Abstract
Worldwide, osteoarthritis (OA) is the most common cause of joint pain in older people. Many factors contribute to osteoarthritis’ development and progression, including secondary osteoarthritis’ underlying causes. It is important to note that osteoarthritis affects all four tissues: cartilage, bone, joint capsule, and articular apparatus. An increasingly prominent area of research in osteoarthritis regulation is microRNAs (miRNAs), a small, single-stranded RNA molecule that controls gene expression in eukaryotes. We aimed to assess and summarize current knowledge about the mechanisms of the action of miRNAs and their clinical significance. Osteoarthritis (OA) is affected by the interaction between miRNAs and inflammatory processes, as well as cartilage metabolism. MiRNAs also influence cartilage cell apoptosis, contributing to the degradation of the cartilage in OA. Studies have shown that miRNAs may have both an inhibitory and promoting effect on osteoporosis progression through their influence on molecular mechanisms. By identifying these regulators, targeted treatments for osteoarthritis may be developed. In addition, microRNA may also serve as a biomarker for osteoarthritis. By using these biomarkers, the disease could be detected faster, and early intervention can be instituted to prevent mobility loss and slow deterioration.
1. Introduction
1.1. Osteoarthritis
Osteoarthritis (OA), also called osteoarthrosis, is the most common progressive chronic joint disease among older people. It leads to significant, chronic pain, the loss of mobility, and disability [1]. Osteoarthritis affects most people over the age of 65 [2]. An increasing trend in the number of people suffering from osteoarthritis has been observed around the world. An estimated 7% of the global population is affected by OA [3]. In 2019, there were over 500 million people with the disease [4]. The prevalence of OA is one in five among the general population and one in three among those over the age of 50 [3,5,6]. There are a variety of synovial joints that can develop OA, but the hands, knees, and hips are the most commonly affected [7]. There is an estimated 16% of the world’s population diagnosed with knee OA, with women suffering from the condition at a higher rate [6]. In the past, osteoarthritis was thought to be solely the result of cartilage “wear and tear”. Currently, it is understood as a complex, joint-wide condition involving matrix proteases and affecting the entire joint [8]. There are very limited non-surgical treatment options, which include physical activity plans, maintaining a healthy weight, and wearing appropriate footwear and supportive equipment [9]. In the advanced stages of the disease, patients who have not received long-term clinical treatment in the early stages of the disease may have to undergo joint replacement surgery. Globally, the number of these surgeries is on the rise by 10% each year, with 95% of them involving patients with osteoarthritis [10,11]. In the face of OA’s increasing incidence of hip and knee replacements, it is imperative to develop non-surgical treatments [12].
1.2. Risk Factors
A combination of cartilage and subchondral damage and other factors contribute to osteoarthritis pathophysiology. One of the key factors that contribute to the development of osteoarthritis is overweight and obesity [13,14,15,16,17,18,19]. Other factors include age >60 years and female gender [13,15,16,17,18,19,20,21,22,23]. Furthermore, mechanical factors contribute to osteoarthritis, such as professional works that place strain on the knee joints, injuries and previous joint surgeries, excessive exercise, running, and sedentary lifestyles [16,17,18,21,22,24,25,26,27,28,29]. Moreover, genetic mutations are also involved, as well as factors such as high bone mineral density (BMD) and diseases that impair deep sensation [17,21,24,29,30,31,32,33]—Table 1.

Table 1.
Factors increasing osteoarthritis risk.
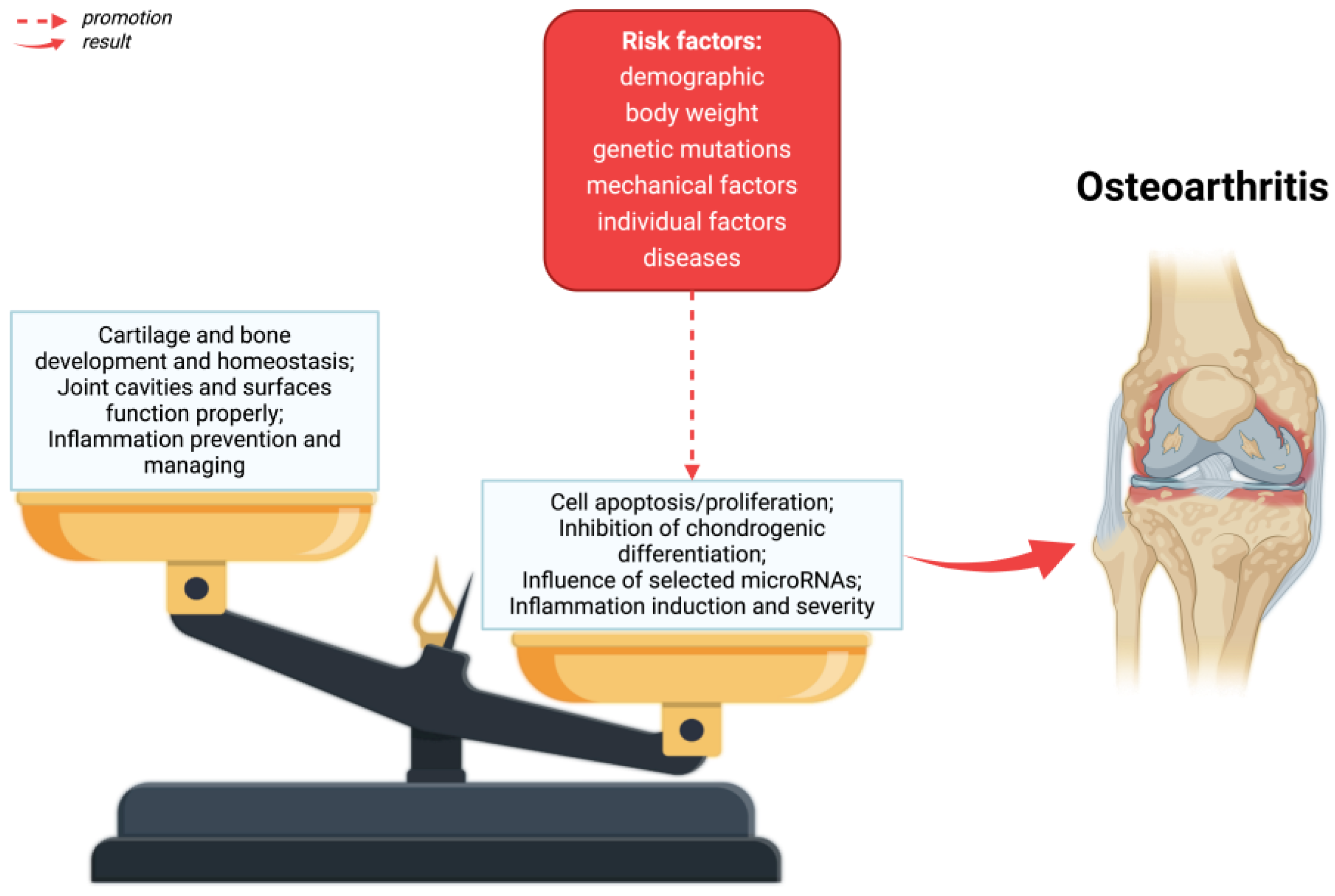
The main symptoms of osteoarthritis are joint pain, limited mobility, crepitations, and inflammatory changes of varying severity without systemic symptoms [34]. As a result of OA, the entire articular joint is affected by disorders [35]—Figure 1. A number of structural defects can be seen in articular cartilage, as well as bone loss in the sublymphatic region, as well as an increase in synovium and tissue hypertrophy. Ties and ligaments may also be unstable [10].
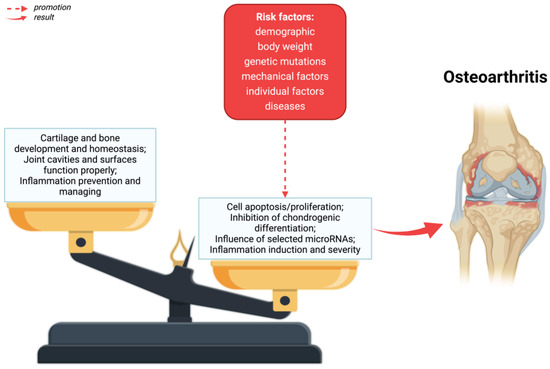
Figure 1.
The pathophysiology of osteoarthritis. As a result of risk factors, alterations in homeostasis in the formation and destruction of cartilage and bones, as well as disorders of the joint cavity and joint surfaces can take place. Created with BioRender.com (accessed on 4 June 2024).
Two types of osteoarthritis exist: primary, which is more common and has an unknown cause; and secondary, which results from local damage to joints and abnormalities of the joint structure or systemic illnesses [24]—Table 2.

Table 2.
Classification of secondary osteoarthritis according to the American College of Rheumatology (ACR) [24,36,37].
1.3. Clinical Relevance
MicroRNAs, also called miRNAs, are small single-stranded RNA molecules consisting of 19 to 25 nucleotides [38]. Human genes are estimated to be regulated by them to the extent of two-thirds [39]. By repressing translation or degrading target messenger RNAs, they are the key regulators of post-transcriptional gene expression [40,41,42,43]. By controlling cell differentiation, proliferation, apoptosis, and immune response, miRNAs can play an important role in many biological processes [44]. The dysregulation of miRNA expression, for example, resulting from mutations in the miRNA genes, may lead to serious disorders and contribute to the pathogenesis of many diseases, including osteoarthritis [45].
There is growing evidence that miRNA may play an important role in osteoarthritis pathogenesis [2,10,46,47,48].
In the context of osteoarthritis, miRNAs may affect various aspects of disease pathogenesis. As an example, some miRNAs play a role in modulating inflammatory processes that contribute to the development of OA and its progression. There is also the possibility that some miRNAs can influence cartilage metabolism by influencing the balance between anabolism and catabolism within cartilage cells. Moreover, some miRNAs can influence cartilage cell apoptosis, which contributes to OA cartilage degradation. Besides its effects on chondrocytes, miRNA also modulates osteoarthritis synovial fibroblasts (OASF) [49,50].
