Rhamnolipid Self-Aggregation in Aqueous Media: A Long Journey toward the Definition of Structure–Property Relationships
Abstract
1. Introduction
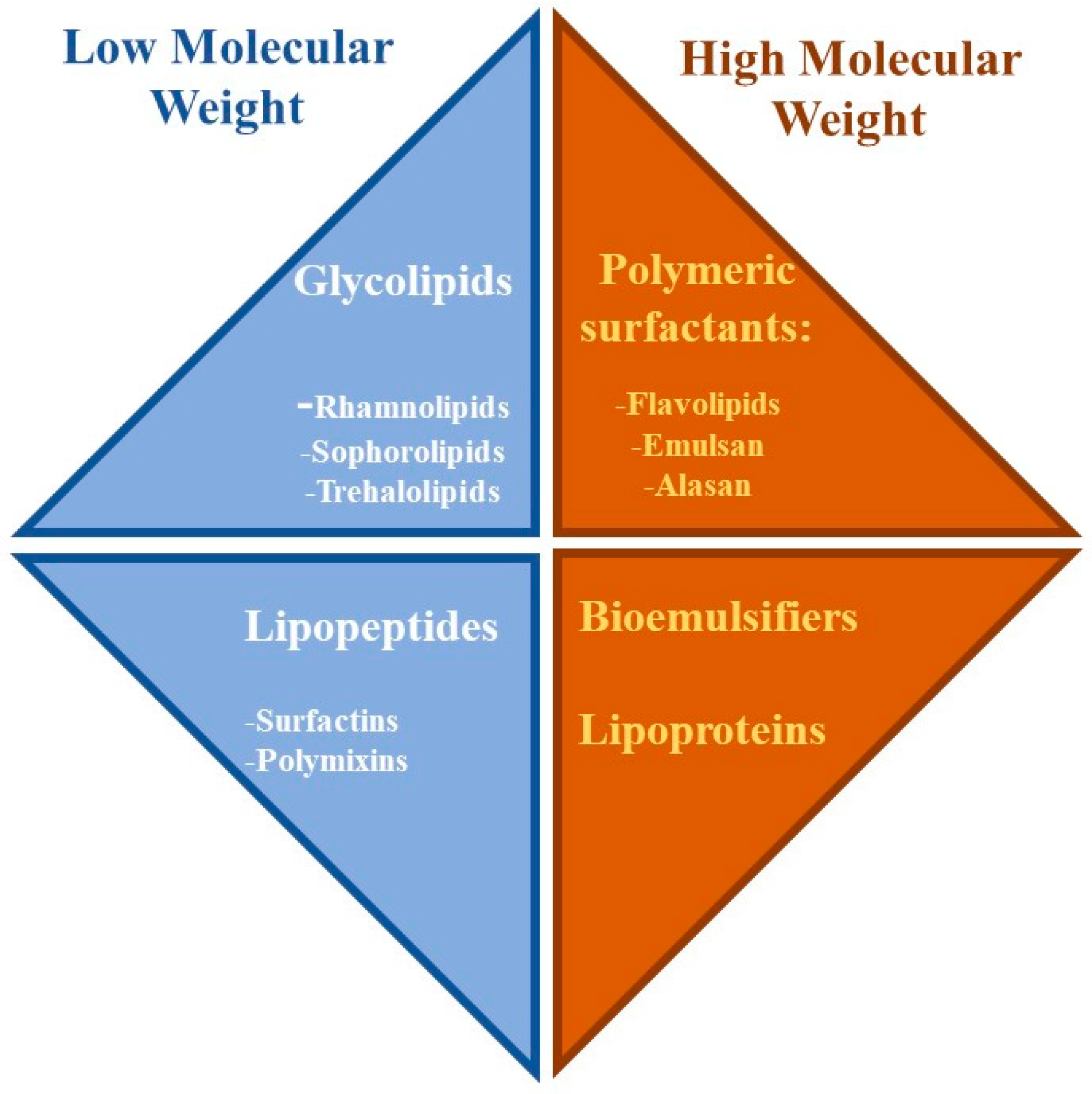
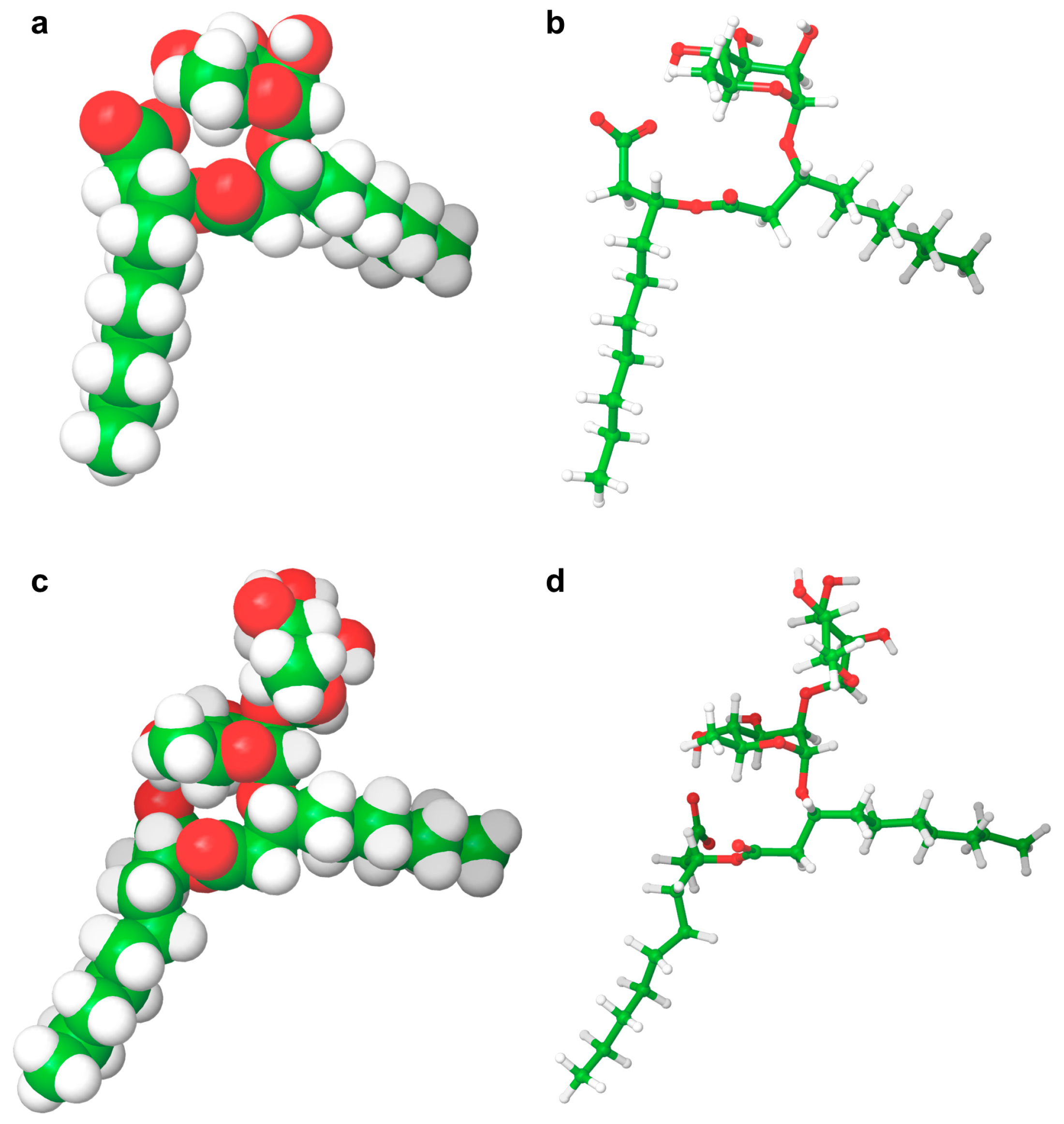
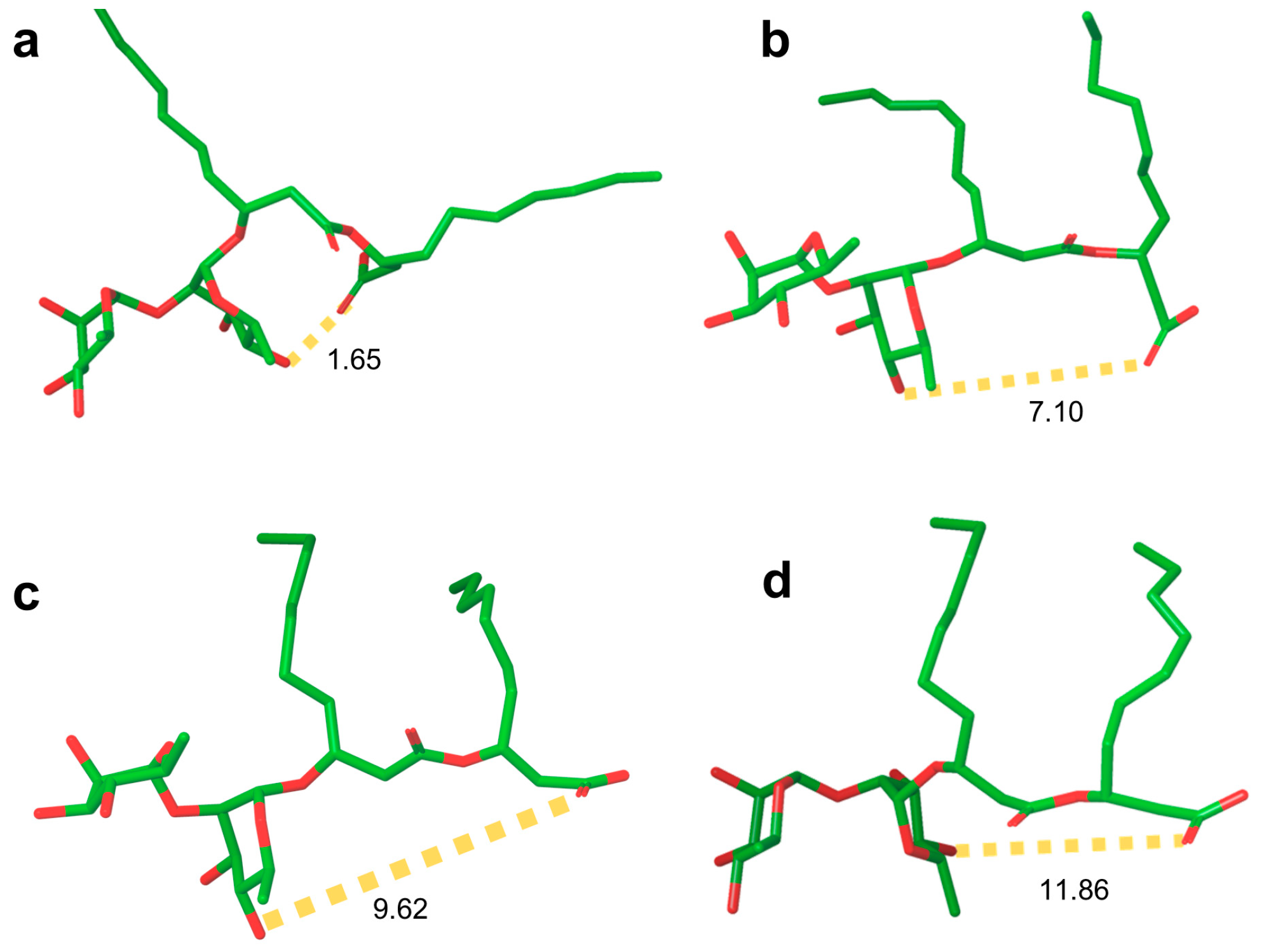
Rhamnolipid Structure
2. Surface Properties and Micellization
2.1. Crude Extracts
2.2. “Pure” Rhamnolipids
2.2.1. Critical Micellar Concentration
2.2.2. Surface Tension Reduction
2.2.3. Surface Adsorption
2.3. Synthetic Rhamnolipids
2.4. Computational Results
3. Aggregation Behavior
3.1. “Pure” Rhamnolipids
| Rhamnolipid | Purity (%) | Concentration (mM) | T | pH | Additive | Aggregate | Dimensions (R, nm) | Experimental Technique § | cpp | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Rha-C10-C10 | 96 | 0.02–0.1 | 25 | 6.8 | Micelles | 75 * 25 ** | DLS | 0.62 | [179] | |
| 96 | 0.5–1 | 25 | 6.8 | Micelles | 80 * 35 ** | DLS | 0.62 | [179] | ||
| 96 | 10 | 25 | 6.8 | Micelles | 100 * 50 ** | DLS | 0.62 | [179] | ||
| 96 | 15–20 | 25 | 6.8 | Vesicles | 30, 125 * 20 ** | DLS | 0.62 | [179] | ||
| 96 | 45 | 25 | 6.8 | Vesicles | 30, 150 * 20 ** | DLS | 0.62 | [179] | ||
| 96 | 1 | 25 | 6.8 | Elliptical vesicles | 75 | SEM | 0.62 | [179] | ||
| 98 † | 5 | RT | 8 | Ellipsoidal micelles Vesicles | 2.2, 10.5, 100 * 2.2 ** | DLS | 0.48 | [174] | ||
| 98 † | 20 | RT | 8 | Ellipsoidal micelles Vesicles | 2.8, 10.2, 90 * 2.8 ** | DLS | [174] | |||
| 98 † | 0.05–15 | RT | 8 | 250 nM prodan | Lamellar aggregates | Fluorescence | [174] | |||
| 98 † | 0.05–15 | RT | 4 | 250 nM prodan | Lamellar aggregates | Fluorescence | [174] | |||
| 98 † | 0.05–15 | RT | 8 | 250 nM prodan | Micelles | Fluorescence | [174] | |||
| 98 † | 10 | RT | 8 | 250 nM pyrene | Micelles | Fluorescence | [174] | |||
| 96 | >5% (w/v) | RT | 6.8 | - | Spherical micelles/lamellar | POM | 0.24 | [172] | ||
| 96 | >5% (w/v) | RT | 6.8 | 0.05 M NaCl | Spherical micelles/lamellar | POM | 0.50 | [172] | ||
| 96 | >5% (w/v) | RT | 6.8 | 0.5 M NaCl | Lamellar/bilayers or multilamellar | POM | 0.61 | [172] | ||
| 96 | >5% (w/v) | RT | 6.8 | 1.0 M NaCl | Isotropic hexagonal | POM | 0.41 | [172] | ||
| Purified | 20 | 9 | Borax 0.023 M HCl 0.008 M | Micelles | SANS | [176] | ||||
| Purified | 30–100 | 9 | Borax 0.023 M HCl 0.008 M | Lamellar structures | SANS | [176] | ||||
| Purified | 0.02 | RT | 6.8 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.05 | RT | 6.8 | Micelles | 28 | DLS | [170] | |||
| Purified | 0.1 | RT | 6.8 | Micelles | 28 | DLS | [170] | |||
| Purified | 0.5 | RT | 6.8 | Micelles | 39 | DLS | [170] | |||
| Purified | 1.0 | RT | 6.8 | Micelles | 45 | DLS | [170] | |||
| Purified | 0.5 | 293 | 6.8 | Micelles | 29 | DLS | [170] | |||
| Purified | 0.5 | 298 | 6.8 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.5 | 303 | 6.8 | Micelles | 26 | DLS | [170] | |||
| Purified | 0.5 | 308 | 6.8 | Micelles | 34 | DLS | [170] | |||
| Purified | 0.5 | 313 | 6.8 | Micelles | 30 | DLS | [170] | |||
| Purified | 0.5 | 318 | 6.8 | Vesicles | 130 | DLS | [170] | |||
| Purified | 0.5 | 323 | 6.8 | Vesicles | 115 | DLS | [170] | |||
| Purified | 0.5 | RT | 2.5 | Vesicles | 170 | DLS | [170] | |||
| Purified | 0.5 | RT | 3.5 | Micelles/vesicles | 70 | DLS | [170] | |||
| Purified | 0.5 | RT | 4.5 | Micelles/vesicles | 70 | DLS | [170] | |||
| Purified | 0.5 | RT | 5.5 | Micelles | 35 | DLS | [170] | |||
| Purified | 0.5 | RT | 6.5 | Micelles | 38 | DLS | [170] | |||
| Purified | 0.5 | RT | 7.5 | Micelles | 38 | DLS | [170] | |||
| Purified | 0.5 | RT | 8.5 | Micelles | 30 | DLS | [170] | |||
| Purified | 0.5 | RT | 9.5 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.5 | RT | 10.5 | Micelles | 23 | DLS | [170] | |||
| Purified | 20 | RT | 9 | Borax 0.023 M HCl 0.008 M | Ellipsoidal micelles | 1.4 a 1.7 b Nagg = 47 | SANS | [199] | ||
| Purified | 20 | RT | 9 | Borax 0.023 M HCl 0.008 M 2 mM Ca2+ | Ellipsoidal micelles | 1.5 a 1.8 b Nagg = 55 | SANS | [199] | ||
| 95 †† | 0.05 | RT | 7.4 | Hepes 5 mM/NaCl 100 mM | Micelles | 25 | DLS | [71] | ||
| 95 †† | 0.5 | RT | 7.4 | Hepes 5 mM/Na Cl 100 mM | Cylindrical micelles | 105 | DLS | [71] | ||
| 90 | 20 | RT | 9 | Borax 0.023 M HCl 0.008 M | Ellipsoidal micelles | SANS | [175] | |||
| 90 | 50 | RT | 9 | Borax 0.023 M HCl 0.008 M | Lamellar structures | SANS | [175] | |||
| 1 | RT | 7.17 | Vesicles | 130 | DLS | [149] | ||||
| 1 | RT | 3.20 | Large aggregates | 1250 | DLS | [149] | ||||
| Rha-Rha-C10-C10 | 99 | >5% (w/v) | RT | 6.8 | - | Spherical micelles/ lamellar | POM | 0.27 | [172] | |
| 99 | >5% (w/v) | RT | 6.8 | 0.05 M NaCl | Spherical micelles/ lamellar | POM | 0.52 | [172] | ||
| 99 | >5% (w/v) | RT | 6.8 | 0.5 M NaCl | Lamellar/bilayers or multilamellar | POM | 0.52 | [172] | ||
| 99 | >5% (w/v) | RT | 6.8 | 1.0 M NaCl | Isotropic hexagonal | POM | 0.40 | [172] | ||
| 50% ††† | 0.125 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Cylindrical micelles Vesicles | 20–30 175–275 | DLS | [59] | ||
| 50% ††† | 0.25 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Cylindrical micelles Vesicles | 20–30 175–275 | DLS | [59] | ||
| 50% ††† | 0.5 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Cylindrical micelles Vesicles | 20–30 175–275 | DLS | [59] | ||
| 50% ††† | 1 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Cylindrical micelles Vesicles | 20–30 175–275 | DLS | [59] | ||
| 50% ††† | 1.5 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Vesicles | 175–275 >750 | DLS | [59] | ||
| 50% ††† | 2.5 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Vesicles | >750 | DLS | [59] | ||
| 50% ††† | 5 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Vesicles | >750 | DLS | [59] | ||
| 50% ††† | >2.5 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | MLV Elongated vesicles | >75–100 >500 | TEM | [59] | ||
| 50% ††† | 20 | 25 | 7.4 | 5 mM Hepes 0.15 M NaCl | Lamellar multilayers | SAXS | [59] | |||
| Purified | 50 | RT | 9 | Borax 0.023 M 0.008 M HCl | Ellipsoidal micelles | 1.15 a 1.5 b Nagg = 34 | SANS | [176] | ||
| Purified | 20 | RT | 9 | Borax 0.023 M 0.008 M HCl | Ellipsoidal micelles | 1.2 a 1.5 b Nagg = 26 | SANS | [175] | ||
| Purified | 50 | RT | 9 | Borax 0.023 M HCl 0.008 M | Ellipsoidal micelles | 1.2 a 1.5 b Nagg = 34 | SANS | [175] | ||
| Purified | 100 | RT | 9 | Borax 0.023 M 0.008 M HCl | Ellipsoidal micelles | 1.1 a 1.5 b Nagg = 86 | SANS | [175] | ||
| Purified | 20 | RT | 9 | Borax 0.023 M 0.008 M HCl | Ellipsoidal micelles | 1.2 a 1.5 b Nagg = 26 | SANS | [199] | ||
| Purified | 20 | RT | 9 | Borax 0.023 M 0.008 M HCl 2 mM Ca2+ | Ellipsoidal micelles | 1.2 a 1.6 b Nagg = 28 | SANS | [199] | ||
| Purified | 50 | RT | 9 | Borax 0.023 M HCl 0.008 M | Ellipsoidal micelles | 1.2 a 1.5 b Nagg = 34 | SANS | [199] | ||
| Purified | 50 | RT | 9 | Borax 0.023 M 0.008 M HCl 2 mM Ca2 | Ellipsoidal micelles | 1.2 a 1.6 b Nagg = 38 | SANS | [199] | ||
| Purified | 100 | RT | 9 | Borax 0.023 M HCl 0.008 M | Ellipsoidal micelles | 1.1 a 1.5 b Nagg = 86 | SANS | [199] | ||
| Purified | 100 | RT | 9 | Borax 0.023 M HCl 0.008 M 2 mM Ca2 | Ellipsoidal micelles | 1.1 a 1.4 b Nagg = 91 | SANS | [199] | ||
| Purified | 0.02 | RT | 6.8 | Micelles/vesicles | 75 | DLS | [170] | |||
| Purified | 0.05 | RT | 6.8 | Micelles | 35 | DLS | [170] | |||
| Purified | 0.1 | RT | 6.8 | Micelles | 35 | DLS | [170] | |||
| Purified | 0.5 | RT | 6.8 | Micelles | 22 | DLS | [170] | |||
| Purified | 1.0 | RT | 6.8 | Micelles | 30 | DLS | [170] | |||
| Purified | 0.5 | 293 | 6.8 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.5 | 298 | 6.8 | Micelles | 30 | DLS | [170] | |||
| Purified | 0.5 | 303 | 6.8 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.5 | 308 | 6.8 | Micelles | 20 | DLS | [170] | |||
| Purified | 0.5 | 313 | 6.8 | Micelles | 15 | DLS | [170] | |||
| Purified | 0.5 | 318 | 6.8 | Micelles/vesicles | 90 | DLS | [170] | |||
| Purified | 0.5 | 323 | 6.8 | Vesicles | 95 | DLS | [170] | |||
| Purified | 0.5 | RT | 2.5 | Vesicles | 230 | DLS | [170] | |||
| Purified | 0.5 | RT | 3.5 | Vesicles | 145 | DLS | [170] | |||
| Purified | 0.5 | RT | 4.5 | Vesicles | 140 | DLS | [170] | |||
| Purified | 0.5 | RT | 5.5 | Micelles/vesicles | 80 | DLS | [170] | |||
| Purified | 0.5 | RT | 6.5 | Micelles/vesicles | 70 | DLS | [170] | |||
| Purified | 0.5 | RT | 7.5 | Micelles/vesicles | 65 | DLS | [170] | |||
| Purified | 0.5 | RT | 8.5 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.5 | RT | 9.5 | Micelles | 25 | DLS | [170] | |||
| Purified | 0.5 | RT | 10.5 | Micelles | 25 | DLS | [170] | |||
| 99 | 0.02 | 25 | 6.8 | Vesicles | 100 * 85 ** | DLS | 0.73 | [179] | ||
| 99 | 0.04 | 25 | 6.8 | Vesicles | 85 * 70 ** | DLS | 0.73 | [179] | ||
| 99 | 0.1 | 25 | 6.8 | Micelles/vesicles | 85 * 50 ** | DLS | 0.73 | [179] | ||
| 99 | 0.5 | 25 | 6.8 | Micelles/vesicles | 85 * 25 ** | DLS | 0.73 | [179] | ||
| 99 | 1 | 25 | 6.8 | Micelles/vesicles | 80 * 30 ** | DLS | 0.73 | [179] | ||
| 99 | 10 | 25 | 6.8 | Micelles/vesicles | 75 * 50 ** | DLS | 0.73 | [179] | ||
| 99 | 20–100 | 25 | 6.8 | Micelles/vesicles | 2–4, 75–90 * 2–4 ** | DLS | 0.73 | [179] |
3.2. Synthetic Rhamnolipids
3.3. Computational Results
4. Open Issues
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otzen, D.E. Biosurfactants and Surfactants Interacting with Membranes and Proteins: Same but Different? Biochim. Biophys. Acta-Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Silva, M.D.G.C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, Properties, Applications, Trends, and General Perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Müller, M.M.; Kügler, J.H.; Henkel, M.; Gerlitzki, M.; Hörmann, B.; Pöhnlein, M.; Syldatk, C.; Hausmann, R. Rhamnolipids—Next Generation Surfactants? J. Biotechnol. 2012, 162, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Baccile, N.; Seyrig, C.; Poirier, A.; Castro, S.A.-D.; Roelants, S.L.K.W.; Abel, S. Self-Assembly, Interfacial Properties, Interactions with Macromolecules and Molecular Modelling and Simulation of Microbial Bio-Based Amphiphiles (Biosurfactants)—A Tutorial Review. Green Chem. 2021, 23, 3842–3944. [Google Scholar] [CrossRef]
- Hargreaves, A.E. Chemical Formulation: An Overview of Surfactant Based Chemical Preparations Used in Everyday Life; Royal Society of Chemistry: London, UK, 2007; ISBN 978-1-84755-038-5. [Google Scholar]
- Falbe, J. Surfactants in Consumer Products: Theory, Technology and Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-71545-7. [Google Scholar]
