Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease
Abstract
1. Introduction
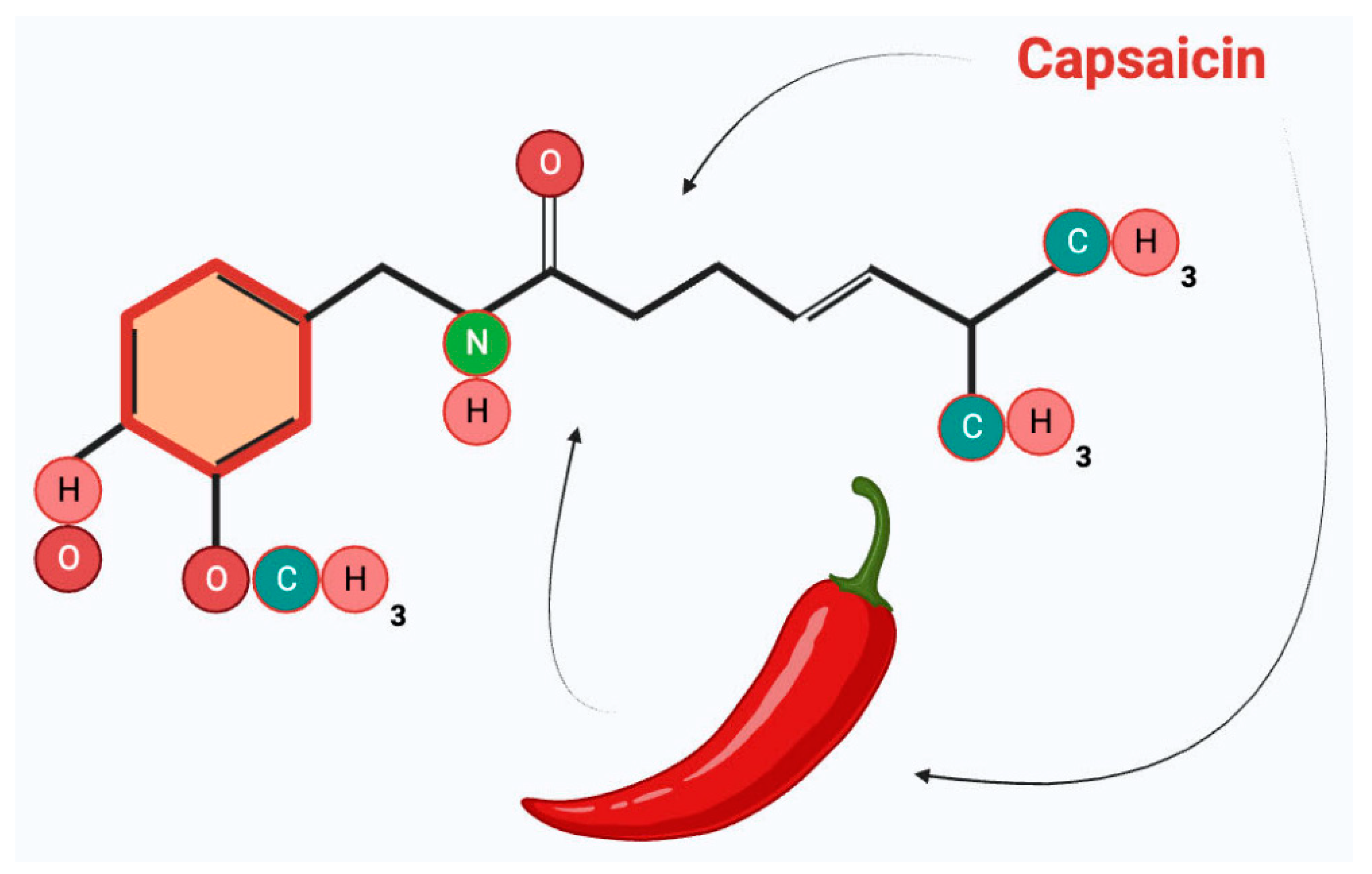
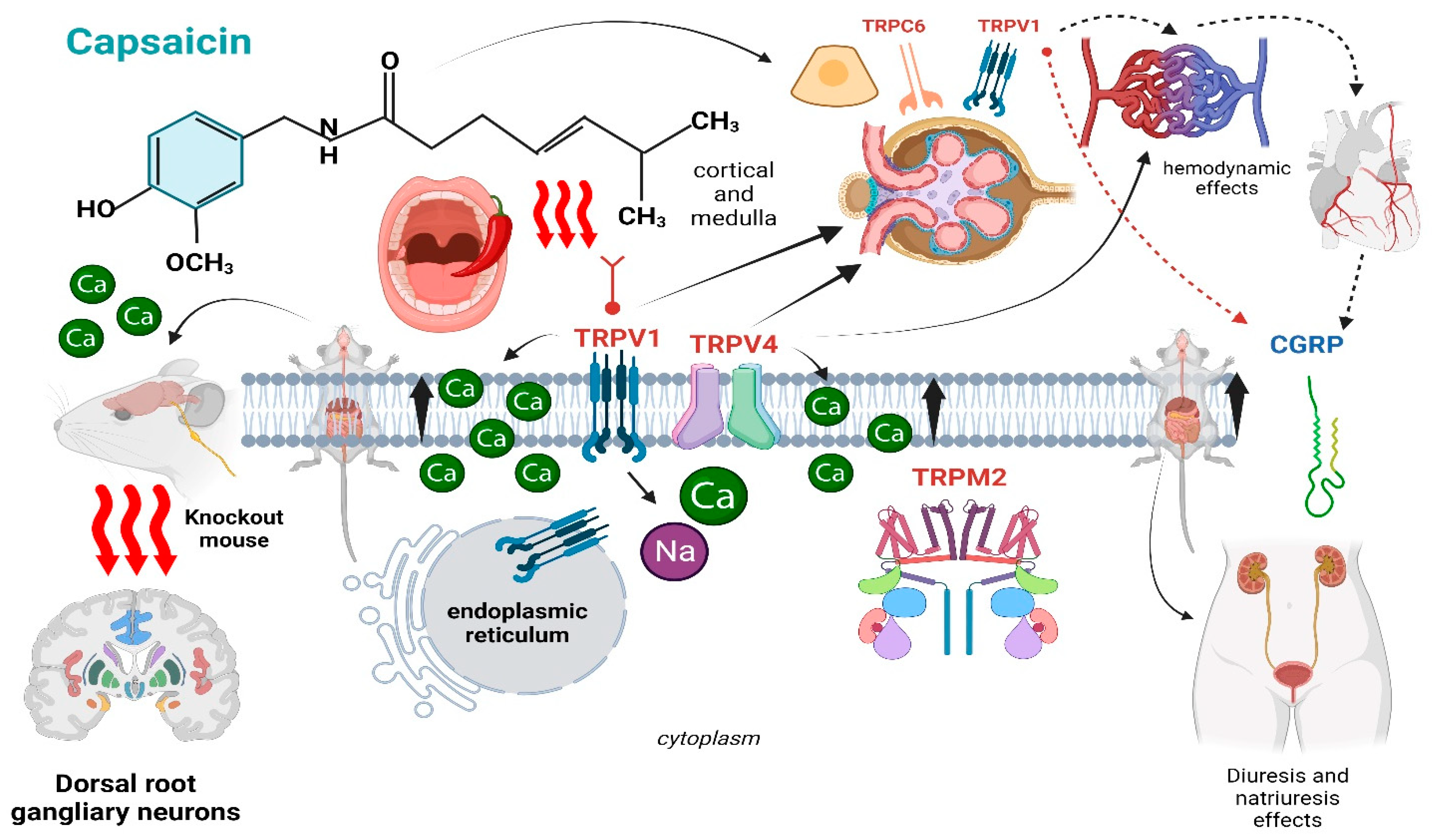
2. Capsaicin—Biochemical Properties and Mechanism of Action
3. Functional and Structural Effects of Capsaicin on the Kidney
4. Capsaicin Modulates Renal Nerves’ Activity
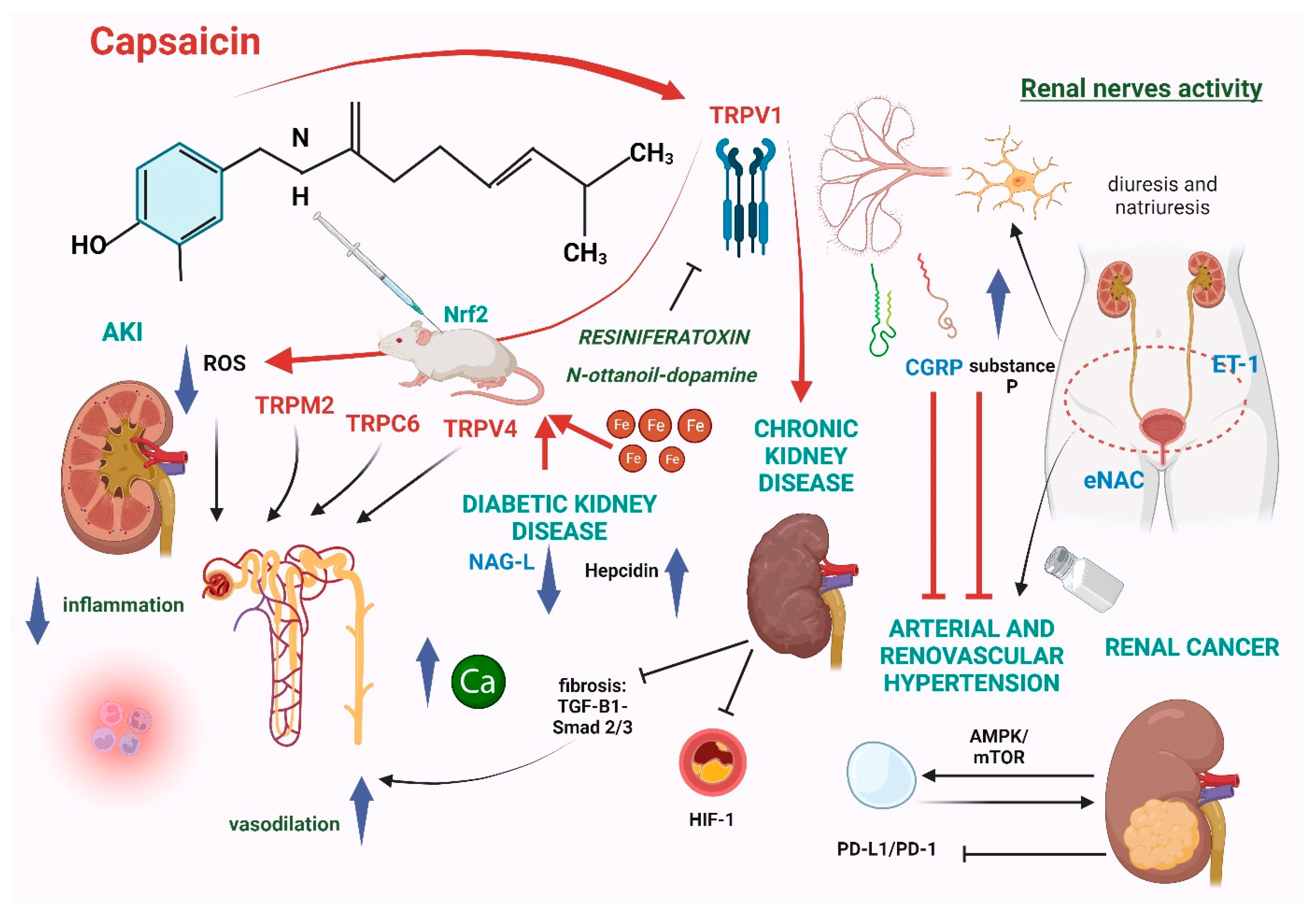
5. Possible Beneficial Effects of Capsaicin in Kidney Diseases
5.1. Acute Kidney Injury
5.2. Diabetic Kidney Disease
5.3. Chronic Kidney Disease
5.4. Arterial and Renovascular Hypertension
5.5. Renal Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonio, A.S.; Wiedemann, L.S.M.; Veiga Junior, V.F. The genus Capsicum: A phytochemical review of bioactive secondary metabolites. RSC Adv. 2018, 8, 25767–25784. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Sabnis, A.S.; Johansen, M.E.; Lanza, D.L.; Moos, P.J.; Yost, G.S.; Reilly, C.A. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J. Pharmacol. Exp. Ther. 2007, 321, 830–838. [Google Scholar] [CrossRef]
- Stueber, T.; Eberhardt, M.J.; Caspi, Y.; Lev, S.; Binshtok, A.; Leffler, A. Differential cytotoxicity and intracellular calcium-signalling following activation of the calcium-permeable ion channels TRPV1 and TRPA1. Cell Calcium 2017, 68, 34–44. [Google Scholar] [CrossRef]
- Vyklicky, L.; Novakova-Tousova, K.; Benedikt, J.; Samad, A.; Touska, F.; Vlachova, V. Calcium-dependent desensitization of vanilloid receptor TRPV1: A mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol. Res. 2008, 57 (Suppl. S3), S59–S68. [Google Scholar] [CrossRef]
- Oh, U.; Hwang, S.W.; Kim, D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J. Neurosci. 1996, 16, 1659–1667. [Google Scholar] [CrossRef]
- Wood, J.N.; Winter, J.; James, I.F.; Rang, H.P.; Yeats, J.; Bevan, S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 1988, 8, 3208–3220. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Braga Ferreira, L.G.; Faria, J.V.; Dos Santos, J.P.S.; Faria, R.X. Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 2020, 887, 173356. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.I.; Olah, A.; Szollosi, A.G.; Biro, T. TRP channels in the skin. Br. J. Pharmacol. 2014, 171, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Brayden, J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef] [PubMed]
- Kassmann, M.; Harteneck, C.; Zhu, Z.; Nurnberg, B.; Tepel, M.; Gollasch, M. Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol. 2013, 207, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Marko, L.; Mannaa, M.; Haschler, T.N.; Kramer, S.; Gollasch, M. Renoprotection: Focus on TRPV1, TRPV4, TRPC6 and TRPM2. Acta Physiol. 2017, 219, 589–612. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.A.; Jansen, C.J.; Hafmans, T.G.; Verhaegh, G.W.; Hoenderop, J.G.; Heesakkers, J.P.; Schalken, J.A. TRPV4 channels in the human urogenital tract play a role in cell junction formation and epithelial barrier. Acta Physiol. 2016, 218, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef]
- Ambrus, L.; Kelemen, B.; Szabo, T.; Biro, T.; Toth, B.I. Human podocytes express functional thermosensitive TRPV channels. Br. J. Pharmacol. 2017, 174, 4493–4507. [Google Scholar] [CrossRef]
