Abstract
Integrins are a group of heterodimers consisting of α and β subunits that mediate a variety of physiological activities of immune cells, including cell migration, adhesion, proliferation, survival, and immunotolerance. Multiple types of integrins act differently on the same immune cells, while the same integrin may exert various effects on different immune cells. In the development of cancer, integrins are involved in the regulation of cancer cell proliferation, invasion, migration, and angiogenesis; conversely, integrins promote immune cell aggregation to mediate the elimination of tumors. The important roles of integrins in cancer progression have provided valuable clues for the diagnosis and targeted treatment of cancer. Furthermore, many integrin inhibitors have been investigated in clinical trials to explore effective regimens and reduce side effects. Due to the complexity of the mechanism of integrin-mediated cancer progression, challenges remain in the research and development of cancer immunotherapies (CITs). This review enumerates the effects of integrins on four types of immune cells and the potential mechanisms involved in the progression of cancer, which will provide ideas for more optimal CIT in the future.
1. Introduction
Integrins, a superfamily of cell adhesion receptors, bind to extracellular matrix (ECM) ligands and cell surface ligands to mediate physiological activities [1]. Integrins are composed of a transmembrane α subunit and β subunit, with 18 α subunits and 8 β subunits currently known, constituting 24 heterodimers in humans that are divided into four categories: RGD receptors, leucocyte-specific receptors, collagen receptors, and laminin receptors [1] (Figure 1). Each heterodimer is generated from a combination of a large extracellular domain, a transmembrane domain, and a short cytoplasmic domain linked to the cytoskeleton [2]. Integrins induce inside-out and outside-in signaling pathways by transitioning from an inactive to an active state based on their structure [1]. Indeed, the binding and dissociation of the cytoplasmic domain of α and β subunits are confirmed to determine the state of integrins [1]. In the inside-out pathway, chemokines transmit signals through G protein-coupled receptors to phosphorylate the cytoplasmic domain of the β subunit [1]. Meanwhile, binding of the cytoskeletal cohesion protein talin to the cytoplasmic tail of a β subunit results in the dissociation of α and β tails and induces conformational changes in the extracellular region that increase the affinity of integrins for their ligands [1]. In the outside-in pathway, ligand binding induces conformational changes to integrins clustered on the plasma membrane and separates their heads and tails, leading to the interaction of the cytoplasmic tail domain with intracellular signaling molecules [1].
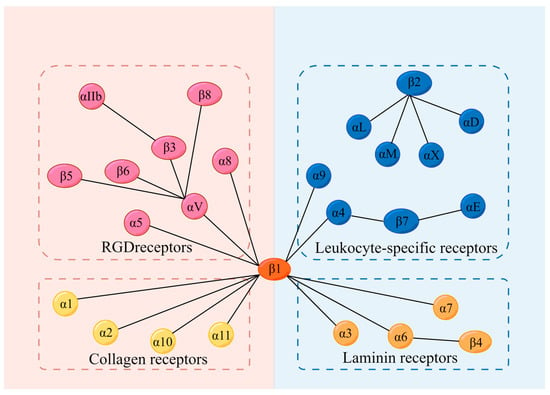
Figure 1.
Integrins and their 4 categories of ligands; 18 α subunits and 8 β subunits constitute 24 integrins in humans, which can be divided into 4 categories according to their receptors. They are RGD receptors, leucocyte-specific receptors, collagen receptors, and laminin receptors. RGD: Arg-Gly-Asp.
Integrins regulate various physiological or pathological processes in diverse cells [1,3]. Immune cells contain integrins that regulate cellular activity to maintain immunity and homeostasis [4,5,6,7]. However, dysregulation of integrins contributes to immune-related diseases such as multiple sclerosis (MS), arthritis, cancer, and certain diseases involving the interaction of immune cell integrins with their receptors, including Alzheimer’s and Parkinson’s diseases, pulmonary pathologies (such as asthma and chronic obstructive pulmonary disease), and atherosclerosis [8,9,10,11,12,13]. Notably, the binding of integrins to various ligands on the surface of immune cells or cancer cells promotes or inhibits the occurrence and development of cancers. Based on comprehensive analyses of numerous studies, this paper summarizes three precancerous mechanisms related to integrins: mediating the overgrowth of cancer cells, promoting invasion and metastasis of cancer cells, and generating immune tolerance effects by the inhibition of immune cell responses [14,15,16]. However, integrins also inhibit cancer through diverse mechanisms [17,18]. The pronounced correlation of integrins with underlying cancer mechanisms has led to substantial research on integrins in the field of cancer therapy. Currently, three main integrin-based therapies are in development: chimeric antigen receptor (CAR) T-cell therapy, oncolytic virotherapy, and immune checkpoint inhibitor (ICI) therapy. With the role of integrins in the development of immune cells and cancer becoming clear, more cancer therapeutic approaches will be likely to be developed to support the expansion of oncotherapy (Table 1).

Table 1.
Integrins, their ligands, and major functions.
2. Potential Influence of Integrins on Immune Cells
2.1. B Cells
Multiple types of integrins regulate the activities of B cells in humoral immunity, such as adhesion, migration, and proliferation. As an outside-in signaling mechanism, it has been reported that αX integrins on tonsillar B cells bound to fibrinogen induce cell proliferation, adhesion, and migration [87]. As an inside-out signaling mechanism, integrins were shown to be activated by talin-1, a key integrin-coactivator, which is critical in the migration of B cells into lymph nodes [88]. Similarly, Hart et al. indicated that αLβ2 integrins that are stimulated by the integrin-activated protein kindlin enhanced the binding of αLβ2 integrins to the intercellular adhesion molecule 1 (ICAM-1), thereby mediating the movement of B cells [89]. In addition, some experiments have confirmed the role of integrins in lymphocyte intestinal homing, which eliminates pathogenic microorganisms and maintains intestinal immune tolerance via intestinal mucosal immunity. Research has shown that the recruitment of Shp1 by CD22, a lectin of B cells, inhibited β7 integrin endocytosis to promote the binding of β7 integrins with mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which is expressed on postcapillary high endothelial venules in Peyer’s patches and mesenteric lymph nodes, thus initiating the intestinal homing of B cells [90,91]. Moreover, Ballet et al. highlighted that β7 integrins played a significant role in homing lymphocytes to gut-associated lymphoid tissue (GALT) via the CD22-Shp1 phosphatase axis, which ultimately facilitated the movement of B cells to the lamina propria of GALT [90].
Numerous studies have shown that integrins mediate the immune response of B cells, such as the synthesis of antibodies and antigen presentation. Through the function of the actin-binding protein vinculin, integrins on the B-cell surface maintain the stabilization of immune synapses, a transient structure centered on the TCR-MHC-antigenic peptide, to favor antigen presentation between B cells and T cells [7]. Additionally, Waffarn et al. demonstrated that the activation of αM integrins by type I interferon directly stimulated the adhesion and rapid redistribution of B lymphocytes to regional mediastinal lymph nodes, and this effect mediated the synthesis and accumulation of protective IgM at the site of infection [92]. Furthermore, the distribution of α4β1 integrins in the plasma membrane is induced by CD37, which is found in the germinal center (GC) of the spleen [93]. The gathered α4β1 integrins bind to vascular cell adhesion molecule 1 (VCAM-1) to activate the phosphatidylinositol-3-kinase (PI3K) signaling pathway and then phosphorylate the lipids on the plasma membrane to form the second messenger phosphatidylinositol (3,4,5)-triphosphate (PIP3), which activates the AKT signaling pathway [94]. The AKT signaling pathway ultimately mediates cell survival, thereby inhibiting apoptosis and increasing the production of plasma cells [93,94]. Likewise, toll-like receptor 9 (TLR9) also triggers the interaction of α4β1 integrins with VCAM-1, which promotes the retention and homing of circulating B cells in secondary lymphoid organs, and homing of the post-germinal central memory B cells into bone marrow or mucosal tissues to differentiate into plasma cells [95]. Research showed that IgA was secreted into the enteric cavity directly by the docking between αEβ7+ plasma cells and E-cadherin-expressing enterocytes, which identified αEβ7 integrins as an important target for the treatment of inflammatory bowel disease and provides new approaches for drug research and development, such as with natalizumab and vedolizumab. However, integrins also inhibit B-cell-mediated immune processes. It has been demonstrated that the deficiency of αV integrins promoted TLR signaling in GC B cells and enhanced the response to TLR ligand-containing antigens and influenza viruses, which led to affinity maturation of GC B cells and facilitated the generation of memory and plasma B cells, resulting in strong, high-affinity, and long-lived antibody responses [96].
Several autoimmune diseases have close relationships with integrins. As the most common central nervous system (CNS) demyelinating disease, MS is stimulated by the strong recruitment activity of β1 integrins. High levels of β1 integrins facilitate the accumulation of B cells in the central nervous system and the recruitment of Th17 and macrophages. These cells attack the myelin essential proteins and cause the destruction of the insulating sheath of nerve tissues, thereby leading to the onset of MS [8]. Nonetheless, some integrins also inhibit the occurrence of autoimmune diseases. In systemic lupus erythematosus (SLE), binding of αM integrins with CD22 downregulated B-cell receptor signaling to maintain autoreactive B-cell tolerance and further inhibited SLE progression [97]. Additionally, αV integrins prevented the development of SLE by inhibiting the interaction of TLRs on B cells with self-DNA and RNA [98]. Moreover, in non-autoimmune diseases, the fibronectin on bone marrow stromal cells in non-Hodgkin’s lymphoma patients adhered to α4β1 integrins on B cells, which can protect B cells from rituximab-induced apoptosis and conferred drug resistance [99]. Integrins in normal lymphocytes can also increase the susceptibility to viral infections. In Epstein-Barr virus (EBV) infection, the EBV adheres to nasal mucosa-associated lymphoid tissue memory B cells through the binding of glycoprotein BMRF-2 with α5β1 integrins, which inhibits the immunological function of B cells [100].
Integrins participate in regulating the activities of B cells in humoral immunity, such as adhesion, migration, and proliferation. Furthermore, they also mediate the immune response of B cells, including the synthesis of antibodies and antigen presentation.
2.2. T Cells
Many significant activities of T cells, as a pivotal cell type in humoral and cellular immunity, are induced by integrins. Recent studies have shown that integrins mediate T-cell production and persistence [101]. It has been demonstrated that α4β1 integrins within tetraspanin-enriched microdomains shifted into a high-affinity state and accumulated through regulation of both CD9 and CD151, which promoted the activation of T cells [102]. Bromley et al. indicated that α1 integrins promoted the formation and persistence of CD8+ tissue-resident memory T cells and their abilities to secrete cytokines [101]. Moreover, the interaction of α5β1 integrins with CD154, which is an important marker of CD4+ T-cell activation, inhibited Fas/FasL signaling pathway-mediated CD4+ T and CD8+ T-cell death [103,104], and it decreased the production of caspase-8, all of which indicates that α5β1 integrins play an important role in T-cell survival and persistence in inflammatory diseases [103,104].
Research has elucidated that integrins can induce the movement of T cells, including their adhesion, migration, and accumulation at different sites. It has been reported that the flow of actin within T cells is regulated by the interaction of TCRs, integrins, and extrinsic signals [105]. T-cell activation mediated by TCR and ligand binding promotes actin centripetal movement, initiating T-cell movement [105]. This centripetal movement promoted integrin-ligand binding (αLβ2 integrins to ICAM-1 and α4β1 integrins to VCAM-1), thus reducing tyrosine in the TCR signaling pathway, which slowed actin centripetal flow and cell movement [105]. Numerous studies demonstrated that αLβ2 integrins facilitated the adhesion and movement of different T-cell types. Adel et al. showed that αLβ2 integrins were regulated by Galectin-9 to support naive CD4+ and CD8+ T-cell migration across endothelial cells in a glycan-dependent manner [106]. Similarly, research indicated that αLβ2 integrins regulated naive T-cell homing and controlled the inflammatory response through the Janus kinases 1 and 2 signaling pathways [107]. The binding of αLβ2 integrins with ICAM-1 induced the phosphorylation of protein tyrosine kinases (Lck) and zeta chain associated protein of 70 kDa (ZAP-70), which further promoted the conversion of intermediate-affinity αLβ2 integrins to high-affinity αLβ2 integrins, thereby increasing their binding with ICAM-1 [108,109]. Through the regulation of high-affinity αLβ2 integrins, the αLβ2/Lck/ZAP-70 complex initiated rapid adhesion and migration of T lymphocytes after encountering infection or injury stimuli [108,109]. In addition, β1 integrins are essential for memory CD4+ T-cell retention at different sites [110]. DeNucci et al. indicated that α1β1 integrins promoted the retention of CD4+ T cells in the bone marrow, lung, and epidermis [110]. Similarly, the synthesis of β1 and β7 integrins was increased by the stimulation of the proinflammatory cytokine HMGB1, which promotes the migration and adhesion of CD4+ T cells to endothelial cells [10]. Integrins facilitate CD4+ T-cell migration away from the lymph node subcapsular sinus and homing to lymph nodes [111]. Additionally, some regulating factors, such as kindlin-3 and all-trans-retinoic acid (ATRA), can activate integrins or upregulate the expression of integrins. The interaction between talin-1 and kindlin-3 and the tension generated by actin polymerization induced and stabilized the activities of β2 integrins and mediated the binding of integrins to ICAM-1, thereby facilitating the activation and migration of T cells [112,113]. Moreover, ATRA induced the upregulation of α4β7 integrins and the mucosal-homing receptor CCR9 by binding to retinoic acid receptors, which promoted T-cell migration and homing to the mucosal surface [114]. Furthermore, integrins also play an important role in regulating T-cell immunological function. Studies showed that the binding of αV integrins and αLβ2 integrins to ICAM-1 can favor antigen presentation [5,115]. αEβ7 integrins expressed on T cells regulate the migration of intestinal stem cells to interact with different types of immune cells and maintain intestinal homeostasis by interacting with E-cadherin [116].
The effect of integrins on T-cell immune activities determines its association with the occurrence and development of certain diseases. It was confirmed that β1 integrins caused the entry of Th17 cells into the CNS, which contributes to MS [117]. TNF-α and IL-1α induced the expression of melanoma cell adhesion molecules (MCAM), and the MCAM/CD146 signaling pathway triggered the phosphorylation of PLCg1, which led to the activation of β1 integrins on memory T cells and enhanced the infiltration of Th17 cells into the brain [117]. Similarly, it has been reported that α4 integrins promoted the entry of Th1 cells into the CNS to cause MS [118]. Additionally, Kanayama et al. indicated that the binding of tenascin-C and osteopontin with α9 integrins on dendritic cells (DCs) and macrophages in cooperation with TLR signaling promoted the production of IL-6 and IL-23, leading to the differentiation of naive CD4+ cells into Th17 cells and the development of arthritis [9]. Additionally, Bachsais et al. showed that α5β1 integrins on T-cell leukemia-lymphoma cells bound to CD154 and inhibited apoptotic events in leukemia cell lines through the activation of pro-survival pathways, including the p38, ERK1/2, and PI3K/Akt signaling pathways [119]. However, certain types of integrins prevent damage caused by immune cells or immunocompetent substances by promoting the generation, maintenance, and function of Tregs or by inhibiting the function of effector T cells. It has been demonstrated that integrins were linked to the cytoskeleton upon activation by talin and further recruited phosphatidylinositol phosphate kinase to maintain Treg homeostasis and peripheral tolerance, preventing autoimmunity [120]. Braun et al. indicated that the lack of αE integrins reduced the expression of FoxP3 in FoxP3+ Treg cells, which increased and recruited hapten-primed effector CD8+ T cells to sites of skin inflammation and draining lymph nodes, and enhanced the contact hypersensitivity reaction [121]. In addition, the expression of αV integrins on Tregs mediates TGF-β activation and inhibits the autoimmune response of effector T cells, promoting the accumulation of Tregs in the inflammatory gut, which plays an important role in inhibiting the development of colitis [122,123] (Figure 2).
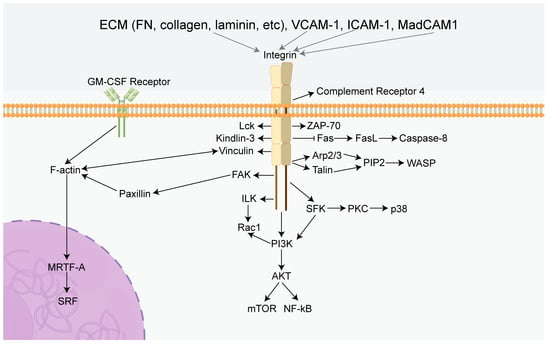
Figure 2.
