted publications released between January 2020 and December 2023. Search strategies were adapted specifically for the Web of Science (WoS) database, focusing on the topic “gene therapy in neurodegenerative diseases”.
2.1. Search Strategy
To optimize search effectiveness and ensure relevant results, the PICO (patient, intervention, comparison, and outcome) framework was employed. Boolean operators OR (to combine free terms) and AND (to connect different search concepts) were utilized to refine the search. Keywords included combinations of terms associated with gene therapy and specific neurodegenerative diseases, such as “neurodegenerative diseases” OR “Alzheimer’s disease” OR “amyotrophic lateral sclerosis” OR “Huntington’s disease” OR “Parkinson’s disease” OR “spinal muscular atrophy” AND “gene therapy”.
The inclusion criteria for this review consisted of full-text, peer-reviewed articles published in English between January 2020 and December 2023. Eligible studies focused on gene therapy interventions for NDDs, including AD, ALS, HD, PD, and SMA. Studies were required to assess the therapeutic efficacy, safety, or clinical outcomes of gene therapies in either clinical or preclinical settings. Both clinical trials and descriptive studies were considered if they evaluated the impact of gene therapy on disease pathophysiology or progression.
Exclusion criteria included studies that were not peer-reviewed, not available in English, or not published in indexed academic journals. Additionally, studies were excluded if they focused on nongenetic therapeutic approaches or addressed diagnostic methods or administration routes without direct evaluation of gene therapy. Publications that did not match the search descriptors or that examined diseases outside the scope of interest were also excluded. Finally, studies published before 2020 were excluded from the review.
2.2. Selection Process and Critical Appraisal
The initial search yielded 1465 articles. After removing duplicates and screening titles and abstracts for relevance, 84 articles remained for full-text review. Based on the application of inclusion and exclusion criteria, 26 articles were excluded due to their primary focus on administration methods, diagnostic techniques, or unrelated clinical trials. Finally, 58 articles met the inclusion criteria and were critically appraised by two independent reviewers to ensure methodological quality and relevance. From this pool, 32 studies were selected, comprising preclinical and clinical trials (n = 28) and descriptive studies (n = 4), all of which focused on assessing the effectiveness of gene therapies in neurodegenerative diseases. A flow diagram illustrating the steps taken during the literature review process was created (Figure 1). Each selected study was evaluated independently by the researchers for methodological rigor, with particular attention to research design, statistical analysis, and the reported outcomes. Discrepancies between reviewers were resolved through discussion, ensuring consistency in the selection of high-quality studies for inclusion in the final review. A qualitative synthesis of the results was then prepared to inform the discussion section.
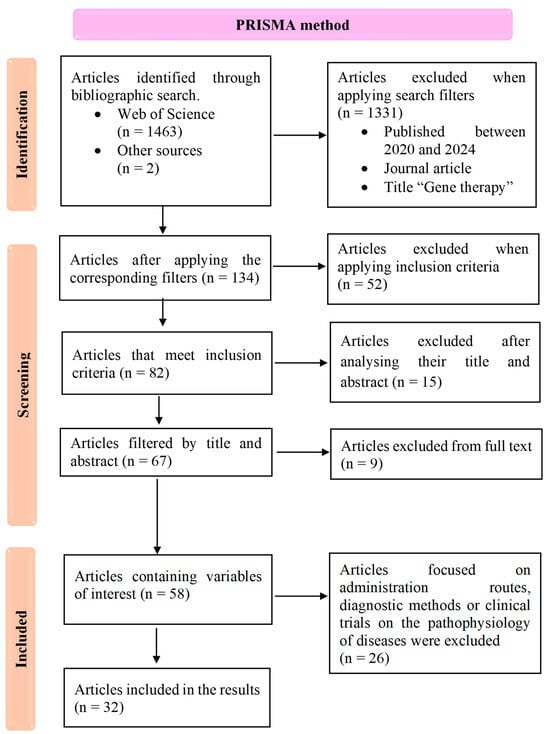
Figure 1.
Study selection flowchart following the PRISMA method, detailing the number of studies identified, included, and excluded at each stage, along with the applied exclusion criteria.
For clarity, summary tables were constructed for each disease studied. These tables presented the authors, year of publication, and the key characteristics of the selected studies, including methodology, country of origin, and participant information. Special emphasis was placed on the curative potential of the gene therapies reviewed.
3. Results
The results of this systematic review are based on a final selection of 32 articles, identified through the PRISMA method (Figure 1). These included 17 preclinical studies, 11 clinical trials, and 4 descriptive studies, all focused on evaluating the effectiveness of gene therapy interventions for various NDDs. Our review aimed to assess the therapeutic potential of gene therapies targeting specific NDDs, with a working hypothesis that these therapies could either provide curative effects by addressing underlying genetic mutations or promote cell survival to slow disease progression. The studies reviewed encompass gene therapy approaches for spinal muscular atrophy (n = 8, Table 1), Huntington’s disease (n = 3, Table 2), amyotrophic lateral sclerosis (n = 6, Table 3), Parkinson’s disease (n = 12, Table 4), and Alzheimer’s disease (n = 3, Table 5). The tables below summarize key findings from these studies, including study design, methodology, and reported outcomes, to provide a comprehensive overview of current progress in gene therapy for NDDs.

Table 1.
Results: spinal muscular atrophy (SMA).

Table 2.
Results: Huntington’s disease (HD).

Table 3.
Results: amyotrophic lateral sclerosis (ALS).

Table 4.
Results: Parkinson’s disease (PD).

Table 5.
Results: Alzheimer’s disease (AD).
Based on the review of selected articles, we identified two principal approaches in gene therapy for NDDs, summarized in Figure 2. The first approach is mutation-specific therapies with curative intent, which aim to directly target and correct specific genetic mutations responsible for monogenic NDDs. These therapies involve the introduction of corrective genes or oligonucleotides using AAV vectors or gene-editing technologies, such as CRISPR-Cas9 and antisense oligonucleotides (ASOs). Examples include targeting the HTT gene in Huntington’s disease or the SMN1 gene in spinal muscular atrophy (Figure 2). These therapies are designed for curative outcomes by addressing the root cause of the disease at the genetic level.