- Mathieson, P.W. The podocyte as a target for therapies—New and old. Nat. Rev. Nephrol. 2011, 8, 52–56. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Mitsui, R.; Takano, H.; Hashitani, H. Mechanosensitive modulation of peristaltic contractions in the mouse renal pelvis. Eur. J. Pharmacol. 2022, 920, 174834. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Chalapala, S.; Gorzelanny, C.; Perali, R.S.; Goycoolea, F.M. The Effect of Capsaicin Derivatives on Tight-Junction Integrity and Permeability of Madin-Darby Canine Kidney Cells. J. Pharm. Sci. 2016, 105, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kassmann, M.; Sendeski, M.; Tsvetkov, D.; Marko, L.; Michalick, L.; Riehle, M.; Liedtke, W.B.; Kuebler, W.M.; Harteneck, C.; et al. Functional transient receptor potential vanilloid 1 and transient receptor potential vanilloid 4 channels along different segments of the renal vasculature. Acta Physiol. 2015, 213, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.H. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol. Res. 2008, 57, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.H. Segmental regulation of sodium and water excretion by TRPV1 activation in the kidney. J. Cardiovasc. Pharmacol. 2008, 51, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Wang, D.H. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension 2005, 46, 992–997. [Google Scholar] [CrossRef]
- Geppetti, P.; Baldi, E.; Castellucci, A.; Del Bianco, E.; Santicioli, P.; Maggi, C.A.; Lippe, I.T.; Amann, R.; Skofitsch, G.; Theodorsson, E.; et al. Calcitonin gene-related peptide in the rat kidney: Occurrence, sensitivity to capsaicin, and stimulation of adenylate cyclase. Neuroscience 1989, 30, 503–513. [Google Scholar] [CrossRef]
- Lazzeri, M.; Barbanti, G.; Beneforti, P.; Maggi, C.A.; Taddei, I.; Andrea, U.; Cantini, C.; Castellani, S.; Turini, D. Vesical-renal reflex: Diuresis and natriuresis activated by intravesical capsaicin. Scand. J. Urol. Nephrol. 1995, 29, 39–43. [Google Scholar] [CrossRef]
- Barajas, L.; Liu, L.; Powers, K. Anatomy of the renal innervation: Intrarenal aspects and ganglia of origin. Can. J. Physiol. Pharmacol. 1992, 70, 735–749. [Google Scholar] [CrossRef]
- Guo, A.; Vulchanova, L.; Wang, J.; Li, X.; Elde, R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999, 11, 946–958. [Google Scholar] [CrossRef]
- Ye, C.; Qiu, Y.; Zhang, F.; Chen, A.D.; Zhou, H.; Wang, J.J.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Chemical Stimulation of Renal Tissue Induces Sympathetic Activation and a Pressor Response via the Paraventricular Nucleus in Rats. Neurosci. Bull. 2020, 36, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Ye, C.; Wan, G.W.; Zhou, B.; Tong, Y.; Lei, J.Z.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Interleukin-1beta in hypothalamic paraventricular nucleus mediates excitatory renal reflex. Pflug. Arch. 2020, 472, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- DeLalio, L.J.; Stocker, S.D. Sympathoexcitatory responses to renal chemosensitive stimuli are exaggerated at nighttime in rats. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H437–H448. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, D.H. Ablation of transient receptor potential vanilloid 1 abolishes endothelin-induced increases in afferent renal nerve activity: Mechanisms and functional significance. Hypertension 2009, 54, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, A.F.; Wang, D.H. ET(A) receptor blockade prevents renal dysfunction in salt-sensitive hypertension induced by sensory denervation. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H2005–H2011. [Google Scholar] [CrossRef]
