Abstract
Bacillus species isolated from Polish bee pollen (BP) and bee bread (BB) were characterized for in silico probiotic and safety attributes. A probiogenomics approach was used, and in-depth genomic analysis was performed using a wide array of bioinformatics tools to investigate the presence of virulence and antibiotic resistance properties, mobile genetic elements, and secondary metabolites. Functional annotation and Carbohydrate-Active enZYmes (CAZYme) profiling revealed the presence of genes and a repertoire of probiotics properties promoting enzymes. The isolates BB10.1, BP20.15 (isolated from bee bread), and PY2.3 (isolated from bee pollen) genome mining revealed the presence of several genes encoding acid, heat, cold, and other stress tolerance mechanisms, adhesion proteins required to survive and colonize harsh gastrointestinal environments, enzymes involved in the metabolism of dietary molecules, antioxidant activity, and genes associated with the synthesis of vitamins. In addition, genes responsible for the production of biogenic amines (BAs) and D-/L-lactate, hemolytic activity, and other toxic compounds were also analyzed. Pan-genome analyses were performed with 180 Bacillus subtilis and 204 Bacillus velezensis genomes to mine for any novel genes present in the genomes of our isolates. Moreover, all three isolates also consisted of gene clusters encoding secondary metabolites.
1. Introduction
Honeybees are social insects that live in large communities [1]. These honeybees in their hives produce and store several products that are beneficial for human health [2]. Indubitably, honey is the most prominent and widely valued product of the honeybee [2]. Ancient civilizations have relied on these products for centuries to treat various ailments, including wound healing, gut diseases, gastric ulcers, coughs, and sore throats in traditional medicine since ancient times [3]. A comprehensive review discussing the health effects of these bee products can be found elsewhere [2,4]. However, other products, including bee pollen (BP), propolis, bee bread (BB), royal jelly (RJ), and beeswax (BW), have also attracted the interest of the scientific community worldwide over the last few years [2]. Numerous studies have found beneficial effects of these natural products on human health, highlighting their potential use as active pharmaceutical ingredients [5,6,7,8,9,10,11,12].
Raw honey serves as a reservoir for several microbial species, including molds, yeasts, and spore-forming bacteria [13], with Bacillus and Lactobacillus species predominantly found [14,15,16]. On the other hand, bee pollen (BP) and, particularly, bee bread (BB) are less known and less popular among consumers. Recent research has uncovered the impressive health benefits of these bee products, which include antimicrobial, antioxidant, anti-radiation, anti-inflammatory, anti-tumor, hepatoprotective, and chemoprotective activities [17,18,19,20,21].
BB is fermented flower pollen collected by bees and stored in honeycombs, where it undergoes a transformation process facilitated by enzymes from bee glands and gut microbiota, particularly lactic acid bacteria (LAB), Bacillus sp., and yeasts [18,22,23]. Rich in carbohydrates, protein, lipids, and various micronutrients such as minerals, vitamins, phenolic compounds, and essential amino acids, BB serves as a food source, primarily for young bees and larvae within the hive [2]. The fermentation process acts as a natural preservative, preventing spoilage and the growth of pathogenic bacteria, ensuring the safety of both bees and humans who consume this product [17]. BP and BB exert several health benefits. For example, BP and BB ameliorate blood sugar, amend diabetic testicular–pituitary system dysfunction, prevent obesity, combat liver disorders, have cardio-protective effects, lower uric acid, etc. [24,25,26,27]. Several bacterial and yeast species have been isolated from BP and BB [28,29]. However, limited efforts have been made to explore the potential of isolates as probiotics.
Probiotics are live microorganisms that, when consumed in adequate amounts, provide health benefits to the host [30]. Probiotic microorganisms have been reported to improve gastrointestinal health, facilitate vitamin production, and make these essential compounds available to their host. Their beneficial impact extends to several health disorders, encompassing improvements in blood pressure, the sustained maintenance of blood cholesterol profiles, and positive associations with conditions like allergies, inflammatory bowel disease, bacterial vaginosis, dental caries, etc. [31]. Honey serves as a rich source of microorganisms, including fungi, lactic acid bacteria, and Bacillus, some of which exhibit probiotic properties [32,33,34,35]. Recent studies have identified bacteria from bee bread as potential probiotics [28,36,37,38]. Furthermore, certain strains of Bacillus and Lactobacillus isolated from fermented foods have shown promising probiotic potential [39]. Bacillus spp. are Gram-positive and spore-forming bacteria commonly found in soil and plants, have the advantage of being highly stable in acidic environments, and can thrive in various food matrices [40,41,42,43,44]. In our earlier study, we assessed ten bacterial isolates obtained from bee bread (BB) and bee pollen (BP) to determine their potential as probiotics. Following extensive wet lab experiments, only three isolates met the established probiotic criteria. Subsequently, we conducted whole-genome sequencing on these three strains, identifying them taxonomically as members of Bacillus [28]. In this study, our primary focus was to conduct a comprehensive probiogenomic analysis on three distinct Bacillus strains—BB10.1 and BP20.15 derived from bee bread and PY2.3 from bee pollen. The main objective was to assess the safety profile of these strains by investigating the presence of virulence genes, toxins, and genes responsible for conferring probiotic characteristics.
2. Results
2.1. Genome Assessment and Synteny
BUSCO analysis revealed a 99.6%, 99.5%, and 100% completeness of the genome for isolates BB10.1, BP20.15, and PY2.3, respectively. The percentages represent the completeness of our genome assemblies, indicating the proportion of conserved genes in a benchmark dataset. Higher percentages indicate better genome completeness. A total of 99.6%, 99.3%, and 99.7% of genes were recognized as single-copy BUSCO for BB10.1, BP20.15, and PY2.3, respectively. The high percentages indicate that a significant portion of genes are present as single-copy orthologs. Fragmented BUSCOs represent genes that are partially present or fragmented in our assemblies. A total of 0.2% and 0.4% of genes were present as fragmented copies for BB10.1 and BP20.15, respectively, while 0% of genes were missing for all three strains. A missing BUSCO indicates that a gene is absent from the assembly. The fact that there are no missing BUSCOs in our results suggests that our assemblies contain a comprehensive set of genes from the benchmark dataset (Table 1), which also provides confidence in our data for exploring the probiotic potential of these Bacillus strains. Furthermore, D-GENIES analyzed the genome synteny by pairwise genome alignment of the isolate with their closely related strains: BB10.1 with B. subtilis strain 168, BP20.15 with B. subtilis 75, and PY2.3 with B. velezensis S4. The dot plots revealed a few genomic region inversions between reference B. subtilis strain 168 and BB10.1, while several were observed between B. subtilis 75 and BP20.15 (Figure 1a,b). For PY2.3 and B. velezensis S4, minor variations were observed (Figure 1c).

Table 1.
BUSCO assembly analysis for strains BB10.1, BP20.15, and PY2.3 assemblies.

Figure 1.
Synteny analysis of (a) BB10.1, (b) BP20.15, and (c) PY2.3. Pairwise genome alignment for the selected strains is shown in the dot plots generated by D-GENIES tool.
2.2. Pan-Genome Analysis
B. subtilis pan-genomic analysis analyzed 752,925 genes, 10,810 total orthologous groups, and 2664 total unique genes, while the count for average core genes was 3499, the average gene count was 4207, and the average unique gene count was 15 (Supplementary Figure S1). Similarly, pan-genomic analysis of B. velezensis analyzed 790,409 genes, 11,759 total orthologous groups, and 3831 total unique genes, while the count for average core genes was 3346, the average gene count was 3821.5, and the average unique gene count was 10 (Supplementary Figure S2). Nevertheless, we observed that the number of new genes is progressively decreasing, proportionally to the number of genomes included in the B. subtilis (Figure 2a) and B. velezensis analyses (Figure 2b). The heatmap (Figure 2c) represents the absence and presence of genes in the BB10.1 and BP20.15 pan-genomes, whereas the gene presence/absence heatmap for PY2.3 is presented in Supplementary Figure S3. The phylogenetic inferences based on the whole genome variation of isolates BB10.1, BP20.15, and PY2.3 are presented in Supplementary Figure S4a and S4b, respectively.
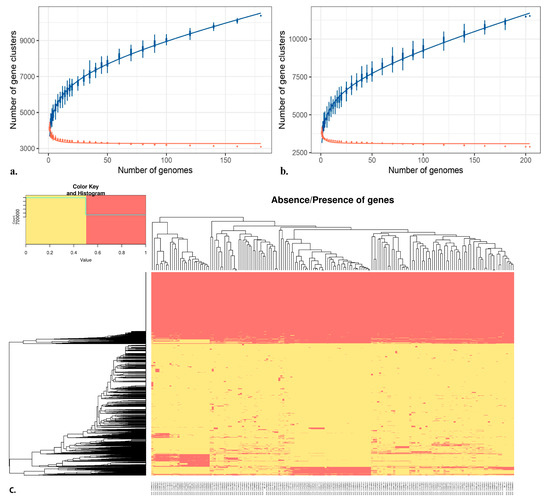
Figure 2.
Representation of (a) BB10.1 and BP20.15 pan-genome and (b) PY2.3 gene content (extrapolated median-based line) according to how the pan-genome varies as genomes are added in random order to the analysis. The blue line represents unique genes; the red line represents new genes. The heatmap (c) represents the presence and absence of particular genes in BB10.1 and BP20.15 pan-genome. The X-axis denotes the particular species name. The yellow color indicates gene presence; the red color indicates gene absence. The Y-axis represents individual gene clustering, while the topmost axis represents genomes clustering (high-quality figure in Supplementary File).
Functional Annotation and Carbohydrate-Active Enzyme (CAZyme) Profiling
The KEGG functional annotation by BlastKOALA annotated and categorized the genes into 22 different functional categories (Supplementary Figure S5). For isolate BB10.1, 2455 entries (60.0%) were annotated out of 4092 and mostly related to protein families: genetic information processing (13.07%), carbohydrate metabolism (10.79%), protein families: signaling and cellular processes (10.54%), environmental information processing (9.04%), genetic information processing (7.37%), amino acid metabolism (5.45%), nucleotide metabolism (3.09%), metabolism of cofactors and vitamins (4.76%), and others. Similarly, 2437 entries (61.2%) out of 3980 were annotated for isolate BP20.15, and the most related were protein families: genetic information processing (13.00%), carbohydrate metabolism (10.91%), protein families: signaling and cellular processes (10.79%), environmental information processing (9.02%), genetic information processing (7.38%), amino acid metabolism (5.37%), nucleotide metabolism (3.03%), metabolism of cofactors and vitamins (4.84%), and others. For isolate PY2.3, 2335 entries were annotated out of 3729 (62.6%), mostly related to protein families: genetic information processing (13.19%), carbohydrate metabolism (10.32%), protein families: signaling and cellular processes (9.89%), environmental information processing (8.39%), genetic information processing (7.45%), amino acid metabolism (5.31%), nucleotide metabolism (3.08%), metabolism of cofactors and vitamins (5.01%), and others.
Clusters of orthologous groups (COGs) functional group analysis was performed using EggNOG Mapper v2. For the isolate BB10.1, 3843 genes out of 4092 (93%) were assigned to 20 clusters. The function unknown (S: 1009) category had the highest numbers, implicating the uniqueness and yet-to-explore potential of this strain. The remaining proteins were categorized under functional groups such as transcription (K: 307); amino acid transport and metabolism (E: 262); carbohydrate transport and metabolism (G: 204); inorganic ion transport and metabolism (P: 197); energy production and conversion (C: 175); translation, ribosomal structure, and biogenesis (J: 173); cell wall/membrane/envelope biogenesis (M: 196); replication, recombination, and repair (L: 146); coenzyme transport and metabolism (H: 107); nucleotide transport and metabolism (F: 93); signal transduction mechanisms (T: 113); post-translational modification, protein turnover, chaperones (O: 82); lipid transport and metabolism (I: 86); defense mechanisms (V: 59); chromosome partitioning (D: 44); cell cycle control, cell division, secondary metabolites biosynthesis, transport, and catabolism (Q: 44); cell motility (N: 32); intracellular trafficking, secretion, and vesicular transport (U: 41); and RNA processing and modification (A: 2) (Figure 3a). A total of 3747 entries out of 3980 (94%) for strain BP20.15 were classified into 20 different COG categories (Figure 3b) and, for PY2.3, 3559 out of 3729 entries (95%) were assigned to 20 different COG categories (Figure 3c).
