Abstract
Activation of the renin–angiotensin–aldosterone system (RAAS) plays an important pathophysiological role in hypertension. Increased mRNA levels of the angiotensinogen angiotensin-converting enzyme, angiotensin type 1 receptor gene, Agtr1a, and the aldosterone synthase gene, CYP11B2, have been reported in the heart, blood vessels, and kidneys in salt-sensitive hypertension. However, the mechanism of gene regulation in each component of the RAAS in cardiovascular and renal tissues is unclear. Epigenetic mechanisms, which are important for regulating gene expression, include DNA methylation, histone post-translational modifications, and microRNA (miRNA) regulation. A close association exists between low DNA methylation at CEBP-binding sites and increased AGT expression in visceral adipose tissue and the heart of salt-sensitive hypertensive rats. Several miRNAs influence AGT expression and are associated with cardiovascular diseases. Expression of both ACE and ACE2 genes is regulated by DNA methylation, histone modifications, and miRNAs. Expression of both angiotensinogen and CYP11B2 is reversibly regulated by epigenetic modifications and is related to salt-sensitive hypertension. The mineralocorticoid receptor (MR) exists in cardiovascular and renal tissues, in which many miRNAs influence expression and contribute to the pathogenesis of hypertension. Expression of the 11beta-hydroxysteroid dehydrogenase type 2 (HSD11B2) gene is also regulated by methylation and miRNAs. Epigenetic regulation of renal and vascular HSD11B2 is an important pathogenetic mechanism for salt-sensitive hypertension.
1. Introduction
The renin–angiotensin–aldosterone system (RAAS) plays a pivotal role in the overall pathophysiology of hypertension [1,2,3]. Angiotensinogen (AGT) is the only known substrate of renin and is the rate-limiting enzyme of the RAAS. The levels of AGT are able to control the activity of the renin–angiotensin system; its upregulation may lead to elevated angiotensin II levels and blood pressure and has been implicated in cardiovascular and renal injuries [4]. There is growing evidence that adipose AGT has a potent role in the development of hypertension [5,6].
Angiotensin-converting enzyme (ACE) plays a major role in the RAAS (Figure 1). The central function of ACE is the conversion of angiotensin I to II. Tissue ACE is recognized as a key factor in cardiovascular and renal diseases. Pathological activation of local ACE has harmful effects on the cardiovascular tissues and kidneys [7,8]. By generating the vasodilator Ang-(1–7) and hydrolyzing portion of angiotensin II, ACE2 counterbalances the vasopressive effect of ACE [9]. ACE2 is the receptor for entry of SARS-CoV-2, which is the cause of COVID-19 in humans. It is expressed in cardiovascular and renal tissues and is related to the complications of COVID-19 infection [10,11].
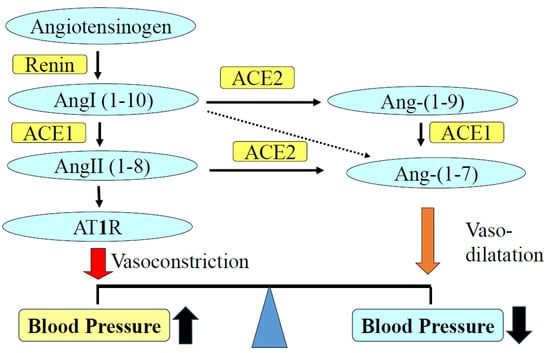
Figure 1.
Role of the ACE and ACE2/Ang-(1–7) in hypertension. ACE converts angiotensin I to angiotensin II. Angiotensin II increases blood pressure as well as injuries to cardiovascular and renal tissues via AT1R. ACE promotes vasodilation by degrading angiotensin II and generating vasodilator Ang 1–7. ACE, angiotensin-converting enzyme; Ang, angiotensin; AT1R, angiotensin II type 1 receptor.
Both angiotensin II type 1 receptor (AT1R) and mineralocorticoid receptor (MR) have well-founded functions in vasoconstriction, cellular proliferation, inflammation, and fibrosis. Treatment with AT1R blockers or MR antagonists (MRAs) protects against cardiovascular and renal injuries in patients with hypertension or diabetes mellitus [12,13,14]. Aldosterone is produced in the zona glomerulosa of the adrenal cortex by aldosterone synthase (CYP11B2) and is known to promote cardiac fibrosis and hypertrophy with concurrent elevation of inflammatory and oxidant signaling [15]. Patients with primary aldosteronism have a higher incidence of myocardial infarction and stroke than patients with essential hypertension [16,17]. Experimental animal data support the role of aldosterone in mediating cardiovascular and renal injury. In the salt-sensitive hypertensive (SSH) rat, administration of the mineralocorticoid receptor antagonist (MRA) greatly attenuated cardiac hypertrophy [18]. An important pathological effect of aldosterone in the heart has also been reported in experimental models of mineralocorticoid hypertension [19]. In these studies, prolonged exposure to aldosterone was associated with the development of myocardial hypertrophy and fibrosis. Local synthesis of aldosterone or angiotensin II has been reported [20,21]. Aldosterone produced in cardiovascular or renal tissues contributes to the development of or complications resulting from hypertension [22,23].
In large populations, significant correlations between salt intake, blood pressure, and hypertension incidence have been reported [24]. Salt-sensitive hypertension (SSH) is defined as a 10% increase in mean blood pressure due to a high-salt diet [25]. The proportion of salt-sensitive hypertension (SSH) is about 50% of hypertension and is associated with an increased risk of cardiovascular and renal injuries [26].
2. Epigenetic Regulation of Gene Expression
Epigenetic changes are inherited modifications that are not part of the DNA sequence. Gene expression is regulated at various levels, including via DNA modifications. Of these modifications, histone acetylation regulates gene expression [27], and DNA hypermethylation induces gene silencing [28]. Gene expression is also regulated by RNA modifications, which mediate RNA metabolism [29].
2.1. DNA Methylation
DNA methylation is generally involved in stabilizing the silent state of genes by either blocking DNA-binding transcription factors or recruiting methyl-CpG-binding domain (MBD) proteins, which favor the formation of transcriptionally inactive forms of chromatin (heterochromatin) [30]. Among the MBD proteins (methyl-CpG-binding proteins), MBD1 and MBD2 repress the transcription from methylated gene promoters. DNA methylation at the 5′-cytosine of CpG dinucleotides is a major epigenetic modification in eukaryotic genomes that is required for mammalian development [28], and it is associated with the formation of heterochromatin and gene silencing.
Dysregulated DNA methylation of renin–angiotensin system genes is involved in the pathogenesis of hypertension and cardiovascular diseases [30]. DNA methylation is established during normal development as well as disease progression. However, the DNA methylation pattern often changes dynamically in response to environmental changes [28,31]. Cardiovascular disorders, diabetes mellitus, and dyslipidemia, as well as lifestyle changes, affect DNA methylation dynamically.
2.2. Histone Modifications
Histone modifications are epigenetic modifications characterized by the addition of an acetyl group to histone proteins, specifically at lysine residues within the histone N-terminal tail [27]. Histone modifications are catalyzed by histone acetyl transferases (HATs) and histone deacetylases (HDACs), which are associated with transcription factors (TFs) [32]. Huang et al. [33] reported increased aldosterone production in rodents deficient in histone demethylase lysine-specific demethylase 1.
2.3. Micro RNAs (miRNAs)
miRNAs are small, non-coding RNA molecules, approximately 22 nucleotides in length, that regulate gene function at the post-transcriptional level [29]. These small RNAs act via complementary binding to the 3′-UTR and occasionally the 5′-UTR or coding regions of target miRNAs [29]. miRNAs are associated with several cardiovascular and renal diseases [34,35]. For example, miRNA-21 and miRNA-155 are associated with atherosclerosis, neovascularization, and vascular remodeling.
3. Epigenetic Regulation of the AGT Gene
The human AGT promoter possesses a number of CpG dinucleotides that are targets of DNA methylation. The human AGT promoter, which is located near a CCAAT enhancer-binding protein (CEBP)-binding site containing a CpG dinucleotide at positions −218/−217, is hypomethylated in the liver, heart, and HepG2 hepatocytes with high AGT expression [30]. In cultured human cells, interleukin 6 stimulation induced DNA demethylation near a CEBP-binding site and a transcription start site; this demethylation was accompanied by increased CEBP-β recruitment and chromatin accessibility of the AGT promoter. The methylation status of the CpG dinucleotide within the CEBP-binding site is inversely associated with AGT expression [30]. DNA demethylation causes a shift in the AGT expression phenotype from the inactive to the active state.