We aimed to summarize and evaluate the current understanding of the mechanism of action of miRNAs and their clinical significance. Understanding miRNAs’ role in OA pathophysiology may lead to new therapeutic targets and biomarkers. For example, if a specific miRNA is overexpressed in OA and contributes to cartilage degradation, it would be possible to develop a therapy that inhibits that miRNA to halt the progression of the disease.
2. Pathophysiology of Osteoarthritis
In recent years, the pathophysiology of osteoarthritis has been intensively studied. Given the complexity of the process, the initiation, development, and severity of osteoarthritis are determined by numerous factors. Although risk factors for the development of osteoarthritis have been identified, the molecular mechanisms that cause the development of this disease are not fully established [10].
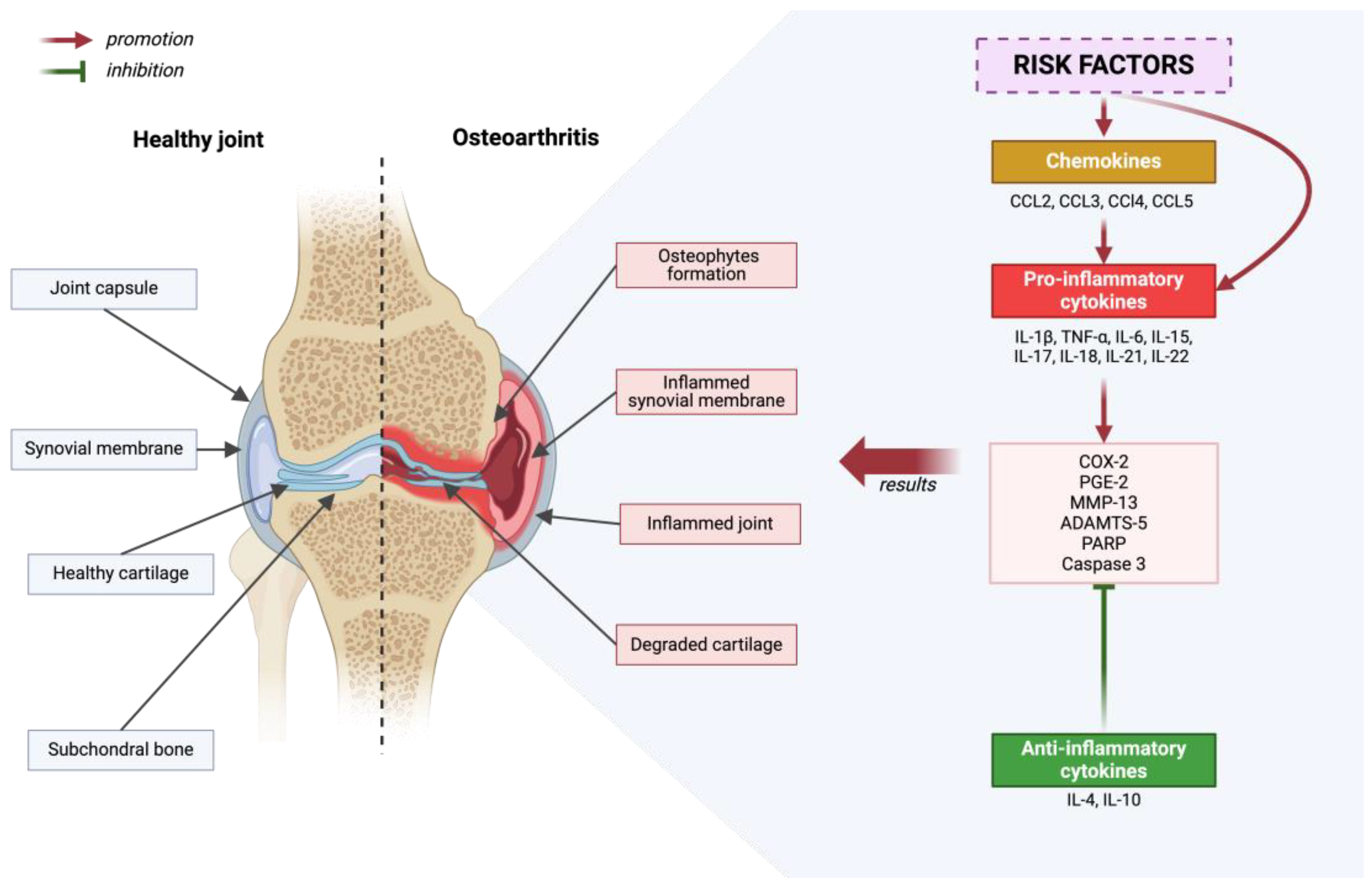
The pathogenesis of osteoarthritis is a multi-aspect process that includes metabolic changes occurring in articular cartilage, subchondral bone, and synovial membrane. In the initial phase of osteoarthritis, increased porosity and reconstruction of the subchondral bone are observed [10]. Generally speaking, the development of osteoarthritis is initiated by disruption of the homeostasis of anti-inflammatory and pro-inflammatory factors. This condition is influenced by, among others, risk factors for osteoarthritis. As a result of many processes, cartilage degradation, bone remodeling and synovium proliferation occur [51]—Figure 2.
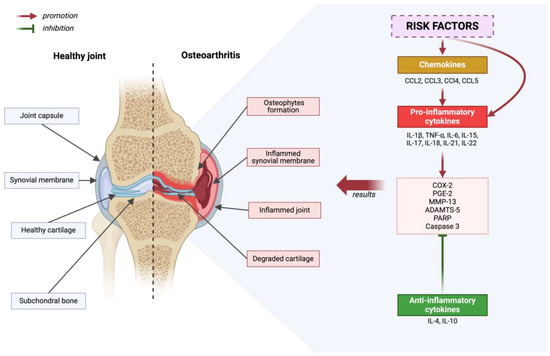
Figure 2.
Diagram depicting a healthy joint in comparison to a joint that has been affected by degenerative disease. Inflammatory processes are outlined, considering the promoting and inhibiting effects of molecular factors. Created with BioRender.com (accessed on 4 June 2024).
In osteoarthritis (OA), initial pathological changes manifest themselves first, mainly on the surface of the articular cartilage, especially in regions subjected to maximum load, where it is damaged [52,53]. Chondrocytes, which are the only cells in cartilage, exhibit increased proliferation in response to matrix degradation [54,55,56]. Some of these cells undergo a phenotypic transformation into hypertrophic chondrocytes, which resemble cells in the hypertrophic zones of the growth plate [57]. As the disease progresses, there is an intense degradation of the matrix caused by proteases that are induced by pro-inflammatory cytokines [54,58,59,60,61]. The pro-inflammatory cytokines involved in the pathogenesis of osteoarthritis include the following: IL-1β, TNF-α, IL-6, IL-15, IL-17, IL-18, IL-21, IL-22 [62,63,64,65,66,67,68,69,70,71]. These cytokines activate chondrocytes for the autocrine and paracrine production of further cytokines and proteases [1]. As a result, chondrocyte apoptosis and regions completely devoid of cells are observed in areas with significant matrix damage [59,72]. Osteoarthritis is also influenced by chemokines: CCL2, CCL3, CCl4, and CCL5, which contribute to pro-inflammatory cytokine production, and anti-inflammatory cytokines such as IL-4 and IL-10 [73,74,75,76,77,78]. The role of IL-4 and IL-10 is to suppress the progression of osteoarthritis [79]. Various cytokines, by activating numerous signaling pathways, lead to an increase in the expression of COX-2, which in turn results in an increase in the production of PGE-2. This phenomenon affects the breakdown of cartilage tissue and the formation of osteophytes. Although COX-2 inhibitors, such as nonsteroidal anti-inflammatory agents, are widely recommended as first-line treatment, they do not stop disease progression [80]. The focus on key cytokines influencing the development of osteoarthritis, such as IL-1β and TNF-α, and their suppression did not meet the expectations placed on them in the results of clinical trials [79,81,82,83,84]. In the case of OA, the action of individual cytokines may be independent, therefore blocking one of them is not always sufficient to effectively inhibit inflammation and the production of matrix-destroying enzymes [79].
As a result of many scientific studies, it has been found that subchondral bone sclerosis may be one of the main causes of osteoarthritis associated with aging [85]. In the place where the cartilage connects with the bone, a phenomenon has been observed where changes in the subchondral bone are inversely proportional to the degree of degeneration of the articular cartilage. It is noticed that the more the subchondral bone thickens, the more advanced the cartilage degeneration process is [1,86]. Moreover, abnormal bone remodeling processes resulting from disorders in the functioning of osteoblasts and osteoclasts play a key role in the initiation and progression of this disease [87,88,89,90]. A change in the subchondral bone plate occurs during both the early and advanced stages of osteoarthritis. In the early stages of the disease, the trabecular structure degrades, and bones become more porous, resulting in a larger distance between trabeculae and a decrease in bone mass [91]. As a result, the subchondral nuclear bone is more likely to deform under load. Bone loss is intensified as a result of microcracks that accumulate. In the advanced stage of the disease, the subchondral bone plate and trabeculae become thicker, but the bone loses mineralization and elasticity [91,92,93]. Moreover, bone cysts and osteophytes also form at the edges of joints, resulting in a flattened and deformed joint contour, which is known as bone abrasion [91,92,93,94].
Osteoarthritis is also accompanied by decreased bone density and mineralization, as well as irregularity in the structure of the bone matrix. These changes are believed to be induced by the transmission of signals from the costal cartilage through subchondral pores and by the process of vascular invasion. These changes occurring in bone and cartilage are considered crucial in the development of osteoarthritis [90]. Unfortunately, the exact mechanisms influencing the initiation and development of osteoarthritis through structural changes in bones and cartilage have not yet been fully explored. This is therefore the basis for conducting further, in-depth research in this area.