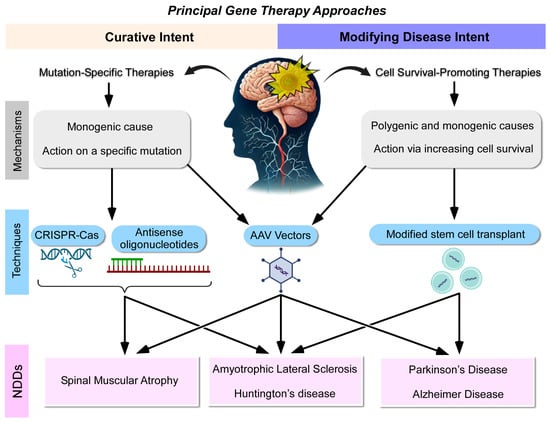
Figure 2.
The review analysis highlights two principal gene therapy approaches for neurodegenerative diseases (NDDs). Therapies with curative intent involve the introduction of a corrective gene or oligonucleotide using adeno-associated viral (AAV) vectors or gene-editing techniques (CRISPR-Cas and antisense oligonucleotides), targeting diseases caused by specific genetic mutations. In contrast, therapies with a cell-survival-promoting intent utilize adenoviral vectors or stem cell transplantation to deliver factors that enhance cellular survival. This latter approach can be applied to both monogenic and polygenic neurodegenerative diseases, offering broader therapeutic potential.
In contrast, the second approach involves cell-survival-promoting therapies with disease-modifying intent, which aim to enhance neuronal survival and slow disease progression without directly correcting the underlying genetic mutation. These therapies utilize adenoviral vectors or stem cell transplantation to deliver neuroprotective factors, inhibit toxic protein aggregation, or modulate gene expression. This broader approach is applicable to both monogenic and polygenic neurodegenerative diseases, offering therapeutic potential for complex diseases, such as Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis. This classification helps clarify the distinction between direct mutation-targeting therapies and broader strategies aimed at promoting cell survival and mitigating disease progression (Figure 2). These recent therapies, classified under curative and disease-modifying categories, are detailed in the sections below.
3.1. Therapies with Curative Intent
In the case of SMA, onasemnogene abeparvovec (Zolgensma®) is an approved gene therapy drug that delivers a functional copy of the SMN1 gene, which is mutated in SMA, via a viral vector [18]. Clinical trials have demonstrated its efficacy, particularly in motor function, as measured by scales, like CHOP INTEND, when compared to historical cohorts [8,9,12,13]. The therapeutic success is influenced by two key factors: (1) the dose administered and (2) the age and weight at the time of administration. The START:I trial [11], demonstrated that higher doses of Zolgensma® correlate with greater efficacy. In addition, some studies have evaluated the effect of these factors [9,10,12,13,18] and have confirmed that asymptomatic lower-weight patients achieve better motor and survival outcomes. For this reason, a treatment protocol has been established in asymptomatic patients with prenatal diagnosis [10]. While intrauterine administration of the therapy has been explored in animal models, it has not yet proven viable [15].
Years later, nusinersen (Spinraza®) was developed, showing significant results in the acquisition of motor milestones for patients with SMA [11]. However, onasemnogene abeparvovec is typically prioritized over nusinersen for SMA treatment, as Zolgensma® requires only a single intravenous dose; whereas, nusinersen involves repeated intrathecal administrations [18,60].
In the case of ALS, tofersen (Qalsody®) is an antisense oligonucleotide-based intrathecal therapy designed to treat ALS patients with mutations in the SOD1 gene. Treatment with tofersen has been shown to decrease the concentration of the SOD1 protein in the cerebrospinal fluid; although, no significant impact on disease progression has been demonstrated [37]. Gene-editing approaches targeting the SOD1 gene have shown modest outcomes. For instance, gene editing in an ALS mouse model resulted in increased survival, improved muscle atrophy, and reduced SOD1 protein inclusions [39].
Therefore, future research is required to optimize these approaches, and hence, it could be an important line of treatment that may develop further.
Finally, in Huntington’s disease, the administration of Tominersen (developed by ROCHE) has been inconclusive due to the allelic heterogeneity presented in the HTT gene mutation [75]. Current research efforts are aimed at blocking mRNA transcription of the HTT gene using noncoding RNAs, such as shRNA and miRNA [16]. Although these approaches have yielded promising results in terms of molecular outcomes, they have not yet translated into significant improvements in clinical parameters [6].
3.2. Therapies with Modifying Intent
Disease-modifying gene therapies focus on delivering neurotrophic factors or neuroprotective agents through vectors or stem cells, particularly in NDDs lacking a specific genomic target. These therapies are also being explored in disorders with known genetic alterations where targeted interventions have proven insufficient.
In Huntington’s disease, stem-cell-based therapies have produced more significant benefits than strategies using adeno-associated viral vectors [4,7]. Notably, a lower dose of cells seems to be enough to confer neuroprotection, as evidenced by superior outcomes in the lower dose group in a rat model [4].
It has been suggested that PD [36] and AD [22] may share a common therapeutic target involving the neuroprotective functions of astrocytes and microglia. In PD, intracerebral vector administration is the most extensively studied approach in preclinical animal models. Moreover, this strategy has shown better clinical and biochemical outcomes [31,32,35,36,45]. Additionally, DOPA-synthesizing and microglia-restoring stem cells have also provided significant results. However, there is no consensus regarding the optimal administration route, with some studies favoring intracerebral delivery [34], while others report encouraging outcomes from systemic, intravenous administration [28].
In AD, the results have been less favorable. Although gene therapies have enhanced cell survival, they have not consistently restored microglial function or significantly reduced β-amyloid plaque accumulation [24].