Several miRNAs influence AGT and cause cardiovascular diseases. Sharma et al. [36] reported that in heart failure, AGT expression was upregulated in the hypothalamus via a post-transcriptional mechanism mediated by miRNA-133a. Wang et al. [37] reported that miRNA-149-5p affected AGT expression, which promoted inflammatory responses. miRNA-133b induces proliferation and inhibits apoptosis in retinal endothelial cells by targeting AGT [38]. However, miRNA-29a inhibits retinal neovascularization to prevent the development and progression of retinopathy via downregulation of AGT [39]. miRNA-31/-584 binds to the AGT and influences coronary artery disease [40].
3.1. Salt-Sensitive Hypertension (SSH)
We showed that high salt intake reduces the levels of circulating RAAS but increases those of tissue RAAS in SSH rats [18,41]. Transgenic mice expressing human aldosterone synthase exhibit SSH [42]. Tissue AGT is an important effector molecule for blood pressure regulation.
Overexpression of AGT in the heart increases blood pressure and cardiac hypertrophy, and young spontaneously hypertensive rats show elevated tissue AGT expression [43]. High salt intake increases the cardiac mRNA levels of AGT as well as AT1R in SSH rats [18]. Treatment with MRAs decreases tissue AGT levels and improves cardiovascular injuries independent of blood pressure [18].
High salt intake demethylates the AGT promoter in the heart of SSH rats. In treatments with the MRA, eplerenone decreases the AGT mRNA level and methylates the AGT promoter in SSH rats [41]. DNA demethylation occurs around the transcription start site and CEBP-binding sites. These results suggest that a salt-associated stimulatory signal may recruit CEBP to its binding sites within the first exon to activate AGT transcription. This mechanism explains the beneficial effects of MRAs on cardiovascular diseases. Based on the role of epigenetics in the development of chronic cardiovascular and metabolic diseases, it is presumed that epigenetic intervention may be an effective strategy for the treatment of these diseases.
3.2. Primary Aldosteronism
More and more studies are being conducted on primary aldosteronism (PA), which accounts for 5–10% of the hypertensive population [44]. Increased prevalences of diabetes mellitus and metabolic syndrome in patients with PA were reported in a large cohort study [45,46]. A high level of aldosterone in individuals without insulin resistance at baseline was found to predict the development of insulin resistance 10 years later [47]. Experimental and clinical evidence indicates that a high aldosterone level impairs glucose metabolism by inhibiting insulin secretion and increasing insulin resistance [48]. Wu et al. [49] demonstrated increased inflammation and fibrosis in peripheral adipose tissues in patients with aldosterone-producing adenoma. Kalupahara et al. [50] reported that overproduction of AGT in adipose tissues induces adipose inflammation, glucose intolerance, and insulin resistance.
Excess circulating aldosterone upregulated AGT expression and was accompanied by DNA hypomethylation around a CEBP-binding site and a transcription start site in human visceral adipose tissue [30]. Increased AGT expression from visceral adipose tissue may contribute, in part, to the development of hypertension and metabolic abnormalities in PA [28].
4. Epigenetic Regulation of the Angiotensin-Converting Enzyme (ACE)
ACE plays a central role in the generation of angiotensin II and the degradation of bradykinin, thereby influencing blood pressure regulation and vascular remodeling [51]. Alterations in endothelial ACE expression or activity are associated with inflammatory cardiovascular diseases, including hypertension, diabetes, and atherosclerosis [52]. The human ACE promoter contains CpG islands [53], and hypomethylation of the ACE has been linked to fetal programming and the potential development of future diseases [54].
Somatic ACE is crucial in cardiovascular homeostasis and displays a tissue-specific profile [55]. We have reported an increased ACE mRNA level in the heart and kidney of SSH rats [18]. Epigenetic patterns modulate gene expression, the alterations of which have been implicated in pathologies, including hypertension. The effects of a maternal low-protein diet on the development of hypertension and cardiovascular diseases during adulthood have been documented extensively [56]. Goyal et al. [57]. reported that a maternal low-protein diet increased the levels of ACE mRNA and demethylated CpG islands of ACE promoter in the brain. Riviere et al. [58] reported that the methylation of the ACE promoter influenced mRNA levels in the rat lung and liver but not the kidney. They concluded that the basal methylation pattern of the ACE promoter correlates with somatic ACE transcription.
The expression of ACE in tissues is also controlled by histone modifications and miRNAs. Lee et al. [59] reported that ACE is upregulated in the heart and kidney of spontaneously hypertensive rats (SHRs) via histone code modifications. Hu et al. [60] reported that mechanical stretch suppresses miRNA-145 expression and promotes ACE expression to alter the vascular smooth muscle cell phenotype. miRNAs act as critical regulators of major cellular functions and are considered in the pathogenesis of hypertension. Kohlstedt et al. [61] reported that upregulation of miRNA-143/145 suppressed endothelial ACE expression. Post-transcriptional regulation of miRNAs in the blood vessels may contribute to the pathogenesis of hypertension.
5. Epigenetic Regulation of ACE2
Angiotensin II is an important vasoconstrictor, whereas angiotensin-converting enzyme 2 (ACE2) promotes vasodilation by degrading angiotensin II and generating the vasodilator Ang 1–7 [62]. Increased expression of ACE2 protects against elevated blood pressure, whereas ACE2 inhibition or deletion promotes hypertension [63].
DNA methylation is an important mechanism of ACE2 regulation. Goyal et al. [64] showed hypomethylation together with high expression of ACE2 in lung epithelial cells. We found that the ACE2 mRNA level was significantly decreased in the hearts of SSH rats compared with control rats [18]. However, the methylation ratio did not differ between SSH rats and control rats (Figure 2) [65].
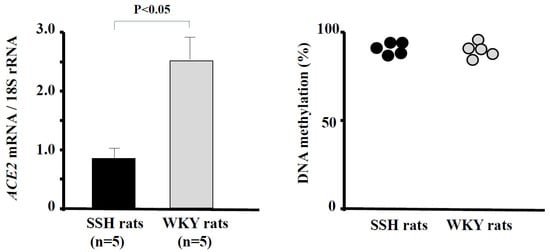
Figure 2.
ACE2 mRNA levels and methylation ratios in the hearts of SSH rats and control rats. The ACE2 mRNA level was significantly lower in SSH rats than in control rats (p < 0.05). The methylation ratio of CpGs of promoter of ACE2 in the hearts did not differ between SSH rats and control rats. (Data were cited from [65]). ACE, angiotensin-converting enzyme; SSH, salt-sensitive hypertension.
Histone modifications within the ACE2 gene region have been reported in COVID-19 infection [66,67,68]. Pinto et al. [69] reported an elevated ACE2 mRNA level with histone modifications such as histone acetyltransferase 1 and adenosine deaminase 2 in the lungs of patients with severe COVID-19 infection. There are no reports of histone modifications of ACE2 in cardiovascular diseases, including hypertension.
There have been several reports on the roles of miRNAs in the regulation of ACE2 [70,71,72]. Gu et al. [73] reported that exercise training decreased blood pressure and increased ACE2 and miRNA-143 expression levels in the aorta in SHRs. Wang et al. [74] reported that angiotensin-(1–7) decreased vascular inflammation and improved vascular function by modulating the expression of miRNA-146a in human aortic endothelial cells. Treatment with an angiotensin II receptor blocker was reported to modulate the level of miRNA-146a/b, along with improvement of the ACE2 level and attenuation of vascular remodeling in hypertension [75]. We have reported that treatment with MRAs improved cardiac hypertrophy and increased ACE2 mRNA in the hearts of SSH rats [18]. These data suggest that RAAS blockers exert cardiovascular protective effects via ACE2 signaling and miRNA levels.
6. Epigenetic Regulation of AT1R
Angiotensin II increases blood pressure as well as cardiovascular and renal tissue injuries via AT1R. AT1R expression is regulated by DNA methylation and miRNAs [76,77]. Kawakami-Mori et al. [78] reported that in the offspring of pregnant rats receiving a low-protein diet or dexamethasone, the mRNA level of the AT1R gene (Agtr1a) was increased in the hypothalamus, concurrent with hypomethylation of the Agtr1a promoter. These offspring showed SSH. Ghosh et al. [79] found that Agtr1a expression in the hypothalamus progressively increased, while the methylation status of the Agtr1a promoter decreased in SHRs compared with Wistar–Kyoto rats. Thus, epigenetic modulation of hypothalamic Agtr1a contributes to SSH or essential hypertension.