Research emerging in recent years indicates an important role of metabolism in maintaining the energy balance of articular chondrocytes. Metabolic dysfunctions of these cells are increasingly recognized as potential factors initiating and accelerating the development of osteoarthritis [95,96,97]. These metabolic changes affect metabolic pathways in chondrocytes, synoviocytes, and bone cells. The abnormally accelerated catabolism of articular chondrocytes, which promotes ECM degradation and preempts ECM synthesis, is a major feature of OA cartilage [10,98,99]. As a result of these processes, there is an interaction between these cells and the immune system, and the mediated regulation of inflammation can be considered a key element in understanding the pathogenesis of osteoarthritis [96].
Molecular pathways contributing to the development of osteoarthritis include the increased activity of extracellular matrix (ECM)-degrading enzymes, such as matrix metalloproteinase 13 (MMP-13) and ADAMTS-5, as well as the increased production of inflammatory cytokines, including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor (TNF). Additionally, apoptotic pathways are activated, including caspase 3 and poly (ADP-ribose) polymerase (PARP), with a simultaneous reduction in the expression of genes responsible for the synthesis of ECM components, such as COL2A1 and ACAN, and changes in processes maintaining cellular balance, including autophagy [100,101,102,103].
3. The Role of miRNAs in the Pathogenesis of Osteoarthritis
The currently published results indicate the high importance of miRNAs in the pathogenesis of osteoarthritis. Data indicate increased concentrations of most miRNAs in patients with osteoarthritis, and therefore, in most cases, the increased promotion of osteoarthritis by various potential targets—Table 3. The reports are mostly innovative and indicate more and more miRs, the increased concentrations of which are observed in osteoarthritis.
There are many potential mechanisms by which microRNAs promote or prevent osteoarthritis.
By far the best-studied microRNA in cartilage is miR-140 [104]. Studies have shown that miR-140-5p is crucial in directly regulating the levels of genes including, but not limited to: IGFBP-5, MMP13, Hdac4, Cxcl12, Bmp2, and SMAD3. These genes play an important role in the development of chondrocytes and in maintaining the balance of joint cartilage [105,106,107]. However, it appears that miR-140-3p is most abundantly expressed in cartilage, and perhaps miR-140-5p is most important in the development of osteoarthritis, while miR-140-3p potentially plays a greater role in the homeostasis of joint tissues [108,109]. It was found that the level of miR-140 in the knee cartilage of people suffering from osteoarthritis is lower compared to healthy cartilage. The complete elimination of miR-140 in mice resulted in a mild form of dwarfism and impaired chondrocyte differentiation and proliferation [110,111]. Additionally, the complete removal of miR-140 increased the tendency of mice to spontaneous age-related cartilage degeneration and to intensify cartilage damage in the case of surgically induced osteoarthritis [111]. The deletion of miR-140 combined with the inhibition of the let-7 microRNA in mice in turn leads to more severe changes in skeletal structure than were observed with any single mutation [112].
MiR-146a is another microRNA that has been extensively studied and is known to be stimulated by a variety of inflammatory factors. It plays an important role in the functioning of the immune system and inflammatory processes [113]. miR-146a is highly expressed in the cartilage tissue of people suffering from early-stage osteoarthritis and has the potential to regulate and modulate pain associated with this disease [114,115]. It has been reported that it may affect inflammatory processes, autophagy, and apoptosis mechanisms in cartilage cells, as well as the activity of genes responsible for the composition of the extracellular matrix [116,117,118]. In their study, Zhang et al. analyzed miR-146a-5p and found that it was highly active in the knee cartilage of individuals with osteoarthritis (OA) [119]. Conversely, the NUMB protein exhibited low activity and was suppressed by miR-146a-5p. The rise in miR-146a-5p levels may lead to enhanced cellular apoptosis and reduced autophagy in human and mouse chondrocytes by affecting factors such as active caspase-3, PARP, Bax, Beclin 1, ATG5, p62, LC3-I, and LC3-II. Elevating the level of weakly expressed NUMB could neutralize the effects of miR-146a-5p on chondrocyte apoptosis and autophagy. Furthermore, the injection of miR-146a-5p antagomir directly into the joint could reverse the effects of miR-146a-5p on apoptosis and autophagy in chondrocytes in OA mice. As a result of these studies, it was shown that reducing miR-146a-5p activity inhibited apoptosis and supported autophagy in chondrocytes, targeting NUMB in both in vivo and in vitro studies [119]. These findings are also supported by another study using miR-146a-5p. Qin et al. demonstrated that knockdown of miR-146a-5p in chondrocytes antagonizes IL-1β-mediated inflammatory responses and increases catabolism in vitro and attenuates cartilage degeneration in injury-induced OA in mice [120].
However, different results were obtained by Guan et al., where they found reduced miR-146a expression in areas affected by osteoarthritis (OA) compared to healthy cartilage. These researchers also described mice genetically lacking miR-146a that showed spontaneous OA symptoms early in the disease development, while mice overexpressing miR-146a in chondrocytes were resistant to OA. Additionally, mice lacking miR-146a were more sensitive to joint instability-induced OA, whereas mice with controlled overexpression of miR-146a were protected from the disease. It appears that miR-146a can protect against OA by affecting the Notch1 protein, and the delivery of Notch1 inhibitors to the joints of mice lacking miR-146a prevented joint destruction [121]. However, genes targeting miR-146a-3p currently have less evidence to correlate with osteoarthritis [122]. Although studies on the single nucleotide polymorphism (sNP) of miR-146a did not confirm an increased risk of osteoarthritis (OA) as a result of the mutation, they did indicate a decrease in the expression level of miR-146a caused by the mutation, which in turn led to an increase in the activity of the IRAK1 and TRAF6 genes [123]. These findings, while promising, require further research to clarify the contradictions.
In a study that detected the presence of miR-9-5p in patients with osteoarthritis, it was shown that this miR promoted cell proliferation and suppressed chondrocyte apoptosis by affecting, among other things, matrix metalloproteinase-13 (MMP-13), which is the primary MMP involved in in the degradation of cartilage through its special ability to cleave type II collagen [124,125]. The second target turned out to be protogenin (PRTG), the overexpression of which induces the activation of caspase-3 signaling and increases apoptosis. Since in OA patients, the expression of PRTG negatively correlated with high miR-9 expression and therefore turned out to be reduced, it did not show an apoptotic effect [124,126]. It turns out that this selected microRNA can reduce the progression of osteoarthritis.
Another microRNA present in patients with osteoarthritis turned out to be miR-10a-5p. Its specific target is HOXA3, the silencing of which significantly inhibited chondrocyte proliferation, and promoted chondrocyte apoptosis and cartilage matrix degradation [127].
Another one, miR-22, activates metalloproteinases and aggrecanases and downregulates the structural proteins of cartilage, which leads to its degradation. PPARα and BMP-7 are potential targets [128]. PPARs are ligand-activated receptors in the nuclear hormone receptor family. The human gene for PPARα is located on chromosome 22. The activation of PPARγ and PPARα has been shown to effectively modulate the NF-κB, AP-1, and other stress-responsive oxidative signaling channels, leading to the inhibition of inflammatory responses. Furthermore, the activation of PPARγ and PPARα may provide protection to chondrocytes by exerting control over their autophagic behavior [129]. The overexpression of miR-22 inhibited BMP-7 and PPARα protein expression, helping to promote osteoarthritis. Moreover, miR-22 expression was positively correlated with BMI [128].
As numerous studies have shown, miR-27b is also involved in promoting osteoarthritis by targeting, among others, MMP-13, COL1A1, and ADAMTS8. Metalloproteinase with thrombospondin motifs (ADAMTS) causes the degradation of extracellular matrix (ECM) collagen and aggrecan II [130]. ADAMTS is also one of the targets of miR-34a-5p, miR-140, and miR-140-5p [114,131,132].
The findings also indicate that OA synovial fibroblasts (OASFs) are characterized by increased levels of proinflammatory cytokines relative to normal synovial fibroblasts (NSF). Moreover, it was noticed that miR-149-5p, which was decreased in patients with osteoarthritis compared to the control group, plays a role in reducing the expression level of IL-1β, IL-6, and TNF-α [133]. Thus, significantly reduced levels of miR-149-5p interfere with the synthesis of connective protein and proteoglycan [133,134]. Furthermore, Jiang et al. showed that increased miR-149 or suppressed vascular cell adhesion molecule 1 (VCAM-1) reduced inflammation and apoptosis in the cartilage tissues of OA mice, which was associated with the inactivation of the PI3K/AKT pathway [135]. In addition, miR-149 suppressed the chondrocyte inflammatory response that was induced by IL-1β by downregulating the activation of TAKI/NF-κB signaling pathway also counteracted osteoarthritis [136]. The therapeutic effect of the anti-inflammatory drugs used in the study was related to their ability to suppress the expression of miR-149-5p, suggesting that the regulation of these miRNAs may constitute an innovative method of reducing inflammation in OA.
Studies have shown that miR-128 is also involved in the pathogenesis of osteoarthritis [137]. According to Lian et al., miR-128a inhibits the autophagy process in cartilage cells and worsens the symptoms of osteoarthritis in the knee joint by affecting Atg12 [137]. Mice with the miR-128a gene deleted presented less abnormalities in microcomputer tomographic and kinematic measurements after DMM surgery and showed less advanced changes in cartilage loss at the histological level [138].
MiR-210 acts as a positive regulator of osteoblastic differentiation by inhibiting the TGF-β/activin signaling pathway through the inhibition of AcvR1b [139]. Additionally, studies have shown that miR-210 acts as an inhibitor of the production of pro-inflammatory cytokines [140]. Recent reports indicate that miR-210 is associated with the NF-κB signaling pathway, which plays an important role in regulating the immune response, inflammatory processes, and cell survival, and therefore has a protective effect against the development of osteoarthritis [140,141].