- Scheibel, J.J. The Evolution of Anionic Surfactant Technology to Meet the Requirements of the Laundry Detergent Industry. J. Surfactants Deterg. 2004, 7, 319–328. [Google Scholar] [CrossRef]
- Cornwell, P.A. A Review of Shampoo Surfactant Technology: Consumer Benefits, Raw Materials and Recent Developments. Int. J. Cosmet. Sci. 2018, 40, 16–30. [Google Scholar] [CrossRef]
- Tadros, T.F. Applied Surfactants: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-3-527-60453-1. [Google Scholar]
- Farn, R.J. Chemistry and Technology of Surfactants; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-7179-3. [Google Scholar]
- Chowdhury, S.; Shrivastava, S.; Kakati, A.; Sangwai, J.S. Comprehensive Review on the Role of Surfactants in the Chemical Enhanced Oil Recovery Process. Ind. Eng. Chem. Res. 2022, 61, 21–64. [Google Scholar] [CrossRef]
- Pradip; Rai, B. Design of Tailor-Made Surfactants for Industrial Applications Using a Molecular Modelling Approach. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 139–148. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Tadros, T.F. Polymeric Surfactants: Dispersion Stability and Industrial Applications; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2017; ISBN 978-3-11-048728-2. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-54194-4. [Google Scholar]
- Castro, M.J.L.; Ojeda, C.; Cirelli, A.F. Surfactants in Agriculture. In Green Materials for Energy, Products and Depollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Environmental Chemistry for a Sustainable World; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 287–334. ISBN 978-94-007-6836-9. [Google Scholar]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-Based Remediation as an Effective Approach for Removal of Environmental Pollutants—A Review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef]
- Salager, J.-L.; Antón, R.; Bullón, J.; Forgiarini, A.; Marquez, R. How to Use the Normalized Hydrophilic-Lipophilic Deviation (HLDN) Concept for the Formulation of Equilibrated and Emulsified Surfactant-Oil-Water Systems for Cosmetics and Pharmaceutical Products. Cosmetics 2020, 7, 57. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Fabrication, Characterization, Pharmaceutical and Cosmetic Applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic Surfactants as Antifungal Agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic Surfactants Derived from Lysine: Effects of Their Structure and Charge Type on Antimicrobial and Hemolytic Activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef]
- Freeling, F.; Alygizakis, N.A.; von der Ohe, P.C.; Slobodnik, J.; Oswald, P.; Aalizadeh, R.; Cirka, L.; Thomaidis, N.S.; Scheurer, M. Occurrence and Potential Environmental Risk of Surfactants and Their Transformation Products Discharged by Wastewater Treatment Plants. Sci. Total Environ. 2019, 681, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Lin, J. Biosurfactant: A New Frontier for Greener Technology and Environmental Sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.; Pour, A.K.; Beach, E.S.; Zimmerman, J.B. Derivation and Synthesis of Renewable Surfactants. Chem. Soc. Rev. 2012, 41, 1499–1518. [Google Scholar] [CrossRef]
- Pardhi, D.S.; Panchal, R.R.; Raval, V.H.; Joshi, R.G.; Poczai, P.; Almalki, W.H.; Rajput, K.N. Microbial Surfactants: A Journey from Fundamentals to Recent Advances. Front. Microbiol. 2022, 13, 982603. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, A. Interactions between Surfactants and the Skin—Theory and Practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.L.; Militello, M.; Barber, C.; Ladd, R.; Laughter, M.; Ferguson, H.; Dewey, J.; Pulsipher, K.J.; Rundle, C.W.; Dunnick, C.A. The History of Surfactants and Review of Their Allergic and Irritant Properties. Dermatitis 2021, 32, 289. [Google Scholar] [CrossRef]
- Weinhammer, A.P.; Scheman, A.; Reeder, M.J. Prevalence of Surfactant in the Contact Allergen Management Program. Dermatitis 2019, 30, 358. [Google Scholar] [CrossRef]
- Han, J.-H.; Jung, S.-K. Toxicity Evaluation of Household Detergents and Surfactants Using Zebrafish. Biotechnol. Bioproc. E 2021, 26, 156–164. [Google Scholar] [CrossRef]
- Olkowska, E.; Ruman, M.; Polkowska, Ż. Occurrence of Surface Active Agents in the Environment. J. Anal. Methods Chem. 2014, 2014, e769708. [Google Scholar] [CrossRef]
- Belanger, S.E.; Boeije, G.; Federle, T.W.; McAvoy, D.C.; Morrall, S.W.; Eckhoff, W.S.; Dunphy, J.C.; Itrich, N.R.; Price, B.B.; Matthijs, E.; et al. Special Issue on the Environmental Risk Assessment of Alcohol Ethoxylate Nonionic Surfactant. Ecotoxicol. Environ. Saf. 2006, 64, 1–2. [Google Scholar] [CrossRef]
- Rebello, S.; Anoopkumar, A.N.; Sindhu, R.; Binod, P.; Pandey, A.; Aneesh, E.M. 23—Comparative Life-Cycle Analysis of Synthetic Detergents and Biosurfactants—An Overview. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 511–521. ISBN 978-0-12-818996-2. [Google Scholar]
- Bognolo, G. Biosurfactants as Emulsifying Agents for Hydrocarbons. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 41–52. [Google Scholar] [CrossRef]
- Makkar, R.S.; Rockne, K.J. Comparison of Synthetic Surfactants and Biosurfactants in Enhancing Biodegradation of Polycyclic Aromatic Hydrocarbons. Environ. Toxicol. Chem. 2003, 22, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A Review on Natural Surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Rodrigues, L.R. Microbial Surfactants: Fundamentals and Applicability in the Formulation of Nano-Sized Drug Delivery Vectors. J. Colloid Interface Sci. 2015, 449, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Deleu, M.; Paquot, M. From Renewable Vegetables Resources to Microorganisms: New Trends in Surfactants. C. R. Chim. 2004, 7, 641–646. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ron, E.Z. High- and Low-Molecular-Mass Microbial Surfactants. Appl. Microbiol. Biotechnol. 1999, 52, 154–162. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, Properties, and Applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, Natural Alternatives to Synthetic Surfactants: Physicochemical Properties and Applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.P.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current Advances in the Classification, Production, Properties and Applications of Microbial Biosurfactants—A Critical Review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef] [PubMed]
- Bioemulsifiers Derived from Microorganisms: Applications in the Drug and Food Industry. Available online: https://apb.tbzmed.ac.ir/Article/apb-19533 (accessed on 19 December 2022).
- Sałek, K.; Euston, S.R. Sustainable Microbial Biosurfactants and Bioemulsifiers for Commercial Exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.; Cao, M.; Wang, J.; Lu, J.R.; Xu, H. Rational Design, Properties, and Applications of Biosurfactants: A Short Review of Recent Advances. Curr. Opin. Colloid Interface Sci. 2020, 45, 57–67. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Yang, X.; Liu, Y.; Shao, B.; Liu, Z. Advances in Applications of Rhamnolipids Biosurfactant in Environmental Remediation: A Review. Biotechnol. Bioeng. 2018, 115, 796–814. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Critical Review on Biosurfactant Analysis, Purification and Characterization Using Rhamnolipid as a Model Biosurfactant. Bioresour. Technol. 2017, 232, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Li, Q. Microbial Production of Rhamnolipids: Opportunities, Challenges and Strategies. Microb. Cell Factories 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M.; Soberón-Chávez, G. Pseudomonas Aeruginosa Rhamnolipids: Biosynthesis and Potential Applications. Appl. Microbiol. Biotechnol. 2000, 54, 625–633. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids Produced by Pseudomonas: From Molecular Genetics to the Market. Microb. Biotechnol. 2020, 14, 136–146. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of Structures, Microbial Origins and Roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Lang, S. Biological Amphiphiles (Microbial Biosurfactants). Curr. Opin. Colloid Interface Sci. 2002, 7, 12–20. [Google Scholar] [CrossRef]
- Jarvis, F.G.; Johnson, M.J. A Glyco-Lipide Produced by Pseudomonas Aeruginosa. J. Am. Chem. Soc. 1949, 71, 4124–4126. [Google Scholar] [CrossRef]
- Zhong, H.; Zeng, G.M.; Liu, J.X.; Xu, X.M.; Yuan, X.Z.; Fu, H.Y.; Huang, G.H.; Liu, Z.F.; Ding, Y. Adsorption of Monorhamnolipid and Dirhamnolipid on Two Pseudomonas Aeruginosa Strains and the Effect on Cell Surface Hydrophobicity. Appl. Microbiol. Biotechnol. 2008, 79, 671–677. [Google Scholar] [CrossRef]
- Sánchez, M.; Aranda, F.J.; Espuny, M.J.; Marqués, A.; Teruel, J.A.; Manresa, Á.; Ortiz, A. Aggregation Behaviour of a Dirhamnolipid Biosurfactant Secreted by Pseudomonas Aeruginosa in Aqueous Media. J. Colloid Interface Sci. 2007, 307, 246–253. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Cambria, M.G.; Impallomeni, G.; Rizzo, M.G.; Pellicorio, C.; Ballistreri, A.; Guglielmino, S.P.P. Carbon Source Effects on the Mono/Dirhamnolipid Ratio Produced by Pseudomonas Aeruginosa L05, a New Human Respiratory Isolate. New Biotechnol. 2017, 39, 36–41. [Google Scholar] [CrossRef]
- Deepika, K.V.; Ramu Sridhar, P.; Bramhachari, P.V. Characterization and Antifungal Properties of Rhamnolipids Produced by Mangrove Sediment Bacterium Pseudomonas Aeruginosa Strain KVD-HM52. Biocatal. Agric. Biotechnol. 2015, 4, 608–615. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Hu, Y.-Y.; Gu, R.R.; Lin, H. Characterization and Micellization of Rhamnolipidic Fractions and Crude Extracts Produced by Pseudomonas Aeruginosa Mutant MIG-N146. J. Colloid Interface Sci. 2009, 331, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Charles Oluwaseun, A.; Julius Kola, O.; Mishra, P.; Ravinder Singh, J.; Kumar Singh, A.; Singh Cameotra, S.; Oluwasesan Micheal, B. Characterization and Optimization of a Rhamnolipid from Pseudomonas Aeruginosa C1501 with Novel Biosurfactant Activities. Sustain. Chem. Pharm. 2017, 6, 26–36. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.-H. Characterization of Rhamnolipid Produced by Pseudomonas Aeruginosa Isolate Bs20. Appl. Biochem. Biotechnol. 2009, 157, 329–345. [Google Scholar] [CrossRef]