Lastly, a potential neuroprotective system based on the homeostatic regulation of autophagy and vesicular trafficking has been discovered in ALS. This system could be modulated either by increasing lysosomal activity [42] or by delivering transport-related proteins, such as synaptotagmin-13 [40]. Nonetheless, research with neuro-protective molecules has achieved better survival outcomes so far [41].
4. Discussion
The current treatments based on the gene therapy of five types of NDDs have been addressed, including both therapies that have already been approved and potential therapeutic approaches.
Diseases, from the perspective of gene therapy, can be broadly divided into two categories: those with actionable genetic mutations and those with polygenic inheritance, where no single actionable gene target exists. In NDDs with an actionable mutation, cells harboring driver mutations exhibit sensitivity in response to targeted therapies. For these cases, gene editing or gene therapy techniques, which consist of the introduction or removal of a gene or oligonucleotide, are employed.
However, extrapolating findings from animal models to human patients remains challenging, as only humans possess the complete mutational and pathogenic burden. The utility of any given animal model must be evaluated within the context of the specific research question being addressed. To enhance translational success to clinical applications, therapies should, whenever possible, be tested across multiple preclinical models. This approach is crucial given our limited understanding of NDDs and the fact that no model fully replicates human disease. Moreover, alignment is essential between the timing of observed therapeutic effects in the preclinical model and the stage of pathology in the intended clinical trial population [76].
In diseases without actionable mutations, or in cases where allelic heterogeneity is too extensive to develop patient-specific therapies, disease-modifying treatments are used. These therapies aim to enhance cellular survival and reduce the toxicity derived from protein inclusions or oxidative stress (free radicals). Notably, some NDDs share pathogenic mechanisms, which suggest common therapeutic targets. For instance, both Alzheimer’s and Parkinson’s exhibit microglia dysfunction. Besides, in Huntington’s, ALS and SMA defects in lysosomal transport and cellular autophagy mechanisms contribute to protein aggregation and inclusion. Regulating these processes may help alleviate cellular toxicity and improve outcomes in these diseases [42].
It is worth noting that gene therapy approaches for diseases with more advanced research and preclinical data tend to focus heavily on biodistribution, safety, and pharmacovigilance. For example, extensive studies have been conducted in PD [34] and SMA [10,13,14,60], underscoring the importance of these aspects in therapeutic development.
Regarding the routes of administration, one major challenge in NDDs, unlike other diseases, is overcoming the BBB [1,62,77]. Direct delivery methods, such as intracerebral administration, appear to be the most effective [14]. While subcutaneous administration of neuroprotective factors has shown significant results in PD animal models, these effects have not been replicated in humans, highlighting the superiority of intracerebral routes [44]. However, NDDs do not consist only of local pathological processes, but rather, they tend to spread throughout the central nervous system.
In gene therapy for NDDs, intracerebral administration requires highly precise delivery devices to overcome the BBB and achieve therapeutic efficacy. Stereotactic injection systems are widely employed for this purpose, both in clinical trials and preclinical studies. These systems enable precise delivery of therapeutic agents to specific brain regions but are invasive and require specialized surgical expertise, posing certain risks. For instance, intracerebral injections in adeno-associated virus gene therapy have shown promising improvements in patient symptoms; yet, they are associated with complications, such as intracranial hemorrhage and transient headaches [78]. Thus, stereotactic intracranial approaches can accommodate a variety of gene therapy vehicles, including adeno-associated viral vectors, lentiviral and adenoviral vectors, as well as antisense oligonucleotides and other small molecules. This approach also allows for lower doses of adeno-associated viral vectors, reducing immune responses compared to intravenous or cerebrospinal-fluid-based deliveries [79]. Convection-enhanced delivery is commonly integrated with stereotactic systems, where a catheter is precisely positioned in the brain using stereotactic guidance. A continuous pressure gradient is then applied to facilitate the infusion of therapeutic agents over an extended area of brain tissue, thereby improving distribution and penetration. This approach enhances gene therapy efficacy by promoting more effective delivery of therapeutic agents, such as viral vectors, across larger brain regions [78,80,81,82]. Although effective, this method is invasive and carries risks, such as catheter misplacement and potential tissue damage. In preclinical studies, microinjection pumps and intracerebral microinfusion devices have been used for long-term or localized delivery of gene therapies in animal models. However, their complexity and invasiveness limit their application in human studies. Emerging techniques, such as focused ultrasound with microbubbles, offer a noninvasive alternative by temporarily opening the BBB. This approach facilitates gene delivery without the direct penetration of the brain tissue and is currently in early testing phases for gene therapy applications [83]. Intranasal delivery is another novel, noninvasive method with experimental potential, as it allows vectors to reach the brain along the olfactory pathway [84]. However, its limited efficiency in delivering larger genetic constructs constrains its applicability in gene therapy for NDDs. Despite this, the intranasal administration of neurotrophin in AD [23] or the opening of the BBB using microbubbles and ultrasounds in PD [73] are being explored showing promising results. This latter technique has successfully enabled the intravenous delivery of therapeutic agents, such as the GDNF protein [30] and the bioactive compound “gastrodin” [85], both of which have been shown to enhance dopamine synthesis by astrocytes. Each of these delivery methods has unique implications for precision, patient safety, and therapeutic outcomes, underscoring the need for continued innovation and optimization of gene therapy delivery systems for NDD treatment. Indeed, each delivery route presents its own challenges, and there might be adverse effects derived from the transfer technique used.
Each of these delivery methods has distinct implications for precision, patient safety, and therapeutic outcomes, emphasizing the need for ongoing innovation and optimization of gene therapy delivery systems for treating NDDs. Importantly, each route poses specific challenges, and adverse effects may arise from the delivery technique itself. Recently, there has been a shift toward using adeno-associated virus serotype 9 (AAV9) vectors via intravenous and intraperitoneal administration, which might reduce risks and potential side effects compared to more invasive methods, offering safer alternative gene therapy applications [14,86].
