The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions
Abstract
1. Introduction
2. Overview of Arbuscular Mycorrhizal Fungi
2.1. Molecular Signaling
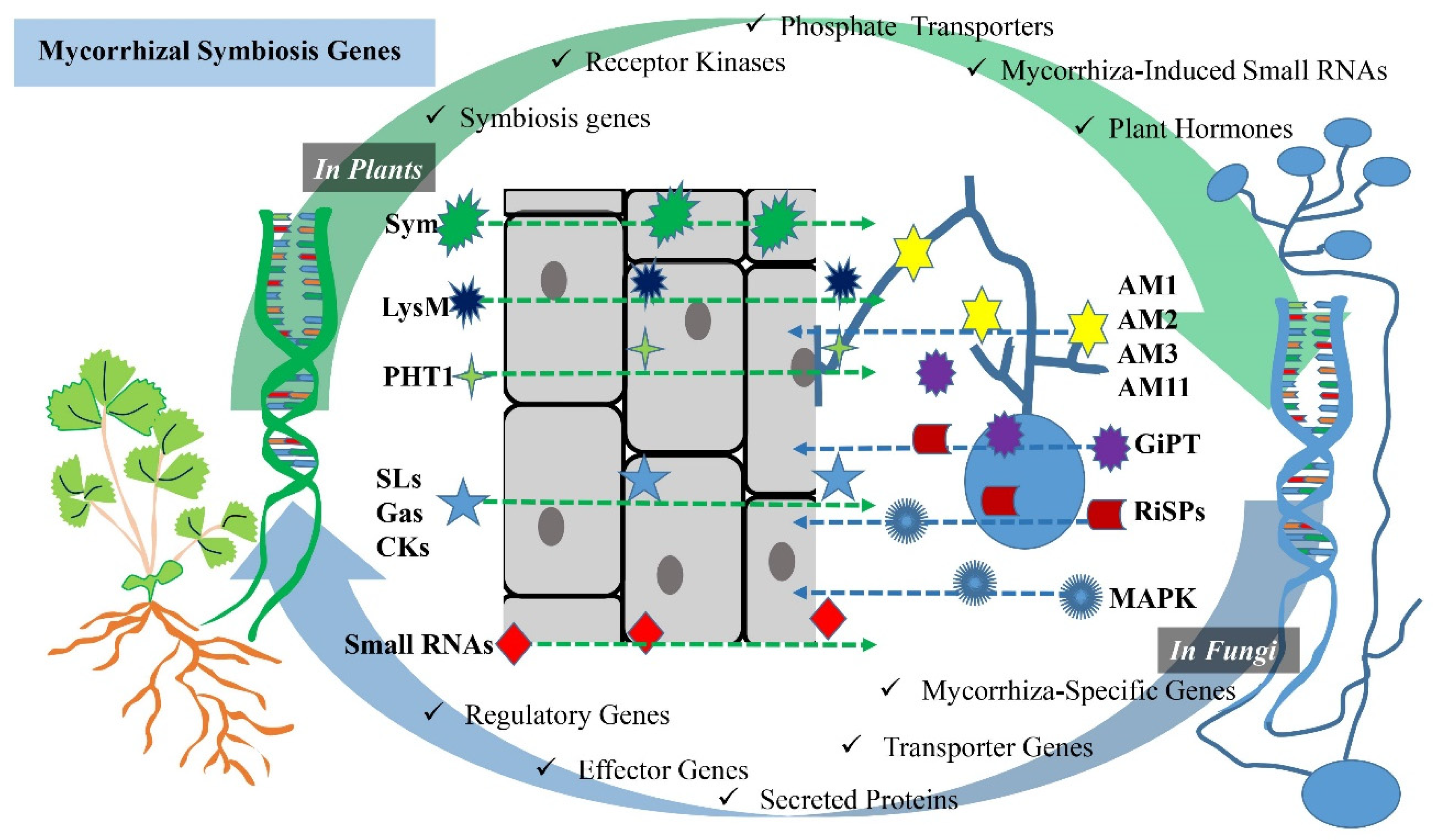
2.2. Mycorrhizal Symbiosis Genes
2.2.1. In Plants
2.2.2. In Mycorrhizal Fungi
| AMF-Induced Genes | References | ||
|---|---|---|---|
| Mycorrhiza-Specific Genes | AM1, AM2, AM3, AM11 | Genes active in the AMF-induced pre-symbiotic stage | [48] |
| AM10, AM14, AM15, AM20, AM24, AM25, AM26, AM29 | Genes active in the AMF-induced early and mature symbiotic stages | [49] | |
| Transporter Genes | GiPT | AMF-induced plant P transporter genes | [52] |
| StPT3 | [61] | ||
| OsPT11 | [62] | ||
| MtPT4 | [63] | ||
| PT11 | [49] | ||
| MtZIP5 | AMF-induced plant Zn transporter gene | [64] | |
| Secreted Proteins | LbMiSSP7 | Secreted proteins regulated by AMF | [65] |
| LjCLE19, LjCLE20 | [66] | ||
| RiSP | [55] | ||
| Effector Genes | RiSLM | AMF-induced effector genes | [67] |
| RirG175680, RirG165580, RirG263220, RirG200050, jgi.p|Gloin1|346360, RirG013260, RirG267270, jgi.p|Gloin1|154898, RirG043250, RirG045350, RirG101100, RirG043650, RirG257590, RirG187640, RirG180400, jgi.p|Gloin1|161262 | [68] | ||
| PvRxLR18, PvAVH52, PvRxLR28, PvRxLR67 | Effector genes against AMF-induced pathogen | [69] | |
| Regulatory Genes | 14-3-3 | Gene regulating AMF-induced ABA-related signaling pathway | [58] |
| MAPK | Genes regulated by AMF to enhance drought tolerance | [59] | |
| miR167, miR394, miR156 | [60] | ||
3. Strigolactones
3.1. Biosynthetic Pathway
3.2. Physiological Functions
4. Synergistic Interaction
4.1. The Evolution of the Synergistic Interaction
4.2. The Mechanisms Underlying the Synergistic Interaction
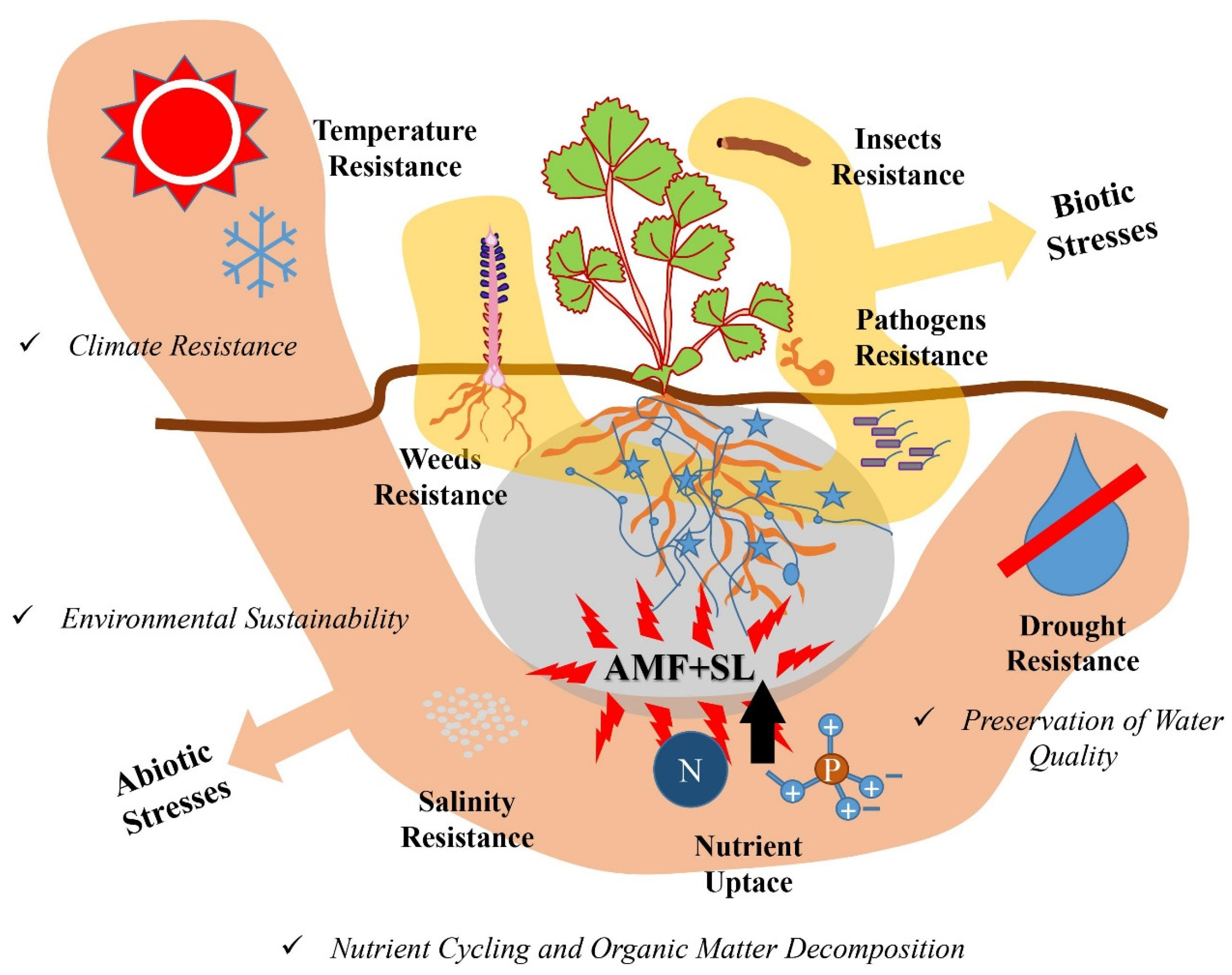
5. Significance of the Interaction
5.1. Influence on Plant–Microbe Symbiosis and Rhizosphere Dynamics
5.2. The Effects against Biotic Stresses
5.3. The Effects against Challenging Environmental Conditions
5.4. The Effects on Sustainable Agro-Ecosystems
6. Agricultural Applications
6.1. Application Methods
6.2. Agricultural Application Areas
7. Future Directions and Conclusion
7.1. Unexplored Aspects and Knowledge Gaps in the Field
7.2. Future Research Directions to Advance the Understanding of the AMF–SL Interaction
7.3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: Rijeka, Croatia, 2019; pp. 1–19. [Google Scholar]
- Demir, S.; Danesh, Y.R.; Boyno, G.; Najafi, S. Arbuscular mycorrhizal fungi in biotic and abiotic stress conditions: Function and management in horticulture. In Sustainable Horticulture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 157–183. [Google Scholar]
- Teniz, N.; Demirer Durak, E. Van’ın Erciş, Gevaş ve Edremit ilçelerinde Biber, Domates ve Kavundan Fusarium spp. ve Rhizoctonia spp.’nin Teşhisi ve Patojeniteleri. Yüzüncü Yıl Üniversitesi Fen Bilim. Enstitüsü Derg. 2023, 28, 704–714. [Google Scholar] [CrossRef]
- Taïbi, K.; Del Campo, A.; Mulet, J.; Flors, J.; Aguado, A. Testing Aleppo pine seed sources response to climate change by using trial sites reflecting future conditions. New For. 2014, 45, 603–624. [Google Scholar] [CrossRef][Green Version]
- Chevilly, S.; Dolz-Edo, L.; Martínez-Sánchez, G.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Blanca, J.; Yenush, L.; Mulet, J.M. Distinctive traits for drought and salt stress tolerance in melon (Cucumis melo L.). Front. Plant Sci. 2021, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; BE, G.S.; Khoshru, B.; De Los Santos Villalobos, S.; Belz, C.; Chaudhary, P.; Shahri, F.N.; Djebaili, R.; Adeyemi, N.O.; El-Ballat, E.M. Impacts of arbuscular mycorrhizal fungi on rice growth, development, and stress management with a particular emphasis on strigolactone effects on root development. Commun. Soil Sci. Plant Anal. 2021, 52, 1591–1621. [Google Scholar] [CrossRef]
- Boyno, G.; Demir, S. Plant-mycorrhiza communication and mycorrhizae in inter-plant communication. Symbiosis 2022, 86, 155–168. [Google Scholar] [CrossRef]
- Kalamulla, R.; Karunarathna, S.C.; Tibpromma, S.; Galappaththi, M.C.; Suwannarach, N.; Stephenson, S.L.; Asad, S.; Salem, Z.S.; Yapa, N. Arbuscular mycorrhizal fungi in sustainable agriculture. Sustainability 2022, 14, 12250. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Boyno, G.; Demir, S.; Danesh, Y.R. Effects of some biological agents on the growth and biochemical parameters of tomato plants infected with Alternaria solani (Ellis & Martin) Sorauer. Eur. J. Plant Pathol. 2022, 162, 19–29. [Google Scholar]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant hormones with promising features. Angew Chem. Int. Ed. 2019, 58, 12778–12786. [Google Scholar] [CrossRef]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Badri, D.V.; Weir, T.L.; van der Lelie, D.; Vivanco, J.M. Rhizosphere chemical dialogues: Plant–Microbe interactions. COBIOT 2009, 20, 642–650. [Google Scholar] [CrossRef]
- Yoneyama, K.; Brewer, P.B. Strigolactones, how are they synthesized to regulate plant growth and development? Curr. Opin. Plant Biol. 2021, 63, 102072. [Google Scholar] [CrossRef] [PubMed]
- Rochange, S.; Goormachtig, S.; Lopez-Raez, J.A.; Gutjahr, C. The role of strigolactones in plant–microbe interactions. In Strigolactones-Bilogy and Aplications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–142. [Google Scholar]
- Bagyaraj, D.; Sharma, M.P.; Maiti, D. Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr. Sci. 2015, 108, 1288–1293. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Boyno, G.; Demir, S.; Rezaee Danesh, Y.; Durak, E.D.; Çevik, R.; Farda, B.; Djebaili, R.; Pellegrini, M. A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere. J. Fungi 2023, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Chen, Y.; Liu, Z.; Meng, J. Biochar and arbuscular mycorrhizal fungi stimulate rice root growth strategy and soil nutrient availability. Eur. J. Soil Biol. 2022, 113, 103448. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Peng, Z.; Yang, Y.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Vogelsang, K.M.; Hartley, A.E.; Bever, J.D.; Schultz, P. Variable responses of old-field perennials to arbuscular mycorrhizal fungi and phosphorus source. Oecologia 2006, 147, 348–358. [Google Scholar] [CrossRef]
