Abstract
Acute pancreatitis (AP) is a significant cause of morbidity, even in children, and is frequently associated with systemic manifestations. There are many cytokines involved in the inflammatory response characteristic of this disease. Interleukin 6 (IL-6) is one of the most important cytokines involved in AP, beginning from cellular injury and continuing to the systemic inflammatory response and distant organ involvement. IL-6 is a multifunctional cytokine that regulates acute-phase response and inflammation. It is produced by various cells and exerts its biological role on many cells through its high-affinity complex receptor. IL-6 has been investigated as a predicting maker for severe forms of AP. Many studies have validated the use of IL-6 serum levels in the first 48 h as a reliable marker for severe evolution and multisystemic involvement. Still, it has not been used in daily practice until now. This review discusses the main binding mechanisms by which IL-6 triggers cellular response and the AP pathogenetic mechanisms in which IL-6 is involved. We then emphasize the promising role of IL-6 as a prognostic marker, which could be added as a routine marker at admission in children with AP.
1. Introduction
Acute pancreatitis (AP) is a rare cause of abdominal pain with the possibility of severe evolution. In recent years, AP incidence has increased in adults and children [1,2].
The AP episodes are divided based on severity according to the revised Atlanta classification, which recognizes three forms of AP: mild (without local or systemic complications), moderate (with local complications like pancreatic/peripancreatic collections and/or organ dysfunction with resolution in less than 48 h), and severe (with organ dysfunction for more than 48 h or developing multiple organ dysfunction syndrome, MODS) [3].
Acute injury to the pancreas occurs after the exposure of acinar cells to various aggressions (gallstones, infections, trauma, toxins, or metabolites [4]. In daily practice, the existence of a pancreatic injury and its severity are monitored by performing specific tests (amylase and lipase). The increase in the serum level of these parameters confirms pancreatic suffering. It gives us an overview of the severity of the injury but does not provide a precise picture of the evolutive potential of the disease. For this reason, new markers that could predict the unfavorable evolution of AP early-on have been studied. Some scoring systems have been developed but are hard to apply and do not have a good predictive value [5]. Interleukin 6 (IL-6) is a biological marker that has been intensively analyzed to determine the severity of pancreatic damage, with promising results.
This review describes the involvement of IL-6 in the pathogenesis of AP and summarizes the data regarding using IL-6 as a prognostic marker for severe forms.
2. IL-6 and IL-6 Receptor
IL-6 is a multifunctional cytokine involved in many pathophysiological processes, such as infections; inflammation; and neuroendocrine, vascular, and malignant diseases [6,7,8]. It represents the primary molecule of the IL-6 family of cytokines, alongside nine other cytokines (IL-11, IL-27, IL-35, IL-39, oncostatin M, leukemia inhibitory factor, cardiotrophin 1, cardiotrophin-like cytokine factor 1, and ciliary neurotrophic factor) [7]. The serum levels of IL-6 in a physiological state are very low, but these levels increase extremely rapidly in the first phases of infections and inflammation [6].
Since the demonstration of the important role of the liver in acute-phase protein synthesis by Miller et al., the existence of some hormone-like molecules that can induce liver response has been proposed [9,10]. Initially, a monocyte-derived polypeptide involved in acute-phase protein synthesis was described and named hepatocyte-stimulating factor [9,10]. After the discrimination of IL-1 and TNFα from hepatocyte-stimulating factor, B-cell stimulatory factor 2 was described as a 26 kDa molecule and later named interleukin 6 [9,11]. The first strong evidence for the significant role of IL-6 in acute-phase reaction came from studies on cellular cultures. In human hepatocyte cultures, IL-6 determined the synthesis of C-reactive protein and serum amyloid in a dose- and time-dependent mode [9,12].
IL-6 represents a key mediator in the regulation and coordination of the immune response, with most immune cells being able to produce IL-6. In the initial stage of inflammation, IL-6 is synthesized locally. Subsequently, it quickly migrates to the liver, favoring the synthesis of acute-phase proteins (C-reactive protein, serum amyloid A, fibrinogen, haptoglobin, and α1-antichymotrypsin) [9].
Besides the effect on hepatocytes, IL-6 affects other cells, like B cells. IL-6 acts directly on B cells previously activated by IL-4 and IL-5 and leads to immunoglobulin A, M, and G secretion. IL-6 cannot act directly on normal resting B cells because they do not express IL-6 receptors [9,13,14].