Intravenous and intrathecal administration can cause systemic toxicity, partly due to the hepatotoxicity of commonly used viral vectors and the high doses required for efficacy. This is the case of the nusinersen for the intrathecal treatment of SMA, which requires a minimum of five doses to increase neuronal survival by 10% [11]. Animal model results, particularly in nonhuman primates, are difficult to apply to humans due to differences in administration volume [60]. On the other hand, although the safety of the intrathecal route has been demonstrated in some studies [54], others raised concerns as intrathecal administration worsened the disease progression in ALS rat models [41].
Evaluating and assessing the effectiveness of gene therapies must also include consideration of adverse side effects. Pharmacovigilance studies on SMA gene therapies have revealed a high risk–benefit ratio, since, although they cause an improvement in motor and respiratory function, they are also associated with short-term [10] and long-term side effects due to an increase in the incidence of tumors [19] and skeletal anomalies [72].
Finally, this study has several limitations. While it aimed to comprehensively evaluate the efficacy and safety of gene therapies for the most prominent NDDs, it was not always feasible to investigate the specific reasons behind the ineffectiveness of certain gene therapies. Additionally, important factors, such as gender differences, which play a significant role in the pathophysiology and progression of NDDs, were not addressed in this review. Another key limitation is the lack of in-depth analysis regarding the timing of therapy administration, a critical determinant of therapeutic success in NDDs. Despite these limitations, our review offers valuable insights by systematically categorizing the latest gene therapy approaches, highlighting both curative and disease-modifying strategies and providing a framework for understanding their therapeutic potential.
This work underscores the importance of continuous research and paves the way for future studies to address these gaps, ultimately advancing the translation of gene therapies into clinical practice. These diseases remain without a cure, and specific biomarkers for diagnosing and tracking the progression of most NDDs are still under investigation. While biomarkers are often measured in CSF, this approach has limitations. However, the development of new, high-sensitivity techniques is enabling the detection of biomarkers at very low concentrations in blood or saliva, offering promising alternatives for clinical use [87].
In the future, continuous and collaborative research efforts will be essential to overcome the scientific, ethical, and clinical challenges associated with gene therapy interventions, facilitating their successful translation into clinical practice. Addressing the adverse effects of these therapies remains a critical priority, as they can significantly impact the risk–benefit ratio for patients. Moreover, it is crucial to further refine our understanding of (1) the natural history and key clinical outcomes of various neurodegenerative disorders and (2) the underlying mechanisms driving neurodegeneration. These advancements will be pivotal in optimizing gene therapy strategies and improving therapeutic efficacy.
5. Conclusions
In conclusion, this review highlights several key insights into the potential of gene therapy for neurodegenerative diseases (NDDs):
- -
- Gene therapy for NDDs offers significant promise for developing interventions that can either slow disease progression or, in some cases, provide curative outcomes;
- -
- Therapies with curative intent focus on addressing specific genetic mutations, while disease-modifying therapies aim to enhance cell survival through the administration of neurotrophic factors or neuroprotective agents;
- -
- Therapies with curative intent that have shown promising results in terms of clinical significance include the introduction of a gene or oligonucleotide, particularly for SMA and gene editing techniques for ALS. However, these approaches have not yet achieved meaningful clinical results for HD;
- -
- Therapies with modifying intent have demonstrated significant clinical results in PD, using both stem cells and the administration of a neurotrophic factor using vectors. Advances have also been made in HD with stem cell therapies and in ALS with the introduction of protective factors through viral vectors or oligonucleotides. However, in AD, the results obtained so far are more limited, highlighting the complexity of targeting polygenic diseases;
- -
- Our analysis identified shared pathogenic pathways among certain NDDs, which may present viable targets for gene therapy. This is particularly evident between PD and AD, as well as HD, ALS, and SMA;
- -
- According to the characteristics described of each administration route for gene therapy and based on their ability to effectively cross the BBB and target the central nervous system, the optimal routes for gene therapy administration in NDDs appear to be the following: intra-arterial, intravenous with ultrasound facilitation, as well as intrathecal delivery;
- -
- Overall, gene therapy offers a promising avenue for both curative and disease-modifying treatments, but further research is necessary to refine these approaches and achieve more widespread clinical success.