Data also report a role for miR-335-5p in inhibiting osteogenic and adipogenic differentiation and promoting extracellular matrix (ECM) degradation. The Wnt and IFNγ signaling pathways and the HBP1 gene have been indicated as targets of miR-335-5p [124,142]. The obtained data also indicate the activation of the NF-κB pathway and a significant increase in the levels of IL-1α and IL-6 in cells after transfection with miR-335-5p mimics, compared to control cells [142].
Finally, miR-485-5p, which is overexpressed in osteoarthritis, can downregulate SOX9. By doing so, it can inhibit the differentiation of BMSCs into chondroblasts and promote the expression of inflammatory factors to accelerate the development of osteoarthritis [143].

Table 3.
The role of selected microRNAs in the osteoarthritis process—potential effect, impact on potential target genes/pathways, and attitude towards osteoarthritis.
Table 3.
The role of selected microRNAs in the osteoarthritis process—potential effect, impact on potential target genes/pathways, and attitude towards osteoarthritis.
| MicroRNA | Level in OA | Potentially Action | Potential Target | Role in OA Pathogenesis | References |
|---|---|---|---|---|---|
| miR-9-5p | ↓ 1 | intensifying proliferation and suppressing chondrocyte apoptosis | MMP-13, PRTG | − 3 | [124] |
| miR-10a-5p | ↑ 2 | inhibiting chondrocyte proliferation, promoting chondrocyte apoptosis, and promoting cartilage matrix degradation | HOXA3 | + 4 | [127] |
| miR-22 | ↑ | the activation of metalloproteinases and aggrecanases and downregulation of cartilage structural proteins, cartilage degradation | PPARα, BMP-7 | + | [128] |
| miR-27b | ↑ | the fibrosis of the synovial membrane, influence on inflammatory processes, cartilage metabolism, and apoptosis of cartilage cells | MMP-13, COL1A1, α-SMA2, ADAMTS8, and CBFB | + | [144,145,146] |
| miR-34a-5p | ↑ | cell cycle arrest, promoting apoptosis, senescence, and proliferation | COL2A1, ACAN, ATG5, MMP13, ADAMTS5, IL-1β, and COL10A1 | + | [131] |
| miR-127-5p | ↓ | increasing the synthesis of cartilage extracellular matrix (ECM) | Osteoponin and MMP-13 | − | [147,148] |
| miR-128a | ↑ | impaired chondrocyte autophagy, the suppression of extracellular matrix deposition | Atg12, Bax, Bcl2, and cleaved caspase-3 | + | [137,138] |
| miR-138-5p | ↑ | the degradation of cartilage extracellular matrix (ECM) | FOXC1 and increase in IL-1β | + | [124] |
| miR-140 | ↓ | promoting chondrocyte differentiation | ADAMTS5 and AGGRECAN | − | [132] |
| miR-140-3p | ↓ | increase in the viability and migration capacity of chondrocytes | increase: SOX-9, COL2, ACAN, RUNX2, and SCX, decrease: COL1, COL6, COMP, TNC, and FMOD | − | [149] |
| miR-140-5p | ↓ | inhibits inflammation in the joint cavity, inhibits the progression of OA, promotes chondrogenesis, inhibits chondrocyte apoptosis, inhibits chondrocyte hypertrophy | IGFBP-5, IL-1β, IL-6, Syndecan-4, ADAMTS5, MMP-13, SMAD3, HMGB1, RALA, FUT1, HDAC4, and SMAD1 | − | [105,109,111,150,151,152,153,154,155,156] |
| mi-146 | ↑ | promoting the inflammatory response in the joint | TRAF6 and IRAQ1, | + | [157,158] |
| miR-146a-5p | ↑ | cartilage degradation, synovitis, neoangiogenesis, and osteoclastogenesis | TNF α, IL-1β, TRAF6 and IRAK1 genes, and MMP-13 | + | [114,124,159] |
| miR-149 | ↓ | promoting the synthesis of connective protein and proteoglycan, suppressing the inflammatory process | TNFα, IL1β, IL6, VCAM-1, and TAK1 | − | [134,135] |
| miR-210 | ↓ | promoting osteoblastic differentiation, anti-apoptotic effect, anti-inflammatory effect | AcvR1b and DR6 | − | [139,140,141,160] |
| miR-335-5p | ↑ | osteogenic and adipogenic differentiation, promoting ECM degradation | Wnt signaling pathway, IFNγ, HBP1, ACAN, MMP13, collagen X, and collagen II | + | [124,142] |
| miR-485-5p | ↑ | inhibiting the differentiation of BMSCs into chondroblasts and promoting the expression of inflammatory factors | SOX9 | + | [143] |
1 ↓—downregulation. 2 ↑—upregulation. 3 −—impairment. 4 +—intensification.
These results indicate a complex role of miRNAs in the regulation of pathological processes in OA, including cartilage degradation, inflammatory processes, cell differentiation, and tissue homeostasis. The most-studied miRNAs in cartilage are miR-140 and miR-146-5p.
Most miRNAs show increased levels in OA patients, which may contribute to its progression through various biological targets. However, changes in miRNA expression are also observed in osteoarthritis, some of which are downregulated, such as miR-127-5p and miR-140-5p. The reduced expression of the mentioned miRs correlates with the formation of changes in the joints and thus promotes osteoarthritis. However, miR-127-5p and miR-140-5p themselves have a protective effect on cartilage, promoting the differentiation of chondrocytes, preventing the formation of a planar state, and increasing the synthesis of the extracellular matrix. Changes in the expression of these miRNAs may therefore constitute potential targets for new therapies in the treatment of OA.
According to the analysis, some miRNAs are upregulated in OA, such as miR-146a-5p and miR-34a-5p, while others are downregulated, such as miR-140-5p. The osteoarthritis-related miRNA-22, for instance, induces inflammation and catabolism in joint cells. Other miRNAs, such as miRNA-9 and miRNA-98, can inhibit the secretion of matrix metalloproteinase-13 (MMP-13) and the inflammatory factors TNF and IL1β, suggesting their potential role in inhibiting cartilage degradation.
4. Potential Therapeutic Targets
Studies have shown that miRNAs may play a key role in the pathogenesis of osteoarthritis by regulating the expression of genes related to articular cartilage homeostasis and inflammatory processes. For example, miR-146a-5p and miR-34a-5p are upregulated in OA and may serve as diagnostic biomarkers and potential therapeutic targets. On the other hand, miR-127-5p, and miR-140-5p are downregulated and may have a protective function in maintaining cartilage integrity [161].
The use of miRNAs in OA therapy may include strategies to modulate their expression to restore articular cartilage homeostasis. For example, the delivery of synthetic miRNA mimetics or inhibitors may help restore the normal miRNA expression profile in diseased joints. Additionally, miRNA therapy can be combined with other approaches, such as cell therapy using mesenchymal stem cells, which are also regulated by miRNAs [161].
However, there are challenges associated with miRNA therapy, including the specificity of delivery, stability of miRNA molecules in the body, and potential adverse effects resulting from the modulation of multiple gene expression [162]. MiRNAs are characterized by a short lifespan, limited stability in the body, problems with distribution in tissues, interference with natural RNA processes, and the possibility of causing undesirable effects [163]. Further research is necessary to understand the complex interactions of miRNAs with their target genes and to develop effective and safe methods for miRNA delivery to joint tissues.
According to some studies, osteoarthritis patients have a much lower expression of certain miRNAs, such as miRNA-27b, than the patients without the disease [29]. It has also been found that miRNA-27b regulates MMP-13 expression in human chondrocytes, indicating that miRNAs may be used therapeutically for the treatment of osteoarthritis. Understanding the interactions between miRNAs and their multiple target genes may be crucial for regulating homeostasis and control pathways in osteoarthritis. As such, these miRNAs may serve as biomarkers for osteoarthritis early diagnosis.
In summary, miRNAs present promising potential as therapeutic targets in OA, offering new opportunities for the treatment of this disease. The development of miRNA therapy may contribute to improving the quality of life of OA patients, but this requires further research and development of miRNA delivery technologies. Understanding the role of miRNAs in the pathophysiology of osteoarthritis may lead to the identification of new therapeutic targets and biomarkers of the disease. For example, if a specific miRNA is overexpressed in OA and contributes to cartilage degradation, it would be possible to develop a therapy that inhibits that miRNA to halt the progression of the disease. Alternatively, if a specific miRNA is deficient in OA and its absence contributes to disease progression, it could be possible to develop a therapy that increases the expression of that miRNA to inhibit disease progression.
5. Discussion
MiRNAs play a critical role in osteoarthritis (OA), influencing cartilage degradation, inflammation, cell differentiation, and tissue homeostasis. Patients with OA generally have increased miRNA concentrations, which generally promote the disease through various biological mechanisms.
One of the most extensively studied miRNAs in cartilage is miR-140. As a direct regulator of chondrocyte development and cartilage balance, MiR-140-5p is known to regulate genes such as IGFBP-5, MMP13, HDAC4, CXCL12, BMP2, and Smad3 [105,106,107]. MiR-140-3p is abundant in cartilage, maintaining joint tissue homeostasis, while miR-140-5p is implicated in OA development [111]. It has been shown that miR-140 levels are diminished in OA cartilage, resulting in impaired chondrocyte differentiation and proliferation, and increased cartilage degeneration [105,111].
OA cartilage expresses high levels of MiR-146a, which targets the NUMB protein to modulate inflammation, autophagy, and apoptosis in chondrocytes [119]. By suppressing MiR-146a-5p, chondrocytes are able to mitigate apoptosis and enhance autophagy [119,164]. OA symptoms are linked to miR-146a downregulation, while overexpression provides resistance to OA by affecting the Notch1 protein [121]. In light of miR-146a’s complex role in OA, further research is needed to resolve contradictory findings.