- Christova, N.; Tuleva, B.; Cohen, R.; Ivanova, G.; Stoev, G.; Stoilova-Disheva, M.; Stoineva, I. Chemical Characterization and Physical and Biological Activities of Rhamnolipids Produced by Pseudomonas Aeruginosa BN10. Z. Nat. C 2011, 66, 394–402. [Google Scholar] [CrossRef]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical Structure, Surface Properties and Biological Activities of the Biosurfactant Produced by Pseudomonas Aeruginosa LBI from Soapstock. Antonie Leeuwenhoek 2004, 85, 1–8. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Core Flood Study for Enhanced Oil Recovery through Ex-Situ Bioaugmentation with Thermo- and Halo-Tolerant Rhamnolipid Produced by Pseudomonas Aeruginosa NCIM 5514. Bioresour. Technol. 2016, 220, 175–182. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, R.; Zhao, J.; Li, G.; Bai, X.; Han, S.; Zhang, Y. Heterologous Production of Pseudomonas Aeruginosa Rhamnolipid under Anaerobic Conditions for Microbial Enhanced Oil Recovery. J. Appl. Microbiol. 2015, 118, 379–389. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Haddad, R.; Gonçalves, L.A.G.; Eberlin, M.N.; Contiero, J. Oil Wastes as Unconventional Substrates for Rhamnolipid Biosurfactant Production by Pseudomonas Aeruginosa LBI. Biotechnol. Prog. 2005, 21, 1562–1566. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, R.; Ma, F.; Han, S.; Zhang, Y. Oxygen Effects on Rhamnolipids Production by Pseudomonas Aeruginosa. Microb. Cell Factories 2018, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Noghabi, K.A.; Hamedi, M.M.; Zahiri, H.S.; Moosavi-Movahedi, A.A.; Amanlou, M.; Teruel, J.A.; Ortiz, A. Physicochemical Characterization of a Monorhamnolipid Secreted by Pseudomonas Aeruginosa MA01 in Aqueous Media. An Experimental and Molecular Dynamics Study. Colloids Surf. B Biointerfaces 2013, 101, 256–265. [Google Scholar] [CrossRef]
- Bharali, P.; Konwar, B.K. Production and Physico-Chemical Characterization of a Biosurfactant Produced by Pseudomonas Aeruginosa OBP1 Isolated from Petroleum Sludge. Appl. Biochem. Biotechnol. 2011, 164, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Müller, M.M.; Syldatk, C.; Hausmann, R. Production of Microbial Rhamnolipid by Pseudomonas Aeruginosa MM1011 for Ex Situ Enhanced Oil Recovery. Appl. Biochem. Biotechnol. 2013, 170, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Pornsunthorntawee, O.; Chavadej, S.; Rujiravanit, R. Solution Properties and Vesicle Formation of Rhamnolipid Biosurfactants Produced by Pseudomonas Aeruginosa SP4. Colloids Surf. B Biointerfaces 2009, 72, 6–15. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Structural and Physicochemical Characterization of Rhamnolipids Produced by Pseudomonas Aeruginosa P6. AMB Express 2020, 10, 201. [Google Scholar] [CrossRef]
- Costa, S.G.V.A.O.; Nitschke, M.; Lépine, F.; Déziel, E.; Contiero, J. Structure, Properties and Applications of Rhamnolipids Produced by Pseudomonas Aeruginosa L2-1 from Cassava Wastewater. Process Biochem. 2010, 45, 1511–1516. [Google Scholar] [CrossRef]
- Onbasli, D.; Aslim, B. Biosurfactant Production in Sugar Beet Molasses by Some Pseudomonas spp. J. Environ. Biol. 2009, 30, 161–163. [Google Scholar]
- Gunther, N.W.; Nuñez, A.; Fett, W.; Solaiman, D.K.Y. Production of Rhamnolipids by Pseudomonas Chlororaphis, a Nonpathogenic Bacterium. Appl. Environ. Microbiol. 2005, 71, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Gunther, N.W.; Nuñez, A.; Fortis, L.; Solaiman, D.K.Y. Proteomic Based Investigation of Rhamnolipid Production by Pseudomonas Chlororaphis Strain NRRL B-30761. J. Ind. Microbiol. Biotechnol. 2006, 33, 914–920. [Google Scholar] [CrossRef]
- Nayak, A.S.; Vijaykumar, M.H.; Karegoudar, T.B. Characterization of Biosurfactant Produced by Pseudoxanthomonas Sp. PNK-04 and Its Application in Bioremediation. Int. Biodeterior. Biodegrad. 2009, 63, 73–79. [Google Scholar] [CrossRef]
- Rooney, A.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.-M. Isolation and Characterization of Rhamnolipid-Producing Bacterial Strains from a Biodiesel Facility. FEMS Microbiol. Lett. 2009, 295, 82–87. [Google Scholar] [CrossRef]
- Vasileva-Tonkova, E.; Gesheva, V. Biosurfactant Production by Antarctic Facultative Anaerobe Pantoea Sp. during Growth on Hydrocarbons. Curr. Microbiol. 2007, 54, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hošková, M.; Ježdík, R.; Schreiberová, O.; Chudoba, J.; Šír, M.; Čejková, A.; Masák, J.; Jirků, V.; Řezanka, T. Structural and Physiochemical Characterization of Rhamnolipids Produced by Acinetobacter Calcoaceticus, Enterobacter Asburiae and Pseudomonas Aeruginosa in Single Strain and Mixed Cultures. J. Biotechnol. 2015, 193, 45–51. [Google Scholar] [CrossRef]
- Hošková, M.; Schreiberová, O.; Ježdík, R.; Chudoba, J.; Masák, J.; Sigler, K.; Řezanka, T. Characterization of Rhamnolipids Produced by Non-Pathogenic Acinetobacter and Enterobacter Bacteria. Bioresour. Technol. 2013, 130, 510–516. [Google Scholar] [CrossRef]
- Dubeau, D.; Déziel, E.; Woods, D.E.; Lépine, F. Burkholderia Thailandensis Harbors Two Identical Rhl Gene Clusters Responsible for the Biosynthesis of Rhamnolipids. BMC Microbiol. 2009, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Rademann, J.; Howe, J.; Koch, M.H.J.; Heine, H.; Zähringer, U.; Brandenburg, K. Endotoxin-like Properties of a Rhamnolipid Exotoxin from Burkholderia (Pseudomonas) Plantarii: Immune Cell Stimulation and Biophysical Characterization. Biol. Chem. 2006, 387, 301–310. [Google Scholar] [CrossRef]
- Häußler, S.; Nimtz, M.; Domke, T.; Wray, V.; Steinmetz, I. Purification and Characterization of a Cytotoxic Exolipid of Burkholderia Pseudomallei. Infect. Immun. 1998, 66, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from Non-Pathogenic Burkholderia Thailandensis E264: Physicochemical Characterization, Antimicrobial and Antibiofilm Efficacy against Oral Hygiene Related Pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Arino, S.; Marchal, R.; Vandecasteele, J.-P. Production of New Extracellular Glycolipids by a Strain of Cellulomonas Cellulans (Oerskovia Xanthineolytica) and Their Structural Characterization. Can. J. Microbiol. 1998, 44, 238–243. [Google Scholar] [CrossRef]
- Christova, N.; Tuleva, B.; Lalchev, Z.; Jordanova, A.; Jordanov, B. Rhamnolipid Biosurfactants Produced by Renibacterium Salmoninarum 27BN during Growth on N-Hexadecane. Z. Nat. C 2004, 59, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, M.K.; Vancanneyt, M.; Swings, J.; Kim, S.-H.; Kang, M.S.; Lee, S.-T. Tetragenococcus Koreensis Sp. Nov., a Novel Rhamnolipid-Producing Bacterium. Int. J. Syst. Evol. Microbiol. 2005, 55, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Vasileva-Tonkova, E.; Gesheva, V. Glycolipids Produced by Antarctic Nocardioides Sp. during Growth on n-Paraffin. Process Biochem. 2005, 40, 2387–2391. [Google Scholar] [CrossRef]
- Roy, S.; Chandni, S.; Das, I.; Karthik, L.; Kumar, G.; Bhaskara Rao, K.V. Aquatic Model for Engine Oil Degradation by Rhamnolipid Producing Nocardiopsis VITSISB. 3Biotech 2015, 5, 153–164. [Google Scholar] [CrossRef]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential Agents for Controlling Cellular Communication, Motility, and Antagonism. Front. Mol. Biosci. 2021, 8, 727070. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, Ł.; Ławniczak, Ł.; Czaczyk, K. Why Do Microorganisms Produce Rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological Aspects: Part 1 in a Series of Papers Devoted to Surfactants in Microbiology and Biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef]
- Wittgens, A.; Kovacic, F.; Müller, M.M.; Gerlitzki, M.; Santiago-Schübel, B.; Hofmann, D.; Tiso, T.; Blank, L.M.; Henkel, M.; Hausmann, R.; et al. Novel Insights into Biosynthesis and Uptake of Rhamnolipids and Their Precursors. Appl. Microbiol. Biotechnol. 2017, 101, 2865–2878. [Google Scholar] [CrossRef]
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; García, F.; Manresa, A. Physicochemical and Antimicrobial Properties of New Rhamnolipids Produced by Pseudomonas a Eruginosa AT10 from Soybean Oil Refinery Wastes. Langmuir 2001, 17, 1367–1371. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Déziel, E.; Lépine, F.; Dennie, D.; Boismenu, D.; Mamer, O.A.; Villemur, R. Liquid Chromatography/Mass Spectrometry Analysis of Mixtures of Rhamnolipids Produced by Pseudomonas Aeruginosa Strain 57RP Grown on Mannitol or Naphthalene. Biochim. Biophys. Acta 1999, 1440, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Geys, R.; Soetaert, W.; Van Bogaert, I. Biotechnological Opportunities in Biosurfactant Production. Curr. Opin. Biotechnol. 2014, 30, 66–72. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Ebadipour, N.; RoostaAzad, R. Evaluation of a Recycling Bioreactor for Biosurfactant Production by Pseudomonas Aeruginosa MR01 Using Soybean Oil Waste. J. Chem. Technol. Biotechnol. 2016, 91, 1368–1377. [Google Scholar] [CrossRef]
- Lang, S.; Wullbrandt, D. Rhamnose Lipids—Biosynthesis, Microbial Production and Application Potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Marangon, C.A. Microbial Surfactants in Nanotechnology: Recent Trends and Applications. Crit. Rev. Biotechnol. 2022, 42, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Factors Influencing the Economics of Biosurfactants. Available online: https://www.taylorfrancis.com/chapters/mono/10.1201/9780585355702-22/factors-influencing-economics-biosurfactants-naim-kosaric-fazilet-vardar-sukan (accessed on 19 December 2022).
- Müller, M.M.; Hausmann, R. Regulatory and Metabolic Network of Rhamnolipid Biosynthesis: Traditional and Advanced Engineering towards Biotechnological Production. Appl. Microbiol. Biotechnol. 2011, 91, 251–264. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost Effective Technologies and Renewable Substrates for Biosurfactants’ Production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [PubMed]
- Moya Ramírez, I.; Altmajer Vaz, D.; Banat, I.M.; Marchant, R.; Jurado Alameda, E.; García Román, M. Hydrolysis of Olive Mill Waste to Enhance Rhamnolipids and Surfactin Production. Bioresour. Technol. 2016, 205, 1–6. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rodrigues, A.I.; de Freitas, V.; Azevedo, Z.; Teixeira, J.A.; Rodrigues, L.R. Valorization of Agro-Industrial Wastes towards the Production of Rhamnolipids. Bioresour. Technol. 2016, 212, 144–150. [Google Scholar] [CrossRef]
