Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation
Abstract
1. Introduction
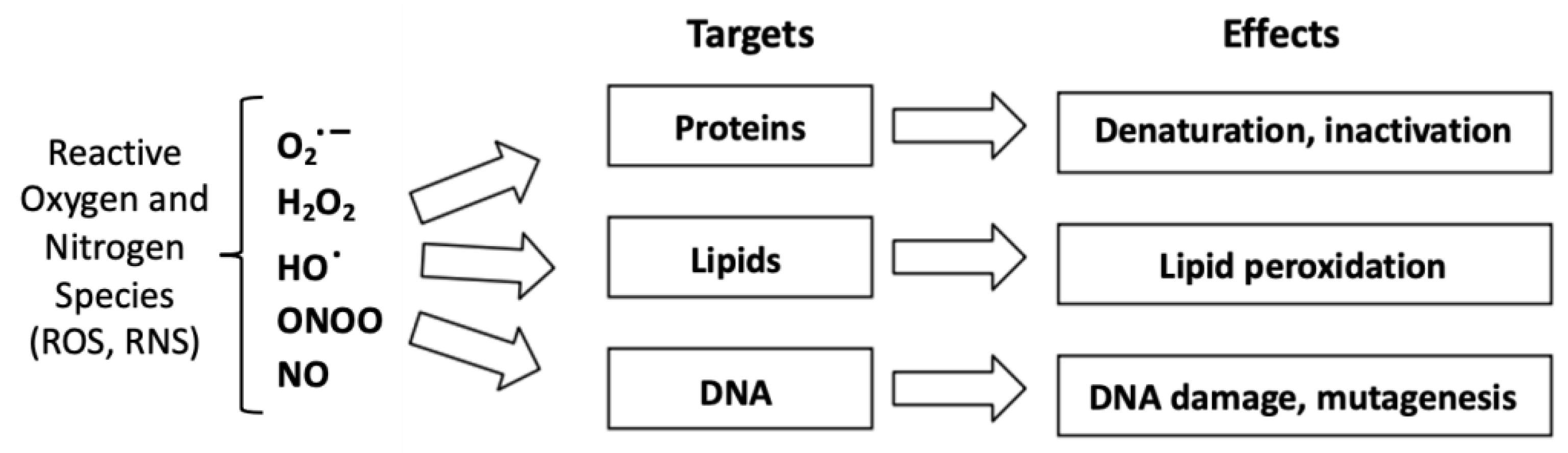
2. Reactive Oxygen Species and Lipid Peroxidation
2.1. Definition of a Reactive Oxygen Species
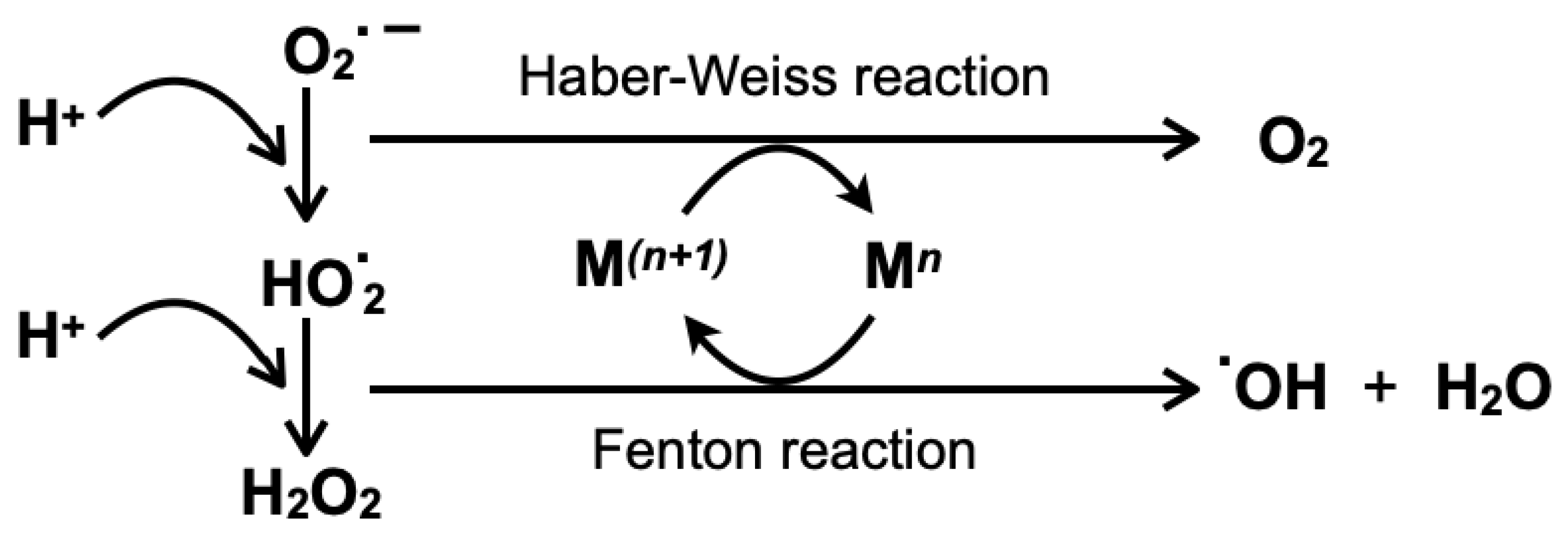
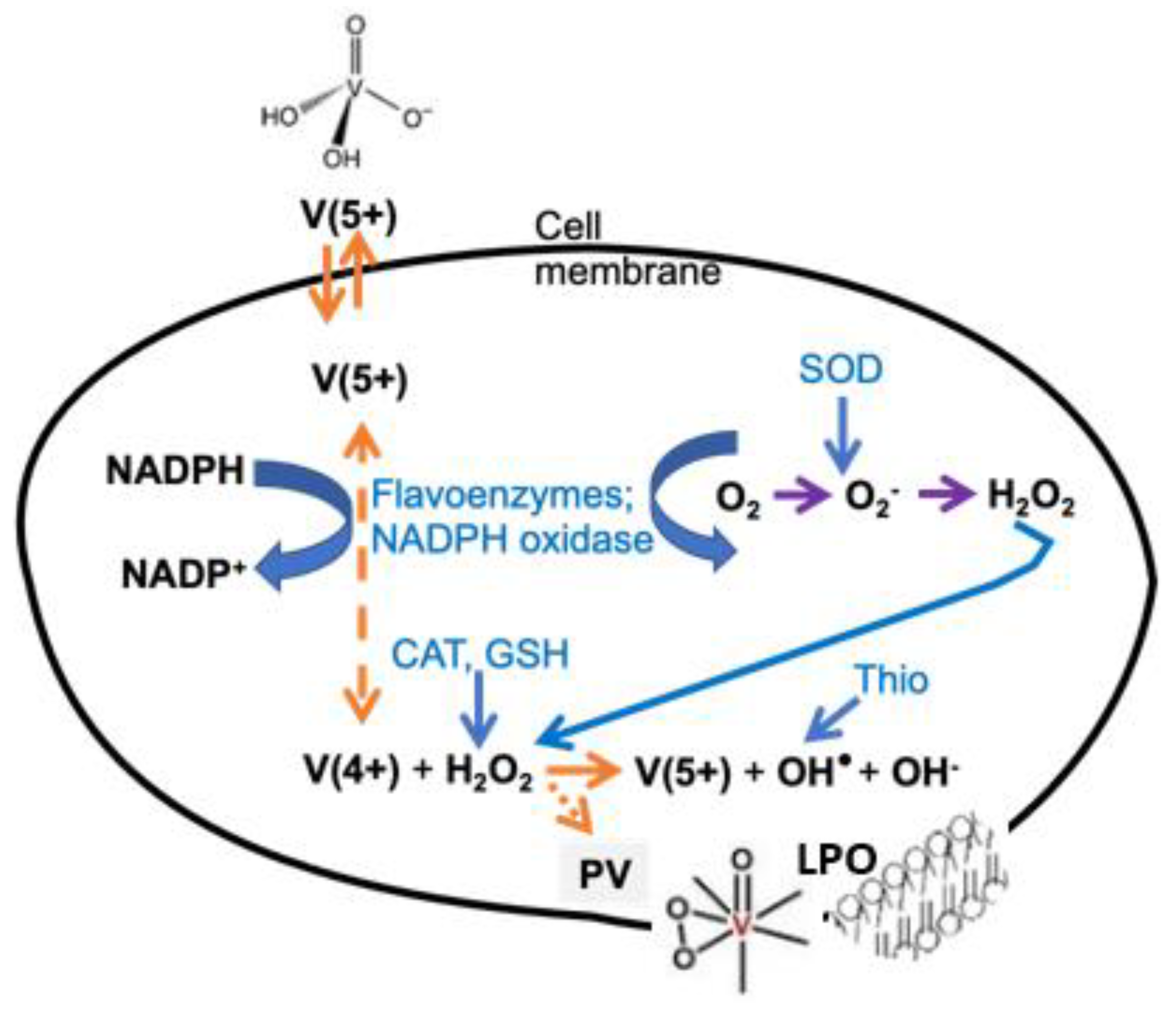
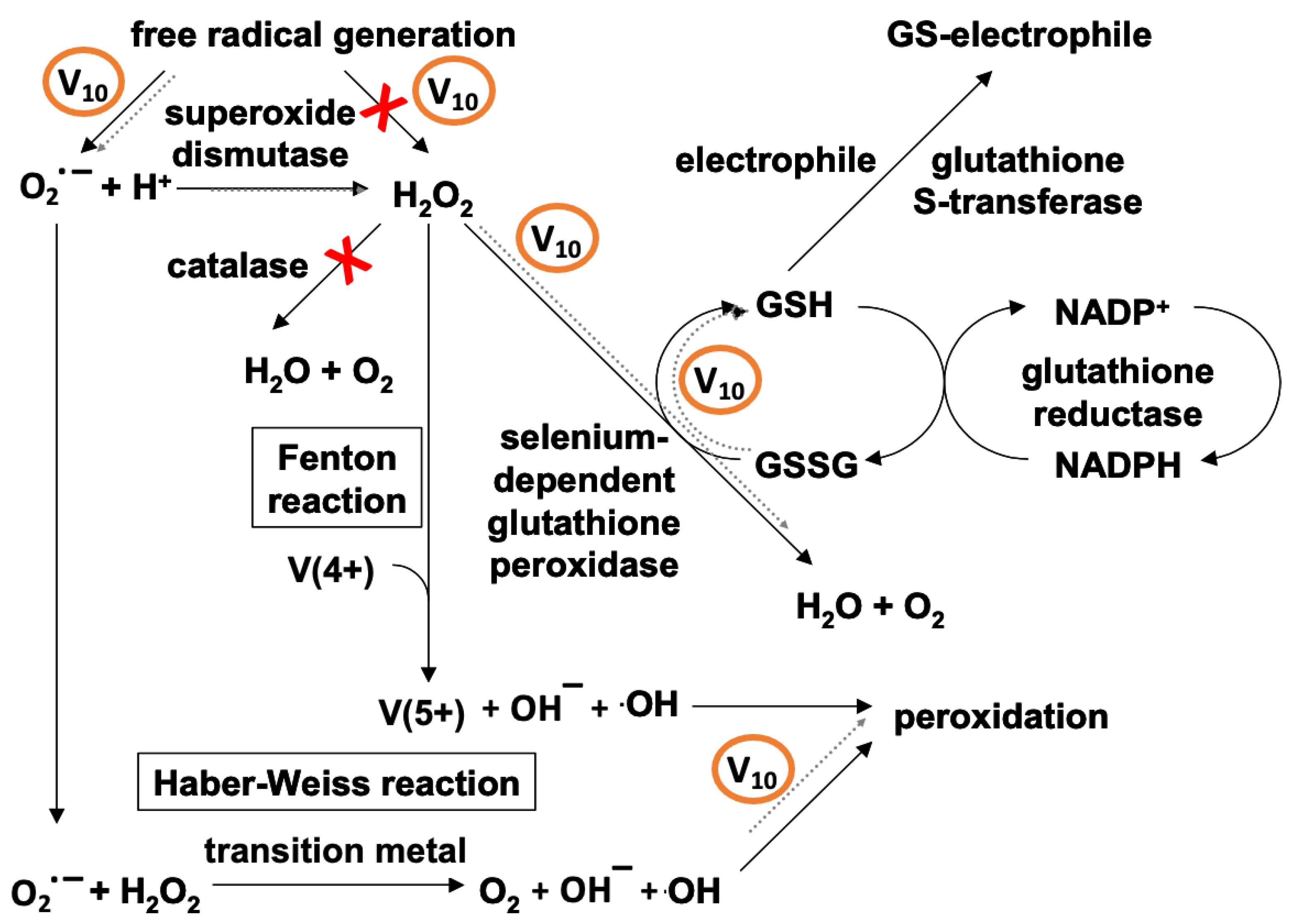
2.2. ROS Formed by Metal Calalysis
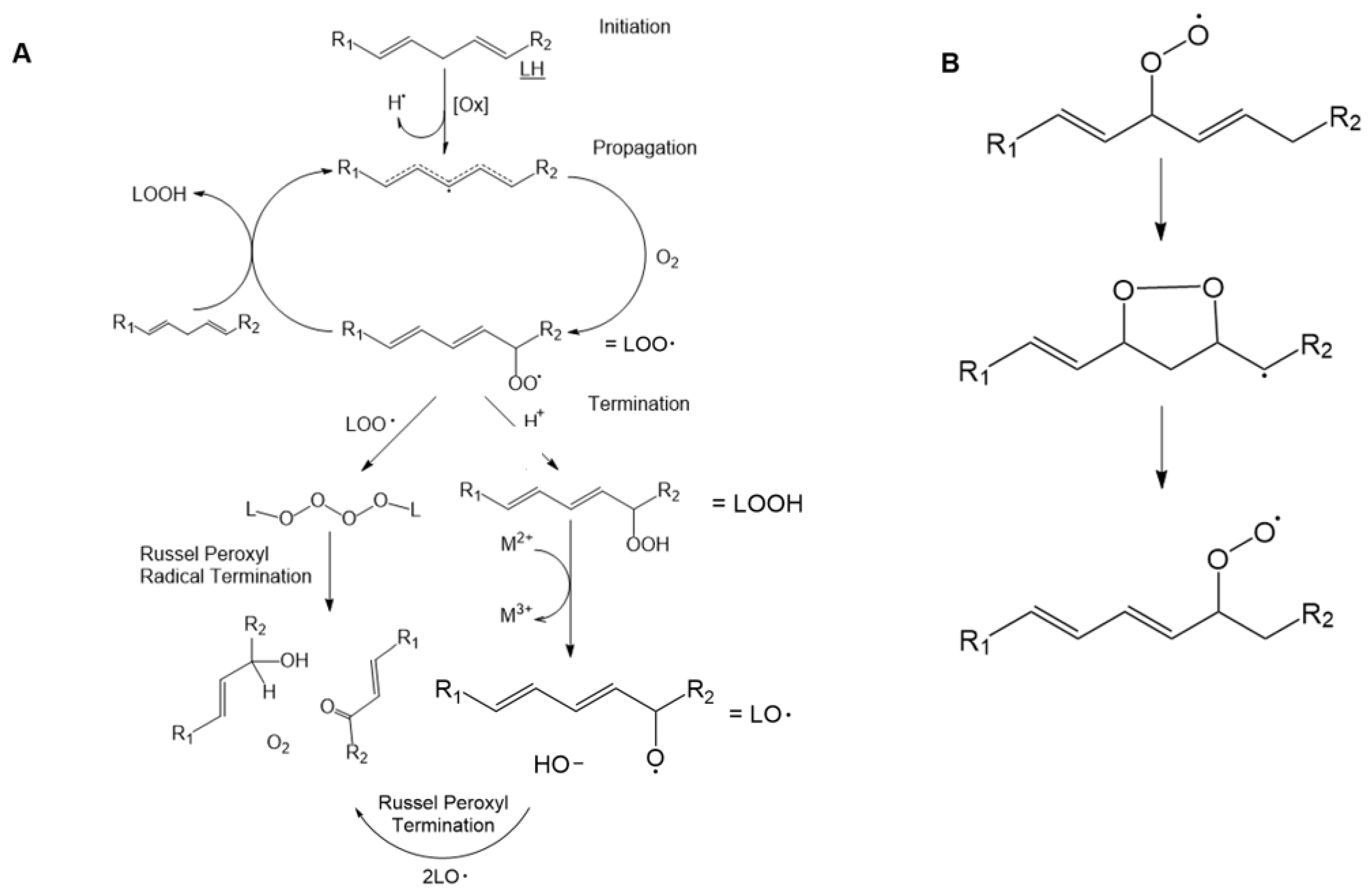
2.3. Lipid Peroxidation and Oxidized Lipid Species
2.4. Effects of Vanadium Compounds and Speciation on Lipid Peroxidation
2.5. Biomarkers Associated with Lipid Peroxidation
2.6. The Effects of Vanadium on LPO of Proteins and Enzymes
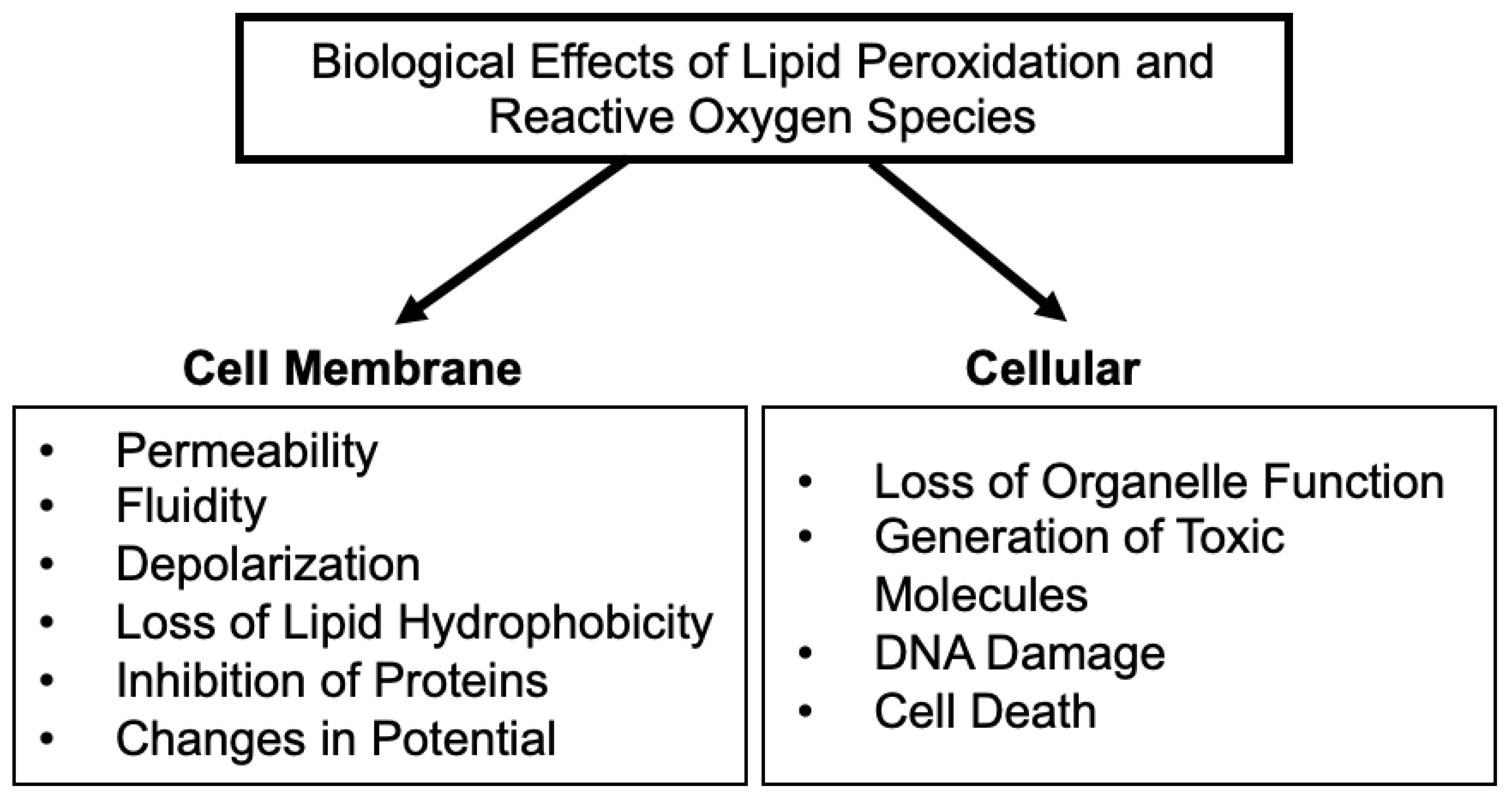
3. Effects of Vanadium and LPO on Plasma Membranes, Organelles, Mitochondria and DNA
3.1. Effects of LPO and Vanadium on the Plasma Membrane
3.2. The Effect of LPO and Vanadium on the Endomembrane System
3.3. The Effects of Vanadium and LPO in the Mitochondria
3.3.1. LPO in the Mitochondria
3.3.2. The Effect of Vanadium Salts and Complexes on the Mitochondria
3.3.3. The Effect of V10 on the Mitochondria
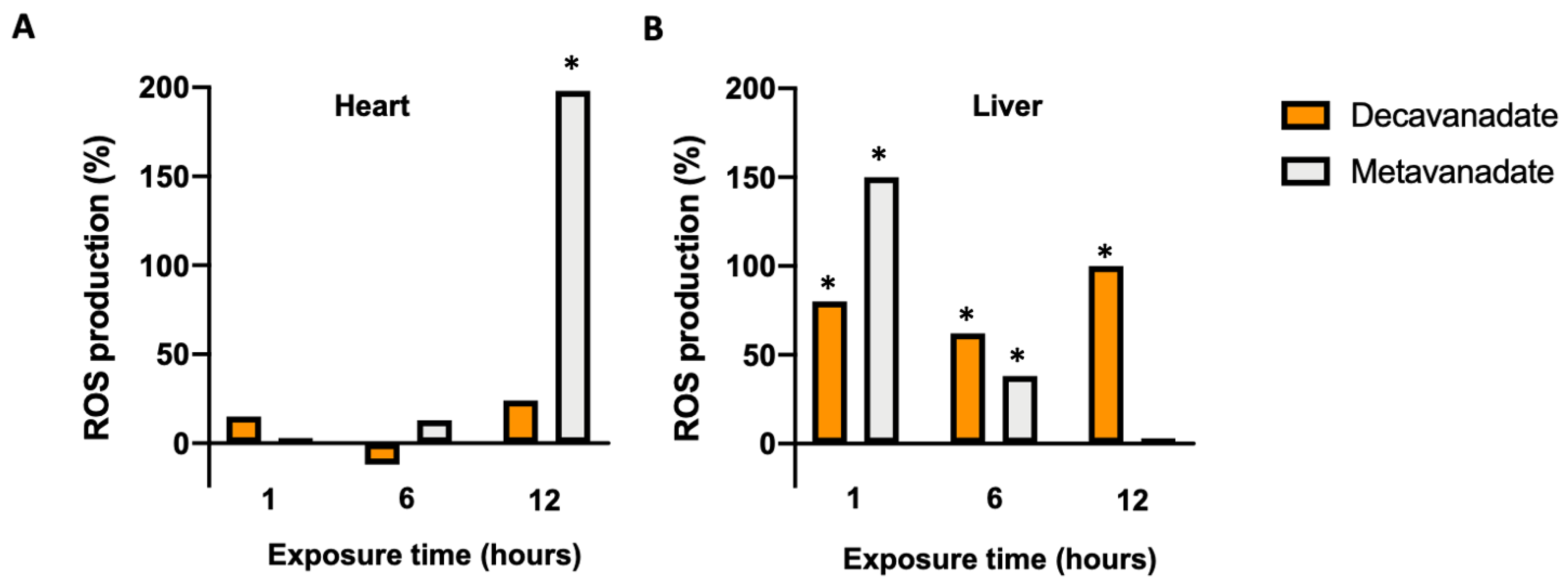
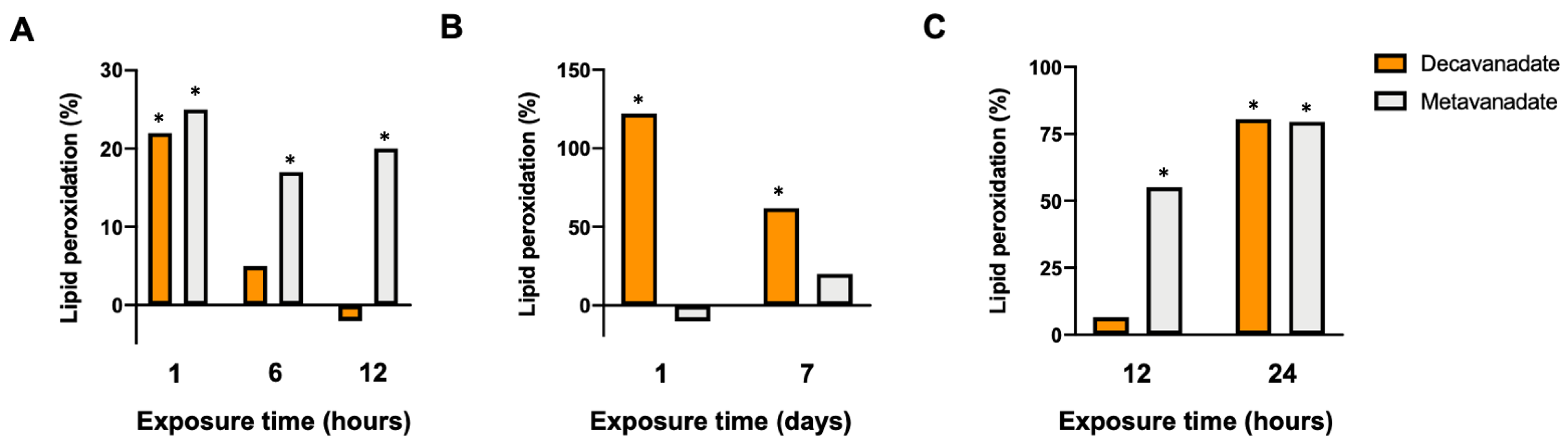
3.3.4. Effects of V10 on LPO in Mitochondria
3.4. The Effect of LPO on DNA and Apoptosis
3.5. The Oxidative Damage by V10 in Biological Systems
4. Vanadium Effects on Lipid Peroxidation and Disease Processes
4.1. Role of Vanadium in Lipid Peroxidation Related to Cancer
| Vanadium Compound | Combined/Complexed | Carcinogenic/Toxic Agent or Cell Lines | Tissue/Model | Main Results/Outcome | Ref. |
|---|---|---|---|---|---|
| V1V; VO | Aspirin; polymeric film | Osteosarcoma UMR106 cells in culture | Bone | Cytotoxic effects | [172,173] |
| V1V derivatives | Naproxen (Nap-VO); Glucose (GluVO) | Apoptosis mediated by lipid peroxidation | [157] | ||
| Ammonium monovanadate (NH4VO3, +V oxidation state) (vanadium supplemented in drinking water) | 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinogenesis in rats | Mammary gland | Prevention of mammary cancer | [138] | |
| Vanadium (in the form of ammonium vanadate) | 2-acetylaminofluorene (2-AAF)-induced hepatocarcinogenesis in rats | Liver | Vanadium was chemopreventive; inhibition of lipid peroxidation | [177] | |
| Oxovanadium(IV)-L-cysteine methyl ester (VC-IV) | Cyclophosphamide (CP)-induced hepatotoxicity in mice | Liver | Protective role of VC-IV against CP-induced toxicity | [179] | |
| Vanadium(III)-L-cysteine complex (VC-III) | Protective role of VC-III against CP- and CDDP-induced toxicity | [178] | |||
| Cisplatin (CDDP)-induced nephrotoxicity in mice | Kidney | [180] | |||
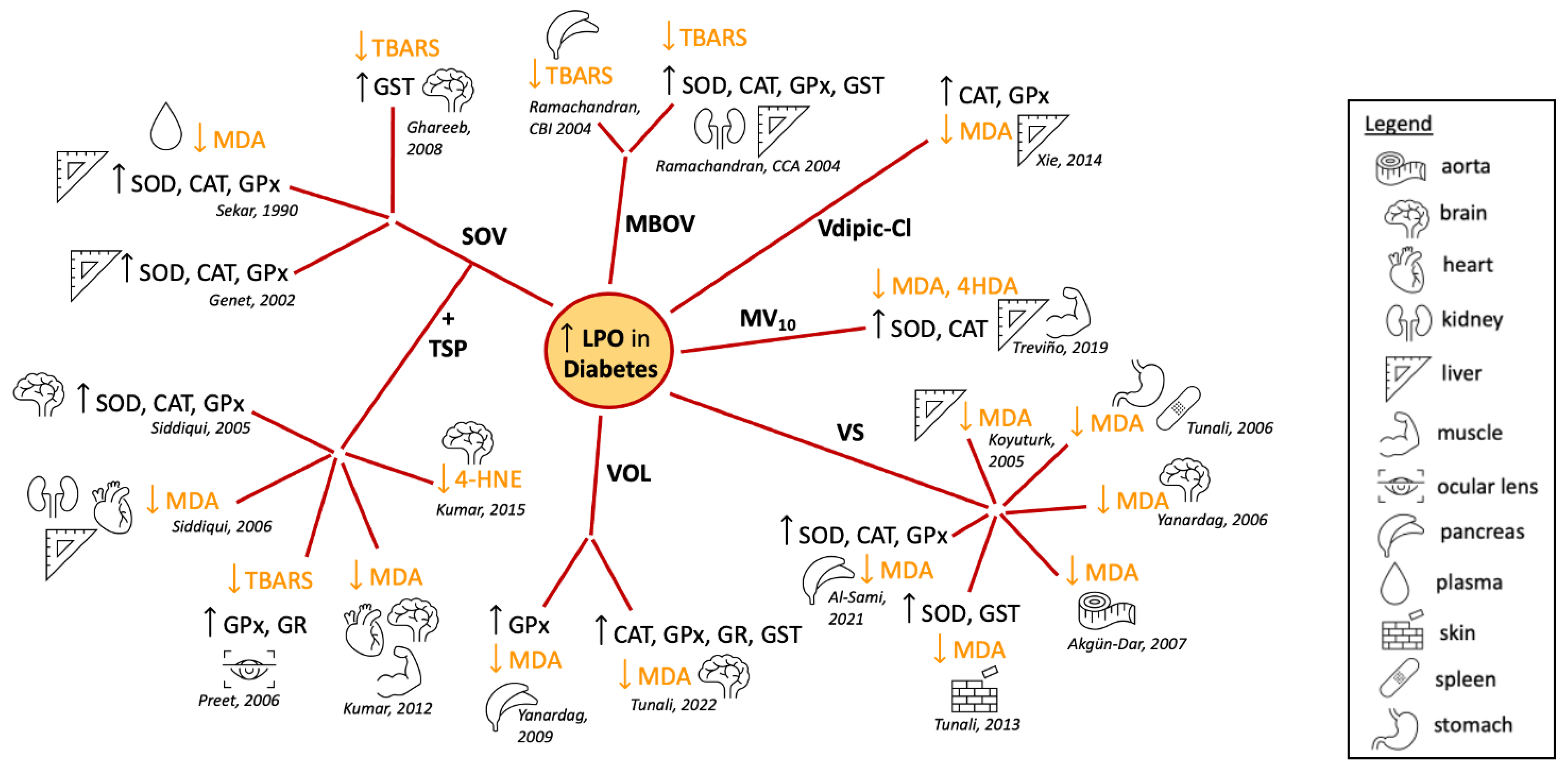
4.2. Effect of Vanadium in Diabetes-Induced Lipid Peroxidation
4.3. Vanadium Lipid Peroxidation and Neurodegenerative Diseases
4.3.1. Parkinson’s Disease
4.3.2. Alzheimer’s Disease
4.4. The Potential for LPO as a Future Target for Therapeutic Treatments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ADP | Adenosine diphosphate |
| AOX | Antioxidant |
| ATP | Adenosine triphosphate |
| BEOV | Bis(ethylmaltolato) oxidovanadium (IV) |
| Ca2+ | Calcium |
| Caco-2 | Colorectal adenocarcinoma cells |
| CAL-33 | Centre Antoine Lacassagne-33 |
| CAT | Catalase |
| CDDP | Cisplatin |
| CP | Cyclophosphamide |
| CsA | Cyclosporine A |
| Cu | Copper |
| CYP | Cytochrome P450 |
| Cyt | Cytochrome |
| DNA | Deoxyribonucleic acid |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FA | Fatty acids |
| Fe | Iron |
| GluVO | Glucose vanadate |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| HeLa | Henrietta Lacks cell line |
| HOO· | Hydroperoxyl radical |
| HO· | Hydroxyl radical |
| H2O2 | Hydrogen peroxide |
| HPLC | High-performance liquid chromatography |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| K+ | Potassium |
| LOOH | Lipid hydroperoxide |
| LOO· | Lipid peroxyl radical |
| L· | Lipid pentadienyl radical LPO |
| LPO | Lipid Peroxidation |
| MAPK | Mitogen-activated protein kinase |
| MBOV | Macrocyclic binuclear oxovanadium complex |
| MEK | Mitogen-activated protein kinase ERK kinase |
| MDA | Malondialdehyde |
| MMTP | Membrane transition pore inhibitor |
| MV10 | Metformin-decavanadate |
| Na+ | Sodium cation |
| NADH | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NapVO | Naproxen vanadate |
| NaVO3 | Sodium metavanadate |
| NF-kB | Nuclear factor kappa B |
| NH4VO3 | Ammonium metavanadate |
| NH4+ | Ammonium |
| NMR | Nuclear magnetic resonance |
| NO· | Nitric oxide radical |
| NO3− | Peroxynitrite |
| O2.− | Superoxide radical |
| O2 | Molecular oxygen |
| Ox | Oxidizers |
| PβPL | Poly(β-propiolactone) |
| POMs | Polyoxometalate |
| PUFA | Polyunsaturated fatty acid |
| RAF | Rapidly accelerated fibrosarcoma |
| Ras | Rat sarcoma |
| RCR | Respiratory control ratio |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SOV | Sodium orthovanadate |
| STZ | Streptozotocin |
| TBA | Thiobarbituric acid |
| TBARS | Thiobarbituric acid reactivity |
| TSP | Trigonella graecum seed powder |
| UV | Ultraviolet |
| V | Vanadium |
| VIV | Vanadyl |
| VV | Vanadate |
| V1 | Monomeric vanadate |
| V10 | Decavanadate |
| VC-III | Vanadium(III)-L-cysteine complex |
| VC-IV | Oxovanadium(IV)-L-cysteine methyl ester |
| VO(acac)2 | Vanadyl acetylacetonate |
| VOcitrate | Vanadyl citrate |
| VOdipic | Vanadate dipicolinate |
| VOL | N(1)-2,4-dihydroxybenzylidene-N(4)-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium (IV) |
| VOSO4 | Vanadyl sulfate |
| 2-AAF | 2-Acetylaminofluorene |
| 4-HNE | 4-Hydroxy-2-nonenal |
| 4HDA | 4-Hydroxyalkenals |
References
- Niki, E. Antioxidants in relation to lipid peroxidation. Chem. Phys. Lipids 1987, 44, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Cheeseman, K.H.; Ingold, K.U.; Slater, T.F. Lipid antioxidants and products of lipid peroxidation as potential tumour protective agents. Biochem. Soc. Trans. 1983, 11, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Vinqvist, M.R. Membrane peroxidation: Inhibiting effects of water-soluble antioxidants on phospholipids of different charge types. Free Radic. Biol. Med. 1994, 16, 779–788. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo 1999, 13, 295–309. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Cai, Z. Lipid Peroxidation. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 730–734. [Google Scholar]
- Dotan, Y. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog. Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef]
- Ścibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef]
- Vasilaki, A.T.; McMillan, D.C. Lipid Peroxidation. In Encyclopedia of Cancer; Springer: Berlin/Heidelberg, Germany, 2011; pp. 2054–2055. [Google Scholar]
- Byczkowski, J.Z.; Kulkarni, A.P. Oxidative stress and pro-oxidant biological effects of vanadium. In Vanadium in the Environment, Part 1: Chemistry and Biochemistry; John Wiley & Sons, Inc.: New York City, NY, USA, 1998; pp. 235–263. [Google Scholar]
- Loizou, M.; Hadjiadamou, I.; Drouza, C.; Keramidas, A.D.; Simos, Y.V.; Peschos, D. Vanadium(V) Complexes with Siderophore Vitamin E-Hydroxylamino-Triazine Ligands. Inorganics 2021, 9, 73. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Francik, R.; Kryczyk-Kozioł, J.; Krośniak, M.; Francik, S.; Hebda, T.; Pedryc, N.; Knapczyk, A.; Berköz, M.; Ślipek, Z. The Influence of Organic Vanadium Complexes on an Antioxidant Profile in Adipose Tissue in Wistar Rats. Materials 2022, 15, 1952. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.; Ozden, T.Y.; Ozsoy, N.; Tunali, S.; Can, A.; Akev, N.; Yanardag, R. Influence of vanadium supplementation on oxidative stress factors in the muscle of STZ-diabetic rats. BioMetals 2011, 24, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Soares, S.S.; Aureliano, M.; Joaquim, N.; Coucelo, J.M. Cadmium and vanadate oligomers effects on methaemoglobin reductase activity from Lusitanian toadfish: In vivo and in vitro studies. J. Inorg. Biochem. 2003, 94, 285–290. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate Toxicity Effects Following In Vivo Administration; Aureliano, M., Ed.; Research Signpost: Kerala, India, 2007; ISBN 978-81-308-0184-1. [Google Scholar]
- Aureliano, M.; Dorinda, M.-S.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Polyoxometalates with Anticancer, Antibacterial and Antiviral Activities. In Polyoxometalates; Rubio, L.R., Artetxe, B., Gutiérrez-Zorrilla, J.M., Vilas, J., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2022; pp. 309–358. ISBN 9781003277446/9789814968140. [Google Scholar]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Aureliano, M.; Gândara, R.M.C. Decavanadate effects in biological systems. J. Inorg. Biochem. 2005, 99, 979–985. [Google Scholar] [CrossRef]
- Aureliano, M.; Joaquim, N.; Sousa, A.; Martins, H.; Coucelo, J.M. Oxidative stress in toadfish (Halobactrachus didactylus) cardiac muscle. J. Inorg. Biochem. 2002, 90, 159–165. [Google Scholar] [CrossRef] [PubMed]
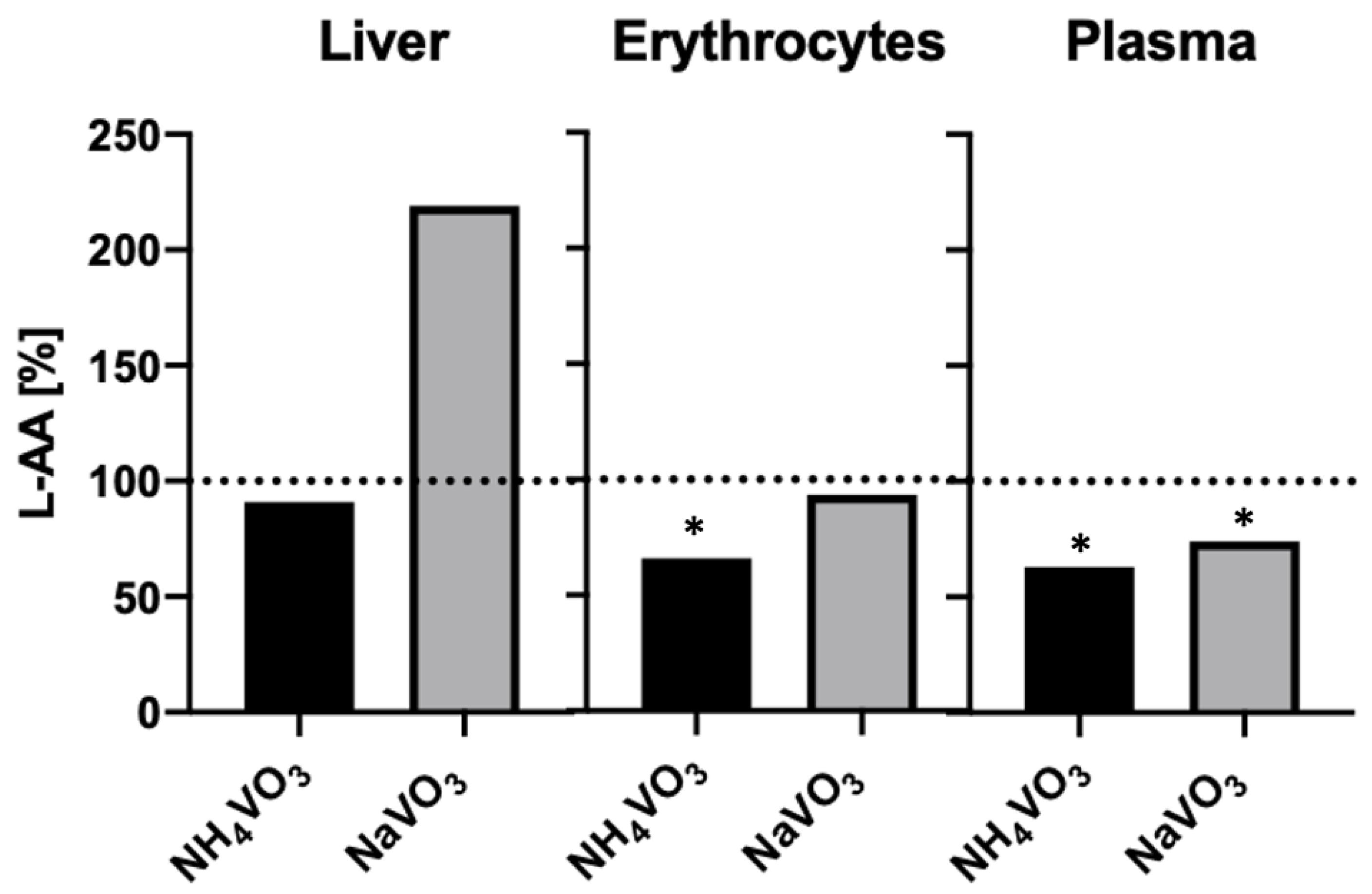
- Gândara, R.M.C.; Soares, S.S.; Martins, H.; Gutiérrez-Merino, C.; Aureliano, M. Vanadate oligomers: In vivo effects in hepatic vanadium accumulation and stress markers. J. Inorg. Biochem. 2005, 99, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.S.; Martins, H.; Duarte, R.O.; Moura, J.J.G.; Coucelo, J.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium distribution, lipid peroxidation and oxidative stress markers upon decavanadate in vivo administration. J. Inorg. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.S.; Martins, H.; Aureliano, M. Vanadium Distribution Following Decavanadate Administration. Arch. Environ. Contam. Toxicol. 2006, 50, 60–64. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Crans, D.C.; Woll, K.A.; Prusinskas, K.; Johnson, M.D.; Norkus, E. Metal Speciation in Health and Medicine Represented by Iron and Vanadium. Inorg. Chem. 2013, 52, 12262–12275. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Biochemical and medical importance of vanadium compounds. Acta Biochim. Pol. 2012, 59, 195–200. [Google Scholar] [CrossRef]
- Soriano-Agueda, L.A.; Ortega-Moo, C.; Garza, J.; Guevara-García, J.A.; Vargas, R. Formation of reactive oxygen species by vanadium complexes. Comput. Theor. Chem. 2016, 1077, 99–105. [Google Scholar] [CrossRef]
- Shi, X.L.; Dalal, N.S. Vanadate-Mediated Hydroxyl Radical Generation from Superoxide Radical in the Presence of NADH: Haber-Weiss vs. Fenton Mechanism. Arch. Biochem. Biophys. 1993, 307, 336–341. [Google Scholar] [CrossRef]
- Cheeseman, K.H. Mechanisms and effects of lipid peroxidation. Mol. Aspects Med. 1993, 14, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Comporti, M. Lipid peroxidation. Biopathological significance. Mol. Aspects Med. 1993, 14, 199–207. [Google Scholar] [CrossRef]
- Storey, K.B. Oxidative stress: Animal adaptations in nature. Brazilian J. Med. Biol. Res. 1996, 29, 1715–1733. [Google Scholar]
- Stohs, S.J. The Role of Free Radicals in Toxicity and Disease. J. Basic Clin. Physiol. Pharmacol. 1995, 6, 205–228. [Google Scholar] [CrossRef]
- Stohs, S. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Suman, S.G.; Gretarsdottir, J.M. Chemical and clinical aspects of metal-containing antidotes for poisoning by cyanide. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; De Gruyter: Berlin, Germany, 2019; pp. 359–392. [Google Scholar]
- Dix, T.A.; Aikens, J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem. Res. Toxicol. 1993, 6, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–725S. [Google Scholar] [CrossRef]
- Girotti, A.W. Mechanisms of lipid peroxidation. J. Free Radic. Biol. Med. 1985, 1, 87–95. [Google Scholar] [CrossRef]
- Bielski, B.H.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J. Biol. Chem. 1983, 258, 4759–4761. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, S.E.; Porter, N.A. Oxidation of arachidonic acid in micelles by superoxide and hydrogen peroxide. J. Biol. Chem. 1981, 256, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A.; Lehman, L.S.; Weber, B.A.; Smith, K.J. Unified mechanism for polyunsaturated fatty acid autoxidation. Competition of peroxy radical hydrogen atom abstraction, beta.-scission, and cyclization. J. Am. Chem. Soc. 1981, 103, 6447–6455. [Google Scholar] [CrossRef]
- Howard, J.A.; Ingold, K.U. Self-reaction of sec-butylperoxy radicals. Confirmation of the Russell mechanism. J. Am. Chem. Soc. 1968, 90, 1056–1058. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- de Groot, H.; Noll, T. The role of physiological oxygen partial pressures in lipid peroxidation. Theoretical considerations and experimental evidence. Chem. Phys. Lipids 1987, 44, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.W.-S.; Levett, G.; Matthew, J.A. The mechanism of the rearrangement of linoleate hydroperoxides. Chem. Phys. Lipids 1979, 24, 245–256. [Google Scholar] [CrossRef]
- Porter, N.A.; Wolf, R.A.; Yarbro, E.M.; Weenen, H. The autoxidation of arachidonic acid: Formation of the proposed SRS-A intermediate. Biochem. Biophys. Res. Commun. 1979, 89, 1058–1064. [Google Scholar] [CrossRef]