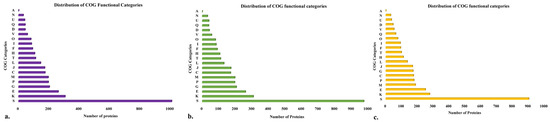
Figure 3.
Distribution of cluster of orthologous group (COG) functional categories to the proteins of isolate (a) BB10.1; (b) BP20.15; (c) PY2.3. The X-axis denotes the number of proteins and Y-axis denotes COG categories. Each alphabet represents a unique COG category. A—RNA processing and modification, C—energy production and conversion, D—cell cycle control, cell division, and chromosome partitioning; E—amino acid transport and metabolism; F—nucleotide transport and metabolism; G—carbohydrate transport and metabolism; H—coenzyme transport and metabolism; I—lipid transport and metabolism; J—translation, ribosomal structure, and biogenesis; K—transcription; L—replication, recombination, and repair; M—cell wall/membrane/envelope biogenesis; N—cell motility; O—post-translational modification, protein turnover, chaperones; P—inorganic ion transport and metabolism; Q—secondary metabolites biosynthesis, transport, and catabolism; S—function unknown; T—signal transduction mechanisms; U—intracellular trafficking, secretion, and vesicular transport; and V—defense mechanisms.
PGAP [45] analysis resulted in 4092, 3980, and 3729 annotated protein sequences for BB10.1, BP20.15, and PY2.3, respectively. The meta server, dbCAN2, classified and annotated enzymes via all three tools. HMMER: dbCAN, DIAMOND: CAZy, and HMMER: dbCAN-sub. Enzymes classified by at least two tools were considered. From the isolate BB10.1 genome, 147 were identified by dbCAN as belonging to carbohydrate-active enzyme families. These included 35 conserved carbohydrate-active enzyme (CAZy) domains whose genes had signal peptides. These CAZy domains represented CAZy families, including 23 from the carbohydrate-binding module (CBM) family, 6 from the carbohydrate esterase (CE) family, 53 from the glycoside hydrolase (GH) family, 38 from the glycosyl transferase (GT) family, and 7 from the polysaccharide lyase (PL) family (Figure 4a). The isolate BP20.15 genome encoded 135 CAZy families, with 35 having conserved signal peptides. These CAZy domains include 23 from the CBM family, 11 from the CE family, 53 from the GH family, 38 from the GT family, 7 from the PL family, and 4 from the auxiliary activities (AAs) family (Figure 4b). On the other hand, the PY2.3 genome consists of 109 CAZy domains, and 31 of those 109 CAZy domains had conserved signal peptides. There was a total of 15 entries for the CBM family, 10 from the CE family, 61 from the GH family, 33 from the GT family, 3 from the PL family, and 1 from the AA family (Figure 4c). The signal-peptide-containing glycoside hydrolase family was the largest group of carbohydrate-active enzymes.
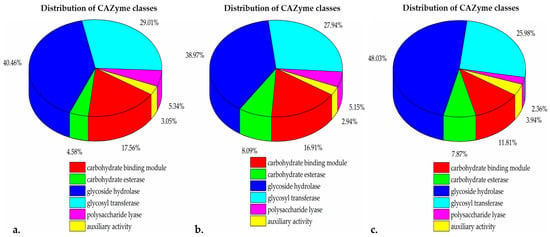
Figure 4.
The percent distribution of CAZymes classes. (a). BB10.1, (b). BP20.15, (c). PY2.3. The color scheme at the bottom represents different CAZyme classes: red, CBM—carbohydrate-binding module; green, CE carbohydrate esterases; blue, GH—glycoside hydrolases; cyan, GT—glycosyl transferases; pink, PL—polysaccharide lyases; and yellow, AA—auxiliary activity.
2.3. Probiotic- and Stress-Related Genes
Probiotic strains usually consist of an arsenal of genes encoding stress proteins in response to temperature, pH, bile, osmotic pressure, and oxidative stress that regulate their adaptability to the gastrointestinal (GI) tract (Journey of the Probiotic Bacteria: Survival of the Fittest). A literature-mining approach was used, and Bacillus and other related probiotic species’ published data were analyzed. The annotated genomes were analyzed to search for various probiotic-properties-related genes (stress resistance, bile salt hydrolase activity, adhesion ability, immunomodulatory activities) to determine the probiotic functions at genomic levels. Several genes encoding for stress-related proteins were identified in the genomes of isolate BB10.1, BP20.15, and PY2.3 as presented in (Table 2) and BLASTed in Uniprot.

Table 2.
Stress response genes mined from BB10.1, BP20.15, and PY2.3 annotated genomes.
The genomes of all the tree isolates were found to encode genes related to heat shock, including heat-shock-related regulators (hrcA, ctsR), molecular chaperones (dnaK, dnaJ, grpE, groEL, groES), and protease-encoding genes (hslO, lon1, lon2, clpC, clpE, clpP, clpQ, clpX, and clpY) [46,47,48,49,50,51]. These genes are considered to play a major role in intracellular protein aggregation and membrane stabilization to resist higher temperatures. Genes (cspB, cspC, cspD) coding for cold shock proteins [52,53] related to survival under low temperatures were also observed in the genomes of the isolates. The CSP family genes are synthesized by several bacillus strains to overcome the deleterious effect under cold stress and hence may provide resistance to the isolates. In addition, genes conferring resistance to low pH conditions were also observed. Out of the 14 genes observed, 9 genes encode a cluster of ATP synthase subunits A–I, which serve as a key regulator of cytoplasmic pH to favor acid tolerance [54,55]. Moreover, Nhac and nhaK genes, coding for sodium–proton (Na+/H+) antiporters and sodium, potassium, lithium, and rubidium/H(+) antiporters, maintaining pH and Na+ homeostasis, were present [56,57,58]. The alkaline shock response genes sigW and rsiW were also identified [59,60,61]. In addition, the Mrp (multiple resistance and pH) operon coding for seven hydrophobic gene products (mrpA-mrpG) was also observed [62]. Regarding bile salt resistance, mpr genes (A–G) [62], ppaC, which codes for inorganic pyrophosphatase (to maintain surface tension and keep membrane integrity) [63,64], oppA (oligopeptide-binding protein OppA) [65,66], and ppaX (pyrophosphatase) were observed [67]. Further, genes known to shield against osmotic stress environments (opuD, opuE, opuAA, opuAB, opuAC, opuBA, opuBB, opuBC, opuBD, opuCA, opuCB, opuCC, opuCD) were also seen in isolates [68,69,70]. The presence of these genes confirms the ability of the strain to adapt to an acidic environment.
The adherence ability of probiotic strains to the host epithelium is due to their cell surface proteins. The isolate genomes consisted of 11 genes that may encode adhesion-related proteins, including maltose phosphorylase (mdxK), sporulation and biofilm formation (spo0A) [71,72], lipoprotein signal peptidase I (lspA), elongation factor Tu (tuf) [73,74,75], sortase A (srtA) [76,77], and putative glycosyltransferase (EpsH) [78,79,80,81]. Another gene, xylA, linked with gut persistence was also found [80,82].
B. subtilis suffers from unavoidable oxidative stress during the exponential phase. Isolates harbor genes related to oxidative stress and, out of them, thioredoxin (tpx, trxA, trxB) [83,84,85], BsaA (glutathione peroxidase homolog) [86,87,88], and ahpF (NADH dehydrogenase) are antioxidant systems involved in ROS scavenging [89,90] (Table 2). The thioredoxin system can remove both ROS and RNS at a higher reaction rate by donating electrons to thiol-dependent peroxidases. The glutathione system detoxifies hydrogen peroxide and lipid peroxyl radicals by regulating the protein dithiol/disulfide balance [80]. In addition, genes for catalase (KatA and KatE) and glutaredoxin (ytnI) were also observed [91,92,93]. Other genes like sodA (superoxide dismutase [Mn]) [94,95,96], sodF (probable superoxide dismutase [Fe]) [97], yojM (superoxide dismutase-like protein) [98], and also the genes [manganese transport systems (mntA-C) and protein (mntH)] coding for the Mn2+ accumulation system were present [80]. Interestingly, methionine sulfoxide reductase genes (msrA and msrB) that can repair oxidized methionine residues by ROS in proteins were detected [99,100]. Moreover, the genes (dlt A–D) coding for immunomodulatory activities and some additional general stress genes were also identified [101]. These findings suggest that strains may withstand multiple stress conditions and are consistent with the adaptability characteristics of the gastrointestinal tract. Further, the adhesion-related protein contributes to the effective colonization of the intestinal environment and can eliminate unwanted gut microorganisms.
2.4. Genome Plasticity Analysis and Safety Assessment
Insertion Sequences
In the isolate BB10.1 genome, a total of eight insertion sequence (IS) elements belonging to two families (IS1182 and IS3) were predicted in the genome with a threshold E-value of 0.00001 (Supplementary Table S1). Among the predicted eight IS elements, six copies belonged to ISBpu1, one to ISBsu1, and one to ISBpe1. The isolate BP20.15 genome contained seven IS belonging to two families (one to IS3, and six to IS1182) (Supplementary Table S1). In the genome of isolate PY2.3, nine ISs were observed, all belonging to the IS3 family. Of the above-mentioned IS elements, only one of the insertion sequences (ISBsu1; IS3) from strain PY2.3 exhibited a score bit >1000 and an E-value of zero (Supplementary Table S1). However, none of the IS elements so far have been associated with safety risks. Moreover, in-depth analyses of genomes using Island Viewer 4 did not predict any virulence factors or pathogenicity-associated genes in any of the strains. The predicted genes for strains BB10.1 and BP20.15 were mapped to different genomic islands (Supplementary File S2). The majority of genes annotated from isolates genomes were hypothetical proteins, antioxidant genes, bacteriocins, insertion sequences, stress-related proteins, sporulation proteins, enzymes related to carbohydrate metabolism, transporters, etc., assisting the organism’s adaptability in the environmental niche [102]. No genomic islands were observed in the isolate PY2.3 genome.
The CRISPR-CasFinder tool identified three CRISPR arrays in the isolate BB10.1 genome; however, all the predicted arrays matched the consensus sequence with evidence level 1 (potentially invalid), and evidence level ≥3 is considered significant (Table 3). Five CRISPR arrays were identified in the genome of isolate BP20.15, but all the predicted arrays were disregarded as they were potentially invalid (evidence level 1). Moreover, no arrays with potential evidence were observed in the PY2.3 genome.

Table 3.
Crispr array and Cas type detection within BB10.1, BP20.15, and PY2.3 genomes using CRISPRFinder tool.
Full-length genome assemblies for each sample allowed for the prediction of the closest putative prophage (Table 4) using the PHASTER server. A total of six prophages were detected in the BB10.1 genome, which included one intact (region 3), one questionable (region 1), and four incomplete (regions 2, 4, 5, and 6) prophages (Table 4). Isolate BP20.15 yielded four incomplete prophage candidates. Whereas, in the PY2.3 genome, three prophage candidates were predicted: one intact (region 1), one questionable (region 2), and one incomplete (region 3). The intact phage (33.7 kb) of isolate BB10.1 (region 3; 662,637–696,369 bp) showed a maximum (46) protein matching and resembled PHAGE_Brevib_Osiris_NC_028969 (8). The other intact phage (31.7 kb) in isolate PY2.3 (region 1; 262,623–294,418 bp) showed a maximum (42) protein matching and resembled PHAGE_Brevib_Jimmer1_NC_029104 (8).

Table 4.
Prophage regions of isolates BB10.1, BP20.15, and PY2.3 identified by the PHASTER tool.