There are two distinct pathways for the IL-6 action in the different cells (classical and trans-signaling). The first one is represented by the IL-6-specific cell receptors (IL-6R). This receptor has two subunits: an 80 kDa ligand-binding subunit (gp80) and a 130 kDa signal-transducing protein (gp130). The extracellular portion of the 80 kDa IL-6 receptor does not directly contribute to IL-6 binding. Still, it increases the affinity of the ligand for the gp130 subunit, which binds IL-6 and mediates the transduction of the signal. This mechanism is called the classical signaling pathway. It depends on both receptor subunits on the cell’s surface, which are expressed only on hepatocytes and certain subpopulations of leukocytes [6,15,16]. In the second pathway, IL-6 binds to a soluble form of the IL-6R (sIL-6R) and forms a complex (IL-6/sIL-6R) that increases the bioavailability of circulating IL-6, allowing its action on cells that lack IL-6R but express gp130. Thus, any cell that expresses gp130 can gain responsiveness to IL-6 through the trans-signaling pathway. Given that gp130 is largely expressed in immune and nonimmune cells, it explains the involvement of IL-6 in many pathologies. Both mechanisms activate the Janus kinase/STAT (JAK/STAT) signal transduction pathway [6,16]. The JAK/STAT pathways are important in immune response, contributing to the body’s defense against infections, maintaining immune tolerance, and strengthening barrier function [17]. JAK is a non-receptor tyrosine-protein kinase activated by different cytokines, which plays an important role in regulatory signal transmission. The JAK family has four main members (JAK1, JAK2, JAK3, and TYK2) expressed in almost all tissues. The STAT family is composed of seven members (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6) [17].
JAK/STAT is a common signaling pathway that transduces signals from the extracellular to the intracellular (nucleus). More than 50 cytokines, growth factors, and hormones are implicated in the JAK/STAT pathway, interfering with hematopoiesis, immune response, tissue repair, inflammation, apoptosis, and adipogenesis [17]. The two signaling pathways for IL-6 are represented in Figure 1A.
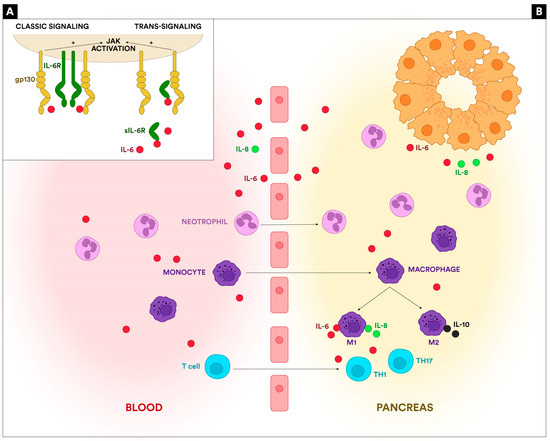
Figure 1.
(A): The IL-6 signaling. The classic pathway can be activated on cells that express both IL-6R and gp130. The trans-signaling pathway occurs through a soluble form of IL-6R that binds IL-6 and forms a complex that can act on cells that lack the IL-6R but express gp130. Both pathways activate the JAK/STAT3 cascade. (B): The involvement of IL-6 in AP pathogenesis. IL-6 is released by the necrotic acinar cells, triggers the recruitment of inflammatory cells in the pancreas (neutrophils, monocytes, T lymphocytes), and activates the resident macrophages. IL-6 also determines the activation of Th1 and Th17 cells.