- Crans, D.C.; Yang, L.; Allison Haase, X.Y. Health Benefits of Vanadium and Its Potential as an Anticancer Agent. Met. Ions Life Sci. 2018, 18, 251–279. [Google Scholar] [CrossRef]
- Crans, D.C.; Henry, L.; Gabriel Cardiff, B.I.P. Developing Vanadium as an Antidiabetic or Anticancer Drug: A Clinical and Historical Perspective. Met Ions Life Sci. 2019, 19, 203–230. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Rivas-García, L.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J.; Aranda, P.; Montes-Bayón, M.; Llopis, J. Vanadium Decreases Hepcidin mRNA Gene Expression in STZ-Induced Diabetic Rats, Improving the Anemic State. Nutrients 2021, 13, 1256. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Dennis Chasteen, N. The biochemistry of vanadium. In Copper, Molybdenum, and Vanadium in Biological Systems; Springer: Berlin/Heidelberg, Germany, 1983; pp. 105–138. [Google Scholar]
- Horton, D.C.; VanDerveer, D.; Krzystek, J.; Telser, J.; Pittman, T.; Crans, D.C.; Holder, A.A. Spectroscopic Characterization of L-ascorbic Acid-induced Reduction of Vanadium(V) Dipicolinates: Formation of Vanadium(III) and Vanadium(IV) Complexes from Vanadium(V) Dipicolinate Derivatives. Inorg. Chim. Acta 2014, 420, 112–119. [Google Scholar] [CrossRef]
- Keller, R.J.; Sharma, R.P.; Grover, T.A.; Piette, L.H. Vanadium and lipid peroxidation: Evidence for involvement of vanadyl and hydroxyl radical. Arch. Biochem. Biophys. 1988, 265, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Kirkova, M.; Karakashev, P.; Russanov, E. Hydroxyl Radicals Production in the Vanadium Ions/Dialuric Acid Systems. Gen. Pharmacol. Vasc. Syst. 1998, 31, 247–251. [Google Scholar] [CrossRef]
- Willsky, G.R.; White, D.A.; McCabe, B.C. Metabolism of added orthovanadate to vanadyl and high-molecular-weight vanadates by Saccharomyces cerevisiae. J. Biol. Chem. 1984, 259, 13273–13281. [Google Scholar] [CrossRef]
- Vlasiou, M.C.; Pafiti, K.S. Cell Arrest and Apoptosis Induced by the Next Generation of Vanadium Based Drugs: Action Mechanism to Structure Relation and Future Perspectives. Anticancer. Agents Med. Chem. 2021, 21, 2111–2116. [Google Scholar] [CrossRef]
- Crans, D.C.; Bunch, R.L.; Theisen, L.A. Interaction of trace levels of vanadium(IV) and vanadium(V) in biological systems. J. Am. Chem. Soc. 1989, 111, 7597–7607. [Google Scholar] [CrossRef]
- Zhang, Z.; Leonard, S.S.; Huang, C.; Vallyathan, V.; Castranova, V.; Shi, X. Role of reactive oxygen species and MAPKs in vanadate-induced G2/M phase arrest. Free Radic. Biol. Med. 2003, 34, 1333–1342. [Google Scholar] [CrossRef]
- Capella, L.S.; Gefé, M.R.; Silva, E.F.; Affonso-Mitidieri, O.; Lopes, A.G.; Rumjanek, V.M.; Capella, M.A. Mechanisms of vanadate-induced cellular toxicity: Role of cellular glutathione and NADPH. Arch. Biochem. Biophys. 2002, 406, 65–72. [Google Scholar] [CrossRef]
- Yang, X.-G.; Yang, X.-D.; Yuan, L.; Wang, K.; Crans, D.C. The Permeability and Cytotoxicity of Insulin-Mimetic Vanadium Compounds. Pharm. Res. 2004, 21, 1026–1033. [Google Scholar] [CrossRef]
- Taso, O.V.; Philippou, A.; Moustogiannis, A.; Zevolis, E.; Koutsilieris, M. Lipid peroxidation products and their role in neurodegenerative diseases. Ann. Res. Hosp. 2019, 3, 2. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxidesin human plasma. Free Radic. Biol. Med. 2001, 31, 331–335. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium and cadmium in vivo effects in teleost cardiac muscle: Metal accumulation and oxidative stress markers. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 168–178. [Google Scholar] [CrossRef]
- Laranjinha, J.A.N.; Almeida, L.M.; Madeira, V.M.C. Lipid peroxidation and its inhibition in low density lipoproteins: Quenching of cis-parinaric acid fluorescence. Arch. Biochem. Biophys. 1992, 297, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Nnama, A.U.; Ekeh, F.N.; Aguzie, I.O.; Udegbunam, S.O.; Nwani, C.D. Vanadium pentoxide induces hematological, oxidative stress and histological changes in Oryctolagus cuniculus. J. Hazard. Mater. Adv. 2022, 5, 100048. [Google Scholar] [CrossRef]
- Willsky, G.R.; Chi, L.-H.; Godzala, M.; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Crans, D.C.; Tracey, A.S. The Chemistry of Vanadium in Aqueous and Nonaqueous Solution. In ACS Symposium Series; Oxford University Press: Washington, DC, USA, 1998; pp. 2–29. [Google Scholar]
- Chasteen, N.D. Vanadyl(IV) EPR Spin Probes Inorganic and Biochemical Aspects. In Biological Magnetic Resonance; Springer: Boston, MA, USA, 1981; pp. 53–119. [Google Scholar]
- McLauchlan, C.C.; Peters, B.J.; Willsky, G.R.; Crans, D.C. Vanadium–phosphatase complexes: Phosphatase inhibitors favor the trigonal bipyramidal transition state geometries. Coord. Chem. Rev. 2015, 301–302, 163–199. [Google Scholar] [CrossRef]
- Huyer, G.; Liu, S.; Kelly, J.; Moffat, J.; Payette, P.; Kennedy, B.; Tsaprailis, G.; Gresser, M.J.; Ramachandran, C. Mechanism of Inhibition of Protein-tyrosine Phosphatases by Vanadate and Pervanadate. J. Biol. Chem. 1997, 272, 843–851. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Zhang, B.; Gaidamauskas, E.; Keramidas, A.D.; Willsky, G.R.; Roberts, C.R. Is Vanadate Reduced by Thiols under Biological Conditions? Changing the Redox Potential of V(V)/V(IV) by Complexation in Aqueous Solution. Inorg. Chem. 2010, 49, 4245–4256. [Google Scholar] [CrossRef]
- Crans, D.C.; Willging, E.M.; Butler, S.R. Vanadate tetramer as the inhibiting species in enzyme reactions in vitro and in vivo. J. Am. Chem. Soc. 1990, 112, 427–432. [Google Scholar] [CrossRef]
- Ramos, S.; Manuel, M.; Tiago, T.; Duarte, R.; Martins, J.; Gutiérrez-Merino, C.; Moura, J.J.G.; Aureliano, M. Decavanadate interactions with actin: Inhibition of G-actin polymerization and stabilization of decameric vanadate. J. Inorg. Biochem. 2006, 100, 1734–1743. [Google Scholar] [CrossRef]
- Ramos, S.; Duarte, R.O.; Moura, J.J.G.; Aureliano, M. Decavanadate interactions with actin: Cysteine oxidation and vanadyl formation. Dalt. Trans. 2009, 38, 7985. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, G.; Batista de Carvalho, L.A.E.; Marques, M.P.M.; Maia, L.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Decavanadate, decaniobate, tungstate and molybdate interactions with sarcoplasmic reticulum Ca2+-ATPase: Quercetin prevents cysteine oxidation by vanadate but does not reverse ATPase inhibition. Dalt. Trans. 2012, 41, 12749. [Google Scholar] [CrossRef] [PubMed]