Summary diagram of the effects of integrins in immune cells. Integrins can mediate several classical signaling pathways, including PI3K/AKT/mTOR, ILK/Rac1, and Fas/FasL. Activation of these specific pathways leads to a cascade reaction inducing the characteristics of immune cells, including proliferation, survival, migration, adhesion, and immunotolerance. FN: Fibronectin, GM-CSF Receptor: Granulocyte-macrophage colony-stimulating factor receptor, FAK: Focal adhesion kinase, PI3K: Phosphatidylinositol 3-phosphokinase, AKT: Protein kinase B, mTOR: Mammalian target of rapamycin, ILK: Integrin-linked kinase, Rac1: Rac Family Small GTPase, Fas: TNF receptor superfamily, FasL: Ligand of TNF receptor superfamily, PIP2: phosphatidylinositol (1,2,3) -triphosphate, Arp2/3: Actin nucleating protein complex, WASP: Wiscott aldridge syndrome protein, MRTF-A: Myocardin-related transcription factor-A, SRF: Serum response factor, SFK: SRC-Family protein tyrosine kinase, VCAM-1: Vascular cell adhesion molecule 1, ICAM-1: intercellular adhesion molecule 1, MadCAM1: Mucosal address in cell-adhesion molecule-1, Lck: Protein tyrosine kinase, ZAP-70: Zeta chain associated protein of 70 kDa, PKC: Protein kinase C, NF-kB: Nuclear factor-k-gene binding.
Integrins play an important role in T-cell production and movement, including adhesion, migration, and gathering in various regions. At the same time, they are closely related to certain diseases, such as MS, arthritis, and T-cell leukemia.
2.3. Dendritic Cells
It is widely acknowledged that DCs are the most functional specialized antigen-presenting cells (APC) in the human body, and integrins play a significant role in their functional implementation, including migration, adhesion, maturation, and immunoregulation [124,125,126,127,128,129,130]. Previous studies showed that β2 integrins affected the podosome formation of DCs through their Ser 745 and Ser 756 amino acid residues [131], which correlated with actin-linkage of the GM-CSF receptor on DCs in the presence of kindlin-3 [130], and further influenced the migration of DCs. Similarly, Sie et al. indicated that α4 integrins differentially promoted the accumulation of DCs in the CNS and gut but had no effects on other parts of the body [127]. In addition, it has been reported that deficiency of kindlin-3 disrupted F-actin polymerization in DCs and inhibited MRTF-A nuclear shuttling, which prevented MRTF-A from co-activating genes with SRF in the nucleus, thereby affecting the adherent DC phenotype and integrin-mediated traction production [132]. Moreover, studies have confirmed that FAK and its downstream signaling molecules promoted β1 integrin-mediated production of mature ALDH1A2high DCs, which indicates the role of β1 integrins in the differentiation and maturation of DCs activated by retinoic acid (RA) [133].
Numerous studies highlighted that integrins make substantial contributions to the immunological function of DCs, specifically in antigen uptake, antigen presentation, immunoregulation, and immunotolerance induction, which plays a critical role in connecting innate immunity with adaptive immunity. Researchers have indicated that αXβ2 integrins mediated phagocytosis of DCs activated by CCL21 in lymphatic endothelium by binding with the complement iC3b, which caused endocytosis and presentation of antigens [134]. Furthermore, it has been demonstrated that apoptotic cells promoted the expression of αV integrins on myeloid DC precursors, which activated TGF-β1 and further promoted CD103 expression and immunomodulatory functions [124]. Similarly, Duhan et al. have shown that αE integrins blocked virus-induced production of IFN-1 in conventional dendritic cells by inhibiting AKT activation and the mTOR signaling pathway to perform immunoregulatory functions [128].
Additionally, the involvement of integrins in various autoimmune diseases induced by abnormalities of DCs has been confirmed [126,133,135,136,137,138]. β1 integrins have been reported to promote the production of RA enzyme ALDH1A2 in GM-CSF-induced DCs through linkage with TLRs to effectively alleviate colitis [133]. Moreover, αVβ8 integrins on DCs initiated a CD4+ T-cell type 2 immune response activated by a high level of TGF-β and RA factors in the intestinal microenvironment and the TLR signaling pathway [125,139], thereby exposing mice to chronic intestinal parasite infection [135]. αVβ8 integrins also promoted IL-1β-induced and allergen-induced airway remodeling and secretion of CCL2 and CCL20 in adult fibroblasts through activation of TGF-β, leading to chronic obstructive pulmonary disease [136]. During the pathogenesis of inflammation, integrins function differentially [137,138,140]. In neuroinflammation, α4β1 integrins promoted the accumulation of plasmacytoid DCs into the CNS to relieve symptoms [126]. In Rotavirus infection, intestinal ΒATF3-dependent cDCs were shown to express α5β8 integrins, which enhanced IgA response by activating TGF-β [138]. Based on the importance of integrins to DCs, integrins are a promising target in immunotherapy.
Integrins play a significant role in the functional activities of DCs, including migration, adhesion, maturation, and immunoregulation. Furthermore, integrins also make great contributions to the immunological function of DCs, specifically in antigen uptake, antigen presentation, immunoregulation, and immunotolerance induction.
2.4. Natural Killer Cells
Numerous studies have shown that natural killer (NK) cells are a type of lymphocyte that can kill target cells without relying on antigens and antibodies and have an important role in the body’s innate immunity. It has been reported that integrins have significant effects on NK cell functions, including synthesis, proliferation, secretion, migration, natural killing, and immunosurveillance. Research has indicated that β2 integrins induced the phosphorylation of T-cell receptor ζ (TCRζ) and spleen tyrosine kinase (Syk) by binding to ICAM-1, which ultimately led to phospholipase C phosphorylation and paxillin-dependent perforin polarization [141]. Additionally, it has been shown that when ICAM-1 is bound to β2 integrins, talin is redistributed to the β2 integrin junction site and initiates two signaling pathways, which promotes the adhesion and migration of NK cells [142,143]. By binding to ICAM-1, β2 integrins induce the recruitment of talin and the actin nucleating protein complex Arp2/3, the result of which was a local increase in PIP2 levels. Furthermore, increased PIP2 recruited WASP to the β2 integrin junction sites, and WASP promoted Arp2/3-mediated actin polymerization, which led to NK cell adhesion to target cells and generated activation signals that resulted in the polarization of the actin cytoskeleton [144]. Intriguingly, it has been demonstrated that the tetraspanin CD53 regulated the response of activated NK cell receptors while promoting the activation of β2 integrins, which inhibited the effector function of NK cells [145].
Notably, integrins play a vital role in the immunoregulatory processes of NK cells, such as natural killing and immunotolerance [141,146,147]. Studies have shown that β1 integrins and NKp30 together acted as specific recognition receptors to activate the SFK-PI3K signaling pathway; at the same time, β1 integrins acted through the integrin-linked kinase (ILK)-Rac1 signaling pathway, thereby allowing NK cells to kill Cryptococcus neoformans [146]. Furthermore, it has been reported that αVβ3 integrins inhibited the inflammatory responses of NK cells, which are essential for the maintenance of decidua homeostasis and immunotolerance [148].
The expression of integrins on NK cells is intimately associated with pathological states. Numerous studies showed that α2β1 integrins contribute to the accumulation and proliferation of NK cells, indicating a crucial role in antiviral resistance [149]. In addition, during chronic inflammation, β1 integrins combined with the NKG2D and TRAIL pathways to mediate cytotoxicity against autoreactive CD4+ T cells and to exert immunomodulatory effects resulting from the inhibitory functions of the CD94/NKG2A pathway [147]. Furthermore, previous research showed that IL-2 stimulated the expression of α4β7 integrins on NK cells, which participated in the myocardial infarction repair process via promoting TNF-α-stimulated endothelial cell proliferation, enhancing collagen synthesis and angiogenesis, thereby reducing fibrosis within the infarcted myocardium, which may provide a novel approach for the treatment of myocardial infarction [150].
Integrins have significant effects on NK cells, including synthesis, proliferation, secretion, migration, natural killing, and immunosurveillance.
2.5. Neutrophils
Numerous studies indicate that integrins play a role in adhesion, aggregation, and movement. Lai Wen et al. reported that activated β2 integrins are involved in many immune functions, including the formation of inflammatory synapses at the neutrophil and endothelial interface, cell rolling, transport, and microparticle generation during transport [151]. It has been demonstrated that integrins drove the activation of PI3K and ARAP3, key regulators of integrin inactivation, promoting local integrin inactivation and turnover of substrate adhesion [152]. In addition, β2 integrins mediated neutrophil cross-cell migration induced by Rap1b deletion, PI3K-AKT activation, and the enhancement of invadopodia-like protrusions [153]. Bromberger et al. showed that β1 and β3 integrins on neutrophils were activated by talin-1, which was recruited to the plasma membrane through direct Rap1/talin-1 interaction [154]. The activation of β1 and β2 integrins was found to be facilitated by Radil, a novel Rap 1 effector, which rapidly translocates from the cytoplasm to the plasma membrane in Rap1a-GTP dependent manner [155]. Additionally, research indicated that β2 integrins bind to talin-1 and kindlin-3 to mediate the aggregation of neutrophils [156,157]. Skap2 was shown to regulate actin polymerization as well as the binding of talin-1 and kindlin-3 to the cytoplasmic domain of β2 integrins, thus promoting the activation of β2 integrins and neutrophil recruitment [156]. Integrin-linked kinase (ILK) promoted the membrane targeting of protein kinase C (PKC) and chemokine-induced kinase activity, thereby promoting the binding of kindlin-3 to the cytoplasmic domain of β2 integrins, which induced binding of extend (E+) headpiece (H-) β2 integrins to ICAM-1 expressed on activated endothelial cells in cis [157,158]. β2 integrins facilitate the arrest, polarization, and crawling of neutrophils on ICAM-1 and ICAM-2, which is a prerequisite for neutrophils to cross the inflamed blood-brain barrier [159]. The binding activity of αMβ2 integrins to their ligand is regulated by protein disulfide isomerase (PDI), which promotes the recruitment of neutrophils to the sites of inflammation during vascular inflammation [160]. Silva et al. reported that the involvement of αMβ2 integrins in neutrophil accumulation was mediated by the activation of fibrin [161]. Moreover, several studies highlighted that the interaction of β2 integrins with cytohesin-1 and galectin-9 promoted neutrophil capture in human blood and adhesion to endothelial cells [162,163]. Pruenster et al. demonstrated that β2 integrin-dependent extracellular regulator MRP8/14 induced slow rolling and adhesion of neutrophils during inflammation in vivo [164]. Previous studies elucidated that the expression of β2 integrins on the plasma membrane of neutrophils is regulated by CD37, which promotes the cytoskeletal function of integrin-mediated adhesion downstream [165]. Furthermore, the dynamic redistribution of β2 integrins and non-muscle myosin (IIA) was shown to be coordinated by chemotactic signals generated by the eicosanoid leukotriene B4 (LTB4) and its receptor BLT1 to promote the arrest and extravasation of neutrophils and migration to the sites of inflammation [166]. In addition, numerous studies indicated that β1 and β3 integrins activated by vitronectin suppressed the apoptosis of neutrophils [167]. Roberta et al. showed that the binding of α9β1 integrins to VLO5, a snake venom disintegrin, induced the degradation of the pro-apoptotic protein Bad and the expression of the anti-apoptotic protein Bcl-xL, effectively reducing the spontaneous apoptosis of neutrophils [168].
Numerous studies have elucidated that integrins regulate neutrophil-associated inflammatory responses and immune killing, which can be the etiology of some diseases. It was reported that αLβ2 integrins triggered the extravasation of neutrophils into areas of the CNS with amyloid-β (Aβ) deposition, thereby initiating Alzheimer’s disease [169]. Research showed that αM integrins regulated by Rho GTPase Cdc42 in the uropod of neutrophils controlled the polarity of neutrophils and mediated neutrophil migration to the inflamed lungs [170]. Similarly, Herbert et al. reported that β2 integrins bound to ICAM-1 on epithelial cells, which mediated transepithelial migration of neutrophils during respiratory syncytial virus infection, causing airway damage [171]. It was demonstrated that the integrin-related G-protein coupled receptor (GPCR) signaling pathway activated neutrophils and induced the formation of neutrophil extracellular traps in acute lung injury [172]. Additionally, β2 integrins promoted the recruitment of neutrophils to lung tissue to cause inflammatory lung disease, which was inhibited by Siglec-E [173,174].
Integrins make great contributions to the adhesion, aggregation, and movement of neutrophils. Moreover, they also participate in neutrophil-associated inflammatory responses and immune killing.
2.6. Macrophages
Numerous studies have elucidated that integrins play a critical role in macrophages, a type of leukocyte belonging to the mononuclear phagocytic system, including their activation, adhesion, migration, phagocytosis, proinflammatory, and anti-inflammatory effects [175]. Research has shown that αV integrins contribute to the development of dry eye disease (DED) by binding with vitronectin (VTN) and activating NF-kB, which induces inflammatory gene expression in the bone marrow-derived macrophages [176]. Moreover, previous studies indicated that integrins controlled the activation and differentiation of macrophages. For example, α2β1 integrins inhibited the induction of M2 macrophages and maintained the macrophage shape [177,178]. Experiments have elucidated that αDβ2 and αMβ2 integrins are regarded as important targets controlling the balance between M1 and M2 macrophages, which is critical in the development of chronic inflammation [179]. In addition, it was reported that α1β1 integrins mediated adhesive interactions between macrophages and inflammatory lesions by binding with collagen IV in the ECM [180]. For example, a lipotoxic insult stimulated the phosphorylation of p38 in hepatocytes, causing hepatocytes to release β1 integrins as extracellular vesicles, which mediated macrophage adhesion to liver sinusoidal endothelial cells by binding with VCAM-1, leading to hepatic inflammation [181]. Additionally, numerous studies have shown that integrins participate in the molecular mechanism of macrophage migration to inflammatory sites through outside-in signaling pathways. For example, it has been reported that β1 integrins interacted with hydrogen sulfide (H2S) and activated the Src/FAK/Pyk2/Rac signaling pathways in macrophages, thereby promoting macrophage migration into an infarct area [182]. Additionally, α4β1 integrins have been reported to mediate macrophage recruitment to atherosclerotic plaques by binding with VCAM-1 on vascular endothelial cells [183]. Similarly, insulin-like growth factor-1 (IGF-1) expressed in atheroma bound with αVβ3 integrins activated the PI3 kinase/PKC/p38 signaling pathway, an essential step in the progression of atherosclerosis [184]. Osteopontin (OPN), as a potent chemokine, has been confirmed to affect macrophages via two distinct pathways. OPN binds with αVβ5 integrins on macrophages, which is critical for maintaining the M2 macrophage gene signature and development of mesenchymal glioblastoma; conversely, OPN could interact with α4 integrins to mediate macrophage chemotaxis [185,186].
Previous experiments highlighted that integrins have both pro- and anti-inflammatory roles in the inflammatory responses related to diseases. It has been reported that αDβ2 integrins on M1 macrophages interacted with the ECM of peripheral tissues, thereby enhancing the accumulation of pro-inflammatory macrophages at atheromas [187,188]. In the molecular mechanism of cardiac inflammation and hypertrophy, β2 integrin transcription was enhanced by interacting with integrin β2 (ITGβ2), which is a complex composed of KDM3A, MRTF-A, and Sp1, and thereby promoted the inflammatory responses [189]. In addition, studies have indicated that bile acids secreted by damaged biliary epithelial cells (BECs) promoted the recruitment of CCL2 and the activation of DAMPs. CCL2 then bound to CCR2 on macrophages and induced inflammation, which promoted the activation of TGF-β1 and led to portal fibrosis; at the same time, ITGF-β6 and ITGF-β6 ligands were upregulated, resulting in the proliferation of BECs [190]. Additionally, it has been reported that integrins in macrophages combine with other immune cells to contribute to diseases. For example, the expression of β2 integrins on macrophages induces the Rac1/reactive oxygen species (ROS)/non-canonical NLRP3 inflammasome/Prp-IL-1β/IL-1β signaling pathway. IL-1β binding to IL-1R on the surface of Group 3 innate cells promoted the expression of IL-22, which participated in the development of C.rodentium-induced colitis [191]. In contrast, numerous studies have indicated that integrins enhanced the anti-inflammatory functions of M2 macrophages. For example, β1 integrins were shown to induce anti-inflammatory effects on lung alveolar epithelial cells by inhibiting ROS production and downregulating NF-kB-dependent chemokines, such as CCL2, which mediated the recruitment of macrophages to attenuate lung inflammation and emphysematous remodeling [192]. In addition, previous experiments showed that αVβ5 integrins are involved in M2 macrophage polarization by interacting with PPARγ stimulated by convallatoxin, which increased the level of IL-10 and decreased the level of pro-inflammatory factors, such as IL-6 and TGF-α, thereby attenuating atherosclerosis [193]. It is also worth mentioning that αVβ3 integrins were shown to mediate anti-inflammatory functions of M2 macrophages via the PI3 kinase/AKT-dependent NF-kB signaling pathway, which is critical for the prevention of and recovery from inflammatory states [194].