- Selwal, N.; Wani, A.K.; Akhtar, N.; Kaur, M.; Jassal, P.S. Molecular insights of Strigolactone biosynthesis, signalling pathways, regulatory roles, and hormonal crosstalks in plant systems. S. Afr. J. Bot. 2023, 160, 9–22. [Google Scholar] [CrossRef]
- Khan, M.H.; Meghvansi, M.; Panwar, V.; Gogoi, H.; Singh, L. Arbuscular mycorrhizal fungi-induced signalling in plant defence against phytopathogens. J. Phytol. 2010, 2, 53–69. [Google Scholar]
- Pozo, M.J.; Jung, S.C.; López-Ráez, J.A.; Azcón-Aguilar, C. Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: The role of plant defence mechanisms. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–207. [Google Scholar]
- Gianinazzi-Pearson, V.; Séjalon-Delmas, N.; Genre, A.; Jeandroz, S.; Bonfante, P. Plants and arbuscular mycorrhizal fungi: Cues and communication in the early steps of symbiotic interactions. Adv. Bot. Res. 2007, 46, 181–219. [Google Scholar]
- Schmitz, A.M.; Harrison, M.J. Signaling events during initiation of arbuscular mycorrhizal symbiosis. J. Integr. Plant Biol. 2014, 56, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Crosino, A.; Genre, A. Peace talks: Symbiotic signaling molecules in arbuscular mycorrhizas and their potential application. J. Plant Interact. 2022, 17, 824–839. [Google Scholar] [CrossRef]
- Requena, N.; Serrano, E.; Ocón, A.; Breuninger, M. Plant signals and fungal perception during arbuscular mycorrhiza establishment. Phytochemistry 2007, 68, 33–40. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef] [PubMed]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Li, S.; He, X. Interplant carbon and nitrogen transfers mediated by common arbuscular mycorrhizal networks: Beneficial pathways for system functionality. Front. Plant Sci. 2023, 14, 1169310. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Druzhinina, I.S.; Xie, X.; Wang, E.; Martin, F.; Yuan, Z. Phosphorus/nitrogen sensing and signaling in diverse root–fungus symbioses. Trends Microbiol. 2023. [Google Scholar] [CrossRef]
- Vos, C.; Schouteden, N.; Van Tuinen, D.; Chatagnier, O.; Elsen, A.; De Waele, D.; Panis, B.; Gianinazzi-Pearson, V. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 2013, 60, 45–54. [Google Scholar] [CrossRef]
- Zhuang, X.; Gao, J.; Ma, A.; Fu, S.; Zhuang, G. Bioactive molecules in soil ecosystems: Masters of the underground. Int. J. Mol. Sci. 2013, 14, 8841–8868. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Banba, M.; Gutjahr, C.; Miyao, A.; Hirochika, H.; Paszkowski, U.; Kouchi, H.; Imaizumi-Anraku, H. Divergence of evolutionary ways among common sym genes: CASTOR and CCaMK show functional conservation between two symbiosis systems and constitute the root of a common signaling pathway. Plant Cell Physiol. 2008, 49, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, W.; Sun, J.; Feng, F.; Deng, Y.; He, Z.; Oldroyd, G.E.; Wang, E. The receptor kinase CERK 1 has dual functions in symbiosis and immunity signalling. Plant J. 2015, 81, 258–267. [Google Scholar] [CrossRef]
- Kelly, S.; Radutoiu, S.; Stougaard, J. Legume LysM receptors mediate symbiotic and pathogenic signalling. Curr. Opin. Plant Biol. 2017, 39, 152–158. [Google Scholar] [CrossRef]
- Walder, F.; Brulé, D.; Koegel, S.; Wiemken, A.; Boller, T.; Courty, P.E. Plant phosphorus acquisition in a common mycorrhizal network: Regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol. 2015, 205, 1632–1645. [Google Scholar] [CrossRef]
- Christophersen, H.; Smith, F.; Smith, S. Arbuscular mycorrhizal colonization reduces arsenate uptake in barley via downregulation of transporters in the direct epidermal phosphate uptake pathway. New Phytol. 2009, 184, 962–974. [Google Scholar] [CrossRef]
- Devers, E.A.; Branscheid, A.; May, P.; Krajinski, F. Stars and symbiosis: microRNA-and microRNA*-mediated transcript cleavage involved in arbuscular mycorrhizal symbiosis. Plant Physiol. 2011, 156, 1990–2010. [Google Scholar] [CrossRef]
- Waldie, T.; McCulloch, H.; Leyser, O. Strigolactones and the control of plant development: Lessons from shoot branching. Plant J. 2014, 79, 607–622. [Google Scholar] [CrossRef]
- Wang, G.-L.; Que, F.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Foo, E.; Ross, J.J.; Jones, W.T.; Reid, J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013, 111, 769–779. [Google Scholar] [CrossRef]
- Giron, D.; Frago, E.; Glevarec, G.; Pieterse, C.M.; Dicke, M. Cytokinins as key regulators in plant–microbe–insect interactions: Connecting plant growth and defence. Funct. Ecol. 2013, 27, 599–609. [Google Scholar] [CrossRef]
- Colard, A.; Angelard, C.; Sanders, I.R. Genetic exchange in an arbuscular mycorrhizal fungus results in increased rice growth and altered mycorrhiza-specific gene transcription. AEM 2011, 77, 6510–6515. [Google Scholar] [CrossRef]
- Gutjahr, C.; Banba, M.; Croset, V.; An, K.; Miyao, A.; An, G.; Hirochika, H.; Imaizumi-Anraku, H.; Paszkowski, U. Arbuscular mycorrhiza–specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 2008, 20, 2989–3005. [Google Scholar] [CrossRef]
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.B.; Xu, G.; Jakobsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. MPMI 2001, 14, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Pieterse, C.M. Modulation of host immunity by beneficial microbes. MPMI 2012, 25, 139–150. [Google Scholar] [CrossRef]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef]
- Kamel, L.; Tang, N.; Malbreil, M.; San Clemente, H.; Le Marquer, M.; Roux, C.; Frei dit Frey, N. The comparison of expressed candidate secreted proteins from two arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Front. Plant Sci. 2017, 8, 124. [Google Scholar] [CrossRef]
- De Wit, P.J.; Mehrabi, R.; Van den Burg, H.A.; Stergiopoulos, I. Fungal effector proteins: Past, present and future. Mol. Plant Pathol. 2009, 10, 735–747. [Google Scholar] [CrossRef]
- Sędzielewska Toro, K.; Brachmann, A. The effector candidate repertoire of the arbuscular mycorrhizal fungus Rhizophagus clarus. BMC Genom. 2016, 17, 101. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Ruan, Y.; Xu, L.; Hu, Y.; Hao, Z.; Zhang, X.; Li, H.; Wang, Y.; Yang, L. Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 2016, 26, 879–893. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Fileccia, V.; Ingraffia, R.; Amato, G.; Giambalvo, D.; Martinelli, F. Identification of microRNAS differentially regulated by water deficit in relation to mycorrhizal treatment in wheat. Mol. Biol. Rep. 2019, 46, 5163–5174. [Google Scholar] [CrossRef]