Author Contributions
Conceptualization, D.E.L.; methodology, N.G.-G.; investigation, N.G.-G.; writing—original draft preparation, J.G.-S.; writing—review and editing, J.M.G.-E., D.E.L. and R.G.-N.; visualization, J.M.G.-E., N.G.-G. and R.G.-N.; supervision, D.E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McMillan, H.J.; Proud, C.M.; Farrar, M.A.; Alexander, I.E.; Muntoni, F.; Servais, L. Onasemnogene abeparvovec for the treatment of spinal muscular atrophy. Expert. Opin. Biol. Ther. 2022, 22, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J.; Boer, G.J. Parkinson’s Disease; a Tale of Many Players. Med. Princ. Pract. 2023, 32, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Fischell, J.M.; Fishman, P.S. A Multifaceted Approach to Optimizing AAV Delivery to the Brain for the Treatment of Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 747726. [Google Scholar] [CrossRef] [PubMed]
- Wenceslau, C.V.; de Souza, D.M.; Mambelli-Lisboa, N.C.; Ynoue, L.H.; Araldi, R.P.; da Silva, J.M.; Pagani, E.; Haddad, M.S.; Kerkis, I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells 2022, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.; Pagani, E.; Wenceslau, C.V.; Ferrara, L.; Kerkis, I. A Phase I clinical trial on intravenous administration of immature human dental pulp stem cells (Nestacell HDTM) to Huntington’s Disease patients. Cytotherapy 2021, 23, 1. [Google Scholar] [CrossRef]
- Kotowska-Zimmer, A.; Przybyl, L.; Pewinska, M.; Suszynska-Zajczyk, J.; Wronka, D.; Figiel, M.; Olejniczak, M. A CAG repeat-targeting artificial miRNA lowers the mutant huntingtin level in the YAC128 model of Huntington’s disease. Mol. Ther.-Nucleic Acids 2022, 28, 702–715. [Google Scholar] [CrossRef]
- Ferlazzo, G.M.; Gambetta, A.M.; Amato, S.; Cannizzaro, N.; Angiolillo, S.; Arboit, M.; Diamante, L.; Carbognin, E.; Romani, P.; La Torre, F.; et al. Genome-wide screening in pluripotent cells identifies Mtf1 as a suppressor of mutant huntingtin toxicity. Nat. Commun. 2023, 14, 3962. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Lehman, K.J.; McColly, M.; Lowes, L.P.; Alfano, L.N.; Reash, N.F.; Iammarino, M.A.; Church, K.R.; Kleyn, A.; et al. Five-Year Extension Results of the Phase 1 START Trial of Onasemnogene Abeparvovec in Spinal Muscular Atrophy. JAMA Neurol. 2021, 78, 834–841. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1381. [Google Scholar] [CrossRef]
- Stettner, G.M.; Hasselmann, O.; Tscherter, A.; Galiart, E.; Jacquier, D.; Klein, A. Treatment of spinal muscular atrophy with Onasemnogene Abeparvovec in Switzerland: A prospective observational case series study. BMC Neurol. 2023, 23, 88. [Google Scholar] [CrossRef]
- Pane, M.; Coratti, G.; Pera, M.C.; Sansone, V.A.; Messina, S.; d’Amico, A.; Bruno, C.; Salmin, F.; Albamonte, E.; De Sanctis, R.; et al. Nusinersen efficacy data for 24-month in type 2 and 3 spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2022, 9, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Berti, B.; Capasso, A.; Coratti, G.; Varone, A.; D’Amico, A.; Bruno, C.; Ricci, F.; Pini, A.; Gagliardi, D.; et al. Onasemnogene abeparvovec in spinal muscular atrophy: Predictors of efficacy and safety in naive patients with spinal muscular atrophy and following switch from other therapies. EClinicalMedicine 2023, 59, 101997. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.H.; Mitchell, S.; Sun, R.; LaMarca, N.; Reyna, S.P.; Sutter, T. Research Paper Safety of Onasemnogene Abeparvovec for Patients With Spinal Muscular Atrophy 8.5 kg or Heavier in a Global Managed Access Program. Pediatr. Neurol. 2022, 132, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Besse, A.; Astord, S.; Marais, T.; Roda, M.; Giroux, B.; Lejeune, F.X.; Relaix, F.; Smeriglio, P.; Barkats, M.; Biferi, M.G. AAV9- Mediated Expression of SMN Restricted to Neurons Does Not Rescue the Spinal Muscular Atrophy Phenotype in Mice. Mol. Ther. 2020, 28, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Rich, K.A.; Wier, C.G.; Russo, J.; Kong, L.; Heilman, P.L.; Reynolds, A.; Knapp, A.; Pino, M.G.; Keckley, E.; Mattox, L.; et al. Premature delivery in the domestic sow in response to in utero delivery of AAV9 to fetal piglets. Gene Ther. 2022, 29, 513–519. [Google Scholar] [CrossRef]
- Morais, R.D.V.S.; Sogorb-Gonzalez, M.; Bar, C.; Timmer, N.C.; Van der Bent, M.L.; Wartel, M.; Vallès, A. Functional Intercellular Transmission of miHTT via Extracellular Vesicles: An In Vitro Proof-of-Mechanism Study. Cells 2022, 11, 2748. [Google Scholar] [CrossRef]
- Cummings, D.M.; Alaghband, Y.; Hickey, M.A.; Joshi, P.R.; Hong, S.C.; Zhu, C.; Ando, T.K.; André, V.M.; Cepeda, C.; Watson, J.B.; et al. A critical window of CAG repeat-length correlates with phenotype severity in the R6/2 mouse model of Huntington’s disease. J. Neurophysiol. 2012, 107, 677–691. [Google Scholar] [CrossRef]
- Day, J.W.; Mendell, J.R.; Mercuri, E.; Finkel, R.S.; Strauss, K.A.; Kleyn, A.; Tauscher-Wisniewski, S.; Tukov, F.F.; Reyna, S.P.; Chand, D.H. Clinical Trial and Postmarketing Safety of Onasemnogene Abeparvovec Therapy. Drug Saf. 2021, 44, 1109–1119. [Google Scholar] [CrossRef]