Integrins play a critical role in macrophage biology, including activation, adhesion, migration, phagocytosis, proinflammatory and anti-inflammation effects, which are related to several diseases, such as hypertension, atherosclerosis, and lung inflammation.
3. The Roles of Integrins in Cancer
3.1. Positive Modulation of Integrins in Cancer Development
3.1.1. Evading Growth Suppressors
Integrins have been shown to play an important role in the overgrowth characteristics of cancer, which are mediated by a combination of genetic and environmental factors. Previous studies showed that αVβ3 integrins, as a receptor of thyroid hormone, mediated malignant T-cell angiogenesis by enhancing the expression and secretion of VEGFB and VEGFA, which in turn promoted endothelial cell migration and T-cell lymphoma proliferation [14]. In addition, it has been reported that α4β1 integrins on cancer cells promoted tumor growth by interacting with talin and paxillin, which are induced by the pro-inflammatory cytokine IL-1β and the chemokine stromal cell-derived growth factor 1 alpha (SDF-1α) [195]. Furthermore, β4 integrins promote the differentiation of cancer stem cells (CSCs) induced by differentiation antigen, which furthers cancer cell proliferation [196]. Notably, studies have elucidated that αVβ6 integrins were rapidly upregulated on epithelial cells during tissue injury and facilitated the differentiation of hepatic progenitor cells into bile duct cells and hepatocytes by activating TGF-β1, thereby promoting ductal fibrosis and, consequently, tumorigenesis [197,198]. Moreover, previous studies showed that αV integrins on M2-macrophages were activated via the TGF-β/smad2/3 signaling pathway, thereby mediating the stemness traits of pancreatic cancer cells [199]. Similarly, αVβ5 integrins on M2-macrophages secreted TGF-β and activated the Src/Stat3 signaling pathway, which promoted the maintenance of glioma stem cells (GSCs) and glioma growth [200] (Figure 3).
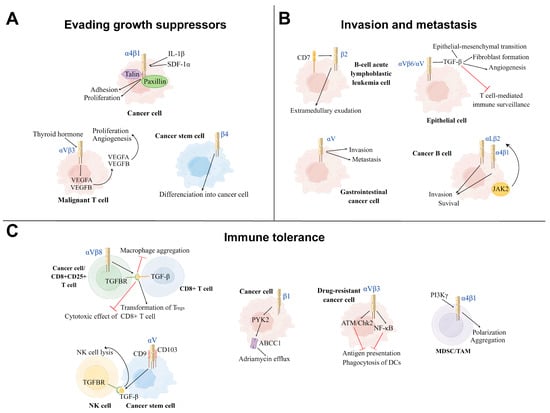
Figure 3.
Three main mechanisms of positive modulation of integrins on cancer development. (A): Evading growth suppressor: Integrins can achieve evading growth suppressor by promoting cancer cell proliferation, differentiation, aggregation, and angiogenesis in the tumor microenvironment (TME). Under the influence of thyroid hormone, αVβ3 Integrins promote the proliferation and angiogenesis of the malignant T cell. The cytokines IL-1β and SDF-1α induce α4β1 integrins to interact with talin and paxillin to facilitate tumor growth. β4 integrins promote the differentiation of cancer stem cells into tumor cells. (B): Invasion and metastasis: Integrins mediate the invasion and metastasis of different types of cancer cells to other sites, leading to disease deterioration. β2 integrins and αV integrins promote, respectively, the invasion and metastasis of B-cell acute lymphoblastic leukemia cells and gastrointestinal cancer cells. αV integrins on the surface of epithelial cells can reshape the TME via activating TGF-β. The cancer B-cell in B-cell chronic lymphocytic leukemia (B-CLL) increases its survival and invasion by the activation of αLβ2 and α4β1 integrins via the JAK2 pathway. (C): Immune tolerance: Part of integrins on cancer cells inhibit the function of immune cells from achieving immune tolerance. αVβ3 integrins on the drug-resistant cancer cells inhibit the function of DCs via ATM/Chk2 and NFKB-mediated pathways. αVβ3 and αV integrins induce the process of TGF-β and TGFBR binding to suppress the immune killing effect of CD8+ T cell and NK cell, respectively. α4 integrins activated by PI3Kγ induce the polarization and aggregation of myeloid-derived suppressor cells (MDSCs) and TAMs to inhibit T-cell-mediated anti-tumor immune responses. In human T-cell acute lymphoblastic leukemia (ALL), β1 integrins activate the ABCC1 drug transporter of adriamycin via the PYK2 pathway. TH: Thyroid hormone, SDF-1α: Stromal cell-derived growth factor 1 alpha, DCs: Dendritic cells, NK cell: Natural killer cell, TGFBR: TGF-β receptor, MDSC: myeloid-derived suppressor cell, TAM: Tumor-associated macrophage, PYK2: Proline-rich tyrosine kinase 2.
3.1.2. Invasion and Metastasis
Integrins are essential to cancer metastatic characteristics, which is one of the major challenges in the treatment of cancer. Research has shown that β2 integrins increased the adhesion and invasiveness of B-cell acute lymphoblastic leukemia cells by promoting extramedullary exudation [16]. In addition, αV integrins have been reported to be overexpressed in gastrointestinal cancer cells, which promote cancer cell invasion and metastasis, resulting in a poor prognosis for patients [201]. In addition, research demonstrated that αV integrins reshape the tumor microenvironment by activating TGF-β, resulting in increased epithelial-mesenchymal transition, angiogenesis and invasion, cancer-associated fibroblast formation, and suppression of T-cell-mediated immune surveillance [202]. One study showed that cancer cells also changed the tissue-specific homing patterns and the characteristics of immune cells by secreting exosomes that were rich in specific integrins, which contributed to the formation of a microenvironment that induced tumor occurrence. This process induced several biological functions, such as increased vascular permeability, angiogenesis, and chronic inflammation, which cultivated a microenvironment that allowed subsequent cancer-homing retention and growth [203]. Other findings revealed that in B-cell chronic lymphocytic leukemia (B-CLL), JAK2 on the surface of B cells promoted cell adhesion and survival in bone marrow stromal niches by activating αLβ2 and α4β1 integrins, mediating B-cell-dependent adhesion and depression in secondary lymphoid-like organs [204].
3.1.3. Immune Tolerance
The immune tolerance properties of cancer cells remain challenging in terms of clinical antitumor drug therapy, including immunological escape and drug efflux. Investigations elaborating the immune escape characteristics of cancer cells showed that αVβ3 integrins were upregulated on a variety of drug-resistant cancer cells, which induced immune tolerance and promoted immune escape via activating ATM/Chk2 and NF-kB-mediated pathways, thereby impairing the ability of DCs to cross-prime antigen-specific T lymphocytes [15]. Moreover, research demonstrated that the αVβ8 integrins on tumor cells promoted tumor-immune evasion via activating latent TGF-β on the surface of T cells, subsequently inducing the transformation of Tregs and thereby producing an immunosuppressive effect [93,205]. Moreover, it has been reported that αVβ6 integrins on CD4+CD25+ T cells also inhibited the cytotoxic effects of CD8+ T cells by activating TGF-β, which reduced pro-inflammatory tumor-associated macrophage aggregation towards tumors, thereby suppressing anti-tumor immunity [198,206,207]. Additionally, Tregs were shown to work in tandem with cancer cells to produce biologically active TGF-β and create an immunosuppressive microenvironment, the biological effect of which is tumor growth [208]. In the case of NK cells, as αV integrins on GSCs interacted with CD9 and CD103 on their surface, GSCs were induced to produce and release TGF-β due to the cell-to-cell contact, leading to impaired NK cell lysis and increased tumor infiltration. Additionally, several studies highlighted that α4 integrins activated by PI3Kγ promoted the polarization and aggregation of myeloid-derived suppressor cells (MDSCs) with immunosuppressive properties and tumor-associated macrophages in tumors during tumor progression, thereby inhibiting T-cell-mediated anti-tumor immune responses and promoting tumor progression [209]. Moreover, it has been reported that β1 integrins promoted adriamycin resistance in human T-cell acute lymphoblastic leukemia by activating Fak-related proline-rich tyrosine kinase 2 (PYK2), which in turn activated the ABCC1 drug transporter and facilitated adriamycin efflux [210].
Integrins positively modulate cancer development by supporting growth suppressor evasion, invasion, metastasis, and immune tolerance.
3.2. Negative Modulation of Integrins on Cancer Development
Integrins can serve as immune-binding targets for APCs. Previous research showed that αVβ3 integrins are upregulated in tumor cells resistant to various drugs in which ATM/Chk2-and NFκB-mediated pathways are activated and act as an immune target for DCs [15]. Several studies have highlighted that integrins promote the recruitment and infiltration of CD8+ T cells and CD8+ tissue-resident memory T cells (TRMs) in tumor tissues, enabling T cells to release cytokines and cytotoxic particles, which induce the apoptosis of tumor cells [211,212]. Additionally, αLβ2 and α4β1 integrins contribute to CD8+ T-cell recruitment, adhesion, spreading, cytolytic activity, co-stimulation, and infiltration in tumor tissues [18,213]. Mahurin et al. indicated that αLβ2 integrins colocalized with and were activated by layilin (a c-type membrane glycoprotein containing the lectin domain) on CD8+ T cells in human melanoma, which enhanced cell adhesion and CD8+ T-cell accumulation in tumors, and inhibited tumor growth [214]. It has been confirmed that TGF-β produced by activated immune cells activated αEβ7 integrins on CD8+ T cells, which enhanced αEβ7-dependent T-cell adhesion and signal transduction to induce the recruitment of CD8+ T cells to epithelial tumor islets [215]. TGF-β activates through αEβ7 integrins by binding between TGF-β and its receptors (TGFBR), which induces the recruitment and phosphorylation of ILK; ILK then interacts with the intracellular domain of αEβ7 integrins, leading to protein kinase B (PKB) activation, thus initiating integrin-based inside-out and outside-in signaling pathways [215]. Similarly, αEβ7 integrins facilitate the retention of CD3+ CD8+ TRMs, the cytotoxic immune synapses of which bind to specific cancer cells in epithelial tumor islands, leading to TCR-dependent target cell killing [211,212]. In addition, integrins can promote the interaction between TCRs of CD8+ T cells and tumor cells to enhance the function of cytotoxic T-lymphocytes. It has been demonstrated that αLβ2 integrins on tumor cells increase the binding power between cytotoxic T cells and tumor cells by binding to TCRs, thus promoting cytotoxic T cells to exert anti-tumor immune effects [216] (Figure 4).
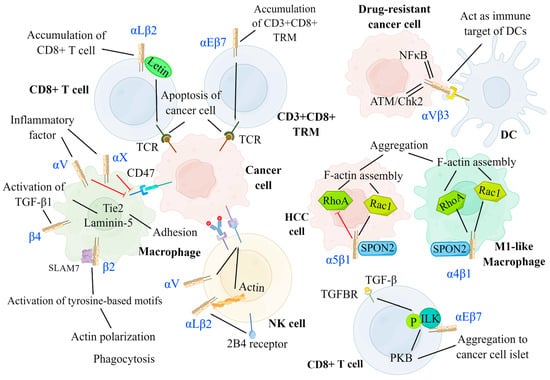
Figure 4.
Negative modulation of integrins on cancer development. Integrins can serve as an immune target for DCs to promote antigen presentation. By interacting with several signaling molecules, integrins facilitate the migration, aggregation, and killing of immune cells, including DCs, T cells, NK cells, and macrophages. DCs: αVβ3 integrins on drug-resistant cancer cells have increased expression for ATM/Chk2-and NF-κB-mediated pathways and act as the immune target of DCs. T cell: αLβ2 integrins on CD8+ T cell and αEβ7 integrins on CD3+CD8+ TRM separately induce the accumulation of CD8+ T cell and CD3+CD8+ TRM at the cancer region and promote the binding between TCR and cancer cell to result in cancer cell apoptosis. αEβ7 integrins on CD8+ T cells are activated by TGF-β via phosphorylated ILK and facilitate the aggregation of CD8+ T cells to cancer islets. NK cell: αLβ2 and αV integrins can enhance the cytotoxicity of NK cells by promoting the adhesion between NK cells and tumor cells and promoting ADCC, respectively. Macrophage: The expression of αL and αX integrins are promoted by inflammatory factors, which inhibit the amount of CD47 on cancer cells, thus preventing the function of checkpoint SIRPa-CD47. The signal of SPON2-α4β1 on M1-like macrophage activates both RhoA and Rac1 to facilitate its aggregation. Conversely, the signal of SPON2-α5β1 on HCC cells inhibits the aggregation of HCC cells by suppressing RhoA. CD3+CD8+ TRM: CD3+CD8+ tissue-resident memory T cells, NK cell: Natural killer cell, DC: Dendritic cell, TGFBR: TGF-β receptor, PKB: Protein kinase B, ILK: Integrin-linked kinase, ADCC: Antibody-dependent cell-mediated cytotoxicity, HCC: Hepatoma carcinoma cell.
Integrins make great contributions to the negative modulation of cancer development. They can not serve as immune-binding targets for APCs, but they can enhance T-cell recruitment in tumors, which inhibits tumor growth.
Moreover, numerous studies demonstrated that integrins can inhibit tumor growth by promoting the cytotoxic effects of NK cells. Research has elucidated that αLβ2 integrins and actins located in NK cells are activated by the expression of 2B4 receptors on NK cells, mediating their rapid adhesion to tumor cells and participation in the early stage of cytotoxicity [17]. Anikeeva et al. reported that αV integrins on tumor cells promoted antibody-dependent cell-mediated cytotoxicity in NK cells in the absence of pro-inflammatory cytokines, resulting in the production of particles and the dissolution of cancer cells [217]. Integrins also promote the recruitment and phagocytosis of macrophages. It has been reported that inflammation promoted the expression of αL and αX integrins in macrophages, thereby inhibiting CD47 overexpression in tumor cells, which prevented checkpoint SIRPa-CD47 from exerting anti-phagocytic effects [218]. In addition, previous studies showed that β2 integrins on macrophages interacted with signaling lymphocytic activation molecule-7 (SLAM7) and activated immunoreceptor tyrosine-based activation motifs, which initiated actin polarization and induced phagocytosis [218]. Moreover, it has been reported that β4 integrins promoted the activation of TGF-β1 and induced the upregulation of laminin-5 and Tie2, which led to enhanced macrophage adhesion [219].
3.3. Integrin-Targeted Immunotherapy
3.3.1. Chimeric Antigen Receptor T-Cell Immunotherapy
CAR T-cell immunotherapy relies on the combination of tumor CAR and patient-derived T cells to enhance the targeting specificity of T cells, which is thus widely applied for the treatment of hematological tumors, such as B-cell lymphoblastic leukemia and lymphoma [220,221]. αvβ6-Binding peptide (A20) fusion with a second-generation CD28/CD3ζ signaling domain or with a fourth-generation CD28/4-1BB/CD27/CD3ζ signaling domain to form A20-2G CAR and A20-4G CAR, respectively, achieves highly selective targeting of αvβ6 [222,223]. Moreover, Whilding et al. demonstrated that the addition of cytokine IL-4 in vitro amplified CAR T cells, which then targeted the tumor-associated αVβ6 integrins to improve T-cell efficacy and availability [223]. Similarly, the activated conformation of β7 integrins has been demonstrated to be a new and specific target of CAR T cells for the treatment of multiple myeloma (MM) [224]. Additionally, CAR T-cell therapy has achieved remarkable results in the treatment of hematological malignancies [221]. The unique specificity of CAR T cells allows them to accurately destroy cancer cells containing corresponding tumor-associated antigens, antigens without damage to normal tissues. Moreover, the augmentation of the immune surveillance function of CAR T cells mediates the recognition of cell-surface molecules without the aid of HLA expression [221]. However, numerous challenges still exist with CAR T-cell immunotherapy, limiting the therapeutic efficacy of CAR T cells in solid tumors, including severe life-threatening toxicities, modest anti-tumor activity, antigen escape, restricted trafficking, and limited tumor infiltration. In order to overcome these significant challenges, innovative approaches will need to be implemented to produce more powerful CAR T cells with anti-tumor activity and decreased toxicity in the near future [225].