Exercise is one of the most effective treatments for hypertension. Shan et al. [80] reported that maternal exercise upregulates the DNA methylation of Agtr1a and decreases gene expression in mesenteric arteries in offspring SHRs. Maternal exercise reduces blood pressure and cardiovascular reactivity of the offspring from SHRs.
Several miRNAs negatively regulate Agtr1a expression at the post-transcriptional level. Zheng et al. [81] demonstrated that miRNA-155 suppressed the activity of an Agtr1a 3′-UTR reporter construct via a luciferase assay. They also reported that miRNA-155 plays an important role in regulating adventitial fibroblast differentiation and contributes to the suppression of Agtr1a expression. Cross-talk between aldosterone and angiotensin II has been proposed in the pathogenesis of cardiovascular and renal diseases [82,83]. DuPont et al. [84] reported that the vascular MR regulates miRNA-155 and Agtr1a to promote vasoconstriction and elevate blood pressure.
AT1R-associated protein is a direct binding protein of AT1R that acts as an endogenous inhibitor of hypertension pathogenesis in cardiovascular and renal tissues [85,86,87,88]. Hirota et al. [88] reported that miRNA-125a-5b/125b-5b contributes to the pathological activation of AT1R-associated protein in mouse distal convoluted tubule cells.
7. Epigenetic Regulation of CYP11B2
CYP11B2 expression is epigenetically regulated by DNA methylation and miRNAs. Angiotensin II increases CYP11B2 expression and aldosterone synthesis both in the adrenal gland and cardiovascular and renal tissues. We have reported that angiotensin II infusion in rats induced hypomethylation of the CYP11B2 promoter and increased gene expression in the adrenal gland [89]. A low-salt diet decreases the methylation ratio of rat CYP11B2 and increases the CYP11B2 mRNA level in parallel with aldosterone synthesis. Interestingly, switching from a low-salt diet to a high-salt diet resulted in a change from a CYP11B2 hypomethylated to a hypermethylated state [89]. These results suggest that angiotensin II regulates aldosterone synthesis by the mechanism of DNA methylation.
Both miRNA-10b and miRNA-24 are negative regulators of the cortisol synthase genes CYP11B1 and CYP11B2 [90,91]. Zhang et al. [92] reported that miRNA-193a-3p not only downregulated CYP11B2 expression but also acted as a tumor suppressor in aldosterone-producing adenoma. miRNA-124a-5p and miRNA-124b-5p are also negative regulators of CYP11B2 [91]. Syed et al. [93] reported that excess aldosterone increased miRNA-21 expression in the rat heart.
7.1. Epigenetics and Aldosterone-Producing Adenoma (APA)
The most common clinical subtypes of PA are APA and bilateral adrenocortical hyperplasia [94]. We and others have reported a lower level of CYP11B2 methylation in APAs than in adrenal tissues or non-functioning adrenal adenomas. A negative correlation between the CYP11B2 methylation ratio and mRNA level was found [95,96,97]. Epigenetic control of CYP11B2 expression may play an important role in aldosterone synthesis in APAs. We found a KCNJ5 mutation in aldosterone-producing microadenoma and aldosterone-producing cell clusters, in which the methylation rate of CYP11B2 was decreased compared with adjacent adrenal tissues [98]. Further study is necessary to clarify the mechanism of aldosterone overproduction, including the epigenome and metabolome, in aldosterone-producing cell clusters and APA.
7.2. Epigenetic Regulation of Mineralocorticoid-Related Genes in SSH
Mineralocorticoids, including aldosterone, are an important pathological factor in SSH [99]. Epigenetic mechanisms involved in the development of SSH have been reported [100]. Maternal lipopolysaccharide exposure during pregnancy induces upregulation of Rac1 via histone modifications mediated by H3K9me2 across generations, resulting in salt-induced activation of the Rac1-/MR pathway in the kidney and development of SSH [101]. We reported that local RAAS activation caused SSH. A high-salt diet-induced hypomethylation of CYP11B2 in the hearts of SSH rats and increased cardiac aldosterone synthesis and hypertrophy [102]. MRA treatment not only decreased blood pressure but also induced hypermethylation of CYP11B2 in the heart [102].
Aldosterone synthesis and CYP11B2 expression are upregulated in cardiac tissues during hypertrophic cardiomyopathy (HCM) and are recognized as major HCM phenotype modifiers [102]. Aldosterone directly affects cardiac hypertrophy and fibrosis. We previously reported that aldosterone, locally produced in cardiovascular tissues, exerts its effects via paracrine or intracrine mechanisms [103]. Garnier et al. [104] reported coronary endothelium-independent dysfunction without hypertrophy in the hearts of transgenic mice overexpressing CYP11B2. Alesutan et al. [105] showed CYP11B2 expression in human coronary arteries as well as smooth muscle cells. In their study, the CYP11B2 mRNA level was higher in aortic tissues in klotho-hypomorphic mice than in control mice, and spironolactone ameliorated aortic osteoinductive reprogramming in adrenalectomized klotho-hypomorphic mice. We found that treatment with spironolactone improved cardiac hypertrophy in adrenalectomized hypertensive rats [106]. Yoshimura et al. [107] reported increased CYP11B2 expression in the hearts of patients with cardiac failure, while we found a clear association between CpG methylation and CYP11B2 expression in the cardiac tissues of HCM patients [102]. Hypomethylation of the CYP11B2 promoter aberrantly increases CYP11B2 expression, which induces cardiac hypertrophy or cardiomyopathy. The molecular mechanisms regulating demethylation of CYP11B2 in the heart remain unclear.
7.3. Epigenetic Control of Mineralocorticoid Receptors
Mineralocorticoid receptors (MRs) exist in both epithelial and non-epithelial cells. MRs in vascular endothelial cells and smooth muscle cells (VSMCs) are involved in vascular smooth muscle hypertrophy and endothelial dysfunction [108]. Cardiac MR contributes to cardiac tissue inflammation, fibrosis, and cardiac dysfunction [109]. We detected MR mRNA in VSMCs, and aldosterone increased the incorporation of tritiated leucine into these VSMCs; this incorporation was inhibited by a specific aldosterone antagonist [110]. MR activation in VSMCs or endothelial cells increased oxidative stress mediated by activation of NADPH oxidases [108]. Oxidative stress promotes the proliferation of VSMCs and regulates blood pressure. Mesquita et al. [111] reported that MR signaling activates long cardiac Ca1,2 N-terminal mRNA expression via P1-promoter activation, leading to hypertension. MR activation in VSMCs also induces the expression of collagens 1 and 3, IL-16, CTLA4, and genes associated with vascular calcification [112].
MRs are epigenetically controlled by methylation, histone modifications, and miRNAs. The histone deacetylase 3/4 complex stimulates the transcriptional activity of MRs [113]. A maternal high-fat diet upregulates MR function in the offspring’s blood vessels via an epigenetic mechanism [114]. Camarda et al. [115] reported that MR-knockout mice or blockade increased the levels of the enhancer of zeste homolog 2 and histone-H3 lysine-27-specific methyltransferase and prevented vascular stiffness and fibrosis.
microRNAs have been implicated in multiple MR-related cardiovascular and renal injuries. Soeber et al. [116] reported that miRNA-124 and miRNA-135a are potential regulators of MR gene expression. miRNA-21 expression is upregulated in the heart by excess aldosterone. Genetic ablation of miRNA-21 exacerbates cardiac hypertrophy and injury in mineralocorticoid-excess mice [93]. miRNA-31 targets the 3′-UTR of MR as well as cardiac troponin-T. Inhibition of miRNA-31 improves cardiac dysfunction and prevents the development of post-ischemic adverse remodeling [117]. Garg et al. [118] found that miRNA-181a is a novel regulator of MR-mediated cardiac remodeling. MR activation increases miRNA-204 expression, which induces T-type calcium channel expression in cardiomyocytes [119]. DuPont et al. [84] reported that the age-associated decrease in miRNA-155 in mesenteric arteries was associated with increased expression of MRs and L-type calcium channels, which cause hypertension. miRNA-34 has been reported to be dysregulated in various human cancers and to play a tumor-suppressive role because of its synergistic effect with the well-known tumor suppressor p53. The role of miRNA-34b/C in MR-mediated VSMC calcification has been reported. Treatment with the MR antagonist upregulates miRNA-34b/c and inhibits vascular calcification [120]. miRNA-766 targets MR genes directly and induces an anti-inflammatory effect via inhibition of NF-kB signaling [121]. NF-kB binds to MR promoters and decreases MR isoform levels.
