Abstract
Cardiac glycosides (CGs) constitute a group of steroid-like compounds renowned for their effectiveness in treating cardiovascular ailments. In recent times, there has been growing recognition of their potential use as drug leads in cancer treatment. In our prior research, we identified three highly promising CG compounds, namely lanatoside C (LC), peruvoside (PS), and strophanthidin (STR), which exhibited significant antitumor effects in lung, liver, and breast cancer cell lines. In this study, we investigated the therapeutic response of these CGs, with a particular focus on the MCF-7 breast cancer cell line. We conducted transcriptomic profiling and further validated the gene and protein expression changes induced by treatment through qRT-PCR, immunoblotting, and immunocytochemical analysis. Additionally, we demonstrated the interactions between the ligands and target proteins using the molecular docking approach. The transcriptome analysis revealed a cluster of genes with potential therapeutic targets involved in cytotoxicity, immunomodulation, and tumor-suppressor pathways. Subsequently, we focused on cross-validating the ten most significantly expressed genes, EGR1, MAPK1, p53, CCNK, CASP9, BCL2L1, CDK7, CDK2, CDK2AP1, and CDKN1A, through qRT-PCR, and their by confirming the consistent expression pattern with RNA-Seq data. Notably, among the most variable genes, we identified EGR1, the downstream effector of the MAPK signaling pathway, which performs the regulatory function in cell proliferation, tumor invasion, and immune regulation. Furthermore, we substantiated the influence of CG compounds on translational processes, resulting in an alteration in protein expression upon treatment. An additional analysis of ligand–protein interactions provided further evidence of the robust binding affinity between LC, PS, and STR and their respective protein targets. These findings underscore the intense anticancer activity of the investigated CGs, shedding light on potential target genes and elucidating the probable mechanism of action of CGs in breast cancer.
1. Introduction
Breast cancer (BC) continues to be the most common cancer in women worldwide. A comprehensive global cancer survey reported a significant shift, with breast cancer surpassing lung cancer in terms of the highest incidence in 2020 [1]. Thus, there is an urgent need to curb this growing crisis of breast cancer incidence and associated mortality. Over the years, significant advancements have been made in treatment strategies, substantially improving patients’ life expectancy. However, BC is a heterogeneous disease with a high degree of variance in cellular, genome, and transcriptome profiles [2], which affects its progression by impeding the therapeutic response. Therefore, there is a high demand for standard therapeutic options analyzing biological mechanisms and tracing molecular footprints in the disease [3,4]. Several researchers worldwide are focused on developing precision therapies utilizing molecules that specifically target malignant cells. A significant challenge in developing novel compounds lies in the expected time redundancy and resources required to conduct comprehensive research and navigate the complex regulatory processes. This draws attention to the importance of drug repurposing, which uses existing or approved drugs to treat a different ailment, defining newer targets and mechanisms [5].
Cardiac glycosides are an emerging class of compounds that have demonstrated potential anticancer activity by selectively targeting oncogenic proteins and signaling pathways, which makes them valuable candidates for drug repurposing in the treatment of cancer [6,7]. With a history of over a century, these naturally derived compounds have been well-accepted medications for heart failure and arrhythmic disorders. The compounds within this family are notably recognized for their ability to bind and inhibit Na+/K+-ATPase pumps regulating various cellular processes [8]. A decade of numerous intensive studies has been conducted to unravel the potential use of these compounds as front-line cancer medicines. However, the last five years have witnessed a remarkable surge in global interest in unveiling the anticancer activity of cardiac glycosides.
Extensively studied glycosides with promising potential as anticancer agents include digitoxin, digoxin, bufalin, ouabain, peruvoside, and lanatoside C. Ouabain is currently undergoing clinical trials to evaluate its effectiveness in cancer treatment [9]. Assessing the anticancer mechanism triggered by CGs includes several hypotheses and aspects, including modulation of signal transduction, inhibition of angiogenesis, immunoregulation, induction of apoptosis, and cell cycle arrest [10,11]. A central shared characteristic of all CGs is their ability to inhibit the Na/K-ATPase pump, and several theories suggest that these compounds induce cellular cytotoxicity through this primary target [12]. Nevertheless, an understanding of the influential downstream genes and a central regulatory mechanism driving cancer cell death is still lacking.