- Retson, L.; Tiwari, N.; Vaughn, J.; Bernes, S.; Adelson, P.D.; Mansfield, K.; Libertini, S.; Kuzmiski, B.; Alecu, I.; Gabriel, R. Epithelioid neoplasm of the spinal cord in a child with spinal muscular atrophy treated with onasemnogene abeparvovec. Mol. Ther. 2023, 31, 2991–2998. [Google Scholar] [CrossRef]
- Hirunagi, T.; Sahashi, K.; Meilleur, K.G.; Katsuno, M. Nucleic Acid-Based Therapeutic Approach for Spinal and Bulbar Muscular Atrophy and Related Neurological Disorders. Genes 2022, 13, 109. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, S.; Duan, N.; Xie, M.; Li, Z.; Feng, M.; Wu, L.; Hu, Z.; Liang, D. Targeted-Deletion of a Tiny Sequence via Prime Editing to Restore SMN Expression. Int. J. Mol. Sci. 2022, 23, 7941. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.T.; Moon, G.J.; Kim, S.; Choi, M.; Oh, Y.S.; Kim, D.W.; Kim, H.J.; Lee, K.J.; Choe, Y.; Ha, C.M.; et al. Neurotrophic interactions between neurons and astrocytes following AAV1-Rheb(S16H) transduction in the hippocampus in vivo. Br. J. Pharmacol. 2020, 177, 668–686. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, S.; Cattaneo, A. Getting Into the Brain: The Intranasal Approach to Enhance the Delivery of Nerve Growth Factor and Its Painless Derivative in Alzheimer’s Disease and Down Syndrome. Front. Neurosci. 2022, 16, 773347. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Silva, A.; Sharma, J.; Nguyen, J.; Pizzo, D.P.; Hinz, D.; Sahoo, D.; Cherqui, S. Rescue of Alzheimer’s disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells. Cell Rep. 2023, 42, 112956. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.T.; Akhter, A.S.; Ehrlich, D.J.; Scott, G.C.; Lungu, C.; Munjal, V.; Aquino, A.; Lonser, R.R.; Fiandaca, M.S.; Hallett, M.; et al. Long-term safety of MRI-guided administration of AAV2-GDNF and gadoteridol in the putamen of individuals with Parkinson’s disease. Mol. Therapy 2022, 30, 3632–3638. [Google Scholar] [CrossRef]
- Onuki, Y.; Ono, S.; Nakajima, T.; Kojima, K.; Taga, N.; Ikeda, T.; Kuwajima, M.; Kurokawa, Y.; Kato, M.; Kawai, K.; et al. Dopaminergic restoration of prefrontal cortico-putaminal network in gene therapy for aromatic l-amino acid decarboxylase deficiency. Brain Commun. 2021, 3, fcab078. [Google Scholar] [CrossRef]
- Tai, C.H.; Lee, N.C.; Chien, Y.H.; Byrne, B.J.; Muramatsu, S.H.; Tseng, S.H.; Hwu, W.L. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol. Ther. 2022, 30, 509–518. [Google Scholar] [CrossRef]
- Chen, C.; Guderyon, M.J.; Li, Y.; Ge, G.; Bhattacharjee, A.; Ballard, C.; He, Z.; Masliah, E.; Clark, R.A.; O’Connor, J.C.; et al. Non-toxic HSC Transplantation-Based Macrophage/Microglia-Mediated GDNF Delivery for Parkinson’s Disease. Mol. Ther.-Methods Clin. Dev. 2020, 17, 83–98. [Google Scholar] [CrossRef]
- Gupta, R.; Santiago-Lopez, A.J.; Berglund, K.; Gross, R.E.; Gutekunst, C.A.N. In Vivo Assessment of Cell Death and Nigrostriatal Pathway Integrity Following Continuous Expression of C3 Transferase. Neuroscience 2020, 442, 183–192. [Google Scholar] [CrossRef]
- Wang, F.; Li, N.; Hou, R.; Wang, L.; Zhang, L.; Li, C.; Zhang, Y.; Yin, Y.; Chang, L.; Cheng, Y.; et al. Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood-brain barrier. Open Chem. 2020, 18, 882–889. [Google Scholar] [CrossRef]
- Cui, K.; Yang, F.; Tufan, T.; Raza, M.U.; Zhan, Y.; Fan, Y.; Zeng, F.; Brown, R.W.; Price, J.B.; Jones, T.C.; et al. Restoration of Noradrenergic Function in Parkinson’s Disease Model Mice. ASN Neuro 2021, 13, 17590914211009730. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Parrilla, M.A.; Reyes-Corona, D.; Flores-Martinez, Y.M.; Nadella, R.; Bannon, M.J.; Escobedo, L.; Maldonado-Berny, M.; Santoyo-Salazar, J.; Soto-Rojas, L.O.; Luna-Herrera, C.; et al. Cerebral dopamine neurotrophic factor transfection in dopamine neurons using neurotensin-polyplex nanoparticles reverses 6-hydroxydopamine-induced nigrostriatal neurodegeneration. Neural Regen. Res. 2022, 17, 854. [Google Scholar] [PubMed]
- Kip, E.; Bentall, L.; Underwood, C.F.; Hughes, S.M.; Parr-Brownlie, L.C. Patterned Stimulation of the Chrimson Opsin in Glutamatergic Motor Thalamus Neurons Improves Forelimb Akinesia in Parkinsonian Rats. Neuroscience 2022, 507, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Wei, J.; Feng, C.; Chen, Y.; Chen, T.; Ai, Z.; Zhu, X.; Ji, W.; Li, T. Genetically engineered mesenchymal stem cells with dopamine synthesis for Parkinson’s disease in animal models. NPJ Park. Dis. 2022, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.D.; Prince, N.; Humbert-Claude, M.; Mesa-Infante, V.; Jeanneret, C.; Golzne, V.; De Matos, K.; Jamot, B.B.; Magara, F.; Gonzalez-Hernandez, T.; et al. Oxidative stress induced by sustained supraphysiological intrastriatal GDNF delivery is prevented by dose regulation. Mol. Ther.-Methods Clin. Dev. 2023, 31, 101106. [Google Scholar] [CrossRef]
- Narvaez-Perez, L.F.; Paz-Bermudez, F.; Avalos-Fuentes, J.A.; Campos-Romo, A.; Floran-Garduno, B.; Segovia, J. CRISPR/sgRNA-directed synergistic activation mediator (SAM) as a therapeutic tool for Parkinson′s disease. Gene Ther. 2023, 31, 31–44. [Google Scholar] [CrossRef]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1–2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef]
- Mueller, C.; Berry, J.D.; McKenna-Yasek, D.M.; Gernoux, G.; Owegi, M.A.; Pothier, L.M.; Douthwright, C.L.; Gelevski, D.; Luppino, S.D.; Blackwood, M.; et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. N. Engl. J. Med. 2020, 383, 151–158. [Google Scholar] [CrossRef]
