Abstract
Cellular membranes are essential for compartmentalization, maintenance of permeability, and fluidity in all three domains of life. Archaea belong to the third domain of life and have a distinct phospholipid composition. Membrane lipids of archaea are ether-linked molecules, specifically bilayer-forming dialkyl glycerol diethers (DGDs) and monolayer-forming glycerol dialkyl glycerol tetraethers (GDGTs). The antifungal allylamine terbinafine has been proposed as an inhibitor of GDGT biosynthesis in archaea based on radiolabel incorporation studies. The exact target(s) and mechanism of action of terbinafine in archaea remain elusive. Sulfolobus acidocaldarius is a strictly aerobic crenarchaeon thriving in a thermoacidophilic environment, and its membrane is dominated by GDGTs. Here, we comprehensively analyzed the lipidome and transcriptome of S. acidocaldarius in the presence of terbinafine. Depletion of GDGTs and the accompanying accumulation of DGDs upon treatment with terbinafine were growth phase-dependent. Additionally, a major shift in the saturation of caldariellaquinones was observed, which resulted in the accumulation of unsaturated molecules. Transcriptomic data indicated that terbinafine has a multitude of effects, including significant differential expression of genes in the respiratory complex, motility, cell envelope, fatty acid metabolism, and GDGT cyclization. Combined, these findings suggest that the response of S. acidocaldarius to terbinafine inhibition involves respiratory stress and the differential expression of genes involved in isoprenoid biosynthesis and saturation.
1. Introduction
Crenarchaeal membranes are highly dynamic, as their composition is dependent on environmental factors, such as nutrient availability, pH, and temperature. Ether-linked archaeal lipids have been extensively studied because of their unique chemical properties, rendering membranes more robust with reduced proton permeability. In recent years, the lipid biosynthesis pathway in archaea has been well-characterized. It commences with the isoprenoid building blocks, isopentenyl pyrophosphate (IPP) or dimethylallyl pyrophosphate (DMAPP), from the alternate or classical mevalonate pathway [1]. Sulfolobales utilize the classic mevalonate pathway (MVA) for this step [2]. IPP and DMAPP undergo sequential condensation reactions with geranylgeranyl pyrophosphate synthase (GGPPS) to form GGPP (Figure 1) [1]. The glycerol-1-phosphate (G1P) backbone is synthesized by G1P dehydrogenase (EgsA) in Sulfolobus acidocaldarius (Figure 1) [3]. GGPP is further processed by geranylgeranyl glycerol phosphate (GGGP) synthase (GGGPS) to form GGGP with a glycerol-1-phosphate backbone (Figure 1) [1]. The formation of the diether 2,3-O-geranylgeranylglyceryl diphosphate (DGGGP) is catalyzed by DGGGP synthase (DGGGPS) [1,4]. CDP Archaeol synthase or CarS then activates DGGGP through cytidine triphosphate (CTP) to produce CDP-archaeol [5]. In the next step, the cytidine diphosphate (CDP) head group is replaced with polar head groups such as inositol, glycerol, or ethanolamine [5]. Geranylgeranyl reductase (GGR) is responsible for the hydrogenation of the unsaturated DGGGP to yield a saturated archaeol or 2,3-di-O-phytanyl-sn-glyceryl phosphate or archaetidic acid (AA) [6,7]. The substrate specificity of GGR remains unclear; however, it has been demonstrated that CarS is specific for unsaturated substrates, suggesting that saturation is a downstream process [8]. In archaea, tetraether lipids can be formed by tail-to-tail condensation of two diether lipids [9]. These tetraether lipids, termed glycerol dialkyl glycerol tetraethers (GDGTs), span the membrane. Recently, the enzyme involved in this process was identified, tetraether synthase (Tes), which belongs to the family of radical S-adenosylmethionine (rSAM) and catalyzes reactions through the formation of free radicals [10]. GDGTs can incorporate up to eight cyclopentane rings into their structures. Two GDGT ring synthases, GrsA and GrsB, have been identified in S. acidocaldarius (Figure 1) [11]. These proteins belong to the rSAM family. GrsA and GrsB introduce rings at the C-7 or C-3 position in GDGTs, and their expression is regulated by pH or temperature [11,12]. These GDGTs can be modified by the addition of headgroups. The calditol or nonnitol headgroup of GDGTs is synthesized by Cds, another rSAM enzyme that yields glycerol dialkyl nonnitol tetraethers (GDNTs) [13].
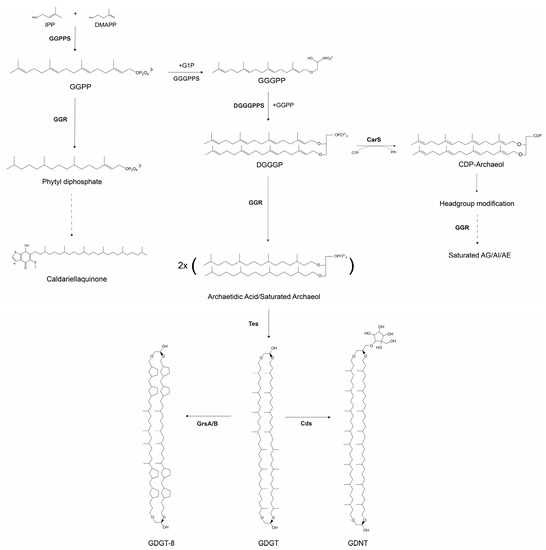
Figure 1.
Schematic overview of the archaeal lipid biosynthesis pathway. The dashed arrows indicate a multi-step pathway that has not yet been characterized. AG-archaetidylglycerol, AE-archaetidylethanoamine, AI-archaetidylinositol.
The cellular membrane of S. acidocaldarius predominantly consists of monolayer-forming GDGTs with a lower abundance of GDNTs [14,15]. Inositol-phosphate dialkyl glycerol diether (IP-DGD) is the most abundant bilayer forming dialkyl glycerol diether lipid (DGD) in the Sulfolobus membrane [15]. The membrane composition of S. acidocaldarius is modulated by environmental factors, such as growth phase, growth rate, temperature, pH, and starvation [11,15,16,17]. Common adaptations include altering the ratio of DGDs to GDGTs and incorporating cyclopentane rings into tetraether lipids. These adaptations presumably lead to a reduction in the permeability of crenarchaeal membranes at higher temperatures [18]. Various headgroups can be found in GDGT lipids of Sulfolobus including inositol-phosphate, monohexose, dihexose, calditol, or nonnitol [15,19]. The calditol headgroup has an adaptive function for survival in acidic environments [13]. Apart from ether-linked lipids, crenarchaeal membranes contain other isoprenoids such as respiratory quinones. The abundance of these quinones is correlated with pH, temperature, and salinity in archaea [20]. Essentially, quinones with short acyl chains are correlated with thermophiles, whereas the presence of long-acyl chains is correlated with salinity [20]. Based on these correlations, quinones have been proposed as putative membrane regulators of archaea [20]. The respiratory quinones of S. acidocaldarius include caldariellaquinones (CQs) and sulfoquinones (SQs) [21]. Biochemical studies have recognized CQs as a pool of reducing factors for cytochromes in the electron transport chain [22,23,24]. Notably, the distribution of CQs is restricted to Sulfolobales, and their higher mid-redox potential (compared to menaquinones) could be an adaptation to strictly aerobic metabolism [20,21]. The complete biosynthetic pathway for CQs remains to be elucidated; however, GGR from S. acidocaldarius is known to accept GGPP as a substrate (Figure 1) [6]. This leads to the formation of phytyl diphosphate, which has been proposed as a precursor for CQs [6].