- Rausch, C.; Daram, P.; Brunner, S.; Jansa, J.; Laloi, M.; Leggewie, G.; Amrhein, N.; Bucher, M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 2001, 414, 462–465. [Google Scholar] [CrossRef]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: A physiological and molecular assessment. Sci. Rep. 2019, 9, 14880. [Google Scholar] [CrossRef]
- Plett, J.M.; Kemppainen, M.; Kale, S.D.; Kohler, A.; Legué, V.; Brun, A.; Tyler, B.M.; Pardo, A.G.; Martin, F. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 2011, 21, 1197–1203. [Google Scholar] [CrossRef]
- Handa, Y.; Nishide, H.; Takeda, N.; Suzuki, Y.; Kawaguchi, M.; Saito, K. RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant Cell Physiol. 2015, 56, 1490–1511. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.M.; Pawlowska, T.E.; Harrison, M.J. A short LysM protein with high molecular diversity from an arbuscular mycorrhizal fungus, Rhizophagus irregularis. Mycoscience 2018, 60, 63–70. [Google Scholar] [CrossRef]
- Zeng, T.; Holmer, R.; Hontelez, J.; te Lintel-Hekkert, B.; Marufu, L.; de Zeeuw, T.; Wu, F.; Schijlen, E.; Bisseling, T.; Limpens, E. Host-and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant J. 2018, 94, 411–425. [Google Scholar] [CrossRef]
- Cruz-Silva, A.; Figueiredo, A.; Sebastiana, M. First insights into the effect of mycorrhizae on the expression of pathogen effectors during the infection of grapevine with Plasmopara viticola. Sustainability 2021, 13, 1226. [Google Scholar] [CrossRef]
- Cook, C.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Li, C.; Dong, L.; Durairaj, J.; Guan, J.-C.; Yoshimura, M.; Quinodoz, P.; Horber, R.; Gaus, K.; Li, J.; Setotaw, Y.B. Maize resistance to witchweed through changes in strigolactone biosynthesis. Science 2023, 379, 94–99. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant. Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, S.; Shukla, R.K. The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front. Physiol. 2017, 7, 691. [Google Scholar] [CrossRef]
- Boyer, F.-D.; de Saint Germain, A.; Pillot, J.-P.; Pouvreau, J.-B.; Chen, V.X.; Ramos, S.; Stévenin, A.; Simier, P.; Delavault, P.; Beau, J.-M. Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: Molecule design for shoot branching. Plant Physiol. 2012, 159, 1524–1544. [Google Scholar] [CrossRef]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef]
- Beveridge, C.A.; Symons, G.M.; Turnbull, C.G. Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 2000, 123, 689–698. [Google Scholar] [CrossRef]
- Simons, J.L.; Napoli, C.A.; Janssen, B.J.; Plummer, K.M.; Snowden, K.C. Analysis of the decreased apical dominance genes of petunia in the control of axillary branching. Plant Physiol. 2007, 143, 697–706. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Zhang, W.; Li, G.; Chen, Z.; Zhai, W.; Zhao, X.; Pan, X.; Xie, Q.; Zhu, L. The rice HIGH—TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006, 48, 687–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; Van Der Krol, S.; Leyser, O. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef]
- Baz, L.; Mori, N.; Mi, J.; Jamil, M.; Kountche, B.A.; Guo, X.; Balakrishna, A.; Jia, K.-P.; Vermathen, M.; Akiyama, K. 3-Hydroxycarlactone, a novel product of the strigolactone biosynthesis core pathway. Mol. Plant 2018, 11, 1312–1314. [Google Scholar] [CrossRef]
- Yoneyama, K.; Akiyama, K.; Brewer, P.B.; Mori, N.; Kawano-Kawada, M.; Haruta, S.; Nishiwaki, H.; Yamauchi, S.; Xie, X.; Umehara, M. Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct. 2020, 4, e00219. [Google Scholar] [CrossRef]
- Butt, H.; Jamil, M.; Wang, J.Y.; Al-Babili, S.; Mahfouz, M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Bari, V.K.; Nassar, J.A.; Kheredin, S.M.; Gal-On, A.; Ron, M.; Britt, A.; Steele, D.; Yoder, J.; Aly, R. CRISPR/Cas9-mediated mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in tomato provides resistance against the parasitic weed Phelipanche aegyptiaca. Sci. Rep. 2019, 9, 11438. [Google Scholar] [CrossRef]
- Hao, J.; Yang, Y.; Futrell, S.; Kelly, E.A.; Lorts, C.M.; Nebie, B.; Runo, S.; Yang, J.; Alvarez, S.; Lasky, J.R. CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase (CCD) genes in Sorghum alters strigolactone biosynthesis and plant biotic interactions. Phytobiomes J. 2023, 7, 339–351. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.-i.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Liu, W.; Kohlen, W.; Lillo, A.; Op den Camp, R.; Ivanov, S.; Hartog, M.; Limpens, E.; Jamil, M.; Smaczniak, C.; Kaufmann, K. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 2011, 23, 3853–3865. [Google Scholar] [CrossRef]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kalliola, M.; Jakobson, L.; Davidsson, P.; Pennanen, V.; Waszczak, C.; Yarmolinsky, D.; Zamora, O.; Palva, E.T.; Kariola, T.; Kollist, H. Differential role of MAX2 and strigolactones in pathogen, ozone, and stomatal responses. Plant Direct. 2020, 4, e00206. [Google Scholar] [CrossRef]
- Nasir, F.; Tian, L.; Shi, S.; Chang, C.; Ma, L.; Gao, Y.; Tian, C. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Biochem. 2019, 142, 106–116. [Google Scholar] [CrossRef]
- Xu, X.; Fang, P.; Zhang, H.; Chi, C.; Song, L.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Strigolactones positively regulate defense against root-knot nematodes in tomato. J. Exp. Bot. 2019, 70, 1325–1337. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944. [Google Scholar] [CrossRef]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef]
- Toh, S.; Kamiya, Y.; Kawakami, N.; Nambara, E.; McCourt, P.; Tsuchiya, Y. Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol. 2012, 53, 107–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, S.; Wang, G. Strigolactones are common regulators in induction of stomatal closure in planta. Plant Signal. Behav. 2018, 13, e1444322. [Google Scholar] [CrossRef]
- Tariq, A.; Ullah, I.; Sardans, J.; Graciano, C.; Mussarat, S.; Ullah, A.; Zeng, F.; Wang, W.; Al-Bakre, D.A.; Ahmed, Z. Strigolactones can be a potential tool to fight environmental stresses in arid lands. Environ. Res. 2023, 229, 115966. [Google Scholar] [CrossRef]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Waters, M.T.; Smith, S.M.; Nelson, D.C. Smoke signals and seed dormancy: Where next for MAX2? Plant Signal. Behav. 2011, 6, 1418–1422. [Google Scholar] [CrossRef][Green Version]