- Tiago, T.; Aureliano, M.; Gutiérrez-Merino, C. Decavanadate binding to a high affinity site near the myosin catalytic centre inhibits F-actin-stimulated myosin ATPase activity. Biochemistry 2004, 43, 5551–5561. [Google Scholar] [CrossRef]
- Tiago, T.; Martel, P.; Gutiérrez-Merino, C.; Aureliano, M. Binding modes of decavanadate to myosin and inhibition of the actomyosin ATPase activity. Biochim. Biophys. Acta Proteins Proteom. 2007, 1774, 474–480. [Google Scholar] [CrossRef]
- Althumairy, D.; Murakami, H.A.; Zhang, D.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Effects of vanadium(IV) compounds on plasma membrane lipids lead to G protein-coupled receptor signal transduction. J. Inorg. Biochem. 2020, 203, 110873. [Google Scholar] [CrossRef]
- Samart, N.; Althumairy, D.; Zhang, D.; Roess, D.A.; Crans, D.C. Initiation of a novel mode of membrane signaling: Vanadium facilitated signal transduction. Coord. Chem. Rev. 2020, 416, 213286. [Google Scholar] [CrossRef]
- Al-Qatati, A.; Fontes, F.L.; Barisas, B.G.; Zhang, D.; Roess, D.A.; Crans, D.C. Raft localization of Type I Fcε receptor and degranulation of RBL-2H3 cells exposed to decavanadate, a structural model for V2O5. Dalt. Trans. 2013, 42, 11912. [Google Scholar] [CrossRef]
- Garner, M.; Reglinski, J.; Smith, W.E.; McMurray, J.; Abdullah, I.; Wilson, R. A1 H spin echo and 51V NMR study of the interaction of vanadate with intact erythrocytes. J. Biol. Inorg. Chem. 1997, 2, 235–241. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Lu, J.; Crans, D. Membrane transport of vanadium compounds and the interaction with the erythrocyte membrane. Coord. Chem. Rev. 2003, 237, 103–111. [Google Scholar] [CrossRef]
- Althumairy, D.; Postal, K.; Barisas, B.G.; Nunes, G.G.; Roess, D.A.; Crans, D.C. Polyoxometalates function as indirect activators of a G protein-coupled receptor. Metallomics 2020, 12, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Kostenkova, K.; Althumairy, D.; Rajan, A.; Kortz, U.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Polyoxidovanadates V9Mo and V9Pt interact with CHO cell plasma membrane lipids causing aggregation and activation of a G proteincoupled receptor. Front. Chem. Biol. 2023, 2. in press. [Google Scholar] [CrossRef]
- Lima, L.M.A.; Murakami, H.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Levina, A.; Pires Vieira, A.; Wijetunga, A.; Kaur, R.; Koehn, J.T.; Crans, D.C.; Lay, P.A. A Short-Lived but Highly Cytotoxic Vanadium(V) Complex as a Potential Drug Lead for Brain Cancer Treatment by Intratumoral Injections. Angew. Chemie 2020, 132, 15968–15972. [Google Scholar] [CrossRef]
- Tiago, T.; Simão, S.; Aureliano, M.; Martín-Romero, F.J.; Gutiérrez-Merino, C. Inhibition of Skeletal Muscle S1-Myosin ATPase by Peroxynitrite. Biochemistry 2006, 45, 3794–3804. [Google Scholar] [CrossRef]
- Tiago, T.; Palma, P.S.; Gutierrez-Merino, C.; Aureliano, M. Peroxynitrite-mediated oxidative modifications of myosin and implications on structure and function. Free Radic. Res. 2010, 44, 1317–1327. [Google Scholar] [CrossRef]
- Tiago, T.; Ramos, S.; Aureliano, M.; Gutiérrez-Merino, C. Peroxynitrite induces F-actin depolymerization and blockade of myosin ATPase stimulation. Biochem. Biophys. Res. Commun. 2006, 342, 44–49. [Google Scholar] [CrossRef]
- Aureliano, M.; Simão, S. Peroxynitrite versus decavanadate protein oxidative modifications: The case of myosin. Free Radic. Biol. Med. 2018, 120, S69. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Doi, O.; Doi, F.; Schroeder, F.; Alberts, A.W.; Vagelos, P.R. Manipulation of fatty acid composition of membrane phospholipid and its effects on cell growth in mouse LM cells. Biochim. Biophys. Acta Biomembr. 1978, 509, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Peter Tieleman, D.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef]
- Ścibior, A.; Zaporowska, H.; Ostrowski, J.; Banach, A. Combined effect of vanadium(V) and chromium(III) on lipid peroxidation in liver and kidney of rats. Chem. Biol. Interact. 2006, 159, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Martonosi, A.; Levy, G.C.; Ejchart, A.J. 51V-n.m.r. analysis of the binding of vanadium(V) oligoanions to sarcoplasmic reticulum. Biochem. J. 1985, 230, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase activities by phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, G.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J. Inorg. Biochem. 2012, 107, 82–89. [Google Scholar] [CrossRef]
- Liochev, S.; Fridovich, I. The oxidation of NADH by tetravalent vanadium. Arch. Biochem. Biophys. 1987, 255, 274–278. [Google Scholar] [CrossRef]
- Thomas, J.P.; Maiorino, M.; Ursini, F.; Girotti, A.W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J. Biol. Chem. 1990, 265, 454–461. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Academic press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar] [CrossRef]
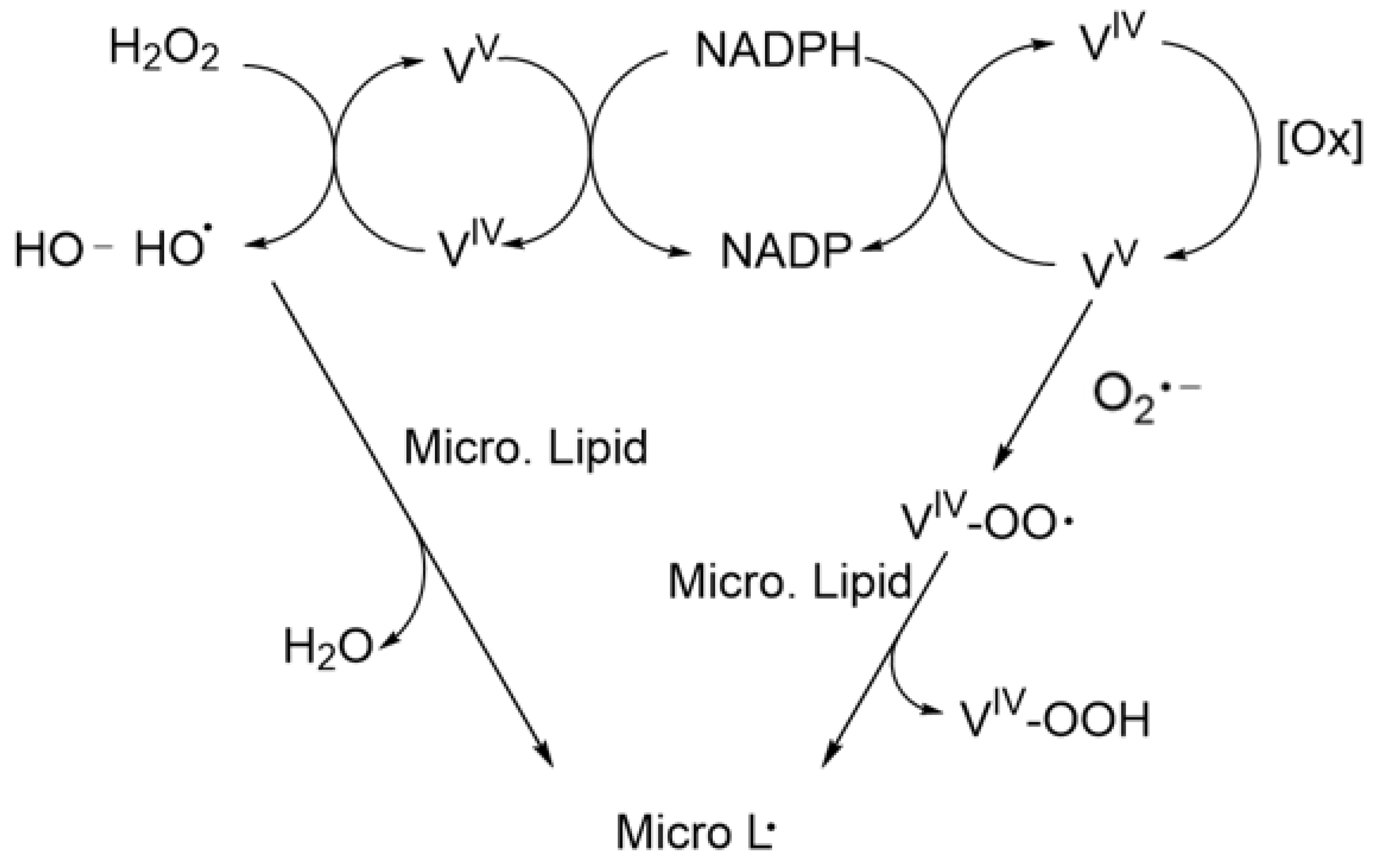
- Byczkowski, J.Z.; Wan, B.; Kulkarni, A.P. Vanadium-mediated lipid peroxidation in microsomes from human term placenta. Bull. Environ. Contam. Toxicol. 1988, 41, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Patole, M.S.; Ramasarma, T. Occurrence of Lipid Peroxidation in Brain Microsomes in the Presence of NADH and Vanadate. J. Neurochem. 1988, 51, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, L.; Liu, H.; Xia, Q.; Zhang, Y.; Yang, X.; Wang, K. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem. 2010, 104, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.-J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of vanadium on isolated rat liver mitochondria: A new mechanistic approach. Metallomics 2013, 5, 152. [Google Scholar] [CrossRef]
- Bandara, A.B.; Drake, J.C.; Brown, D.A. Complex II subunit SDHD is critical for cell growth and metabolism, which can be partially restored with a synthetic ubiquinone analog. BMC Mol. Cell Biol. 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Tesmar, A.; Sikorski, A.; Inkielewicz-Stępniak, I. Oxidovanadium(IV) Complex Disrupts Mitochondrial Membrane Potential and Induces Apoptosis in Pancreatic Cancer Cells. Anticancer. Agents Med. Chem. 2020, 21, 71–83. [Google Scholar] [CrossRef]
- Soares, S.S.; Henao, F.; Aureliano, M.; Gutiérrez-Merino, C. Vanadate Induces Necrotic Death in Neonatal Rat Cardiomyocytes Through Mitochondrial Membrane Depolarization. Chem. Res. Toxicol. 2008, 21, 607–618. [Google Scholar] [CrossRef]