Radiolabeling assays with the crenarchaeon Thermoplasma acidophilum have indicated the accumulation of DGDs upon treatment with terbinafine, a squalene epoxidase inhibitor in fungi [25]. This effect was not observed in Halobacterium salinarum, which has a membrane lacking GDGTs [25]. It has been suggested that this compound inhibits tetraether synthase, which is now identified as Tes [25]. The target of terbinafine in the fungal membrane is squalene epoxidase, an FAD-dependent enzyme involved in the ergosterol biosynthesis pathway [26]. Molecular dynamics simulations have indicated that the lipophilic part of terbinafine binds to the central cavity of squalene epoxidase and induces conformational changes that block the substrate from entering the binding site [26]. Since archaea do not synthesize sterols, the target(s) and mechanism of action of terbinafine remain elusive. Additionally, studies with a comprehensive lipidome analysis and the cellular responses of archaea to terbinafine are absent from the literature. In this study, the impact of terbinafine on the lipidome of S. acidocaldarius was determined, and transcriptomic analysis was conducted to gain insights into the cellular responses of crenarchaeotes to terbinafine. Furthermore, the high concentrations of terbinafine required for growth inhibition suggest that the inhibitory mechanism might be more complex than originally anticipated in targeting specifically the tetraether synthase.
2. Results
2.1. Terbinafine Causes the Accumulation of DGDs and Depletion of DGTs in S. acidocaldarius Membranes
S. acidocaldarius MW001 was grown in the presence of various concentrations of terbinafine in Brock medium at 75 °C, pH 3.0 with aeration. Terbinafine inhibited S. acidocaldarius growth in a concentration-dependent manner (Figure 2). The lipidome of the MW001 strain was analyzed during the exponential and stationary phases of growth in the absence and presence of terbinafine (Figure 3, Table 1). The accumulation of inositol phosphate dialkyl glycerol diether lipid (IP-DGD, m/z 893.685 [M-H]−) was observed in exponential and stationary phase cells (Figure 3 and Figure 4) [15,27]. IP-DGD was the only diether lipid that showed consistent accumulation across growth phases (Figure 3 and Figure 4). The levels of DGGGP (m/z 715.507 [M-H]−) and archaetidic acid (AA, m/z 731.632 [M-H]−) were slightly elevated during the exponential phase; however, they were depleted during the stationary phase (Figure 3 and Figure 4) [15,27]. The lipidome of S. acidocaldarius was examined for GDGTs with various cyclopentane rings (0–8) [27]. A slight decrease in the levels of GDGT-1 to 3 was observed in the exponential phase of growth with terbinafine (Supplementary Figure S1). Meanwhile, GDGT-1 to 6 showed slightly decreased levels in the stationary phase of growth with terbinafine (Supplementary Figure S1).
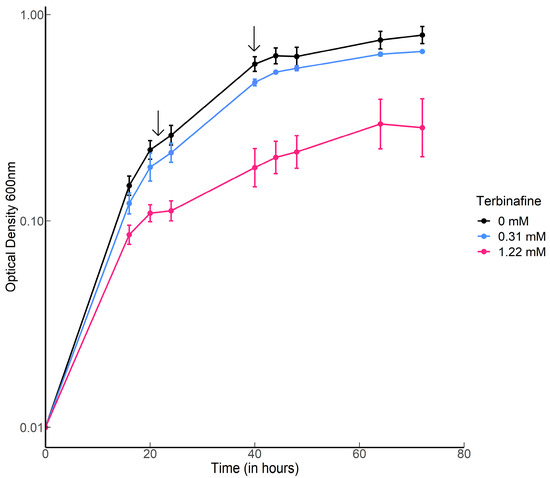
Figure 2.
Growth curves of S. acidocaldarius with and without terbinafine: Error bars represent the standard error of the mean. Black arrows indicate the time points for harvesting the cells for lipid extraction.
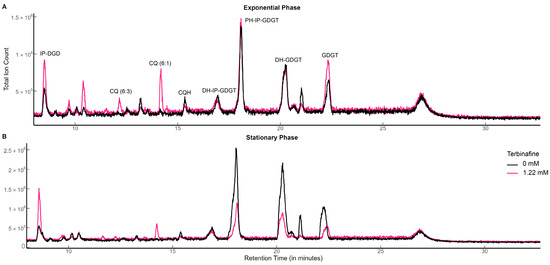
Figure 3.
Total ion chromatograms of S. acidocaldarius lipid extracts with and without terbinafine: (A) exponential phase and (B) stationary phase. TIC was normalized to the amount of lipids using the internal standard DOPG.

Table 1.
Ppm error of the lipid species observed in this study.
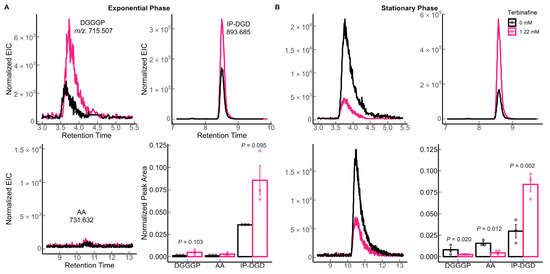
Figure 4.
Effect of terbinafine on the bilayer-forming membrane lipids (DGDs) of S. acidocaldarius: Extracted ion chromatograms and relative quantification of DGGGP (digeranylgeranylglyceryl phosphate), AA (archaetidic acid), IP-DGD (inositol phosphate dialkyl glycerol diether) in (A) exponential phase and (B) stationary phase of growth. Error bars represent the standard error of the mean and p-values are indicated for each lipid species. The dots represent biological replicates. The theoretical m/z [M-H]– values are listed in the figure. The extracted ion chromatograms (EICs) were normalized to the amount of lipids using the internal standard DOPG.
A reduction in levels of all abundant GDGTs including di-hexose inositol phosphate GDGT 0–6 (m/z 1866.433–1854.339 [M-H]−), penta-hexose inositol phosphate GDGT-0 to 6 (m/z 1542.327–1530.233, [M-H]−), di-hexose GDGT—0 to 6 (m/z 1670.419–1658.293, [M + CHO2−]−), and GDGT- 0 to 6 (m/z 1346.314–1334.220, [M + CHO2−]−) was observed only in the stationary phase (Figure 5B) [15,27]. Interestingly, this relative reduction in GDGT levels was not observed during the exponential growth phase (Figure 5A).