- García-Garrido, J.; Lendzemo, V.; Castellanos-Morales, V.; Steinkellner, S.; Vierheilig, H. Strigolactones, signals for parasitic plants and arbuscular mycorrhizal fungi. Mycorrhiza 2009, 19, 449–459. [Google Scholar] [CrossRef]
- Omoarelojie, L.; Kulkarni, M.; Finnie, J.; Van Staden, J. Strigolactones and their crosstalk with other phytohormones. Ann. Bot. 2019, 124, 749–767. [Google Scholar] [CrossRef]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Kloosterman, B.; Oortwijn, M.; Kohlen, W.; Bouwmeester, H.J.; Visser, R.G.; Bachem, C.W. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 2012, 63, 4539–4547. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Sami, F.; Siddiqui, H.; Yusuf, M.; Gruszka, D.; Hayat, S. Role of strigolactones: Signalling and crosstalk with other phytohormones. Open Life Sci. 2020, 15, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2010; pp. 169–190. [Google Scholar]
- Pons, S.; Fournier, S.; Chervin, C.; Bécard, G.; Rochange, S.; Frei Dit Frey, N.; Puech Pagès, V. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef]
- Mishev, K.; Dobrev, P.I.; Lacek, J.; Filepová, R.; Yuperlieva-Mateeva, B.; Kostadinova, A.; Hristeva, T. Hormonomic changes driving the negative impact of broomrape on plant host interactions with arbuscular mycorrhizal fungi. Int. J. Mol. Sci. 2021, 22, 13677. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Aroca, R. Evaluation of the possible participation of drought-induced genes in the enhanced tolerance of arbuscular mycorrhizal plants to water deficit. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 185–205. [Google Scholar]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Duan, X.; Neuman, D.S.; Reiber, J.M.; Green, C.D.; Saxton, A.M.; Augé, R.M. Mycorrhizal influence on hydraulic and hormonal factors implicated in the control of stomatal conductance during drought. J. Exp. Bot. 1996, 47, 1541–1550. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; Davies, F.T., Jr. Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid and growth of micropropagated chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. J. Plant Physiol. 2003, 160, 1073–1083. [Google Scholar] [CrossRef]
- Fernández, I.; Merlos, M.; López-Ráez, J.; Martínez-Medina, A.; Ferrol, N.; Azcón, C.; Bonfante, P.; Flors, V.; Pozo, M. Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. J. Chem. Ecol. 2014, 40, 791–803. [Google Scholar] [CrossRef]
- Lendzemo, V.W.; Kuyper, T.W.; Matusova, R.; Bouwmeester, H.J.; Ast, A.V. Colonization by arbuscular mycorrhizal fungi of sorghum leads to reduced germination and subsequent attachment and emergence of Striga hermonthica. Plant Signal. Behav. 2007, 2, 58–62. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; García-Garrido, J.; Ocampo, J.A.; Rubiales, D. Colonisation of field pea roots by arbuscular mycorrhizal fungi reduces Orobanche and Phelipanche species seed germination. Weed Res. 2010, 50, 262–268. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Pozo, M.J.; García-Garrido, J.M. Strigolactones: A cry for help in the rhizosphere. Botany 2011, 89, 513–522. [Google Scholar] [CrossRef]
- Marro, N.; Lidoy, J.; Chico, M.Á.; Rial, C.; García, J.; Varela, R.M.; Macías, F.A.; Pozo, M.J.; Janoušková, M.; López-Ráez, J.A. Strigolactones: New players in the nitrogen–phosphorus signalling interplay. Plant Cell Environ. 2022, 45, 512–527. [Google Scholar] [CrossRef]
- Jiang, L.; Matthys, C.; Marquez-Garcia, B.; De Cuyper, C.; Smet, L.; De Keyser, A.; Boyer, F.-D.; Beeckman, T.; Depuydt, S.; Goormachtig, S. Strigolactones spatially influence lateral root development through the cytokinin signaling network. J. Exp. Bot. 2016, 67, 379–389. [Google Scholar] [CrossRef]
- Choi, J.; Lee, T.; Cho, J.; Servante, E.K.; Pucker, B.; Summers, W.; Bowden, S.; Rahimi, M.; An, K.; An, G. The negative regulator SMAX1 controls mycorrhizal symbiosis and strigolactone biosynthesis in rice. Nat. Commun. 2020, 11, 2114. [Google Scholar] [CrossRef]
- Yoshida, S.; Kameoka, H.; Tempo, M.; Akiyama, K.; Umehara, M.; Yamaguchi, S.; Hayashi, H.; Kyozuka, J.; Shirasu, K. The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol. 2012, 196, 1208–1216. [Google Scholar] [CrossRef]
- Rapparini, F.; Peñuelas, J. Mycorrhizal fungi to alleviate drought stress on plant growth. In Use of Microbes for the Alleviation of Soil Stresses; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1, pp. 21–42. [Google Scholar]
- Jia-Dong, H.; Tao, D.; Hui-Hui, W.; Ying-Ning, Z.; Qiang-Sheng, W.; Kamil, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar] [CrossRef]
- Cheng, H.-Q.; Ding, Y.-E.; Shu, B.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Plant Aquaporin Responses to Mycorrhizal Symbiosis under Abiotic Stress. Int. J. Agric. Biol. 2020, 23, 786–794. [Google Scholar]
- Aroca, R.; Bago, A.; Sutka, M.; Paz, J.A.; Cano, C.; Amodeo, G.; Ruiz-Lozano, J.M. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. MPMI 2009, 22, 1169–1178. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hijikata, N.; Ohtomo, R.; Handa, Y.; Kawaguchi, M.; Saito, K.; Masuta, C.; Ezawa, T. Aquaporin-mediated long-distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: Application of virus-induced gene silencing. New Phytol. 2016, 211, 1202–1208. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.-N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef]
- Lanfranco, L.; Novero, M.; Bonfante, P. The mycorrhizal fungus Gigaspora margarita possesses a CuZn superoxide dismutase that is up-regulated during symbiosis with legume hosts. Plant Physiol. 2005, 137, 1319–1330. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Cano, C.; Azcón-Aguilar, C.; Ferrol, N. GintMT1 encodes a functional metallothionein in Glomus intraradices that responds to oxidative stress. Mycorrhiza 2007, 17, 327–335. [Google Scholar] [CrossRef]
- Benabdellah, K.; Merlos, M.-Á.; Azcón-Aguilar, C.; Ferrol, N. GintGRX1, the first characterized glomeromycotan glutaredoxin, is a multifunctional enzyme that responds to oxidative stress. Fungal Genet. Biol. 2009, 46, 94–103. [Google Scholar] [CrossRef]