- Gutiérrez-Gómez, U.; Soto-Aceves, M.P.; Servín-González, L.; Soberón-Chávez, G. Overproduction of Rhamnolipids in Pseudomonas Aeruginosa PA14 by Redirection of the Carbon Flux from Polyhydroxyalkanoate Synthesis and Overexpression of the RhlAB-R Operon. Biotechnol. Lett. 2018, 40, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Bator, I.; Karmainski, T.; Tiso, T.; Blank, L.M. Killing Two Birds with One Stone—Strain Engineering Facilitates the Development of a Unique Rhamnolipid Production Process. Front. Bioeng. Biotechnol. 2020, 8, 899. [Google Scholar] [CrossRef]
- Tiso, T.; Ihling, N.; Kubicki, S.; Biselli, A.; Schonhoff, A.; Bator, I.; Thies, S.; Karmainski, T.; Kruth, S.; Willenbrink, A.-L.; et al. Integration of Genetic and Process Engineering for Optimized Rhamnolipid Production Using Pseudomonas Putida. Front. Bioeng. Biotechnol. 2020, 8, 976. [Google Scholar] [CrossRef] [PubMed]
- Dobler, L.; Vilela, L.F.; Almeida, R.V.; Neves, B.C. Rhamnolipids in Perspective: Gene Regulatory Pathways, Metabolic Engineering, Production and Technological Forecasting. New Biotechnol. 2016, 33, 123–135. [Google Scholar] [CrossRef]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent Advancements in the Production of Rhamnolipid Biosurfactants by Pseudomonas Aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef]
- Invally, K.; Sancheti, A.; Ju, L.-K. A New Approach for Downstream Purification of Rhamnolipid Biosurfactants. Food Bioprod. Process. 2019, 114, 122–131. [Google Scholar] [CrossRef]
- Anic, I.; Apolonia, I.; Franco, P.; Wichmann, R. Production of Rhamnolipids by Integrated Foam Adsorption in a Bioreactor System. AMB Express 2018, 8, 122. [Google Scholar] [CrossRef]
- Jadhav, J.; Dutta, S.; Kale, S.; Pratap, A. Fermentative Production of Rhamnolipid and Purification by Adsorption Chromatography. Prep. Biochem. Biotechnol. 2018, 48, 234–241. [Google Scholar] [CrossRef]
- Microbial Biosurfactants. Available online: https://www.marketresearch.com/Global-Industry-Analysts-v1039/Microbial-Biosurfactants-32495863/ (accessed on 20 December 2022).
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Yang, X.; Li, Z.; Fang, Z.; et al. Effects of Rhamnolipids on Microorganism Characteristics and Applications in Composting: A Review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Inès, M.; Dhouha, G. Glycolipid Biosurfactants: Potential Related Biomedical and Biotechnological Applications. Carbohydr. Res. 2015, 416, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, D.; Morita, T.; Fukuoka, T.; Konishi, M.; Imura, T. Self-Assembling Properties of Glycolipid Biosurfactants and Their Potential Applications. Curr. Opin. Colloid Interface Sci. 2009, 14, 315–328. [Google Scholar] [CrossRef]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising Molecules for Petroleum Biotechnology Advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Le Guenic, S.; Chaveriat, L.; Lequart, V.; Joly, N.; Martin, P. Renewable Surfactants for Biochemical Applications and Nanotechnology. J. Surfactants Deterg. 2019, 22, 5–21. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Bothra, N.; Singh, R.; Sai, M.C.; Nedungadi, S.V.; Sarangi, P.K. Microbial Originated Surfactants with Multiple Applications: A Comprehensive Review. Arch Microbiol. 2022, 204, 452. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Sabatini, D.A. Characterization and Emulsification Properties of Rhamnolipid and Sophorolipid Biosurfactants and Their Applications. Int. J. Mol. Sci. 2011, 12, 1232–1244. [Google Scholar] [CrossRef]
- Pornsunthorntawee, O.; Wongpanit, P.; Rujiravanit, R. Rhamnolipid Biosurfactants: Production and Their Potential in Environmental Biotechnology. In Biosurfactants; Sen, R., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; pp. 211–221. ISBN 978-1-4419-5979-9. [Google Scholar]
- Kumar, R.; Das, A.J. Rhamnolipid Biosurfactant: Recent Trends in Production and Application; Springer: New York, NY, USA, 2018; ISBN 9789811312908. [Google Scholar]
- Kiran, G.S.; Ninawe, A.S.; Lipton, A.N.; Pandian, V.; Selvin, J. Rhamnolipid Biosurfactants: Evolutionary Implications, Applications and Future Prospects from Untapped Marine Resource. Crit. Rev. Biotechnol. 2016, 36, 399–415. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipid Surfactants: An Update on the General Aspects of These Remarkable Biomolecules. Biotechnol. Prog. 2005, 21, 1593–1600. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Hua, Y.; Chen, J.; Zhang, H.; Wang, H. Potential Applications of Biosurfactant Rhamnolipids in Agriculture and Biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. [Google Scholar] [CrossRef]
- Chebbi, A.; Franzetti, A.; Formicola, F.; Ambaye, T.G.; Gomez, F.H.; Murena, B.; De Marco, E.; Beltrani, T.; Sbaffoni, S.; Vaccari, M. Insights into Rhamnolipid-Based Soil Remediation Technologies by Safe Microorganisms: A Critical Review. J. Clean. Prod. 2022, 367, 133088. [Google Scholar] [CrossRef]
- Long, X.; Zhang, G.; Shen, C.; Sun, G.; Wang, R.; Yin, L.; Meng, Q. Application of Rhamnolipid as a Novel Biodemulsifier for Destabilizing Waste Crude Oil. Bioresour. Technol. 2013, 131, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-J.; Invally, K.; Ju, L.-K. Chapter 5—Rhamnolipids: Pathways, Productivities, and Potential. In Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Urbana, IL, USA, 2019; pp. 169–203. ISBN 978-0-12-812705-6. [Google Scholar]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging Trends and Promising Strategies in the Field of Biotechnology and Biomedicine. Microb. Cell Factories 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C.; Romão-Dumaresq, A.S. Microbial Biosurfactants: A Broad Analysis of Properties, Applications, Biosynthesis, and Techno-Economical Assessment of Rhamnolipid Production. Biotechnol. Prog. 2021, 37, e3093. [Google Scholar] [CrossRef]
- Whang, L.-M.; Liu, P.-W.G.; Ma, C.-C.; Cheng, S.-S. Application of Biosurfactants, Rhamnolipid, and Surfactin, for Enhanced Biodegradation of Diesel-Contaminated Water and Soil. J. Hazard. Mater. 2008, 151, 155–163. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental Applications of Biosurfactants: Recent Advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Patowary, R.; Patowary, K.; Kalita, M.C.; Deka, S. Application of Biosurfactant for Enhancement of Bioremediation Process of Crude Oil Contaminated Soil. Int. Biodeterior. Biodegrad. 2018, 129, 50–60. [Google Scholar] [CrossRef]
- Mulligan, C.N. Recent Advances in the Environmental Applications of Biosurfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 372–378. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R.; Janek, T. Phase Behaviour, Functionality, and Physicochemical Characteristics of Glycolipid Surfactants of Microbial Origin. Front. Bioeng. Biotechnol. 2022, 10, 816613. [Google Scholar] [CrossRef]
- Herzog, M.; Li, L.; Blesken, C.C.; Welsing, G.; Tiso, T.; Blank, L.M.; Winter, R. Impact of the Number of Rhamnose Moieties of Rhamnolipids on the Structure, Lateral Organization and Morphology of Model Biomembranes. Soft Matter. 2021, 17, 3191–3206. [Google Scholar] [CrossRef]
- Zhang, Y.; Placek, T.L.; Jahan, R.; Alexandridis, P.; Tsianou, M. Rhamnolipid Micellization and Adsorption Properties. Int. J. Mol. Sci. 2022, 23, 11090. [Google Scholar] [CrossRef]
- Zhao, F.; Han, S.; Zhang, Y. Comparative Studies on the Structural Composition, Surface/Interface Activity and Application Potential of Rhamnolipids Produced by Pseudomonas Aeruginosa Using Hydrophobic or Hydrophilic Substrates. Bioresour.Technol. 2020, 295, 122269. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. A Stereospecific Pathway Diverts β-Oxidation Intermediates to the Biosynthesis of Rhamnolipid Biosurfactants. Chem. Biol. 2014, 21, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Palos Pacheco, R.; Eismin, R.J.; Coss, C.S.; Wang, H.; Maier, R.M.; Polt, R.; Pemberton, J.E. Synthesis and Characterization of Four Diastereomers of Monorhamnolipids. J. Am. Chem. Soc. 2017, 139, 5125–5132. [Google Scholar] [CrossRef]
- Palos Pacheco, R.; Kegel, L.L.; Pemberton, J.E. Interfacial and Solution Aggregation Behavior of a Series of Bioinspired Rhamnolipid Congeners Rha-C14-C x (x = 6, 8, 10, 12, 14). J. Phys. Chem. B 2021, 125, 13585–13596. [Google Scholar] [CrossRef]
- Lebrón-Paler, A.; Pemberton, J.E.; Becker, B.A.; Otto, W.H.; Larive, C.K.; Maier, R.M. Determination of the Acid Dissociation Constant of the Biosurfactant Monorhamnolipid in Aqueous Solution by Potentiometric and Spectroscopic Methods. Anal. Chem. 2006, 78, 7649–7658. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, Y.; Gama, Y.; Nagahora, H.; Yamaguchi, M.; Nakahara, H.; Kamata, T. The PH-Sensitive Conversion of Molecular Aggregates of Rhamnolipid Biosurfactant. Chem. Lett. 1987, 16, 763–766. [Google Scholar] [CrossRef]
- Mohammed, I.U.; Deeni, Y.; Hapca, S.M.; McLaughlin, K.; Spiers, A.J. Predicting the Minimum Liquid Surface Tension Activity of Pseudomonads Expressing Biosurfactants. Lett. Appl. Microbiol. 2015, 60, 37–43. [Google Scholar] [CrossRef]
- Youssef, N.H.; Duncan, K.E.; Nagle, D.P.; Savage, K.N.; Knapp, R.M.; McInerney, M.J. Comparison of Methods to Detect Biosurfactant Production by Diverse Microorganisms. J. Microbiol. Methods 2004, 56, 339–347. [Google Scholar] [CrossRef]
- Georgiou, G.; Lin, S.-C.; Sharma, M.M. Surface–Active Compounds from Microorganisms. Nat. Biotechnol. 1992, 10, 60–65. [Google Scholar] [CrossRef]
- Das, P.; Yang, X.-P.; Ma, L.Z. Analysis of Biosurfactants from Industrially Viable Pseudomonas Strain Isolated from Crude Oil Suggests How Rhamnolipids Congeners Affect Emulsification Property and Antimicrobial Activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Del Regno, A.; Warren, P.B.; Bray, D.J.; Anderson, R.L. Critical Micelle Concentrations in Surfactant Mixtures and Blends by Simulation. J. Phys. Chem. B 2021, 125, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar Solubilization of Poorly Water-Soluble Drugs: Effect of Surfactant and Solubilizate Molecular Structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Ghosh, S.; Ray, A.; Pramanik, N. Self-Assembly of Surfactants: An Overview on General Aspects of Amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 1st ed.; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-0-471-49883-4. [Google Scholar]
- Kronberg, B.; Holmberg, K. Surface Chemistry of Surfactants and Polymers, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Prosser, A.J.; Franses, E.I. Adsorption and Surface Tension of Ionic Surfactants at the Air–Water Interface: Review and Evaluation of Equilibrium Models. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 1–40. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Adsorption of Ionic Surfactants on Water/Air Interface: One More Transformation of the Gibbs Equation. Surf. Eng. Appl. Electrochem. 2014, 50, 173–182. [Google Scholar] [CrossRef]