Our prior research primarily focused on investigating the anticancer efficacy of CGs. In these studies, we have conducted a broad screening of CGs to identify the most promising anticancer compounds through in silico and in vitro studies in three human cancer cell lines, specifically MCF-7 breast cancer cell line, A549 lung cancer cell line, and HepG2 liver cancer cell line. The study screened out three efficient compounds, including lanatoside C (LC), peruvoside (PS), and strophanthidin (STR), with spectacular cytotoxic effects on the mentioned cancer cells with an IC50 ranging from 100 nM to 2 µM concentrations. The compounds showed significant anticancer activity, substantiated by comprehensive validations from biochemical assays, molecular analysis, and gene and protein expression assessments. The study concluded by mentioning a considerable influence of MAPK/PI3K/AKT/mTOR signaling pathway regulation upon treatment [13,14,15]. The findings strongly support the hypothesis that LC, PS, and STR can effectively impede cancer proliferation by modulating key proteins involved in vital cellular processes, including cell cycle regulation, apoptosis, and autophagy.
Irrespective of the growing body of evidence regarding the potent anticancer efficacy of CGs, the lack of comprehensive data on their mode of action and genome expression prevents their characterization as effective cancer drugs. In order to address this limitation and delve deeper into the details of our earlier observations, we performed RNA-seq analysis on MCF-7 breast cancer cells treated with LC, PS, and STR. We compared its effects with the untreated control sample. Furthermore, we validated the obtained transcriptome results using qRT-PCR. Following RNA-seq confirmation at the transcript expression level, we attempted to study the interactions between the compounds and crucial proteins using the molecular docking technique. Thus, the current study holds significant importance as it provides transcriptomic data analyzing sequence information and insights into the genes and pathways influenced by CG treatment.
2. Results
2.1. Transcriptome Profiling of Cardiac Glycoside Induction in MCF-7 Cells Reveals Variability in Genes Associated with Cytotoxicity
To evaluate transcriptional alteration induced by CGs in breast cancer, we performed RNA sequencing on MCF-7 cell lines corresponding to the untreated sample as a control group and the treatment groups, consisting of LC, PS, and STR. The effect of individual treatments compared to control samples is depicted through volcano plots in Figure 1A–C, showing LC vs. Control (CTRL), PS vs. CTRL, and STR vs. CTRL, respectively. LC treatment resulted in 6135 differentially expressed genes, of which 2311 are downregulated and 3824 are upregulated. PS treatment differentially expressed 6079 genes compared to the control, including 2643 downregulated and 3436 upregulated genes. A similar result was observed on treatment with STR, whereby 6143 genes were differentially expressed, of which 2500 were downregulated and 3643 were upregulated. The degree of expressional changes in the control and treatment group is illustrated through a heat map (Figure 1D), where each row represents genes while the column indicates samples. Subsequently, all three treatments were compared to identify the genes that are commonly differentially expressed by LC, PS, and STR groups (Figure 1E). For further analysis, we have compiled and pruned 5813 differentially expressed genes common to all three treatments for downstream functional enrichment analysis.
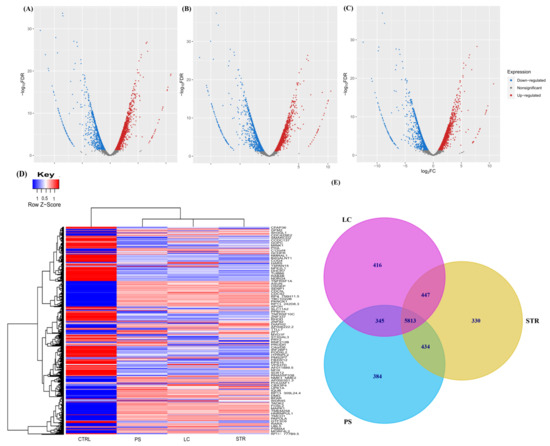
Figure 1.
Analysis of differential gene expression from Cardiac-glycoside-treated MCF7 cells using RNA-Seq pipelines. Volcano plot showing differentially expressed genes on treatment relative to control sample, (A) Downregulated genes (blue; n = 2311), upregulated genes (red; n = 3824) on LC treatment. (B) Downregulated genes (blue; n = 2643) and upregulated genes (red; n = 3436) on PS treatment. (C) Downregulated genes (blue; n = 2500) and upregulated genes (red; n = 3643) on STR treatment. (D) Heat map representation of normalized and filtered differentially expressed genes between treated (LC, PS, and STR) and untreated MCF7 cells. (E) Venn diagram illustrating DEG in LC, PS, and STR.