3.3.2. Oncolytic Virotherapy
Oncolytic virotherapy is conducted by modifying the specific virus to mediate immune killing or to disrupt the tumor blood supply in the tumor microenvironment (TME) [226,227]. It has been demonstrated that Ad5NULL-A20, a target-modified αVβ6 integrin-selective oncolytic adenovirus, inserted an A20 peptide into its capsid protein to target αVβ6 integrins for the treatment of pancreatic and breast cancer [228,229]. Additionally, Pesonen et al. reported that the RGD-4C-modified oncolytic adenovirus Ad5D24-RGD entered tumor cells through αV integrins highly expressed in advanced tumors [230]. Ad5D24-RGD facilitated immune-killing by incorporating granulocyte-macrophage colony-stimulating factor (GM-CSF), a powerful immune activator [230]. Other research indicated that the oncolytic herpes simplex virus-1 (oHSV) produces OS2966, a therapeutic antibody that blocks the migration of macrophages by targeting the β1 integrins receptor on macrophages [227]. Compared with many other treatments, oncolytic virotherapy produces fewer adverse effects [231] (Table 2). However, tumor cells make use of the body’s self-protection mechanism to block the specific virus from entering and replicating in cells, thereby attenuating the therapeutic effects of oncolytic virotherapy. To overcome these limitations, having a deeper understanding of the mechanism by which tumor cells protect themselves from oncolytic viruses is necessary.

Table 2.
Integrin-targeted immunotherapies in clinical trials for cancer therapeutics.
3.3.3. Immune Checkpoint Inhibitor Therapy
ICI therapy refers to the reduction of T-cell immune suppression by inhibiting the immune checkpoints on the surface of T cells and enhancing the function of T-cell immune surveillance and tumor killing [202,236]. Several studies have confirmed that downregulation of αVβ3 integrins can inhibit the expression of PD-L1 mediated by the BRAF/TAK1/ERK/ETV4 signaling pathway, thus inhibiting the immune escape of cancer cells [235,236,242]. Other research showed that the inhibition of αVβ3 integrins downregulated the production of active TGF-β, thus promoting the invasion and infiltration of local cytotoxic T cells and tumor cell killing [202]. Similarly, Busenhart et al. showed that the inhibition of αVβ6 integrins stimulated T-cell antitumor responses and enhanced immune checkpoint blockade therapy in colorectal cancers [207]. Currently, ICI has a widely applied as a first and second-line treatment of advanced hepatocellular carcinoma. Nevertheless, there are still several limitations that hinder the therapeutic potency of ICI therapy, including the immuno-oncology (IO) bubble phenomenon, drug hyperinfiltration cycle, peculiar side effects, and paradoxical responses. Based on these challenges, many attempts have been made to improve this cancer treatment in the clinic. Changing to more rational, biology-based trials that study meaningful biomarkers tied to IO-specific outcomes might improve the clinical outcomes. Moreover, it may also be helpful to integrate modeling and simulation strategies into trial designs, which would be a prerequisite for improving therapeutic efficacy and survival [243].
3.3.4. Other Strategies
In addition to the three therapies mentioned above, there are other cancer treatments available. It has been indicated that CSCs can be used as immunotherapy targets [196,244]. The expression of β4 integrins on CSCs promotes tumor growth and metastasis, and CSCs also influence the maturation of DCs [196,244]. In addition, inhibition of JAK2 or BTK, downstream effectors of CXCL12, inhibits integrin activation and suppresses tumor cell adhesion, survival, and spread in B-cell chronic lymphocytic leukemia (B-CLL) [204]. Furthermore, Nadine et al. demonstrated that NKG2A blockers prevented the ligand Qa-1/HLA-E on tumor cells from binding to the inhibitory receptor NKG2A on the surface of CD8 + T cells, avoiding adaptive immune tolerance development [245]. Similarly, OPN blockers prevent plasma OPN from interacting with tumor-derived integrins to inhibit tumor growth, invasion, and metastasis [246]. Nanotherapy is a therapeutic approach in which nanoparticles are loaded with antineoplastic drugs and delivered to tumors. αVβ3 integrin-specific cyclic arginine–glycine–aspartate nanoparticles actively bind to neutrophils in the blood before migrating to the tumor, hitchhiking to the tumor location to deliver drugs to the tumor islet [237]. Remarkably, natalizumab is widely used to treat MS; however, when present in the body for a long time, natalizumab affects the degranulation of the melanoma cells by α4β1 integrins, thereby promoting the development of melanoma [206]. Moreover, strategies involving the use of integrin-simulating targeting molecules (in particular, targeting peptides) are used to develop drug delivery systems targeting cancer cells. For example, the well-known VHPKQHR peptide was identified by Kelly et al. through a phage display technique and is homologous to α4β1 integrins [247,248,249,250].
Integrin-targeted immunotherapies are shown to be suitable therapeutic approaches for cancer treatment, including CAR T-cell immunotherapy, oncolytic virotherapy, and ICI therapy.
4. Discussion
Integrins, as universal transmembrane heterodimeric proteins, play a crucial role in both intracellular and extracellular signaling pathways [8]. Numerous studies indicated that integrins have an important role in the regulation of immune cell structures and functions, including migration, adhesion, proliferation, immunotolerance, and immunosurveillance. At the same time, abnormalities of immune cell functions can lead to cancer, the mechanisms of which are twofold. On the one hand, integrins are involved in the regulation of cancer cell proliferation, invasion, migration, and angiogenesis; on the other hand, integrins promote immune cell aggregation to mediate the elimination of tumors. These findings on the underlying mechanisms of integrins in immune cell functions and cancer progression will provide new ideas for combined immunotherapy for cancer patients in the near future.
Based on the effects of integrins on immune cells and cancer progression discussed in this review, a wide range of prospects can be investigated to discover suitable therapeutic methods. CITs, currently a hotspot of cancer treatment nowadays research, have been studied extensively, such as in CAR T-cell immunotherapy, oncolytic virotherapy, and ICI therapy. Compared to traditional cancer treatments, CITs show the advantages of strong effects, good tolerance, and lower toxicity. Although much progress has been made in the field of CIT, there are still numerous challenges to achieving a good prognosis due to the wide variety of integrins, their poor specificity, the varying activity of immune cells, and the nature of cancer cells themselves [251]. For example, the activation of anti-tumor immunity in CAR T cells results in a strong driving effect of cytokines, potentially culminating in macrophage activation syndrome and hemophagocytic lymphohistiocytosis [221]. Similarly, neurotoxicity and endothelial dysfunction can be caused by CAR T-cell therapy [221]. In addition, great challenges have been faced in the field of solid tumor treatment due to the insufficient and atypical variety of molecular targets of the TME in which CAR T cells act and due to the existence of immunosuppressive cytokines in the TME [221,231]. Furthermore, research showed that the number of circulating activated T cells targeting autoantigens can be increased by ICI therapy, which may lead to autoimmunity [252]. This autoinflammatory toxicity contributes to diseases of the CNS and peripheral nervous system, such as encephalitis, MS, myasthenia gravis, and peripheral neuropathy [252].
Although CIT is not clinically ideal, it can be improved in several ways. First, experimental models must be improved because current models may not accurately represent clinical effects. In preclinical models, both animal models and tumor transplants cultured from cell lines cannot replace and mimic human tumors in their biological function. Moreover, preclinical trials do not fully simulate conditions in which an integrin inhibitor is used as the main therapeutic approach to eliminate most tumor tissues or as adjuvant therapy for the reduction of residual diseases and circulating cancer cells. Second, a greater understanding of how molecular and cellular drivers function during primary versus secondary immune escape would make CIT more effective. For example, the effects of integrin expression at different stages of various cancers are not well understood. Third, personalized approaches can be maximized through composite biomarkers. The advantages of integrin inhibitors, such as their low side effects, have led to their rapid development as a popular treatment for cancer today, and their combination with conventional drugs could be further investigated. Moreover, the gene therapies that target various integrins are potentially highly effective cancer treatments. Advances in technology and treatment methods have made it possible to gain a better understanding of immune cells and cancer. Moreover, these initiatives provide further opportunities to promote more effective and less costly care for cancer patients. Consequently, we conclude that integrins have a great impact on immunity and cancer development, providing new clues into clinical CITs.
Author Contributions
Conceptualization, Z.X. and J.C.; investigation, J.C. and Q.Z.; writing—original draft preparation, Q.Z. and S.Z.; writing—review and editing, Q.Z., J.C., Z.X. and S.Z.; figure design and editing, Q.Z. and S.Z.; visualization, Q.Z. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the fund of the National Natural Science Foundation of China (41966006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Servier Home for Researchers. Parts of the figures were drawn by using pictures from Servier Home for Researchers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Integrin diversity brings specificity in mechanotransduction. Biol. Cell 2018, 110, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lietha, D.; Izard, T. Roles of Membrane Domains in Integrin-Mediated Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 5531. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Overstreet, M.G.; Gaylo, A.; Angermann, B.R.; Hughson, A.; Hyun, Y.M.; Lambert, K.; Acharya, M.; Billroth-Maclurg, A.C.; Rosenberg, A.F.; Topham, D.J.; et al. Inflammation-induced interstitial migration of effector CD4⁺ T cells is dependent on integrin αV. Nat. Immunol. 2013, 14, 949–958. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, Y.; Yuan, Z.; Qin, G. Research advances on structure and biological functions of integrins. SpringerPlus 2016, 5, 1094. [Google Scholar] [CrossRef]
- Saez de Guinoa, J.; Barrio, L.; Carrasco, Y.R. Vinculin arrests motile B cells by stabilizing integrin clustering at the immune synapse. J. Immunol. 2013, 191, 2742–2751. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Kanayama, M.; Morimoto, J.; Matsui, Y.; Ikesue, M.; Danzaki, K.; Kurotaki, D.; Ito, K.; Yoshida, T.; Uede, T. α9β1 integrin-mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J. Immunol. 2011, 187, 5851–5864. [Google Scholar] [CrossRef]
- Yu, Y.; Ou-Yang, W.; Zhang, H.; Jiang, T.; Cho, W.C.; Zhu, H.; Xiao, Z.; Li, S. High-mobility Group Box 1 Facilitates CD4 T Cell Self-aggregation Via Integrin and STAT3 Activation Before Homing. Inflamm. Bowel Dis. 2020, 26, 1188–1198. [Google Scholar] [CrossRef]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Yang, C.M. Inflammatory signalings involved in airway and pulmonary diseases. Mediat. Inflamm. 2013, 2013, 791231. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, F.; Díaz Flaqué, M.C.; Fernando, T.; Yang, S.N.; Sterle, H.A.; Bolontrade, M.; Amorós, M.; Isse, B.; Farías, R.N.; Ahn, H.; et al. Integrin αvβ3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant T cells. Blood 2015, 125, 841–851. [Google Scholar] [CrossRef]
- Jinushi, M.; Chiba, S.; Baghdadi, M.; Kinoshita, I.; Dosaka-Akita, H.; Ito, K.; Yoshiyama, H.; Yagita, H.; Uede, T.; Takaoka, A. ATM-mediated DNA damage signals mediate immune escape through integrin-αvβ3-dependent mechanisms. Cancer Res. 2012, 72, 56–65. [Google Scholar] [CrossRef]
- Kondoh, T.; Kuribayashi, K.; Tanaka, M.; Kobayashi, D.; Yanagihara, N.; Watanabe, N. CD7 promotes extramedullary involvement of the B-cell acute lymphoblastic leukemia line Tanoue by enhancing integrin β2-dependent cell adhesiveness. Int. J. Oncol. 2014, 45, 1073–1081. [Google Scholar] [CrossRef]
- Hoffmann, S.C.; Cohnen, A.; Ludwig, T.; Watzl, C. 2B4 engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy. J. Immunol. 2011, 186, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, T.; Mocevicius, P.; Deduchovas, O.; Salnikova, O.; Castro-Santa, E.; Büchler, M.W.; Schmidt, J.; Ryschich, E. αL β2 integrin is indispensable for CD8+ T-cell recruitment in experimental pancreatic and hepatocellular cancer. Int. J. Cancer 2012, 130, 2067–2076. [Google Scholar] [CrossRef]
- Han, Z.; Ma, Y.; Cao, G.; Ma, Z.; Chen, R.; Cvijic, M.E.; Cheng, D. Integrin αVβ1 regulates procollagen I production through a non-canonical transforming growth factor β signaling pathway in human hepatic stellate cells. Biochem. J. 2021, 478, 1689–1703. [Google Scholar] [CrossRef]
- Hendesi, H.; Barbe, M.F.; Safadi, F.F.; Monroy, M.A.; Popoff, S.N. Integrin mediated adhesion of osteoblasts to connective tissue growth factor (CTGF/CCN2) induces cytoskeleton reorganization and cell differentiation. PLoS ONE 2015, 10, e0115325. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T.D.; DeGrado, W.F.; Sheppard, D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015, 7, 288ra279. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905. [Google Scholar] [CrossRef]
- Bishop, G.G.; McPherson, J.A.; Sanders, J.M.; Hesselbacher, S.E.; Feldman, M.J.; McNamara, C.A.; Gimple, L.W.; Powers, E.R.; Mousa, S.A.; Sarembock, I.J. Selective α(v)β(3)-receptor blockade reduces macrophage infiltration and restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation 2001, 103, 1906–1911. [Google Scholar] [CrossRef]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Uchida, H.; Choi, E.T. Integrin α(v)β(3) as a target in the prevention of neointimal hyperplasia. J. Vasc. Surg. 2007, 45 (Suppl. A), A33–A38. [Google Scholar] [CrossRef]