- Jones, R.O.; Matsuda, Y.; Koyama, S.; Uematsu, H.; Fujita, T.; Shibamoto, T. Reflex responses on blood pressure and renal nerve activity to local intra-arterial injection of capsaicin in anesthetized dogs. Jpn. J. Physiol. 1990, 40, 491–502. [Google Scholar] [CrossRef]
- Ma, H.J.; Wu, Y.M.; Ma, H.J.; Zhang, L.H.; He, R.R. Intrarenal artery injection of capsaicin activates spontaneous activity of renal afferent nerve fibers. Sheng Li Xue Bao 2003, 55, 505–510. [Google Scholar]
- Lang, I.; Skofitsch, G. Pharmacological effects of capsaicin treatment on innervation of the rat kidney with calcitonin gene-related peptide. Ann. N. Y. Acad. Sci. 1992, 657, 481–483. [Google Scholar] [CrossRef]
- Jancso, G.; Kiraly, E.; Joo, F.; Such, G.; Nagy, A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci. Lett. 1985, 59, 209–214. [Google Scholar] [CrossRef]
- Perfumi, M.; Massi, M. Resiniferatoxin provides further evidence for a role of capsaicin-sensitive sensory neurons in the control of the kidney function. Arch. Int. Pharmacodyn. Ther. 1994, 327, 232–245. [Google Scholar]
- Maggi, C.A.; Giuliani, S. Non-adrenergic non-cholinergic excitatory innervation of the guinea-pig isolated renal pelvis: Involvement of capsaicin-sensitive primary afferent neurons. J. Urol. 1992, 147, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Knels, L.; Kummer, W.; Koch, T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J. Histochem. Cytochem. 2006, 54, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.; Loesch, A.; Burnstock, G. Endothelin immunoreactivity and mRNA expression in sensory and sympathetic neurones following selective denervation. Int. J. Dev. Neurosci. 2000, 18, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.T.; Chao, C.T.; Lin, S.H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Negi, S.; Koreeda, D.; Kobayashi, S.; Yano, T.; Tatsuta, K.; Mima, T.; Shigematsu, T.; Ohya, M. Acute kidney injury: Epidemiology, outcomes, complications, and therapeutic strategies. Semin. Dial. 2018, 31, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sohaney, R.; Yin, H.; Shahinian, V.; Saran, R.; Burrows, N.R.; Pavkov, M.E.; Banerjee, T.; Hsu, C.Y.; Powe, N.; Steffick, D.; et al. In-Hospital and 1-Year Mortality Trends in a National Cohort of US Veterans with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2022, 17, 184–193. [Google Scholar] [CrossRef]
- Han, J.; Wu, J.; Liu, H.; Huang, Y.; Ju, W.; Xing, Y.; Zhang, X.; Yang, J. Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro. Open Life Sci. 2023, 18, 20220647. [Google Scholar] [CrossRef]
- Ran, F.; Yang, Y.; Yang, L.; Chen, S.; He, P.; Liu, Q.; Zou, Q.; Wang, D.; Hou, J.; Wang, P. Capsaicin Prevents Contrast-Associated Acute Kidney Injury through Activation of Nrf2 in Mice. Oxid. Med. Cell. Longev. 2022, 2022, 1763922. [Google Scholar] [CrossRef]
- Aldossary, S.A.; Chohan, M.S.; Mohaini, M.A.; Tasleem Rasool, S. Capsaicin ameliorate the nephrotoxicity induced by methotrexate. Pak. J. Pharm. Sci. 2021, 34, 2191–2195. [Google Scholar]