2.5. Safety-Associated Genes
The TABD v2 Wu-BLAST results were analyzed closely and most of the proteins in the genome of the isolates recognized by the database as toxins were predominantly the transporter proteins, other housekeeping proteins, and several hypothetical proteins. To search for the AMR genes, the Resfinder 4.1 database was used with default parameters (90% threshold and 60% minimum length). No AMR genes were detected in the genome of PY2.3; however, three AMR genes, aadK (streptomycin), mph(K) (spiramycin, telithromycin), and tet(L) (doxycycline, tetracycline), were detected in the genomes of isolates BB10.1 and BP20.15 (Table 5).

Table 5.
AMR (antimicrobial resistance) genes identified and their location in the isolates’ genomes.
The KEGG database search also yielded AMR-related genes in all three isolates’ genomes (Supplementary Table S2). In the genome of all the isolates (BB10.1, BP20.15, and PY2.3), the identified genes were related to vancomycin (vanY, vanX) (map01502), beta-lactam (penP) resistance (map01501), and cationic antimicrobial peptides (CAMPs) (map01503). Likewise, the CARD database search under default parameters (only perfect and strict hits) resulted in 18 hits (10 strict and 8 perfect hits) from the isolate BB10.1 genome. Isolate BP20.15 yielded 15 hits (14 strict hits and 1 perfect hit), and PY2.3 returned with 9 strict hits (Supplementary Table S3). The perfect hits in the BB10.1 genome had an identity of 100%, and the strict hits had an identity of 32–75% and included resistance genes to antibiotic target alteration (6), antibiotic target protection (1), antibiotic efflux (7), reduced permeability to antibiotics (1), and antibiotic inactivation (3). In isolate BB20.15, 14 strict hits (identity range 32–99%) and 1 perfect hit (100% identity) were found. The resistance genes included those for antibiotic target alteration (4), antibiotic target protection (1), antibiotic efflux (6), reduced permeability to antibiotics (1), and antibiotic inactivation (3). Whereas, in the genome of PY2.3, nine strict hits (identity 33–99%) were observed. The resistance genes included genes for antibiotic target alteration (4), antibiotic efflux (4), and antibiotic inactivation (1). Since the AMR genes of pathogenic bacteria are the major focus of both of the aforementioned databases, the AMR genes of non-pathogenic bacteria, such as B. subtilis and others, are often not incorporated in the databases.
VirulenceFinder detected no virulence genes under the BLASTn search. Sixty-nine (69) virulence genes were predicted by VFDB in the isolate BB10.1 genome, mainly associated with adherence, enzymes, immune evasion, iron acquisition, regulation, secretion system, and toxins (Supplementary Table S4). A total of 86 virulence genes were predicted by VFDB in the isolate BP20.15 genome, mainly associated with adherence, enzymes, immune evasion, iron acquisition, regulation, secretion system, toxin, acid resistance, anti-phagocytosis, copper uptake, iron uptake, peptidoglycan modification, stress adaptation, and surface protein anchoring (Supplementary Table S4). Seventy (70) virulence genes were predicted by VFDB in the isolate PY2.3 genome, mainly associated with adherence, enzymes, immune evasion, iron acquisition, regulation, secretion system, toxin, and cell surface components (Supplementary Table S4). The genes characterized as “virulence factors” in pathogens usually help them survive in the host environment under physiological stresses and can supposedly favor probiotic strain survival in the gut. OriTfinder did not detect any OriT in BB10.1 and BP20.15; however, in the genome of PY2.3, a 24 bp (AACCCCCCCACGCTAACAGGGGGG) DNA relaxase was observed having an 84% BlastP identity to the ICEBs1 mobile element of B. subtilis.
2.5.1. Determination of Toxins, Biogenic Amines, and Undesirable Genes
The genes coding for undesirable properties were identified using BlastKoala and KAAS servers. Hemolysin (hlyIII; K11068, tlyc; K03699) was identified. Only L-lactate dehydrogenase (K00016) was present in all three strains and no gene for D-lactate dehydrogenase was found.
Biogenic amine (BA) production is another essential trait related to probiotic safety issues. In all three genomes of isolates BB10.1, BP20.15, and PY2.3, genes related to spermidine synthase (speE, SPE3, SRM) [EC:2.5.1.16] (K00797; polyamine biosynthesis, arginine -> agmatine -> putrescine -> spermidine), arginine decarboxylase (speA) [EC:4.1.1.19] (K01585; polyamine biosynthesis, arginine -> agmatine -> putrescine -> spermidine), agmatinase (speB) [EC:3.5.3.11] (K01480; polyamine biosynthesis, agmatine -> putrescine), and arginase (E3.5.3.1, rocF, arg) [EC:3.5.3.1] (K01476; polyamine biosynthesis, arginine -> ornithine -> putrescine) were present.
Furthermore, no plasmids were detected in the genomes using the PlasmidFinder web tool [103], and the probability of being a human pathogen assessed using Pathogen Finder [104] was near zero (isolate BB10.1; 0.24; isolate BP20.15; 0.129; and isolate PY2.3; 0.118), indicating the safety of these strains. iProbiotics analysis revealed the portions of the genome of our isolates encoding for probiotics-properties-associated genes (Figure 5).
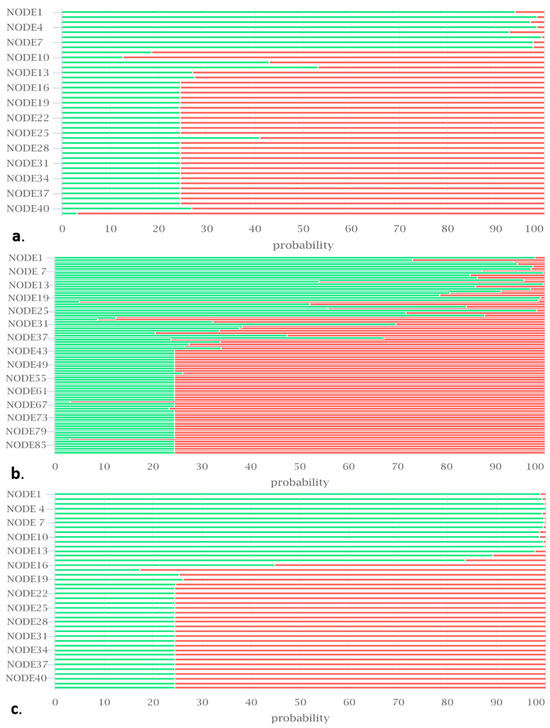
Figure 5.
The predicted probability of probiotic genes in the genomes of isolates (a) BB10.1, (b) BP20.15, and (c) PY2.3. The green color indicates probiotic genes, and the red color indicates non-probiotic genes. The x-axis values (0–100) represent the probability of a gene being a probiotic gene. The y-axis represents the contig number in the genome.
2.5.2. Antimicrobial Peptides and Secondary Metabolites Analysis
The Antismash tool detected 18 biosynthetic gene clusters (BGCs) in the BB10.1 genome, of which 13 encode antimicrobial peptides (Table 6). One cluster was found for bacillaene, subtilosin A, bacilysin, sporulation killing factor, and thailanstatin A. Two clusters for fengycin, plipastatin, and bacillibactin, and three were detected for surfactin. Additionally, one cluster encoding 1-carbapen-2-em-3-carboxylic acid and pulcherriminic acid was also identified. In the BP20.15 genome, 15 BCGs were detected (Supplementary Table S5a). One cluster was observed for fengycin, bacillaene, subtilosin A, bacilysin, sporulation killing factor, and bacillibactin. Two clusters were observed for surfactin and plipastatin. Similarly, one cluster encoding 1-carbapen-2-em-3-carboxylic acid and pulcherriminic acid was also identified. The PY2.3 genome revealed 13 BCGs (Supplementary Table S5b). One gene cluster was observed for macrolactin H, difficidin, fengycin, bacillaene, bacilysin, surfactin, bacillibactin, and plipastatin. Two clusters were observed for fengycin and terpene, and one for T3PKS.

Table 6.
List of identified secondary metabolite clusters of isolate BB10.1 using strictness ‘strict’.
3. Discussion
In this study, the probiotic potential of Bacillus strains isolated from two essential natural bee products, bee bread (BB) and bee pollen (BP), was assessed [28]. BP and BB are increasingly gaining attention in the food industry as they can consist of rich macro- and micronutrients content with therapeutic properties, satisfying consumers’ trends for natural and functional foods.
The Generally Recognized As Safe (GRAS) status [105] of several Bacillus strains has increased their importance and role as probiotic starter cultures for developing functional foods with probiotic benefits for consumers [106,107,108,109]. However, before probiotic strains can exert their beneficial effects, they need to meet certain requirements. Based on the guidelines provided by the World Health Organization (WHO), some essential requirements for assessing a strain as an effective probiotic microorganism include their ability to tolerate gastrointestinal conditions, survive gastric acid and bile concentrations, adhere to intestinal epithelial cells, demonstrate antimicrobial actions, and lack antibiotic-resistant genes (FAO/WHO, 2006) [30]. Moreover, Chokesajjawatee et al., 2020 recommended some prerequisites for probiotic strains [110].
In pangenome analysis, the entire gene set of the selected Bacillus strains was compared, enabling the assessment of the genomic diversity of the entire repertoire of genes and the identification of core genomic elements [111]. The pan-genome is categorized into three categories: the core genome, the dispensable genome, and strain-specific unique genes [112]. To understand the relationships between pan-genome size, the core gene number, and the strain numbers, the fitted curves of the pan-genome profile were plotted for isolates. To determine the core genome, the number of conserved genes observed upon the sequential addition of new genomes was inferred by fitting a decaying function, implying that the average number of core genes converged to a relatively constant number. The core gene number in each genome varied slightly because of the involvement of duplicated genes and paralogs in the shared clusters. As illustrated in (Figure 2a,b), the blue curve increased with the addition of a new strain and was far from saturation, suggesting that the genetic repertoire of the species was nevertheless growing despite suitable adaptation to their diverse ecological niches. Thus, the pangenome of these strains was found to be open. This is in accordance with previous Bacillus pan-genome studies showing that environmental samples usually have open pan-genomes [113,114,115,116]. In other words, the isolated strains are expected to gain genes and evolve in the future. Thus, the availability of a large genetic repertoire might be an advantage for these strains to survive when facing environmental challenges. However, no novel genes were observed in any of our isolates that could confer any new functions, particularly in terms of safety and pathogenicity.
CAZyme profiling of the isolates revealed glycoside hydrolases (GHs) to be the most abundant CAZyme group. GH enzymes have significant potential to hydrolyze complex carbohydrates and are considered key enzymes in carbohydrate metabolism. They are commonly found in nature and can degrade abundant biomasses such as starch, cellulose, and hemicellulose [117]. An in-depth analysis for differentiating GH enzyme families in the isolates revealed the presence of several GH families, including GH1, GH101, GH105, GH11, GH126, GH13, GH13_11, GH13_23, GH13_29, GH13_3, GH13_30, GH13_31, GH16_21, GH171, GH18, GH23, GH24, GH26, GH28, GH3, GH30_3, GH30_8, GH32, GH36, GH38, GH4, GH42, GH43_11, GH43_4, GH43_5, GH46, GH5_11, GH51, GH53, GH65, GH68, GH73, and GH84. Some of these families are reported to be key enzymes in oligosaccharide degradation [118]. Oligosaccharides, as complex carbohydrates, are a major source of prebiotics, particularly galactans, and fructans, which have been linked to human gut health [119,120]. Galactans include galacto-oligosaccharides (GOSs), while fructans comprise fructo-oligosaccharides (FOSs) and inulin. Inulin, a well-known prebiotic, is a linear fructosyl polymer linked by β-(2,1) bonds (n = 3–65), attached to a terminal glucosyl residue by an α-(1,2) bond [121]. The hydrolysis of inulin is typically carried out by inulinase, an enzyme belonging to the GH32 family [122]. Interestingly, we detected genes related to GH32, which could be associated with inulinase production and its strong ability to consume inulin [123]. Moreover, it also suggests that the presence of these enzymes can positively affect the availability of bee pollen and bee bread compounds for young bees and humans.