- Asano, Y.; Ihn, H.; Jinnin, M.; Mimura, Y.; Tamaki, K. Involvement of αvβ5 integrin in the establishment of autocrine TGF-β signaling in dermal fibroblasts derived from localized scleroderma. J. Investig. Dermatol. 2006, 126, 1761–1769. [Google Scholar] [CrossRef]
- Kumawat, A.K.; Yu, C.; Mann, E.A.; Schridde, A.; Finnemann, S.C.; Mowat, A.M. Expression and characterization of αvβ5 integrin on intestinal macrophages. Eur. J. Immunol. 2018, 48, 1181–1187. [Google Scholar] [CrossRef]
- Oishi, Y.; Hashimoto, K.; Abe, A.; Kuroda, M.; Fujii, A.; Miyamoto, Y. Vitronectin regulates the axon specification of mouse cerebellar granule cell precursors via αvβ5 integrin in the differentiation stage. Neurosci. Lett. 2021, 746, 135648. [Google Scholar] [CrossRef]
- Porte, J.; Jenkins, G.; Tatler, A.L. Myofibroblast TGF-β Activation Measurement In Vitro. Methods Mol. Biol. 2021, 2299, 99–108. [Google Scholar] [CrossRef]
- Schiesser, J.V.; Loudovaris, T.; Thomas, H.E.; Elefanty, A.G.; Stanley, E.G. Integrin αvβ5 heterodimer is a specific marker of human pancreatic β cells. Sci. Rep. 2021, 11, 8315. [Google Scholar] [CrossRef] [PubMed]
- Tatler, A.L.; John, A.E.; Jolly, L.; Habgood, A.; Porte, J.; Brightling, C.; Knox, A.J.; Pang, L.; Sheppard, D.; Huang, X.; et al. Integrin αvβ5-mediated TGF-β activation by airway smooth muscle cells in asthma. J. Immunol. 2011, 187, 6094–6107. [Google Scholar] [CrossRef]
- Ansar, M.; Jan, A.; Santos-Cortez, R.L.; Wang, X.; Suliman, M.; Acharya, A.; Habib, R.; Abbe, I.; Ali, G.; Lee, K.; et al. Expansion of the spectrum of ITGB6-related disorders to adolescent alopecia, dentogingival abnormalities and intellectual disability. Eur. J. Hum. Genet. EJHG 2016, 24, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, L.; Bi, J.; Häkkinen, L.; Larjava, H. Integrin αvβ6: Structure, function and role in health and disease. Int. J. Biochem. Cell Biol. 2018, 99, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Madala, S.K.; Korfhagen, T.R.; Schmidt, S.; Davidson, C.; Edukulla, R.; Ikegami, M.; Violette, S.M.; Weinreb, P.H.; Sheppard, D.; Hardie, W.D. Inhibition of the αvβ6 integrin leads to limited alteration of TGF-α-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L726–L735. [Google Scholar] [CrossRef]
- White, J.B.; Hu, L.Y.; Boucher, D.L.; Sutcliffe, J.L. ImmunoPET Imaging of α(v)β(6) Expression Using an Engineered Anti-α(v)β(6) Cys-diabody Site-Specifically Radiolabeled with Cu-64: Considerations for Optimal Imaging with Antibody Fragments. Mol. Imaging Biol. 2018, 20, 103–113. [Google Scholar] [CrossRef]
- McCarty, J.H. αvβ8 integrin adhesion and signaling pathways in development, physiology and disease. J. Cell Sci. 2020, 133, jcs239434. [Google Scholar] [CrossRef]
- Zhou, M.; Niu, J.; Wang, J.; Gao, H.; Shahbaz, M.; Niu, Z.; Li, Z.; Zou, X.; Liang, B. Integrin αvβ8 serves as a Novel Marker of Poor Prognosis in Colon Carcinoma and Regulates Cell Invasiveness through the Activation of TGF-β1. J. Cancer 2020, 11, 3803–3815. [Google Scholar] [CrossRef]
- Hou, J.; Yan, D.; Liu, Y.; Huang, P.; Cui, H. The Roles of Integrin α5β1 in Human Cancer. OncoTargets Ther. 2020, 13, 13329–13344. [Google Scholar] [CrossRef]
- López-Luppo, M.; Catita, J.; Ramos, D.; Navarro, M.; Carretero, A.; Mendes-Jorge, L.; Muñoz-Cánoves, P.; Rodriguez-Baeza, A.; Nacher, V.; Ruberte, J. Cellular Senescence Is Associated With Human Retinal Microaneurysm Formation During Aging. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2832–2842. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Guan, J.; Hu, S.; Zhang, J.; Lan, Y.; Zhao, K.; Lu, H.; Song, D.; He, H.; et al. Porcine Hemagglutinating Encephalomyelitis Virus Activation of the Integrin α5β1-FAK-Cofilin Pathway Causes Cytoskeletal Rearrangement To Promote Its Invasion of N2a Cells. J. Virol. 2019, 93, e01736-18. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, J.W.; Kim, Y.; Lee, M.N.; Kook, M.S.; Choi, E.Y.; Im, S.Y.; Koh, J.T. The extracellular matrix protein Edil3 stimulates osteoblast differentiation through the integrin α5β1/ERK/Runx2 pathway. PLoS ONE 2017, 12, e0188749. [Google Scholar] [CrossRef] [PubMed]
- Renner, G.; Noulet, F.; Mercier, M.C.; Choulier, L.; Etienne-Selloum, N.; Gies, J.P.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. Expression/activation of α5β1 integrin is linked to the β-catenin signaling pathway to drive migration in glioma cells. Oncotarget 2016, 7, 62194–62207. [Google Scholar] [CrossRef]
- Zargham, R. Tensegrin in context: Dual role of α8 integrin in the migration of different cell types. Cell Adhes. Migr. 2010, 4, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef]
- Guenther, C. β2-Integrins—Regulatory and Executive Bridges in the Signaling Network Controlling Leukocyte Trafficking and Migration. Front. Immunol. 2022, 13, 809590. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, A.R.; Mamdouh, Z.; Muller, W.A. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 2004, 5, 393–400. [Google Scholar] [CrossRef]
- Yuki, K.; Hou, L. Role of β2 Integrins in Neutrophils and Sepsis. Infect. Immun. 2020, 88, e00031-20. [Google Scholar] [CrossRef]
- Gaither, T.A.; Vargas, I.; Inada, S.; Frank, M.M. The complement fragment C3d facilitates phagocytosis by monocytes. Immunology 1987, 62, 405–411. [Google Scholar]
- Torres-Gomez, A.; Cabanas, C.; Lafuente, E.M. Phagocytic Integrins: Activation and Signaling. Front. Immunol. 2020, 11, 738. [Google Scholar] [CrossRef]
- Le Cabec, V.; Cols, C.; Maridonneau-Parini, I. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect. Immun. 2000, 68, 4736–4745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bajic, G.; Andersen, G.R.; Christiansen, S.H.; Vorup-Jensen, T. The cationic peptide LL-37 binds Mac-1 (CD11b/CD18) with a low dissociation rate and promotes phagocytosis. Biochim. Biophys. Acta 2016, 1864, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Guenther, C.; Faisal, I.; Fusciello, M.; Sokolova, M.; Harjunpää, H.; Ilander, M.; Tallberg, R.; Vartiainen, M.K.; Alon, R.; Gonzalez-Granado, J.M.; et al. β2-Integrin Adhesion Regulates Dendritic Cell Epigenetic and Transcriptional Landscapes to Restrict Dendritic Cell Maturation and Tumor Rejection. Cancer Immunol. Res. 2021, 9, 1354–1369. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Pluskota, E.; Cao, W.; Plow, E.F.; Soloviev, D.A. Distinct Effects of Integrins αXβ2 and αMβ2 on Leukocyte Subpopulations during Inflammation and Antimicrobial Responses. Infect. Immun. 2017, 85, e00644-16. [Google Scholar] [CrossRef] [PubMed]
- Schack, L.; Stapulionis, R.; Christensen, B.; Kofod-Olsen, E.; Skov Sorensen, U.B.; Vorup-Jensen, T.; Sorensen, E.S.; Hollsberg, P. Osteopontin enhances phagocytosis through a novel osteopontin receptor, the αXβ2 integrin. J. Immunol. 2009, 182, 6943–6950. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Vieira-de-Abreu, A.; Harris, E.S.; Shah, A.M.; Weyrich, A.S.; Castro-Faria-Neto, H.C.; Zimmerman, G.A. Integrin αDβ2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling. PLoS ONE 2014, 9, e112770. [Google Scholar] [CrossRef]
- Fukui, T.; Fukaya, T.; Uto, T.; Takagi, H.; Nasu, J.; Miyanaga, N.; Nishikawa, Y.; Koseki, H.; Choijookhuu, N.; Hishikawa, Y.; et al. Pivotal role of CD103 in the development of psoriasiform dermatitis. Sci. Rep. 2020, 10, 8371. [Google Scholar] [CrossRef]
- Schreiber, T.D.; Steinl, C.; Essl, M.; Abele, H.; Geiger, K.; Müller, C.A.; Aicher, W.K.; Klein, G. The integrin α9β1 on hematopoietic stem and progenitor cells: Involvement in cell adhesion, proliferation and differentiation. Haematologica 2009, 94, 1493–1501. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, T.; Cao, Z.; Zhong, W.; Zhang, C.; Li, H.; Song, J. Integrin-α9β1 as a Novel Therapeutic Target for Refractory Diseases: Recent Progress and Insights. Front. Immunol. 2021, 12, 638400. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Nawaz, F.; Byrareddy, S.N.; Villinger, F.; Santangelo, P.J.; Ansari, A.A.; Fauci, A.S. The Role of Integrin α(4)β(7) in HIV Pathogenesis and Treatment. Curr. HIV/AIDS Rep. 2018, 15, 127–135. [Google Scholar] [CrossRef]
- Li, H.; Huang, S.Y.; Shi, F.H.; Gu, Z.C.; Zhang, S.G.; Wei, J.F. α(4)β(7) integrin inhibitors: A patent review. Expert Opin. Ther. Pat. 2018, 28, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.; Meehan, D.T.; Delimont, D.; Zallocchi, M.; Perry, G.A.; O’Brien, S.; Tu, H.; Pihlajaniemi, T.; Cosgrove, D. Collagen XIII induced in vascular endothelium mediates α1β1 integrin-dependent transmigration of monocytes in renal fibrosis. Am. J. Pathol. 2010, 177, 2527–2540. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H. Integrin α1β1. Adv. Exp. Med. Biol. 2014, 819, 21–39. [Google Scholar] [CrossRef]
- Hamaia, S.W.; Pugh, N.; Raynal, N.; Némoz, B.; Stone, R.; Gullberg, D.; Bihan, D.; Farndale, R.W. Mapping of potent and specific binding motifs, GLOGEN and GVOGEA, for integrin α1β1 using collagen toolkits II and III. J. Biol. Chem. 2012, 287, 26019–26028. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, C.F.; Cerwinka, W.H.; Sprague, A.G.; Laroux, F.S.; Grisham, M.B.; Koteliansky, V.E.; Senninger, N.; Granger, D.N.; de Fougerolles, A.R. Collagen-binding integrin α1β1 regulates intestinal inflammation in experimental colitis. J. Clin. Investig. 2002, 110, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okuno, T.; Yamamoto, M.; Pasterkamp, R.J.; Takegahara, N.; Takamatsu, H.; Kitao, T.; Takagi, J.; Rennert, P.D.; Kolodkin, A.L.; et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1 integrin. Nature 2007, 446, 680–684. [Google Scholar] [CrossRef]
- El Azreq, M.A.; Arseneault, C.; Boisvert, M.; Pagé, N.; Allaeys, I.; Poubelle, P.E.; Tessier, P.A.; Aoudjit, F. Cooperation between IL-7 Receptor and Integrin α2β1 (CD49b) Drives Th17-Mediated Bone Loss. J. Immunol. 2015, 195, 4198–4209. [Google Scholar] [CrossRef] [PubMed]
- Grenache, D.G.; Zhang, Z.; Wells, L.E.; Santoro, S.A.; Davidson, J.M.; Zutter, M.M. Wound healing in the α2β1 integrin-deficient mouse: Altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J. Investig. Dermatol. 2007, 127, 455–466. [Google Scholar] [CrossRef]
- Madamanchi, A.; Santoro, S.A.; Zutter, M.M. α2β1 Integrin. Adv. Exp. Med. Biol. 2014, 819, 41–60. [Google Scholar] [CrossRef]
- Zweers, M.C.; Davidson, J.M.; Pozzi, A.; Hallinger, R.; Janz, K.; Quondamatteo, F.; Leutgeb, B.; Krieg, T.; Eckes, B. Integrin α2β1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J. Investig. Dermatol. 2007, 127, 467–478. [Google Scholar] [CrossRef]
- Camper, L.; Holmvall, K.; Wängnerud, C.; Aszódi, A.; Lundgren-Akerlund, E. Distribution of the collagen-binding integrin α10β1 during mouse development. Cell Tissue Res. 2001, 306, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lundgren-Åkerlund, E.; Aszòdi, A. Integrin α10β1: A collagen receptor critical in skeletal development. Adv. Exp. Med. Biol. 2014, 819, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.M.; Olsen, L.H.; da Franca, P.; Loos, B.G.; Mustafa, K.; Gullberg, D.; Bolstad, A.I. A role for α11β1 integrin in the human periodontal ligament. J. Dent. Res. 2009, 88, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Erusappan, P.; Alam, J.; Lu, N.; Zeltz, C.; Gullberg, D. Integrin α11 cytoplasmic tail is required for FAK activation to initiate 3D cell invasion and ERK-mediated cell proliferation. Sci. Rep. 2019, 9, 15283. [Google Scholar] [CrossRef]
- Ramovs, V.; Te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 57–58, 213–243. [Google Scholar] [CrossRef]
- Delwel, G.O.; de Melker, A.A.; Hogervorst, F.; Jaspars, L.H.; Fles, D.L.; Kuikman, I.; Lindblom, A.; Paulsson, M.; Timpl, R.; Sonnenberg, A. Distinct and overlapping ligand specificities of the α 3A β 1 and α 6A β 1 integrins: Recognition of laminin isoforms. Mol. Biol. Cell 1994, 5, 203–215. [Google Scholar] [CrossRef]
- Fujiwara, H.; Kataoka, N.; Honda, T.; Ueda, M.; Yamada, S.; Nakamura, K.; Suginami, H.; Mori, T.; Maeda, M. Physiological roles of integrin α 6 β 1 in ovarian functions. Horm. Res. 1998, 50 (Suppl. 2), 25–29. [Google Scholar] [CrossRef]
- Georges-Labouesse, E.; Mark, M.; Messaddeq, N.; Gansmüller, A. Essential role of α 6 integrins in cortical and retinal lamination. Curr. Biol. CB 1998, 8, 983–986. [Google Scholar] [CrossRef]
- Reynolds, L.E.; D’Amico, G.; Lechertier, T.; Papachristodoulou, A.; Muñoz-Félix, J.M.; De Arcangelis, A.; Baker, M.; Serrels, B.; Hodivala-Dilke, K.M. Dual role of pericyte α6β1-integrin in tumour blood vessels. J. Cell Sci. 2017, 130, 1583–1595. [Google Scholar] [CrossRef]
- Soung, Y.H.; Clifford, J.L.; Chung, J. Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep. 2010, 43, 311–318. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Z.; Zhang, Y.; Miao, J. The roles of integrin β4 in vascular endothelial cells. J. Cell. Physiol. 2012, 227, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Flintoff-Dye, N.L.; Welser, J.; Rooney, J.; Scowen, P.; Tamowski, S.; Hatton, W.; Burkin, D.J. Role for the α7β1 integrin in vascular development and integrity. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 234, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.K.; Chou, F.L.; Engvall, E.; Ogawa, M.; Matsuda, C.; Hirabayashi, S.; Yokochi, K.; Ziober, B.L.; Kramer, R.H.; Kaufman, S.J.; et al. Mutations in the integrin α7 gene cause congenital myopathy. Nat. Genet. 1998, 19, 94–97. [Google Scholar] [CrossRef]
- Belkin, A.M.; Stepp, M.A. Integrins as receptors for laminins. Microsc. Res. Technol. 2000, 51, 280–301. [Google Scholar] [CrossRef]
- Munksgaard Thorén, M.; Chmielarska Masoumi, K.; Krona, C.; Huang, X.; Kundu, S.; Schmidt, L.; Forsberg-Nilsson, K.; Floyd Keep, M.; Englund, E.; Nelander, S.; et al. Integrin α10, a Novel Therapeutic Target in Glioblastoma, Regulates Cell Migration, Proliferation, and Survival. Cancers 2019, 11, 587. [Google Scholar] [CrossRef]
- Sasaki, T.; Timpl, R. Domain IVa of laminin α5 chain is cell-adhesive and binds β1 and αVβ3 integrins through Arg-Gly-Asp. FEBS Lett. 2001, 509, 181–185. [Google Scholar] [CrossRef]