- Zwolak, I.; Wnuk, E. Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants 2022, 11, 909. [Google Scholar] [CrossRef]
- Inouye, B.; Morita, K.; Ishida, T.; Ogata, M. Cooperative effect of sulfite and vanadium compounds on lipid peroxidation. Toxicol. Appl. Pharmacol. 1980, 53, 101–107. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J. Inorg. Biochem. 2007, 101, 789–796. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Mitochondria as a target for decavanadate toxicity in Sparus aurata heart. Aquat. Toxicol. 2007, 83, 1–9. [Google Scholar] [CrossRef]
- Kalyani, P.; Ramasarma, T. A novel phenomenon of burst of oxygen uptake during decavanadate-dependent oxidation of NADH. Mol. Cell. Biochem. 1993, 121, 21–29. [Google Scholar] [CrossRef] [PubMed]
- DeMaster, E.G.; Mitchell, R.A. Comparison of arsenate and vanadate as inhibitors or uncouplers of mitochondrial and glycolytic energy metabolism. Biochemistry 1973, 12, 3616–3621. [Google Scholar] [CrossRef]
- Zychlinski, L.; Byczkowski, J.Z. Inhibitory effects of vanadium pentoxide on respiration of rat liver mitochondria. Arch. Environ. Contam. Toxicol. 1990, 19, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Krivánek, J.; Nováková, L. Differential sensitivity of the brain ATP-dependent and GTP-dependent succinyl-CoA synthetase to vanadium ions. Developmental aspects. Physiol. Res. 1992, 41, 345–350. [Google Scholar]
- Kalyani, P.; Vijaya, S.; Ramasarma, T. Characterization of oxygen free radicals generated during vanadate-stimulated NADH oxidation. Mol. Cell. Biochem. 1992, 111, 33–40. [Google Scholar] [CrossRef]
- Kalyani, P.; Ramasarma, T. Polyvanadate-stimulated NADH oxidation by plasma membranes—The need for a mixture of deca and meta forms of vanadate. Arch. Biochem. Biophys. 1992, 297, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Ramasarma, T. NADH-dependent decavanadate reductase, an alternative activity of NADP-specific isocitrate dehydrogenase protein. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, B.V.; Ravishankar, H.N.; Rao, A.V.S.; Kalyani, P.; Sharada, G.; Namboodiri, K.; Gabor, B.; Ramasarma, T. Decavanadate possesses α-adrenergic agonist activity and a structural motif common with trans-β form of noradrenaline. Mol. Cell. Biochem. 1997, 169, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zurgil, N.; Shafran, Y.; Fixler, D.; Deutsch, M. Analysis of Early Apoptotic Events in Individual Cells by Fluorescence Intensity and Polarization Measurements. Biochem. Biophys. Res. Commun. 2002, 290, 1573–1582. [Google Scholar] [CrossRef]
- Matarrese, P.; Gambardella, L.; Cassone, A.; Vella, S.; Cauda, R.; Malorni, W. Mitochondrial Membrane Hyperpolarization Hijacks Activated T Lymphocytes Toward the Apoptotic-Prone Phenotype: Homeostatic Mechanisms of HIV Protease Inhibitors. J. Immunol. 2003, 170, 6006–6015. [Google Scholar] [CrossRef]
- Ganote, C. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J. Mol. Cell. Cardiol. 2003, 35, 749–759. [Google Scholar] [CrossRef]
- Sharov, V.G.; Todor, A.V.; Imai, M.; Sabbah, H.N. Inhibition of Mitochondrial Permeability Transition Pores by Cyclosporine A Improves Cytochrome c Oxidase Function and Increases Rate of ATP Synthesis in Failing Cardiomyocytes. Heart Fail. Rev. 2005, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Votyakova, T.V.; Reynolds, I.J. ΔΨm-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2008, 79, 266–277. [Google Scholar] [CrossRef]
- Genet, S.; Kale, R.K.; Baquer, N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonellafoenum graecum). Mol. Cell. Biochem. 2002, 236, 7–12. [Google Scholar] [CrossRef]
- Bishayee, A.; Oinam, S.; Basu, M.; Chatterjee, M. Vanadium chemoprevention of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis: Probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res. Treat. 2000, 63, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Feng, J.; Yue, L.; Tu, S.; Zhu, H. Synthesis, characterization and Ct-DNA interaction study of two vanadium complexes. Acta Chim. Sin. 2009, 67, 1297–1302. [Google Scholar]
- Patel, M.N.; Chhasatia, M.R.; Patel, S.H.; Bariya, H.S.; Thakkar, V.R. DNA cleavage, binding and intercalation studies of drug-based oxovanadium(IV) complexes. J. Enzyme Inhib. Med. Chem. 2009, 24, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, M.A.; Balakrishnan, C.; Selvarani, V.; Theetharappan, M. DNA/BSA binding interactions and VHPO mimicking potential of vanadium(IV) complexes: Synthesis, structural characterization and DFT studies. Appl. Organomet. Chem. 2018, 32, e4125. [Google Scholar] [CrossRef]
- Mato-López, L.; Sar-Rañó, A.; Fernández, M.R.; Díaz-Prado, M.L.; Gil, A.; Sánchez-González, Á.; Fernández-Bertólez, N.; Méndez, J.; Valdiglesias, V.; Avecilla, F. Relationship between structure and cytotoxicity of vanadium and molybdenum complexes with pyridoxal derived ligands. J. Inorg. Biochem. 2022, 235, 111937. [Google Scholar] [CrossRef] [PubMed]
- Kothandan, S.; Sheela, A. Design of oxoperoxovanadium(V) complexes and their DNA interaction studies. J. Coord. Chem. 2020, 73, 1147–1158. [Google Scholar] [CrossRef]
- Kothandan, S.; Sheela, A. DNA Interaction and Cytotoxic studies on Mono/Di-Oxo and Peroxo-Vanadium (V) complexes—A Review. Mini-Reviews Med. Chem. 2021, 21, 1909–1924. [Google Scholar] [CrossRef]
- Dash, S.P.; Panda, A.K.; Dhaka, S.; Pasayat, S.; Biswas, A.; Maurya, M.R.; Majhi, P.K.; Crochet, A.; Dinda, R. A study of DNA/BSA interaction and catalytic potential of oxidovanadium(v) complexes with ONO donor ligands. Dalt. Trans. 2016, 45, 18292–18307. [Google Scholar] [CrossRef] [PubMed]
- Aliabad, H.B.; Mohamadi, M.; Falahati-Pour, S.K.; Hajizadeh, M.R.; Abdollahdokht, D.; Nematollahi, M.H.; Mahmoodi, M. Interaction of a Vanadyl Schiff Base Complex with DNA and BSA: A Combination of Experimental and Computational Studies. Anticancer. Agents Med. Chem. 2021, 21, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Palmajumder, E.; Sepay, N.; Mukherjea, K.K. Development of oxidovanadium and oxido-peroxido vanadium-based artificial DNA nucleases via multi spectroscopic investigations and theoretical simulation of DNA binding. J. Biomol. Struct. Dyn. 2018, 36, 919–927. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Sam, M.; Hwang, J.H.; Chanfreau, G.; Abu-Omar, M.M. Hydroxyl Radical is the Active Species in Photochemical DNA Strand Scission by Bis(peroxo)vanadium(V) Phenanthroline. Inorg. Chem. 2004, 43, 8447–8455. [Google Scholar] [CrossRef]