- Lim, C.K.W.; Gapinske, M.; Brooks, A.K.; Woods, W.S.; Powell, J.E.; Zeballos, M.A.C.; Winter, J.; Perez-Pinera, P.; Gaj, T. Treatment of a Mouse Model of ALS by In Vivo Base Editing. Mol. Ther. 2020, 28, 1177–1189. [Google Scholar] [CrossRef]
- Nizzardo, M.; Taiana, M.; Rizzo, F.; Benitez, J.A.; Nijssen, J.; Allodi, I.; Melzi, V.; Bresolin, N.; Comi, G.P.; Hedlund, E.; et al. Synaptotagmin 13 is neuroprotective across motor neuron diseases. Acta Neuropathol. 2020, 139, 837–853. [Google Scholar] [CrossRef]
- Wang, S.; Ichinomiya, T.; Savchenko, P.; Wang, D.; Sawada, A.; Li, X.; Duong, T.; Li, W.; Bonds, J.A.; Kim, E.J.; et al. Subpial delivery of adeno-associated virus 9-synapsin- caveolin-1 (AAV9-SynCavl) preserves motor neuron and neuromuscular junction morphology, motor function, delays disease onset, and extends survival in hSOD1G93A mice. Theranostics 2022, 12, 5389–5403. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, L.; Jung, Y.; Kokubu, H.; Sowmithra, S.; Ni, L.; Lee, C.; Sanders, B.; Lee, P.J.; Xiang, Y.; Luttik, K.; et al. Reduction of nemo-like kinase increases lysosome biogenesis and ameliorates TDP-43-related neurodegeneration. J. Clin. Investig. 2023, 133, e138207. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Jiang, H.L.; Ashraf, G.M.; Li, Z.R.; Liu, R. MicroRNA and mRNA profiling of cerebral cortex in a transgenic mouse model of Alzheimer’s disease by RNA sequencing. Neural Regen. Res. 2021, 16, 2099. [Google Scholar] [PubMed]
- Renko, J.M.; Mahato, A.K.; Visnapuu, T.; Valkonen, K.; Karelson, M.; Voutilainen, M.H.; Saarma, M.; Tuominen, R.K.; Sidorova, Y.A. Neuroprotective Potential of a Small Molecule RET Agonist in Cultured Dopamine Neurons and Hemiparkinsonian Rats. J. Park. Dis. 2021, 11, 1023–1046. [Google Scholar] [CrossRef] [PubMed]
- Soner, B.C.; Acikgoz, E.; Inan, S.Y.; Ayla, S.; Sahin, A.S.; Oktem, G. Neuroprotective Effect of Intrastriatal Caffeic Acid Phenethyl Ester Treatment in 6-OH Dopamine Model of Parkinson’s Disease in Rats. Park. Dis. 2021, 2021, 5553480. [Google Scholar] [CrossRef]
- Feher, M.; Marton, Z.; Szabo, A.; Kocsa, J.; Kormos, V.; Hunyady, A.; Kovács, L.A.; Ujvári, B.; Berta, G.; Farkas, J.; et al. Downregulation of PACAP and the PAC1 Receptor in the Basal Ganglia, Substantia Nigra and Centrally Projecting Edinger-Westphal Nucleus in the Rotenone model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 11843. [Google Scholar] [CrossRef]
- Dommershuijsen, L.J.; Heshmatollah, A.; Darweesh, S.K.L.; Koudstaal, P.J.; Ikram, M.A.; Ikram, M.K. Life expectancy of parkinsonism patients in the general population. Park. Relat. Disord. 2020, 77, 94–99. [Google Scholar] [CrossRef]
- Ishihara, L.S.; Cheesbrough, A.; Brayne, C.; Schrag, A. Estimated life expectancy of Parkinson’s patients compared with the UK population. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1304–1309. [Google Scholar] [CrossRef]
- Song, J.; Liu, L.; Li, Z.; Mao, T.; Zhang, J.; Zhou, L.; Chen, X.; Shang, Y.; Sun, T.; Luo, Y.; et al. Lycium barbarum polysaccharide improves dopamine metabolism and symptoms in an MPTP-induced model of Parkinson’s disease. BMC Med. 2022, 20, 412. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.H.; Joe, E.H.; Jou, I. Small heterodimer partner (SHP) aggravates ER stress in Parkinson’s disease-linked LRRK2 mutant astrocyte by regulating XBP1 SUMOylation. J. Biomed. Sci. 2021, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Srikanth, M.P.; Deleidi, M.; Hallett, P.J.; Isacson, O.; Feldman, R.A. Acid ceramidase involved in pathogenic cascade leading to accumulation of α-synuclein in iPSC model of GBA1-associated Parkinson’s disease. Hum. Mol. Genet. 2023, 32, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, S.; Valiente, N.; Sesena, S.; Cabrera-Pinto, M.; Rodriguez, A.; Aranda, A.; Palop, L.; Fernández-Martos, C.M. Ozone modified hypothalamic signaling enhancing thermogenesis in the TDP-43A315T transgenic model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2022, 12, 20814. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Huo, J.; Xin, C.; Yang, J.; Liu, Q.; Dong, H.; Li, R.; Liu, Y. Protective effects of intrathecal injection of AAV9-RabGGTB-GFP+ in SOD1G93A mice. Front. Aging Neurosci. 2023, 15, 1092607. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Holodkov, N.; Klima, R.; Feiguin, F. TDP-43 regulates GAD1 mRNA splicing and GABA signaling in Drosophila CNS. Sci. Rep. 2021, 11, 18761. [Google Scholar] [CrossRef]
- Kay, M.A.; Glorioso, J.C.; Naldini, L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef]
- Akkewar, A.; Mahajan, N.; Kharwade, R.; Gangane, P. Liposomes in the Targeted Gene Therapy of Cancer: A Critical Review. Curr. Drug Deliv. 2023, 20, 350–370. [Google Scholar] [CrossRef]
- Luiz, M.T.; Tofani, L.B.; Araújo, V.H.S.; Di Filippo, L.D.; Duarte, J.L.; Marchetti, J.M.; Chorilli, M. Gene Therapy Based on Lipid Nanoparticles as Non-viral Vectors for Glioma Treatment. Curr. Gene Ther. 2021, 21, 452–463. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Hinderer, C.; Katz, N.; Dyer, C.; Goode, T.; Johansson, J.; Bell, P.; Richman, L.; Buza, E.; Wilson, J.M. Translational Feasibility of Lumbar Puncture for Intrathecal AAV Administration. Mol. Ther.-Methods Clin. Dev. 2020, 17, 969–974. [Google Scholar] [CrossRef]