OA pathogenesis is also influenced by other miRNAs. Through its targeting of MMP-13 and PRTG, MiR-9-5p promotes chondrocyte proliferation and inhibits apoptosis [124]. Through targeting HOXA3, MiR-10a-5p inhibits chondrocyte proliferation and promotes apoptosis and cartilage degradation [127]. By activating metalloproteinases and aggrecanases, MiR-22 downregulates structural proteins in cartilage and targets PPARα and BMP-7 [128]. The miR-27b promotes osteoarthritis by targeting MMP-13, COL1A1, and ADAMTS8, which results in the degradation of the ECM [130]. It is reported that miR-34a-5p induces apoptosis and senescence by interacting with several genes, including MMP13 and IL-1β [131].
MiR-149-5p, reduced in OA, plays a role in decreasing IL-1β, IL-6, and TNF-α expression, with potential anti-inflammatory effects [133]. MiR-128a inhibits autophagy in cartilage cells, aggravating OA symptoms [137]. MiR-210 inhibits pro-inflammatory cytokine production and regulates osteoblastic differentiation [140,141]. There has been evidence that MiR-335-5p promotes the degradation of ECM and may be associated with the NF-B pathway activation, increasing IL-1α and IL-6 levels [142]. MiR-485-5p, overexpressed in OA, inhibits chondroblast differentiation by downregulating SOX9 [143].
There is a complex regulatory network in the body in which one miRNA can influence multiple molecular targets at the same time, while multiple miRNAs can affect the same target at the same time. The same miRNAs may be responsible for regulating different molecular pathways and pathogenic mechanisms in osteoarthritis. For example, there are several miRNAs that influence IL-1β levels, including miR-140-5p, miR-149-5p, and miR-146a-5p. A similar situation is in the case of IL-6, which is acted upon by miR-140-5p and miR-149-5p. MMP13 is also a target of several miRNAs. It is a target of, among others, miR-335-5p, miR-9, miR-98, miR-27b, miR-34a-5p, miR-140, and miR-140-5p. In some cases, miRNAs can regulate the same molecular target together. This is known as cooperative miRNA interaction, which may lead to a synergistic effect in regulating osteoarthritis-related gene expression [165]. There may be diversity in gene regulation by miRNAs in individuals with osteoarthritis, resulting in differential gene expression and genetic variants [166]. Because of this, individual genetic and epigenetic characteristics may determine which miRNAs regulate the same molecular targets.
There is potential for the therapeutic targeting of miRNAs in OA. MiRNA expression can be modulated to restore cartilage homeostasis. A synthetic miRNA mimetic or inhibitor, for example, could normalize miRNA profiles in diseased joints. Combining miRNA therapy with other treatments, like mesenchymal stem cell therapy, could enhance therapeutic outcomes. However, challenges such as delivery specificity, miRNA stability, and potential adverse effects must be addressed. Further research is essential to develop effective miRNA delivery methods and understand miRNA/gene interactions in OA.
6. Conclusions
In articular cartilage degeneration, enzymes from the metalloproteinase family play a critical role, whose activity is regulated by pro-inflammatory cytokines, transcription factors, and miRNAs. MiRNAs are small, non-structural RNA molecules that regulate gene expression post-transcriptionally. The miRNAs affect protein synthesis by binding to complementary sequences in mRNAs, thereby causing degradation or blocking translation. In the context of osteoarthritis (OA), miRNAs are considered important regulators of anabolic and catabolic processes in articular cartilage, as well as mediators in the inflammatory response and degenerative processes.
Furthermore, miRNAs may prove useful as diagnostic biomarkers in osteoarthritis. Despite many studies suggesting that miRNAs play an important role in OA pathogenesis, some results remain controversial. Different test results can be influenced primarily by the type of sample used: cartilage, synovial fluid, and blood may affect miRNA expression profiles [161]. As well, the complexity of OA resulting from the interaction of many factors such as genetics, age, gender, lifestyle, and overall health may lead to differences in miRNA expression. Studies may also use different research techniques to measure miRNA expression, which may result in different results. Last but not least, statistical analysis methods used in different studies may influence the interpretation of the results.
It is important to remember that treating OA will likely require a multipronged approach that addresses the genetic, biological, and environmental factors contributing to the disease. We may be able to use miRNAs as part of our treatment arsenal against OA in this context.
Thus, miRNAs are critical in the pathophysiology of OA, influencing the processes of degradation and repair of the cartilage. It may be possible to develop new therapeutic and diagnostic strategies by understanding their function. To improve patient outcomes, miRNA therapies may be able to synergize with existing osteoporosis treatments, such as bisphosphonates and monoclonal antibodies. There is, however, still much work to be done to better understand the complex interactions between miRNAs and their target genes. Specifically, further research is needed to determine how miRNA therapies can be delivered to joints, how to ensure target specificity, and how to avoid potential side effects. To mitigate the possibility of adverse effects of miRNA-based therapies, comprehensive studies are needed on their long-term effects and safety.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACAN | Aggrecan |
| ACR | American College of Rheumatology |
| ADAMTS5 | A Disintegrin and Metalloproteinase with Thrombospondin Motifs 5 |
| ADAMTS8 | A Disintegrin and Metalloproteinase with Thrombospondin Motifs 8 |
| Atg12 | Autophagy Related 12 |
| ATG5 | Autophagy Related 5 |
| Bax | BCL2 Associated X, Apoptosis Regulator |
| Bcl2 | B-Cell Lymphoma 2 |
| BMP-7 | Bone Morphogenetic Protein 7 |
| CBFB | Core-Binding Factor Subunit Beta |
| COL10A1 | Collagen Type X Alpha 1 Chain |
| COL1A1 | Collagen Type I Alpha 1 Chain |
| COL2 | Collagen Type II |
| COL2A1 | Collagen Type II Alpha 1 Chain |
| COL6 | Collagen Type VI |
| COMP | Cartilage Oligomeric Matrix Protein |
| DR6 | Death Receptor 6 |
| ECM | Extracellular matrix |
| FMOD | Fibromodulin |
| FOXC1 | Forkhead Box C1 |
| FUT1 | Fucosyltransferase 1 |
| HDAC4 | Histone Deacetylase 4 |
| HMGB1 | High Mobility Group Box 1 |
| HOXA3 | Homeobox A3 |
| HBP1 | HMG-Box Transcription Factor 1 |
| IFNγ | Interferon Gamma |
| IGFBP-5 | Insulin-Like Growth Factor Binding Protein 5 |
| IL | interleukins |
| IRAK1 | Interleukin-1 Receptor-Associated Kinase 1 |
| MiRNA, MiR | microRNA |
| MMP-13 | Matrix Metallopeptidase 13 |
| NF-κB | Nuclear factor kappa B |
| OA | osteoarthritis |
| PARP | Poly (ADP-Ribose) Polymerase |
| PPARα/γ | Peroxisome Proliferator-Activated Receptor α/γ |
| PRTG | Protogenin |
| RALA | Ras-Like Proto-Oncogene A |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SCX | Scleraxis |
| SMAD1 | SMAD Family Member 1 |
| SMAD3 | SMAD Family Member 3 |
| SOX9 | SRY-Box Transcription Factor 9 |
| TAK1 | TGF-Beta Activated Kinase 1 |
| TNC | Tenascin C |
| TNFα | Tumor Necrosis Factor Alpha |
| TRAF6 | TNF Receptor Associated Factor 6 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| α-SMA2 | α Smooth Muscle Actin 2 |
References
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis Pathogenesis: A Review of Molecular Mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Thudium, C.S.; Bay-Jensen, A.C.; Maleitzke, T.; Geissler, S.; Duda, G.N.; Winkler, T. Biomarkers for Osteoarthritis: Current Status and Future Prospects. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101852. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. eClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. Br. Med. J. 2006, 332, 639. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR Recommendations for the Non-Pharmacological Core Management of Hip and Knee Osteoarthritis: 2023 Update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Little, C.B.; Hunter, D.J. Post-Traumatic Osteoarthritis: From Mouse Models to Clinical Trials. Nat. Rev. Rheumatol. 2013, 9, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; van der Esch, M.; Hinman, R.S.; Peat, G.; de Zwart, A.; Quicke, J.G.; Runhaar, J.; Knoop, J.; van der Leeden, M.; de Rooij, M.; et al. How Does Hip Osteoarthritis Differ from Knee Osteoarthritis? Osteoarthr. Cartil. 2022, 30, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Z.; Chen, Y.; Zhang, Y.; Xing, D.; Zhao, L.; Lin, J.; Mei, Y.; Lin, H.-Y.; Zheng, Y.; et al. Development and Formulation of the Classification Criteria for Osteoarthritis. Ann. Transl. Med. 2020, 8, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of Metabolic Abnormalities in Osteoarthritis: A New Perspective to Understand Its Pathological Mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chung, C.Y.; Sung, K.H.; Lee, S.Y.; Won, S.H.; Kim, T.G.; Choi, Y.; Kwon, S.S.; Kim, Y.H.; Park, M.S. Risk Factors for Osteoarthritis and Contributing Factors to Current Arthritic Pain in South Korean Older Adults. Yonsei Med. J. 2015, 56, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Karpiński, R. Knee Joint Osteoarthritis Diagnosis Based on Selected Acoustic Signal Discriminants Using Machine Learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Lee, R.; Kean, W.F. Obesity and Knee Osteoarthritis. Inflammopharmacology 2012, 20, 53–58. [Google Scholar] [CrossRef]