- Shimeda, Y.; Hirotani, Y.; Akimoto, Y.; Shindou, K.; Ijiri, Y.; Nishihori, T.; Tanaka, K. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biol. Pharm. Bull. 2005, 28, 1635–1638. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Xu, Q.; Huang, F.; Xu, R.; Liu, Q.; Lv, Y. Role of capsaicin sensitive sensory nerves in ischemia reperfusion-induced acute kidney injury in rats. Biochem. Biophys. Res. Commun. 2018, 506, 176–182. [Google Scholar] [CrossRef]
- Tsagogiorgas, C.; Wedel, J.; Hottenrott, M.; Schneider, M.O.; Binzen, U.; Greffrath, W.; Treede, R.D.; Theisinger, B.; Theisinger, S.; Waldherr, R.; et al. N-octanoyl-dopamine is an agonist at the capsaicin receptor TRPV1 and mitigates ischemia-induced [corrected] acute kidney injury in rat. PLoS ONE 2012, 7, e43525. [Google Scholar] [CrossRef]
- Chen, L.; Marko, L.; Kassmann, M.; Zhu, Y.; Wu, K.; Gollasch, M. Role of TRPV1 channels in ischemia/reperfusion-induced acute kidney injury. PLoS ONE 2014, 9, e109842. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Q.; Ma, S.; Wang, D.H. Activation of TRPV1 Prevents Salt-Induced Kidney Damage and Hypertension after Renal Ischemia-Reperfusion Injury in Rats. Kidney Blood Press. Res. 2018, 43, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Pechman, K.R.; De Miguel, C.; Lund, H.; Leonard, E.C.; Basile, D.P.; Mattson, D.L. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R1358–R1363. [Google Scholar] [CrossRef]
- Yu, S.Q.; Ma, S.; Wang, D.H. TRPV1 Activation Prevents Renal Ischemia-Reperfusion Injury-Induced Increase in Salt Sensitivity by Suppressing Renal Sympathetic Nerve Activity. Curr. Hypertens. Rev. 2020, 16, 148–155. [Google Scholar] [CrossRef]
- Ueda, K.; Tsuji, F.; Hirata, T.; Takaoka, M.; Matsumura, Y. Preventive effect of TRPV1 agonists capsaicin and resiniferatoxin on ischemia/reperfusion-induced renal injury in rats. J. Cardiovasc. Pharmacol. 2008, 51, 513–520. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Martinez-Castelao, A. Diabetes Mellitus and Diabetic Kidney Disease: The Future Is Already Here. J. Clin. Med. 2023, 12, 2914. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Cirilli, I.; Marcheggiani, F.; Mabhida, S.E.; Ziqubu, K.; Ntamo, Y.; Jack, B.; Nyambuya, T.M.; Hanser, S.; et al. Capsaicin, its clinical significance in patients with painful diabetic neuropathy. Biomed. Pharmacother. 2022, 153, 113439. [Google Scholar] [CrossRef] [PubMed]
- Rios-Silva, M.; Santos-Alvarez, R.; Trujillo, X.; Cardenas-Maria, R.Y.; Lopez-Zamudio, M.; Bricio-Barrios, J.A.; Leal, C.; Saavedra-Molina, A.; Huerta-Trujillo, M.; Espinoza-Mejia, K.; et al. Effects of Chronic Administration of Capsaicin on Biomarkers of Kidney Injury in Male Wistar Rats with Experimental Diabetes. Molecules 2018, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bellini, S.; Gruden, G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin. Sci. 2022, 136, 493–520. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wei, X.; Lu, Z.; Li, L.; Hu, Y.; Sun, F.; Jiang, Y.; Ma, H.; Zheng, H.; Yang, G.; et al. Activation of TRPV1 channel antagonizes diabetic nephropathy through inhibiting endoplasmic reticulum-mitochondria contact in podocytes. Metabolism 2020, 105, 154182. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C.; Acton, R.T. Diabetes in HFE Hemochromatosis. J. Diabetes Res. 2017, 2017, 9826930. [Google Scholar] [CrossRef]