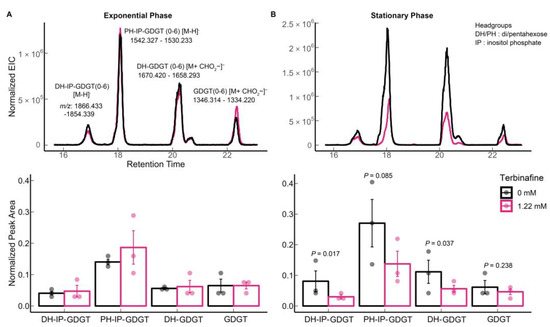
Figure 5.
Effect of terbinafine on monolayer forming membrane lipids (GDGTs) of S. acidocaldarius: Extracted ion chromatograms and relative quantification of tetraether lipids in (A) exponential phase and (B) stationary phase of growth. Error bars represent the standard error of the mean, and p-values are indicated for each lipid species. The numbers in parentheses indicate the number of cyclopentane rings. The dots represent biological replicates. The theoretical m/z values are shown in the figure. The EICs values were normalized to the amount of lipids using the internal standard DOPG.
2.2. Terbinafine Interferes with the Respiratory Complex in S. acidocaldarius
To examine the early cellular responses of S. acidocaldarius on terbinafine-induced growth inhibition, the transcriptomic response was evaluated after three hours of growth with terbinafine at concentrations of 0.31 mM and 1.22 mM. At 0.31 mM terbinafine, only five genes were upregulated (at p-adjusted values < 0.05): aceA (isocitrate lyase), argG-H (argininosuccinate synthase and lyase), leu2 (3-isopropylmalate dehydratase large subunit), and saci_2320 (predicted glutamate synthase) (Supplementary Figure S2) [28]. In S. solfataricus, the uptake of glutamate, leucine, and isoleucine from the medium is the highest among all observed amino acids [28]. Simulations indicated that leucine undergoes incomplete degradation to 3-methyl-2-butenoate, which was detected in the culture supernatant [28]. The biosynthesis and degradation of amino acids may be potential targets for lower concentrations of terbinafine.
Meanwhile, 260 genes were differentially expressed with 1.22 mM terbinafine. These genes were mapped to the arCOG database. The density plot (Figure 6A) illustrates the categorization of these genes based on arCOGs. Most of the differentially expressed genes (p-adjusted value < 0.05) were mapped to the arCOG pathway ‘Energy production and conversion’, as illustrated by the column graph (Figure 6B). S. acidocaldarius is a strict aerobe which conserves energy by a proton driven chemiosmotic gradient [29]. Transcript levels of sdhA, sdhC, and nuoD were elevated, which are part of the succinate dehydrogenase (SDH) and NADH complexes responsible for reducing quinones [22,30] (Figure 7). This respiratory chain has three terminal oxidase complexes: SoxABCDL, SoxEFGHIM, and DoxBCE [31]. The transcript levels of soxA-C, soxG-I, and doxB-C were also elevated (Figure 7). The ATP synthase complex consists of nine subunits: atpA-I [23]. Transcript levels of atpA and atpB were elevated (Figure 7). This was accompanied by depleted transcript levels of proposed acyl-CoA dehydrogenases, elevated levels of acetoacyl-CoA acetyltransferase and enoyl-CoA hydratase (Supplementary Figure S3).
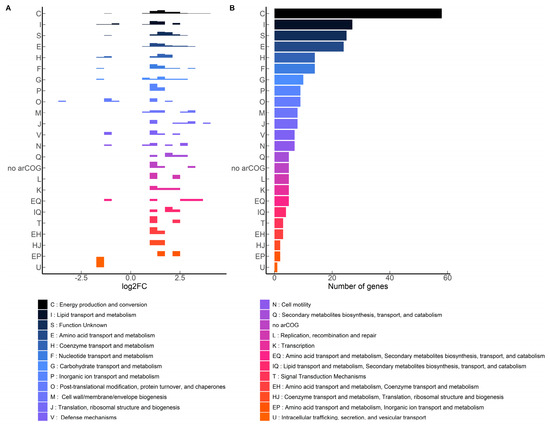
Figure 6.
arCOG mapping of significantly affected MW001 genes with terbinafine (p-adjusted < 0.05): (A) Density plot illustrating log2 fold change distribution of genes, (B) Tally of genes mapping to the arCOG categories.
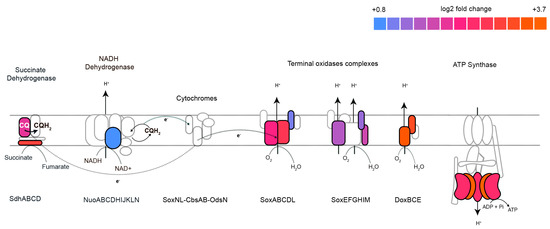
Figure 7.
Effect of terbinafine on the transcription of the S. acidocaldarius respiratory complex: Colors represent log2 fold change values. No color indicates significantly unaffected transcript levels. All log2 fold-change values are p-adjusted < 0.05. CQ refers to caldariellaquinone.
2.3. A Shift in the Saturation Levels of Caldariellaquinone
Caldariellaquinones (CQ) act as electron and proton carriers in the respiratory chain of thermoacidophiles [29,31]. Sulfolobus predominantly synthesizes two classes of benzothiophenones: sulfoquinones and CQs [32]. They are found only in saturated forms of crenarchaeotes [21]. S. solfataricus alters its quinone composition depending on temperature, carbon source, and availability of oxygen [32]. Specifically, the total quinone content increases with increasing aeration [32]. Only the saturated species of caldariellaquinone (CQH, m/z: 630.451 [M]) were detected in the lipid extracts of S. acidocaldarius without terbinafine (Figure 8) [21]. Monosaturated (CQ 6:1, m/z 627.428, [M-H-]−), di-saturated (CQ 6:2, m/z 625.412, [M-H-]−), and tri-saturated (CQ 6:3, m/z 623.396, [M-H-]−) species and reduced levels of CQH were observed in the exponential and stationary phase lipid extracts after growth with terbinafine (Figure 8). For CQH, fragments corresponding to the loss of the headgroup were observed, as reported previously (Figure 8C) [21]. The polyunsaturated species, CQ 6:1 and CQ 6:3, showed additional product ions related to the loss of parts of the isoprenoid chain (Figure 8C), which has been reported for other quinones, such as menaquinone (MK) [21].