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of Selected Classification Methods Based on Machine Learning as a Diagnostic Tool for Knee Joint Cartilage Damage Based on Generated Vibroacoustic Processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Felson, D.T. Clinical Practice. Osteoarthritis of the Knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Górska, I.; Szczepański, L. Osteoarthritis. In Interna Szczeklika 2023; Szczeklik, A., Gajewski, P., Eds.; Medycyna Praktyczna: Kraków, Poland, 2023; pp. 2162–2170. [Google Scholar]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk Factors and Burden of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours That Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the Epidemiology, Risk Factors and Disease Outcomes of Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, T.; Angelov, A.K. Modifiable Risk Factors in Knee Osteoarthritis: Treatment Implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, M.; Kwapisz, A.; Synder, M.; Szemraj, J. Osteoarthritis: Etiology, Risk Factors, Molecular Mechanisms. Postep. Hig. Med. Dosw. 2014, 68, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, M.; Rego, I.; Carreira-Garcia, V.; Blanco, F.J. Genetics in Osteoarthritis. Curr. Genom. 2008, 9, 542–547. [Google Scholar] [CrossRef]
- Hardcastle, S.A.; Dieppe, P.; Gregson, C.L.; Davey Smith, G.; Tobias, J.H. Osteoarthritis and Bone Mineral Density: Are Strong Bones Bad for Joints? Bonekey Rep. 2015, 4, 624. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wang, Y.; Wluka, A.E.; Strauss, B.J.; Proietto, J.; Dixon, J.B.; Jones, G.; Cicuttini, F.M. Associations between Systemic Bone Mineral Density and Early Knee Cartilage Changes in Middle-Aged Adults without Clinical Knee Disease: A Prospective Cohort Study. Arthritis Res. Ther. 2017, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, M.C.; Zhang, Y.; Javaid, M.K.; Neogi, T.; Curtis, J.R.; Niu, J.; McCulloch, C.E.; Segal, N.A.; Felson, D.T. High Systemic Bone Mineral Density Increases the Risk of Incident Knee OA and Joint Space Narrowing, but Not Radiographic Progression of Existing Knee OA: The MOST Study. Ann. Rheum. Dis. 2010, 69, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA—J. Am. Med. Assoc. 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R.; et al. The American College of Rheumatology Criteria for the Classification and Reporting of Osteoarthritis of the Hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of Criteria for the Classification and Reporting of Osteoarthritis: Classification of Osteoarthritis of the Knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, O.; Hayashi, M.; Horikawa, A.; Owada, H.; Miyamoto, R.; Mizukami, N.; Inui, T. The Role of MiR-217-5p in the Puromycin Aminonucleoside-Induced Morphological Change of Podocytes. Noncoding RNA 2022, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Strmsek, Z.; Kunej, T. MicroRNA Silencing by DNA Methylation in Human Cancer: A Literature Analysis. Noncoding RNA 2015, 1, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Grenda, A.; Budzyński, M.; Filip, A. Biogenesis of MicroRNAs and Their Role in the Development and Course of Selected Hematologic Disorders. Postep. Hig. Med. Dosw. 2013, 67, 174–185. [Google Scholar] [CrossRef]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory Network of MiRNA on Its Target: Coordination between Transcriptional and Post-Transcriptional Regulation of Gene Expression. Cell. Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of Gene Silencing by Double-Stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Wakiyama, M.; Takimoto, K.; Ohara, O.; Yokoyama, S. Let-7 MicroRNA-Mediated MRNA Deadenylation and Translational Repression in a Mammalian Cell-Free System. Genes Dev. 2007, 21, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Alwani, A.; Baj-Krzyworzeka, M. MiRNAs—Targets in Cancer Therapy. Postep. Biochem. 2021, 67. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Alahari, S.K. MicroRNA and Breast Cancer: Understanding Pathogenesis, Improving Management. Noncoding RNA 2015, 1, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.J.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef] [PubMed]
- Caldo, D.; Massarini, E.; Rucci, M.; Deaglio, S.; Ferracini, R. Epigenetics in Knee Osteoarthritis: A 2020–2023 Update Systematic Review. Life 2024, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, P.; Goljanek-Whysall, K.; Clegg, P.D.; Fang, Y.; Cremers, A.; Smagul, A.; Welting, T.J.M.; Peffers, M.J. MicroRNA Signatures in Cartilage Ageing and Osteoarthritis. Biomedicines 2023, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Chuang, S.M.; Hsu, C.J.; Tsai, C.H.; Wang, S.W.; Tang, C.H. CTGF Increases Vascular Endothelial Growth Factor-Dependent Angiogenesis in Human Synovial Fibroblasts by Increasing MiR-210 Expression. Cell Death Dis. 2014, 5, e1485. [Google Scholar] [CrossRef]
- Moran-Moguel, M.C.; Rio, S.P.D.; Mayorquin-Galvan, E.E.; Zavala-Cerna, M.G. Rheumatoid Arthritis and MiRNAs: A Critical Review through a Functional View. J. Immunol. Res. 2018, 2018, 2474529. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of Obesity Is Directly Associated with the Clinical and Functional Consequences of Knee Osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Krawczyk-Marć, I.; Wawrzyniak, A.; Orkisz, S. Chondrocytes Apoptosis in Osteoarthritis. Postep. Hig. Med. Dosw. 2018, 72, 875–883. [Google Scholar] [CrossRef]
- Sandell, L.J.; Aigner, T. Articular Cartilage and Changes in Arthritis An Introduction: Cell Biology of Osteoarthritis. Arthritis Res. 2001, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, C.C.; Wilson, W. Mechanics of Chondrocyte Hypertrophy. Biomech. Model. Mechanobiol. 2012, 11, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Dreier, R. Hypertrophic Differentiation of Chondrocytes in Osteoarthritis: The Developmental Aspect of Degenerative Joint Disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Murakami, T.; Hata, K.; Nishimura, R. Molecular Mechanisms Involved in the Progression and Protection of Osteoarthritis. Curr. Mol. Pharmacol. 2020, 14, 165–169. [Google Scholar] [CrossRef]
- Yamamoto, K.; Wilkinson, D.; Bou-Gharios, G. Targeting Dysregulation of Metalloproteinase Activity in Osteoarthritis. Calcif. Tissue Int. 2021, 109, 277–290. [Google Scholar] [CrossRef]
- Mehana, E.S.E.; Khafaga, A.F.; El-Blehi, S.S. The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Statnikov, A.; Samuels, J.; Li, Z.; Alekseyenko, A.V.; Greenberg, J.D.; Krasnokutsky, S.; Rybak, L.; Lu, Q.A.; Todd, J.; et al. Plasma Levels of Interleukin-1 Receptor Antagonist (IL1Ra) Predict Radiographic Progression of Symptomatic Knee Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; DiCarlo, E.; Potter, H.G.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local Cytokine Profiles in Knee Osteoarthritis: Elevated Synovial Fluid Interleukin-15 Differentiates Early from End-Stage Disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Steultjens, M. Classification of Patients with Knee Osteoarthritis in Clinical Phenotypes: Data from the Osteoarthritis Initiative. PLoS ONE 2018, 13, e019104. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, J.Y.; Baldwin, M.J.; Cribbs, A.P.; Philpott, M.; Carr, A.J.; Dakin, S.G.; Snelling, S.J.B. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front. Immunol. 2021, 12, 676173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, D.; Long, L.; Deng, X.; Tao, R.; Huang, G. Correlation between Plasma, Synovial Fluid and Articular Cartilage Interleukin-18 with Radiographic Severity in 33 Patients with Osteoarthritis of the Knee. Clin. Exp. Med. 2014, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Qi, C.; Liu, Y.; Gao, H.; Zhao, D.; Jiang, Y. Increased Frequency of Peripheral Blood Follicular Helper T Cells and Elevated Serum IL-21 Levels in Patients with Knee Osteoarthritis. Mol. Med. Rep. 2017, 15, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential Expression of Interleukin-17 and Interleukin-22 in Inflamed and Non-Inflamed Synovium from Osteoarthritis Patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef]
- Yi, C.; Yi, Y.; Wei, J.; Jin, Q.; Li, J.; Sacitharan, P.K. Targeting IL-22 and IL-22R Protects against Experimental Osteoarthritis. Cell. Mol. Immunol. 2021, 18, 1329–1331. [Google Scholar] [CrossRef]
- Xiao, S.Q.; Cheng, M.; Wang, L.; Cao, J.; Fang, L.; Zhou, X.P.; He, X.J.; Hu, Y.F. The Role of Apoptosis in the Pathogenesis of Osteoarthritis. Int. Orthop. 2023, 47, 1895–1919. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Chemokines and Inflammation in Osteoarthritis: Insights from Patients and Animal Models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Nishida, K.; Yorimitsu, M.; Komiyama, T.; Kadota, Y.; Tetsunaga, T.; Yoshida, A.; Kubota, S.; Takigawa, M.; Ozaki, T. Interleukin-4 Downregulates the Cyclic Tensile Stress-Induced Matrix Metalloproteinases-13 and Cathepsin b Expression by Rat Normal Chondrocytes. Acta Med. Okayama 2008, 62, 119–126. [Google Scholar] [PubMed]