2.2. The Biological Significance of DEGs in P53, MAPK, and Immune Regulatory Pathway
We further performed functional enrichment analysis with the common DEG of the LC, PS, and STR treatment to evaluate the biological relevance of the traced genes through the FunRich algorithm. First, we visualized the gene–gene interactions between the DEG and the enriched genes in the associated pathways, as depicted in Figure 2. This paved the way for understanding the fundamental role of the TP53 (P53) gene, which represented a central regulatory role in integrating multiple interaction networks.
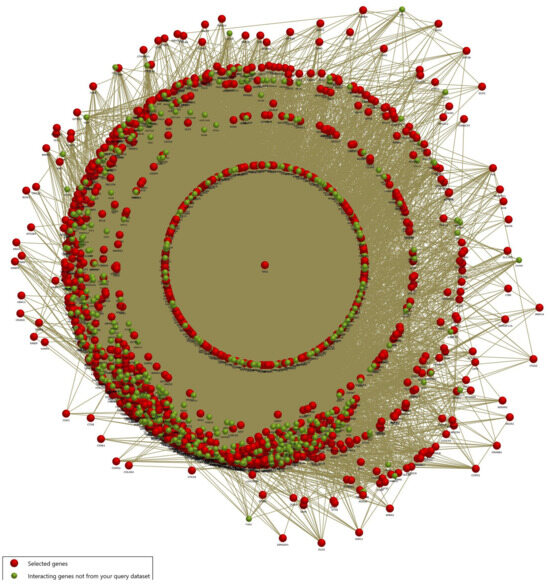
Figure 2.
Interactome network of differentially expressed genes common across all treatments (LC, PS, STR). The red node indicates DEGs, while the green node emphasizes interacting genes in enriched pathways from the query.
Additionally, we carried out a detailed exploration of the data to gain deeper biological insights into the DEG through functional enrichment analysis of GO terms, including cellular component or localization (CC), biological process (BP), and molecular function (MF). The study revealed that the genes played a significant role in transcription regulation, cell growth, cell cycle progression, immune response modulation, and apoptosis (Figure 3A,B). A detailed analysis of each treatment’s biological influence is shown in Supplementary Figure S1. We then performed KEGG pathway analysis on the upregulated DE genes, demonstrating their involvement in the P53 pathway, apoptotic pathway, and immune system. Likewise, the corresponding KEGG analysis of the downregulated DE genes revealed their participation in the p38-MAPK pathway, PI3K signaling events mediated by Akt, immune system, and suppression of ER receptors on the plasma membrane (Figure 3C). We then identified a set of ten common Significantly Differentially Expressed (SDE) Genes, namely EGR1, MAPK1, CCNK, CASP9, BCL2L1, CDK2, CDK7, CDK2AP1, CDKN1A, and P53, that were involved in regulating these enriched signaling pathways (Table 1). According to whole transcriptome analysis, we found that genes associated with cell cytotoxicity, cell growth, and differentiation were amongst the most highly differentially expressed on treatment with CG. We previously reported that CGs induce cell cycle arrest by attenuating MAPK, PI3K/AKT/mTOR signaling pathways in cancer cells [13,14,15]. Confirming our previous findings, we observed transcriptional changes and influential cytotoxicity-related genes associated with the MAPK/PI3K pathway and P53-dependent apoptosis pathways through RNA-Seq analysis.
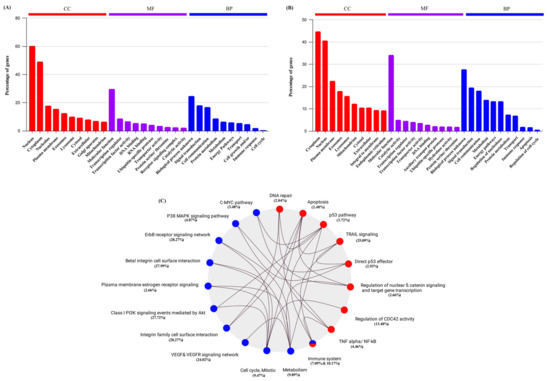
Figure 3.