- Martínez-Balbuena, L.; Arteaga-Jiménez, A.; Hernández-Zapata, E.; Márquez-Beltrán, C. Applicability of the Gibbs Adsorption Isotherm to the Analysis of Experimental Surface-Tension Data for Ionic and Nonionic Surfactants. Adv. Colloid Interface Sci. 2017, 247, 178–184. [Google Scholar] [CrossRef]
- Bae, S.; Haage, K.; Wantke, K.; Motschmann, H. On the Factor in Gibbs Equation for Ionic Surfactants. J. Phys. Chem. B 1999, 103, 1045–1050. [Google Scholar] [CrossRef]
- Cohen, R.; Exerowa, D. Surface Forces and Properties of Foam Films from Rhamnolipid Biosurfactants. Adv. Colloid Interface Sci. 2007, 134–135, 24–34. [Google Scholar] [CrossRef]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and Characterisation of Short Chain Rhamnolipid Production in a Previously Uninvestigated, Non-Pathogenic Marine Pseudomonad. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. [Google Scholar] [CrossRef]
- Hung, H.; Shreve, G.S. Effect of the Hydrocarbon Phase on Interfacial and Thermodynamic Properties of Two Anionic Glycolipid Biosurfactants in Hydrocarbon/Water Systems. J. Phys. Chem. B 2001, 105, 12596–12600. [Google Scholar] [CrossRef]
- Tripathi, L.; Twigg, M.S.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Biosynthesis of Rhamnolipid by a Marinobacter Species Expands the Paradigm of Biosurfactant Synthesis to a New Genus of the Marine Microflora. Microb. Cell Factories 2019, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lai, L.; Lu, Q.; Mei, P.; Wang, Y.; Cheng, L.; Liu, Y. Comparative Studies on the Surface/Interface Properties and Aggregation Behavior of Mono-Rhamnolipid and Di-Rhamnolipid. Colloids Surf. B Biointerfaces 2019, 181, 593–601. [Google Scholar] [CrossRef]
- Özdemir, G.; Peker, S.; Helvaci, S.S. Effect of PH on the Surface and Interfacial Behavior of Rhamnolipids R1 and R2. Colloids Surf. A Physicochem. Eng. Asp. 2004, 234, 135–143. [Google Scholar] [CrossRef]
- Helvacı, Ş.Ş.; Peker, S.; Özdemir, G. Effect of Electrolytes on the Surface Behavior of Rhamnolipids R1 and R2. Colloids Surf. B Biointerfaces 2004, 35, 225–233. [Google Scholar] [CrossRef]
- Peker, S.; Helvacı, Ş.; Özdemir, G. Interface—Subphase Interactions of Rhamnolipids in Aqueous Rhamnose Solutions. Langmuir 2003, 19, 5838–5845. [Google Scholar] [CrossRef]
- Eismin, R.J.; Munusamy, E.; Kegel, L.L.; Hogan, D.E.; Maier, R.M.; Schwartz, S.D.; Pemberton, J.E. Evolution of Aggregate Structure in Solutions of Anionic Monorhamnolipids: Experimental and Computational Results. Langmuir 2017, 33, 7412–7424. [Google Scholar] [CrossRef]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Solution Self-Assembly and Adsorption at the Air–Water Interface of the Monorhamnose and Dirhamnose Rhamnolipids and Their Mixtures. Langmuir 2010, 26, 18281–18292. [Google Scholar] [CrossRef]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Mixing Behavior of the Biosurfactant, Rhamnolipid, with a Conventional Anionic Surfactant, Sodium Dodecyl Benzene Sulfonate. Langmuir 2010, 26, 17958–17968. [Google Scholar] [CrossRef]
- Mańko, D.; Zdziennicka, A.; Jańczuk, B. Thermodynamic Properties of Rhamnolipid Micellization and Adsorption. Colloids Surf. B Biointerfaces 2014, 119, 22–29. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, L.; Yang, X.; Zeng, G.; Liu, Z.; Liu, Y.; Yuan, X. Aggregation of Low-Concentration Dirhamnolipid Biosurfactant in Electrolyte Solution. RSC Adv. 2015, 5, 88578–88582. [Google Scholar] [CrossRef]
- İkizler, B.; Arslan, G.; Kipcak, E.; Dirik, C.; Çelenk, D.; Aktuğlu, T.; Helvacı, Ş.Ş.; Peker, S. Surface Adsorption and Spontaneous Aggregation of Rhamnolipid Mixtures in Aqueous Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 125–136. [Google Scholar] [CrossRef]
- Ishigami, Y.; Gama, Y.; Ishii, F.; Choi, Y.K. Colloid Chemical Effect of Polar Head Moieties of a Rhamnolipid-Type Biosurfactant. Langmuir 1993, 9, 1634–1636. [Google Scholar] [CrossRef]
- De Castro, C.; Klose, T.; Speciale, I.; Lanzetta, R.; Molinaro, A.; Van Etten, J.L.; Rossmann, M.G. Structure of the Chlorovirus PBCV-1 Major Capsid Glycoprotein Determined by Combining Crystallographic and Carbohydrate Molecular Modeling Approaches. Proc. Natl. Acad. Sci. USA 2018, 115, E44–E52. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Rosés, M.; Bosch, E. Critical Micelle Concentration of Surfactants in Aqueous Buffered and Unbuffered Systems. Anal. Chim. Acta 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Dutkiewicz, E.; Jakubowska, A. Effect of Electrolytes on the Physicochemical Behaviour of Sodium Dodecyl Sulphate Micelles. Colloid Polym. Sci. 2002, 280, 1009–1014. [Google Scholar] [CrossRef]
- Naskar, B.; Dey, A.; Moulik, S.P. Counter-Ion Effect on Micellization of Ionic Surfactants: A Comprehensive Understanding with Two Representatives, Sodium Dodecyl Sulfate (SDS) and Dodecyltrimethylammonium Bromide (DTAB). J. Surfactants Deterg. 2013, 16, 785–794. [Google Scholar] [CrossRef]
- Santos, F.K.G.; Neto, E.L.B.; Moura, M.C.P.A.; Dantas, T.N.C.; Neto, A.A.D. Molecular Behavior of Ionic and Nonionic Surfactants in Saline Medium. Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 156–162. [Google Scholar] [CrossRef]
- Eastoe, J.; Nave, S.; Downer, A.; Paul, A.; Rankin, A.; Tribe, K.; Penfold, J. Adsorption of Ionic Surfactants at the Air—Solution Interface. Langmuir 2000, 16, 4511–4518. [Google Scholar] [CrossRef]
- Munusamy, E.; Luft, C.M.; Pemberton, J.E.; Schwartz, S.D. Unraveling the Differential Aggregation of Anionic and Nonionic Monorhamnolipids at Air–Water and Oil–Water Interfaces: A Classical Molecular Dynamics Simulation Study. J. Phys. Chem. B 2018, 122, 6403–6416. [Google Scholar] [CrossRef]
- Wang, H.; Coss, C.S.; Mudalige, A.; Polt, R.L.; Pemberton, J.E. A PM-IRRAS Investigation of Monorhamnolipid Orientation at the Air–Water Interface. Langmuir 2013, 29, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Luft, C.M.; Munusamy, E.; Pemberton, J.E.; Schwartz, S.D. A Classical Molecular Dynamics Simulation Study of Interfacial and Bulk Solution Aggregation Properties of Dirhamnolipids. J. Phys. Chem. B 2020, 124, 814–827. [Google Scholar] [CrossRef]
- Euston, S.R.; Banat, I.M.; Salek, K. Congener-Dependent Conformations of Isolated Rhamnolipids at the Vacuum-Water Interface: A Molecular Dynamics Simulation. J. Colloid Interface Sci. 2021, 585, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Söderman, O.; Johansson, I. Polyhydroxyl-Based Surfactants and Their Physico-Chemical Properties and Applications. Curr. Opin. Colloid Interface Sci. 1999, 4, 391–401. [Google Scholar] [CrossRef]
- Imura, T.; Hikosaka, Y.; Worakitkanchanakul, W.; Sakai, H.; Abe, M.; Konishi, M.; Minamikawa, H.; Kitamoto, D. Aqueous-Phase Behavior of Natural Glycolipid Biosurfactant Mannosylerythritol Lipid A: Sponge, Cubic, and Lamellar Phases. Langmuir 2007, 23, 1659–1663. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Srinivasan, V.; Blankschtein, D. Effect of Counterion Binding on Micellar Solution Behavior: 2. Prediction of Micellar Solution Properties of Ionic Surfactant−Electrolyte Systems. Langmuir 2003, 19, 9946–9961. [Google Scholar] [CrossRef]
- Nagarajan, R. Constructing a Molecular Theory of Self-Assembly: Interplay of Ideas from Surfactants and Block Copolymers. Adv. Colloid Interface Sci. 2017, 244, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.I.; Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Sodium Chloride Effect on the Aggregation Behaviour of Rhamnolipids and Their Antifungal Activity. Sci. Rep. 2017, 7, 12907. [Google Scholar] [CrossRef]
- Champion, J.T.; Gilkey, J.C.; Lamparski, H.; Retterer, J.; Miller, R.M. Electron Microscopy of Rhamnolipid (Biosurfactant) Morphology: Effects of PH, Cadmium, and Octadecane. J. Colloid Interface Sci. 1995, 170, 569–574. [Google Scholar] [CrossRef]
- Chen, M.; Dong, C.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; et al. Influence of Calcium Ions on Rhamnolipid and Rhamnolipid/Anionic Surfactant Adsorption and Self-Assembly. Langmuir 2013, 29, 3912–3923. [Google Scholar] [CrossRef]
- Arabadzhieva, D.; Soklev, B.; Mileva, E. Amphiphilic Nanostructures in Aqueous Solutions of Triethyleneglycol Monododecyl Ether. Colloids Surf. A Physicochem. Eng. Asp. 2013, 419, 194–200. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Liu, H.-S.; Lin, S.-Y.; Huang, H.-F.; Wang, Y.-Y.; Chou, L.-W. An Observation of the Coexistence of Multimers and Micelles in a Nonionic Surfactant C10E4 Solution by Dynamic Light Scattering. J. Chin. Inst. Chem. Eng. 2008, 39, 75–83. [Google Scholar] [CrossRef]
- LeBard, D.N.; Levine, B.G.; DeVane, R.; Shinoda, W.; Klein, M.L. Premicelles and Monomer Exchange in Aqueous Surfactant Solutions above and below the Critical Micelle Concentration. Chem. Phys. Lett. 2012, 522, 38–42. [Google Scholar] [CrossRef]
- D’Errico, G.; Ortona, O.; Paduano, L.; Vitagliano, V. Transport Properties of Aqueous Solutions of Alkyltrimethylammonium Bromide Surfactants at 25 Degrees C. J. Colloid Interface Sci. 2001, 239, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, L.; Costantino, L.; D’errico, G.; Vitagliano, V. Thermodynamic and Dynamic Properties of Micellar Aggregates of Nonionic Surfactants with Short Hydrophobic Tails. J. Colloid Interface Sci. 1997, 190, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Hayter, J.B.; Penfold, J. Determination of Micelle Structure and Charge by Neutron Small-Angle Scattering. Colloid Polym. Sci. 1983, 261, 1022–1030. [Google Scholar] [CrossRef]