- Nagy-Baló, Z.; Kiss, R.; Menge, A.; Bödör, C.; Bajtay, Z.; Erdei, A. Activated Human Memory B Lymphocytes Use CR4 (CD11c/CD18) for Adhesion, Migration, and Proliferation. Front. Immunol. 2020, 11, 565458. [Google Scholar] [CrossRef]
- Manevich-Mendelson, E.; Grabovsky, V.; Feigelson, S.W.; Cinamon, G.; Gore, Y.; Goverse, G.; Monkley, S.J.; Margalit, R.; Melamed, D.; Mebius, R.E.; et al. Talin1 is required for integrin-dependent B lymphocyte homing to lymph nodes and the bone marrow but not for follicular B-cell maturation in the spleen. Blood 2010, 116, 5907–5918. [Google Scholar] [CrossRef]
- Hart, R.; Stanley, P.; Chakravarty, P.; Hogg, N. The kindlin 3 pleckstrin homology domain has an essential role in lymphocyte function-associated antigen 1 (LFA-1) integrin-mediated B cell adhesion and migration. J. Biol. Chem. 2013, 288, 14852–14862. [Google Scholar] [CrossRef]
- Ballet, R.; Brennan, M.; Brandl, C.; Feng, N.; Berri, J.; Cheng, J.; Ocón, B.; Alborzian Deh Sheikh, A.; Marki, A.; Bi, Y.; et al. A CD22-Shp1 phosphatase axis controls integrin β(7) display and B cell function in mucosal immunity. Nat. Immunol. 2021, 22, 381–390. [Google Scholar] [CrossRef]
- Schippers, A.; Kochut, A.; Pabst, O.; Frischmann, U.; Clahsen, T.; Tenbrock, K.; Müller, W.; Wagner, N. β7 integrin controls immunogenic and tolerogenic mucosal B cell responses. Clin. Immunol. 2012, 144, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Waffarn, E.E.; Hastey, C.J.; Dixit, N.; Soo Choi, Y.; Cherry, S.; Kalinke, U.; Simon, S.I.; Baumgarth, N. Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat. Commun. 2015, 6, 8991. [Google Scholar] [CrossRef] [PubMed]
- van Spriel, A.B.; de Keijzer, S.; van der Schaaf, A.; Gartlan, K.H.; Sofi, M.; Light, A.; Linssen, P.C.; Boezeman, J.B.; Zuidscherwoude, M.; Reinieren-Beeren, I.; et al. The tetraspanin CD37 orchestrates the α(4)β(1) integrin-Akt signaling axis and supports long-lived plasma cell survival. Sci. Signal 2012, 5, ra82. [Google Scholar] [CrossRef] [PubMed]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Lundborg, L.R.; Yeasmin, S.; Tyler, C.J.; Zgajnar, N.R.; Taupin, V.; Dobaczewska, K.; Mikulski, Z.; Bamias, G.; Rivera-Nieves, J. An integrin αEβ7-dependent mechanism of IgA transcytosis requires direct plasma cell contact with intestinal epithelium. Mucosal Immunol. 2021, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Raso, F.; Sagadiev, S.; Du, S.; Gage, E.; Arkatkar, T.; Metzler, G.; Stuart, L.M.; Orr, M.T.; Rawlings, D.J.; Jackson, S.W.; et al. αv Integrins regulate germinal center B cell responses through noncanonical autophagy. J. Clin. Investig. 2018, 128, 4163–4178. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Ma, Y.; Chen, X.; Liu, M.; Cai, Y.; Hu, X.; Xiang, D.; Nath, S.; Zhang, H.G.; Ye, H.; et al. Integrin CD11b negatively regulates BCR signalling to maintain autoreactive B cell tolerance. Nat. Commun. 2013, 4, 2813. [Google Scholar] [CrossRef]
- Acharya, M.; Raso, F.; Sagadiev, S.; Gilbertson, E.; Kadavy, L.; Li, Q.Z.; Yan, M.; Stuart, L.M.; Hamerman, J.A.; Lacy-Hulbert, A. B Cell αv Integrins Regulate TLR-Driven Autoimmunity. J. Immunol. 2020, 205, 1810–1818. [Google Scholar] [CrossRef]
- Mraz, M.; Zent, C.S.; Church, A.K.; Jelinek, D.F.; Wu, X.; Pospisilova, S.; Ansell, S.M.; Novak, A.J.; Kay, N.E.; Witzig, T.E.; et al. Bone marrow stromal cells protect lymphoma B-cells from rituximab-induced apoptosis and targeting integrin α-4-β-1 (VLA-4) with natalizumab can overcome this resistance. Br. J. Haematol. 2011, 155, 53–64. [Google Scholar] [CrossRef]
- Dorner, M.; Zucol, F.; Alessi, D.; Haerle, S.K.; Bossart, W.; Weber, M.; Byland, R.; Bernasconi, M.; Berger, C.; Tugizov, S.; et al. β1 integrin expression increases susceptibility of memory B cells to Epstein-Barr virus infection. J. Virol. 2010, 84, 6667–6677. [Google Scholar] [CrossRef]
- Bromley, S.K.; Akbaba, H.; Mani, V.; Mora-Buch, R.; Chasse, A.Y.; Sama, A.; Luster, A.D. CD49a Regulates Cutaneous Resident Memory CD8(+) T Cell Persistence and Response. Cell Rep. 2020, 32, 108085. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Perugini, V.; González-Granado, J.M.; Tejera, E.; López-Martín, S.; Yañez-Mó, M.; Sánchez-Madrid, F. Tetraspanins CD9 and CD151 at the immune synapse support T-cell integrin signaling. Eur. J. Immunol. 2014, 44, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Bachsais, M.; Salti, S.; Zaoui, K.; Hassan, G.S.; Aoudjit, F.; Mourad, W. CD154 inhibits death of T cells via a Cis interaction with the α5β1 integrin. PLoS ONE 2020, 15, e0235753. [Google Scholar] [CrossRef]
- Gitlin, A.D.; Heger, K.; Schubert, A.F.; Reja, R.; Yan, D.; Pham, V.C.; Suto, E.; Zhang, J.; Kwon, Y.C.; Freund, E.C.; et al. Integration of innate immune signalling by caspase-8 cleavage of N4BP1. Nature 2020, 587, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, K.I.; Williamson, E.K.; Roy, N.H.; Blumenthal, D.; Chandra, V.; Baumgart, T.; Burkhardt, J.K. Integrins Modulate T Cell Receptor Signaling by Constraining Actin Flow at the Immunological Synapse. Front. Immunol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Raucci, F.; Sevim, M.; Saviano, A.; Begum, J.; Zhi, Z.; Pezhman, L.; Tull, S.; Maione, F.; Iqbal, A.J. Galectin-9 supports primary T cell transendothelial migration in a glycan and integrin dependent manner. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 151, 113171. [Google Scholar] [CrossRef]
- Pérez-Rivero, G.; Cascio, G.; Soriano, S.F.; Sanz, Á.G.; de Guinoa, J.S.; Rodríguez-Frade, J.M.; Gomariz, R.P.; Holgado, B.L.; Cabañas, C.; Carrasco, Y.R.; et al. Janus kinases 1 and 2 regulate chemokine-mediated integrin activation and naïve T-cell homing. Eur. J. Immunol. 2013, 43, 1745–1757. [Google Scholar] [CrossRef]
- Evans, R.; Lellouch, A.C.; Svensson, L.; McDowall, A.; Hogg, N. The integrin LFA-1 signals through ZAP-70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood 2011, 117, 3331–3342. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Cheng, Y.-J.; Huang, J.-Y.; Lin, H.-C.; Yang, B.-C. Zap70 controls the interaction of talin with integrin to regulate the chemotactic directionality of T-cell migration. Mol. Immunol. 2010, 47, 2022–2029. [Google Scholar] [CrossRef]
- DeNucci, C.C.; Shimizu, Y. β1 integrin is critical for the maintenance of antigen-specific CD4 T cells in the bone marrow but not long-term immunological memory. J. Immunol. 2011, 186, 4019–4026. [Google Scholar] [CrossRef]
- Martens, R.; Permanyer, M.; Werth, K.; Yu, K.; Braun, A.; Halle, O.; Halle, S.; Patzer, G.E.; Bošnjak, B.; Kiefer, F.; et al. Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nat. Commun. 2020, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.L.; MacPherson, M.; Savinko, T.; Lek, H.S.; Prescott, A.; Fagerholm, S.C. The β2 integrin-kindlin-3 interaction is essential for T-cell homing but dispensable for T-cell activation in vivo. Blood 2013, 122, 1428–1436. [Google Scholar] [CrossRef]
- Nordenfelt, P.; Elliott, H.L.; Springer, T.A. Coordinated integrin activation by actin-dependent force during T-cell migration. Nat. Commun. 2016, 7, 13119. [Google Scholar] [CrossRef] [PubMed]
- Manhas, K.R.; Marshall, P.A.; Wagner, C.E.; Jurutka, P.W.; Mancenido, M.V.; Debray, H.Z.; Blattman, J.N. Rexinoids Modulate Effector T Cell Expression of Mucosal Homing Markers CCR9 and α4β7 Integrin and Direct Their Migration In Vitro. Front. Immunol. 2022, 13, 746484. [Google Scholar] [CrossRef] [PubMed]
- Garçon, F.; Okkenhaug, K. PI3Kδ promotes CD4(+) T-cell interactions with antigen-presenting cells by increasing LFA-1 binding to ICAM-1. Immunology and cell biology 2016, 94, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, Y.; Ran, X.; Du, H.; Feng, H.; Yang, L.; Wen, Y.; Lin, C.; Wang, S.; Huang, M.; et al. Integrin αEβ7(+) T cells direct intestinal stem cell fate decisions via adhesion signaling. Cell Res. 2021, 31, 1291–1307. [Google Scholar] [CrossRef]
- Zondler, L.; Herich, S.; Kotte, P.; Körner, K.; Schneider-Hohendorf, T.; Wiendl, H.; Schwab, N.; Zarbock, A. MCAM/CD146 Signaling via PLCγ1 Leads to Activation of β(1)-Integrins in Memory T-Cells Resulting in Increased Brain Infiltration. Front. Immunol. 2020, 11, 599936. [Google Scholar] [CrossRef]
- Glatigny, S.; Duhen, R.; Arbelaez, C.; Kumari, S.; Bettelli, E. Integrin α L controls the homing of regulatory T cells during CNS autoimmunity in the absence of integrin α 4. Sci. Rep. 2015, 5, 7834. [Google Scholar] [CrossRef]
- Bachsais, M.; Naddaf, N.; Yacoub, D.; Salti, S.; Alaaeddine, N.; Aoudjit, F.; Hassan, G.S.; Mourad, W. The Interaction of CD154 with the α5β1 Integrin Inhibits Fas-Induced T Cell Death. PloS ONE 2016, 11, e0158987. [Google Scholar] [CrossRef]
- Klann, J.E.; Kim, S.H.; Remedios, K.A.; He, Z.; Metz, P.J.; Lopez, J.; Tysl, T.; Olvera, J.G.; Ablack, J.N.; Cantor, J.M.; et al. Integrin Activation Controls Regulatory T Cell-Mediated Peripheral Tolerance. J. Immunol. 2018, 200, 4012–4023. [Google Scholar] [CrossRef]
- Braun, A.; Dewert, N.; Brunnert, F.; Schnabel, V.; Hardenberg, J.H.; Richter, B.; Zachmann, K.; Cording, S.; Claßen, A.; Brans, R.; et al. Integrin αE(CD103) Is Involved in Regulatory T-Cell Function in Allergic Contact Hypersensitivity. J. Investig. Dermatol. 2015, 135, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Mair, I.; Zandee, S.E.J.; Toor, I.S.; Saul, L.; McPherson, R.C.; Leech, M.D.; Smyth, D.J.; O’Connor, R.A.; Henderson, N.C.; Anderton, S.M. A Context-Dependent Role for αv Integrins in Regulatory T Cell Accumulation at Sites of Inflammation. Front. Immunol. 2018, 9, 264. [Google Scholar] [CrossRef]
- Worthington, J.J.; Kelly, A.; Smedley, C.; Bauché, D.; Campbell, S.; Marie, J.C.; Travis, M.A. Integrin αvβ8-Mediated TGF-β Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity 2015, 42, 903–915. [Google Scholar] [CrossRef]
- Zhang, A.; Paidassi, H.; Lacy-Hulbert, A.; Savill, J. Apoptotic cells induce CD103 expression and immunoregulatory function in myeloid dendritic cell precursors through integrin αv and TGF-β activation. PLoS ONE 2020, 15, e0232307. [Google Scholar] [CrossRef] [PubMed]
- Boucard-Jourdin, M.; Kugler, D.; Endale Ahanda, M.L.; This, S.; De Calisto, J.; Zhang, A.; Mora, J.R.; Stuart, L.M.; Savill, J.; Lacy-Hulbert, A.; et al. β8 Integrin Expression and Activation of TGF-β by Intestinal Dendritic Cells Are Determined by Both Tissue Microenvironment and Cell Lineage. J. Immunol. 2016, 197, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Laffont, S.; Garnier, L.; Kaba, E.; Deutsch, U.; Engelhardt, B.; Guéry, J.C. CD49d/CD29-integrin controls the accumulation of plasmacytoid dendritic cells into the CNS during neuroinflammation. Eur. J. Immunol. 2019, 49, 2030–2043. [Google Scholar] [CrossRef] [PubMed]
- Sie, C.; Perez, L.G.; Kreutzfeldt, M.; Potthast, M.; Ohnmacht, C.; Merkler, D.; Huber, S.; Krug, A.; Korn, T. Dendritic Cell Accumulation in the Gut and Central Nervous System Is Differentially Dependent on α4 Integrins. J. Immunol. 2019, 203, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Duhan, V.; Khairnar, V.; Kitanovski, S.; Hamdan, T.A.; Klein, A.D.; Lang, J.; Ali, M.; Adomati, T.; Bhat, H.; Friedrich, S.K.; et al. Integrin α E (CD103) Limits Virus-Induced IFN-I Production in Conventional Dendritic Cells. Front. Immunol. 2020, 11, 607889. [Google Scholar] [CrossRef]
- Ling, G.S.; Bennett, J.; Woollard, K.J.; Szajna, M.; Fossati-Jimack, L.; Taylor, P.R.; Scott, D.; Franzoso, G.; Cook, H.T.; Botto, M. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat. Commun. 2014, 5, 3039. [Google Scholar] [CrossRef]
- Morrison, V.L.; James, M.J.; Grzes, K.; Cook, P.; Glass, D.G.; Savinko, T.; Lek, H.S.; Gawden-Bone, C.; Watts, C.; Millington, O.R.; et al. Loss of β2-integrin-mediated cytoskeletal linkage reprogrammes dendritic cells to a mature migratory phenotype. Nat. Commun. 2014, 5, 5359. [Google Scholar] [CrossRef]
- Gawden-Bone, C.; West, M.A.; Morrison, V.L.; Edgar, A.J.; McMillan, S.J.; Dill, B.D.; Trost, M.; Prescott, A.; Fagerholm, S.C.; Watts, C. A crucial role for β2 integrins in podosome formation, dynamics and Toll-like-receptor-signaled disassembly in dendritic cells. J. Cell. Sci. 2014, 127, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
- Guenther, C.; Faisal, I.; Uotila, L.M.; Asens, M.L.; Harjunpaa, H.; Savinko, T.; Ohman, T.; Yao, S.; Moser, M.; Morris, S.W.; et al. A β2-Integrin/MRTF-A/SRF Pathway Regulates Dendritic Cell Gene Expression, Adhesion, and Traction Force Generation. Front. Immunol. 2019, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yokota-Nakatsuma, A.; Ohoka, Y.; Takeuchi, H.; Song, S.-Y.; Iwata, M. β 1-integrin ligation and TLR ligation enhance GM-CSF–induced ALDH1A2 expression in dendritic cells, but differentially regulate their anti-inflammatory properties. Sci. Rep. 2016, 6, 37914. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, H.; An, J.; Ballantyne, C.M.; Cyster, J.G. Critical role of integrin CD11c in splenic dendritic cell capture of missing-self CD47 cells to induce adaptive immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 6786–6791. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Klementowicz, J.E.; Rahman, S.; Czajkowska, B.I.; Smedley, C.; Waldmann, H.; Sparwasser, T.; Grencis, R.K.; Travis, M.A. Loss of the TGFβ-activating integrin αvβ8 on dendritic cells protects mice from chronic intestinal parasitic infection via control of type 2 immunity. PLoS Pathog. 2013, 9, e1003675. [Google Scholar] [CrossRef]
- Kitamura, H.; Cambier, S.; Somanath, S.; Barker, T.; Minagawa, S.; Markovics, J.; Goodsell, A.; Publicover, J.; Reichardt, L.; Jablons, D.; et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Investig. 2011, 121, 2863–2875. [Google Scholar] [CrossRef]
- Altorki, T.; Muller, W.; Brass, A.; Cruickshank, S. The role of β2 integrin in dendritic cell migration during infection. BMC Immunol. 2021, 22, 2. [Google Scholar] [CrossRef]
- Nakawesi, J.; This, S.; Hutter, J.; Boucard-Jourdin, M.; Barateau, V.; Muleta, K.G.; Gooday, L.J.; Thomsen, K.F.; Lopez, A.G.; Ulmert, I.; et al. αvβ8 integrin-expression by BATF3-dependent dendritic cells facilitates early IgA responses to Rotavirus. Mucosal Immunol. 2021, 14, 53–67. [Google Scholar] [CrossRef]