Functional enrichment analysis of Differentially expressed genes. (A) Gene ontology (GO) analysis of commonly downregulated genes in LC, PS, and STR treatment. (B) Gene ontology (GO) analysis of commonly upregulated genes in LC, PS, and STR treatment. (C) KEGG pathway enrichment analysis of commonly downregulated (blue) and upregulated (red) genes in LC, PS, and STR treatment and their interactions.

Table 1.
Significantly differentially expressed genes selected for downstream analysis and validation.
2.3. Protein–Protein Interaction (PPI) Analysis
The analysis of gene interaction profiles and protein–protein interrelations of SDE genes was carried out using the STRING platform and further explored using Cytoscape software (version 3.9.0). The 10 differentially expressed genes were queried through STRING, and we retrieved molecular networks and functional associations from curated pathways. Additionally, the correlation of DEG was analyzed using the k-means clustering method. The analysis provided two cluster groups, the cyclin-dependent kinase proteins, and the cytotoxic-associated pathways, as depicted in Figure 4.
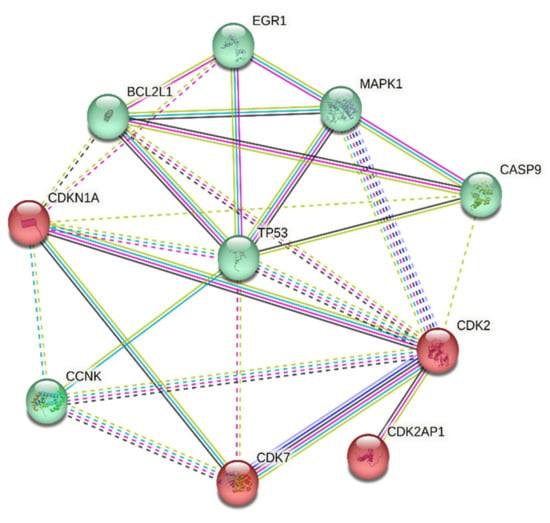
Figure 4.
STRING analysis of candidate differentially expressed genes, characterized into two clusters of pathway regulatory mechanism.
2.4. Confirmation of RNA-Seq Data through qRT-PCR
To further validate the results of RNA-Seq analysis, qRT-PCR assay was performed for the 10 SDE genes, EGR1, MAPK1, CCNK, CASP9, BCL2L1, CDK7, CDK2, CDKN1A, CDK2AP1, and P53. The outcomes of the RT-PCR analysis mirrored those of RNA-Seq data, demonstrating that the cardiac glycoside treatment enhanced the expression of CASP9, BCL2L1 (BAX), CDKN1A (P21), and P53 while turning down the expression of EGR1, MAPK1, CCNK, CDK7, CDK2, and CDK2AP1 genes, as shown in Figure 5.
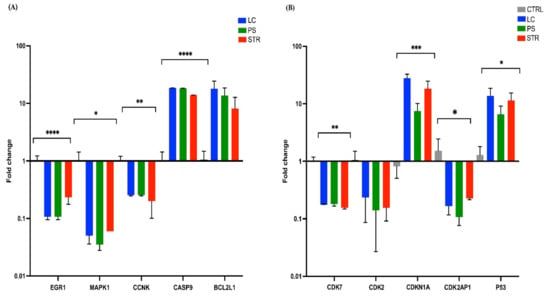
Figure 5.
RT-PCR analysis of the 10 candidate gene expressions in control, LC, PS, and STR. (A) Bar graph representing the fold change of EGR1, MAPK1, CCNK, CASP9, and BCL2L1 (B) Bar graph representing the fold change of CDK7, CDK2, CDKN1A, CDKN2AP1, and P53. All p-values are summarized with asterisks (ns p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001).
2.5. Cardiac Glycosides Exhibit Cell Growth Arrest in Breast Cancer Cells Altering p53 Dependent MAPK Signaling Pathway
In cancer cells, MAPK signaling facilitates mitosis entry and extends cell growth and division while ensuring the downregulation of cancer suppressor and pro-apoptotic proteins such as p53 and caspase-9 [16]. To elucidate the interdependence of MAPK, p53, and apoptotic signaling pathways in response to cardiac glycoside treatment, we performed Western blot analysis to quantify protein expression. The findings revealed significant downregulation of MAPK1 protein, and its downstream effector protein EGR1, upon treatment (Figure 6). This reduction likely influences the activation of the cyclin cascade of proteins, subsequently leading to cell death. Additionally, we observed upregulation of the p53 and caspase-9 protein safeguarding cells to undergo cell death. Over the years, it has become evident that the transcription factor EGR1 directly controls several tumor suppressor genes like PTEN and p53 [17]. The protein expression analysis confirms the specificity of the tested compounds in inhibiting MAPK and associated signaling cascades, thereby turning off EGR1 gene expression.