Glycosyltransferases (GTs) were the second most abundant group, and the cellulose synthase GT2, an important enzyme for cellulose biosynthesis, was identified in the genomes of all three isolates. It stores cellulose on the cell wall surface as an extracellular matrix for cell adhesion and biofilm formation, providing protection from the surrounding environment [124]. Glycosyltransferases catalyze the transfer of sugars from activated donor molecules to specific acceptors, playing an essential role in the formation of surface structures recognized by host immune systems [125]. Analysis by the dbCAN server also revealed the presence of carbohydrate binding modules (CBMs) in all the isolates. CBM enzymes are mostly associated with GHs, binding to carbohydrate ligands and enhancing the catalytic efficiency of carbohydrate-active enzymes [126]. In BB10.1, a total of four auxiliary activity (AA) enzymes were observed, consisting of one from AA4, two from AA6, and one from AA7. Similarly, in BP20.15, four AA enzymes were identified, comprising one from family AA4, two from AA6, and one from AA7. Additionally, in PY2.3, a single auxiliary activity (AA) enzyme, AA10, was observed. Therefore, the high number of GH and GT genes, along with other CAZyme genes in these strains, suggests their probiotic potential, particularly for immune stimulation, pathogen defense, and the production of essential fermentation end-products in fermented foods.
KEGG analysis revealed that most of the genes in BB10.1, BP20.15, and PY2.3 are involved in genetic information processing, carbohydrate metabolism, protein signaling and cellular processes, environmental information processing, amino acid metabolism, nucleotide metabolism, and, notably, the metabolism of cofactors and vitamins. BlastKOALA revealed genes related to riboflavin metabolism, vitamin B6 metabolism, biotin metabolism, and folate biosynthesis. The gut microbiota plays a crucial role in aiding the host by contributing to nutrient digestion and energy recovery [100]. The capacity to produce folate has been investigated in various probiotic strains due to its potentially relevant applications [127,128]. Previous studies have shown that the B. subtilis genome contains all the pathways and components necessary for folate biosynthesis and has been engineered for folate production [128,129,130].
Probiotic strains encounter various harsh environmental conditions during transport in the gastrointestinal tract, including the acidic conditions of the stomach, the bile juice environment in the small intestine, oxidative stress, and osmotic stress [131]. The F0F1 ATP synthase pump helps to maintain H+ homeostasis when bacteria face an acidic environment. It hydrolyzes ATP to pump protons (H+) from the cytoplasm [132]. We found that this synthase complex is present in the genomes of our isolates. Bacteria must withstand the toxicity of bile salts, which induce intracellular acidification and act as detergents that disrupt biological membranes [133]. Proteins involved in bile tolerance mechanisms, such as the Na(+)/H(+) antiporter, manganese-dependent inorganic pyrophosphatase, pyrophosphatase PpaX, and oligopeptide-binding protein OppA, were also identified. Molecular chaperones that impart resistance against environmental stress, such as the chaperonins GroES and GroEL [134,135], six Clp proteases, and HtpX protease heat shock proteins, were present. Three copies of cold shock proteins (CSPs) were also found. These proteins play important roles in basic cellular functions, including growth, DNA and RNA stability, and the prevention of inclusion body formation [136,137,138].
To handle hyperosmotic stress and heat resistance, the isolates possess one copy of the chaperone protein DnaJ, the chaperone protein DnaK, and the nucleotide exchange factor GrpE. Additionally, two methionine sulfoxide reductases [139], along with others, were present in the genomes of the isolates, providing resistance to oxidative stress. Annotation analysis also revealed the presence of proteins involved in adhesion. These adhesion proteins may facilitate the binding of probiotic bacteria and enable direct interactions with the intestinal mucosa layer. All the isolates also harbored general stress adaptation proteins. Universal stress proteins are important for survival during cellular growth arrest and help to reprogram the cell toward defense and escape during cellular stress [140,141]. This suggests that our isolates have proteins that can handle stress and harsh conditions in the human gut and improve adhesion to the intestinal mucosa.
Concerning the safety assessment, KEGG analysis revealed the presence of hemolysin (hlyIII) in the genomes of all three strains. However, our wet lab studies found the activity to be γ-hemolysis [142], which is generally considered safe [44,143]. Moreover, the KEGG analysis indicated antibiotic resistance to vancomycin (vanY, vanX), beta-lactam (penP), and cationic antimicrobial peptides. Resfinder 4.1 predicted streptomycin, spiramycin/telithromycin, and doxycycline/tetracycline resistance in the isolates BB10.1 and BP20.15. Nevertheless, in the antibiotic susceptibility tests conducted in the wet lab with chloramphenicol, azithromycin, linezolid, rifampicin, penicillin, trimethoprim, clindamycin, ciprofloxacin, gentamycin, kanamycin, and streptomycin (results not shown), resistance was observed only for penicillin. While tetracycline- and vancomycin-resistant genes were predicted computationally, we did not perform any susceptibility studies to substantiate or confirm any intrinsic resistance. Different classes of beta-lactamase presence in one or the other Bacillus probiotic suggest the presence of penicillin resistance in the Bacillus probiotic [100,144]. Moreover, previous studies have shown that an organism may exhibit intrinsic resistance to a few antibiotics that cannot be attributed to its genotype [144].
Conjugative elements and phage-mediated insertions play significant roles in bacterial evolution [145] by contributing to genetic variability among closely related bacterial strains [146]. This variability often leads to phenotypical differences, such as in bacterial pathogenesis [146,147]. Bacteriophage-mediated horizontal gene transfer enhances bacterial adaptive responses to environmental changes, including the rapid spread of antibiotic resistance [148]. Furthermore, phages facilitate inversions, deletions, and chromosomal rearrangements, which help to transfer genes that can directly impact the phenotype between related or phylogenetically distant strains through horizontal gene transfer (HGT), and all of these evolutionary events have implications for selection and fitness [146,147]. Brevibacillus phage Osiris, a temperate phage, was reported in the genome of isolate BB10.1 [149]. The BB10.1 genome had a GC content of 43.67%, and the Brevibacillus phage Osiris had a GC content of 44.85%. Similarly, Brevibacillus phage Jimmer 1, another temperate phage reported in the genome of isolate PY2.3, specifically targets Paenibacillus larvae, a Firmicute bacterium, as its host [150]. Isolate PY2.3 had a GC content of 46.52%, while the Brevibacillus phage Jimmer1 region had a GC content of 47.00%. The distribution of phage elements is not consistently associated with resistance properties. The Jimmer1 phage includes many genes found in all strains, whether resistant or not [151]. Incomplete prophage regions are considered as defective prophages, lacking complete structural prophage genes compared to active, functional phages. Moreover, defective prophages often carry genes beneficial to the host, such as recombination, virulence, stress resistance, or toxin genes, that can inhibit the growth of competing bacteria in the environment [152].
Insertion sequences (ISs) are short DNA sequences that function as simple transposable elements. They are often smaller than other transposable elements and encode only proteins involved in transposition [153]. In the genomes of isolates BB10.1 and BP20.15, copies of the insertion sequences ISBsu1 and ISBpu1 were found, while BB10.1 also had a copy of ISBspe1. PY2.3, along with copies of ISBsu1, also had one copy of IS665 and ISLmo1. These elements, along with others, have been reported in several other Bacillus species [154,155,156,157]. However, none of these elements have been associated with pathogenicity or virulence in Bacillus spp., but they have been linked to stress tolerance [158]. ICEs (integrative and conjugative elements) are mobile genetic elements that significantly contribute to genome evolution [159]. In the genome of isolate PY2.3, a mobile genetic element called ICEBs1 was found. ICEBs1 is an integral part of the global DNA damage SOS response and has also been reported in other Bacillus genomes [160]. It has been associated with gene activation and inactivation, recombination and rearrangement, evolution of new functions, antibiotic resistance and virulence, horizontal gene transfer, adaptation to environmental changes, stress response, resistance to radiation, and desiccation [151].
Biogenic amines (BAs) are naturally occurring low-molecular-weight organic nitrogen bases found in living organisms. These compounds serve as metabolic intermediates and products, synthesized and degraded during the metabolism of animals, plants, and microorganisms. BAs are primarily formed through the decarboxylation of amino acids or the amination and transamination of aldehydes and ketones [161,162]. They possess chemical structures that can be classified as aliphatic (such as putrescine, cadaverine, spermine, and spermidine), aromatic (tyramine and phenylethylamine), or heterocyclic (histamine and tryptamine) [163]. BAs play critical roles in various human physiological functions, including cerebral activity, gastric acid secretion, and immune responses [164]. The accumulation of BAs in food can occur at high concentrations due to the activities of microorganisms possessing decarboxylation enzymes. Excessive oral intake of BAs can result in symptoms such as nausea, headaches, rashes, and changes in blood pressure [164]. Consequently, it is essential to prevent BA accumulation in food to avoid adverse health effects [165]. In our isolates, genes associated with the synthesis of spermidine and putrescine were detected. Spermidine plays a critical role in the robust formation of biofilms in B. subtilis [166]. In B. subtilis, the triamine spermidine is formed from the diamine putrescine through the transfer of an aminopropyl group to putrescine by spermidine synthase, which is encoded by the speE gene [166]. However, genes related to other biogenic amines (cadaverine, ornithine, histamine, tyramine, and tryptamine) were not detected in any of the isolate genomes, as described by Chokesajjawatee et al., 2020 [110].
4. Materials and Methods
4.1. Genomic Sequences
The genomic material in this in silico investigation comprised three Bacillus strains: BB10.1 and BP20.15 isolated from bee bread, and PY2.3 obtained from bee pollen. These genomic sequences were publicly accessible via the Sequence Read Archive (SRA) and GenBank, associated with BioProject IDs PRJNA949953, PRJNA949979, and PRJNA949984, respectively. The SRA accession numbers for BB10.1, BP20.15, and PY2.3 were cataloged as SRR24003043, SRR24003725, and SRR24005238, respectively [28].
4.2. Genome Synteny and Completeness
To assess the completeness of the genome assembly and the quality of the protein annotation, Benchmarking Universal Single-Copy Orthologs (BUSCO) tool 5.4.6 [167] with bacilli_odb10 as reference lineage dataset was used, and D-Genies web server4 [168] was used to evaluate bacterial genome synteny. Dot plots generated were used for visual analysis.
4.3. Pan-Genome Analysis
To obtain an insight into the genomes of other isolates, pan-genome analysis was performed with 180 B. subtilis genomes and 204 Bacillus velezensis genomes (accession number Supplementary File S1) using IPGA v1.09 (integrated prokaryotes genome and pan-genome analysis service) [169]. The following parameters were used: genome filter; completeness 90; contamination 5; and, for pan-genome analysis procedures, modules, PANOCT, OrthoMCL, Roary, panX, OrthoFinder, Panaroo, and PPanGGoLiN were selected. Further downstream analyses were performed in the R studio using R modules ggplot2 and heatmap.2. Whole-genome-variation-based phylogenetic inference tree was generated and the binary matrix file, gene presence, and absence across all strains were used to estimate the sizes of the pan-genome and core genome.
4.4. Probiogenomics Analysis
4.4.1. Functional Annotation and Carbohydrate-Active Enzyme (CAZyme) Profiling
The Kyoto Encyclopedia of Genes and Genomes (KEGG) Database and Links Annotation (BlastKOALA) (https://www.kegg.jp/blastkoala/ (accessed on 5 August 2023)) and EGGNOG2-mapper V2 [170] were used for the functional annotation of the proteins. Carbohydrate-active enzymes (CAZymes) were annotated by the dbCAN database (https://bcb.unl.edu/dbCAN2/index.php (accessed on 5 August 2023)) [171], a Meta server for automated carbohydrate-active enzyme annotation. CAZymes predicted by any two of the tools out of three were considered.
4.4.2. Genome Plasticity Analysis and Safety Assessment
To analyze the genome plasticity, the web server PHASTER was used for the rapid identification and annotation of prophage sequences within bacterial genomes [172]. The insertion elements in the genome were detected with the ISfinder database [173] using BLASTn v2.2.31 with an E-value threshold of 1 × 10−5. The Island Viewer 4 server was used for determining the genomic islands and the presence of genes related to pathogenicity [174]. The CRISPRCasFinder tool was used to determine the sequences coding for clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated genes (Cas) using default parameters [175]. To detect the presence of plasmids in the genome, the tool Plasmid Finder was used [103]. To search for the toxin–antitoxin proteins in the genomes of our selected strains, a WU-BLAST 2.0 search was carried out against the TADB v2.0 finder database [176] with the following parameters: (1) E-value for BLAST = 0.01, (2) E-value for HMMer = 1, (3) maximum length of potential toxin/antitoxin = 300, (4) maximum distance (or overlap) between potential toxin and antitoxin = 20–150 nucleotides. The comparison matrix was BLOSUM62, the cutoff score (S value) was default, the word length (W value) was default (default = 11 for BLASTN, 3 for all others), and the expected threshold (E threshold) was default.