- Janek, T.; Krasowska, A.; Czyżnikowska, Ż.; Łukaszewicz, M. Trehalose Lipid Biosurfactant Reduces Adhesion of Microbial Pathogens to Polystyrene and Silicone Surfaces: An Experimental and Computational Approach. Front. Microbiol. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Kuddushi, M.; Kumar, A.; Ray, D.; Aswal, V.K.; El Seoud, O.A.; Malek, N.I. Concentration- and Temperature-Responsive Reversible Transition in Amide-Functionalized Surface-Active Ionic Liquids: Micelles to Vesicles to Organogel. ACS Omega 2020, 5, 24272–24284. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, K.; Zhou, J.; Zhang, Y.; Di Serio, M.; Yang, X.; Li, Y. Concentration-Induced Micelle-to-Vesicle Transitions in Aqueous Sodium Ricinate Branched Polyoxyethylene Ether Solutions. J. Dispers. Sci. Technol. 2021, 42, 1099–1105. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, J.; Sun, K.; Dong, J.; Li, X.; Mao, S.; Du, Y.; Liu, M. PH- and Concentration-Induced Micelle-to-Vesicle Transitions in Pyrrolidone-Based Gemini Surfactants. Colloid Polym. Sci. 2014, 292, 739–747. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, J.; Shi, Q.; Chen, W.; Zhao, M. Transition from Micelle to Vesicle in Aqueous Mixtures of Anionic/Zwitterionic Surfactants Studied by Fluorescence, Conductivity, and Turbidity Methods. J. Colloid Interface Sci. 2005, 284, 698–703. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Z.; Huang, J.; Zheng, R.; Zhang, Y. Temperature-Induced Micelle to Vesicle Transition in the Sodium Dodecylsulfate/Dodecyltriethylammonium Bromide System. Angew. Chem. Int. Ed. 2003, 42, 2188–2191. [Google Scholar] [CrossRef]
- Yin, H.; Huang, J.; Lin, Y.; Zhang, Y.; Qiu, S.; Ye, J. Heating-Induced Micelle to Vesicle Transition in the Cationic—Anionic Surfactant Systems: Comprehensive Study and Understanding. J. Phys. Chem. B 2005, 109, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Kroll, P.; Benke, J.; Enders, S.; Brandenbusch, C.; Sadowski, G. Influence of Temperature and Concentration on the Self-Assembly of Nonionic CiEj Surfactants: A Light Scattering Study. ACS Omega 2022, 7, 7057–7065. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Kato, T. Influence of Temperature and Headgroup Size on Condensed-Phase Patterns in Langmuir Monolayers of Some Oxyethylenated Nonionic Surfactants. Langmuir 2005, 21, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Baccile, N.; Cuvier, A.-S.; Prévost, S.; Stevens, C.V.; Delbeke, E.; Berton, J.; Soetaert, W.; Van Bogaert, I.N.A.; Roelants, S. Self-Assembly Mechanism of PH-Responsive Glycolipids: Micelles, Fibers, Vesicles, and Bilayers. Langmuir 2016, 32, 10881–10894. [Google Scholar] [CrossRef]
- Zaru, M.; Sinico, C.; De Logu, A.; Caddeo, C.; Lai, F.; Manca, M.L.; Fadda, A.M. Rifampicin-Loaded Liposomes for the Passive Targeting to Alveolar Macrophages: In Vitro and in Vivo Evaluation. J. Liposome Res. 2009, 19, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bellare, J.R.; Davis, H.T.; Miller, W.G.; Scriven, L.E. Polarized Optical Microscopy of Anisotropic Media: Imaging Theory and Simulation. J. Colloid Interface Sci. 1990, 136, 305–326. [Google Scholar] [CrossRef]
- Horbaschek, K.; Hoffmann, H.; Thunig, C. Formation and Properties of Lamellar Phases in Systems of Cationic Surfactants and Hydroxy-Naphthoate. J. Colloid Interface Sci. 1998, 206, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Makai, M.; Csányi, E.; Németh, Z.; Pálinkás, J.; Erős, I. Structure and Drug Release of Lamellar Liquid Crystals Containing Glycerol. Int. J. Pharm. 2003, 256, 95–107. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Imperatore, R.; De Santis, A.; Luchini, A.; Paduano, L.; D’Errico, G. Structure and Dynamics of Cetyltrimethylammonium Chloride-Sodium Dodecylsulfate (CTAC-SDS) Catanionic Vesicles: High-Value Nano-Vehicles from Low-Cost Surfactants. J. Colloid Interf Sci. 2017, 501, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Munusamy, E.; Luft, C.M.; Pemberton, J.E.; Schwartz, S.D. Structural Properties of Nonionic Monorhamnolipid Aggregates in Water Studied by Classical Molecular Dynamics Simulations. J. Phys. Chem. B 2017, 121, 5781–5793. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.; Gelatt, C.D.; Vecchi, M.P. Optimization by Simulated Annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, A.; Hammond, O.S.; Jackson, A.J.; Arnold, T.; Doutch, J.; Edler, K.J. Surfactant–Solvent Interaction Effects on the Micellization of Cationic Surfactants in a Carboxylic Acid-Based Deep Eutectic Solvent. Langmuir 2017, 33, 14304–14314. [Google Scholar] [CrossRef]
- Mańko, D.; Zdziennicka, A.; Jańczuk, B. Adsorption and Aggregation Activity of Sodium Dodecyl Sulfate and Rhamnolipid Mixture. J. Surfactants Deterg. 2017, 20, 411–423. [Google Scholar] [CrossRef]
- Esposito, R.; Ingenito, L.; Cavasso, D.; Siciliano, A.; Alfieri, M.L.; Chiappisi, L.; Fragneto, G.; Ottaviani, M.F.; Guida, M.; Paduano, L.; et al. Rhamnolipid–SLES Aqueous Mixtures: From the Molecular Self-Aggregation to the Functional and Ecotoxicological Properties. J. Mol. Liq. 2022, 367, 120547. [Google Scholar] [CrossRef]
- Song, D.; Li, Y.; Liang, S.; Wang, J. Micelle Behaviors of Sophorolipid/Rhamnolipid Binary Mixed Biosurfactant Systems. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 201–206. [Google Scholar] [CrossRef]
- Haidar, C.N.; Martini, G.; Pellegrini Malpiedi, L.; Nerli, B.B. Rhamnolipids Biosurfactants as Potential Modulators of Phase and Partitioning Behavior in Micellar Systems of Aliphatic Alcohol Ethoxylate Surfactants. J. Mol. Liq. 2020, 309, 113125. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, H. Synergistic Solubilization of Phenanthrene by Mixed Micelles Composed of Biosurfactants and a Conventional Non-Ionic Surfactant. Molecules 2020, 25, 4327. [Google Scholar] [CrossRef] [PubMed]
- Rekiel, E.; Zdziennicka, A.; Szymczyk, K.; Jańczuk, B. Thermodynamic Analysis of the Adsorption and Micellization Activity of the Mixtures of Rhamnolipid and Surfactin with Triton X-165. Molecules 2022, 27, 3600. [Google Scholar] [CrossRef] [PubMed]
- Phaodee, P.; Sabatini, D.A. Anionic and Cationic Surfactant Synergism: Minimizing Precipitation, Microemulsion Formation, and Enhanced Solubilization and Surface Modification. J. Surfactants Deterg. 2021, 24, 551–562. [Google Scholar] [CrossRef]
- Xu, J.; Sun, S.; Wang, Z.; Peng, S.; Hu, S.; Zhang, L. PH-Induced Evolution of Surface Patterns in Micelles Assembled from Dirhamnolipids: Dissipative Particle Dynamics Simulation. Phys. Chem. Chem. Phys. 2018, 20, 9460–9470. [Google Scholar] [CrossRef]
- Safinya, C.R.; Radler, J. (Eds.) Handbook of Lipid Membranes: Molecular, Functional, and Materials Aspects; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-429-19407-8. [Google Scholar]
- Khunpetch, P.; Majee, A.; Podgornik, R. Curvature Effects in Charge-Regulated Lipid Bilayers. Soft Matter 2022, 18, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Hossein, A.; Deserno, M. Spontaneous Curvature, Differential Stress, and Bending Modulus of Asymmetric Lipid Membranes. Biophys. J. 2020, 118, 624–642. [Google Scholar] [CrossRef]
- Zhou, Y.; Pellouchoud, L.A.; Reed, E.J. The Potential for Fast van Der Waals Computations for Layered Materials Using a Lifshitz Model. 2D Mater. 2017, 4, 025005. [Google Scholar] [CrossRef]
- Fabozzi, A.; Russo Krauss, I.; Vitiello, R.; Fornasier, M.; Sicignano, L.; King, S.; Guido, S.; Jones, C.; Paduano, L.; Murgia, S.; et al. Branched Alkyldimethylamine Oxide Surfactants: An Effective Strategy for the Design of High Concentration/Low Viscosity Surfactant Formulations. J. Colloid Interface Sci. 2019, 552, 448–463. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Cavasso, D.; Ciccarelli, D.; Heenan, R.K.; Ortona, O.; D’Errico, G.; Paduano, L. A Hofmeister Series Perspective on the Mixed Micellization of Cationic and Non-Ionic Surfactants. J. Mol. Liq. 2021, 335, 116205. [Google Scholar] [CrossRef]
- Okur, H.I.; Hladílková, J.; Rembert, K.B.; Cho, Y.; Heyda, J.; Dzubiella, J.; Cremer, P.S.; Jungwirth, P. Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. J. Phys. Chem. B 2017, 121, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Vlachy, N.; Jagoda-Cwiklik, B.; Vácha, R.; Touraud, D.; Jungwirth, P.; Kunz, W. Hofmeister Series and Specific Interactions of Charged Headgroups with Aqueous Ions. Adv. Colloid Interface Sci. 2009, 146, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef]
- Gregory, K.P.; Elliott, G.R.; Robertson, H.; Kumar, A.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Andersson, G.G.; Page, A.J. Understanding Specific Ion Effects and the Hofmeister Series. Phys. Chem. Chem. Phys. 2022, 24, 12682–12718. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.B.; Slavchov, R.I.; Basheva, E.S.; Sidzhakova, D.; Karakashev, S.I. Hofmeister Effect on Micellization, Thin Films and Emulsion Stability. Adv. Colloid Interface Sci. 2011, 168, 93–104. [Google Scholar] [CrossRef]
- Budroni, M.A.; Rossi, F.; Marchettini, N.; Wodlei, F.; Lo Nostro, P.; Rustici, M. Hofmeister Effect in Self-Organized Chemical Systems. J. Phys. Chem. B 2020, 124, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.F.; Salis, A. Hofmeister Effects at Low Salt Concentration Due to Surface Charge Transfer. Curr. Opin. Colloid Interface Sci. 2016, 23, 41–49. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and Mechanisms of Hofmeister Effects in Electrolyte Solutions, and Colloid and Protein Systems Revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef]
- Pollard, T.P.; Beck, T.L. Toward a Quantitative Theory of Hofmeister Phenomena: From Quantum Effects to Thermodynamics. Curr. Opin. Colloid Interface Sci. 2016, 23, 110–118. [Google Scholar] [CrossRef]
| Mono-Rhamnolipid Mono-Lipidic | ||||||||
|---|---|---|---|---|---|---|---|---|
| Congener | Molar Mass (g·mol−1) | Relative Abundance (wt.%) Glucose Substrate | Relative Abundance (wt.%) Soy Substrate | R1 | n1 | n2 | R2 | 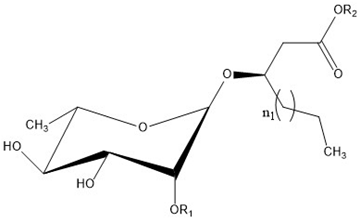 |
| Rha-C6 | 277 | 2 | - | - | - | - | - | |
| Rha-C8 | 305 | 15 | 4 | H | 1 | - | H | |
| Rha-C8:2 | 302 | - | * | H | 1(−4H) | - | H | |
| Rha-C10 | 333 | 7 | 6 | H | 3 | - | H | |
| Rha-C12:2 | 358 | - | * | H | 5(−4H) | - | H | |
| Rha-C12 | 362 | - | 7 | H | 5 | H | ||
| Rha-C14:2 | 386 | 2 | - | H | 7(−4H) | - | H | |
| Rha-C16:1 | 415 | 15 | 4 | - | - | - | - | |
| Mono-Rhamnolipid Di-Lipidic | ||||||||
| Congener | Molar Mass (g·mol−1) | Relative Abundance (wt.%) Glucose Substrate | Relative Abundance (wt.%) Soy Substrate |  | ||||
| Rha-C6-C8 | 419 | - | 2 | - | - | - | - | |
| Rha-C8-C8 | 447 | 5 | 2 | H | 1 | 3(−2H) | H | |
| Rha-C8-C8:1 | 445 | - | 3 | - | - | - | - | |
| Rha-C8-C10 | 475 | 18 | - | H | 1 | 3 | H | |
| Rha-C8-C10:1 | 473 | - | 18 | |||||
| Rha-C10-C10 | 503 | 7 | 14 | H | 3 | 3 | H | |
| Rha-C10-C12 | 531 | 4 | 5 | H | 3 | 5 | H | |
| Rha-C12-C12:1 | 557 | 4 | - | H | 5 | 5(−2H) | H | |
| Di-Rhamnolipid Mono-Lipidic | ||||||||
| Congener | Molar Mass (g·mol−1) | Relative Abundance (wt.%) Glucose Substrate | Relative Abundance (wt.%) Soy Substrate |  | ||||
| Rha-Rha-C10 | 479 | 13 | 2 | |||||
| Di-Rhamnolipid Di-Lipidic | ||||||||