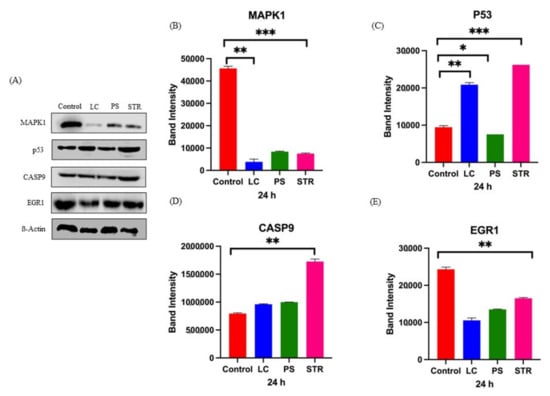
Figure 6.
Western blot expression of influenced protein on treatment with cardiac glycosides. (A) Representative Western blot showing the expression of crucial influenced proteins including MAPK1, p53, CASP9, and EGR1 on treatment with lanatoside C (LC), peruvoside (PS), and strophanthidin (STR). (B) Quantification of Western blot, showing decreased expression of MAPK1 protein on treatment with respective cardiac glycosides. (C) Quantification of Western blot, showing increased expression of p53 protein on treatment with respective cardiac glycosides. (D) Quantification of Western blot, showing increased expression of caspase-9 protein on treatment with respective cardiac glycosides. (E) Quantification of Western blot, showing decreased expression of EGR1 protein on treatment with respective cardiac glycosides. All p-values are summarized with asterisks (ns p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001).
2.6. Cardiac Glycosides Effectively Downregulated EGR1 Expression Via Influencing MAPK Signaling Cascade Controlling Cell Fate Decision
To provide further evidence supporting the dependence of the MAPK pathway and the p53 pathway in regulating cell growth, we performed immunostaining of MAPK1, p53, and EGR1 proteins studying the co-regulation and expressional changes on treatment with CG. We prioritized the key proteins of MAPK signaling, the p53 pathway, and downstream effectors through a combination of our transcriptomic analysis and subsequent assays. The fluorescent image (Figure 7A) shows that the level of MAPK1 protein is significantly reduced on three treatments compared to control cells. Whereas, p53 has lower expression in control than treatments (Figure 7B). Figure 7C clearly depicts the effect of CG on MCF-7 cells, where the cytoplasmic expression of EGR1 protein is highly reduced upon treatment. The analysis revealed that cardiac glycoside treatment significantly reduced the expression of MAPK1 and the downstream effector protein EGR1. The downregulation of MAPK1 protein is further promoted by p53 expression, suggesting that MAPK dynamics is crucial to establishing a balance between cell cycle arrest and cell progression in a p53-dependent manner.
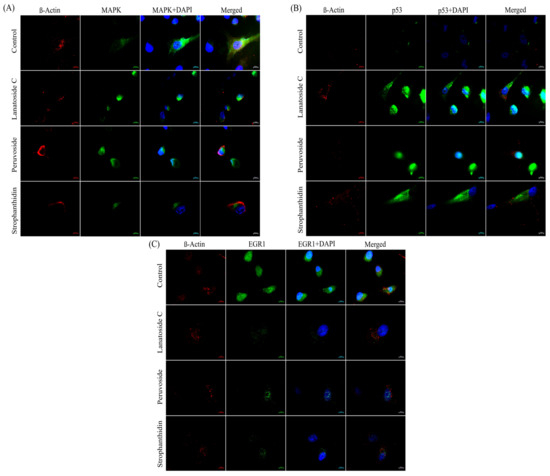
Figure 7.
Immunofluorescence imaging of subcellular localization of target proteins in MCF-7 cell lines. (A) Inhibition of MAPK1 protein expression (MAPK1 antibody—green) on treatment condition compared to control, where the protein is highly expressed. (B) Enhanced expression of p53 protein (p53 antibody- green) on treatment. (C) Immunoblotting image analysis of EGR1 protein (EGR1 antibody- green) showing significant downregulation on treatment in accordance with the expressional alteration of MAPK1 protein. The β-Actin antibody is indicated in red colour while the blue colous is hoechst 33342 staining nu-clear portion of the cell.