- Roe, M.M.; Swain, S.; Sebrell, T.A.; Sewell, M.A.; Collins, M.M.; Perrino, B.A.; Smith, P.D.; Smythies, L.E.; Bimczok, D. Differential regulation of CD103 (αE integrin) expression in human dendritic cells by retinoic acid and Toll-like receptor ligands. J. Leukoc. Biol. 2017, 101, 1169–1180. [Google Scholar] [CrossRef]
- Lukacsi, S.; Gerecsei, T.; Balazs, K.; Francz, B.; Szabo, B.; Erdei, A.; Bajtay, Z. The differential role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in the adherence, migration and podosome formation of human macrophages and dendritic cells under inflammatory conditions. PLoS ONE 2020, 15, e0232432. [Google Scholar] [CrossRef]
- March, M.E.; Long, E.O. β2 integrin induces TCRzeta-Syk-phospholipase C-gamma phosphorylation and paxillin-dependent granule polarization in human NK cells. J. Immunol. 2011, 186, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Brzostowski, J.A.; Liu, D.; Long, E.O. Tethering of intercellular adhesion molecule on target cells is required for LFA-1-dependent NK cell adhesion and granule polarization. J. Immunol. 2010, 185, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Urlaub, D.; Hofer, K.; Muller, M.L.; Watzl, C. LFA-1 Activation in NK Cells and Their Subsets: Influence of Receptors, Maturation, and Cytokine Stimulation. J. Immunol. 2017, 198, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.M.; Zhang, J.; Siminovitch, K.A.; Takei, F. Elucidation of the integrin LFA-1-mediated signaling pathway of actin polarization in natural killer cells. Blood 2010, 116, 1272–1279. [Google Scholar] [CrossRef]
- Todros-Dawda, I.; Kveberg, L.; Vaage, J.T.; Inngjerdingen, M. The tetraspanin CD53 modulates responses from activating NK cell receptors, promoting LFA-1 activation and dampening NK cell effector functions. PLoS ONE 2014, 9, e97844. [Google Scholar] [CrossRef]
- Xiang, R.F.; Li, S.; Ogbomo, H.; Stack, D.; Mody, C.H. β1 Integrins Are Required To Mediate NK Cell Killing of Cryptococcus neoformans. J. Immunol. 2018, 201, 2369–2376. [Google Scholar] [CrossRef]
- Nielsen, N.; Odum, N.; Urso, B.; Lanier, L.L.; Spee, P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS ONE 2012, 7, e31959. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Yang, J. Integrin αvβ3 Is Essential for Maintenance of Decidua Tissue Homeostasis and of Natural Killer Cell Immune Tolerance During Pregnancy. Reprod Sci. 2018, 25, 1424–1430. [Google Scholar] [CrossRef]
- Stotesbury, C.; Alves-Peixoto, P.; Montoya, B.; Ferez, M.; Nair, S.; Snyder, C.M.; Zhang, S.; Knudson, C.J.; Sigal, L.J. α2β1 Integrin Is Required for Optimal NK Cell Proliferation during Viral Infection but Not for Acquisition of Effector Functions or NK Cell-Mediated Virus Control. J. Immunol. 2020, 204, 1582–1591. [Google Scholar] [CrossRef]
- Bouchentouf, M.; Forner, K.A.; Cuerquis, J.; Michaud, V.; Zheng, J.; Paradis, P.; Schiffrin, E.L.; Galipeau, J. Induction of cardiac angiogenesis requires killer cell lectin-like receptor 1 and α4β7 integrin expression by NK cells. J. Immunol. 2010, 185, 7014–7025. [Google Scholar] [CrossRef]
- Wen, L.; Marki, A.; Wang, Z.; Orecchioni, M.; Makings, J.; Billitti, M.; Wang, E.; Suthahar, S.S.A.; Kim, K.; Kiosses, W.B.; et al. A humanized β(2) integrin knockin mouse reveals localized intra- and extravascular neutrophil integrin activation in vivo. Cell Rep. 2022, 39, 110876. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.; Craig, H.E.; Chu, J.Y.; Carlin, L.M.; Canel, M.; Wollweber, F.; Toivakka, M.; Michael, M.; Astier, A.L.; Norton, L.; et al. A Negative Feedback Loop Regulates Integrin Inactivation and Promotes Neutrophil Recruitment to Inflammatory Sites. J. Immunol. 2019, 203, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Xu, J.; Kumar, R.S.; Lakshmikanthan, S.; Kapur, R.; Kofron, M.; Chrzanowska-Wodnicka, M.; Filippi, M.D. The small GTPase Rap1b negatively regulates neutrophil chemotaxis and transcellular diapedesis by inhibiting Akt activation. J. Exp. Med. 2014, 211, 1741–1758. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, T.; Klapproth, S.; Rohwedder, I.; Zhu, L.; Mittmann, L.; Reichel, C.A.; Sperandio, M.; Qin, J.; Moser, M. Direct Rap1/Talin1 interaction regulates platelet and neutrophil integrin activity in mice. Blood 2018, 132, 2754–2762. [Google Scholar] [CrossRef]
- Liu, L.; Aerbajinai, W.; Ahmed, S.M.; Rodgers, G.P.; Angers, S.; Parent, C.A. Radil controls neutrophil adhesion and motility through β2-integrin activation. Mol. Biol. Cell 2012, 23, 4751–4765. [Google Scholar] [CrossRef]
- Boras, M.; Volmering, S.; Bokemeyer, A.; Rossaint, J.; Block, H.; Bardel, B.; Van Marck, V.; Heitplatz, B.; Kliche, S.; Reinhold, A.; et al. Skap2 is required for β(2) integrin-mediated neutrophil recruitment and functions. J. Exp. Med. 2017, 214, 851–874. [Google Scholar] [CrossRef]
- Margraf, A.; Germena, G.; Drexler, H.C.A.; Rossaint, J.; Ludwig, N.; Prystaj, B.; Mersmann, S.; Thomas, K.; Block, H.; Gottschlich, W.; et al. The integrin-linked kinase is required for chemokine-triggered high-affinity conformation of the neutrophil β2-integrin LFA-1. Blood 2020, 136, 2200–2205. [Google Scholar] [CrossRef]
- Fan, Z.; McArdle, S.; Marki, A.; Mikulski, Z.; Gutierrez, E.; Engelhardt, B.; Deutsch, U.; Ginsberg, M.; Groisman, A.; Ley, K. Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis. Nat. Commun. 2016, 7, 12658. [Google Scholar] [CrossRef]
- Gorina, R.; Lyck, R.; Vestweber, D.; Engelhardt, B. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 2014, 192, 324–337. [Google Scholar] [CrossRef]
- Hahm, E.; Li, J.; Kim, K.; Huh, S.; Rogelj, S.; Cho, J. Extracellular protein disulfide isomerase regulates ligand-binding activity of αMβ2 integrin and neutrophil recruitment during vascular inflammation. Blood 2013, 121, 3789–3800. [Google Scholar] [CrossRef]
- Silva, L.M.; Doyle, A.D.; Greenwell-Wild, T.; Dutzan, N.; Tran, C.L.; Abusleme, L.; Juang, L.J.; Leung, J.; Chun, E.M.; Lum, A.G.; et al. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science 2021, 374, eabl5450. [Google Scholar] [CrossRef] [PubMed]
- El azreq, M.A.; Bourgoin, S.G. Cytohesin-1 regulates human blood neutrophil adhesion to endothelial cells through β2 integrin activation. Mol. Immunol. 2011, 48, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.J.; Krautter, F.; Blacksell, I.A.; Wright, R.D.; Austin-Williams, S.N.; Voisin, M.B.; Hussain, M.T.; Law, H.L.; Niki, T.; Hirashima, M.; et al. Galectin-9 mediates neutrophil capture and adhesion in a CD44 and β2 integrin-dependent manner. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22065. [Google Scholar] [CrossRef]
- Pruenster, M.; Kurz, A.R.; Chung, K.J.; Cao-Ehlker, X.; Bieber, S.; Nussbaum, C.F.; Bierschenk, S.; Eggersmann, T.K.; Rohwedder, I.; Heinig, K.; et al. Extracellular MRP8/14 is a regulator of β2 integrin-dependent neutrophil slow rolling and adhesion. Nat. Commun. 2015, 6, 6915. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.L.; Schulze, K.E.; Jones, E.L.; Yeung, L.; Cheng, Q.; Pereira, C.F.; Costin, A.; Ramm, G.; van Spriel, A.B.; Hickey, M.J.; et al. Tetraspanin CD37 Regulates β2 Integrin-Mediated Adhesion and Migration in Neutrophils. J. Immunol. 2015, 195, 5770–5779. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.C.; Melis, N.; Chen, D.; Wang, W.; Gallardo, D.; Weigert, R.; Parent, C.A. The LTB4-BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. J. Cell Biol. 2020, 219, e201910215. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.B.; Zmijewski, J.W.; Deshane, J.S.; Zhi, D.; Thompson, L.C.; Peterson, C.B.; Chaplin, D.D.; Abraham, E. Vitronectin inhibits neutrophil apoptosis through activation of integrin-associated signaling pathways. Am. J. Respir. Cell Mol. Biol. 2012, 46, 790–796. [Google Scholar] [CrossRef]
- Saldanha-Gama, R.F.; Moraes, J.A.; Mariano-Oliveira, A.; Coelho, A.L.; Walsh, E.M.; Marcinkiewicz, C.; Barja-Fidalgo, C. α(9)β(1) integrin engagement inhibits neutrophil spontaneous apoptosis: Involvement of Bcl-2 family members. Biochim. Et Biophys. Acta 2010, 1803, 848–857. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Kumar, S.; Xu, J.; Perkins, C.; Guo, F.; Snapper, S.; Finkelman, F.D.; Zheng, Y.; Filippi, M.D. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood 2012, 120, 3563–3574. [Google Scholar] [CrossRef]
- Herbert, J.A.; Deng, Y.; Hardelid, P.; Robinson, E.; Ren, L.; Moulding, D.; Smyth, R.L.; Smith, C.M. β(2)-integrin LFA1 mediates airway damage following neutrophil transepithelial migration during respiratory syncytial virus infection. Eur. Respir. J. 2020, 56, 1902216. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Döring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 2014, 123, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.J.; Sharma, R.S.; McKenzie, E.J.; Richards, H.E.; Zhang, J.; Prescott, A.; Crocker, P.R. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood 2013, 121, 2084–2094. [Google Scholar] [CrossRef]
- McMillan, S.J.; Sharma, R.S.; Richards, H.E.; Hegde, V.; Crocker, P.R. Siglec-E promotes β2-integrin-dependent NADPH oxidase activation to suppress neutrophil recruitment to the lung. J. Biol. Chem. 2014, 289, 20370–20376. [Google Scholar] [CrossRef]
- Patel, S.K.; Janjic, J.M. Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics 2015, 5, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Yeh, S.I.; Chen, S.L.; Tsao, Y.P. Integrin αv and Vitronectin Prime Macrophage-Related Inflammation and Contribute the Development of Dry Eye Disease. Int. J. Mol. Sci. 2021, 22, 8410. [Google Scholar] [CrossRef]
- Cha, B.H.; Shin, S.R.; Leijten, J.; Li, Y.C.; Singh, S.; Liu, J.C.; Annabi, N.; Abdi, R.; Dokmeci, M.R.; Vrana, N.E.; et al. Integrin-Mediated Interactions Control Macrophage Polarization in 3D Hydrogels. Adv. Health Mater. 2017, 6, 1700289. [Google Scholar] [CrossRef]
- Paterson, N.; Lammermann, T. Macrophage network dynamics depend on haptokinesis for optimal local surveillance. Elife 2022, 11, e75354. [Google Scholar] [CrossRef]
- Cui, K.; Ardell, C.L.; Podolnikova, N.P.; Yakubenko, V.P. Distinct Migratory Properties of M1, M2, and Resident Macrophages Are Regulated by α(D)β(2) and α(M)β(2) Integrin-Mediated Adhesion. Front. Immunol. 2018, 9, 2650. [Google Scholar] [CrossRef]
- Becker, H.M.; Rullo, J.; Chen, M.; Ghazarian, M.; Bak, S.; Xiao, H.; Hay, J.B.; Cybulsky, M.I. α1β1 integrin-mediated adhesion inhibits macrophage exit from a peripheral inflammatory lesion. J. Immunol. 2013, 190, 4305–4314. [Google Scholar] [CrossRef]
- Guo, Q.; Furuta, K.; Lucien, F.; Gutierrez Sanchez, L.H.; Hirsova, P.; Krishnan, A.; Kabashima, A.; Pavelko, K.D.; Madden, B.; Alhuwaish, H.; et al. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J. Hepatol. 2019, 71, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Xin, X.; Xin, H.; Shen, X.; Zhu, Y.Z. Hydrogen Sulfide Recruits Macrophage Migration by Integrin β1-Src-FAK/Pyk2-Rac Pathway in Myocardial Infarction. Sci. Rep. 2016, 6, 22363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Che, J.; Chang, L.; Guo, M.; Bao, X.; Mu, D.; Sun, X.; Zhang, X.; Lu, W.; Xie, J. CD47- and Integrin α4/β1-Comodified-Macrophage-Membrane-Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv. Health Mater. 2022, 11, e2101788. [Google Scholar] [CrossRef] [PubMed]
- Furundzija, V.; Fritzsche, J.; Kaufmann, J.; Meyborg, H.; Fleck, E.; Kappert, K.; Stawowy, P. IGF-1 increases macrophage motility via PKC/p38-dependent αvβ3-integrin inside-out signaling. Biochem. Biophys. Res. Commun. 2010, 394, 786–791. [Google Scholar] [CrossRef]
- Wei, J.; Marisetty, A.; Schrand, B.; Gabrusiewicz, K.; Hashimoto, Y.; Ott, M.; Grami, Z.; Kong, L.Y.; Ling, X.; Caruso, H.; et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Investig. 2019, 129, 137–149. [Google Scholar] [CrossRef]
- Lund, S.A.; Wilson, C.L.; Raines, E.W.; Tang, J.; Giachelli, C.M.; Scatena, M. Osteopontin mediates macrophage chemotaxis via α4 and α9 integrins and survival via the α4 integrin. J. Cell. Biochem. 2013, 114, 1194–1202. [Google Scholar] [CrossRef]
- Cui, K.; Podolnikova, N.P.; Bailey, W.; Szmuc, E.; Podrez, E.A.; Byzova, T.V.; Yakubenko, V.P. Inhibition of integrin α(D)β(2)-mediated macrophage adhesion to end product of docosahexaenoic acid (DHA) oxidation prevents macrophage accumulation during inflammation. J. Biol. Chem. 2019, 294, 14370–14382. [Google Scholar] [CrossRef]
- Aziz, M.H.; Cui, K.; Das, M.; Brown, K.E.; Ardell, C.L.; Febbraio, M.; Pluskota, E.; Han, J.; Wu, H.; Ballantyne, C.M.; et al. The Upregulation of Integrin α(D)β(2) (CD11d/CD18) on Inflammatory Macrophages Promotes Macrophage Retention in Vascular Lesions and Development of Atherosclerosis. J. Immunol. 2017, 198, 4855–4867. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Kong, M.; Mao, L.; Yang, Y.; Xu, Y. Myocardin-related transcription factor A regulates integrin β 2 transcription to promote macrophage infiltration and cardiac hypertrophy in mice. Cardiovasc. Res. 2022, 118, 844–858. [Google Scholar] [CrossRef]
- Guillot, A.; Guerri, L.; Feng, D.; Kim, S.J.; Ahmed, Y.A.; Paloczi, J.; He, Y.; Schuebel, K.; Dai, S.; Liu, F.; et al. Bile acid-activated macrophages promote biliary epithelial cell proliferation through integrin αvβ6 upregulation following liver injury. J. Clin. Investig. 2021, 131, e132305. [Google Scholar] [CrossRef]
- Wang, B.; Lim, J.H.; Kajikawa, T.; Li, X.; Vallance, B.A.; Moutsopoulos, N.M.; Chavakis, T.; Hajishengallis, G. Macrophage β2-Integrins Regulate IL-22 by ILC3s and Protect from Lethal Citrobacter rodentium-Induced Colitis. Cell. Rep. 2019, 26, 156–161; discussion 162–163. [Google Scholar] [CrossRef] [PubMed]
- Plosa, E.J.; Benjamin, J.T.; Sucre, J.M.; Gulleman, P.M.; Gleaves, L.A.; Han, W.; Kook, S.; Polosukhin, V.V.; Haake, S.M.; Guttentag, S.H.; et al. β1 Integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight 2020, 5, e129259. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Han, J.; Peng, W.; Fang, Z.; Zhou, Y.; Xu, X.; Lin, J.; Xiao, F.; Zhao, L.; et al. Convallatoxin Promotes M2 Macrophage Polarization to Attenuate Atherosclerosis Through PPARgamma-Integrin α(v)β(5) Signaling Pathway. Drug Des. Devel. Ther. 2021, 15, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Antonova, G.N.; Munn, D.H.; Mivechi, N.; Lucas, R.; Catravas, J.D.; Verin, A.D. αVβ3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-kappaB activation. J. Cell. Physiol. 2011, 226, 469–476. [Google Scholar] [CrossRef]