- Van Meegeren, M.E.R.; Roosendaal, G.; Jansen, N.W.D.; Wenting, M.J.G.; Van Wesel, A.C.W.; Van Roon, J.A.G.; Lafeber, F.P.J.G. IL-4 Alone and in Combination with IL-10 Protects against Blood-Induced Cartilage Damage. Osteoarthr. Cartil. 2012, 20, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Zreiqat, H.; Sabat, R.; Kohl, B.; Halder, A.; Muller, R.; John, T. Interleukin-10 and Articular Cartilage: Experimental Therapeutical Approaches in Cartilage Disorders. Curr. Gene Ther. 2009, 9, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Lynn Rasmussen, G. Circulating IL-10 Is Compromised in Patients Predisposed to Developing and in Patients with Severe Knee Osteoarthritis. Sci. Rep. 2021, 11, 1812. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef]
- Verbruggen, G.; Wittoek, R.; Vander Cruyssen, B.; Elewaut, D. Tumour Necrosis Factor Blockade for the Treatment of Erosive Osteoarthritis of the Interphalangeal Finger Joints: A Double Blind, Randomised Trial on Structure Modification. Ann. Rheum. Dis. 2012, 71, 891–898. [Google Scholar] [CrossRef]
- Cohen, S.B.; Proudman, S.; Kivitz, A.J.; Burch, F.X.; Donohue, J.P.; Burstein, D.; Sun, Y.N.; Banfield, C.; Vincent, M.S.; Ni, L.; et al. A Randomized, Double-Blind Study of AMG 108 (a Fully Human Monoclonal Antibody to IL-1R1) in Patients with Osteoarthritis of the Knee. Arthritis Res. Ther. 2011, 13, R125. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic Agents in Osteoarthritis: Hopes and Disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular Injection of Anakinra in Osteoarthritis of the Knee: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Care Res. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in Articular Cartilage and Subchondral Bone Histomorphometry in Osteoarthritic Knee Joints in Humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cui, J.; Chu, L.; Zhang, W.; Xie, K.; Jiang, X.; He, Z.; Du, J.; Ai, S.; Sun, Q.; et al. Abnormal Subchondral Trabecular Bone Remodeling in Knee Osteoarthritis under the Influence of Knee Alignment. Osteoarthr. Cartil. 2022, 30, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in Subchondral Bone: Predominant Regulator for the Treatment of Osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kirkwood, C.L.; Sohn, J.; Lau, A.; Bayers-Thering, M.; Bali, S.K.; Rachala, S.; Marzo, J.M.; Anders, M.J.; Beier, F.; et al. Expansion of Myeloid-Derived Suppressor Cells Contributes to Metabolic Osteoarthritis through Subchondral Bone Remodeling. Arthritis Res. Ther. 2021, 23, 287. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral Bone Microenvironment in Osteoarthritis and Pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yu, Y.E.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 33, 316–327. [Google Scholar] [CrossRef]
- Yokota, S.; Ishizu, H.; Miyazaki, T.; Takahashi, D.; Iwasaki, N.; Shimizu, T. Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines 2024, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage Bone Crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Wu, X.; Liyanage, C.; Plan, M.; Stark, T.; McCubbin, T.; Barrero, R.A.; Batra, J.; Crawford, R.; Xiao, Y.; Prasadam, I. Dysregulated Energy Metabolism Impairs Chondrocyte Function in Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; Van Der Kraan, P.; Fearon, U. The Role of Metabolism in the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The Role of Metabolism in Chondrocyte Dysfunction and the Progression of Osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoblast Role in Osteoarthritis Pathogenesis. J. Cell. Physiol. 2017, 232, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Maglaviceanu, A.; Wu, B.; Kapoor, M. Fibroblast-like Synoviocytes: Role in Synovial Fibrosis Associated with Osteoarthritis. Wound Repair. Regen. 2021, 29, 642–649. [Google Scholar] [CrossRef]
- Li, T.; Peng, J.; Li, Q.; Shu, Y.; Zhu, P.; Hao, L. The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis. Biomolecules 2022, 12, 959. [Google Scholar] [CrossRef]
- Grässel, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef]
- Salman, L.A.; Ahmed, G.; Dakin, S.G.; Kendrick, B.; Price, A. Osteoarthritis: A Narrative Review of Molecular Approaches to Disease Management. Arthritis Res. Ther. 2023, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021, 13, 101–125. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The Function of MicroRNAs in Cartilage and Osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 40–47. [Google Scholar] [PubMed]
- Tardif, G.; Hum, D.; Pelletier, J.P.; Duval, N.; Martel-Pelletier, J. Regulation of the IGFBP-5 and MMP-13 Genes by the MicroRNAs MiR-140 and MiR-27a in Human Osteoarthritic Chondrocytes. BMC Musculoskelet. Disord. 2009, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Pais, H.; Schwach, F.; Lindow, M.; Kauppinen, S.; Moulton, V.; Dalmay, T. Experimental Identification of MicroRNA-140 Targets by Silencing and Overexpressing MiR-140. RNA 2008, 14, 2513–2520. [Google Scholar] [CrossRef]
- Pais, H.; Nicolas, F.E.; Soond, S.M.; Swingler, T.E.; Clark, I.M.; Chantry, A.; Moulton, V.; Dalmay, T. Analyzing MRNA Expression Identifies Smad3 as a MicroRNA-140 Target Regulated Only at Protein Level. RNA 2010, 16, 489–494. [Google Scholar] [CrossRef]
- Barter, M.J.; Tselepi, M.; Gõmez, R.; Woods, S.; Hui, W.; Smith, G.R.; Shanley, D.P.; Clark, I.M.; Young, D.A. Genome-Wide MicroRNA and Gene Analysis of Mesenchymal Stem Cell Chondrogenesis Identifies an Essential Role and Multiple Targets for MiR-140-5p. Stem Cells 2015, 33, 3266–3280. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Jakobsen, R.B.; Mikkelsen, T.S.; Brinchmann, J.E. MicroRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cells Dev. 2014, 23, 290–304. [Google Scholar] [CrossRef]
- Nakamura, Y.; Inloes, J.B.; Katagiri, T.; Kobayashi, T. Chondrocyte-Specific MicroRNA-140 Regulates Endochondral Bone Development and Targets Dnpep to Modulate Bone Morphogenetic Protein Signaling. Mol. Cell. Biol. 2011, 31, 3019–3028. [Google Scholar] [CrossRef]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S.; et al. MicroRNA-140 Plays Dual Roles in Both Cartilage Development and Homeostasis. Genes Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef]
- Papaioannou, G.; Inloes, J.B.; Nakamura, Y.; Paltrinieri, E.; Kobayashi, T. Let-7 and MiR-140 MicroRNAs Coordinately Regulate Skeletal Development. Proc. Natl. Acad. Sci. USA 2013, 110, E3291–E3300. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.R.; Kriegel, A.J. MiR-146a/b: A Family with Shared Seeds and Different Roots. Physiol. Genom. 2017, 49, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Nakasa, T.; Miyaki, S.; Ishikawa, M.; Deie, M.; Adachi, N.; Yasunaga, Y.; Asahara, H.; Ochi, M. Expression of MicroRNA-146a in Osteoarthritis Cartilage. Arthritis Rheum. 2009, 60, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gibson, G.; Kim, J.S.; Kroin, J.; Xu, S.; van Wijnen, A.J.; Im, H.J. MicroRNA-146a Is Linked to Pain-Related Pathophysiology of Osteoarthritis. Gene 2011, 480, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, X.; Wang, J.; Yang, C.; Wang, Y.; Liu, Y.; Zou, W.; Liu, T. Hypoxia-Induced MicroRNA-146a Represses Bcl-2 through Traf6/IRAK1 but Not Smad4 to Promote Chondrocyte Autophagy. Biol. Chem. 2017, 398, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Dai, L.; Yu, D.; Chen, Q.; Zhang, X.; Dai, K. MiR-146a, an IL-1β Responsive MiRNA, Induces Vascular Endothelial Growth Factor and Chondrocyte Apoptosis by Targeting Smad4. Arthritis Res. Ther. 2012, 14, R75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Chu, J.; Yang, C.; Xiao, H.; Zhao, C.; Sun, Z.; Gao, X.; Chen, G.; Han, Z.; et al. MicroRNA-146a Induced by Hypoxia Promotes Chondrocyte Autophagy through Bcl-2. Cell. Physiol. Biochem. 2015, 37, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, W.; Li, D.; Zheng, J. MiR-146a-5p Promotes Chondrocyte Apoptosis and Inhibits Autophagy of Osteoarthritis by Targeting NUMB. Cartilage 2021, 13, 1467S–1477S. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, C.; He, Y.; Lu, A.; Li, T.; Zhang, B.; Shen, J. Silencing MiR-146a-5p Protects against Injury-Induced Osteoarthritis in Mice. Biomolecules 2023, 13, 123. [Google Scholar] [CrossRef]
- Guan, Y.J.; Li, J.; Yang, X.; Du, S.; Ding, J.; Gao, Y.; Zhang, Y.; Yang, K.; Chen, Q. Evidence That MiR-146a Attenuates Aging- and Trauma-Induced Osteoarthritis by Inhibiting Notch1, IL-6, and IL-1 Mediated Catabolism. Aging Cell 2018, 17, e12752. [Google Scholar] [CrossRef]
- Liu, J.N.; Lu, S.; Fu, C.M. MiR-146a Expression Profiles in Osteoarthritis in Different Tissue Sources: A Meta-Analysis of Observational Studies. J. Orthop. Surg. Res. 2022, 17, 148. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Mourmoura, E.; Balis, C.; Tsezou, A. Impact of MiR-SNP Rs2910164 on MiR-146a Expression in Osteoarthritic Chondrocytes. Adv. Med. Sci. 2020, 65, 78–85. [Google Scholar] [CrossRef]
- Kopańska, M.; Szala, D.; Czech, J.; Gabło, N.; Gargasz, K.; Trzeciak, M.; Zawlik, I.; Snela, S. MiRNA Expression in the Cartilage of Patients with Osteoarthritis. J. Orthop. Surg. Res. 2017, 12, 51. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Song, J.; Kim, D.; Chun, C.H.; Jin, E.J. MicroRNA-9 Regulates Survival of Chondroblasts and Cartilage Integrity by Targeting Protogenin. Cell Commun. Signal. 2013, 11, 66. [Google Scholar] [CrossRef]