- Benabdellah, K.; Azcón-Aguilar, C.; Valderas, A.; Speziga, D.; Fitzpatrick, T.B.; Ferrol, N. GintPDX1 encodes a protein involved in vitamin B6 biosynthesis that is up-regulated by oxidative stress in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2009, 184, 682–693. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Oger, E.; Benabdellah, K.; Azcón-Aguilar, C.; Lanfranco, L.; Ferrol, N. Characterization of a CuZn superoxide dismutase gene in the arbuscular mycorrhizal fungus Glomus intraradices. Curr. Genet. 2010, 56, 265–274. [Google Scholar] [CrossRef]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2018, 19, 3146. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, T.; Zhang, D.; Ding, K.; Li, C.; Ma, F. Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Hohmann, P.; Messmer, M.M. Breeding for mycorrhizal symbiosis: Focus on disease resistance. Euphytica 2017, 213, 113. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Root exudates and their molecular interactions with rhizospheric microbes. In Plant, Soil and Microbes: Volume 2: Mechanisms and Molecular Interactions; Springer: Berlin/Heidelberg, Germany, 2016; pp. 59–77. [Google Scholar]
- Andreo-Jimenez, B.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lopez-Raez, J.A. Ecological relevance of strigolactones in nutrient uptake and other abiotic stresses, and in plant-microbe interactions below-ground. Plant Soil 2015, 394, 1–19. [Google Scholar] [CrossRef]
- López-Ráez, J.A. How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 2016, 243, 1375–1385. [Google Scholar] [CrossRef]
- Thula, S.; Moturu, T.R.; Salava, H.; Balakhonova, V.; Berka, M.; Kerchev, P.; Mishra, K.B.; Nodzynski, T.; Simon, S. Strigolactones stimulate high light stress adaptation by modulating photosynthesis rate in Arabidopsis. J. Plant Growth Regul. 2022, 42, 4818–4833. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.S.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant. Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef]
- Mathesius, U.; Watt, M. Rhizosphere signals for plant–microbe interactions: Implications for field-grown plants. In Progress in Botany 72; Springer: Berlin/Heidelberg, Germany, 2010; pp. 125–161. [Google Scholar]
- Hallett, P.D.; Feeney, D.S.; Bengough, A.G.; Rillig, M.C.; Scrimgeour, C.M.; Young, I.M. Disentangling the impact of AM fungi versus roots on soil structure and water transport. Plant Soil 2009, 314, 183–196. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.-C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef]
- Boyno, G.; Yerli, C.; Çakmakcı, T.; Şahin, Ü.; Demir, S. Effects of arbuscular mycorrhizal fungi on carbon dioxide (CO2) and water (H2O) emissions in turfgrass soil under different sainity irrigation levels. Environ. Eng. Manag. J. 2023, 22, 1081–1090. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Prasad, K.; Khare, A.; Rawat, P. Glomalin arbuscular mycorrhizal fungal reproduction, lifestyle and dynamic role in global sustainable agriculture for future generation. In Fungal Reproduction and Growth; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–22. [Google Scholar]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638. [Google Scholar] [CrossRef]
- Uwamungu, J.Y.; Shi, G.; Wang, Y.; Paliwal, A.; Jadhav, R.R.; Wani, A.W. Arbuscular mycorrhizal fungi (AMF) for sustainable soil and plant health. In Microbial and Biotechnological Interventions in Bioremediation and Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 135–152. [Google Scholar]
- Demir, S.; Durak, E.D.; Güneş, H.; Boyno, G.; Mulet, J.M.; Rezaee Danesh, Y.; Porcel, R. Biological control of three fungal diseases in strawberry (Fragaria× ananassa) with arbuscular mycorrhizal fungi. Agronomy 2023, 13, 2439. [Google Scholar] [CrossRef]
- Cordier, C.; Pozo, M.J.; Barea, J.-M.; Gianinazzi, S.; Gianinazzi-Pearson, V. Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. MPMI 1998, 11, 1017–1028. [Google Scholar] [CrossRef]
- Bansal, M.; Mukerji, K. Positive correlation between VAM-induced changes in root exudation and mycorrhizosphere mycoflora. Mycorrhiza 1994, 5, 39–44. [Google Scholar] [CrossRef]
- Azaizeh, H.; Marschner, H.; Römheld, V.; Wittenmayer, L. Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition and root exudation of soil-grown maize plants. Mycorrhiza 1995, 5, 321–327. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.E.; Higashi, R.M. Root exudation and physiological status of a root-colonizing fluorescent pseudomonad in mycorrhizal and non-mycorrhizal pepper (Capsicum annuum L.). Plant Soil 1997, 189, 11–20. [Google Scholar] [CrossRef]
- Gupta Sood, S. Chemotactic response of plant-growth-promoting bacteria towards roots of vesicular-arbuscular mycorrhizal tomato plants. FEMS Microbiol. Ecol. 2003, 45, 219–227. [Google Scholar] [CrossRef]
- Pivato, B.; Gamalero, E.; Lemanceau, P.; Berta, G. Colonization of adventitious roots of Medicago truncatula by Pseudomonas fluorescens C7R12 as affected by arbuscular mycorrhiza. FEMS Microbiol. Lett. 2008, 289, 173–180. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Charnikhova, T.; Fernández, I.; Bouwmeester, H.; Pozo, M.J. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J. Plant Physiol. 2011, 168, 294–297. [Google Scholar] [CrossRef]
- Vos, C.; Claerhout, S.; Mkandawire, R.; Panis, B.; De Waele, D.; Elsen, A. Arbuscular mycorrhizal fungi reduce root-knot nematode penetration through altered root exudation of their host. Plant Soil 2012, 354, 335–345. [Google Scholar] [CrossRef]
- Pozo, M.J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J.M.; Azcón-Aguilar, C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 2002, 53, 525–534. [Google Scholar] [CrossRef]
- Zhu, H.; Yao, Q. Localized and systemic increase of phenols in tomato roots induced by Glomus versiforme inhibits Ralstonia solanacearum. J. Phytopathol. 2004, 152, 537–542. [Google Scholar] [CrossRef]
- Khaosaad, T.; Garcia-Garrido, J.; Steinkellner, S.; Vierheilig, H. Take-all disease is systemically reduced in roots of mycorrhizal barley plants. Soil Biol. Biochem. 2007, 39, 727–734. [Google Scholar] [CrossRef]