- Sankar Ray, R.; Roy, S.; Ghosh, S.; Kumar, M.; Chatterjee, M. Suppression of cell proliferation, DNA protein cross-links, and induction of apoptosis by vanadium in chemical rat mammary carcinogenesis. Biochim. Biophys. Acta Gen. Subj. 2004, 1675, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, V.A.; Nersesyan, A.K.; Hoelzl, C.; Ferk, F.; Bichler, J.; Valic, E.; Schaffer, A.; Schulte-Hermann, R.; Fenech, M.; Wagner, K.-H.; et al. Inhalative Exposure to Vanadium Pentoxide Causes DNA Damage in Workers: Results of a Multiple End Point Study. Environ. Health Perspect. 2008, 116, 1689–1693. [Google Scholar] [CrossRef]
- Desaulniers, D.; Cummings-Lorbetskie, C.; Leingartner, K.; Xiao, G.-H.; Zhou, G.; Parfett, C. Effects of vanadium (sodium metavanadate) and aflatoxin-B1 on cytochrome p450 activities, DNA damage and DNA methylation in human liver cell lines. Toxicol. Vitr. 2021, 70, 105036. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mercado, J.J.; Mateos-Nava, R.A.; Altamirano-Lozano, M.A. DNA damage induction in human cells exposed to vanadium oxides in vitro. Toxicol. Vitr. 2011, 25, 1996–2002. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.M.; Bernstein, C.; Bernstein, H. Apoptosis Overview Emphasizing the Role of Oxidative Stress, DNA Damage and Signal-Transduction Pathways. Leuk. Lymphoma 1995, 19, 43–93. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Molinuevo, M.S.; Barrio, D.A.; Cortizo, A.M.; Etcheverry, S.B. Antitumoral properties of two new vanadyl(IV) complexes in osteoblasts in culture: Role of apoptosis and oxidative stress. Cancer Chemother. Pharmacol. 2004, 53, 163–172. [Google Scholar] [CrossRef]
- Woo, E.S.; Rice, R.L.; Lazo, J.S. Cell cycle dependent subcellular distribution of Cdc25B subtypes. Oncogene 1999, 18, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, C.; Li, J.; Leonard, S.S.; Lanciotti, R.; Butterworth, L.; Shi, X. Vanadate-Induced Cell Growth Regulation and the Role of Reactive Oxygen Species. Arch. Biochem. Biophys. 2001, 392, 311–320. [Google Scholar] [CrossRef]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the Decavanadate(V) and Oxidovanadium(IV) Binding to G-Actin and the Competition with Decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef]
- Aureliano, M. The Future Is Bright for Polyoxometalates. BioChem 2022, 2, 8–26. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Fraqueza, G.; Lagoa, R.; Vannathan, A.A.; Mal, S.S.; Aureliano, M. Polyoxovanadate inhibition of Escherichia coli growth shows a reverse correlation with Ca2+-ATPase inhibition. New J. Chem. 2019, 43, 17577–17587. [Google Scholar] [CrossRef]
- Treviño, S.; Velázquez-Vázquez, D.; Sánchez-Lara, E.; Diaz-Fonseca, A.; Flores-Hernandez, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Metforminium Decavanadate as a Potential Metallopharmaceutical Drug for the Treatment of Diabetes Mellitus. Oxid. Med. Cell. Longev. 2016, 2016, 6058705. [Google Scholar] [CrossRef]
- French, R.J.; Jones, P.J.H. Role of vanadium in nutrition: Metabolism, essentiality and dietary considerations. Life Sci. 1993, 52, 339–346. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Harland, B.F.; Harden-Williams, B.A. Is vanadium of human nutritional importance yet? J. Am. Diet. Assoc. 1994, 94, 891–894. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Anwar, M.J.; Qureshi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Byczkowski, J.Z.; Kulkarni, A.P. Lipid peroxidation and benzo(a)pyrene derivative co-oxygenation by environmental pollutants. Bull. Environ. Contam. Toxicol. 1990, 45, 633–640. [Google Scholar] [CrossRef]
- Byczkowski, J.Z.; Kulkarni, A.P. Vanadium redox cycling, lipid peroxidation and co-oxygenation of benzo(a)pyrene-7,8-dihydrodiol. Biochim. Biophys. Acta Lipids Lipid Metab. 1992, 1125, 134–141. [Google Scholar] [CrossRef]
- Younes, M.; Strubelt, O. Vanadate-induced toxicity towards isolated perfused rat livers: The role of lipid peroxidation. Toxicology 1991, 66, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, A.M.; Bruzzone, L.; Molinuevo, S.; Etcheverry, S.B. A possible role of oxidative stress in the vanadium-induced cytotoxicity in the MC3T3E1 osteoblast and UMR106 osteosarcoma cell lines. Toxicology 2000, 147, 89–99. [Google Scholar] [CrossRef]
- Cortizo, M.S.; Alessandrini, J.L.; Etcheverry, S.B.; Cortizo, A.M. A vanadium/aspirin complex controlled release using a poly(β-propiolactone) film. Effects on osteosarcoma cells. J. Biomater. Sci. Polym. Ed. 2001, 12, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Wyrzykowski, D.; Hac, S.; Rychlowski, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. New Oxidovanadium(IV) Coordination Complex Containing 2-Methylnitrilotriacetate Ligands Induces Cell Cycle Arrest and Autophagy in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 261. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chatterjee, A.; Rana, A.; Rana, B.; Palanisamy, A.; Madhappan, R.; Chatterjee, M. Suppression of Early Stages of Neoplastic Transformation in a Two-Stage Chemical Hepatocarcinogenesis Model: Supplementation of Vanadium, a Dietary Micronutrient, Limits Cell Proliferation and Inhibits the Formations of 8-Hydroxy-2′-deoxyguanosines and DN. Nutr. Cancer 2007, 59, 228–247. [Google Scholar] [CrossRef]
- Basu, A.; Bhattacharjee, A.; Samanta, A.; Bhattacharya, S. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: Effect of an l-cysteine based oxovanadium(IV) complex on oxidative stress and DNA damage. Environ. Toxicol. Pharmacol. 2015, 40, 747–757. [Google Scholar] [CrossRef]
- Basu, A.; Bhattacharjee, A.; Roy, S.S.; Ghosh, P.; Chakraborty, P.; Das, I.; Bhattacharya, S. Vanadium as a chemoprotectant: Effect of vanadium(III)-l-cysteine complex against cyclophosphamide-induced hepatotoxicity and genotoxicity in Swiss albino mice. J. Biol. Inorg. Chem. 2014, 19, 981–996. [Google Scholar] [CrossRef]
- Basu, A.; Singha Roy, S.; Bhattacharjee, A.; Bhuniya, A.; Baral, R.; Biswas, J.; Bhattacharya, S. Vanadium(III)-L-cysteine protects cisplatin-induced nephropathy through activation of Nrf2/HO-1 pathway. Free Radic. Res. 2016, 50, 39–55. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. 1), S19–S40. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gibbons, C.H.; Giurini, J.M.; Hilliard, M.E.; et al. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. 1), S203–S215. [Google Scholar] [CrossRef]
- Wright, E.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Turk, H.M.; Sevinc, A.; Camci, C.; Cigli, A.; Buyukberber, S.; Savli, H.; Bayraktar, N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002, 39, 117–122. [Google Scholar] [CrossRef]