- Lopez, M.; Quintero-Macias, L.; Huerta, M.; Rodriguez-Hernandez, A.; Melnikov, V.; Cardenas, Y.; Bricio-Barrios, J.A.; Sanchez-Pastor, E.; Gamboa-Dominguez, A.; Leal, C.; et al. Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study. Molecules 2022, 27, 7764. [Google Scholar] [CrossRef]
- Panizo, S.; Martinez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martin-Carro, B.; Fernandez-Martin, J.L.; Naves-Diaz, M.; Carrillo-Lopez, N.; Cannata-Andia, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Huang, Z.; Sharma, M.; Dave, A.; Yang, Y.; Chen, Z.S.; Radhakrishnan, R. The Antifibrotic and the Anticarcinogenic Activity of Capsaicin in Hot Chili Pepper in Relation to Oral Submucous Fibrosis. Front. Pharmacol. 2022, 13, 888280. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Li, X.; Tang, S.; Meng, D.; Xia, W.; Wang, H.; Wu, Y.; Zhou, X.; Zhang, J. Capsaicin ameliorates renal fibrosis by inhibiting TGF-beta1-Smad2/3 signaling. Phytomedicine 2022, 100, 154067. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, S.; Li, D.; Zhang, Y.; Tang, B.; Qiu, C.; Yang, Y.; Yang, D. Dietary capsaicin ameliorates pressure overload-induced cardiac hypertrophy and fibrosis through the transient receptor potential vanilloid type 1. Am. J. Hypertens. 2014, 27, 1521–1529. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. Protective effect of TRPV1 against renal fibrosis via inhibition of TGF-beta/Smad signaling in DOCA-salt hypertension. Mol. Med. 2011, 17, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qian-Hui, S. A7970 Dietary capsaicin improve high salt-induced renal tubular-interstitial fibrosis by inhibiting TGF-β1/EMT pathway. J. Hypertens. 2018, 36, e51–e52. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C. Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease. Circ. Res. 2023, 132, 993–1012. [Google Scholar] [CrossRef]
- Gross, M.L.; Meyer, H.P.; Ziebart, H.; Rieger, P.; Wenzel, U.; Amann, K.; Berger, I.; Adamczak, M.; Schirmacher, P.; Ritz, E. Calcification of coronary intima and media: Immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin. J. Am. Soc. Nephrol. 2007, 2, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, I.; Moraca, F.M.; Sciangula, A.; Aquila, S.; Mazzulla, S. HIF-1α and VEGF: Immunohistochemical Profile and Possible Function in Human Aortic Valve Stenosis. Ultrastruct. Pathol. 2015, 39, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, W.; Xie, C.; Yin, L.; Su, X.; Chen, J.; Huang, H. Capsaicin Attenuates Arterial Calcification Through Promoting SIRT6-Mediated Deacetylation and Degradation of Hif1α (Hypoxic-Inducible Factor-1 Alpha). Hypertension 2022, 79, 906–917. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.T.; Joo, Y.S.; Lee, C.; Yun, H.R.; Yoo, T.H.; Kang, S.W.; Choi, K.H.; Ahn, C.; Oh, K.H.; et al. Association of Blood Pressure with the Progression of CKD: Findings from KNOW-CKD Study. Am. J. Kidney Dis. 2021, 78, 236–245. [Google Scholar] [CrossRef]
- Deng, P.Y.; Li, Y.J. Calcitonin gene-related peptide and hypertension. Peptides 2005, 26, 1676–1685. [Google Scholar] [CrossRef]