3. IL-6 in the Pathogenesis of Acute Pancreatitis
The initiating mechanism in AP is the damage to the acinar cells. Necrotic acinar cells release damage-associated molecular patterns (DAMPs) and proinflammatory cytokines represented by IL-6, tumor necrosis factor (TNF-alpha), and interleukin-8 (IL-8), as well as anti-inflammatory cytokines, including interleukin-10 (IL-10). IL-6 is a multifunctional cytokine with pro- and anti-inflammatory properties and is one of the first cytokines involved in the inflammation associated with AP [15,18]. The cytokines released initiate the recruitment of phagocytes (neutrophils and macrophages) in the pancreatic tissue. IL-6 is closely involved in neutrophil migration by trans-signaling on endothelial, smooth muscle, and epithelial cells and by upregulating the expression of adhesion molecules like ICAM-1 and VCAM-1 [6,19]. After the neutrophil influx, IL-6 is involved in their burst, thus participating in the proinflammatory activity mediated by elastase, reactive oxygen species (ROS), and myeloperoxidase (MPO). More importantly, IL-6 is involved in neutrophil apoptosis, participating in the prevention of excessive inflammation [6,20]. IL-6 is leading to the shift from neutrophil recruitment in the pancreas to mononuclear cell recruitment and macrophage differentiation [6]. Macrophage differentiation is one of the most important mechanisms determining AP’s severity. The imbalance between M1 (proinflammatory) and M2 (anti-inflammatory) macrophage subtypes determines increased severity through extreme inflammation [21]. IL-6 plays a bivalent role: on the one hand, it is one of the cytokines secreted by M1-activated macrophages that induce a Th1-type response and inflammatory augmentation, secreting reactive oxygen species, chemokines, and cytokines; on the other hand, it can promote alternative activation of macrophages to the wound-healing phenotype [6,22]. The role of IL-6 in macrophage stimulation is not limited to the pancreatic resident macrophages. IL-6 and other proinflammatory cytokines migrate to the bloodstream and, through the portal vein, reach the liver and, afterward, the lung, activating Kupffer cells and alveolar macrophages, thus increasing inflammation and participating in the systemic progression of AP [22].
Furthermore, IL-6 plays an essential role in the acquired immune mechanisms involved in AP. IL-6 promotes the differentiation of naïve CD4 T-cells, thus being involved in the imbalance of the Th1/Th2 ratio, one of the most important mechanisms involved in AP severity [23,24]. IL-6 is also an important promotor of Th17 cell proliferation and IL-17 secretion, increasing inflammatory response, primarily through activating STAT3 in naïve CD4 cells [18]. The activation of the JAK/STAT signaling pathway via the signal transducing gp130 leads to the expression of various critical mediators of inflammation. It has been demonstrated that the JAK/STAT pathway activation promotes the progression of acute or chronic pancreatitis and can be the trigger for pancreatic tumors [25]. Another essential function of IL-6 is the stimulation of mature B cells, thus increasing levels of B-cell-activating factor (BAFF), an important marker of inflammation and severity in AP [6,26]. IL-6 is not only an inflammatory cytokine but also has anti-inflammatory action on T cells, stimulating the production of IL-10 [6]. The involvement of IL-6 in AP pathogenesis is shown in Figure 1B.
4. IL-6 as a Prognosis Marker for Acute Pancreatitis
Predicting the severity of AP episodes is one of the most critical concerns due to the high mortality and complications associated with severe, necrotic forms. There are several scoring systems developed for adults that can be useful but do not have sufficient accuracy. Another inconvenience of these scores is that they cannot be used in the pediatric population. Thus, finding a good marker with a high predictive value that is easy to use in children is desirable [27]. IL-6 is one of the most studied cytokines for this purpose. Since IL-6 is released beginning with the phase of cellular injury, it is one of the first inflammatory markers to be detected in the blood.
A study conducted by Dambrauskas et al. on 108 patients with AP demonstrated the utility of IL-6 as a prognostic marker compared with other cytokines. The evaluation of these markers was made at admission and was compared to the evolution based on imaging findings on CT on days four and seven. This study analyzed the prediction value of five cytokines (IL-6, IL-8, IL-10, MIF—macrophage migration inhibitory factor, and LTB4- leukotriene B4) for the local and systemic complications and fatal outcomes. IL-6 and MIF had the best predicting value in discriminating mild from severe forms, as well as the development of systemic complications (systemic inflammatory response syndrome—SIRS and multiple organ failure—MOF) and fatal outcomes. IL-6 was superior to MIF in differentiating edematous from necrotic forms [28].
Another study conducted by Ceranic et al. showed the superiority of IL-6 compared to other cytokine and severity scores. The study included 96 patients with AP. Severity scores (BISAP and Ranson) were calculated, and IL-6, IL-8, and IL-10 were measured at admission and after 48 h. IL-6 had a higher predictive value for severity at admission but also in the 48-h follow-up compared to IL-8 and IL-10. Furthermore, compared to the Ranson score, IL-6 measured at 48 h after admission exhibited similar predictive values for severe evolution [29].