2.7. Protein–Ligand Interaction Analysis
Following the identification and confirmation of crucial genes influencing treatment outcomes with LC, PS, and STR, there was a compelling interest in further elucidating the precise impact of these compounds on proteins. Molecular docking studies were conducted to investigate this phenomenon to understand the interaction between the compounds and the protein units of the 10 scrutinized genes. The docking studies revealed higher binding efficiency and dominant bond interactions between the compounds and proteins (Table 2). Our particular focus was on comprehending the MAPK-dependent activation of EGR1 in driving transcriptional regulation and subsequently controlling cell proliferation and growth in cancer cells. This significant focus on evaluating the interactions between the EGR1 protein and the ligands revealed a robust binding efficiency, with binding scores of −8.8, −6.8, and −7.2 kcal/mol for LC, PS, and STR, respectively. The active site binding and the major residues involved in the interaction between ligands and EGR1 protein are depicted in Figure 8A–F. Similarly, MAPK1 protein showed significant ligand interaction with binding scores of −9.6, −8.2, and −7.3 kcal/mol for LC, PS, and STR, respectively (Figure 8K–L). The higher binding efficiency of LC with the proteins compared to the other ligands is probably due to its ability to engage in a broader range of H-bond interactions. The docking images and the corresponding interacting residues of p53, CCNK, CASP9, BCL2L1, CDK7, CDK2, CDK2AP1, and CDKN1A protein–ligand interactions are shown in Supplementary Figure S2.

Table 2.
The docking analysis results, portraying the binding energy score and amino acids interacting between the bonds.
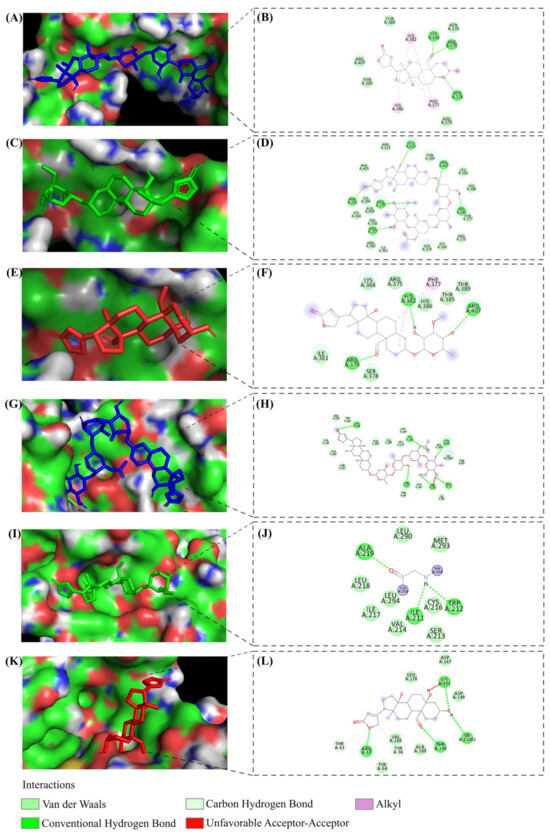
Figure 8.
Molecular docking interactions of LC, PS, and STR ligands. (A–F) Interactions of ligand molecules with EGR1 protein in 3D and 2D images. (G–L) Interactions of ligand molecules with MAPK1 protein in 3D and 2D images.
3. Discussion
Drug repurposing is an accelerated drug development approach that involves the identification of novel clinical possibilities of existing drugs, thereby bypassing prolonged traditional drug development methods. The concept has been effectively studied and utilized across a spectrum of medical conditions combating ailments like viral infections [18], contagious COVID-19 [19], influenza infection [20], antimicrobial resistance [21], etc. The therapeutic strategy was practiced and implemented in cancer treatment for many years. Over time, several candidate compounds have been successfully repositioned, including Doxorubicin, Cyclophosphamide, Everolimus, Tamoxifen, and Anastrozole, as remedial drugs to treat various cancers [22].