- Harada, N.; Okajima, K. Effect of capsaicin on plasma and tissue levels of insulin-like growth factor-I in spontaneously hypertensive rats. Growth Horm. IGF Res. 2008, 18, 75–81. [Google Scholar] [CrossRef]
- Staessen, J.A.; Wang, J.; Bianchi, G.; Birkenhager, W.H. Essential hypertension. Lancet 2003, 361, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Sui, D.; Garavito, R.M.; Worden, R.M.; Wang, D.H. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: Functional significance and implication. Hypertension 2009, 53, 228–235. [Google Scholar] [CrossRef]
- Gao, F.; Wang, D.H. Impairment in function and expression of transient receptor potential vanilloid type 4 in Dahl salt-sensitive rats: Significance and mechanism. Hypertension 2010, 55, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, F.; Wei, X.; Liang, Y.; Cui, Y.; Gao, F.; Zhong, J.; Pu, Y.; Zhao, Y.; Yan, Z.; et al. Transient receptor potential vanilloid 1 activation by dietary capsaicin promotes urinary sodium excretion by inhibiting epithelial sodium channel alpha subunit-mediated sodium reabsorption. Hypertension 2014, 64, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.; Pisano, A.; Rivoli, L.; Bolignano, D. Renal denervation for resistant hypertension. Cochrane Database Syst. Rev. 2017, 2, CD011499. [Google Scholar] [CrossRef]
- Stocker, S.D.; Sullivan, J.B. Deletion of the Transient Receptor Potential Vanilloid 1 Channel Attenuates Sympathoexcitation and Hypertension and Improves Glomerular Filtration Rate in 2-Kidney-1-Clip Rats. Hypertension 2023, 80, 1671–1682. [Google Scholar] [CrossRef]
- Wang, D.H.; Zhao, Y. Increased salt sensitivity induced by impairment of sensory nerves: Is nephropathy the cause? J. Hypertens. 2003, 21, 403–409. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: Role of the transient receptor potential vanilloid type 1. Hypertension 2006, 47, 609–614. [Google Scholar] [CrossRef]
- Xie, C.; Wang, D.H. Effects of a high-salt diet on TRPV-1-dependent renal nerve activity in Dahl salt-sensitive rats. Am. J. Nephrol. 2010, 32, 194–200. [Google Scholar] [CrossRef]
- Segawa, Y.; Hashimoto, H.; Maruyama, S.; Shintani, M.; Ohno, H.; Nakai, Y.; Osera, T.; Kurihara, N. Dietary capsaicin-mediated attenuation of hypertension in a rat model of renovascular hypertension. Clin. Exp. Hypertens. 2020, 42, 352–359. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, F.; Wang, J.X.; Wang, X.L.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Dysregulation of the Excitatory Renal Reflex in the Sympathetic Activation of Spontaneously Hypertensive Rat. Front. Physiol. 2021, 12, 673950. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Q.; Ma, S.; Wang, D.H. Ablation of TRPV1-positive nerves exacerbates salt-induced hypertension and tissue injury in rats after renal ischemia-reperfusion via infiltration of macrophages. Clin. Exp. Hypertens. 2021, 43, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.H. Anticancer Properties of Capsaicin Against Human Cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Kaiser, M.; Pohl, L.; Ketelhut, S.; Kastl, L.; Gorzelanny, C.; Gotte, M.; Schnekenburger, J.; Goycoolea, F.M.; Kemper, B. Nanoencapsulated capsaicin changes migration behavior and morphology of madin darby canine kidney cell monolayers. PLoS ONE 2017, 12, e0187497. [Google Scholar] [CrossRef]