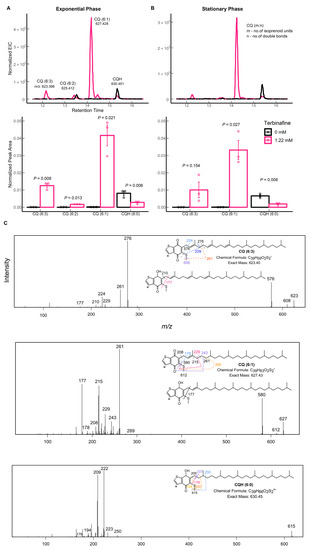
Figure 8.
Altered saturation of caldariellaquinone (CQ) under the influence of terbinafine: (A) extracted ion chromatogram (top) and relative quantification of peak areas (bottom) in the exponential phase of growth in S. acidocaldarius and (B) stationary phase. Error bars represent the standard error of the mean, and p-values are indicated for each lipid species. The dots represent biological replicates. (C) Fragmentation patterns of the observed CQ species. The EICs values were normalized to the amount of lipids using the internal standard DOPG.
An MK-specific reductase from Archaeoglobus fulgidus was shown to alter the MK saturation profile in Escherichia coli [33]. WP_011278262 (saci_1431) in S. acidocaldarius is a homolog of this enzyme with 29% sequence identity. The transcript levels of this gene were unaffected. GGPP is a proposed common precursor of isoprenoids such as CQs and membrane lipids [34]. The transcript levels of GGGPS were unaffected (Figure 9), and GGPP could not be detected in the lipid extracts of S. acidocaldarius.
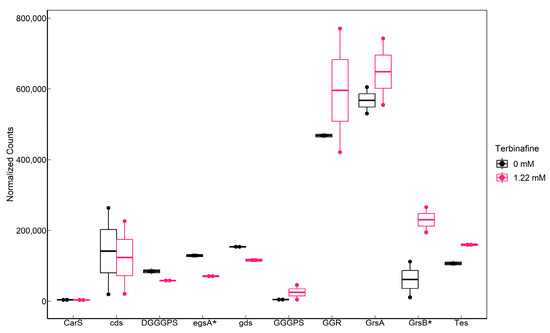
Figure 9.
Transcriptomic response of lipid biosynthesis genes in S. acidocaldarius to terbinafine: Significantly affected genes (p-adjusted < 0.05) are indicated by an asterisk (*) on the x-axis. Counts were normalized for sequencing depth using the mean ratio method in DESeq2. cds: saci_1498 (calditol synthase), carS: Saci_0897 (CDP-archaeol synthase), DGGGPS: Saci_1565 (Digeranylgeranylglycerol phosphate synthase), egsA: Saci_0640 (Glycerol-1-phosphate dehydrogenase), gds: Saci_0092 (geranylgeranylpyrophosphate synthase), GGGPS: Saci_0728 (geranylgeranylglycerolphosphate synthase), GGR: Saci_0986 (geranylgeranyl reductase), grsA: Saci_1585 (GDGT cyclization A), grsB: Saci_0240 (GDGT cyclization B), and Tes: Saci_0703 (tetraether synthase).
2.4. Influence of Terbinafine on the Expression of Phospholipid Biosynthesis Genes
RNA sequencing data indicated that terbinafine caused a significant elevation in saci_0240 (grsB) transcript levels, which has been identified as a GDGT cyclization enzyme in S. acidocaldarius [11] (Figure 9). However, cyclization of GDGTs was not significantly affected. A growth phase-dependent decrease was observed in the levels of GDGT-1 to 6 (Supplementary Figure S1). This could likely be a response of the membrane to energy availability and not necessarily due to the elevated transcript level of grsB [35]. Transcriptomic data also indicated significantly depleted levels of egsA (saci_0640), which is responsible for the NAD(P)H- dependent reduction of dihydroxyacetonephosphate (DHAP) to produce a glycerol-1-phosphate backbone for archaeal lipids (Figure 9). Interestingly, terbinafine did not significantly affect the transcript levels of saci_0703 (Figure 9), which was recently identified as tetraether lipid synthase (Tes) [10].
2.5. Cell Envelope and Motility
In S. acidocaldarius, motility is an ATP–dependent physiological process mediated by the archaellum [36,37]. It is a rotating-type IV pilus consisting of seven proteins (flaBFGHIJX) transcriptionally regulated by arnR, arnR1, and arnB [36,38,39,40]. The flaB promoter is induced by environmental stressors such as nitrogen starvation [41]. ArnR binds to the flaB promoter under nutrient-limitation conditions [40]. Both flaF and flaG are conserved components of the archaellum, localized in the membrane [36]. Meanwhile, flaH and flaI are predicted cytoplasmic components. Transcript levels of flaB, flaF, flaG, flaH, flaI, and arnR were elevated, whereas flaG was reduced in the presence of terbinafine (Supplementary Figure S4). In addition, the transcript levels of slaA in the cell envelope were elevated (Supplementary Figure S4) [42].
3. Discussion
The lipid membranes of thermoacidophilic organisms are highly adaptive owing to the extreme environments in which these organisms thrive [11,13,15,35,43,44]. Terbinafine has been proposed as a tetraether lipid biosynthesis inhibitor in archaea based on radiolabeled [2-14C]mevanolic acid incorporation studies [25]. However, the concentrations needed for inhibition are substantially higher than those required in eukaryotes, i.e., millimolar versus micromolar, respectively. Therefore, it remains uncertain whether terbinafine is a specific inhibitor of tetraether lipid biosynthesis or whether it has global inhibitory effects on cells. Therefore, the cellular response of S. acidocaldarius to this compound was studied. The inhibitory effect of terbinafine on growth and GDGT biosynthesis was consistent with the results of a study in T. acidophilum [25]. However, transcriptomic analysis has shown that growth inhibition is accompanied by altered expression of several subunits of the respiratory system. In addition to altering the distribution of GDGTs over IP-DGD, terbinafine inhibition, the precursor for GDGT biosynthesis, also leads to a shift of the CQs to unsaturated species and, to a lesser extent, a reduced level of cyclization of the GDGTs.
Terbinafine inhibits squalene epoxidase in fungi [26]. Squalene epoxidase is involved in the biosynthesis of ergosterols, a membrane regulator responsible for the fluidity and permeability of membranes [45]. In bacteria, hopanoids have similar function [46]. Both sterols and hopanoids can induce ordered phases in lipid membranes [20]. Sterols and hopanoids have not been reported in archaea. Possible candidates for these functions in archaea could be other polyterpenes: carotenoids, polyprenols, quinones, and polyisoprenoids [20,47]. Therefore, it is possible that isoprenoids like the CQs belong to the targets of terbinafine in archaea. The shift towards unsaturated CQs species starts to occur in the exponential phase and continues in the stationary phase. Respiratory quinones in archaea have been proposed to serve as membrane regulators [20]. Hyperthermophiles, in particular, harbor quinones with short acyl chains, such as CQs, which could aid in membrane packing and the improvement of lipid chain order [20]. It is difficult to assess the significance of saturated CQs in S. acidocaldarius because little is known about these isoprenoids. Unsaturated CQs could influence the fluidity of the membrane as well as the ordered phases of the membrane. So far, the distribution of CQs is known to be restricted to Sulfolobales [20,21,32,48]. The only known archaeal quinone reductase is from Archaeglobus fulgidus which solely synthesizes menaquinones (MK) [21,33]. This class of enzymes is paralogous to archaeal GGRs, and their multiplicity in genomes indicates that such enzymes could be responsible for the saturation of other isoprenoids, such as CQs [33]. S. acidocaldarius harbors five such uncharacterized paralogs to GGR which are clustered into arCOG00570. Thus, these paralogs could be responsible for altering the saturation profile of the CQs.