MRs contribute to hypertension by increasing renal salt reabsorption and promoting kidney dysfunction via direct effects on renal parenchymal cells. We have reported that the MR antagonist, eplerenone, prevented renal injury and decreased blood pressure in salt-sensitive hypertensive rats [122]. The miRNA-466 family targets and regulates the expression of MRs and serum glucocorticoid-regulated kinase 1 (SGK1) [123], which stimulates MR-dependent renal sodium reabsorption, increases blood pressure, and promotes kidney inflammation and fibrosis [124]. Park et al. [125] reported that in aldosterone-treated cells, reduced miRNA-34c-5p level increased Ca2+/calmodulin-dependent protein kinase type II beta-chain expression and stimulated fibronectin and alpha-smooth muscle actin, which play a significant role in the development of fibrosis.
Long non-coding RNAs (lncRNAs) interact with proteins and interfere with miRNAs by acting as molecular sponges to modify the epigenome. Zhang et al. [126] reported that lncRNA Tug1 promotes angiotensin II-induced renal fibrosis by binding MRs and negatively regulating miRNA-29b-3p. Upregulation of lncRNA H19 is reported to contribute to aldosterone-MR complex-promoted vascular smooth muscle calcification by sponging miRNA-106a-5p [127].
Aldosterone inhibits miRNA-192, which increases the serine/threonine kinase with no lysine (WNK1) [128]. Long-form WNK1 is an important regulator of both K+ and Na+ transport [129]. Both miRNA-194 and miRNA-802 regulate renal outer medullary potassium channels [130,131]. Edinger et al. [132] reported that aldosterone downregulated mmu-miRNA-335-3p, mmu-miRNA-290-5p, and mmu-miRNA-1983, which increased epithelial sodium channel-mediated sodium transport in mouse cortical collecting ducts.
7.4. Epigenetic Control of 11ß-Hydroxysteroid Dehydrogenase Type 2
The enzyme 11ß-hydroxysteroid dehydrogenase (11ß-HSD) catalyzes the conversion of glucocorticoids into their inactive metabolites and modulates mineralocorticoid and glucocorticoid activity. (Figure 3) Biochemical studies have revealed the existence of two isoforms of 11ß-HSD, NAD+-dependent and NADP+-dependent. 11ß-HSD2 (the NAD+-dependent isoform) is found in distal portions of the nephron, which co-localizes with MRs. Kidney-specific gene deletion of HSD11B2, which induces human or renal dysfunction, causes hypertension [133,134]. We have reported that vascular 11ß-HSD2 contributes to salt-sensitive hypertension [135].
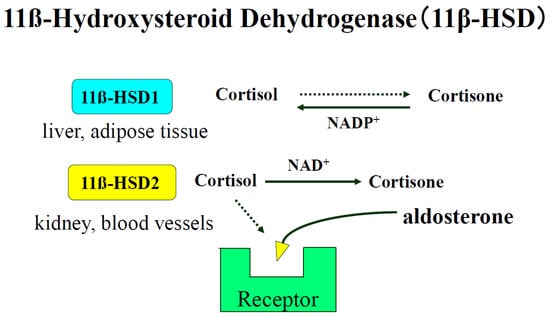
Figure 3.
Biochemical studies have revealed that there are two isoforms of 11ß-HSD, a NAD+-dependent form (11ß-HSD2) and a NADP+-dependent form (11ß-HSD1). 11ß-HSD2 is found in tissues with high levels of MR activity, such as kidney, placenta, and colon [59]. 11ß-HSD2 is also found in blood vessel type 1 11β-HSD (11β-HSD1), which is highly expressed in adipose tissue, liver, and skeletal muscle and plays a central role in obesity, diabetes mellitus, and hypertension.
HSD11B2 is epigenetically controlled by methylation and miRNAs. Alikhani-Koopaei et al. [136] reported that methylation of the promoter region of HSD11B2 regulates HSD11B2 gene expression. Nuclear factor 1 (NF1) is a strong stimulator of the HSD11B2 gene, and this effect is dependent on the position and the combination of methylated CpGs. Apparent mineralocorticoid excess is a rare genetic hypokalemic low-renin hypertension. Mutation of the HSD11B2 gene was reported [137]. Pizzolo et al. [138] reported that the hypertension phenotype of apparent mineralocorticoid excess was associated with higher methylation of the HSD11B2 promoter region compared with normotensive heterozygous relatives.
HSD11B2 expression is regulated by several miRNAs. Rezaei et al. [139] found lower expression of mo-miRNA-20a-5p, mo-miRNA-19b-3p, and mo-miRNA-190a-5p in Sprague Dawley rats compared with Wistar rats, and uninephrectomy decreased the expression of mo-miRNA 26b-5p, mo-miRNA-19b-3p, and mo-miRNA-29b-3p in Sprague Dawley rats. They also showed reduced 11β-HSD2 activity after miRNA-20a overexpression. High fructose consumption is related to hypertension and obesity [140]. Nouchi et al. [141] reported that although maternal high-fructose corn syrup did not affect the methylation status of HSD11B2, it increased miRNA-27a-5p overexpression and decreased mRNA expression in the kidney of the offspring.
8. Conclusions
The epigenetic modifications of local RAAS in cardiovascular, renal, and adipose tissues and their influence on hypertension are described. Gene expression of RAAS is regulated by epigenetic modifications such as DNA methylation, histone modifications, and miRNAs. In salt-sensitive hypertension, hypomethylation of AGT and CYP11B2 increases both mRNA levels in cardiovascular tissues. miRNAs regulate the gene expression of MR and HSD11B2 in the kidney, which controls blood pressure and electrolytes. Epigenesis of RAAS needs to be further clarified both under normal physiological conditions and in pathophysiological states, including hypertension.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACE | angiotensin-converting enzyme |
| AGT | angiotensinogen |
| APA | aldosterone-producing adenoma |
| AT1R | angiotensin II type 1 receptor |
| CEBP | CCAAT enhancer-binding protein |
| DNA | deoxyribonucleic acid |
| HATs | histone acetyl transferases |
| HCM | hypertrophic cardiomyopathy |
| HDACs | histone deacetylases |
| mRNA | messenger ribonucleic acid |
| miRNA | microRNA |
| MBD | methyl-CpG-binding domain |
| MR | mineralocorticoid receptor |
| MRA | mineralocorticoid receptor antagonist |
| PA | primary aldosteronism |
| RAAS | renin–angiotensin–aldosterone system |
| RNA | ribonucleic acid |
| SHR | spontaneously hypertensive rat |
| SSH | salt-sensitive hypertension |
| TFs | transcription factors |
| UTR | untranslated region |
References
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Azushima, K.; Morisawa, N.; Tamura, K.; Nishiyama, A. Recent research advances in renin-angiotensin-aldosterone system receptors. Curr. Hypertens. Rep. 2020, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E.; Pitt, B.J. The renin-angiotensin-aldosterone system and its suppression. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, E.O.; Ye, D.; Wu, C.; Lu, H.S.; Uijl, E.; Mirabito Colafella, K.M.; Danser, A.H.J. Angiotensinogen suppression: A new tool to treat cardiovascular and renal disease. Hypertension 2022, 79, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Yasue, S.; Masuzaki, H.; Okada, S.; Ishii, T.; Kozuka, C.; Tanaka, T.; Fujikura, J.; Ebihara, K.; Hosoda, K.; Katsurada, A.; et al. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: Impact of nutritional status and adipocyte hypertrophy. Am. J. Hypertens. 2010, 23, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, E.O.; Uijl, E.; Danser, A.H.J. Perivascular adipose tissue in vascular function: Does locally synthesized angiotensinogen play a role? J. Cardiovasc. Pharmacol. 2021, 78 (Suppl. S6), S53–S62. [Google Scholar] [CrossRef]
- Khurana, V.; Goswami, B. Angiotensin converting enzyme (ACE). Clin. Chim. Acta 2022, 524, 113–122. [Google Scholar] [CrossRef]
- Le, D.; Brown, L.; Malik, K.; Murakami, S. Two opposing functions of angiotensin-converting enzyme (ACE) that links hypertension, dementia, and aging. Int. J. Mol. Sci. 2021, 22, 13178. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.R.; Khalil, R.A. Regulation of vascular angiotensin II type 1 and type 2 receptor and angiotensin-(1–7)/MasR signaling in normal and hypertensive pregnancy. Biochem. Pharmacol. 2024, 220, 115963. [Google Scholar] [CrossRef]