Cardiac glycosides are among the promising candidates studied and tested through drug repurposing approaches and have demonstrated widespread utility for anticancer and antiviral activities [23]. Notably, during the last 15 years, compelling evidence has increasingly supported the potency of CGs as a potent antiproliferative agent in various forms of cancer [24,25]. Earlier studies have demonstrated the antitumor efficacy, immunomodulatory effects, ability to induce apoptosis, and other notable activities of more than 20 such compounds [26]. Recent research focusing on the anticancer effect of ouabain has uncovered its senolytic property, adding another piece to the spectrum of potential applications of CGs [6,27]. Inspired by this work, our research team has been committed to exploring the efficacy of CGs in combating cancer. Through rigorous in silico and in vitro screening processes, we have identified three potent members of the family, namely Lanatoside C, Peruvoside, and Strophanthidin, with high efficacy in various human cancers, including lung, liver, and breast cancer. Further analysis of each molecule has revealed their antiproliferative mechanism, which involved attenuation of the MAPK, PI3K/AKT/mTOR, and Wnt/β-catenin signaling pathways, causing apoptosis induction and cell cycle arrest [13,14,15,28,29,30].
In the current study, we performed comprehensive RNA sequencing to gain more information on the transcriptional regulation and mechanism of action responsible for the LC, PS, and STR anticancer activity in the MCF-7 cell line. For this purpose, we sequenced four samples of MCF-7 cells, including three treatments (LC, PS, and STR) and an untreated control sample. The RNA-Seq analysis of the corresponding sequence data identified 5813 DEGs common in the three treatment groups compared to the control sample. We also identified a significant correlation between the MAPK1 and EGR1 genes, which are associated with cell growth during differential gene expression analysis, following treatment with CGs (Figure 1D). Additionally, functional enrichment analysis of the curated common genes showed their involvement in oncogenesis, cell cycle regulation, apoptosis, and complex interconnections of associated signaling pathways in immunoregulation. Accumulating evidence supports the efficacy of CGs as potent anti-inflammatory agents in various ailments, including neurodegenerative diseases [31], autoimmune diseases [32], and cancer [11,33] via influencing cellular signaling pathways. Recently, Škubník et al. discussed the broad immunomodulatory actions of CGs in cancer treatment, influencing immune system regulators and thereby inhibiting the transcriptional activity of key oncogenes [34]. Using the FunRich algorithm, we were able to infer the central role of p53 gene alteration during treatment (Figure 2). Moreover, the functional enrichment analysis of upregulated genes revealed them to be members of the P53-dependent apoptosis pathway. Yet another essential finding derived from the transcriptome analysis was the differential expression of cell cycle regulators, including CDK2 (Cyclin Dependent Kinase-2), CDK2AP1 (Cyclin Dependent Kinase-2 Associated Protein), CDK7 (Cyclin Dependent Kinase-7), and CCNK (Cyclin K), pointing towards the potential link between cell growth and the apoptosis pathway through immune regulation. However, the role of CDK2AP1 is still ambiguous, as it has a complex role in different types of cancer. While its exact function is still not fully understood, there are indications of its tumor suppressive activity in breast cancer cells via regulating cell cycle proteins [28]. A recent study on hepatocellular carcinoma (HCC) marked the gene as a prognostic indicator, as its expression was significantly lower in normal tissue than in tumor tissues. These findings confirmed the metastatic effect of CDK2AP1protein and highlighted the potential of CDK2AP1 as a target for immunotherapy in HCC [35].