- Ceriello, A.; Quagliaro, L.; Catone, B.; Pascon, R.; Piazzola, M.; Bais, B.; Marra, G.; Tonutti, L.; Taboga, C.; Motz, E. Role of Hyperglycemia in Nitrotyrosine Postprandial Generation. Diabetes Care 2002, 25, 1439–1443. [Google Scholar] [CrossRef]
- Likidlilid, A.; Patchanans, N.; Peerapatdit, T.; Sriratanasathavorn, C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J. Med. Assoc. Thai. 2010, 93, 682–693. [Google Scholar]
- Davì, G.; Falco, A.; Patrono, C. Lipid Peroxidation in Diabetes Mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef]
- de Souza Bastos, A.; Graves, D.T.; de Melo Loureiro, A.P.; Júnior, C.R.; Corbi, S.C.T.; Frizzera, F.; Scarel-Caminaga, R.M.; Câmara, N.O.; Andriankaja, O.M.; Hiyane, M.I.; et al. Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J. Diabetes Complicat. 2016, 30, 1593–1599. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Tolman, E.L.; Barris, E.; Burns, M.; Pansini, A.; Partridge, R. Effects of vanadium on glucose metabolism. Life Sci. 1979, 25, 1159–1164. [Google Scholar] [CrossRef]
- McNeill, J.H.; Yuen, V.G.; Hoveyda, H.R.; Orvig, C. Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J. Med. Chem. 1992, 35, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]
- Oster, M.H.; Llobet, J.M.; Domingo, J.L.; Bruce German, J.; Keen, C.L. Vanadium treatment of diabetic Sprague-Dawley rats results in tissue vanadium accumulation and pro-oxidant effects. Toxicology 1993, 83, 115–130. [Google Scholar] [CrossRef]
- Sekar, N.; Kanthasamy, A.; William, S.; Balasubramaniyan, N.; Govindasamy, S. Antioxidant effect of vanadate on experimental diabetic rats. Acta Diabetol. Lat. 1990, 27, 285–293. [Google Scholar] [CrossRef]
- Ghareeb, D.A.; Hussen, H.M. Vanadium improves brain acetylcholinesterase activity on early stage alloxan-diabetic rats. Neurosci. Lett. 2008, 436, 44–47. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Taha, A.; Moorthy, K.; Hussain, M.E.; Basir, S.F.; Baquer, N.Z. Amelioration of altered antioxidant status and membrane linked functions by vanadium andTrigonella in alloxan diabetic rat brains. J. Biosci. 2005, 30, 483–490. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Moorthy, K.; Taha, A.; Hussain, M.E.; Baquer, N.Z. Low doses of vanadate and Trigonella synergistically regulate Na+/K+-ATPase activity and GLUT4 translocation in alloxan-diabetic rats. Mol. Cell. Biochem. 2006, 285, 17–27. [Google Scholar] [CrossRef]
- Preet, A.; Siddiqui, M.R.; Taha, A.; Badhai, J.; Hussain, M.E.; Yadava, P.K.; Baquer, N.Z. Long-term effect of Trigonella foenum graecum and its combination with sodium orthovanadate in preventing histopathological and biochemical abnormalities in diabetic rat ocular tissues. Mol. Cell. Biochem. 2006, 289, 137–147. [Google Scholar] [CrossRef]
- Kumar, P.; Taha, A.; Kale, R.K.; McLean, P.; Baquer, N.Z. Beneficial effects of Trigonella foenum graecum and sodium orthovanadate on metabolic parameters in experimental diabetes. Cell Biochem. Funct. 2012, 30, 464–473. [Google Scholar] [CrossRef]
- Kumar, P.; Taha, A.; Kumar, N.; Kumar, V.; Baquer, N.Z. Sodium Orthovanadate and Trigonella Foenum Graecum Prevents Neuronal Parameters Decline and Impaired Glucose Homeostasis in Alloxan Diabetic Rats. Prague Med. Rep. 2015, 116, 122–138. [Google Scholar] [CrossRef]
- Tunali, S.; Yanardag, R. Effect of vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharmacol. Res. 2006, 53, 271–277. [Google Scholar] [CrossRef]
- Yanardag, R.; Tunali, S. Vanadyl Sulfate Administration Protects the Streptozotocin-Induced Oxidative Damage to Brain Tissue in Rats. Mol. Cell. Biochem. 2006, 286, 153–159. [Google Scholar] [CrossRef]
- Akgün-Dar, K.; Bolkent, S.; Yanardag, R.; Tunalı, S. Vanadyl sulfate protects against streptozotocin-induced morphological and biochemical changes in rat aorta. Cell Biochem. Funct. 2007, 25, 603–609. [Google Scholar] [CrossRef]
- Tunali, S.; Yanardag, R. Protective effect of vanadyl sulfate on skin injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2013, 32, 1206–1212. [Google Scholar] [CrossRef]
- Al-Salmi, F.A.; Hamza, R.Z. Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats. Curr. Issues Mol. Biol. 2021, 44, 94–104. [Google Scholar] [CrossRef]
- Tunalı, S.; Yanardağ, R. The effects of vanadyl sulfate on glutathione, lipid peroxidation and nonenzymatic glycosylation levels in various tissues in experimental diabetes. İstanbul J. Pharm. 2021, 51, 73–78. [Google Scholar] [CrossRef]
- Ramachandran, B.; Ravi, K.; Narayanan, V.; Kandaswamy, M.; Subramanian, S. Protective effect of macrocyclic binuclear oxovanadium complex on oxidative stress in pancreas of streptozotocin induced diabetic rats. Chem. Biol. Interact. 2004, 149, 9–21. [Google Scholar] [CrossRef]
- Ramachandran, B.; Ravi, K.; Narayanan, V.; Kandaswamy, M.; Subramanian, S. Effect of macrocyclic binuclear oxovanadium complex on tissue defense system in streptozotocin-induced diabetic rats. Clin. Chim. Acta 2004, 345, 141–150. [Google Scholar] [CrossRef]
- Yanardag, R.; Demirci, T.B.; Ülküseven, B.; Bolkent, S.; Tunali, S.; Bolkent, S. Synthesis, characterization and antidiabetic properties of N1-2,4-dihydroxybenzylidene-N4-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium(IV). Eur. J. Med. Chem. 2009, 44, 818–826. [Google Scholar] [CrossRef]
- Tunali, S.; Bal-Demirci, T.; Ulkuseven, B.; Yanardag, R. Protective effects of N(1)-2,4-dihydroxybenzylidene-N(4)-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium (IV) on oxidative brain injury in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2022, 36, e22991. [Google Scholar] [CrossRef]
- Thompson, K.H.; McNeill, J.H. Effect of vanadyl sulfate feeding on susceptibility to peroxidative change in diabetic rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 80, 187–200. [Google Scholar]
- Koyuturk, M.; Tunali, S.; Bolkent, S.; Yanardag, R. Effects of Vanadyl Sulfate on Liver of Streptozotocin-Induced Diabetic Rats. Biol. Trace Elem. Res. 2005, 104, 233–248. [Google Scholar] [CrossRef]
- Treviño, S.; González-Vergara, E. Metformin-decavanadate treatment ameliorates hyperglycemia and redox balance of the liver and muscle in a rat model of alloxan-induced diabetes. New J. Chem. 2019, 43, 17850–17862. [Google Scholar] [CrossRef]
- Pereira, M.J.; Carvalho, E.; Eriksson, J.W.; Crans, D.C.; Aureliano, M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J. Inorg. Biochem. 2009, 103, 1687–1692. [Google Scholar] [CrossRef]