- Bierstadt, S.; Casaro, E.B.; Range, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Lawal, I.O.; Kgatle, M.M.; Mokoala, K.; Farate, A.; Sathekge, M.M. Cardiovascular disturbances in COVID-19: An updated review of the pathophysiology and clinical evidence of cardiovascular damage induced by SARS-CoV-2. BMC Cardiovasc. Disord. 2022, 22, 93. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney diseasemechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Uehara, T.; Azushima, K.; Wakui, H.; Tamura, K. Updates for cardio-kidney protective effects by angiotensin receptor-neprilysin inhibitor: Requirement for additional evidence of kidney protection. J. Am. Heart Assoc. 2023, 12, e029565. [Google Scholar] [CrossRef]
- Parksook, W.W.; Williamn, G.H. Aldosterone and cardiovascular diseases. Cardiovasc. Res. 2023, 119, 28–44. [Google Scholar] [CrossRef]
- Ohno, Y.; Sone, M.; Inagaki, N.; Yamasaki, T.; Ogawa, O.; Takeda, Y.; Kurihara, I.; Itoh, H.; Umakoshi, H.; Tsuiki, M.; et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: A multicenter study in Japan. Hypertension 2018, 71, 530–537. [Google Scholar] [CrossRef]
- Zennaro, M.C.; Boulkroun, S.; Fernandes-Rosa, F.L. Pathogenesis and treatment of primary aldosteronism. Nat. Rev. Endocrinol. 2020, 16, 578–589. [Google Scholar] [CrossRef]
- Takeda, Y.; Zhu, A.; Yoneda, T.; Usukura, M.; Takata, H.; Yamagishi, M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am. J. Hypertens. 2007, 20, 1119–1124. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, inflammation, immune system, and hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Dzau, V.J. Multiple pathways of angiotensin production in the blood vessel wall: Evidence, possibilities and hypotheses. J. Hypertens. 1989, 7, 933–936. [Google Scholar] [CrossRef]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef]
- Takeda, Y. Role of cardiovascular aldosterone in hypertension. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 261–266. [Google Scholar] [CrossRef]
- Xu, C. Extra-adrenal aldosterone: A mini review focusing on the physiology and pathophysiology of intrarenal aldosterone. Endocrine 2023, 83, 285–301. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.Y.; Cho, Y.; Koh, S.B.; Kim, N.; Choi, J.R. Genetically, dietary sodium intake is causally associated with salt-sensitive hypertension risk in a community-based cohort study: A mendelian randomization approach. Curr. Hypertens. Rep. 2020, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997, 350, 1734–1737. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef]
- Stoll, S.; Wang, C.; Qiu, H. DNA methylation and histone modification in hypertension. Int. J. Mol. Sci. 2018, 19, 1174. [Google Scholar] [CrossRef]
- Demura, M.; Demura, Y.; Takeda, Y.; Saijoh, K. Dynamic regulation of the angiotensinogen gene by DNA methylation, which is influenced by various stimuli experienced in daily life. Hypertens. Res. 2015, 38, 519–527. [Google Scholar] [CrossRef]
- Klimczak-Tomaniak, D.; Haponiuk-Skwarlińska, J.; Kuch, M.; Pączek, L. Crosstalk between microRNA and oxidative stress in heart failure: A systematic review. Int. J. Mol. Sci. 2022, 23, 15013. [Google Scholar] [CrossRef]
- Wang, F.; Demura, M.; Cheng, Y.; Zhu, A.; Karashima, S.; Yoneda, T.; Demura, Y.; Maeda, Y.; Namiki, M.; Ono, K.; et al. Dynamic CCAAT/enhancer binding protein-associated changes of DNA methylation in the angiotensinogen gene. Hypertension 2014, 63, 281–288. [Google Scholar] [CrossRef]
- Guarner-Lans, V.; Ramírez-Higuera, A.; Rubio-Ruiz, M.E.; Castrejón-Téllez, V.; Soto, M.E.; Pérez-Torres, I. Early programming of adult systemic essential hypertension. Int. J. Mol. Sci. 2020, 21, 1203. [Google Scholar] [CrossRef] [PubMed]
- Field, A.; Adelman, K. Evaluating enhancer function and transcription. Annu. Rev. Biochem. 2020, 89, 213–234. [Google Scholar] [CrossRef]
- Huang, Y.; Ting, P.Y.; Yao, T.M.; Homma, T.; Brooks, D.; Rangel, I.K.; Adler, G.K.; Romero, J.R.; Williams, J.S.; Pojoga, L.H.; et al. Histone demethylase LSD1 deficiency and biological sex: Impact on blood pressure and aldosterone production. J. Endocrinol. 2019, 240, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Giacca, M. Small non-coding RNA therapeutics for cardiovascular disease. Eur. Heart J. 2022, 43, 4548–4561. [Google Scholar] [CrossRef]
- Mahtal, N.; Lenoir, O.; Tinel, C.; Anglicheau, D.; Tharaux, P.L. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef]
- Sharma, N.M.; Nandi, S.S.; Zheng, H.; Mishra, P.K.; Patel, K.P. A novel role for miR-133a in centrally mediated activation of the renin-angiotensin system in congestive heart failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H968–H979. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, X.; Zhao, T.; Zhang, X.; Qu, P.; Zhao, H. AGT, targeted by miR-149-5p, promotes IL-6-induced inflammatory responses of chondrocytes in osteoarthritis via activating JAK2/STAT3 pathway. Clin. Exp. Rheumatol. 2020, 38, 1088–1095. [Google Scholar]
- Liu, T.T.; Hao, Q.; Zhang, Y.; Li, Z.H.; Cui, Z.H.; Yang, W. Effects of microRNA-133b on retinal vascular endothelial cell proliferation and apoptosis through angiotensinogen-mediated angiotensin II- extracellular signal-regulated kinase 1/2 signalling pathway in rats with diabetic retinopathy. Acta Ophthalmol. 2018, 96, e626–e635. [Google Scholar] [CrossRef]
- Chen, X.K.; Ouyang, L.J.; Yin, Z.Q.; Xia, Y.Y.; Chen, X.R.; Shi, H.; Xiong, Y.; Pi, L.H. Effects of microRNA-29a on retinopathy of prematurity by targeting AGT in a mouse model. Am. J. Transl. Res. 2017, 9, 791–801. [Google Scholar]
- Novák, J.; Maceková, S.; Héžová, R.; Máchal, J.; Zlámal, F.; Hlinomaz, O.; Rezek, M.; Souček, M.; Vašků, A.; Slabý, O.; et al. Polymorphism rs7079 in miR-31/-584 binding site in angiotensinogen gene associates with earlier onset of coronary artery disease in Central European population. Genes 2022, 13, 1981. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Yoneda, T.; Takeda, Y. DNA methylation of the angiotensinogen gene, agt, and the aldosterone synthase gene, CYP11B2 in cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 4587. [Google Scholar] [CrossRef] [PubMed]
- Mopidevi, B.; Kaw, M.K.; Puri, N.; Ponnala, M.; Jain, S.; Rana, A.; Keetha, N.R.; Khuder, S.A.; Fiering, S.N.; Kumar, A. Variable transcriptional regulation of the human aldosterone synthase gene causes salt-dependent high blood pressure in transgenic mice. Circ. Cardiovasc. Genet. 2015, 8, 30–39. [Google Scholar] [CrossRef]
- Pellieux, C.; Montessuit, C.; Papageorgiou, I.; Pedrazzini, T.; Lerch, R. Differential effects of high-fat diet on myocardial lipid metabolism in failing and nonfailing hearts with angiotensin II-mediated cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1795–H1805. [Google Scholar] [CrossRef]
- Dogra, P.; Bancos, I.; Young, W.F., Jr. Primary aldosteronism: A pragmatic approach to diagnosis and management. Mayo Clin. Proc. 2023, 98, 1207–1215. [Google Scholar] [CrossRef]
- Hanslik, G.; Wallaschofski, H.; Dietz, A.; Riester, A.; Reincke, M.; Allolio, B.; Lang, K.; Quack, I.; Rump, L.C.; Willenberg, H.S.; et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldsoteronism of the German Conn’s registry. Eur. J. Endocrinol. 2015, 173, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Akehi, Y.; Yanase, T.; Motonaga, R.; Umakoshi, H.; Tsuiki, M.; Takeda, Y.; Yoneda, T.; Kurihara, I.; Itoh, H.; Katabami, T.; et al. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: A large, multicenter cohort study in Japan. Diabetes Care. 2019, 42, 938–945. [Google Scholar] [CrossRef]