Sternby et al. investigated the evolution of seven markers (IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12, and TNF-α) over the first 48 h from the onset of the disease. This study concentrated on comparing the mean value of these parameters at the beginning and after the first 24 h of onset to identify the best timing for their predictive value. There were 115 patients included, classified as mild (71), moderate (33), and severe (11) according to the modified Atlanta criteria. All parameters except TNF-α had significant differences between the mild and severe groups. Still, only for IL-6, the delta values (the difference between the first and second mean values) varied significantly between all three severity groups. The main finding of this study was that the mean value of each severity group differed considerably after 24 h for IL-1β, IL-6, IL-8, and IL-10, with the most evident rise for IL-6. This may be useful for the identification of the best timing for the evaluation of these prognostic markers [30].
Kolber et al. also investigated the usefulness of IL-6 as a predicting marker for severity at admission and after 24 h. They enrolled 95 patients with AP, classified according to modified Atlanta criteria: twenty-nine mild, fifty-eight moderate, and eight severe. This study did not find significant differences between IL-6 levels upon admission and after 24 h of hospitalization. Patients with SAP had significantly higher levels of IL-6 on both days compared to the moderate and mild groups. Moreover, higher levels of IL-6 were observed in patients who developed necrosis. The level measured on the second day correlated with hospital length. There was no significant difference between the prediction accuracy of IL-6 compared with multi-variable scores (BISAP, Ranson’s, or APACHE II). This is a very important finding, given that these scores are sometimes hard to determine or cannot be applied, especially if we refer to the pediatric population [31].
The study conducted by Jain et al. demonstrated the utility of IL-6 in association with SIRS for increased precision in detecting severe cases. The study was conducted on 115 patients with mild, moderate, and severe AP. SIRS was diagnosed in the first 72 h of the onset of the disease, and additionally, IL-6, TNF-α, IL-10, MCP-1, GM-CSF, and IL-1β were measured. The study revealed that the combination of SIRS at admission and IL-6 >160 pg/mL had a significantly higher PPV than SIRS alone (85% vs. 56%) and a specificity of 95%. From the other cytokines, only MPC-1 showed good results in differentiating mild from severe forms, but none of them were able to increase the sensitivity in association with SIRS. The association of IL-6 measured at admission and SIRS can be an easy-to-use tool for the early identification of severe forms, especially in children, where the other scores of severity cannot be applied [32].
Tian et al. also investigated the usefulness of the combined detection of four serum markers. They included 153 patients with AP, 81 mild and 72 severe forms. CRP, PCT, IL-6, and LDH levels were measured at admission. Each of them showed significantly different levels between mild and severe forms. However, combining these four parameters measured at admission, the sensitivity and specificity for the early detection of severe forms were significantly increased. This combination could be a simple and cost-efficient test for early severity stratification [33].
Many other studies have evaluated the value of IL-6 as a marker for prognosis in AP, and the results are promising (Table 1). IL-6 serum levels are significantly elevated in patients with AP compared with controls, and there are differences between mild and severe forms [34,35,36,37,38,39,40,41,42,43,44,45,46,47]. IL-6 levels are higher in patients with distant organ failure and MOSF (multiple organ system failure) [44,45,46,47,48]. Some studies have shown a better predictive value for severe forms than other serum markers (CRP, IL-8, IL-1, IL-10, and TNF) [49,50,51,52,53,54,55,56,57,58,59]. Other studies have shown an increased sensibility for the association of IL-6 with other markers like CRP or PCT [60,61,62,63,64].

Table 1.
Summarization of the evidence regarding the importance of IL-6 as a predictor marker for AP.
Although there are other inflammatory markers, like CRP, that are more available and already used for severity prognosis in AP, many studies show a superior predictive value of IL-6, especially in the very early stages [43,53]. It was observed that CRP peaks at 72 h of admission, having a delay of 24-48 h compared to IL-6, making IL-6 a more precocious marker [34,36]. CRP had a relatively high value in sensitivity and accuracy after the second day of admission in predicting severe forms, necrosis, and mortality [44,54]. The study conducted by De Beaux et al. showed there was no significant difference in the median serum levels of CRP on the first day of admission among patients who developed organ failure or a local pancreatic complication and those who had mild disease. By the second day of admission, the median serum level of CRP was greater in patients who developed organ failure than in those with a local pancreatic complication (p = 0.02) or with mild disease alone (p = 0.003) [48]. All these data, alongside other studies showing the usefulness of the combined assessment of IL-6 and CRP, concluded that IL-6 is not a replacement for CRP but rather an earlier or a complementary marker.