- Hao, Z.; Fayolle, L.; van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Alonso, J.M. Ethylene signaling and response: Where different regulatory modules meet. Curr. Opin. Plant Biol. 2009, 12, 548–555. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Li, C.; Liu, Z.; Yang, N.; Pan, L.; Wu, J.; Wang, J.; Yang, J.; Lv, Y. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 2018, 217, 290–304. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Feng, Q.; Zhang, J.; Han, X.; Zhang, L.; Yang, F.; Zhou, J. Effects of exogenous Strigolactone on the physiological and ecological characteristics of Pennisetum purpureum Schum. Seedlings under drought stress. BMC Plant Biol. 2022, 22, 578. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Q.; Jing, G.; Ma, M.; Li, C.; Ma, F. Overexpression of MdIAA24 improves apple drought resistance by positively regulating strigolactone biosynthesis and mycorrhization. Tree Physiol. 2021, 41, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Abderrahim, L.A.; Boussaid, M.; Bissoli, G.; Taïbi, F.; Achir, M.; Souana, K.; Mulet, J.M. Salt-tolerance of Phaseolus vulgaris L. is a function of the potentiation extent of antioxidant enzymes and the expression profiles of polyamine encoding genes. S. Afr. J. Bot. 2021, 140, 114–122. [Google Scholar] [CrossRef]
- Kumar, A.; Dames, J.F.; Gupta, A.; Sharma, S.; Gilbert, J.A.; Ahmad, P. Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: A biotechnological perspective. Crit. Rev. Biotechnol. 2015, 35, 461–474. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Bhatt, D. Strigolactones in overcoming environmental stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; Wiley: Hoboken, NJ, USA, 2020; pp. 327–341. [Google Scholar]
- Kong, C.-C.; Ren, C.-G.; Li, R.-Z.; Xie, Z.-H.; Wang, J.-P. Hydrogen peroxide and strigolactones signaling are involved in alleviation of salt stress induced by arbuscular mycorrhizal fungus in Sesbania cannabina seedlings. J. Plant Growth Regul. 2017, 36, 734–742. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Arif, M.S.; Ahmad, R.; Hasanuzzaman, M.; Ali, B.; Hussain, A. Approaches in enhancing thermotolerance in plants: An updated review. J. Plant Growth Regul. 2020, 39, 456–480. [Google Scholar] [CrossRef]
- Taïbi, K.; Del Campo, A.; Aguado, A.; Mulet, J. Early establishment response of different Pinus nigra ssp. salzmanii seed sources on contrasting environments: Implications for future reforestation programs and assisted population migration. J. Environ. Manag. 2016, 171, 184–194. [Google Scholar] [CrossRef]
- Maya, M.A.; Matsubara, Y.-i. Influence of arbuscular mycorrhiza on the growth and antioxidative activity in cyclamen under heat stress. Mycorrhiza 2013, 23, 381–390. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Song, F.-B.; Liu, S.-Q.; Liu, T.-D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Huang, B. Strigolactones and interaction with auxin regulating root elongation in tall fescue under different temperature regimes. Plant Sci. 2018, 271, 34–39. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Vidaurre, D.; Toh, S.; Hanada, A.; Nambara, E.; Kamiya, Y.; Yamaguchi, S.; McCourt, P. A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat. Chem. Biol. 2010, 6, 741–749. [Google Scholar] [CrossRef]
- Lechat, M.-M.; Brun, G.; Montiel, G.; Véronési, C.; Simier, P.; Thoiron, S.; Pouvreau, J.-B.; Delavault, P. Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parasitic plant, Phelipanche ramosa L. Pomel. J. Exp. Bot. 2015, 66, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the l aw of the m inimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Olubode, A.A.; Ridwan, M.; Adebiyi, A.; Oni, O.; Ibrahim, I. Influence of different arbuscular mycorrhizal fungi isolates in enhancing growth, phosphorus uptake and grain yield of soybean in a phosphorus deficient soil under field conditions. Commun. Soil Sci. Plant Anal. 2021, 52, 1171–1183. [Google Scholar] [CrossRef]
- ud din Khanday, M.; Bhat, R.A.; Haq, S.; Dervash, M.A.; Bhatti, A.A.; Nissa, M.; Mir, M.R. Arbuscular mycorrhizal fungi boon for plant nutrition and soil health. In Soil Science: Agricultural and Environmental Prospectives; Springer: Berlin/Heidelberg, Germany, 2016; pp. 317–332. [Google Scholar]
- Geng, Z.; Chen, J.; Lu, B.; Zhang, F.; Chen, Z.; Liu, Y.; Xia, C.; Huang, J.; Zhang, C.; Zha, M. A Review: Systemic signaling in the regulation of plant responses to low N, P and Fe. Plants 2023, 12, 2765. [Google Scholar] [CrossRef]
- Mayzlish-Gati, E.; De-Cuyper, C.; Goormachtig, S.; Beeckman, T.; Vuylsteke, M.; Brewer, P.B.; Beveridge, C.A.; Yermiyahu, U.; Kaplan, Y.; Enzer, Y. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 2012, 160, 1329–1341. [Google Scholar] [CrossRef]
- Marzec, M.; Muszynska, A.; Gruszka, D. The role of strigolactones in nutrient-stress responses in plants. Int. J. Mol. Sci. 2013, 14, 9286–9304. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Czarnecki, O.; Yang, J.; Weston, D.J.; Tuskan, G.A.; Chen, J.-G. A dual role of strigolactones in phosphate acquisition and utilization in plants. Int. J. Mol. Sci. 2013, 14, 7681–7701. [Google Scholar] [CrossRef]
- Chen, B.; Fang, J.; Piao, S.; Ciais, P.; Black, T.A.; Wang, F.; Niu, S.; Zeng, Z.; Luo, Y. A meta-analysis highlights globally widespread potassium limitation in terrestrial ecosystems. New Phytol. 2023, 240, 1–12. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, K.; Zhou, X.; Wang, L.; Xu, C.; Zhang, H.; Zhu, G.; Si, C.; Wang, J.; Zhang, Y. Interactive impact of potassium and arbuscular mycorrhizal fungi on the root morphology and nutrient uptake of sweet potato (Ipomoea batatas L.). Front. Microbiol. 2023, 13, 1075957. [Google Scholar] [CrossRef]
- Mulet, J.M.; Porcel, R.; Yenush, L. Modulation of potassium transport to increase abiotic stress tolerance in plants. J. Exp. Bot. 2023, 74, erad333. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Matusova, R.; Cardoso, C.; Jamil, M.; Charnikhova, T.; Kohlen, W.; Ruyter-Spira, C.; Verstappen, F.; Bouwmeester, H. Strigolactones: Ecological significance and use as a target for parasitic plant control. Pest Manag. Sci. 2009, 65, 471–477. [Google Scholar] [CrossRef]
- Barman, J.; Samanta, A.; Saha, B.; Datta, S. Mycorrhiza: The oldest association between plant and fungi. Resonance 2016, 21, 1093–1104. [Google Scholar] [CrossRef]
- Soto-Cruz, F.J.; Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.; Igartuburu, J.M.; Macías, F.A. Allelopathic activity of strigolactones on the germination of parasitic plants and arbuscular mycorrhizal fungi growth. Agronomy 2021, 11, 2174. [Google Scholar] [CrossRef]