The FadRsa cluster has predicted acyl-CoA dehydrogenases, 3-hydroxyalkyl-CoA-dehydrogenase, and acetyl-CoA acetyltransferase in S. acidocaldarius which are involved in the synthesis of acetoacetyl-CoA, a precursor of the alternate mevalonate pathway in archaea [2,49]. The altered transcript levels of these genes may indicate a potential bottleneck in the synthesis of the isoprenoid building blocks—IPP and DMAPP (Supplementary Figure S3). However, the transcript levels of most genes involved in the ether lipid biosynthesis pathway remained unaffected by terbinafine inhibition, whereas the precursor GGPP could not be detected in the lipidome (Figure 9).
Our study confirms the growth phase-dependent depletion of GDGTs in the presence of terbinafine in S. acidocaldarius, as previously observed for T. acidophilum [25]. Significant DGT depletion was observed only in stationary-phase cells, indicating that alteration of the lipidome upon terbinafine inhibition is a slow event. Additionally, the transcript levels of the recently identified tetraether lipid synthase, Tes, were not affected after three hours of growth with terbinafine. Taken together, these findings suggest that depletion of GDGTs may be a secondary effect of terbinafine on the lipidome. IP-DGD is the predominant fully saturated diether in the membrane of Sulfolobus, and its amounts are consistent across growth phases [2,17]. Unsaturated archaetidylglycerol (AG) has been proposed as a precursor for tetraether lipids in T. acidophilum based on radiolabeling assays [50]. Remarkably, IP-DGD was the only diether lipid that increased in level upon addition of terbinafine (Figure 4). The discovery of Tes has fully not resolved the question as to whether the precursor molecule needs to be saturated or (partially) unsaturated; however, purified Tes protein from Methanosarcina acetivorans shows a preference for the fully saturated diether species in a lipid extract [9].
The archaeal membrane maintains metabolic processes through energy-transducing processes such as the transfer of electrons/protons or ATPase-dependent translocation of ions [51]. The altered transcript levels of the FadRsa cluster, respiratory chain complex, grsB, archaellum, and slaA in the cell envelope indicate that terbinafine induces a multitude of responses in S. acidocaldarius related to energy metabolism. The synthesis of tetraether lipids and formation of cyclopentane rings are key adaptations that regulate rigidity and homeostasis [2,51,52,53]. Membranes formed with higher levels of IP-DGD, unsaturated CQs, and lower levels of tetraether lipids might be less rigid and have higher basal permeability. The surface protein layer (S-layer) is a major component of archaeal cell walls and is, therefore, critical for survival [42]. SlaA is an impetus for the assembly of the S-layer [42]. An elevation in its transcript levels under the influence of terbinafine could indicate a structural deformity of the cell envelope [54]. This could also explain the terbinafine-induced growth inhibition observed in S. acidocaldarius. The presence of unsaturated CQs in the membrane, along with the upregulation of genes involved in the respiratory complex, indicates that the flow of electrons is disrupted. Furthermore, the altered expression of the F1F0-ATPase subunits suggests a possible problem with ATP synthesis in cells. A possible explanation for these membrane adaptations and transcriptome responses to terbinafine is that the organism adjusts to this respiratory stress by redirecting its metabolic energy to increase motility through the archaellum and not by investing its net reducing power in the saturation of CQs or synthesizing complex GDGTs. However, proteomics analysis and assessment of the impact of terbinafine on the respiratory capabilities of the organism may further support this conclusion.
This study describes the cellular response of S. acidocaldarius to terbinafine. Terbinafine has a multitude of effects on S. acidocaldarius, one of which is the altered DGD: GDGT ratio in the membrane. Considering its effects on the respiratory chain and saturation of CQs, terbinafine appears to be an indirect inhibitor of GDGT biosynthesis. The role of other isoprenoids in archaeal membrane architecture, and their regulatory mechanisms remain elusive. Furthermore, knowledge of quorum sensing in S. acidocaldarius is limited to lactonase, and the sensors responsible for membrane adaptations are not known [55]. Therefore, the present studies on archaeal membrane adaptations rely on the correlation of changes in lipid species with the environments inhabited by these organisms. CQs have not been quantified in the Sulfolobus membrane so far, making it difficult to speculate on any possible structural role of these isoprenoids. This is the first study to show that an allylamine drug—like terbinafine—affects the saturation profile of respiratory quinone (CQ) in the membrane of S. acidocaldarius. This drug causes respiratory stress in organisms, causing a redirection of its metabolism towards energy conservation and motility. This could explain the reduced levels of complex phospholipids, such as GDGTs, in the membrane.
4. Materials and Methods
4.1. Strains and Growth Conditions
S. acidocaldarius MW001 was grown at 75 °C by shaking at 120 rpm in Brock medium, pH 3 supplemented with 0.1% NZ-amine, 0.2% dextrin, and 10 µg/mL uracil. As indicated, various amounts of a stock solution containing 250 mg/mL terbinafine hydrochloride (Sigma-Aldrich Chemie NV, Zwijndrecht, the Netherlands; final concentrations of 0.31, 0.61, and 1.22 mM which are in the same range as the earlier study with T. acidophilum [25]) dissolved in DMSO were added such that its concentration in the medium did not exceed 0.16%. Growth curves were generated from 5 mL cultures (in biological triplicates), and growth was monitored in time at OD600 nm up to 72 h. The initial inoculums were at an OD600 nm of 0.01.
4.2. RNA Isolation, Sequencing and Transcriptome Analysis
For RNA isolation, S. acidocaldarius MW001 cultures (50 mL) were grown in duplicate until the exponential phase (OD600 nm of 0.3) and then subjected to terbinafine (0.31 mM and 1.22 mM) or DMSO treatment (0.16%) for 3 h. Cultures were harvested (Allegra X-R benchtop cooled centrifuge, 3000× g, 20 min, 4 °C), and cells were processed further to isolate RNA using the TRIzol method [43]. The obtained RNA was purified by ethanol precipitation. Ribosomal RNA depletion, library preparation, quantification, and RNA sequencing were performed by GenomeScan BV, Leiden. Ribosomal RNA was depleted using the NEBNext rRNA depletion kit (Bioke, New England BioLabs Inc., Leiden, the Netherlands) for bacteria. Sequencing was performed using paired-end reads of 150 bp, with a sequencing depth of 20 million reads. The sequenced reads were aligned against the S. acidocaldarius DSM639 genome (RefSeq ID: NC_007181.1) using STAR [56]. These reads were mapped and visualized using multiQC for quality control [57]. Differential gene expression analysis was performed using DESeq2 [58]. Adjusted p-values (<0.05) were used for data analysis to reduce the false discovery rate (FDR). The results of differential gene expression from DESeq2 were mapped to the archaeal cluster of orthologous genes (arCOGs) and then manually revised [59]. Figures were constructed using standard graphics editing software based on MetaboMAPS [60].