5. Limitations and Future Directions
As shown above, numerous data support the usefulness of IL-6 as an early predictor for severity in AP. Still, it has not been introduced in clinical practice because of some limitations. Firstly, IL-6 is involved in the very first stages of AP, and it is not stored in cells, meaning that it could have a rapid increase but also a decrease in serum levels. Thus, the measurement of serum levels has to be done close to the onset of the symptoms. In clinical practice, this may prove difficult, as it is sometimes hard to determine the exact timeline. Although all studies discussed above demonstrated the utility of IL-6 as a severity predictor, the timing for measurement is not very well-established. The majority of data indicate the first 48 h after admission as the best timeframe.
Secondly, there are no clear cut-off values. Some of the studies did not evaluate this parameter, or in the studies that are present, there are extremely large variations.
Moreover, the cost and availability of cytokine measurement is another limitation for routine use in daily practice. Although in the past this was the primary limitation, currently more and more methods are widely available with moderate costs. Furthermore, a closer analysis of the other severity scores shows us that they are time-consuming and need other laboratory parameters and imaging evaluation. At the same time, IL-6 measurement is rapid and can be used in children as well as adults.
Serum IL-6 measured in the first 48 h of admission could be used in clinical practice as a single parameter or as part of a new score associated with other serum markers. A point-of-care test for IL-6 has been used in different diseases and could be used in AP for decision-making and treatment strategy. More data is needed to establish unitary cut-off values for severity stages and systemic complications.
6. Conclusions
Severity stratification and prediction of severe, necrotic forms of AP is essential in managing the disease due to its rapid and unpredictable evolution. A reliable serum marker or a combination of markers could be the best approach for more standardized care. IL-6 is the most promising marker, with the best results, and should enter the panel of markers analyzed in patients with AP, including children.
Author Contributions
Conceptualization, A.M., A.G., M.-C.C., G.B., M.-S.P. and T.L.P.; methodology, A.M. and A.G; software, A.M.; resources, A.M. and A.G.; data curation, A.M., A.G., M.-C.C., G.B., M.-S.P. and T.L.P.; writing—original draft preparation, A.M., A.G., M.-C.C. and G.B.; writing—review and editing, A.G., M.-S.P. and T.L.P.; supervision, T.L.P.; project administration, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We would like to thank Marina Perta for the design of the figure.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.-A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Morinville, V.D.; Barmada, M.M.; Lowe, M.E. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: Is greater awareness among physicians responsible? Pancreas 2010, 39, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Wang, G.J.; Gao, C.F.; Wei, D.; Wang, C.; Ding, S.Q. Acute pancreatitis: Etiology and common pathogenesis. World J. Gastroenterol. 2009, 15, 1427–1430. [Google Scholar] [CrossRef]
- Sigounas, D.E.; Tatsioni, A.; Christodoulou, D.K.; Tsianos, E.V.; Ioannidis, J.P. New prognostic markers for outcome of acute pancreatitis: Overview of reporting in 184 studies. Pancreas 2011, 40, 522–532. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457, Correction in Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Colceriu, M.-C.; Aldea, P.L.; Răchişan, A.-L.B.; Bulată, B.; Delean, D.; Grama, A.; Mititelu, A.; Decea, R.M.; Sevastre-Berghian, A.; Clichici, S.; et al. The Utility of Noninvasive Urinary Biomarkers for the Evaluation of Vesicoureteral Reflux in Children. Int. J. Mol. Sci. 2023, 24, 17579. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.L.; Bly, C.G.; Watson, M.L.; Bale, W.F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J. Exp. Med. 1951, 94, 431–453. [Google Scholar] [CrossRef]