| Congener | Molar Mass (g·mol−1) | Relative Abundance (wt.%) Glucose Substrate | Relative Abundance (wt.%) Soy Substrate | 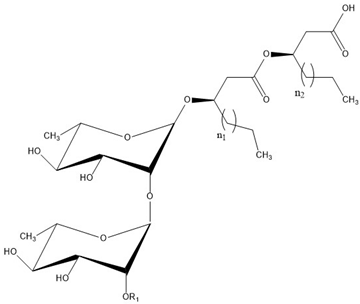 | ||||
| Rha-Rha-C10-C10 | 649 | 13 | 11 | H | 3 | 3 | H | |
| Rha-Rha-C10-C12 | 677 | - | 4 | H | 3 | 5 | H | |
| Rha-Rha-C12-C12 | 705 | 1 | - | H | 5 | 5 | H | |
| Rha-Rha-C6-C12 | 621 | - | 7 | |||||
| Rha-Rha-C6-C14 | 649 | - | 2 | |||||
| Rha-Rha-C8-C10 | 621 | 5 | 4 | H | 1 | 3 | H | |
| Rha-Rha-C8-C12 | 649 | 3 | 6 | |||||
| Rha-Rha-C8-C12:1 | 647 | 3 | 5 | H | 1 | 5(−2H) | H | |
| Organism | Carbon Source | pH | Conditions | cmc (mg/L) | γmin | Predominant Rhamnolipid Homologues | Ref |
|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa L2-1 | Mineral salts medium (MSM) + 2% (w/v) soybean oil | 30 | 30 | Rha-Rha-C10-C10 | [76] | ||
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) | Flask | 50 | 31.4 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | |
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) | Reactor | 30 | 29.0 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | |
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) +5% oil mill wastewater | 38 | 31.3 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | ||
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) +10% oil mill wastewater | 36 | 31.4 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | ||
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) +15% oil mill wastewater | 34 | 31.3 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | ||
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) +20% oil mill wastewater | 15 | 31.0 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | ||
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) +25% oil mill wastewater | Flask | 14 | 31.0 | Rha-C10-C10; Rha-Rha-C10-C10; Rha-C10 | [110] | |
| Pseudomonas aeruginosa #112 | Corn steep liquor (10%, v/v) + sugarcane molasses (10%, w/v) +25% oil mill wastewater | Reactor | 13 | 29.2 | Rha-Rha-C10-C10 | [110] | |
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 4 °C | 36.85 a 38.20 b 39.43 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 30 °C | 34.23 a 37.98 b 39.78 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 37 °C | 34.05 a 38.76 b 40.76 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 40 °C | 33.57 a 37.12 b 39.67 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 50 °C | 33.21 a 35.87 b 40.19 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 60 °C | 32.96 a 34.75 b 41.06 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 70 °C | 31.82 a 34.76 b 37.23 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 80 °C | 32.53 a 35.80 b 42.30 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 90 °C | 32.90 a 36.29 b 43.79 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | T = 100 °C | 33.65 a 36.87 b 44.97 c | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 2.0 | 32.48 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 4.0 | 34.17 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 6.0 | 34.83 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 7.2 | 32.89 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 8.0 | 33.41 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 10.0 | 36.52 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | 12.0 | 39.43 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 0% w/v | 33.56 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 0.5% w/v | 33.12 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 1% w/v | 33.27 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 1.5% w/v | 32.99 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 2% w/v | 32.96 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 2.5% w/v | 33.00 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 3% w/v | 32.85 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 3.5% w/v | 32.53 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 4% w/v | 30.47 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 4.5% w/v | 30.68 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 5% w/v | 29.71 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 6% w/v | 29.84 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 8% w/v | 30.95 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 10% w/v | 31.07 | Mixture Rha-C10-C14:1 and Rha-C8-C1 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 12% w/v | 33.00 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 14% w/v | 33.50 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 15% w/v | 34.61 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 16% w/v | 34.82 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 18% w/v | 35.50 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas aeruginosa NCIM 5514 | Bushnell-Hass (BH) medium (pH 7.2) supplemented with 1% (w/v) glucose | NaCl 20% w/v | 38.57 | Rha-C10-C14:1; Rha-C8-C10 | [67] | ||
| Pseudomonas sp. MCTG214(3b1) | ZMB supplemented with 1% (v/v) rapeseed oil | 30.13 | Rha-Rha-C10-C10; Rha-Rha-C10 | [167] | |||
| Pseudomonas aeruginosa P6 | Glucose mineral salts medium (GMSM) + 2% glycerol | 200 | 36 | Rha-Rha-C10-C10; Rha-Rha-C10-C12:1(Rha-Rha-C12:1-C10); Rha-Rha-C12-C10 (Rha-Rha-C10-C12); Rha-Rha-C10-C8 (Rha-Rha-C8-C10) | [75] | ||
| Pseudomonas aeruginosa AT10 | Soybean oil refinery wastes | Water | 0 | 230 | 27.3 | Rha-C10-C10; Rha-Rha-C10-C12; Rha-C10-C12; Rha-C12:1-C10; Rha-C12:2; R1C8:2 | [99] |
| Pseudomonas aeruginosa AT10 | Soybean oil refinery wastes | Water | 0 | 150 | 26.8 | Rha-Rha-C10C10; Rha-C10-C10; Rha-Rha-C10-C12; Rha-C10-C12; Rha-C12:1-C10; Rha-1C12:2; Rha-C8:2 | [99] |
| Pseudomonas aeruginosa mutant MIG-N146 | Mineral salts medium (MSM) and 10% (v/v) corn oil | 6.8 | NaHCO3 10 mM NaCl | 45 | 28.6 | Rha-Rha-C10-C14:1; Rha- Rha-C10-C12; Rha-Rha-C10-C12:1; Rha-Rha-C10-C10; Rha-Rha-C10- C8; Rha-Rha-C8-C10; Rha-Rha-C10 | [62] |
| Pseudomonas aeruginosa mutant MIG-N146 | Mineral salts medium (MSM) and 10% (v/v) corn oil | 6.8 | NaHCO3 10 mM NaCl | 60 | 27.6 | Rha-Rha-C10-C14:1; Rha- Rha-C10-C12; Rha-Rha-C10-C12:1; Rha-Rha-C10-C10; Rha-Rha-C10- C8; Rha-Rha-C8-C10; Rha-Rha-C10 | [62] |
| Pseudomonas aeruginosa mutant MIG-N146 | Mineral salts medium (MSM) and 10% (v/v) corn oil | 6.8 | NaHCO3 10 mM NaCl | 120 | 28.4 | Rha-C14:2; Rha-C12:2; Rha-C12-C10; Rha- C10-C12:1; Rha-C10-C10; Rha-C10:1-C8; Rha-Rha-C10-C10 | [62] |
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + glucose | 50.7 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | |||
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + glycerol | 47.2 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | |||
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + n-hexadecane | 45 | 31.1 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | ||
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + octadecene | 31.9 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | |||
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + crude oil | 32.7 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | |||
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + sunflower oil | 37.9 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | |||
| Pseudomonas aeruginosa OBP1 | Mineral salts medium (MSM) + 2 g (NH4)2SO4 + 2 g urea + soybean oil | 38.3 | Rha-C10-C10; Rha-Rha-C10-C10 | [72] | |||
| Pseudomonas aeruginosa strain KVD-HM52 | Mineral salts medium (MSM) + molasses 2% | 120 | 33.03 | Rha-C10-C10; Rha-Rha-C10-C10 | [61] | ||
| Pseudomonas aeruginosa MM1011 | Sunflower oil | 120 | 26 | Rha-C10-C10; Rha-Rha-C10-C10 | [73] | ||
| Pseudomonas aeruginosa SG | Glycerol–nitrate (GN) medium | Aerobically | 60 | 27.9 | Rha-C8-C10; Rha-Rha-C10-C12:1; Rha-Rha-C8-C10 | [70] | |
| Pseudomonas aeruginosa SG | Glycerol–nitrate (GN) medium | Anaerobically | 80 | 33.1 | Rha-C10-C12; Rha-C10-C10 | [70] | |
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 13.4 | 30 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 100 °C | 34 d 34 e 35 f 34 g 34 h 35 i | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 121 °C | 35 j | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | NaCl 0.3% w/v | 33 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | NaCl 0.9% w/v | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | NaCl 3% w/v | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | NaCl 6% w/v | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 2 | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 3 | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 4 | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 5 | 34 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 6 | 34 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 7 | 32 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 8 | 32 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 9 | 35 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 10 | 36 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 11 | 36 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 12 | 37.5 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa isolate Bs20 | Soybean oil-mineral salts medium (SMSM) | 13 | 37.5 | Mono-rhamnolipids 57%; di-rhamnolipids 43% | [64] | ||
| Pseudomonas aeruginosa BN10 | Mineral salts medium (MSM) + 2% (w/v) glucose | 33.4 | Rha-Rha-C10-C10; Rha-C10-C10 | [65] | |||
| Pseudomonas aeruginosa BN10 | Mineral salts medium (MSM) + 2% (w/v) glycerol | 40 | 27.5 | Rha-Rha-C10-C10; Rha-C10-C10 | [65] | ||
| Pseudomonas aeruginosa BN10 | Mineral salts medium (MSM) + 2% (w/v) n-hexadecane | 28.3 | Rha-Rha-C10-C10; Rha-C10-C10 | [65] | |||
| Pseudomonas aeruginosa BN10 | Mineral salts medium (MSM) + 2% (w/v) n-alkane | 70–40.3 | Rha-Rha-C10-C10; Rha-C10-C10 | [65] | |||
| Pseudomonas aeruginosa LBI | Soapstock linoleic acid 50%, oleic acid 25%, palmitic acid 7%, and stearic acid 4% | 120 | 24 | Rha-Rha-C10-C10; Rha-Rha-C10-C12:1; Rha-Rha-C10-C12; Rha-C10-C12:1; Rha-C10-C12 | [66] | ||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) glucose | 35.76 | Rha-Rha-C10-C10 | [69] | |||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) glycerol | 34.80 | Rha-Rha-C10-C10 | [69] | |||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) used soybean oil | 30.80 | Rha-C10-C10 | [69] | |||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) chicken fat | 32.76 | Rha-C10-C10 | [69] | |||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) soybean oil soapstock | 32.36 | Rha-C10-C10 | [69] | |||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) cottonseed oil waste | 86.79 | 33.86 | Rha-C10-C10 | [69] | ||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) babassu oil waste | 210.77 | 30.08 | Rha-C10-C10 | [69] | ||