- Li, H.Z.; Xu, X.H.; Lin, N.; Wang, D.W.; Lin, Y.M.; Su, Z.Z.; Lu, H.D. Overexpression of MiR-10a-5p Facilitates the Progression of Osteoarthritis. Aging 2020, 12, 5948–5976. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Malizos, K.N.; Oikonomou, P.; Tsezou, A. Integrative MicroRNA and Proteomic Approaches Identify Novel Osteoarthritis Genes and Their Collaborative Metabolic and Inflammatory Networks. PLoS ONE 2008, 3, e3740. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Wang, Q.; Qin, H.; Cao, S.; Wei, Y.; Weng, J.; Yu, F.; Zeng, H. Osteoarthritis: Role of Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2023, 24, 13137. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM Metalloproteinases in Osteoarthritis—Looking beyond the ‘Usual Suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallaee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a-5p Promotes Joint Destruction during Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439. [Google Scholar] [CrossRef]
- Miyaki, S.; Nakasa, T.; Otsuki, S.; Grogan, S.P.; Higashiyama, R.; Inoue, A.; Kato, Y.; Sato, T.; Lotz, M.K.; Asahara, H. MicroRNA-140 Is Expressed in Differentiated Human Articular Chondrocytes and Modulates Interleukin-1 Responses. Arthritis Rheum. 2009, 60, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.Y.; Lee, W.F.; Hsu, C.J.; Lin, Y.Y.; Tsai, C.H.; Huang, C.C.; Wu, M.H.; Tang, C.H.; Liu, J.F. MiR-Let-7c-5p and MiR-149-5p Inhibit Proinflammatory Cytokine Production in Osteoarthritis and Rheumatoid Arthritis Synovial Fibroblasts. Aging 2021, 13, 17227–17236. [Google Scholar] [CrossRef] [PubMed]
- Santini, P.; Politi, L.; Vedova, P.D.; Scandurra, R.; Scotto D’Abusco, A. The Inflammatory Circuitry of MiR-149 as a Pathological Mechanism in Osteoarthritis. Rheumatol. Int. 2014, 34, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Tian, H. MicroRNA-149 Improves Osteoarthritis via Repression of VCAM-1 and Inactivation of PI3K/AKT Pathway. Exp. Gerontol. 2023, 174, 112103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, S.; Wu, Y.; Chen, L.; Pang, Q. MiR-149 Suppresses the Inflammatory Response of Chondrocytes in Osteoarthritis by down-Regulating the Activation of TAK1/NF-ΚB. Biomed. Pharmacother. 2018, 101, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.S.; Ko, J.Y.; Wu, R.W.; Sun, Y.C.; Chen, Y.S.; Wu, S.L.; Weng, L.H.; Jahr, H.; Wang, F.S. MicroRNA-128a Represses Chondrocyte Autophagy and Exacerbates Knee Osteoarthritis by Disrupting Atg12. Cell Death Dis. 2018, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-Y.; Wang, F.-S.; Lian, W.-S.; Fang, H.-C.; Kuo, S.-J. Cartilage-Specific Knockout of MiRNA-128a Expression Normalizes the Expression of Circadian Clock Genes (CCGs) and Mitigates the Severity of Osteoarthritis. Biomed. J. 2024, 47, 100629. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Tokuzawa, Y.; Ninomiya, Y.; Yagi, K.; Yatsuka-Kanesaki, Y.; Suda, T.; Fukuda, T.; Katagiri, T.; Kondoh, Y.; Amemiya, T.; et al. MiR-210 Promotes Osteoblastic Differentiation through Inhibition of AcvR1b. FEBS Lett. 2009, 583, 2263–2268. [Google Scholar] [CrossRef]
- Qi, J.; Qiao, Y.; Wang, P.; Li, S.; Zhao, W.; Gao, C. MicroRNA-210 Negatively Regulates LPS-Induced Production of Proinflammatory Cytokines by Targeting NF-ΚB1 in Murine Macrophages. FEBS Lett. 2012, 586, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, X.; Li, J.; Zhao, G. MiR-210 Inhibits NF-ΚB Signaling Pathway by Targeting DR6 in Osteoarthritis. Sci. Rep. 2015, 5, 12775. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Chen, H.; Pan, Y.; Lin, R.; Chen, S. MiR-335-5P Contributes to Human Osteoarthritis by Targeting HBP1. Exp. Ther. Med. 2020, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.O.; Zhang, L.; Tang, Z.Y.; Gong, Z.M. MiR-485-5p Promotes the Development of Osteoarthritis by Inhibiting Cartilage Differentiation in BMSCs. Eur. Rev. Med. Pharmacol. Sci. 2021, 22, 3294–3302. [Google Scholar] [CrossRef]
- Akhtar, N.; Rasheed, Z.; Ramamurthy, S.; Anbazhagan, A.N.; Voss, F.R.; Haqqi, T.M. MicroRNA-27b Regulates the Expression of Matrix Metalloproteinase 13 in Human Osteoarthritis Chondrocytes. Arthritis Rheum. 2010, 62, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Tavallaee, G.; Lively, S.; Rockel, J.S.; Ali, S.A.; Im, M.; Sarda, C.; Mitchell, G.M.; Rossomacha, E.; Nakamura, S.; Potla, P.; et al. Contribution of MicroRNA-27b-3p to Synovial Fibrotic Responses in Knee Osteoarthritis. Arthritis Rheumatol. 2022, 74, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Xu, J.; Chen, L.; Wu, H.; Feng, W.; Zheng, Y.; Li, P.; Zhang, H.; Zhang, L.; Chi, G.; et al. MicroRNA-27b Targets CBFB to Inhibit Differentiation of Human Bone Marrow Mesenchymal Stem Cells into Hypertrophic Chondrocytes. Stem Cell Res. Ther. 2020, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Li, Y.; Zeng, C.; Deng, Z.; Gao, S.; Xiao, W.; Luo, W.; Jiang, W.; Li, L.; Lei, G. MicroRNA-127-5p Regulates Osteopontin Expression and Osteopontin-Mediated Proliferation of Human Chondrocytes. Sci. Rep. 2016, 6, 25032. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Cheon, E.J.; Lee, M.H.; Kim, H.A. MicroRNA-127-5p Regulates Matrix Metalloproteinase 13 Expression and Interleukin-1β-Induced Catabolic Effects in Human Chondrocytes. Arthritis Rheum. 2013, 65, 3141–3152. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.X.; Xu, D.; Xue, X.; Xu, X. The Anti-Inflammatory Effect of MiR-140-3p in BMSCs-Exosomes on Osteoarthritis. Acta Chir. Orthop. Traumatol. Cech. 2023, 90, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.A.; de Souza, G.A.; Ødegaard, B.; Engebretsen, L.; Brinchmann, J.E. MicroRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1β-Induced Osteoarthritis. Mol. Ther. Nucleic Acids 2016, 5, e373. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Xiong, J.; Zhu, W.; Liu, Q.; Wang, D.; Liu, W.; Li, Z.; Wang, D. E2 Regulates MMP-13 via Targeting MiR-140 in IL-1β-Induced Extracellular Matrix Degradation in Human Chondrocytes. Arthritis Res. Ther. 2016, 18, 105. [Google Scholar] [CrossRef]
- Li, W.; Zhao, S.; Yang, H.; Zhang, C.; Kang, Q.; Deng, J.; Xu, Y.; Ding, Y.; Li, S. Potential Novel Prediction of TMJ-OA: MiR-140-5p Regulates Inflammation through Smad/TGF-β Signaling. Front. Pharmacol. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, S.; Li, Z.; Li, W.; Weng, X. MIR-140-5p Affects Chondrocyte Proliferation, Apoptosis, and Inflammation by Targeting HMGB1 in Osteoarthritis. Inflamm. Res. 2020, 69, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, J.; Pan, Y.; Shan, Y.; Jiang, L.; Qi, X.; Jia, L. MiR-140-5p/MiR-149 Affects Chondrocyte Proliferation, Apoptosis, and Autophagy by Targeting FUT1 in Osteoarthritis. Inflammation 2018, 41, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The Cartilage Specific MicroRNA-140 Targets Histone Deacetylase 4 in Mouse Cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Q.; Chen, Z.; Shen, B.; Yang, J.; Kang, P.; Zhou, Z.; Pei, F. MicroRNA-140 Suppresses Human Chondrocytes Hypertrophy by Targeting SMAD1 and Controlling the Bone Morphogenetic Protein Pathway in Osteoarthritis. Am. J. Med. Sci. 2018, 355, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-ΚB-Dependent Induction of MicroRNA MiR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Jones, S.W.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.L.; Brockbank, S.M.V.; Needham, M.R.C.; Read, S.J.; Newham, P. The Identification of Differentially Expressed MicroRNA in Osteoarthritic Tissue That Modulate the Production of TNF-α and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Skrzypa, M.; Szala, D.; Gablo, N.; Czech, J.; Pajak, J.; Kopanska, M.; Trzeciak, M.; Gargasz, K.; Snela, S.; Zawlik, I. MiRNA-146a-5p Is Upregulated in Serum and Cartilage Samples of Patients with Osteoarthritis. Pol. J. Surg. 2019, 91, 1–5. [Google Scholar] [CrossRef]
- Kapinas, K.; Delany, A.M. MicroRNA Biogenesis and Regulation of Bone Remodeling. Arthritis Res. Ther. 2011, 13, 220. [Google Scholar] [CrossRef]
- Liu, H.; Yan, L.; Li, X.; Li, D.; Wang, G.; Shen, N.N.; Li, J.J.; Wang, B. MicroRNA Expression in Osteoarthritis: A Meta-Analysis. Clin. Exp. Med. 2023, 23, 3737–3749. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Esbrit, P.; Alcaraz, M.J.; Largo, R. Oxidative Stress, Autophagy, Epigenetic Changes and Regulation by MiRNAs as Potential Therapeutic Targets in Osteoarthritis. Biochem. Pharmacol. 2016, 108, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, A.; Della Porta, G.; Peretti, G.M.; Maffulli, N. MicroRNA in Osteoarthritis: Physiopathology, Diagnosis and Therapeutic Challenge. Br. Med. Bull. 2019, 130, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.B.; Wen, K.; Wu, X.X.; Yao, Z.J. MiR-183 Regulates the Differentiation of Osteoblasts in the Development of Osteoporosis by Targeting Smad4. Acta Histochem. 2021, 123, 151786. [Google Scholar] [CrossRef] [PubMed]
- Briskin, D.; Wang, P.Y.; Bartel, D.P. The Biochemical Basis for the Cooperative Action of MicroRNAs. Proc. Natl. Acad. Sci. USA 2020, 117, 17764–17774. [Google Scholar] [CrossRef]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. Correlation between Gene Expression and Osteoarthritis Progression in Human. Int. J. Mol. Sci. 2016, 17, 1126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).