- Poupart, P.; Vandenabeele, P.; Cayphas, S.; Van Snick, J.; Haegeman, G.; Kruys, V.; Fiers, W.; Content, J. B cell growth modulating and differentiating activity of recombinant human 26-kd protein (BSF-2, HuIFN-beta 2, HPGF). EMBO J. 1987, 6, 1219–1224. [Google Scholar] [CrossRef]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Hirano, T.; Kishimoto, T.; Heinrich, P.C. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett. 1988, 232, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.-I.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Taga, T.; Kawanishi, Y.; Hardy, R.R.; Hirano, T.; Kishimoto, T. Receptors for B cell stimulatory factor 2. Quantitation, specificity, distribution, and regulation of their expression. J. Exp. Med. 1987, 166, 967–981. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Metcalfe, R.D.; Putoczki, T.L.; Griffin, M.D.W. Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11. Front. Immunol. 2020, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.-S.; Zeng, J.; Mei, J.; Wang, P.-Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R. Update on innate immunity and perspectives on metabolite regulation in acute pancreatitis. Curr. Opin. Gastroenterol. 2016, 32, 507–512. [Google Scholar] [CrossRef]
- Habtezion, A.; Algul, H. Immune modulation in acute and chronic pancreatitis. Pancreapedia Exocrine Pancreas Knowl. Base 2016. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Peng, C.; Li, Z.; Yu, X. The Role of Pancreatic Infiltrating Innate Immune Cells in Acute Pancreatitis. Int. J. Med Sci. 2021, 18, 534–545. [Google Scholar] [CrossRef]
- Hu, F.; Lou, N.; Jiao, J.; Guo, F.; Xiang, H.; Shang, D. Macrophages in pancreatitis: Mechanisms and therapeutic potential. Biomed. Pharmacother. 2020, 131, 110693. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Zhuang, Q.; Huang, L.; Zeng, Y.; Wu, X.; Qiao, G.; Liu, M.; Wang, L.; Zhou, Y.; Xiong, Y. Dynamic Monitoring of Immunoinflammatory Response Identifies Immunoswitching Characteristics of Severe Acute Pancreatitis in Rats. Front. Immunol. 2022, 13, 876168. [Google Scholar] [CrossRef]
- Lesina, M.; Wörmann, S.M.; Neuhöfer, P.; Song, L.; Algül, H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin. Immunol. 2014, 26, 80–87. [Google Scholar] [CrossRef]
- Pongratz, G.; Hochrinner, H.; Straub, R.H.; Lang, S.; Brünnler, T. B cell activating factor of the tumor necrosis factor family (BAFF) behaves as an acute phase reactant in acute pancreatitis. PLoS ONE. 2013, 8, e54297. [Google Scholar] [CrossRef]
- Suzuki, M.; Sai, J.K.; Shimizu, T. Acute pancreatitis in children and adolescents. World J. Gastrointest Pathophysiol. 2014, 5, 416–426. [Google Scholar] [CrossRef]
- Dambrauskas, Z.; Giese, N.; Gulbinas, A.; Giese, T.; Berberat, P.O.; Pundzius, J.; Barauskas, G.; Friess, H. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J. Gastroenterol. 2010, 16, 1845–1853. [Google Scholar] [CrossRef]
- Ćeranić, D.B.; Zorman, M.; Skok, P. Interleukins and inflammatory markers are useful in predicting the severity of acute pancreatitis. Bosn. J. Basic Med Sci. 2020, 20, 99–105. [Google Scholar] [CrossRef]
- Sternby, H.; Hartman, H.; Thorlacius, H.; Regnér, S. The Initial Course of IL-1β, IL-6, IL-8, IL-10, IL-12, IFN-γ and TNF-α with Regard to Severity Grade in Acute Pancreatitis. Biomolecules 2021, 11, 591. [Google Scholar] [CrossRef]
- Kolber, W.; Dumnicka, P.; Maraj, M.; Kuśnierz-Cabala, B.; Ceranowicz, P.; Pędziwiatr, M.; Maziarz, B.; Mazur-Laskowska, M.; Kuźniewski, M.; Sporek, M.; et al. Does the Automatic Measurement of Interleukin 6 Allow for Prediction of Complications during the First 48 h of Acute Pancreatitis? Int. J. Mol. Sci. 2018, 19, 1820. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Midha, S.; Mahapatra, S.J.; Gupta, S.; Sharma, M.K.; Nayak, B.; Jacob, T.G.; Shalimar; Garg, P.K. Interleukin-6 significantly improves predictive value of systemic inflammatory re-sponse syndrome for predicting severe acute pancreatitis. Pancreatology 2018, 18, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, H.; Wang, L.; Li, B.; Aibibula, M.; Zhao, H.; Feng, N.; Lv, J.; Zhang, G.; Ma, X. The diagnostic value of serum C-reactive protein, procalcitonin, interleukin-6 and lactate dehydrogenase in patients with severe acute pancreatitis. Clin. Chim. Acta 2020, 510, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Hoshino, M.; Hayakawa, T.; Ohara, H.; Yamada, T.; Yamada, H.; Iida, M.; Nakazawa, T.; Ogasawara, T.; Uchida, A.; et al. Interleukin-6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas 1997, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pooran, N.; Indaram, A.; Singh, P.; Bank, S. Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of severe acute pancreatitis. J. Clin. Gastroenterol. 2003, 37, 263–266. [Google Scholar] [CrossRef]
- Berney, T.; Gasche, Y.; Robert, J.; Jenny, A.; Mensi, N.; Grau, G.; Vermeulen, B.; Morel, P. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas 1999, 18, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kostic, I.; Spasic, M.; Stojanovic, B.; Jurisevic, M.; Radovanovic, D.; Canović, D.; Stefanovic, S.; Jankovic, S. Early cytokine profile changes in interstitial and necrotic forms of acute pancreatitis. Exp. Clin. Res. 2015, 16, 33–37. [Google Scholar] [CrossRef]
- Viedma, J.A.; Pérez-Mateo, M.; Domínguez, J.E.; Carballo, F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut 1992, 33, 1264–1267. [Google Scholar] [CrossRef]
- Park, J.; Chang, J.H.; Park, S.H.; Lee, H.J.; Lim, Y.S.; Kim, T.H.; Kim, C.W.; Han, S.W. Interleukin-6 is associated with obesity, central fat distribution, and disease severity in patients with acute pancreatitis. Pancreatology 2015, 15, 59–63. [Google Scholar] [CrossRef]
- Gunjaca, I.; Zunic, J.; Gunjaca, M.; Kovac, Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation 2012, 35, 758–763. [Google Scholar] [CrossRef]
- Riché, F.C.; Cholley, B.P.; Laisné, M.-J.C.; Vicaut, E.; Panis, Y.H.; Lajeunie, E.J.; Boudiaf, M.; Valleur, P.D. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery 2003, 133, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.B.; Karim, T.; Jain, A.; Arora, S.; Katiyar, V.K.; Patel, G. Role of Serum Interleukin-6 and C-reactive Protein in Early Prediction of Severe Acute Pancreatitis. West Afr. Coll. Surg. 2022, 12, 20–26. [Google Scholar] [CrossRef]
- Leser, H.-G.; Gross, V.; Scheibenbogen, C.; Heinisch, A.; Salm, R.; Lausen, M.; Rückauer, K.; Andreesen, R.; Farthmann, E.; Schölmerich, J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology 1991, 101, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.K.; Meher, S.; Prakash, S.; Tiwary, S.K.; Singh, U.; Srivastava, A.; Dixit, V.K. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013, 2013, 367581. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, M.L.; Hansen, M.B.M.; Andersen, A.M.; Ersbøll, A.K.M.; Nielsen, O.H.M.; Jørgensen, L.N.M.; Novovic, S. Cytokines and organ failure in acute pancreatitis: Inflammatory response in acute pancreatitis. Pancreas 2012, 41, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayan, G.; Garg, P.K.; Prasad, H.; Tandon, R.K. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 2007, 22, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, S.; Zhou, F.; Zhuang, M.; Fei, S. The relationship between inflammatory cytokines and in-hospital complications of acute pancreatitis. Immun. Inflamm. Dis. 2024, 12, e1203. [Google Scholar] [CrossRef] [PubMed]
- De Beaux, A.C.; Goldie, A.S.; Ross, J.A.; Carter, D.C.; Fearon, K.C. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br. J. Surg. 1996, 83, 349–353. [Google Scholar] [CrossRef]
- Gregoric, P.; Sijacki, A.; Stankovic, S.; Radenkovic, D.; Ivancevic, N.; Karamarkovic, A.; Popovic, N.; Karadzic, B.; Stijak, L.; Stefanovic, B.; et al. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepatogastroenterology 2010, 57, 349–353, Correction in Hepatogastroenterology 2011, 58, 263. [Google Scholar] [CrossRef]