| Pseudomonas aeruginosa LBI | Mineral salts medium (MSM) + 2% (w/v) corn oil waste | 43.21 | 30.96 | Rha-C10-C10 | [69] | ||
| Pseudomonas aeruginosa LBI | Soybean oil waste | 51.56 | 26.92 | Rha-C10-C10 | [69] | ||
| Pseudomonas aeruginosa LBI | Palm oil waste | 40.19 | 31.76 | Rha-C10-C10 | [69] | ||
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0 | 200 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0.1 M NaCl | 200 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0.2 M NaCl | 200 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0.4 M NaCl | 200 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0.1 M EtOH | 300 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0.2 M EtOH | 600 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa SP4 | Nutrient broth + 2% (w/v) inoculum + 2% (w/v) palm oil | PBS pH 7.4 | 0.4 M EtOH | 600 | 29 | Rha-C10-C10 | [74] |
| Pseudomonas aeruginosa CCTCC AB93066 | Mineral salts medium (MSM) + 20 g/L glucose | 6.5 | 35 | 32 | Rha-C10-C10 Rha-C10-C12–H2 Rha-C10-C12 | [58] | |
| Pseudomonas aeruginosa CCTCC AB93066 | Mineral salts medium (MSM) + 20 g/L glucose | 6.5 | 70 | 36 | Rha-Rha-C10-C10 Rha-Rha-C10-C12–H2 Rha-Rha-C10-C12 | [58] | |
| Pseudomonas sp. pyr 41 | Proteose peptone glucose ammonium salt (PPGAS) medium + glycerol | 100 | 31.93 | Di-rhamnolipids | [154] | ||
| Pseudomonas aeruginosa LCD12 | Proteose peptone glucose ammonium salt (PPGAS) medium + glycerol | 50 | 29.85 | Mono-rhamnolipids ~50%; di-rhamnolipids ~50% | [154] | ||
| Pseudomonas aeruginosa D2 | Proteose peptone glucose ammonium salt (PPGAS) medium + glycerol | 80 | 31.27 | Di-rhamnolipids | [154] | ||
| Pseudomonas aeruginosa PAO1 | Proteose peptone glucose ammonium salt (PPGAS) medium + glycerol | 60 | 31.23 | Di-rhamnolipids | [154] | ||
| Pseudomonas aeruginosa PG201 | Glycerol 2% (w/v) + hexadecane 1% (w/v) | 25.96 | Mono-rhamnolipids: di-rhamnolipids 2:1 | [168] | |||
| Pseudomonas stutzeri Rhl | Luria–Bertani (LB) medium + glycerol | 60 | [68] | ||||
| Pseudomonas stutzeri Rhl | Luria–Bertani (LB) medium + glycerol | 90 | 30.3 k | [68] | |||
| Pseudomonas stutzeri Rhl | Luria–Bertani (LB) medium + glycerol | pH 2–8 | 32 | [68] | |||
| Pseudomonas stutzeri Rhl | Luria–Bertani (LB) medium + glycerol | NaCl 0–18% | 31.5 | [68] | |||
| Burkholderia thailandensis E264 | Nutrient broth + 4% (w/v) glycerol | pH 7 | Water | 125 | 30 | Mono-rhamnolipids: di-rhamnolipids 1:3 | [88] |
| Marinobacter sp. MCTG107b | ZM/1 medium supplemented with 1% (v/v) rapeseed oil 1% (w/v) glucose | 31 | Rha-Rha-C10-C10 Methyl-Rha-Rha-C10-C10 | [169] |
| Rhamnolipid | pH | Additive | Purity | cmc (mmol/L) | γmin (mN/m) | Amin (Å2) | n | Ref |
|---|---|---|---|---|---|---|---|---|
| Rha-C10-C10 (R1) | 2 | - | purified | 27.5 | [170] | |||
| 4 | - | purified | 27.5 | [170] | ||||
| 4 | Sodium citrate 100 mM | 95 † | 0.04 | [71] | ||||
| 4 | Sodium citrate 100 mM NaCl 0.05 M | 95 † | 0.04 | [71] | ||||
| 4 | Sodium citrate 100 mM NaCl 0.1 M | 95 † | 0.05 | 30 | 63.2 | [71] | ||
| 4 | Sodium citrate 100 mM NaCl 0.2 M | 95 † | 0.04 | [71] | ||||
| 4 | Sodium citrate 100 mM NaCl 0.3 M | 95 † | 0.05 | [71] | ||||
| 4 | Sodium citrate 100 mM NaCl 0.5 M | 95 † | 0.05 | [71] | ||||
| 4 | Sodium citrate 100 mM NaCl 1 M | 95 † | 0.04 | [71] | ||||
| 5 | Sodium acetate buffer | 96 | 0.04 | 28.2 | 59.3 | 1 | [171] | |
| 6 | - | purified | 27.5 | [170] | ||||
| 6.5 | - | 80 | 0.075 | 32 | [58] | |||
| 6.8 | - | 96 | 0.1 | 30/30 | 135.1 | 2 | [171] | |
| 6.8 | - | 96 | 0.1 | 30 | 135.1 | 2 | [29] | |
| 6.8 | NaCl 0.05 M | 96 | 0.1 | 28.6 | 71.4 | 1 | [172] | |
| 6.8 | NaCl 0.5 M | 96 | 0.05 | 28.5 | 66.1 | 1 | [172] | |
| 6.8 | NaCl 1.0 M | 96 | 0.04 | 28.6 | 83.6 | 1 | [172] | |
| 6.8 | - | 96 | 0.1 | 30 | 68 | 1 | [172] | |
| 6.8 | - | purified | 0.40 | 27.44 | 110 | 2 | [170] | |
| 6.8 | NaCl 0.3 M | purified | 0.20 | 27.18 | [170] | |||
| 6.8 | NaCl 0.6 M | purified | 0.16 | 27.00 | [170] | |||
| 6.8 | NaCl 1.4 M | purified | 0.16 | 27.19 | [170] | |||
| 6.8 | NaCl 1.7 M | purified | 0.16 | 27.29 | [170] | |||
| 6.8 | Rhamnose (rhamnose: R1 = 1) | 99 | 0.07 | 28.6 | [173] | |||
| 6.8 | - | 98 †† | 0.108 | 25.2 | 98 | 2 | [174] | |
| 7 | KH2PO4 0.063 M NaOH 0.037 M | 98 †† | 0.130 | 26.3 | 109 | 2 | [174] | |
| 7 | KH2PO4 0.063 M NaOH 0.037 M | purified | 0.18 | 28.7 | 66 | 1 | [175] | |
| 7 | - | purified | 27.5 | [170] | ||||
| 7.4 | Hepes 5 mM | 95 † | 0.25 | [71] | ||||
| 7.4 | Hepes 5 mM NaCl 0.05 M | 95 † | 0.09 | [71] | ||||
| 7.4 | Hepes 5 mM NaCl 0.1 M | 95 † | 0.07 | 36 | 57.2 | 1 | [71] | |
| 7.4 | Hepes 5 mM NaCl 0.2 M | 95 † | 0.06 | [71] | ||||
| 7.4 | Hepes 5 mM NaCl 0.3 M | 95 † | 0.05 | [71] | ||||
| 7.4 | Hepes 5 mM NaCl 0.5 M | 95 † | 0.04 | [71] | ||||
| 7.4 | Hepes 5 mM NaCl 1 M | 95 † | 0.06 | [71] | ||||
| 8 | - | purified | 27.4 | [170] | ||||
| 8 | Phosphate buffer 10 mM | 98 †† | 0.201 | 29.0 | 86 | 2 | [174] | |
| 9 | Borax 0.023 M HCl 0.008 M | purified | 0.36 | 31.2 | 77 | 1 | [175] | |
| 9 | Borax 0.023 M HCl 0.008 M | purified | 0.36 | 31.2 | 75 | [176] | ||
| 10 | - | purified | 27.4 | [170] | ||||
| 12 | - | purified | 31 | [170] | ||||
| * | - | purified | 0.04 | 27.9 | 53 | 1 | [175] | |
| * | NaCl 0.5 M | purified | 0.03 | 27.8 | 52 | 1 | [165] | |
| * | - | 95 ††† | 1.17 a | - | - | - | [144] | |
| * | NaCl 0.8 M | 95 ††† | 0.22 a | - | - | - | [144] | |
| * | - | 95 ††† | 0.052 b 0.048 c 0.051 d 0.050 e | 27.89 | 82.6 | 2 | [177] | |
| R,R | 4 | - | pure | 0.016 | 27.5 | 21 | 1 | [147] |
| R,S | 4 | - | pure | 0.025 | 28.8 | 23 | 1 | [147] |
| S,S | 4 | - | pure | 0.018 | 27.5 | 21 | 1 | [147] |
| S,R | 4 | - | pure | 0.015 | 28.2 | 21 | 1 | [147] |
| R,R | 8 | - | pure | 0.27 | 28.1 | 117 | 2 | [147] |
| R,S | 8 | - | pure | 0.079 | 27.4 | 80 | 2 | [147] |
| S,S | 8 | - | pure | 0.201 | 29.5 | 93 | 2 | [147] |
| S,R | 8 | - | pure | 0.180 | 28.5 | 103 | 2 | [147] |
| Rha-C14 | 8 | - | - | 1.646 | 31.67 | 88.96 | 2 | [148] |
| Rha-C14-C6 | 8 | - | 96 | 0.519 | 35.97 | 113.87 | 2 | [148] |
| Rha-C14-C8 | 8 | - | 95 | 0.02977 | 25.83 | 61.78 | 2 | [148] |
| Rha-C14-C10 | 8 | - | 96 | 0.01034 | 23.84 | 37.78 | 2 | [148] |
| Rha-C14-C12 | 8 | - | 97 | 0.05275 | 26.26 | 63.01 | 2 | [148] |
| Rha-C14-C14 | 8 | - | 98 | 0.15265 | 35.96 | 48.78 | 2 | [148] |
| Rha-Rha-C10-C10 (R2) | 2 | - | purified | 29.5 | [170] | |||
| 4 | - | purified | 34 | [170] | ||||
| 4 | Sodium citrate 100 mM | purified | 0.01 | [59] | ||||
| 4 | Sodium citrate 100 mM NaCl 0.1 M | 50 †††† | 0.01 | 76 | 1 | [59] | ||
| 4 | Sodium citrate 100 mM NaCl 0.2 M | 50 †††† | 0.01 | [59] | ||||
| 4 | Sodium citrate 100 mM NaCl 0.5 M | 50 †††† | 0.01 | [59] | ||||
| 5 | Sodium acetate buffer | 50 †††† | 0.04 | 28.2 | 64.8 | 1 | [171] | |
| 6 | - | purified | 34 | [170] | ||||
| 6 | Phosphate-buffered saline (PBS) | 70 | 0.062 | [178] | ||||
| 6.5 | Phosphate-buffered saline (PBS) | 70 | 0.078 | [178] | ||||
| 6.5 | - | 70 | 0.106 | 35 | [58] | |||
| 6.8 | - | 99 | 0.15 | 31.2 | 131.0 | 2 | [171] | |
| 6.8 | - | 99 | 0.15 | 31.2 | 131 | 2 | [172] | |
| 6.8 | NaCl 0.05 M | 99 | 0.08 | 31.2 | 67.3 | 1 | [172] | |
| 6.8 | NaCl 0.5 M | 99 | 0.08 | 31.2 | 68.2 | 1 | [172] | |
| 6.8 | NaCl 1.0 M | 99 | 0.04 | 31.0 | 79.8 | 1 | [172] | |
| 6.8 | - | 99 | 0.15 | 30 | 56 | 1 | [179] | |
| 6.8 | - | purified | 0.46 | 31.27 | 138 | 2 | [170] | |
| 6.8 | NaCl 0.3 M | purified | 0.15 | 29.36 | [170] | |||
| 6.8 | NaCl 0.6 M | purified | 0.09 | 29.79 | [170] | |||
| 6.8 | NaCl 1.4 M | purified | 0.09 | 29.67 | [170] | |||
| 6.8 | NaCl 1.7 M | purified | 0.08 | 29.71 | [170] | |||
| 6.8 | - | 99 | 0.15 | 32 | 131 | 2 | [173] | |
| 6.8 | Rhamnose (rhamnose: R2 = 1) | 99 | 0.05 | 34 | 2 | [173] | ||
| 7 | Phosphate-buffered saline (PBS) | 70 | 0.082 | [178] | ||||
| 7 | - | purified | 33.5 | [170] | ||||
| 7 | KH2PO4 0.063 M NaOH 0.037 M | purified | 0.11 | 34.7 | 77 | 1 | [175] | |
| 7.4 | Hepes 5 mM NaCl 0 M | 50 †††† | 0.5 | [59] | ||||
| 7.4 | Hepes 5 mM NaCl 0.075 M | 50 †††† | 0.18 | [59] | ||||
| 7.4 | Hepes 5 mM NaCl 0.15 M | 50 †††† | 0.11 | 76 | 1 | [59] | ||
| 7.4 | Hepes 5 mM NaCl 0.3 M | 50 †††† | 0.11 | [59] | ||||
| 7.4 | Hepes 5 mM NaCl 0.5 M | 50 †††† | 0.1 | [59] | ||||
| 7.5 | Phosphate-buffered saline (PBS) | 70 | 0.083 | [178] | ||||
| 8 | Phosphate-buffered saline (PBS) | 70 | 0.082 | [178] | ||||
| 8 | - | purified | 34 | [170] | ||||
| 9 | Borax 0.023 M HCl 0.008 M | purified | 0.18 | 37.4 | 80 | 1 | [175] | |
| 9 | Borax 0.023 M HCl 0.008 M | purified | 0.18 | 37.4 | 79 | 1 | [176] | |
| 10 | - | purified | 33 | [170] | ||||
| 12 | - | purified | 35.5 | [170] | ||||
| * | - | purified | 0.07 | 30.3 | 84 | 1 | [175] | |
| * | NaCl 0.5 M | purified | 0.08 | 30.4 | 79 | 1 | [175] | |
| * | - | purified | 0.17 ** | 28.8 | 100 *** | 2 | [99] | |
| * | purified | 0.315 | 28 | [180] | ||||
| * | - | 95 | 0.97 e | [144] | ||||
| * | NaCl 0.6 M | 95 | 0.17 e | [144] | ||||
| Methyl-Rha-Rha-C10-C10 | * | purified | 0.495 f | 30.6 | [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, R.; Speciale, I.; De Castro, C.; D’Errico, G.; Russo Krauss, I. Rhamnolipid Self-Aggregation in Aqueous Media: A Long Journey toward the Definition of Structure–Property Relationships. Int. J. Mol. Sci. 2023, 24, 5395. https://doi.org/10.3390/ijms24065395
Esposito R, Speciale I, De Castro C, D’Errico G, Russo Krauss I. Rhamnolipid Self-Aggregation in Aqueous Media: A Long Journey toward the Definition of Structure–Property Relationships. International Journal of Molecular Sciences. 2023; 24(6):5395. https://doi.org/10.3390/ijms24065395
Chicago/Turabian StyleEsposito, Rodolfo, Immacolata Speciale, Cristina De Castro, Gerardino D’Errico, and Irene Russo Krauss. 2023. "Rhamnolipid Self-Aggregation in Aqueous Media: A Long Journey toward the Definition of Structure–Property Relationships" International Journal of Molecular Sciences 24, no. 6: 5395. https://doi.org/10.3390/ijms24065395
APA StyleEsposito, R., Speciale, I., De Castro, C., D’Errico, G., & Russo Krauss, I. (2023). Rhamnolipid Self-Aggregation in Aqueous Media: A Long Journey toward the Definition of Structure–Property Relationships. International Journal of Molecular Sciences, 24(6), 5395. https://doi.org/10.3390/ijms24065395