- Que, T.; Ren, B.; Fan, Y.; Liu, T.; Hou, T.; Dan, W.; Liu, B.; Wei, Y.; Lei, Y.; Zeng, J.; et al. Capsaicin inhibits the migration, invasion and EMT of renal cancer cells by inducing AMPK/mTOR-mediated autophagy. Chem. Biol. Interact. 2022, 366, 110043. [Google Scholar] [CrossRef]
- Morelli, M.B.; Marinelli, O.; Aguzzi, C.; Zeppa, L.; Nabissi, M.; Amantini, C.; Tomassoni, D.; Maggi, F.; Santoni, M.; Santoni, G. Unveiling the Molecular Mechanisms Driving the Capsaicin-Induced Immunomodulatory Effects on PD-L1 Expression in Bladder and Renal Cancer Cell Lines. Cancers 2022, 14, 2644. [Google Scholar] [CrossRef]
- Liu, T.; Wang, G.; Tao, H.; Yang, Z.; Wang, Y.; Meng, Z.; Cao, R.; Xiao, Y.; Wang, X.; Zhou, J. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer 2016, 16, 790. [Google Scholar] [CrossRef]
- Makhlough, A.; Ala, S.; Haj-Heydari, Z.; Kashi, Z.; Bari, A. Topical capsaicin therapy for uremic pruritus in patients on hemodialysis. Iran. J. Kidney Dis. 2010, 4, 137–140. [Google Scholar]
- Murphy, M.; Carmichael, A.J. Renal itch. Clin. Exp. Dermatol. 2000, 25, 103–106. [Google Scholar] [CrossRef]
- Hall, O.M.; Broussard, A.; Range, T.; Carroll Turpin, M.A.; Ellis, S.; Lim, V.M.; Cornett, E.M.; Kaye, A.D. Novel Agents in Neuropathic Pain, the Role of Capsaicin: Pharmacology, Efficacy, Side Effects, Different Preparations. Curr. Pain Headache Rep. 2020, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Shamsheer, R.; Sunoqrot, S.; Kasabri, V.; Shalabi, D.; Alkhateeb, R.; Alhiari, Y.; Ababneh, R.; Ikhmais, B.; Abumansour, H. Preparation and Characterization of Capsaicin Encapsulated Polymeric Micelles and Studies of Synergism with Nicotinic Acids as Potential Anticancer Nanomedicines. J. Pharm. Bioallied Sci. 2023, 15, 107–125. [Google Scholar] [CrossRef]
- Jia, Y.; Lee, L.Y. Role of TRPV receptors in respiratory diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 915–927. [Google Scholar] [CrossRef]
| Authors | Models | Results |
|---|---|---|
| Han et al. [48] | HK-2 cells treated with ATP and LPS | Capsaicin preincubation ameliorated LPS-induced cytotoxicity through TRPV1/UCP2 axis activation by reducing IL-1β, IL-18, and ROS release. |
| Ran et al. [49] | Dehydrated C57BL/6J mice treated with the contrast medium iodixanol | Preventive capsaicin administration reduced contrast-induced AKI through Nrf2 activation by decreasing superoxide, renal malondialdehyde, and apoptotic tubular cells and improving mitochondrial function. |
| Shimeda et al. [51] | Male Sprague–Dawley rats treated with cisplatin | Dietary capsaicin reduced cisplatin-induced renal damage by reducing lipid peroxidation. |
| Aldossary et al. [50] | AKI following methotrexate intoxication in rats | Capsaicin administration reduced methotrexate-induced renal damage by anti-inflammatory and antioxidant effects. |
| Tsagogiorgas et al. [53] | Inbred male Lewis rats treated with NOD | Treatment with the synthetic analogue of capsaicin NOD had renoprotective effects against ischemia-induced AKI through TRPV1 activation by inhibiting TNF-α mediated inflammation and through production of the vasodilator peptides CGRP and SP. |
| Yu et al. [55] | Male Wistar rats fed with high-salt diet | Capsaicin injection reduced renal inflammation driven by high-salt diet, oxidative stress, and fibrosis through activation of TRPV1. |