- Barea, J.; Pozo, M.; López-Ráez, J.; Aroca, R.; Ruíz-Lozano, J.; Ferrol, N.; Azcón, R.; Azcón-Aguilar, C. Arbuscular Mycorrhizas and Their Significance in Promoting Soil-Plant Systems Sustainability against Environmental Stresses; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Colombo, R.P.; Fernandez Bidondo, L.; Silvani, V.A.; Godeas, A.M. Impact of genetically modified crops on the biodiversity of arbuscular mycorrhizal fungi. In GMOs: Implications for Biodiversity Conservation and Ecological Processes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 69–86. [Google Scholar]
- Vassileva, M.; Peregrin, E.; Martos, V.; Vassilev, N. Biotechnological strategies aimed at sustainable mineral plant nutrition and food safety. J. Int. Sci. Publ. Ecol. Saf. 2012, 6, 330–340. [Google Scholar]
- Yang, S.; Imran; Ortas, I. Impact of mycorrhiza on plant nutrition and food security. J. Plant Nutr. 2023, 46, 1–26. [Google Scholar] [CrossRef]
- Barea, J. Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbiome interactions. J. Soil Sci. Plant Nutr. 2015, 15, 261–282. [Google Scholar]
- Rasmann, S.; Turlings, T.C. Root signals that mediate mutualistic interactions in the rhizosphere. Curr. Opin. Plant Biol. 2016, 32, 62–68. [Google Scholar] [CrossRef]
- Hodge, A. Interactions between arbuscular mycorrhizal fungi and organic material substrates. Adv. Appl. Microbiol. 2014, 89, 47–99. [Google Scholar]
- Sosa-Hernández, M.A.; Leifheit, E.F.; Ingraffia, R.; Rillig, M.C. Subsoil arbuscular mycorrhizal fungi for sustainability and climate-smart agriculture: A solution right under our feet? Front. Microbiol. 2019, 10, 744. [Google Scholar] [CrossRef]
- Ebbisa, A. Arbuscular mycorrhizal fungi (AMF) in optimizing nutrient bioavailability and reducing agrochemicals for maintaining sustainable agroecosystems. In Mycorrhiza-New Insights; IntechOpen: London, UK, 2022. [Google Scholar]
- Borghi, L.; Screpanti, C.; Lumbroso, A.; Lachia, M.; Gübeli, C.; De Mesmaeker, A. Efficiency and bioavailability of new synthetic strigolactone mimics with potential for sustainable agronomical applications. Plant Soil 2021, 465, 109–123. [Google Scholar] [CrossRef]
- Besserer, A.; Bécard, G.; Jauneau, A.; Roux, C.; Séjalon-Delmas, N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008, 148, 402–413. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospíšil, T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant 2013, 6, 38–62. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef]
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 2021, 254, 28. [Google Scholar] [CrossRef]
- Dag, A.; Yermiyahu, U.; Ben-Gal, A.; Zipori, I.; Kapulnik, Y. Nursery and post-transplant field response of olive trees to arbuscular mycorrhizal fungi in an arid region. Crop. Pasture Sci. 2009, 60, 427–433. [Google Scholar] [CrossRef]
- Davidson, B.E.; Novak, S.J.; Serpe, M.D. Consequences of inoculation with native arbuscular mycorrhizal fungi for root colonization and survival of Artemisia tridentata ssp. wyomingensis seedlings after transplanting. Mycorrhiza 2016, 26, 595–608. [Google Scholar] [CrossRef]
- Yi, F.; Song, A.; Cheng, K.; Liu, J.; Wang, C.; Shao, L.; Wu, S.; Wang, P.; Zhu, J.; Liang, Z. Strigolactones positively regulate Verticillium wilt resistance in cotton via crosstalk with other hormones. Plant Physiol. 2023, 192, 945–966. [Google Scholar] [CrossRef]
- Jamil, M.; Wang, J.Y.; Yonli, D.; Patil, R.H.; Riyazaddin, M.; Gangashetty, P.; Berqdar, L.; Chen, G.-T.E.; Traore, H.; Margueritte, O. A new formulation for strigolactone suicidal germination agents, towards successful Striga management. Plants 2022, 11, 808. [Google Scholar] [CrossRef]
- Jamil, M.; Wang, J.Y.; Yonli, D.; Ota, T.; Berqdar, L.; Traore, H.; Margueritte, O.; Zwanenburg, B.; Asami, T.; Al-Babili, S. Striga hermonthica suicidal germination activity of potent strigolactone analogs: Evaluation from laboratory bioassays to field trials. Plants 2022, 11, 1045. [Google Scholar] [CrossRef]
- Kountche, B.A.; Jamil, M.; Yonli, D.; Nikiema, M.P.; Blanco-Ania, D.; Asami, T.; Zwanenburg, B.; Al-Babili, S. Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants People Planet 2019, 1, 107–118. [Google Scholar] [CrossRef]
- Johnson, A.W.; Gowada, G.; Hassanali, A.; Knox, J.; Monaco, S.; Razavi, Z.; Rosebery, G. The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1981, 1, 1734–1743. [Google Scholar] [CrossRef]
- Nefkens, G.H.; Thuring, J.W.J.; Beenakkers, M.F.; Zwanenburg, B. Synthesis of a phthaloylglycine-derived strigol analogue and its germination stimulatory activity toward seeds of the parasitic weeds Striga hermonthica and Orobanche crenata. J. Agric. Food Chem. 1997, 45, 2273–2277. [Google Scholar] [CrossRef]
- Mwakaboko, A.S.; Zwanenburg, B. Single step synthesis of strigolactone analogues from cyclic keto enols, germination stimulants for seeds of parasitic weeds. Bioorg. Med. Chem. 2011, 19, 5006–5011. [Google Scholar] [CrossRef]
- Mwakaboko, A.S.; Zwanenburg, B. Strigolactone analogs derived from ketones using a working model for germination stimulants as a blueprint. Plant Cell Physiol. 2011, 52, 699–715. [Google Scholar] [CrossRef]
- Jamil, M.; Kountche, B.A.; Haider, I.; Guo, X.; Ntui, V.O.; Jia, K.-P.; Ali, S.; Hameed, U.S.; Nakamura, H.; Lyu, Y. Methyl phenlactonoates are efficient strigolactone analogs with simple structure. J. Exp. Bot. 2018, 69, 2319–2331. [Google Scholar] [CrossRef]
- Chen, C.; Zou, J.; Zhang, S.; Zaitlin, D.; Zhu, L. Strigolactones are a new-defined class of plant hormones which inhibit shoot branching and mediate the interaction of plant-AM fungi and plant-parasitic weeds. Sci. China Ser. C Life Sci. 2009, 52, 693–700. [Google Scholar] [CrossRef]
- Jamil, M.; Charnikhova, T.; Verstappen, F.; Bouwmeester, H. Carotenoid inhibitors reduce strigolactone production and Striga hermonthica infection in rice. Arch. Biochem. Biophys. 2010, 504, 123–131. [Google Scholar] [CrossRef]
- Dor, E.; Joel, D.M.; Kapulnik, Y.; Koltai, H.; Hershenhorn, J. The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta 2011, 234, 419–427. [Google Scholar] [CrossRef]
- Leytem, A.B. Response of Striga-Susceptible and Striga-Resistant Sorghum Genotypes to Soil Phosphorus and Colonization by an Arbuscular Mycorrhizal Fungus. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2012. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/79408135z (accessed on 23 November 2023).