4.3. Lipid Extraction and Analyses
S. acidocaldarius MW001 cultures were inoculated in 200 mL of Brock medium supplemented with either 0.16% DMSO or 1.22 mM terbinafine in biological triplicates. Cultures were harvested by centrifugation (3000× g, 4 °C, 20 min) at the exponential (22 h) and early stationary phases (40 h) of growth. The pellets were washed with fresh Brock medium, centrifuged again, flash-frozen in liquid nitrogen, and freeze-dried at 0.07 mbar, −55 °C for 48 h. Freeze-dried biomass (10 mg) was processed for lipid extraction using a modified acidic Bligh and Dyer method [61] using 0.1 M HCl. Di-oleoylphosphatidylglycerol (DOPG, 5 µg) was added as an internal standard at the beginning of the Bligh and Dyer extraction method. This internal standard was used to normalize the total lipid content of each sample for relative quantification. The chloroform fraction obtained from Bligh and Dyer extraction was evaporated under a N2 stream to form a lipid film, re-extracted with chloroform, and dried again [27]. This step was repeated twice using 1:2 chloroform-methanol and methanol [27]. The obtained lipid film was weighed (MS104TS/00, Mettler Toledo BV, Tiel, the Netherlands) and dissolved in methanol (0.25 mg/mL) for UHPLC-MS analysis. The analysis was performed on an Accela1250 UHPLC system (Thermo Fisher Scientific, Breda, the Netherlands) coupled to a Thermo Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, Breda, the Netherlands) equipped with an ESI ion source in negative mode. Sulfolobus lipids were separated using an Acquity UPLC CSH C18 column (2.1 × 5 mm, 1.7 μm) with an eluent flow rate of 300 µL/min at 55°C. A scan range of m/z 125–2500 was used in the full MS mode. The voltage parameters were 3 kV (spray), −75 V (capillary), −190 V (tube lens), and −46 V (Skimmer). Eluent A was MilliQ water (Millipore Plus System, resistivity 18.2 mΩ.cm): MeCN (40:60), and Eluent B was MilliQ water: MeCN:1-BuOH (0.5:10:90), both containing 5 mM ammonium formate (pH 6.5) [30]. A linear elution gradient was used: 55/45 eluent A/B for 2.5 min, 10/90 eluent A/B from 2.5 to 24.5 min, and returning to 55/45 eluent A/B for 25–33 min. Spectra were acquired in parallel-reaction-monitoring mode for the inclusion list masses (calculated for negative mode) at low resolution, followed by MS/MS fragmentation of the targeted precursor ions with a resolution of 17,500. The collision energy potential was set at 30 or 60 V. Lipid species were identified by their molecular weight, retention time, and fragmentation analysis using an in-house calculated in-silico lipid database for archaea [30]. Peak areas were integrated and calculated with the genesis algorithm using Xcalibur software (Thermo Fisher) with a 5 ppm mass window for peak detection. The obtained fragmentation spectra were compared and validated with published studies [6]. Extracted ion chromatograms were visualized using xcms and MSNbase packages in R [62,63]. Statistical significance was determined using the Student’s T-test in R 4.2.1 [64].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24087328/s1.
Author Contributions
Conceptualization, A.R. and A.J.M.D.; investigation, A.R. and N.A.W.d.K.; formal analysis, A.R. and N.A.W.d.K.; writing—original draft preparation, A.R.; writing—review and editing, all authors; visualization, A.R.; supervision, A.J.M.D.; funding acquisition, A.J.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Groningen and the Netherlands Organization for the Advancement of Science (NWO) through the “BaSyC—Building a Synthetic Cell” Gravitation grant (024.003.019) of the Ministry of Education, Culture, and Science.
Data Availability Statement
All the data in this study have been provided in the manuscript, and the raw data are available as part of the Supplementary Information.
Acknowledgments
The authors thank Hjalmar Permentiar and Wim Huibers for the MS-MS analysis of CQs. Mirthe Hoekzema for technical help during the LC-MS run of S. acidocaldarius lipid samples. Jeroen Nijland for assistance with the experimental setup of the transcriptomic study. Alejandra Recalde and Bianca Wassmer for the growth experiments with S. acidocaldarius.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jain, S.; Caforio, A.; Driessen, A.J.M. Biosynthesis of archaeal membrane ether lipids. Front. Microbiol. 2014, 5, 641. [Google Scholar] [CrossRef]
- Rastädter, K.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. The cell membrane of Sulfolobus spp.—Homeoviscous adaption and biotechnological applications. Int. J. Mol. Sci. 2020, 21, 3935. [Google Scholar] [CrossRef]
- Koga, Y.; Ohga, M.; Tsujimura, M.; Morii, H.; Kawarabayasi, Y. Identification of sn-glycerol-1-phosphate dehydrogenase activity from genomic information on a hyperthermophilic archaeon, Sulfolobus tokodaii strain 7. Biosci. Biotechnol. Biochem. 2006, 70, 282–285. [Google Scholar] [CrossRef][Green Version]
- Ren, S.; de Kok, N.A.W.; Gu, Y.; Yan, W.; Sun, Q.; Chen, Y.; He, J.; Tian, L.; Andringa, R.L.H.; Zhu, X.; et al. Structural and Functional Insights into an Archaeal Lipid Synthase. Cell Rep. 2020, 33, 108294. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Caforio, A.; Fodran, P.; Lolkema, J.S.; Minnaard, A.J.; Driessen, A.J.M. Identification of CDP-Archaeol Synthase, a Missing Link of Ether Lipid Biosynthesis in Archaea. Chem. Biol. 2014, 21, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Murakami, M.; Yoshimura, T.; Hemmi, H. Specific partial reduction of geranylgeranyl diphosphate by an enzyme from the thermoacidophilic archaeon Sulfolobus acidocaldarius yields a reactive prenyl donor, not a dead-end product. J. Bacteriol. 2008, 190, 3923–3929. [Google Scholar] [CrossRef]