- Aureliano, M. Recent perspectives into biochemistry of decavanadate. World J. Biol. Chem. 2011, 2, 215. [Google Scholar] [CrossRef]
- Xie, M.; Chen, D.; Zhang, F.; Willsky, G.R.; Crans, D.C.; Ding, W. Effects of vanadium (III, IV, V)-chlorodipicolinate on glycolysis and antioxidant status in the liver of STZ-induced diabetic rats. J. Inorg. Biochem. 2014, 136, 47–56. [Google Scholar] [CrossRef]
- Usende, I.L.; Leitner, D.F.; Neely, E.; Connor, J.R.; Olopade, J.O. The Deterioration Seen in Myelin Related Morphophysiology in Vanadium Exposed Rats is Partially Protected by Concurrent Iron Deficiency. Niger. J. Physiol. Sci. 2016, 3, 11–22. [Google Scholar]
- Folarin, O.R.; Snyder, A.M.; Peters, D.G.; Olopade, F.; Connor, J.R.; Olopade, J.O. Brain Metal Distribution and Neuro-Inflammatory Profiles after Chronic Vanadium Administration and Withdrawal in Mice. Front. Neuroanat. 2017, 11, 58. [Google Scholar] [CrossRef]
- Garcia, S.J.; Gellein, K.; Syversen, T.; Aschner, M. Iron Deficient and Manganese Supplemented Diets Alter Metals and Transporters in the Developing Rat Brain. Toxicol. Sci. 2007, 95, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Adekeye, A.O.; Irawo, G.J.; Fafure, A.A. Ficus exasperata Vahl leaves extract attenuates motor deficit in vanadium-induced parkinsonism mice. Anat. Cell Biol. 2020, 53, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [PubMed]
- HaMai, D.; Campbell, A.; Bondy, S.C. Modulation of oxidative events by multivalent manganese complexes in brain tissue. Free Radic. Biol. Med. 2001, 31, 763–768. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Anantharam, V.; Kitazawa, M.; Yang, Y.; Kanthasamy, A.; Kanthasamy, A.G. Protein Kinase Cδ Is a Key Downstream Mediator of Manganese-Induced Apoptosis in Dopaminergic Neuronal Cells. J. Pharmacol. Exp. Ther. 2005, 313, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A.G. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef]
- Afeseh Ngwa, H.; Kanthasamy, A.; Anantharam, V.; Song, C.; Witte, T.; Houk, R.; Kanthasamy, A.G. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: Relevance to etiopathogenesis of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2009, 240, 273–285. [Google Scholar] [CrossRef]
- Ohiomokhare, S.; Olaolorun, F.; Ladagu, A.; Olopade, F.; Howes, M.-J.R.; Okello, E.; Olopade, J.; Chazot, P.L. The Pathopharmacological Interplay between Vanadium and Iron in Parkinson’s Disease Models. Int. J. Mol. Sci. 2020, 21, 6719. [Google Scholar] [CrossRef]
- Bush, A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Markesbery, W.; Lovell, M. Four-Hydroxynonenal, a Product of Lipid Peroxidation, is Increased in the Brain in Alzheimer’s Disease. Neurobiol. Aging 1998, 19, 33–36. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Flores, E.; Mejía-García, O.A.; Ordoñez-Librado, J.L.; Gutierrez-Valdez, A.L.; Espinosa-Villanueva, J.; Dorado-Martínez, C.; Reynoso-Erazo, L.; Tron-Alvarez, R.; Rodríguez-Lara, V.; Avila-Costa, M.R. Alzheimer-like cell death after vanadium pentoxide inhalation. Heliyon 2021, 7, e07856. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; You, G.; Liu, Q.; Li, N. Alzheimer’s Disease and Diabetes Mellitus in Comparison: The Therapeutic Efficacy of the Vanadium Compound. Int. J. Mol. Sci. 2021, 22, 11931. [Google Scholar] [CrossRef]
- Dong, Y.; Stewart, T.; Zhang, Y.; Shi, M.; Tan, C.; Li, X.; Yuan, L.; Mehrotra, A.; Zhang, J.; Yang, X. Anti-diabetic vanadyl complexes reduced Alzheimer’s disease pathology independent of amyloid plaque deposition. Sci. China Life Sci. 2019, 62, 126–139. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Zhu, D.; Zhao, C.; Du, W. Methionine oxidation of amyloid peptides by peroxovanadium complexes: Inhibition of fibril formation through a distinct mechanism. Metallomics 2015, 7, 1562–1572. [Google Scholar] [CrossRef]
- He, Z.; Han, S.; Zhu, H.; Hu, X.; Li, X.; Hou, C.; Wu, C.; Xie, Q.; Li, N.; Du, X.; et al. The Protective Effect of Vanadium on Cognitive Impairment and the Neuropathology of Alzheimer’s Disease in APPSwe/PS1dE9 Mice. Front. Mol. Neurosci. 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A. Overview of Research on Vanadium-Quercetin Complexes with a Historical Outline. Antioxidants 2022, 11, 790. [Google Scholar] [CrossRef]
- Crans, D.C.; Kostenkova, K. Open questions on the biological roles of first-row transition metals. Commun. Chem. 2020, 3, 104. [Google Scholar] [CrossRef]
- Ghalichi, F.; Ostadrahimi, A.; Saghafi-Asl, M. Vanadium and biomarkers of inflammation and oxidative stress in diabetes: A systematic review of animal studies. Health Promot. Perspect. 2022, 12, 122–130. [Google Scholar] [CrossRef]
- Kaur, M.; Kaushal, R. Synthesis and in-silico molecular modelling, DFT studies, antiradical and antihyperglycemic activity of novel vanadyl complexes based on chalcone derivatives. J. Mol. Struct. 2022, 1252, 132176. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Fei, Y.; Cheng, Y. Novel Pathway for Vanadium(V) Bio-Detoxification by Gram-Positive Lactococcus raffinolactis. Environ. Sci. Technol. 2021, 55, 2121–2131. [Google Scholar] [CrossRef] [PubMed]

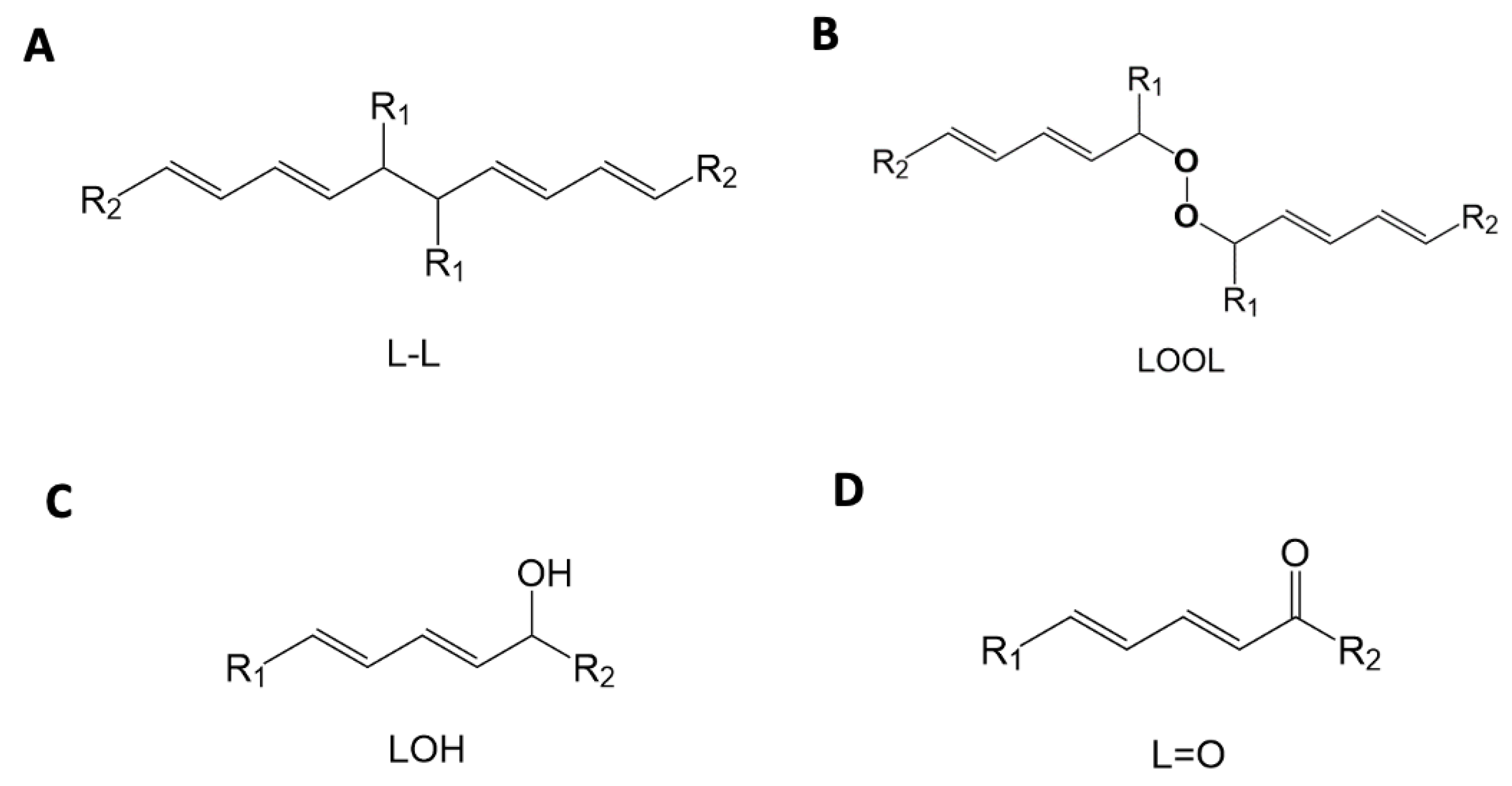

| Mechanism | Products |
|---|---|
| Russell peroxyl radical termination (4 oxygen) | Aldehyde/ketone, alcohol, molecular oxygen |
| Russell peroxyl radical termination (2 oxygen) | Aldehyde/ketone, alcohol, pentane |
| Antioxidant quenching | Lipid hydroperoxide, lipid alcohol |
| Self-termination | Crosslinked lipids |
| Cross-termination | LOOL |
| Metal-mediated | Hydroxyl radical, aldehyde/ketone, pentane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. https://doi.org/10.3390/ijms24065382
Aureliano M, De Sousa-Coelho AL, Dolan CC, Roess DA, Crans DC. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. International Journal of Molecular Sciences. 2023; 24(6):5382. https://doi.org/10.3390/ijms24065382
Chicago/Turabian StyleAureliano, Manuel, Ana Luísa De Sousa-Coelho, Connor C. Dolan, Deborah A. Roess, and Debbie C. Crans. 2023. "Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation" International Journal of Molecular Sciences 24, no. 6: 5382. https://doi.org/10.3390/ijms24065382
APA StyleAureliano, M., De Sousa-Coelho, A. L., Dolan, C. C., Roess, D. A., & Crans, D. C. (2023). Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. International Journal of Molecular Sciences, 24(6), 5382. https://doi.org/10.3390/ijms24065382