- Koltai, H.; Gadkar, V.; Kapulnik, Y. 5 biochemical and practical views of arbuscular mycorrhizal fungus-host association in horticultural crops. Hortic. Rev. 2010, 36, 257. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Nomura, T.; Takahashi, I.; Asami, T.; Mori, N.; Akiyama, K.; Kusajima, M.; Nakashita, H. Regulation of biosynthesis, perception, and functions of strigolactones for promoting arbuscular mycorrhizal symbiosis and managing root parasitic weeds. Pest Manag. Sci. 2019, 75, 2353–2359. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, X.; Zhan, M.; Chen, Z.; Wang, H.; Yao, F.; Chen, J. Effect of an exogenous strigolactone GR24 on the antioxidant capacity and quality deterioration in postharvest sweet orange fruit stored at ambient temperature. Int. J. Food Sci. Technol. 2022, 57, 619–630. [Google Scholar] [CrossRef]
- Mishra, V.; Ellouze, W.; Howard, R.J. Utility of arbuscular mycorrhizal fungi for improved production and disease mitigation in organic and hydroponic greenhouse crops. J. Hortic. 2018, 5, 1000237. [Google Scholar] [CrossRef]
- Yang, M.; Dong, X.; Zhu, Y.; Song, J.; Wei, J.; Wu, Z.; Zhao, Y. Effect of different mixed light-emitting diode light wavelengths on CO2 absorption from biogas and nutrient removal from biogas slurry by microalgae and fungi induced using strigolactone and endophytic bacteria. WER 2022, 94, e10812. [Google Scholar] [CrossRef]
- de Carvalho, A.M.X.; de Castro Tavares, R.; Cardoso, I.M.; Kuyper, T.W. Mycorrhizal associations in agroforestry systems. In Soil Biology and Agriculture in the Tropics; Springer: Berlin/Heidelberg, Germany, 2010; pp. 185–208. [Google Scholar]
- Laurindo, L.K.; de Souza, T.A.F.; da Silva, L.J.R.; Casal, T.B.; de Jesus Conceição Pires, K.; Kormann, S.; Schmitt, D.E.; Siminski, A. Arbuscular mycorrhizal fungal community assembly in agroforestry systems from the Southern Brazil. Biologia 2021, 76, 1099–1107. [Google Scholar] [CrossRef]
- Jingjing, Y.; Huiqin, G.; Fry, E.L.; Jonathan, R.; Shiming, T.; Ting, Y.; Weibo, R. Plant roots send metabolic signals to microbes in response to long-term overgrazing. Sci. Total Environ. 2022, 842, 156241. [Google Scholar] [CrossRef]
- Cordero, A.P.; Vergara, D.E.M.; Mendoza, Y.A. Presence of Gigaspora rosea In rizosphere of pasture in Bothriochloa pertusa (L) A. Camus. J. Posit. Sch. Psychol. 2023, 7, 947–953. [Google Scholar]
- Purakayastha, T.; Chhonkar, P. Influence of vesicular-arbuscular mycorrhizal fungi (Glomus etunicatum L.) on mobilization of zinc in wetland rice (Oryza sativa L.). Biol. Fertil. Soils 2001, 33, 323–327. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Blake, S.N.; Barry, K.M.; Gill, W.M.; Reid, J.B.; Foo, E. The role of strigolactones and ethylene in disease caused by Pythium irregulare. Mol. Plant Pathol. 2016, 17, 680–690. [Google Scholar] [CrossRef]
- Hu, S.; Bidochka, M. Root colonization by endophytic insect-pathogenic fungi. J. Appl. Microbiol. 2021, 130, 570–581. [Google Scholar] [CrossRef]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef]
- Saeed, W.; Naseem, S.; Ali, Z. Strigolactones biosynthesis and their role in abiotic stress resilience in plants: A critical review. Front. Plant Sci. 2017, 8, 1487. [Google Scholar] [CrossRef]
- Kobae, Y.; Kameoka, H.; Sugimura, Y.; Saito, K.; Ohtomo, R.; Fujiwara, T.; Kyozuka, J. Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant Cell Physiol. 2018, 59, 544–553. [Google Scholar] [CrossRef]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef]
- Tulasikorra; Siva Devika, O.; Mounika, K.; Kumar, I.S.; Kumar, S.; Sabina Mary, G.; Kumar, U.; Kumar, M. Current status–enlightens in its biology and omics approach on arbuscular mycorrhizal community. In Symbiotic Soil Microorganisms: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–29. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyno, G.; Rezaee Danesh, Y.; Demir, S.; Teniz, N.; Mulet, J.M.; Porcel, R. The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 16774. https://doi.org/10.3390/ijms242316774
Boyno G, Rezaee Danesh Y, Demir S, Teniz N, Mulet JM, Porcel R. The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions. International Journal of Molecular Sciences. 2023; 24(23):16774. https://doi.org/10.3390/ijms242316774
Chicago/Turabian StyleBoyno, Gökhan, Younes Rezaee Danesh, Semra Demir, Necmettin Teniz, José M. Mulet, and Rosa Porcel. 2023. "The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions" International Journal of Molecular Sciences 24, no. 23: 16774. https://doi.org/10.3390/ijms242316774
APA StyleBoyno, G., Rezaee Danesh, Y., Demir, S., Teniz, N., Mulet, J. M., & Porcel, R. (2023). The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions. International Journal of Molecular Sciences, 24(23), 16774. https://doi.org/10.3390/ijms242316774