- Garg, R.; Adler, G.K. Role of mineralocorticoid receptor in insulin resistance. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 168–175. [Google Scholar] [CrossRef]
- Adler, G.K.; Murray, G.R.; Turcu, A.F.; Nian, H.; Yu, C.; Solorzano, C.C.; Manning, R.; Dungeng Peng, D.; Luther, J.M. Primary aldosteronism decreases insulin secretion and increases insulin clearance in humans. Hypertension 2020, 75, 1251–1259. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, H.; Zhang, J.; Xie, C.; Fan, C.; Zhang, H.; Wu, P.; Wei, Q.; Tan, W.; Xu, L.; et al. Inflammation and fibrosis in peripheral adipose tissue of patients with aldosterone-producing adenoma. Endocrinology 2018, 159, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Massiera, F.; Quignard-Boulange, A.; Ailhaud, G.; Voy, B.H.; Wasserman, D.H.; Moustaid-Moussa, N. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity 2012, 20, 48–56. [Google Scholar] [CrossRef]
- Bader, M.; Steckelings, U.M.; Alenina, N.; Santos, R.A.S.; Ferrario, C.M. Alternative renin-angiotensin system. Hypertension 2024, 81, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Jarajapu, Y.P.R. Targeting angiotensin-converting enzyme-2/angiotensin-(1–7)/mas receptor axis in the vascular progenitor cells for cardiovascular diseases. Mol. Pharmacol. 2021, 99, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mudersbach, T.; Siuda, D.; Kohlstedt, K.; Fleming, I. Epigenetic control of the angiotensin-converting enzyme in endothelial cells during inflammation. PLoS ONE 2019, 14, e0216218. [Google Scholar] [CrossRef]
- Najafipour, R.; Mohammadi, D.; Momeni, A.; Moghbelinejad, S.J. Effect of B12 and folate deficiency in hypomethylation of Angiotensin I converting enzyme 2 gene and severity of disease among the acute respiratory distress syndrome patients. Clin. Lab. Anal. 2023, 37, e24846. [Google Scholar] [CrossRef]
- Ceconi, C.; Francolini, G.; Olivares, A.; Comini, L.; Bachetti, T.; Ferrari, R. Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE. Eur. J. Pharmacol. 2007, 577, 1–6. [Google Scholar] [CrossRef]
- Ryznar, R.J.; Phibbs, L.; Van Winkle, L.J. Epigenetic modifications at the center of the Barker hypothesis and their transgenerational implications. Int. J. Environ. Res. Public Health 2021, 18, 12728. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Goyal, D.; Leitzke, A.; Gheorghe, C.P.; Longo, L.D. Brain renin-angiotensin system: Fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod. Sci. 2010, 17, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rivière, G.; Lienhard, D.; Andrieu, T.; Vieau, D.; Frey, B.M.; Frey, F.J. Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics 2011, 6, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Cho, H.M.; Lee, D.Y.; Kim, K.C.; Han, H.S.; Kim, I.K. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension 2012, 59, 621–626. [Google Scholar] [CrossRef]
- Hu, B.; Song, J.T.; Qu, H.Y.; Bi, C.L.; Huang, X.Z.; Liu, X.X.; Zhang, M. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PLoS ONE 2014, 9, e96338. [Google Scholar] [CrossRef]
- Kohlstedt, K.; Trouvain, C.; Boettger, T.; Shi, L.; Fisslthaler, B.; Fleming, I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ. Res. 2013, 112, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Nalivaeva, N.N. Angiotensin-converting enzyme 2 (ACE2): Two decades of revelations and re-evaluation. Peptides 2022, 151, 170766. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Berdasco, C.; Lazartigues, E. Brain angiotensin converting enzyme-2 in central cardiovascular regulation. Clin. Sci. 2020, 134, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Leitzke, A.; Goyal, D.; Gheorghe, C.P.; Longo, L.D. Antenatal maternal hypoxic stress: Adaptations in fetal lung renin-angiotensin system. Reprod. Sci. 2011, 18, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Demuea, M.; Yoneda, T.; Takeda, Y. Epigenesis of blood pressure regulating hormones. In Proceeding of the 45th Annual Scientific Meeting of Japanese Society of Hypertension, Osaka, Japan, 15–17 September 2023; p. 184. [Google Scholar]
- Sen, R.; Garbati, M.; Bryant, K.; Lu, Y. Epigenetic mechanisms influencing COVID-19. Genome 2021, 64, 372–385. [Google Scholar] [CrossRef]
- Lima, R.S.; Rocha, L.P.C.; Moreira, P.R. Genetic and epigenetic control of ACE2 expression and its possible role in COVID-19. Cell Biochem. Funct. 2021, 39, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Chlamydas, S.; Papavassiliou, A.G.; Piperi, C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics 2021, 16, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.G.G.; Oliveira, A.; Singh, Y.; Jimenez, L.; Gonçalves, A.N.A.; Ogava, R.L.T.; Creighton, R.; Schatzmann Peron, J.P.; Nakaya, H.I. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J. Infect. Dis. 2020, 222, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Rutigliano, G.; Sestito, S.; Bandini, L.; Storti, B.; Bizzarri, R.; Zucchi, R. ACE2 in the era of SARS-CoV-2: Controversies and novel perspectives. Front. Mol. Biosci. 2020, 7, 588618. [Google Scholar] [CrossRef]
- Elemam, N.M.; Hasswan, H.; Aljaibeji, H.; Sulaiman, N. Circulating soluble ace2 and upstream microRNA expressions in serum of type 2 diabetes mellitus patients. Int. J. Mol. Sci. 2021, 22, 5263. [Google Scholar] [CrossRef]
- Hejenkowska, E.D.; Mitash, N.; Donovan, J.E.; Chandra, A.; Bertrand, C.; De Santi, C.; Greene, C.M.; Mu, F.; Swiatecka-Urban, A. TGF-β1 inhibition of ace2 mediated by miRNA uncovers novel mechanism of SARS-CoV-2 pathogenesis. J. Innate Immun. 2023, 15, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, B.; Zhang, X.F.; Ma, Y.P.; Liu, J.D.; Wang, X.Z. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc. Pathol. 2014, 23, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Huang, Y.L.; Shih, Y.Y.; Wu, H.Y.; Peng, C.T.; Lo, W.Y. MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflamm. 2014, 2014, 379537. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Xu, R.; Yu, H.M.; Chang, Q.; Zhong, J.C. The ace2/apelin signaling, microRNAs, and hypertension. Int. J. Hypertens. 2015, 2015, 896861. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fan, X.; Zhao, M.; Wu, M.; Li, H.; Ji, B.; Zhu, X.; Li, L.; Ding, H.; Sun, M.; et al. DNA methylation-reprogrammed ang II (angiotensin II) type 1 receptor-early growth response gene 1-protein kinase C ε axis underlies vascular hypercontractility in antenatal hypoxic offspring. Hypertension 2021, 77, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.I.; Papadopoulou, A.; Aw, T.C. MicroRNA-155 mediates endogenous angiotensin II type 1 receptor regulation: Implications for innovative type 2 diabetes mellitus management. World J. Diabetes 2023, 14, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Kawakami-Mori, F.; Nishimoto, M.; Reheman, L.; Kawarazaki, W.; Ayuzawa, N.; Ueda, K.; Hirohama, D.; Kohno, D.; Oba, S.; Shimosawa, T.; et al. DNA methylation of hypothalamic angiotensin receptor in prenatal programmed hypertension. JCI Insight 2018, 3, e95625. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Zhou, J.J.; Shao, J.Y.; Chen, S.R.; Pan, H.L. DNA demethylation in the hypothalamus promotes transcription of Agtr1a and Slc12a2 and hypertension development. J. Biol. Chem. 2023, 300, 105597. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Li, S.; Zhang, Y.; Chen, Y.; Zhou, Y.; Shi, L. Maternal exercise upregulates the DNA methylation of Agtr1a to enhance vascular function in offspring of hypertensive rats. Hypertens. Res. 2023, 46, 654–666. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, C.C.; Chen, W.D.; Shen, W.L.; Ruan, C.C.; Zhu, L.M.; Zhu, D.L.; Gao, P.J. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem. Biophys. Res. Commun. 2010, 400, 483–488. [Google Scholar] [CrossRef]