- Salafutdinov, I.I.; Gatina, D.Z.; Markelova, M.I.; Garanina, E.E.; Malanin, S.Y.; Gazizov, I.M.; Izmailov, A.A.; Rizvanov, A.A.; Islamov, R.R.; Palotás, A.; et al. A Biosafety Study of Human Umbilical Cord Blood Mononuclear Cells Transduced with Adenoviral Vector Carrying Human Vascular Endothelial Growth Factor cDNA In Vitro. Biomedicines 2023, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, R.N.; DeAndrade, M.P.; Hull, F.; Nguyen, Q.; Peterson, T.; Yan, A.; Loperfido, M.; Baricordi, C.; Barbarossa, L.; Yoon, J.K.; et al. High-throughput analysis of hematopoietic stem cell engraftment after intravenous and intracerebroventricular dosing. Mol. Ther. 2022, 30, 3209–3225. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F.; Setten, R.L.; Cui, X.S.; Jadhav, S.G. Delivery of RNA Therapeutics: The Great Endosomal Escape! Nucleic Acid. Ther. 2022, 32, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Palfi, S.; Gurruchaga, J.M.; Ralph, G.S.; Lepetit, H.; Lavisse, S.; Buttery, P.C.; Watts, C.; Miskin, J.; Kelleher, M.; Deeley, S.; et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: A dose escalation, open-label, phase 1/2 trial. Lancet 2014, 383, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Kakoty, V.; Sarathlal, K.C.; Dubey, S.K.; Yang, C.H. Lentiviral mediated gene delivery as an effective therapeutic approach for Parkinson disease. Neurosci. Lett. 2021, 750, 135769. [Google Scholar] [CrossRef]
- Weiss, A.R.; Liguore, W.A.; Domire, J.S.; Button, D.; McBride, J.L. Intra-striatal AAV2.retro administration leads to extensive retrograde transport in the rhesus macaque brain: Implications for disease modeling and therapeutic development. Sci. Rep. 2020, 10, 6970. [Google Scholar] [CrossRef]
- Jang, S.; Shen, H.K.; Ding, X.; Miles, T.F.; Gradinaru, V. Structural basis of receptor usage by the engineered capsid AAV-PHP.eB. Mol. Ther.-Methods Clin. Dev. 2022, 26, 343–354. [Google Scholar] [CrossRef]
- Blesa, J.; Pineda-Pardo, J.A.; Inoue, K.I.; Gasca-Salas, C.; Balzano, T.; Del Rey, N.L.G.; Reinares-Sebastián, A.; Esteban-García, N.; Rodríguez-Rojas, R.; Márquez, R.; et al. BBB opening with focused ultrasound in nonhuman primates and Parkinson’s disease patients: Targeted AAV vector delivery and PET imaging. Sci. Adv. 2023, 9, eadf4888. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Ereej, N.; Hameed, H.; Khan, M.A.; Faheem, S.; Hameed, A. Nanoparticle-based Gene Therapy for Neurodegenerative Disorders. Mini-Rev. Med. Chem. 2024, 24, 1723–1745. [Google Scholar] [CrossRef] [PubMed]
- Soini, V.; Schreiber, G.; Wilken, B.; Hell, A.K. Early Development of Spinal Deformities in Children Severely Affected with Spinal Muscular Atrophy after Gene Therapy with Onasemnogene Abeparvovec-Preliminary Results. Child 2023, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G.; Nistico, R.; Triaca, V. Exploiting Focused Ultrasound to Aid Intranasal Drug Delivery for Brain Therapy. Front. Pharmacol. 2022, 13, 786475. [Google Scholar] [CrossRef] [PubMed]
- PRISMA Statement. Available online: https://www.prisma-statement.org (accessed on 15 October 2024).
- McColgan, P.; Thobhani, A.; Boak, L.; Schobel, S.A.; Nicotra, A.; Palermo, G.; Trundell, D.; Zhou, J.; Schlegel, V.; Sanwald Ducray, P.; et al. Tominersen in Adults with Manifest Huntington’s Disease. N. Engl. J. Med. 2023, 389, 2203–2205. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Xhima, K.; McMahon, D.; Ntiri, E.E.; Goubran, M.; Hynynen, K.; Aubert, I. Intravenous and Non-invasive Drug Delivery to the Mouse Basal Forebrain Using MRIguided Focused Ultrasound. Bio-Protoc. 2021, 11, e4056. [Google Scholar] [CrossRef]
- Christine, C.W.; Starr, P.A.; Larson, P.S.; Eberling, J.L.; Jagust, W.J.; Hawkins, R.A.; VanBrocklin, H.F.; Wright, J.F.; Bankiewicz, K.S.; Aminoff, M.J. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 2009, 73, 1662–1669. [Google Scholar] [CrossRef]
- Zhou, K.; Han, J.; Wang, Y.; Zhang, Y.; Zhu, C. Routes of administration for adeno-associated viruses carrying gene therapies for brain diseases. Front. Mol. Neurosci. 2022, 15, 988914. [Google Scholar] [CrossRef]
- Bankiewicz, K.S.; Eberling, J.L.; Kohutnicka, M.; Jagust, W.; Pivirotto, P.; Bringas, J.; Cunningham, J.; Budinger, T.F.; Harvey-White, J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000, 164, 2–14. [Google Scholar] [CrossRef]
- Hadaczek, P.; Kohutnicka, M.; Krauze, M.T.; Bringas, J.; Pivirotto, P.; Cunningham, J.; Bankiewicz, K. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum. Gene Ther. 2006, 17, 291–302. [Google Scholar] [CrossRef]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, H.; Aubert, I. Focused ultrasound gene delivery for the treatment of neurological disorders. Trends Mol. Med. 2024, 30, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, K.; Li, J.; Liao, Y.; Liao, C.; Chen, W.S.; Chen, M.; Ao, L. Focused Ultrasound Promotes the Delivery of Gastrodin and Enhances the Protective Effect on Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease. Front. Cell Neurosci. 2022, 16, 884788. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Molecular Biomarkers of Neurodegenerative Disorders: A Practical Guide to Their Appropriate Use and Interpretation in Clinical Practice. Int. J. Mol. Sci. 2024, 25, 4323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).