- Karpavicius, A.; Dambrauskas, Z.; Gradauskas, A.; Samuilis, A.; Zviniene, K.; Kupcinskas, J.; Brimas, G.; Meckovski, A.; Sileikis, A.; Strupas, K. The clinical value of adipokines in predicting the severity and outcome of acute pancreatitis. BMC Gastroenterol. 2016, 16, 99. [Google Scholar] [CrossRef]
- Pezzilli, R.; Billi, P.; Miniero, R.; Fiocchi, M.; Cappelletti, O.; Morselli-Labate, A.M.; Barakat, B.; Sprovieri, G.; Miglioli, M. Serum interleukin-6, interleukin-8, and beta 2-microglobulin in early assessment of severity of acute pancreatitis. Comparison with serum C-reactive protein. Dig. Dis. Sci. 1995, 40, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Curley, P.J.; McMahon, M.J.; Lancaster, F.; E Banks, R.; Barclay, G.R.; Shefta, J.; Boylston, A.W.; Whicher, J.T. Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: Relationship to endotoxin, interleukin 6 and disease severity. Br. J. Surg. 1993, 80, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Morselli-Labate, A.M.; Miniero, R.; Barakat, B.; Fiocchi, M.; Cappelletti, O. Simultaneous serum assays of lipase and interleukin-6 for early diagnosis and prognosis of acute pancreatitis. Clin. Chem. 1999, 45, 1762–1767. [Google Scholar] [CrossRef]
- Jiang, C.F.; Shiau, Y.C.; Ng, K.W.; Tan, S.W. Serum interleukin-6, tumor necrosis factor alpha and C-reactive protein in early prediction of severity of acute pancreatitis. J. Chin. Med. Assoc. 2004, 67, 442–446. [Google Scholar]
- Rao, S.A.; Kunte, A.R. Interleukin-6: An Early Predictive Marker for Severity of Acute Pancreatitis. Indian J. Crit. Care Med. 2017, 21, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Rau, B.; Gansauge, F.; Beger, H.G. Inflammatory mediators in human acute pancreatitis: Clinical and pathophysiological implications. Gut 2000, 47, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wang, S.-S.; Lee, F.-Y.; Chang, F.-Y.; Lee, S.-D. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am. J. Gastroenterol. 1999, 94, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fisic, E.; Poropat, G.; Bilic-Zulle, L.; Licul, V.; Milic, S.; Stimac, D. The Role of IL-6, 8, and 10, sTNFr, CRP, and Pancreatic Elastase in the Prediction of Systemic Complications in Patients with Acute Pancreatitis. Gastroenterol. Res. Pract. 2013, 2013, 282645. [Google Scholar] [CrossRef] [PubMed]
- Bidarkundi, G.K.; Wig, J.D.; Bhatnagar, A.; Majumdar, S. Clinical relevance of intracellular cytokines IL-6 and IL-12 in acute pancreatitis, and correlation with APACHE III score. Br. J. Biomed. Sci. 2002, 59, 85–89. [Google Scholar] [CrossRef]
- Cho, I.R.; Do, M.Y.; Han, S.Y.; Jang, S.I.; Cho, J.H. Comparison of Interleukin-6, C-Reactive Protein, Procalcitonin, and the Computed Tomography Severity Index for Early Prediction of Severity of Acute Pancreatitis. Gut Liver 2023, 17, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Lin, T.L.; Cai, Z.; Yan, C.H.; Gou, S.R.; Zhuang, Y.D. Assessment of acute pancreatitis severity via determination of serum levels of hsa-miR-126-5p and IL-6. Exp. Ther. Med. 2020, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, A.; Maksimow, M.; Mentula, P.; Kyhälä, L.; Kylänpää, L.; Puolakkainen, P.; Kemppainen, E.; Repo, H.; Salmi, M. Circulating cytokines in predicting development of severe acute pancreatitis. Crit. Care 2014, 18, R104. [Google Scholar] [CrossRef] [PubMed]
- Sternby, H.; Hartman, H.; Johansen, D.; Thorlacius, H.; Regnér, S. IL-6 and CRP are superior in early differentiation between mild and non-mild acute pancreatitis. Pancreatology 2017, 17, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Li, L.; Lai, T.; Peng, H.; Gui, L.; He, W. Interleukin-6 is better than C-reactive protein for the prediction of infected pancreatic necrosis and mortality in patients with acute pancreatitis. Front. Cell. Infect. Microbiol. 2022, 12, 933221. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.R.; Jones, E.K.; Hornung, L.; Thompson, T.; Patel, J.; Lin, T.K.; Nathan, J.D.; Vitale, D.S.; Habtezion, A.; Abu-El-Haija, M. Cytokine Profile Elevations on Admission Can Determine Risks of Severe Acute Pancreatitis in Children. J. Pediatr. 2021, 238, 33–41.e4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).