Subsequently, to assess the safety of isolates, the search for antimicrobial resistance (AMR) genes in the genomes of isolates BB10.1, BP20.15, and PY2.3 was carried out in three publicly available databases, i.e., ResFinder tool v.4.1. of the Center for Genomic Epidemiology [177], the Resistance Gene Identifier (RGI) tool in the Comprehensive Antibiotic Resistance Database (CARD) [178], and the BlastKOALA and KAAS tool in the KEGG database [179]. The Virulence Finder v.2.0.3 tool (https://cge.food.dtu.dk/services/VirulenceFinder/ (accessed on 7 August 2023)) and Virulence Factor of Bacterial Pathogen Database (VFDB) [180] were used to determine the putative virulence factors in the compare draft genome of isolates with known Bacillus pathogens. A web-based tool oriTfinder (available at https://bioinfo-mml.sjtu.edu.cn/oriTfinder/ (accessed on 7 August 2023)) [181] was used to identify the origin of transfer (oriT), the essential element for self-transmitted conjugative plasmids. The results from the BlastKOALA and KAAS tool in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database were critically observed, and the genes involved in toxins, biogenic amine (BA) production, and other undesirable properties as stated by Chokesajjawatee et al., 2020 [110] were determined by literature mining and annotated genome analyses. PathogenFinder [104] was used for assessing the probability of the isolates being pathogenic to humans. iProbiotics (http://bioinfor.imu.edu.cn/iprobiotics/public/Home (accessed on 15 August 2023)) [182], a web-based machine learning tool for the detection of probiotics genes, was used to identify probiotics genes in our isolates genomes.
5. Conclusions
We have identified several promising probiotic characteristics in the genomes of our isolates. It is essential to conduct in vitro and in vivo studies to confirm and further investigate specific traits, such as the potential production of biogenic amines. Additionally, more extensive investigations are needed to understand the mechanisms underlying tetracycline and vancomycin resistance, including the role of insertion elements, which warrants a broader range of molecular biology studies. Furthermore, while the iProbiotics tool successfully identified potential probiotic regions, it lacks additional information regarding non-probiotic regions. Specifically, details about the presence of genes contributing to virulence and the similarity of these regions to other pathogenic strains are not provided. This information could be of considerable significance in evaluating the characteristics of non-probiotic regions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25010666/s1.
Author Contributions
Conceptualization, A.B.H. and P.S.; methodology, A.B.H.; formal analysis, A.B.H., P.S. and R.W.; investigation, A.B.H. and K.P.; curation, A.B.H.; writing—original draft preparation, A.B.H. and P.S.; writing—review and editing A.B.H., K.P., P.S. and R.W.; supervision, P.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The studies were financed by the grant UMO-2021/41/B/NZ9/03929 from the National Science Centre, Poland.
Institutional Review Board Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
A.B.H. declares that he has no conflicts of interest. K.P. declares that she has no conflicts of interest. R.W. declares that he has no conflicts of interest. P.S. declares that he has no conflicts of interest.
References
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987; ISBN 0674074092. [Google Scholar]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabri, A.A. Honey, Milk and Antibiotics. Afr. J. Biotechnol. 2013, 4, 1580–1587. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The Roles of Strawberry and Honey Phytochemicals on Human Health: A Possible Clue on the Molecular Mechanisms Involved in the Prevention of Oxidative Stress and Inflammation. Phytomedicine 2021, 86, 153170. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 Signalling by Manuka Honey Protects Human Dermal Fibroblasts against Oxidative Damage by Improving Antioxidant Response and Mitochondrial Function Promoting Wound Healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Maouche, N.; Otmani, A.; Terrab, A.; Madani, K.; Ouchemoukh, S. Evaluation of Algerian’s Honey in Terms of Quality and Authenticity Based on the Melissopalynology and Physicochemical Analysis and Their Antioxidant Powers. Med. J. Nutr. Metab. 2021, 14, 305–324. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry Tree Honey as a New Potential Functional Food. Part 1: Strawberry Tree Honey Reduces Colon Cancer Cell Proliferation and Colony Formation Ability, Inhibits Cell Cycle and Promotes Apoptosis by Regulating EGFR and MAPKs Signaling Pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Pistollato, F.; Zhang, J.J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Bompadre, S.; et al. Strawberry Tree Honey as a New Potential Functional Food. Part 2: Strawberry Tree Honey Increases ROS Generation by Suppressing Nrf2-ARE and NF-KB Signaling Pathways and Decreases Metabolic Phenotypes and Metastatic Activity in Colon Cancer Cells. J. Funct. Foods 2019, 57, 477–487. [Google Scholar] [CrossRef]
- Osés, S.M.; Nieto, S.; Rodrigo, S.; Pérez, S.; Rojo, S.; Sancho, M.T.; Fernández-Muiño, M.Á. Authentication of Strawberry Tree (Arbutus unedo L.) Honeys from Southern Europe Based on Compositional Parameters and Biological Activities. Food Biosci. 2020, 38, 100768. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and Bee Bread as New Health-Oriented Products: A Review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Snowdon, J.A.; Cliver, D.O. Microorganisms in Honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Pełka, K.; Buzun, K.; Worobo, R.; Szweda, P. Whole-Genome Sequencing and Antimicrobial Potential of Bacteria Isolated from Polish Honey. Appl. Microbiol. Biotechnol. 2023, 107, 6389–6406. [Google Scholar] [CrossRef] [PubMed]
- Pajor, M.; Worobo, R.W.; Milewski, S.; Szweda, P. The Antimicrobial Potential of Bacteria Isolated from Honey Samples Produced in the Apiaries Located in Pomeranian Voivodeship in Northern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2002. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. Honey as an Ecological Reservoir of Antibacterial Compounds Produced by Antagonistic Microbial Interactions in Plant Nectars, Honey and Honey Bee. Antibiotics 2021, 10, 551. [Google Scholar] [CrossRef]
- Pełka, K.; Otłowska, O.; Worobo, R.W.; Szweda, P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Didaras, N.A.; Kafantaris, I.; Dimitriou, T.G.; Mitsagga, C.; Karatasou, K.; Giavasis, I.; Stagos, D.; Amoutzias, G.D.; Hatjina, F.; Mossialos, D. Biological Properties of Bee Bread Collected from Apiaries Located across Greece. Antibiotics 2021, 10, 555. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Barać, M.B.; Shariati, M.A.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient-The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically Active Antimicrobial and Antioxidant Substances in the Helianthus annuus L. Bee Pollen. J. Environ. Sci. Health. B 2016, 51, 176–181. [Google Scholar] [CrossRef]
- Gilliam, M. Microbiology of Pollen and Bee Bread: The Yeasts. Apidologie 1979, 10, 43–53. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid. Based. Complement. Alternat. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Strant, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Mohamed, M.; Bakar, A.B.A.; Zakaria, Z.; Othman, Z.A.; Nna, V.U. Therapeutic Effects of Bee Bread on Obesity-Induced Testicular-Derived Oxidative Stress, Inflammation, and Apoptosis in High-Fat Diet Obese Rat Model. Antioxidants 2022, 11, 255. [Google Scholar] [CrossRef]
- Malihah Mohammad, S.; Mahmud-Ab-Rashid, N.-K.; Zawawi, N.; Jembrek, J.; Juszczak, L. Molecules Stingless Bee-Collected Pollen (Bee Bread): Chemical and Microbiology Properties and Health Benefits. Molecules 2021, 26, 957. [Google Scholar] [CrossRef] [PubMed]
- Pełka, K.; Bin Hafeez, A.; Worobo, R.W.; Szweda, P. Probiotic Potential of Bacillus Isolates from Polish Bee Pollen and Bee Bread. Probiotics Antimicrob. Proteins 2023, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pełka, K.; Worobo, R.W.; Walkusz, J.; Szweda, P. Bee Pollen and Bee Bread as a Source of Bacteria Producing Antimicrobials. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. Food Nutr. 2006, 85, 1–56. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Zulkhairi Amin, F.A.; Sabri, S.; Ismail, M.; Chan, K.W.; Ismail, N.; Mohd Esa, N.; Mohd Lila, M.A.; Zawawi, N. Probiotic Properties of Bacillus Strains Isolated from Stingless Bee (Heterotrigona Itama) Honey Collected across Malaysia. Int. J. Environ. Res. Public Health 2019, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Abdelsamad, N.O.; Esawy, M.A.; Mahmoud, Z.E.; El-Shazly, A.I.; Elsayed, T.R.; Gamal, A.A. Evaluation of Different Bacterial Honey Isolates as Probiotics and Their Efficient Roles in Cholesterol Reduction. World J. Microbiol. Biotechnol. 2022, 38, 106. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.B.; Roobia, R.R.; Karthikeyan, M.; Murugappan, R.M. Validation of Nutraceutical Properties of Honey and Probiotic Potential of Its Innate Microflora. LWT-Food Sci. Technol. 2015, 60, 743–750. [Google Scholar] [CrossRef]
- Hasali, N.H.M.; Zamri, A.I.; Lani, M.N.; Mubarak, A.; Suhaili, Z. Identification of Lactic Acid Bacteria from Meliponine Honey and Their Antimicrobial Activity against Pathogenic Bacteria. Am. J. Sustain. Agric. 2015, 9, 1–7. [Google Scholar]
- Vergalito, F.; Testa, B.; Cozzolino, A.; Letizia, F.; Succi, M.; Lombardi, S.J.; Tremonte, P.; Pannella, G.; Di Marco, R.; Sorrentino, E.; et al. Potential Application of Apilactobacillus Kunkeei for Human Use: Evaluation of Probiotic and Functional Properties. Foods 2020, 9, 1535. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.K.; Zawawi, N. Probiotic Properties of Bacteria Isolated from Bee Bread of Stingless Bee Heterotrigona Itama. J. Apic. Res. 2020, 60, 172–187. [Google Scholar] [CrossRef]
- Toutiaee, S.; Mojgani, N.; Harzandi, N.; Moharrami, M.; Mokhberosafa, L. In Vitro Probiotic and Safety Attributes of Bacillus spp. Isolated from Beebread, Honey Samples and Digestive Tract of Honeybees Apis Mellifera. Lett. Appl. Microbiol. 2022, 74, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Dabiré, Y.; Somda, N.S.; Somda, M.K.; Compaoré, C.B.; Mogmenga, I.; Ezeogu, L.I.; Traoré, A.S.; Ugwuanyi, J.O.; Dicko, M.H. Assessment of Probiotic and Technological Properties of Bacillus spp. Isolated from Burkinabe Soumbala. BMC Microbiol. 2022, 22, 228. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, C.Y.; Huang, H.T.; Lu, M.K.; Hu, W.S.; Lee, K.T. Bacillus Subtilis Natto Derivatives Inhibit Enterococcal Biofilm Formation via Restructuring of the Cell Envelope. Front. Microbiol. 2021, 12, 785351. [Google Scholar] [CrossRef]