- Takeda, Y. Effects of eplerenone, a selective mineralocorticoid receptor antagonist, on clinical and experimental salt-sensitive hypertension. Hypertens. Res. 2009, 32, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; McCurley, A.; Davel, A.P.; McCarthy, J.; Bender, S.B.; Hong, K.; Yang, Y.; Yoo, J.K.; Aronovitz, M.; Baur, W.E.; et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016, 1, e88942. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.F.; Sun, Y.L.; Hamet, P.; Inagami, T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001, 11, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Q.; Sun, S.; Qiu, Y.; Li, J.; Liu, W.; Yuan, G.; Ma, H. Renal transplantation increases angiotensin II receptor-mediated vascular contractility associated with changes of epigenetic mechanisms. Int. J. Mol. Med. 2018, 41, 2375–2388. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Azushima, K.; Yamaji, T.; Suzuki, T.; Abe, E.; Tanaka, S.; Hirota, K.; Tsukamoto, S.; Morita, R.; Kobayashi, R.; et al. Angiotensin II type 1 receptor-associated protein deletion combined with angiotensin II stimulation accelerates the development of diabetic kidney disease in mice on a C57BL/6 strain. Hypertens. Res. 2024, 47, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Yamashita, A.; Abe, E.; Yamaji, T.; Azushima, K.; Tanaka, S.; Taguchi, S.; Tsukamoto, S.; Wakui, H.; Tamura, K. miR-125a-5p/miR-125b-5p contributes to pathological activation of angiotensin II-AT1R in mouse distal convoluted tubule cells by the suppression of Atrap. J. Biol. Chem. 2023, 299, 105478. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Wang, F.; Karashima, S.; Yoneda, T.; Kometani, M.; Hashimoto, A.; Aono, D.; Horike, S.I.; Meguro-Horike, M.; et al. Epigenetic regulation of aldosterone synthase gene by sodium and angiotensin II. J. Am. Heart Assoc. 2018, 7, e008281. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; MacKenzie, S.M.; Alvarez-Madrazo, S.; Diver, L.A.; Lin, J.; Stewart, P.M.; Fraser, R.; Connell, J.M.; Davies, E. MicroRNA-24 is a novel regulator of aldosterone and cortisol production in the human adrenal cortex. Hypertension 2013, 62, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Diver, L.A.; Alvarez-Madrazo, S.; Livie, C.; Ejaz, A.; Fraser, R.; Connell, J.M.; MacKenzie, S.M.; Davies, E. Regulation of corticosteroidogenic genes by microRNAs. Int. J. Endocrinol. 2017, 2017, 2021903. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Liu, Q.; Xie, T.; Huang, R.; Kang, H.; Lai, C.; Zhu, J. MiR-193a-3p functions as a tumour suppressor in human aldosterone-producing adrenocortical adenoma by down-regulating CYP11B2. Int. J. Exp. Pathol. 2018, 99, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Ball, J.P.; Mathis, K.W.; Hall, M.E.; Ryan, M.J.; Rothenberg, M.E.; Yanes Cardozo, L.L.; Romero, D.G. MicroRNA-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1154–E1167. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, K.; Ogishima, T.; Sugiura, Y.; Suematsu, M.; Mukai, K. Pathology and gene mutations of aldosterone-producing lesions. Endocr. J. 2023, 70, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Wang, Y.; Xekouki, P.; Faucz, F.R.; Jain, M.; Zhang, L.; Meltzer, P.G.; Stratakis, C.A.; Kebebew, E. Integrated analysis of genome-wide methylation and gene expression shows epigenetic regulation of CYP11B2 in aldosteronomas. J. Clin. Endocrinol. Metab. 2014, 99, E536–E543. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Morandi, L.; Rubin, B.; Pilon, C.; Asioli, S.; Vicennati, V.; De Leo, A.; Ambrosi, F.; Santini, D.; Pagotto, U.; et al. DNA methylation of steroidogenic enzymes in benign adrenocortical tumors: New insights in aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 2020, 105, dgaa585. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Demura, M.; Wang, F.; Karashima, S.; Yoneda, T.; Kometani, M.; Aomo, D.; Hashimoto, A.; Horike, S.; Meguro-Horike, M.; et al. Effect of potassium on DNA methylation of aldosterone synthase gene. J. Hypertens. 2021, 39, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Kometani, M.; Yoneda, T.; Demura, M.; Aono, D.; Gondoh, Y.; Karashima, S.; Nishimoto, K.; Yasuda, M.; Horike, S.; Takeda, Y. Genetic and epigenetic analyses of aldosterone-producing adenoma with hypercortisolemia. Steroids 2019, 151, 108470. [Google Scholar] [CrossRef] [PubMed]
- Ayuzawa, N.; Fujita, T. The mineralocorticoid receptor in salt-sensitive hypertension and renal injury. J. Am. Soc. Nephrol. 2021, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Recent advances in hypertension: Epigenetic mechanism involved in development of salt-sensitive hypertension. Hypertension 2023, 80, 711–718. [Google Scholar] [CrossRef]
- Cao, N.; Lan, C.; Chen, C.; Xu, Z.; Luo, H.; Zheng, S.; Gong, X.; Ren, H.; Li, Z.; Qu, S.; et al. Prenatal lipopolysaccharides exposure induces transgenerational inheritance of hypertension. Circulation 2022, 146, 1082–1095. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Kometani, M.; Karashima, S.; Yoneda, T.; Takeda, Y. Molecular and epigenetic control of aldosterone synthase, CYP11B2 and 11-hydroxylase, CYP11B1. Int. J. Mol. Sci. 2023, 24, 5782. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yoneda, T.; Demura, M.; Furukawa, K.; Miyamori, I.; Mabuchi, H. Effects of high sodium intake on cardiovascular aldosterone synthesis in stroke-prone spontaneously hypertensive rats. J. Hypertens. 2001, 19, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Bendall, J.K.; Fuchs, S.; Escoubet, B.; Rochais, F.; Hoerter, J.; Nehme, J.; Ambroisine, M.L.; De Angelis, N.; Morineau, G.; et al. Cardiac specific increase in aldosterone production induces coronary dysfunction in aldosterone synthase-transgenic mice. Circulation 2004, 110, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Voelkl, J.; Feger, M.; Kratschmar, D.V.; Castor, T.; Mia, S.; Sacherer, M.; Viereck, R.; Borst, O.; Leibrock, C.; et al. Involvement of vascular aldosterone synthase in phosphate-induced osteogenic transformation of vascular smooth muscle cells. Sci. Rep. 2017, 7, 2059. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yoneda, T.; Demura, M.; Usukura, M.; Mabuchi, H. Calcineurin inhibition attenuates mineralocorticoid-induced cardiac hypertrophy. Circulation 2002, 105, 677–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshimura, M.; Nakamura, S.; Ito, T.; Nakayama, M.; Harada, E.; Mizuno, Y.; Sakamoto, T.; Yamamuro, M.; Saito, Y.; Nakao, K.; et al. Expression of aldosterone synthase gene in failing human heart: Quantitative analysis using modified real-time polymerase chain reaction. J. Clin. Endocrinol. Metab. 2002, 87, 3936–3940. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola, J.; Jaffe, I.Z. The mineralocorticoid receptor in the vasculature: Friend or foe? Annu. Rev. Physiol. 2024, 86, 49–70. [Google Scholar] [CrossRef]
- Brown, J.M. Adverse effects of aldosterone: Beyond blood pressure. J. Am. Heart Assoc. 2024, 13, e030142. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Miyamori, I.; Fujita, T.; Takeda, Y.; Takeda, R.; Yamamoto, H. Vascular aldosterone. biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J. Biol. Chem. 1994, 269, 24316–24320. [Google Scholar] [CrossRef]