- Sasaki, D.; Fujihashi, M.; Iwata, Y.; Murakami, M.; Yoshimura, T.; Hemmi, H.; Miki, K. Structure and mutation analysis of archaeal geranylgeranyl reductase. J. Mol. Biol. 2011, 409, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Caforio, A.; Yang, Q.; Sun, B.; Yu, F.; Zhu, X.; Wang, J.; Dou, C.; Fu, Q.; Huang, N.; et al. Structural and mechanistic insights into the biosynthesis of CDP-archaeol in membranes. Cell Res. 2017, 27, 1378–1391. [Google Scholar] [CrossRef]
- Lloyd, C.T.; Iwig, D.F.; Wang, B.; Cossu, M.; Metcalf, W.W.; Boal, A.K.; Booker, S.J. Discovery, structure and mechanism of a tetraether lipid synthase. Nature 2022, 609, 197–203. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, H.; Yang, H.; Chen, Y.; Yang, W.; Feng, X.; Pei, H.; Welander, P.V. Identification of a protein responsible for the synthesis of archaeal membrane-spanning GDGT lipids. Nat. Commun. 2022, 13, 1545. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Delago, A.; Nußbaum, P.; Meyer, B.H.; Albers, S.-V.; Eichler, J. Gene deletions leading to a reduction in the number of cyclopentane rings in Sulfolobus acidocaldarius tetraether lipids. FEMS Microbiol. Lett. 2018, 365, fnx250. [Google Scholar] [CrossRef]
- Yang, W.; Chen, H.; Chen, Y.; Chen, A.; Feng, X.; Zhao, B.; Zheng, F.; Fang, H.; Zhang, C.; Zeng, Z. Thermophilic archaeon orchestrates temporal expression of GDGT ring synthases in response to temperature and acidity stress. Environ. Microbiol. 2022, 25, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, X.-L.; Wei, J.H.; Summons, R.E.; Welander, P.V. Calditol-linked membrane lipids are required for acid tolerance in Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA 2018, 115, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Schouten, S.; Van Der Meer, M.T.J.; Hopmans, E.C.; Rijpstra, W.I.C.; Reysenbach, A.L.; Ward, D.M.; Damsté, J.S.S. Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in hot springs of Yellowstone National Park. Appl. Environ. Microbiol. 2007, 73, 6181–6191. [Google Scholar] [CrossRef]
- Jensen, S.M.; Neesgaard, V.L.; Skjoldbjerg, S.L.N.; Brandl, M.; Ejsing, C.S.; Treusch, A.H. The Effects of Temperature and Growth Phase on the Lipidomes of Sulfolobus islandicus and Sulfolobus tokodaii. Life 2015, 5, 1539–1566. [Google Scholar] [CrossRef]
- Tourte, M.; Schaeffer, P.; Grossi, V.; Oger, P.M. Membrane adaptation in the hyperthermophilic archaeon Pyrococcus furiosus relies upon a novel strategy involving glycerol monoalkyl glycerol tetraether lipids. Environ. Microbiol. 2022, 24, 2029–2046. [Google Scholar] [CrossRef]
- Quehenberger, J.; Pittenauer, E.; Allmaier, G.; Spadiut, O. The influence of the specific growth rate on the lipid composition of Sulfolobus acidocaldarius. Extremophiles 2020, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Driessen, A.J.M.; Albers, S. Membrane Adaptations of (Hyper)Thermophiles to High Temperatures. In Physiology and Biochemistry of Extremophiles; Gerday, C., Glansdorff, N., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 104–116. [Google Scholar]
- Lo, S.-L.; Montague, C.E.; Chang, E.L. Purification of glycerol dialkyl nonitol tetraether from Sulfolobus acidocaldarius. J. Lipid Res. 1989, 30, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Castell, M.; Tourte, M.; Oger, P.M. In search for the membrane regulators of archaea. Int. J. Mol. Sci. 2019, 20, 4434. [Google Scholar] [CrossRef] [PubMed]
- Elling, F.J.; Becker, K.W.; Könneke, M.; Schröder, J.M.; Kellermann, M.Y.; Thomm, M.; Hinrichs, K.-U. Respiratory quinones in Archaea: Phylogenetic distribution and application as biomarkers in the marine environment. Environ. Microbiol. 2016, 18, 692–707. [Google Scholar] [CrossRef]
- Janssen, S.; Schäfer, G.; Anemüller, S.; Moll, R. A succinate dehydrogenase with novel structure and properties from the hyperthermophilic archaeon Sulfolobus acidocaldarius: Genetic and biophysical characterization. J. Bacteriol. 1997, 179, 5560–5569. [Google Scholar] [CrossRef]
- Reviews, F.M.; Femsre, E.; Sch, G.; Anemtiller, S. Electron transport and energy conservation in the archaebacterium Sulfolobus acidocaldarius. FEMS Microbiol. Lett. 1990, 75, 335–348. [Google Scholar]
- Whit, A.V.; Kingston, D.G.I.; Niehaus, W.G. Biosynthesis of Caldariellaquinone in Sulfolobus Acidocaldarius. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 1991. [Google Scholar]
- Kon, T.; Nemoto, N.; Oshima, T.; Yamagishi, A. Effects of a squalene epoxidase inhibitor, terbinafine, on ether lipid biosyntheses in a thermoacidophilic archaeon, Thermoplasma acidophilum. J. Bacteriol. 2002, 184, 1395–1401. [Google Scholar] [CrossRef]
- Nowosielski, M.; Hoffmann, M.; Wyrwicz, L.S.; Stepniak, P.; Plewczynski, D.M.; Lazniewski, M.; Ginalski, K.; Rychlewski, L. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J. Chem. Inf. Model. 2011, 51, 455–462. [Google Scholar] [CrossRef] [PubMed]
- De Kok, N.A.W.; Exterkate, M.; Andringa, R.L.H.; Minnaard, A.J.; Driessen, A.J.M. A versatile method to separate complex lipid mixtures using 1-butanol as eluent in a reverse-phase UHPLC-ESI-MS system. Chem. Phys. Lipids 2021, 240, 105125. [Google Scholar] [CrossRef]
- Stark, H.; Wolf, J.; Albersmeier, A.; Pham, T.K.; Hofmann, J.D.; Siebers, B.; Kalinowski, J.; Wright, P.C.; Neumann-Schaal, M.; Schomburg, D. Oxidative Stickland reactions in an obligate aerobic organism-amino acid catabolism in the Crenarchaeon Sulfolobus solfataricus. FEBS J. 2017, 284, 2078–2095. [Google Scholar] [CrossRef]
- Schäfer, G.; Moll, R.; Schmidt, C.L. Respiratory enzymes from Sulfolobus acidocaldarius. Methods Enzymol. 2001, 331, 369–410. [Google Scholar] [CrossRef]
- Wakao, H.; Wakagi, T.; Oshima, T. Purification and properties of NADH dehydrogenase from a thermoacidophilic archaebacterium, Sulfolobus acidocaldarius. J. Biochem. 1987, 102, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Auernik, K.S.; Kelly, R.M. Identification of components of electron transport chains in the extremely thermoacidophilic crenarchaeon Metallosphaera sedula through iron and sulfur compound oxidation transcriptomes. Appl. Environ. Microbiol. 2008, 74, 7723–7732. [Google Scholar] [CrossRef]