- Li, A.; Wang, M.; Zhang, Y.; Lin, Z.; Xu, M.; Wang, L.; Kulyar, M.F.-e.-A.; Li, J. Complete Genome Analysis of Bacillus Subtilis Derived from Yaks and Its Probiotic Characteristics. Front. Vet. Sci. 2023, 9, 1099150. [Google Scholar] [CrossRef]
- Shangpliang, H.N.J.; Tamang, J.P. Genome Analysis of Potential Probiotic Levilactobacillus Brevis AcCh91 Isolated from Indian Home-Made Fermented Milk Product (Chhurpi). Probiotics Antimicrob. Proteins 2023, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Khullar, G.; Det-udom, R.; Prombutar, P.; Prakitchaiwattana, C. Probiogenomic Analysis and Safety Assessment of Bacillus Isolates Using Omics Approach in Combination with In-Vitro. LWT 2022, 159, 113216. [Google Scholar] [CrossRef]
- Kim, S.H.; Yehuala, G.A.; Bang, W.Y.; Yang, J.; Jung, Y.H.; Park, M.K. Safety Evaluation of Bacillus Subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications. Microorganisms 2022, 10, 2494. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; Dicuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Chastanet, A.; Fert, J.; Msadek, T. Comparative Genomics Reveal Novel Heat Shock Regulatory Mechanisms in Staphylococcus Aureus and Other Gram-Positive Bacteria. Mol. Microbiol. 2003, 47, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Gerth, U.; Wipat, A.; Harwood, C.R.; Carter, N.; Emmerson, P.T.; Hecker, M. Sequence and Transcriptional Analysis of ClpX, a Class-III Heat-Shock Gene of Bacillus Subtilis. Gene 1996, 181, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.A.; Hubbard, T.J.P.; Travers, A.A.; Ellar, D.J. Heat-Shock Proteins during Growth and Sporulation of Bacillus Subtilis. FEBS Lett. 1985, 188, 209–214. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Piechowska, M. Heat-Shock Proteins in Membrane Vesicles of Bacillus Subtilis. Acta Biochim. Pol. 1988, 35, 367–376. [Google Scholar]
- Ghafoori, H.; Askari, M.; Sarikhan, S. Molecular Cloning, Expression and Functional Characterization of the 40-KDa Heat Shock Protein, DnaJ, from Bacillus Halodurans. Process Biochem. 2017, 54, 33–40. [Google Scholar] [CrossRef]
- Endo, A.; Sasaki, M.; Maruyama, A.; Kurusu, Y. Temperature Adaptation of Bacillus Subtilis by Chromosomal GroEL Replacement. Biosci. Biotechnol. Biochem. 2006, 70, 2357–2362. [Google Scholar] [CrossRef]
- Graumann, P.L.; Marahiel, M.A. Cold Shock Proteins CspB and CspC Are Major Stationary-Phase-Induced Proteins in Bacillus Subtilis. Arch. Microbiol. 1999, 171, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, R.; Hao, L.; Wang, C.; Li, M. CspB and CspC Are Induced upon Cold Shock in Bacillus Cereus Strain D2. Can. J. Microbiol. 2021, 67, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Akanuma, G.; Tagana, T.; Sawada, M.; Suzuki, S.; Shimada, T.; Tanaka, K.; Kawamura, F.; Kato-Yamada, Y. C-Terminal Regulatory Domain of the ε Subunit of FoF1 ATP Synthase Enhances the ATP-Dependent H+ Pumping That Is Involved in the Maintenance of Cellular Membrane Potential in Bacillus Subtilis. Microbiologyopen 2019, 8, e00815. [Google Scholar] [CrossRef] [PubMed]
- Keis, S.; Kaim, G.; Dimroth, P.; Cook, G.M. Cloning and Molecular Characterization of the Atp Operon Encoding for the F1F0–ATP Synthase from a Thermoalkaliphilic Bacillus Sp. Strain TA2.A1. Biochim. Biophys. Acta-Gene Struct. Expr. 2004, 1676, 112–117. [Google Scholar] [CrossRef]
- Cheng, J.; Guffanti, A.A.; Krulwich, T.A. The Chromosomal Tetracycline Resistance Locus of Bacillus Subtilis Encodes a Na+/H+ Antiporter That Is Physiologically Important at Elevated PH. J. Biol. Chem. 1994, 269, 27365–27371. [Google Scholar] [CrossRef] [PubMed]
- Kosono, S.; Morotomi, S.; Kitada, M.; Kudo, T. Analyses of a Bacillus Subtilis Homologue of the Na+/H+ Antiporter Gene Which Is Important for PH Homeostasis of Alkaliphilic bacillus sp. C-125. Biochim. Biophys. Acta-Bioenerg. 1999, 1409, 171–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujisawa, M.; Kusumoto, A.; Wada, Y.; Tsuchiya, T.; Ito, M. NhaK, a Novel Monovalent Cation/H+ Antiporter of Bacillus Subtilis. Arch. Microbiol. 2005, 183, 411–420. [Google Scholar] [CrossRef]
- Devkota, S.R.; Kwon, E.; Ha, S.C.; Chang, H.W.; Kim, D.Y. Structural Insights into the Regulation of Bacillus Subtilis SigW Activity by Anti-Sigma RsiW. PLoS ONE 2017, 12, e0174284. [Google Scholar] [CrossRef]
- Wiegert, T.; Homuth, G.; Versteeg, S.; Schumann, W. Alkaline Shock Induces the Bacillus SubtilisσW Regulon. Mol. Microbiol. 2001, 41, 59–71. [Google Scholar] [CrossRef]
- Zweers, J.C.; Nicolas, P.; Wiegert, T.; van Dijl, J.M.; Denham, E.L. Definition of the ΣW Regulon of Bacillus Subtilis in the Absence of Stress. PLoS ONE 2012, 7, e48471. [Google Scholar] [CrossRef][Green Version]
- Masahiro, I.; Guffanti, A.A.; Oudega, B.; Krulwich, T.A. Mrp, A Multigene, Multifunctional Locus in Bacillus Subtilis with Roles in Resistance to Cholate and to Na+ and in PH Homeostasis. J. Bacteriol. 1999, 181, 2394–2402. [Google Scholar] [CrossRef]
- Chi, B.K.; Gronau, K.; Mäder, U.; Hessling, B.; Becher, D.; Antelmann, H. S-Bacillithiolation Protects against Hypochlorite Stress in Bacillus Subtilis as Revealed by Transcriptomics and Redox Proteomics. Mol. Cell. Proteom. 2011, 10, M111.009506. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.K.; Roberts, A.A.; Huyen, T.T.T.; Bäsell, K.; Becher, D.; Albrecht, D.; Hamilton, C.J.; Antelmann, H. S-Bacillithiolation Protects Conserved and Essential Proteins against Hypochlorite Stress in Firmicutes Bacteria. Antioxid. Redox Signal. 2013, 18, 1273–1295. [Google Scholar] [CrossRef] [PubMed]
- Sleator, R.D.; Gahan, C.G.M.; Hill, C. A Postgenomic Appraisal of Osmotolerance in Listeria Monocytogenes. Appl. Environ. Microbiol. 2003, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, D.; Ma, X.; An, H.; Zhai, Z.; Ren, F.; Hao, Y. Functional Role of OppA Encoding an Oligopeptide-Binding Protein from Lactobacillus Salivarius Ren in Bile Tolerance. J. Ind. Microbiol. Biotechnol. 2015, 42, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- McMillan, L.J.; Hepowit, N.L.; Maupin-Furlow, J.A. Archaeal Inorganic Pyrophosphatase Displays Robust Activity under High-Salt Conditions and in Organic Solvents. Appl. Environ. Microbiol. 2016, 82, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.; Bremer, E.; Schmitt, L. Nucleotide Dependent Monomer/Dimer Equilibrium of OpuAA, the Nucleotide-Binding Protein of the Osmotically Regulated ABC Transporter OpuA from Bacillus Subtilis. J. Mol. Biol. 2003, 334, 403–419. [Google Scholar] [CrossRef]
- Horn, C.; Bremer, E.; Schmitt, L. Functional Overexpression and In Vitro Re-Association of OpuA, an Osmotically Regulated ABC-Transport Complex from Bacillus Subtilis. FEBS Lett. 2005, 579, 5765–5768. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bremer, E. Guardians in a Stressful World: The Opu Family of Compatible Solute Transporters from Bacillus Subtilis. Biol. Chem. 2017, 398, 193–214. [Google Scholar] [CrossRef]
- Hamon, M.A.; Lazazzera, B.A. The Sporulation Transcription Factor Spo0A Is Required for Biofilm Development in Bacillus Subtilis. Mol. Microbiol. 2001, 42, 1199–1209. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus Subtilis Biofilm Formation and Social Interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, M.; Sam, S.; Mustafa, S.; Hashim, A.M.; Yusof, T.; Zulkifly, S.; Zuhairi, A.; Malek, A.; Akhmal, M.; Roslan, H.; et al. Mining the Genome of Bacillus Velezensis FS26 for Probiotic Markers and Secondary Metabolites with Antimicrobial Properties against Aquaculture Pathogens. Microb. Pathog. 2023, 181, 106161. [Google Scholar] [CrossRef]
- Van Pijkeren, J.P.; Canchaya, C.; Ryan, K.A.; Li, Y.; Claesson, M.J.; Sheil, B.; Steidler, L.; O’Mahony, L.; Fitzgerald, G.F.; Van Sinderen, D.; et al. Comparative and Functional Analysis of Sortase-Dependent Proteins in the Predicted Secretome of Lactobacillus Salivarius UCC118. Appl. Environ. Microbiol. 2006, 72, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Pérez Montoro, B.; Casimiro-Soriguer, C.S.; Pérez Pulido, A.J.; Knapp, C.W.; Caballero Gómez, N.; Castillo-Gutiérrez, S.; Estudillo-Martínez, M.D.; Gálvez, A.; Benomar, N. Insight into Potential Probiotic Markers Predicted in Lactobacillus Pentosus MP-10 Genome Sequence. Front. Microbiol. 2017, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jaatinen, A.; Rintahaka, J.; Hynönen, U.; Lyytinen, O.; Kant, R.; Åvall-Jääskeläinen, S.; Von Ossowski, I.; Palva, A. Human Gut-Commensalic Lactobacillus Ruminis ATCC 25644 Displays Sortase-Assembled Surface Piliation: Phenotypic Characterization of Its Fimbrial Operon through In Silico Predictive Analysis and Recombinant Expression in Lactococcus Lactis. PLoS ONE 2015, 10, e0145718. [Google Scholar] [CrossRef] [PubMed]
- Von, I.; Id, O. Novel Molecular Insights about Lactobacillar Sortase-Dependent Piliation. Int. J. Mol. Sci. 2017, 18, 1551. [Google Scholar] [CrossRef]
- Roux, D.; Cywes-Bentley, C.; Zhang, Y.F.; Pons, S.; Konkol, M.; Kearns, D.B.; Little, D.J.; Howell, P.L.; Skurnik, D.; Pier, G.B. Identification of Poly-N-Acetylglucosamine as a Major Polysaccharide Component of the Bacillus Subtilis Biofilm Matrix. J. Biol. Chem. 2015, 290, 19261–19272. [Google Scholar] [CrossRef]
- Guttenplan, S.B.; Blair, K.M.; Kearns, D.B. The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Bacillus Subtilis Biofilm Formation. PLOS Genet. 2010, 6, e1001243. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Lee, K.H.; Kang, H.B.; Kim, J.E.; Oh, M.H.; Ham, J.S. Probiogenomic In-Silico Analysis and Safety Assessment of Lactiplantibacillus Plantarum DJF10 Strain Isolated from Korean Raw Milk. Int. J. Mol. Sci. 2022, 23, 14494. [Google Scholar] [CrossRef]
- Zivkovic, M.; Miljkovic, M.; Ruas-Madiedo, P.; Strahinic, I.; Tolinacki, M.; Golic, N.; Kojic, M. Exopolysaccharide Production and Ropy Phenotype Are Determined by Two Gene Clusters in Putative Probiotic Strain Lactobacillus Paraplantarum BGCG11. Appl. Environ. Microbiol. 2015, 81, 1387–1396. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Li, H.; Zhu, J.; Liu, Z.; Hu, X.; Yi, J. Assessments of Probiotic Potentials of Lactiplantibacillus Plantarum Strains Isolated From Chinese Traditional Fermented Food: Phenotypic and Genomic Analysis. Front. Microbiol. 2022, 13, 895132. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Riethdorf, S.; Ernst, H.; Engelmann, S.; Völker, U.; Hecker, M. Thioredoxin Is an Essential Protein Induced by Multiple Stresses in Bacillus Subtilis. J. Bacteriol. 1998, 180, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Smits, W.K.; Dubois, J.Y.F.; Bron, S.; Van Dijl, J.M.; Kuipers, O.P. Tricksy Business: Transcriptome Analysis Reveals the Involvement of Thioredoxin A in Redox Homeostasis, Oxidative Stress, Sulfur Metabolism, and Cellular Differentiation in Bacillus Subtilis. J. Bacteriol. 2005, 187, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Tossounian, M.A.; Baczynska, M.; Dalton, W.; Peak-Chew, S.Y.; Undzenas, K.; Korza, G.; Filonenko, V.; Skehel, M.; Setlow, P.; Gout, I. Bacillus Subtilis YtpP and Thioredoxin A Are New Players in the Coenzyme-A-Mediated Defense Mechanism against Cellular Stress. Antioxidants 2023, 12, 938. [Google Scholar] [CrossRef]