- Mesquita, T.R.; Auguste, G.; Falcón, D.; Ruiz-Hurtado, G.; Salazar-Enciso, R.; Sabourin, J.; Lefebvre, F.; Viengchareun, S.; Kobeissy, H.; Lechène, P.; et al. Specific activation of the alternative cardiac promoter of cacna1c by the mineralocorticoid receptor. Circ. Res. 2018, 122, e49–e61. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Huang, Z.; Fan, X.; Tan, X.; Lu, C.; Yang, J. Aldosterone is a possible new stimulating factor for promoting vascular calcification. Front. Biosci. 2021, 26, 1052–1063. [Google Scholar] [CrossRef]
- Lee, H.A.; Song, M.J.; Seok, Y.M.; Kang, S.H.; Kim, S.Y.; Kim, I. Histone Deacetylase 3 and 4 Complex Stimulates the Transcriptional Activity of the Mineralocorticoid Receptor. PLoS ONE 2015, 10, e0136801. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, N.; Zhang, Y.; Li, J.; Zhang, E.; Xu, Z. Fat-diets in perinatal stages altered nr3c2-mediated Ca(2+) currents in mesenteric arteries of offspring rats. Mol. Nutr. Food Res. 2023, 67, e2200722. [Google Scholar] [CrossRef] [PubMed]
- Camarda, N.D.; Ibarrola, J.; Biwer, L.A.; Jaffe, I.Z. Mineralocorticoid receptors in vascular smooth muscle: Blood pressure and beyond. Hypertension 2024, 81, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Sõber, S.; Laan, M.; Annilo, T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem. Biophys. Res. Commun. 2010, 391, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.C.; Lilyanna, S.; Wang, P.; Vardy, L.A.; Jiang, X.; Armugam, A.; Jeyaseelan, K.; Richards, A.M. MicroRNA-31 promotes adverse cardiac remodeling and dysfunction in ischemic heart disease. J. Mol. Cell. Cardiol. 2017, 112, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Foinquinos, A.; Jung, M.; Janssen-Peters, H.; Biss, S.; Bauersachs, J.; Gupta, S.K.; Thum, T. MiRNA-181a is a novel regulator of aldosterone-mineralocorticoid receptor-mediated cardiac remodelling. Eur. J. Heart Fail. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Koyama, R.; Mannic, T.; Ito, J.; Amar, L.; Zennaro, M.C.; Rossier, M.F.; Maturana, A.D. MicroRNA-204 is necessary for aldosterone-stimulated T-type calcium channel expression in cardiomyocytes. Int. J. Mol. Sci. 2018, 19, 2941. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, L.; Cong, G.; Ren, L.; Hao, L. MicroRNA-34b/c inhibits aldosterone-induced vascular smooth muscle cell calcification via a SATB2/Runx2 pathway. Cell Tissue Res. 2016, 366, 733–746. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kawasaki, M.; Hirai, T.; Yoshida, Y.; Tsushima, H.; Fujishiro, M.; Ikeda, K.; Morimoto, S.; Takamori, K.; Sekigawa, I. MicroRNA-766-3p contributes to anti-inflammatory responses through the indirect inhibition of NF-kappaB signaling. Int. J. Mol. Sci. 2019, 20, 809. [Google Scholar] [CrossRef]
- Zhu, A.; Yoneda, T.; Demura, M.; Karashima, S.; Usukura, M.; Yamagishi, M.; Takeda, Y. Effect of mineralocorticoid receptor blockade on the renal renin-angiotensin system in Dahl salt-sensitive hypertensive rats. J. Hypertens. 2009, 27, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Ozbaki-Yagan, N.; Liu, X.; Bodnar, A.J.; Ho, J.; Butterworth, M.B. Aldosterone-induced microRNAs act as feedback regulators of mineralocorticoid receptor signaling in kidney epithelia. FASEB J. 2020, 4, 11714–11728. [Google Scholar] [CrossRef]
- Sierra-Ramos, C.; Velazquez-Garcia, S.; Keskus, A.G.; Vastola-Mascolo, A.; Rodríguez-Rodríguez, A.E.; Luis-Lima, S.; Hernández, G.; Navarro-González, J.F.; Porrini, E.; Konu, O.; et al. Increased SGK1 activity potentiates mineralocorticoid/NaCl-induced kidney injury. Am. J. Physiol. Ren. Physiol. 2021, 320, F628–F643. [Google Scholar] [CrossRef]
- Park, E.-J.; Jung, H.J.; Choi, H.-J.; Cho, J.-I.; Park, H.-J.; Kwon, T.-H. miR-34c-5p and CaMKII are involved in aldosterone-induced fibrosis in kidney collecting duct cells. Am. J. Physiol. Ren. Physiol. 2018, 314, F329–F342. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Gao, J.; Wang, M.; Li, X.; Cui, Z.; Fu, G. Long noncoding RNA Tug1 promotes angiotensin II-induced renal fibrosis by binding to mineralocorticoid receptor and negatively regulating microR-29b-3p. Hypertension 2021, 78, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Xiong, Z.-C.; Zhang, S.-L.; Hao, Q.-Y.; Liu, Z.-Y.; Zhang, H.-F.; Wang, J.-F.; Gao, J.-W.; Liu, P.-M. Upregulated LncRNA H19 sponges miR-106a-5p and contributes to aldosterone-induced vascular calcification via activating the Runx2-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1684–1699. [Google Scholar] [CrossRef]
- Elvira-Matelot, E.; Zhou, X.; Farman, N.; Beaurain, G.; Henrion-Caude, A.; Hadchouel, J.; Jeunemaitre, X. Regulation of WNK1 expression by miR-192 and aldosterone. J. Am. Soc. Nephrol. 2010, 21, 1724–1731. [Google Scholar] [CrossRef]
- Subramanya, A.R.; Yang, C.L.; McCormick, J.A.; Ellison, D.H. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int. 2006, 70, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Yue, P.; Zhang, C.; Wang, W.H. MicroRNA-194 (miR-194) regulates ROMK channel activity by targeting intersectin 1. Am. J. Physiol. Ren. Physiol. 2014, 306, F53–F60. [Google Scholar] [CrossRef][Green Version]
- Lin, D.H.; Yue, P.; Pan, C.; Sun, P.; Wang, W.H. MicroRNA 802 stimulates ROMK channels by suppressing caveolin-1. J. Am. Soc. Nephrol. 2011, 22, 1087–1098. [Google Scholar] [CrossRef]
- Edinger, R.S.; Coronnello, C.; Bodnar, A.J.; Labarca, M.; Bhalla, V.; LaFramboise, W.A.; Benos, P.V.; Ho, J.; Johnson, J.P.; Butterworth, M.B. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J. Am. Soc. Nephrol. 2014, 25, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Apparent mineralocorticoid excess: Research as an art form. Endocrine 2020, 70, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Nishimoto, M.; Hirohama, D.; Ayuzawa, N.; Kawarazaki, W.; Watanabe, A.; Shimosawa, T.; Loffing, J.; Zhang, M.-Z.; Marumo, T.; et al. Renal dysfunction induced by kidney-specific gene deletion of Hsd11b2 as a primary cause of salt-dependent hypertension. Hypertension 2017, 70, 111–118. [Google Scholar] [CrossRef]
- Takeda, Y. Pathophysiological roles of vascular 11beta-hydroxysteroid dehydrogenase and aldosterone. J. Steroid Biochem. Mol. Biol. 2003, 85, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Alikhani-Koopaei, R.; Fouladkou, F.; Frey, F.J.; Frey, B.M. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J. Clin. Investig. 2004, 114, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- White, P.C.; Mune, T.; Agarwal, A.K. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr. Rev. 1997, 18, 135–156. [Google Scholar] [PubMed]
- Pizzolo, F.; Friso, S.; Morandini, F.; Antoniazzi, F.; Zaltron, C.; Udali, S.; Gandini, A.; Cavarzere, P.; Salvagno, G.; Giorgetti, A.; et al. Apparent mineralocorticoid excess by a novel mutation and epigenetic modulation by HSD11B2 promoter methylation. J. Clin. Endocrinol. Metab. 2015, 100, E1234–E1241. [Google Scholar] [CrossRef]
- Rezaei, M.; Andrieu, T.; Neuenschwander, S.; Bruggmann, R.; Mordasini, D.; Frey, F.J.; Vogt, B.; Frey, B.M. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by microRNA. Hypertension 2014, 64, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of hypertension in metabolic syndrome: The role of fructose and salt. Int. J. Mol. Sci. 2023, 24, 4294. [Google Scholar] [CrossRef]
- Nouchi, Y.; Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Ikeya, M.; Kageyama, I.; Wakasugi, T.; Teshigawara, A.; et al. Maternal high-fructose corn syrup intake impairs corticosterone clearance by reducing renal 11β-Hsd2 activity via miR-27a-mediated mechanism in rat offspring. Nutrients 2023, 15, 2122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).