- Nicolaus, B.; Trincone, A.; Lama, L.; Palmieri, G.; Gambacorta, A. Quinone Composition in Sulfolobus solfataricus Grown under Different Conditions. Syst. Appl. Microbiol. 1992, 15, 18–20. [Google Scholar] [CrossRef]
- Hemmi, H.; Takahashi, Y.; Shibuya, K.; Nakayama, T.; Nishino, T. Menaquinone-specific prenyl reductase from the hyperthermophilic archaeon Archaeoglobus fulgidus. J. Bacteriol. 2005, 187, 1937–1944. [Google Scholar] [CrossRef]
- Hemmi, H.; Ikejiri, S.; Yamashita, S.; Nishino, T. Novel medium-chain prenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 2002, 184, 615–620. [Google Scholar] [CrossRef]
- Zhou, A.; Chiu, B.; Weber, Y.; Elling, F.; Cobban, A.; Pearson, A.; Leavitt, W. Energy flux controls tetraether lipid cyclization in Sulfolobus acidocaldarius. Environ. Microbiol. 2019, 22, 744623. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.V.; Jarrell, K.F. The archaellum: How Archaea swim. Front. Microbiol. 2015, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Hartung, S.; Van Der Does, C.; Tainer, J.A.; Albers, S.V. Archaeal flagellar ATPase motor shows ATP-dependent hexameric assembly and activity stimulation by specific lipid binding. Biochem. J. 2011, 437, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lassak, K.; Peeters, E.; Wróbel, S.; Albers, S.-V. The one-component system ArnR: A membrane-bound activator of the crenarchaeal archaellum. Mol. Microbiol. 2013, 88, 125–139. [Google Scholar] [CrossRef]
- Hoffmann, L.; Anders, K.; Bischof, L.F.; Ye, X.; Reimann, J.; Khadouma, S.; Pham, T.K.; Van Der Does, C.; Wright, P.C.; Essen, L.O.; et al. Structure and interactions of the archaeal motility repression module ArnA-ArnB that modulates archaellum gene expression in Sulfolobus acidocaldarius. J. Biol. Chem. 2019, 294, 7460–7471. [Google Scholar] [CrossRef]
- Bischof, L.F.; Haurat, M.F.; Albers, S.V. Two membrane-bound transcription factors regulate expression of various type-IV-pili surface structures in Sulfolobus acidocaldarius. PeerJ 2019, 2019, e6459. [Google Scholar] [CrossRef]
- Lassak, K.; Neiner, T.; Ghosh, A.; Klingl, A.; Wirth, R.; Albers, S.-V. Molecular analysis of the crenarchaeal flagellum. Mol. Microbiol. 2012, 83, 110–124. [Google Scholar] [CrossRef]
- Gambelli, L.; Meyer, B.H.; McLaren, M.; Sanders, K.; Quax, T.E.F.; Gold, V.A.M.; Albers, S.V.; Daum, B. Architecture and modular assembly of Sulfolobus S-layers revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 2019, 116, 25278–25286. [Google Scholar] [CrossRef]
- Bischof, L.F.; Haurat, M.F.; Hoffmann, L.; Albersmeier, A.; Wolf, J.; Neu, A.; Pham, T.K.; Albaum, S.P.; Jakobi, T.; Schouten, S.; et al. Early Response of Sulfolobus acidocaldarius to Nutrient Limitation. Front. Microbiol. 2019, 9, 3201. [Google Scholar] [CrossRef]
- Shimada, H.; Nemoto, N.; Shida, Y.; Oshima, T.; Yamagishi, A. Effects of pH and temperature on the composition of polar lipids in Thermoplasma acidophilum HO-62. J. Bacteriol. 2008, 190, 5404–5411. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The multifunctional fungal ergosterol. mBio 2018, 9, e01755-18. [Google Scholar] [CrossRef]
- Rohmer, M. A Mevalonate-independent Route to Isopentenyl Diphosphate. In Comprehensive Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 1999; pp. 45–67. [Google Scholar]
- Sáenz, J.P.; Sezgin, E.; Schwille, P.; Simons, K. Functional convergence of hopanoids and sterols in membrane ordering. Proc. Natl. Acad. Sci. USA 2012, 109, 14236–14240. [Google Scholar] [CrossRef]
- Collins, M.D.; Langworthy, T.A. Respiratory Quinone Composition of Some Acidophilic Bacteria. Syst. Appl. Microbiol. 1983, 4, 295–304. [Google Scholar] [CrossRef]
- Wang, K.; Sybers, D.; Maklad, H.R.; Lemmens, L.; Lewyllie, C.; Zhou, X.; Schult, F.; Bräsen, C.; Siebers, B.; Valegård, K.; et al. A TetR-family transcription factor regulates fatty acid metabolism in the archaeal model organism Sulfolobus acidocaldarius. Nat. Commun. 2019, 10, 1542. [Google Scholar] [CrossRef]
- Nemoto, N.; Shida, Y.; Shimada, H.; Oshima, T.; Yamagishi, A. Characterization of the precursor of tetraether lipid biosynthesis in the thermoacidophilic archaeon Thermoplasma acidophilum. Extremophiles 2003, 7, 235–243. [Google Scholar] [CrossRef]
- Van de Vossenberg, J.L.C.M.; Driessen, A.J.M.; Konings, W.N. The essence of being extremophilic: The role of the unique archaeal membrane lipids. Extremophiles 1998, 2, 163–170. [Google Scholar] [CrossRef]
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef]
- Hanford, M.J.; Peeples, T.L. Archaeal Tetraether Lipids: Unique Structures and Applications. Appl. Biochem. Biotechnol. 2002, 97, 45–62. [Google Scholar] [CrossRef]
- Zhang, C.; Phillips, A.P.R.; Wipfler, R.L.; Olsen, G.J.; Whitaker, R.J. The essential genome of the crenarchaeal model Sulfolobus islandicus. Nat. Commun. 2018, 9, 4908. [Google Scholar] [CrossRef]
- Bzdrenga, J.; Hiblot, J.; Gotthard, G.; Champion, C.; Elias, M.; Chabriere, E. SacPox from the thermoacidophilic crenarchaeon Sulfolobus acidocaldarius is a proficient lactonase. BMC Res. Notes 2014, 7, 333. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Archaeal clusters of orthologous genes (arCOGs): An update and application for analysis of shared features between thermococcales, methanococcales, and methanobacteriales. Life 2015, 5, 818–840. [Google Scholar] [CrossRef]
- Neumann-Schaal, M.; Koblitz, J.; Schomburg, D. MetaboMAPS: Pathway sharing and multi-omics data visualization in metabolic context. F1000Research 2020, 9, 288. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Gatto, L.; Gibb, S.; Rainer, J. MSnbase, Efficient and Elegant R-Based Processing and Visualization of Raw Mass Spectrometry Data. J. Proteome Res. 2021, 20, 1063–1069. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2022; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).