- Capuano, V.; Galleron, N.; Pujic, P.; Sorokin, A.; Ehrlich, S.D. Organization of the Bacillus Subtilis 168 Chromosome between Kdg and the Attachment Site of the SPβ Prophage: Use of Long Accurate PCR and Yeast Artificial Chromosomes for Sequencing. Microbiology 1996, 142, 3005–3015. [Google Scholar] [CrossRef][Green Version]
- Gioia, J.; Yerrapragada, S.; Qin, X.; Jiang, H.; Igboeli, O.C.; Muzny, D.; Dugan-Rocha, S.; Ding, Y.; Hawes, A.; Liu, W.; et al. Paradoxical DNA Repair and Peroxide Resistance Gene Conservation in Bacillus Pumilus SAFR-032. PLoS ONE 2007, 2, e928. [Google Scholar] [CrossRef]
- Dhanya Raj, C.; Suryavanshi, M.V.; Kandaswamy, S.; Priyan Ramasamy, K.; Arthur James, R. Whole Genome Sequence Analysis and In-Vitro Probiotic Characterization of Bacillus Velezensis FCW2 MCC4686 from Spontaneously Fermented Coconut Water. Genomics 2023, 115, 110637. [Google Scholar] [CrossRef]
- Broden, N.J.; Flury, S.; King, A.N.; Schroeder, B.W.; Coe, G.D.; Faulkner, M.J. Insights into the Function of a Second, Nonclassical Ahp Peroxidase, AhpA, in Oxidative Stress Resistance in Bacillus Subtilis. J. Bacteriol. 2016, 198, 1044–1057. [Google Scholar] [CrossRef]
- Basu Thakur, P.; Long, A.R.; Nelson, B.J.; Kumar, R.; Rosenberg, A.F.; Gray, M.J. Complex Responses to Hydrogen Peroxide and Hypochlorous Acid by the Probiotic Bacterium Lactobacillus Reuteri. mSystems 2019, 4, 10-1128. [Google Scholar] [CrossRef]
- Engelmann, S.; Hecker, M. Impaired Oxidative Stress Resistance of Bacillus Subtilis SigB Mutants and the Role of KatA and KatE. FEMS Microbiol. Lett. 1996, 145, 63–69. [Google Scholar] [CrossRef]
- Rochat, T.; Miyoshi, A.; Gratadoux, J.J.; Duwatt, P.; Sourice, S.; Azevedo, V.; Langella, P. High-Level Resistance to Oxidative Stress in Lactococcus Lactis Conferred by Bacillus Subtilis Catalase KatE. Microbiology 2005, 151, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.Q.; Ritter, A.C.; Cibulski, S.; Brandelli, A. Functional Genome Annotation Depicts Probiotic Properties of Bacillus Velezensis FTC01. Gene 2019, 713, 143971. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, T.; Matsumura, Y.; Tsuchido, T. SodA and Manganese Are Essential for Resistance to Oxidative Stress in Growing and Sporulating Cells of Bacillus Subtilis. J. Bacteriol. 1999, 181, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.M.; Andrus, J.M.; Bruno-Bárcena, J.M.; Klaenhammer, T.R.; Hassan, H.M.; Threadgill, D.S. Anti-Inflammatory Properties of Lactobacillus Gasseri Expressing Manganese Superoxide Dismutase Using the Interleukin 10-Deficient Mouse Model of Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 729–738. [Google Scholar] [CrossRef]
- Bruno-Bárcena, J.M.; Andrus, J.M.; Libby, S.L.; Klaenhammer, T.R.; Hassan, H.M. Expression of a Heterologous Manganese Superoxide Dismutase Gene in Intestinal Lactobacilli Provides Protection against Hydrogen Peroxide Toxicity. Appl. Environ. Microbiol. 2004, 70, 4702–4710. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, P.; Lata, J.; Devi, N.; Chand, D. Thermostable Fe/Mn Superoxide Dismutase from Bacillus Licheniformis SPB-13 from Thermal Springs of Himalayan Region: Purification, Characterization and Antioxidative Potential. Int. J. Biol. Macromol. 2018, 115, 1026–1032. [Google Scholar] [CrossRef]
- Belaouni, H.A.; Compant, S.; Antonielli, L.; Nikolic, B.; Zitouni, A.; Sessitsch, A. In-Depth Genome Analysis of Bacillus sp. BH32, a Salt Stress-Tolerant Endophyte Obtained from a Halophyte in a Semiarid Region. Appl. Microbiol. Biotechnol. 2022, 106, 3113–3137. [Google Scholar] [CrossRef]
- You, C.; Sekowska, A.; Francetic, O.; Martin-Verstraete, I.; Wang, Y.; Danchin, A. Spx Mediates Oxidative Stress Regulation of the Methionine Sulfoxide Reductases Operon in Bacillus Subtilis. BMC Microbiol. 2008, 8, 128. [Google Scholar] [CrossRef]
- Khatri, I.; Sharma, G.; Subramanian, S. Composite Genome Sequence of Bacillus Clausii, a Probiotic Commercially Available as Enterogermina®, and Insights into Its Probiotic Properties. BMC Microbiol. 2019, 19, 307. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Yang, R.S.; Lin, Y.C.; Xin, W.G.; Zhou, H.Y.; Wang, F.; Zhang, Q.L.; Lin, L.B. Assessment of the Safety and Probiotic Characteristics of Lactobacillus Salivarius CGMCC20700 Based on Whole-Genome Sequencing and Phenotypic Analysis. Front. Microbiol. 2023, 14, 1120263. [Google Scholar] [CrossRef]
- Novick, R.P. Pathogenicity and Other Genomic Islands. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 240–242. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garciá-Fernández, A.; Larsen, M.V.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Voldby Larsen, M.; Møller Aarestrup, F.; Lund, O. PathogenFinder—Distinguishing Friend from Foe Using Bacterial Whole Genome Sequence Data. PLoS ONE 2013, 8, 77302. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Generally Recognized as Safe (GRAS): History and Description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Chang, H.C. Assessment of Bacillus Subtilis SN7 as a Starter Culture for Cheonggukjang, a Korean Traditional Fermented Soybean Food, and Its Capability to Control Bacillus Cereus in Cheonggukjang. Food Control 2017, 73, 946–953. [Google Scholar] [CrossRef]
- Ouoba, L.I.I. Traditional Alkaline Fermented Foods. In Starter Cultures in Food Production; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 370–383. [Google Scholar] [CrossRef]
- Ma, S.; Cao, J.; Liliu, R.; Li, N.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Bacillus Coagulans as an Adjunct Starter Culture on Yogurt Quality and Storage. J. Dairy Sci. 2021, 104, 7466–7479. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, F. Characterization of Lactobacillus, Bacillus and Saccharomyces Isolated from Iranian Traditional Dairy Products for Potential Sources of Starter Cultures. AIMS Microbiol. 2017, 3, 815. [Google Scholar] [CrossRef]
- Chokesajjawatee, N.; Santiyanont, P.; Chantarasakha, K.; Kocharin, K.; Thammarongtham, C.; Lertampaiporn, S.; Vorapreeda, T.; Srisuk, T.; Wongsurawat, T.; Jenjaroenpun, P.; et al. Safety Assessment of a Nham Starter Culture Lactobacillus Plantarum BCC9546 via Whole-Genome Analysis. Sci. Rep. 2020, 10, 10241. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Siles, L.; Karlsson, R.; Schmidt, P.; Salvà-Serra, F.; Jaén-Luchoro, D.; Skovbjerg, S.; Moore, E.R.B.; Gomila, M. A Pangenome Approach for Discerning Species-Unique Gene Markers for Identifications of Streptococcus Pneumoniae and Streptococcus Pseudopneumoniae. Front. Cell. Infect. Microbiol. 2020, 10, 531410. [Google Scholar] [CrossRef]
- Costa, S.S.; Guimarães, L.C.; Silva, A.; Soares, S.C.; Baraúna, R.A. First Steps in the Analysis of Prokaryotic Pan-Genomes. Bioinform. Biol. Insights 2020, 14, 1177932220938064. [Google Scholar] [CrossRef]
- Fu, X.; Gong, L.; Liu, Y.; Lai, Q.; Li, G.; Shao, Z. Bacillus Pumilus Group Comparative Genomics: Toward Pangenome Features, Diversity, and Marine Environmental Adaptation. Front. Microbiol. 2021, 12, 571212. [Google Scholar] [CrossRef]
- Brito, P.H.; Chevreux, B.; Serra, C.R.; Schyns, G.; Henriques, A.O.; Pereira-Leal, J.B. Genetic Competence Drives Genome Diversity in Bacillus Subtilis. Genome Biol. Evol. 2018, 10, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Kim, K.H.; Jeong, S.E.; Jeon, C.O. Genomic and Metabolic Features of the Bacillus Amyloliquefaciens Group—B. amyloliquefaciens, B. velezensis, and B. Siamensis—Revealed by Pan-Genome Analysis. Food Microbiol. 2019, 77, 146–157. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Ben Slama, H.; Bouket, A.C.; Cherif-Silini, H.; Silini, A.; Luptakova, L.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Bacillus Velezensis: A Treasure House of Bioactive Compounds of Medicinal, Biocontrol and Environmental Importance. Forests 2021, 12, 1714. [Google Scholar] [CrossRef]
- Park, Y.J.; Jeong, Y.U.; Kong, W.S. Genome Sequencing and Carbohydrate-Active Enzyme (CAZyme) Repertoire of the White Rot Fungus Flammulina Elastica. Int. J. Mol. Sci. 2018, 19, 2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Xu, H.; Xin, H.; Zhang, Y. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Holstein Cows Fed Different Forage-to-Concentrate Ratios. Front. Microbiol. 2019, 10, 441658. [Google Scholar] [CrossRef]
- Subin, S.R.; Okolie, C.L.; Udenigwe, C.C.; Mason, B. Structural Features Underlying Prebiotic Activity of Conventional and Potential Prebiotic Oligosaccharides in Food and Health. J. Food Biochem. 2017, 41, e12389. [Google Scholar] [CrossRef]
- Egan, M.; Van Sinderen, D. Carbohydrate Metabolism in Bifidobacteria. In The Bifidobacteria and Related Organisms, Biology, Taxonomy, Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 145–164. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, Y.; Zhan, Y.; Zhang, Y.; Sha, Y.; Xu, Z.; Li, S.; Feng, X.; Xu, H. Systematic Unravelling of the Inulin Hydrolase from Bacillus Amyloliquefaciens for Efficient Conversion of Inulin to Poly-(γ-Glutamic Acid). Biotechnol. Biofuels 2019, 12, 145. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Chassard, C.; Clerget, M.; Potrykus, J.; Campbell, G.; Mayer, C.D.; Young, P.; Rucklidge, G.; Ramsay, A.G.; et al. Substrate-Driven Gene Expression in Roseburia Inulinivorans: Importance of Inducible Enzymes in the Utilization of Inulin and Starch. Proc. Natl. Acad. Sci. USA 2011, 108, 4672–4679. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarangi, A.N.; Mukherjee, M.; Bhowmick, S.; Tripathy, S. Reanalysis of Lactobacillus Paracasei Lbs2 Strain and Large-Scale Comparative Genomics Places Many Strains into Their Correct Taxonomic Position. Microorganisms 2019, 7, 487. [Google Scholar] [CrossRef]