- Schmid, M.C.; Avraamides, C.J.; Foubert, P.; Shaked, Y.; Kang, S.W.; Kerbel, R.S.; Varner, J.A. Combined blockade of integrin-α4β1 plus cytokines SDF-1α or IL-1β potently inhibits tumor inflammation and growth. Cancer Res. 2011, 71, 6965–6975. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Lin, M.; Zhu, Y.; Lum, L.; Thakur, A.; Jin, R.; Shao, W.; Zhang, Y.; Hu, Y.; Huang, S.; et al. Integrin β4-Targeted Cancer Immunotherapies Inhibit Tumor Growth and Decrease Metastasis. Cancer Res. 2020, 80, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Ikenaga, N.; Liu, S.B.; Sverdlov, D.Y.; Vaid, K.A.; Dixit, R.; Weinreb, P.H.; Violette, S.; Sheppard, D.; Schuppan, D.; et al. Integrin αvβ6 critically regulates hepatic progenitor cell function and promotes ductular reaction, fibrosis, and tumorigenesis. Hepatology 2016, 63, 217–232. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Gao, D.; Liu, H.; Yu, X.; Lai, J.; Wang, F.; Lin, J.; Liu, Z. Enhanced Anti-Tumor Efficacy through a Combination of Integrin αvβ6-Targeted Photodynamic Therapy and Immune Checkpoint Inhibition. Theranostics 2016, 6, 627–637. [Google Scholar] [CrossRef]
- Zhang, B.; Ye, H.; Ren, X.; Zheng, S.; Zhou, Q.; Chen, C.; Lin, Q.; Li, G.; Wei, L.; Fu, Z.; et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019, 459, 204–215. [Google Scholar] [CrossRef]
- Peng, P.; Zhu, H.; Liu, D.; Chen, Z.; Zhang, X.; Guo, Z.; Dong, M.; Wan, L.; Zhang, P.; Liu, G.; et al. TGFBI secreted by tumor-associated macrophages promotes glioblastoma stem cell-driven tumor growth via integrin αvβ5-Src-Stat3 signaling. Theranostics 2022, 12, 4221–4236. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, G.; Zhu, H.; Xu, A. ITGA5 is a prognostic biomarker and correlated with immune infiltration in gastrointestinal tumors. BMC Cancer 2021, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Malenica, I.; Adam, J.; Corgnac, S.; Mezquita, L.; Auclin, E.; Damei, I.; Grynszpan, L.; Gros, G.; de Montpréville, V.; Planchard, D.; et al. Integrin-α(V)-mediated activation of TGF-β regulates anti-tumour CD8 T cell immunity and response to PD-1 blockade. Nat Commun 2021, 12, 5209. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Park, E.J.; Gaowa, A.; Kawamoto, E.; Shimaoka, M. Targeted remodeling of breast cancer and immune cell homing niches by exosomal integrins. Diagn Pathol. 2020, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Montresor, A.; Toffali, L.; Mirenda, M.; Rigo, A.; Vinante, F.; Laudanna, C. JAK2 tyrosine kinase mediates integrin activation induced by CXCL12 in B-cell chronic lymphocytic leukemia. Oncotarget 2015, 6, 34245–34257. [Google Scholar] [CrossRef]
- Stockis, J.; Lienart, S.; Colau, D.; Collignon, A.; Nishimura, S.L.; Sheppard, D.; Coulie, P.G.; Lucas, S. Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αVβ8. Proc. Natl. Acad. Sci. USA 2017, 114, E10161–E10168. [Google Scholar] [CrossRef]
- Gandoglia, I.; Ivaldi, F.; Carrega, P.; Armentani, E.; Ferlazzo, G.; Mancardi, G.; Kerlero de Rosbo, N.; Uccelli, A.; Laroni, A. In vitro VLA-4 blockade results in an impaired NK cell-mediated immune surveillance against melanoma. Immunol. Lett. 2017, 181, 109–115. [Google Scholar] [CrossRef]
- Busenhart, P.; Montalban-Arques, A.; Katkeviciute, E.; Morsy, Y.; Van Passen, C.; Hering, L.; Atrott, K.; Lang, S.; Garzon, J.F.G.; Naschberger, E.; et al. Inhibition of integrin αvβ6 sparks T-cell antitumor response and enhances immune checkpoint blockade therapy in colorectal cancer. J. Immunother. Cancer 2022, 10, e003465. [Google Scholar] [CrossRef]
- Laine, A.; Labiad, O.; Hernandez-Vargas, H.; This, S.; Sanlaville, A.; Leon, S.; Dalle, S.; Sheppard, D.; Travis, M.A.; Paidassi, H.; et al. Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat. Commun. 2021, 12, 6228. [Google Scholar] [CrossRef]
- Foubert, P.; Kaneda, M.M.; Varner, J.A. PI3Kgamma Activates Integrin α4 and Promotes Immune Suppressive Myeloid Cell Polarization during Tumor Progression. Cancer Immunol. Res. 2017, 5, 957–968. [Google Scholar] [CrossRef]
- Berrazouane, S.; Boisvert, M.; Salti, S.; Mourad, W.; Al-Daccak, R.; Barabe, F.; Aoudjit, F. Beta1 integrin blockade overcomes doxorubicin resistance in human T-cell acute lymphoblastic leukemia. Cell Death Dis 2019, 10, 357. [Google Scholar] [CrossRef]
- Corgnac, S.; Boutet, M.; Kfoury, M.; Naltet, C.; Mami-Chouaib, F. The Emerging Role of CD8(+) Tissue Resident Memory T (T(RM)) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front. Immunol. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Corgnac, S.; Boutet, M.; Gros, G.; Validire, P.; Bismuth, G.; Mami-Chouaib, F. Paxillin Binding to the Cytoplasmic Domain of CD103 Promotes Cell Adhesion and Effector Functions for CD8(+) Resident Memory T Cells in Tumors. Cancer Res. 2017, 77, 7072–7082. [Google Scholar] [CrossRef] [PubMed]
- Hickman, A.; Koetsier, J.; Kurtanich, T.; Nielsen, M.C.; Winn, G.; Wang, Y.; Bentebibel, S.E.; Shi, L.; Punt, S.; Williams, L.; et al. LFA-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with CTLA-4 blockade. J. Clin. Investig. 2022, 132, e154152. [Google Scholar] [CrossRef] [PubMed]
- Mahuron, K.M.; Moreau, J.M.; Glasgow, J.E.; Boda, D.P.; Pauli, M.L.; Gouirand, V.; Panjabi, L.; Grewal, R.; Luber, J.M.; Mathur, A.N.; et al. Layilin augments integrin activation to promote antitumor immunity. J. Exp. Med. 2020, 217, e20192080. [Google Scholar] [CrossRef]
- Boutet, M.; Gauthier, L.; Leclerc, M.; Gros, G.; de Montpreville, V.; Théret, N.; Donnadieu, E.; Mami-Chouaib, F. TGFβ Signaling Intersects with CD103 Integrin Signaling to Promote T-Lymphocyte Accumulation and Antitumor Activity in the Lung Tumor Microenvironment. Cancer Res. 2016, 76, 1757–1769. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Le Floc’h, A.; Boutet, M.; Vergnon, I.; Schmitt, A.; Mami-Chouaib, F. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 2013, 73, 617–628. [Google Scholar] [CrossRef]
- Anikeeva, N.; Steblyanko, M.; Fayngerts, S.; Kopylova, N.; Marshall, D.J.; Powers, G.D.; Sato, T.; Campbell, K.S.; Sykulev, Y. Integrin receptors on tumor cells facilitate NK cell-mediated antibody-dependent cytotoxicity. Eur. J. Immunol. 2014, 44, 2331–2339. [Google Scholar] [CrossRef]
- Tang, Z.; Davidson, D.; Li, R.; Zhong, M.C.; Qian, J.; Chen, J.; Veillette, A. Inflammatory macrophages exploit unconventional pro-phagocytic integrins for phagocytosis and anti-tumor immunity. Cell Rep. 2021, 37, 110111. [Google Scholar] [CrossRef]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P.; et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell. Rep. 2019, 27, 1967–1978.e1964. [Google Scholar] [CrossRef]
- Guha, P.; Cunetta, M.; Somasundar, P.; Espat, N.J.; Junghans, R.P.; Katz, S.C. Frontline Science: Functionally impaired geriatric CAR-T cells rescued by increased α5β1 integrin expression. J. Leukoc. Biol. 2017, 102, 201–208. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Francisco, N.M.; Zhang, Y.; Wu, M. The application of CAR-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm. Sin. B 2018, 8, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Phanthaphol, N.; Somboonpatarakun, C.; Suwanchiwasiri, K.; Chieochansin, T.; Sujjitjoon, J.; Wongkham, S.; Maher, J.; Junking, M.; Yenchitsomanus, P.T. Chimeric Antigen Receptor T Cells Targeting Integrin αvβ6 Expressed on Cholangiocarcinoma Cells. Front. Oncol. 2021, 11, 657868. [Google Scholar] [CrossRef] [PubMed]
- Whilding, L.M.; Parente-Pereira, A.C.; Zabinski, T.; Davies, D.M.; Petrovic, R.M.G.; Kao, Y.V.; Saxena, S.A.; Romain, A.; Costa-Guerra, J.A.; Violette, S.; et al. Targeting of Aberrant αvβ6 Integrin Expression in Solid Tumors Using Chimeric Antigen Receptor-Engineered T Cells. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Hosen, N.; Matsunaga, Y.; Hasegawa, K.; Matsuno, H.; Nakamura, Y.; Makita, M.; Watanabe, K.; Yoshida, M.; Satoh, K.; Morimoto, S.; et al. The activated conformation of integrin β(7) is a novel multiple myeloma-specific target for CAR T cell therapy. Nature medicine 2017, 23, 1436–1443. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Fujii, K.; Kurozumi, K.; Ichikawa, T.; Onishi, M.; Shimazu, Y.; Ishida, J.; Chiocca, E.A.; Kaur, B.; Date, I. The integrin inhibitor cilengitide enhances the anti-glioma efficacy of vasculostatin-expressing oncolytic virus. Cancer Gene Ther. 2013, 20, 437–444. [Google Scholar] [CrossRef]
- Lee, T.J.; Nair, M.; Banasavadi-Siddegowda, Y.; Liu, J.; Nallanagulagari, T.; Jaime-Ramirez, A.C.; Guo, J.Y.; Quadri, H.; Zhang, J.; Bockhorst, K.H.; et al. Enhancing Therapeutic Efficacy of Oncolytic Herpes Simplex Virus-1 with Integrin β1 Blocking Antibody OS2966. Mol. Cancer Ther. 2019, 18, 1127–1136. [Google Scholar] [CrossRef]
- Davies, J.A.; Marlow, G.; Uusi-Kerttula, H.K.; Seaton, G.; Piggott, L.; Badder, L.M.; Clarkson, R.W.E.; Chester, J.D.; Parker, A.L. Efficient Intravenous Tumor Targeting Using the αvβ6 Integrin-Selective Precision Virotherapy Ad5(NULL)-A20. Viruses 2021, 13, 864. [Google Scholar] [CrossRef]
- Uusi-Kerttula, H.; Davies, J.A.; Thompson, J.M.; Wongthida, P.; Evgin, L.; Shim, K.G.; Bradshaw, A.; Baker, A.T.; Rizkallah, P.J.; Jones, R.; et al. Ad5(NULL)-A20: A Tropism-Modified, αvβ6 Integrin-Selective Oncolytic Adenovirus for Epithelial Ovarian Cancer Therapies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4215–4224. [Google Scholar] [CrossRef]
- Pesonen, S.; Diaconu, I.; Cerullo, V.; Escutenaire, S.; Raki, M.; Kangasniemi, L.; Nokisalmi, P.; Dotti, G.; Guse, K.; Laasonen, L.; et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int. J. Cancer 2012, 130, 1937–1947. [Google Scholar] [CrossRef]
- Watanabe, N.; McKenna, M.K.; Rosewell Shaw, A.; Suzuki, M. Clinical CAR-T Cell and Oncolytic Virotherapy for Cancer Treatment. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.S.; National Cancer Institute; Stanford University. 18F-FPPRGD2 PET/CT or PET/MRI in Predicting Early Response in Patients With Cancer Receiving Anti-Angiogenesis Therapy. 2013. Available online: https://ClinicalTrials.gov/show/NCT01806675 (accessed on 21 March 2023).
- Healthcare, G. A Proof-of-concept Study to Assess the Ability of 18F AH-111585 PET Imaging to Detect Tumours and Angiogenesis. 2007. Available online: https://ClinicalTrials.gov/show/NCT00565721 (accessed on 21 March 2023).
- Rigshospitalet, D. PET/CT Imaging of Angiogenesis in Patients With Neuroendocrine Tumors Using 68Ga-NODAGA-E c(RGDyK) 2. 2017. Available online: https://ClinicalTrials.gov/show/NCT03271281 (accessed on 21 March 2023).
- Ma, P.; Jin, X.; Fan, Z.; Wang, Z.; Yue, S.; Wu, C.; Chen, S.; Wu, Y.; Chen, M.; Gu, D.; et al. Super-enhancer receives signals from the extracellular matrix to induce PD-L1-mediated immune evasion via integrin/BRAF/TAK1/ERK/ETV4 signaling. Cancer Biol. Med. 2021, 19, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Leoni, V.; Barboni, C.; Sanapo, M.; Zaghini, A.; Malatesta, P.; Campadelli-Fiume, G.; Gianni, T. αvβ3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc. Natl. Acad. Sci. USA 2019, 116, 20141–20150. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Toner, Y.C.; Meerwaldt, A.E.; van Leent, M.M.T.; Soultanidis, G.; Elschot, M.; Gonai, H.; Grendstad, K.; Flobak, Å.; Neckmann, U.; et al. Tumor Targeting by α(v)β(3)-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano 2020, 14, 7832–7846. [Google Scholar] [CrossRef]
- Biogen. Natalizumab (BG00002, Tysabri) Study in Japanese Participants With Relapsing-Remitting Multiple Sclerosis (RRMS). 2010. Available online: https://ClinicalTrials.gov/show/NCT01440101 (accessed on 21 March 2023).
- Biogen; Pharmaceuticals, E. Safety Study of Natalizumab to Treat Multiple Sclerosis (MS). 2007. Available online: https://ClinicalTrials.gov/show/NCT00559702 (accessed on 21 March 2023).
- Biogen; Pharmaceuticals, E. A Pharmacokinetic (PK) Study of Natalizumab (Tysabri) at Steady State. 2008. Available online: https://ClinicalTrials.gov/show/NCT00744679 (accessed on 21 March 2023).
- Buffalo, U.A. Natalizumab Temporary Discontinuation Study. 2012. Available online: https://ClinicalTrials.gov/show/NCT02775110 (accessed on 21 March 2023).
- Pan, X.; Yi, M.; Liu, C.; Jin, Y.; Liu, B.; Hu, G.; Yuan, X. Cilengitide, an αvβ3-integrin inhibitor, enhances the efficacy of anti-programmed cell death-1 therapy in a murine melanoma model. Bioengineered 2022, 13, 4557–4572. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell. 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Szaryńska, M.; Olejniczak, A.; Kobiela, J.; Łaski, D.; Śledziński, Z.; Kmieć, Z. Cancer stem cells as targets for DC-based immunotherapy of colorectal cancer. Sci. Rep. 2018, 8, 12042. [Google Scholar] [CrossRef]
- van Montfoort, N.; Borst, L.; Korrer, M.J.; Sluijter, M.; Marijt, K.A.; Santegoets, S.J.; van Ham, V.J.; Ehsan, I.; Charoentong, P.; André, P.; et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell 2018, 175, 1744–1755.e15. [Google Scholar] [CrossRef]
- Maeda, N.; Ohashi, T.; Chagan-Yasutan, H.; Hattori, T.; Takahashi, Y.; Harigae, H.; Hasegawa, H.; Yamada, Y.; Fujii, M.; Maenaka, K.; et al. Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology 2015, 12, 99. [Google Scholar] [CrossRef]
- Kelly, K.A.; Nahrendorf, M.; Yu, A.M.; Reynolds, F.; Weissleder, R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol. Imaging Biol. 2006, 8, 201–207. [Google Scholar] [CrossRef]
- Ailuno, G.; Baldassari, S.; Zuccari, G.; Schlich, M.; Caviglioli, G. Peptide-based nanosystems for vascular cell adhesion molecule-1 targeting: A real opportunity for therapeutic and diagnostic agents in inflammation associated disorders. J. Drug Deliv. Sci. Technol. 2020, 55, 101461. [Google Scholar] [CrossRef]
- Koudrina, A.; DeRosa, M.C. Advances in Medical Imaging: Aptamer- and Peptide-Targeted MRI and CT Contrast Agents. ACS Omega 2020, 5, 22691–22701. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Baldassari, S.; Ailuno, G.; Zuccari, G.; Drava, G.; Petretto, A.; Cossu, V.; Marini, C.; Alfei, S.; Florio, T.; et al. Two Novel PET Radiopharmaceuticals for Endothelial Vascular Cell Adhesion Molecule-1 (VCAM-1) Targeting. Pharmaceutics 2021, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Haugh, A.M.; Probasco, J.C.; Johnson, D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin. Drug Safety 2020, 19, 479–488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).