| Yu et al. [57] | Rats fed with high-salt diet after ischemia–reperfusion damage | Capsaicin inhibited renal sympathetic nerve activity by activating TRPV1 receptors, which prevented the appearance of salt sensitivity following renal ischemia–reperfusion damage. |
| Ueda et al. [58] | Uninephrectomized male Sprague–Dawley rats developing AKI following renal artery and vein occlusion | Treatment with capsaicin or its analogue resiniferatoxin reduced ischemia–reperfusion renal damage by reducing neutrophil infiltration, superoxide production, and TNF-α production and by increasing IL-10 production. |
| Authors | Model | Results |
|---|---|---|
| Harada et al. [80] | Spontaneously hypertensive rats and Wistar Kyoto rats | Capsaicin administration increased CGRP and IGF-1 plasma levels in SHR as compared to those reported in WKR. |
| Gao et al. [82] | Male Wistar rats fed with normal sodium diet and high sodium diet | HS diet induced TRPV4 expression in mesenteric arteries and sensory nerves with following increase in CGRP and IGF-1 levels. HS diet induced a marked increase of blood pressure when TRPV4 channel was blocked. |
| Li et al. [84] | C57BL/6 wild-type mice and TRPV1-/- mice | Dietary capsaicin induced natriuretic effect by inhibiting WNK1/SGK1/aENaC pathway with consequent reduction of aENaC expression at the renal level. Dietary capsaicin reduced HS diet-induced hypertension through TRPV1 activation. |
| Stocker et al. [86] | 2-kidney-1-clip (2K1C) wild-type rats and 2K1C TRPV1-/- rats | TRPV1 channels deprivation in presence of capsaicin caused reduction in blood pressure and increase in the glomerular filtration rate due to the lack of sympathetic activity. |
| Ye et al. [91] | Spontaneously hypertensive rats and Wistar Kyoto rats | Renal infusion of capsaicin increased contralateral renal sympathetic nerve activation, causing an increase in blood pressure through a renal nerve reflex mediated by the paraventricular nucleus. |
| Segawa et al. [90] | 2K1C rats and sham-operated rats | Dietary capsaicin reduced nephrovascular hypertension by promoting phosphorylation of Akt and eNOS, thus enhancing NO release. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musolino, M.; D’Agostino, M.; Zicarelli, M.; Andreucci, M.; Coppolino, G.; Bolignano, D. Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease. Int. J. Mol. Sci. 2024, 25, 791. https://doi.org/10.3390/ijms25020791
Musolino M, D’Agostino M, Zicarelli M, Andreucci M, Coppolino G, Bolignano D. Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease. International Journal of Molecular Sciences. 2024; 25(2):791. https://doi.org/10.3390/ijms25020791
Chicago/Turabian StyleMusolino, Michela, Mario D’Agostino, Mariateresa Zicarelli, Michele Andreucci, Giuseppe Coppolino, and Davide Bolignano. 2024. "Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease" International Journal of Molecular Sciences 25, no. 2: 791. https://doi.org/10.3390/ijms25020791
APA StyleMusolino, M., D’Agostino, M., Zicarelli, M., Andreucci, M., Coppolino, G., & Bolignano, D. (2024). Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease. International Journal of Molecular Sciences, 25(2), 791. https://doi.org/10.3390/ijms25020791