- Chung, W.H.; Kang, J.; Lim, M.Y.; Lim, T.J.; Lim, S.; Roh, S.W.; Nam, Y. Do Complete Genome Sequence and Genomic Characterization of Lactobacillus Acidophilus LA1 (11869BP). Front. Pharmacol. 2018, 9, 311400. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-Binding Modules: Fine-Tuning Polysaccharide Recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Zhu, T.; Pan, Z.; Domagalski, N.; Koepsel, R.; Ataai, M.M.; Domach, M.M. Engineering of Bacillus Subtilis for Enhanced Total Synthesis of Folic Acid. Appl. Environ. Microbiol. 2005, 71, 7122–7129. [Google Scholar] [CrossRef] [PubMed]
- De Crécy-Lagard, V. Variations in Metabolic Pathways Create Challenges for Automated Metabolic Reconstructions: Examples from the Tetrahydrofolate Synthesis Pathway. Comput. Struct. Biotechnol. J. 2014, 10, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.R.; Pattison, J.R.; Foster, M.A. Folic Acid and the Methylation of Homocysteine by Bacillus Subtilis. Biochem. J. 1972, 126, 993–1004. [Google Scholar] [CrossRef][Green Version]
- AFRC, R.F. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the Acid Test: Responses of Gram-Positive Bacteria to Low PH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.M.; Hill, C. The Interaction between Bacteria and Bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Zink, R.; Fitzgerald, G.F.; Van Sinderen, D. Characterization of the GroEL and GroES Loci in Bifidobacterium Breve UCC 2003: Genetic, Transcriptional, and Phylogenetic Analyses. Appl. Environ. Microbiol. 2004, 70, 6197–6209. [Google Scholar] [CrossRef]
- Susin, M.F.; Baldini, R.L.; Gueiros-Filho, F.; Gomes, S.L. GroES/GroEL and DnaK/DnaJ Have Distinct Roles in Stress Responses and during Cell Cycle Progression in Caulobacter Crescentus. J. Bacteriol. 2006, 188, 8044–8053. [Google Scholar] [CrossRef] [PubMed]
- Veinger, L.; Diamant, S.; Buchner, J.; Goloubinoff, P. The Small Heat-Shock Protein IbpB from Escherichia Coli Stabilizes Stress-Denatured Proteins for Subsequent Refolding by a Multichaperone Network. J. Biol. Chem. 1998, 273, 11032–11037. [Google Scholar] [CrossRef] [PubMed]
- Narberhaus, F. α-Crystallin-Type Heat Shock Proteins: Socializing Minichaperones in the Context of a Multichaperone Network. Microbiol. Mol. Biol. Rev. 2002, 66, 64–93. [Google Scholar] [CrossRef] [PubMed]
- Jakob, U.; Gaestel, M.; Engel, K.; Buchner, J. Small Heat Shock Proteins Are Molecular Chaperones. J. Biol. Chem. 1993, 268, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Adams, Z.; Liu, R.; Hepowit, N.L.; Wu, Y.; Bowmann, C.F.; Moskovitz, J.; Maupin-Furlow, J.A. Methionine Sulfoxide Reductase a (MsrA) and Its Function in Ubiquitin-like Protein Modification in Archaea. MBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Nachin, L.; Nannmark, U.; Nyström, T. Differential Roles of the Universal Stress Proteins of Escherichia Coli in Oxidative Stress Resistance, Adhesion, and Motility. J. Bacteriol. 2005, 187, 6265–6272. [Google Scholar] [CrossRef]
- Seifart Gomes, C.; Izar, B.; Pazan, F.; Mohamed, W.; Mraheil, M.A.; Mukherjee, K.; Billion, A.; Aharonowitz, Y.; Chakraborty, T.; Hain, T. Universal Stress Proteins Are Important for Oxidative and Acid Stress Resistance and Growth of Listeria Monocytogenes EGD-e In Vitro and In Vivo. PLoS ONE 2011, 6, e24965. [Google Scholar] [CrossRef]
- Berkowitz, F.E. Hemolysis and Infection: Categories and Mechanisms of Their Interrelationship. Rev. Infect. Dis. 1991, 13, 1151–1162. [Google Scholar] [CrossRef]
- Jeon, H.L.; Lee, N.K.; Yang, S.J.; Kim, W.S.; Paik, H.D. Probiotic Characterization of Bacillus Subtilis P223 Isolated from Kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic Resistance in Probiotic Bacteria. Front. Microbiol. 2013, 4, 51661. [Google Scholar] [CrossRef]
- Salmond, G.P.C.; Fineran, P.C. A Century of the Phage: Past, Present and Future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H.; Canchaya, C.; Hardt, W.-D. Phages and the Evolution of Bacterial Pathogens: From Genomic Rearrangements to Lysogenic Conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602. [Google Scholar] [CrossRef] [PubMed]
- Canchaya, C.; Fournous, G.; Chibani-Chennoufi, S.; Dillmann, M.L.; Brüssow, H. Phage as Agents of Lateral Gene Transfer. Curr. Opin. Microbiol. 2003, 6, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Brown-Jaque, M.; Calero-Cáceres, W.; Muniesa, M. Transfer of Antibiotic-Resistance Genes via Phage-Related Mobile Elements. Plasmid 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Berg, J.A.; Merrill, B.D.; Crockett, J.T.; Esplin, K.P.; Evans, M.R.; Heaton, K.E.; Hilton, J.A.; Hyde, J.R.; McBride, M.S.; Schouten, J.T.; et al. Characterization of Five Novel Brevibacillus Bacteriophages and Genomic Comparison of Brevibacillus Phages. PLoS ONE 2016, 11, e0156838. [Google Scholar] [CrossRef] [PubMed]
- Merrill, B.D.; Grose, J.H.; Breakwell, D.P.; Burnett, S.H. Characterization of Paenibacillus Larvae Bacteriophages and Their Genomic Relationships to Firmicute Bacteriophages. BMC Genomics 2014, 15, 745. [Google Scholar] [CrossRef] [PubMed]
- Tirumalai, M.R.; Stepanov, V.G.; Wünsche, A.; Montazari, S.; Gonzalez, R.O.; Venkateswaran, K.; Fox, G.E. Bacillus Safensis FO-36b and Bacillus Pumilus SAFR-032: A Whole Genome Comparison of Two Spacecraft Assembly Facility Isolates. BMC Microbiol. 2018, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Minnullina, L.; Pudova, D.; Shagimardanova, E.; Shigapova, L.; Sharipova, M.; Mardanova, A. Comparative Genome Analysis of Uropathogenic Morganella Morganii Strains. Front. Cell. Infect. Microbiol. 2019, 9, 458792. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, M.; Mao, C.; Wang, C.; Yuan, P.; Wang, T.; Sun, D. A Type I Restriction Modification System Influences Genomic Evolution Driven by Horizontal Gene Transfer in Paenibacillus Polymyxa. Front. Microbiol. 2021, 12, 709571. [Google Scholar] [CrossRef]
- Diabankana, R.G.C.; Shulga, E.U.; Validov, S.Z.; Afordoanyi, D.M. Genetic Characteristics and Enzymatic Activities of Bacillus Velezensis KS04AU as a Stable Biocontrol Agent against Phytopathogens. Int. J. Plant Biol. 2022, 13, 201–222. [Google Scholar] [CrossRef]
- Niazi, A.; Manzoor, S.; Asari, S.; Bejai, S.; Meijer, J.; Bongcam-Rudloff, E. Genome Analysis of Bacillus amyloliquefaciens subsp. Plantarum UCMB5113: A Rhizobacterium That Improves Plant Growth and Stress Management. PLoS ONE 2014, 9, e104651. [Google Scholar] [CrossRef]
- Nanjani, S.; Soni, R.; Paul, D.; Keharia, H. Genome Analysis Uncovers the Prolific Antagonistic and Plant Growth-Promoting Potential of Endophyte Bacillus Velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, J.; de Oliveira, B.F.R.; Vigoder, F.d.M.; Muricy, G.; Dobson, A.D.W.; Laport, M.S. Peeling the Layers Away: The Genomic Characterization of Bacillus Pumilus 64-1, an Isolate with Antimicrobial Activity From the Marine Sponge Plakina Cyanorosea (Porifera, Homoscleromorpha). Front. Microbiol. 2021, 11, 592735. [Google Scholar] [CrossRef] [PubMed]
- Takami, H.; Matsuki, A.; Takaki, Y. Wide-Range Distribution of Insertion Sequences Identified in B. halodurans among Bacilli and a New Transposon Disseminated in Alkaliphilic and Thermophilic Bacilli. DNA Res. 2004, 11, 153–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guglielmini, J.; Quintais, L.; Garcillán-Barcia, M.P.; de la Cruz, F.; Rocha, E.P.C. The Repertoire of ICE in Prokaryotes Underscores the Unity, Diversity, and Ubiquity of Conjugation. PLOS Genet. 2011, 7, e1002222. [Google Scholar] [CrossRef] [PubMed]
- Auchtung, J.M.; Lee, C.A.; Monson, R.E.; Lehman, A.P.; Grossman, A.D. Regulation of a Bacillus Subtilis Mobile Genetic Element by Intercellular Signaling and the Global DNA Damage Response. Proc. Natl. Acad. Sci. USA 2005, 102, 12554–12559. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S.; Grant, G.; Brown, D.S.; Ralph, A.; Pusztai, A. Polyamines in Food—Implications for Growth and Health. J. Nutr. Biochem. 1993, 4, 66–71. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of Biogenic Amines to Food Safety and Human Health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.; Alvarez, M.A. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Daniel Collins, J.; Noerrung, B.; Budka, H.; Andreoletti, O.; Buncic, S.; Griffin, J.; Hald, T.; Havelaar, A.; Hope, J.; Klein, G.; et al. Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Hobley, L.; Li, B.; Wood, J.L.; Kim, S.H.; Naidoo, J.; Ferreira, A.S.; Khomutov, M.; Khomutov, A.; Stanley-Wall, N.R.; Michael, A.J. Spermidine Promotes Bacillus Subtilis Biofilm Formation by Activating Expression of the Matrix Regulator SlrR. J. Biol. Chem. 2017, 292, 12041–12053. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Cabanettes, F.; Klopp, C. D-GENIES: Dot Plot Large Genomes in an Interactive, Efficient and Simple Way. PeerJ 2018, 2018, e4958. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Fan, G.; Sun, D.; Zhang, X.; Yu, Z.; Wang, J.; Wu, L.; Shi, W.; Ma, J. IPGA: A Handy Integrated Prokaryotes Genome and Pan-Genome Analysis Web Service. iMeta 2022, 1, e55. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. EggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. DbCAN2: A Meta Server for Automated Carbohydrate-Active Enzyme Annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The Reference Centre for Bacterial Insertion Sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded Prediction of Genomic Islands for Larger-Scale Datasets. Nucleic Acids Res. 2017, 45, W30. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an Update of CRISRFinder, Includes a Portable Version, Enhanced Performance and Integrates Search for Cas Proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wei, Y.; Shen, Y.; Li, X.; Zhou, H.; Tai, C.; Deng, Z.; Ou, H.Y. TADB 2.0: An Updated Database of Bacterial Type II Toxin–Antitoxin Loci. Nucleic Acids Res. 2018, 46, D749–D753. [Google Scholar] [CrossRef] [PubMed]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.T.L.C.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder—An Open Online Resource for Identification of Antimicrobial Resistance Genes in next-Generation Sequencing Data and Prediction of Phenotypes from Genotypes. Microb. Genomics 2022, 8, 000748. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The Comprehensive Antibiotic Resistance Database. Antimicrob. Agents Chemother. 2013, 57, 3348. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Liu, M.; Tai, C.; Sun, J.; Deng, Z.; Ou, H.Y. OriTfinder: A Web-Based Tool for the Identification of Origin of Transfers in DNA Sequences of Bacterial Mobile Genetic Elements. Nucleic Acids Res. 2018, 46, W229–W234. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Zheng, L.; Li, J.; Hong, Y.; Liang, P.; Kwok, L.Y.; Zuo, Y.; Zhang, W.; Zhang, H. IProbiotics: A Machine Learning Platform for Rapid Identification of Probiotic Properties from Whole-Genome Primary Sequences. Brief. Bioinform. 2022, 23, bbab477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).