The transcriptome profiling further validated our previous findings, demonstrating significant downregulation of key genes associated with MAPK- PI3K oncogenic signaling pathways on treatment with CGs. However, the downstream effectors responsible for the cytolysis of cancer cells on treatment with CGs, have not been previously identified. Following the identification of crucial genes associated with cancer, we quantitated the variability in mRNA expression through qRT-PCR (Figure 5). We then extended our investigations to assess the impact of the selected CGs on translational processes using immunoblotting (Figure 6) and immunocytochemical analysis (Figure 7). Our findings revealed significant alterations in the expression of the target proteins upon treatment with CGs. Notably, we observed a coordinated downregulation of the MAPK pathway and an upregulation of p53 expression, which jointly exerted control over cell progression at the translational level. These results shed light on the dynamic interplay between MAPK and p53 within cancer cells and highlight the potential for targeted interventions using CGs. In particular, our observations pointed to substantial suppression of EGR1, a downstream transcription factor activated by the MAPK-ERK signaling pathway in cancer. It is widely accepted that induction of the p38-MAPK pathway leads to activation of EGR1 through cyclic AMP-response element binding protein (CREB) transcription factor [29]. A recent report by Wang et al. highlighted the significance of the EGR1 transcription factor in cancer. The authors illustrated the impact of the EGR1 gene in tumor initiation, cell death, immune response, and its potential role in the tumor microenvironment. The significance of the EGR1 protein as the downstream regulator of the MAPK signaling pathway gains higher importance as it can be targeted for cancer therapy [30,36]. Studies have also indicated that EGR1 plays a regulatory role in angiogenic and osteoclastogenic factors, which contribute to metastasis by influencing tumor suppressors such as p53 and PTEN [37,38]. A recent study by Shan reported the significant effect of EGR1 response interconnecting three principal signaling pathways. This study provides essential evidence for an interdependent effect of endoplasmic reticulum stress on the MAPK pathway, transcription regulation through the EGR1 gene, and cell death mechanism in HepG2 cells [39]. At the same time, a similar study reported EGR1 expression through the p38 MAPK pathway modulating immune response and finally limiting transcription [40]. Previous works identified EGR1 as a target gene getting promptly activated by various mitogens, and apoptotic signaling pathways [41] in various cancers including lung [42], prostate [43], and breast [44]. Thus, our results discuss a concordance similar to these studies, where we report the influential role of cell cycle key regulators, CDKs, MAPK pathway genes, and apoptosis regulators on treatment with cardiac glycosides.
Through the utilization of RNA-Seq data, we have substantially enhanced our understanding of the potential mechanistic action of CGs on breast cancer cell lines. We have identified differentially expressed genes with substantial relevance to breast cancer, thereby augmenting our understanding of their significant roles. In addition to exploring the transcriptome, we have uncovered the translational effect and critical pathway interconnections that underlie the cytolytic properties of the compounds. These results also suggest that EGR1 dysregulation defines the inflammatory and immunosuppressive effects in breast cancer, causing loss of cell cycle control and apoptosis induction [45,46,47]. This study identifies the downstream effector genes and transcriptional regulators that contribute cytotoxic properties to the compound. These findings have enabled us to pinpoint potential targets that may exert a significant impact on CG treatment in breast cancer.
4. Materials and Methods
4.1. Cell Culture Reagents and Chemicals
A human breast cancer cell line (MCF-7) was obtained from the National Centre for Cell Science (NCCS), Pune, India. The obtained MCF-7 cells were cultured in DMEM medium (Himedia) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Cambridge, MA, USA), 100 µ/mL antibiotic–antimycotic solution (Himedia Pvt., Ltd., Mumbai, India) and maintained at 37 °C with 5% CO2. The CG compounds, including lanatoside C and strophanthidin, were purchased from Sigma Aldrich (St. Louis, MO, USA), while peruvoside was procured from Toronto Research Chemicals (North York, ON, Canada) and dissolved in dimethyl sulphoxide (DMSO) by maintaining the overall DMSO concentration not exceeding 0.01%.
4.2. RNA Isolation and Quality Assessment
The MCF-7 cells were seeded in 6-well plates to isolate RNA and divided into control and treatment groups. The treatment group was exposed to lethal concentrations of IC50 of CGs, determined based on our previously published studies, specifically, LC (1.2 µM), PS (100 nM), and STR (2 µM), respectively, and incubated for 24 h at 37 °C. After incubation, total RNA was isolated using TRIzolTM reagent (Invitrogen, Waltham, MA, USA.; Thermo Fisher Scientific, Inc., Boston, MA, USA) following the manufacturer’s instructions. Next, the concentration and purity of isolates were measured using a NanoDrop 2000C spectrophotometer (Thermo Scientific, Boston, MA, USA), and the isolated total RNA was then subjected to sequencing to obtain expression data.
4.3. Library Preparation and Sequencing
The genome-wide transcriptome library preparation and high-throughput RNA sequencing were performed at Eurofins Scientific (Bangalore, India). The extracted total RNA was used for preparing sequence-read libraries using the Illumina TrueSeq RNA sample prep kit. Subsequently, quality assessment and quantification of the prepared RNA libraries were evaluated using an Agilent 2100 bioanalyzer (Agilent, Santa Clara, CA, USA). The samples were also sequenced on the Illumina HiSeq250 platform, obtaining 100 bp paired-end (PE) sequences with a mean sequencing depth of 45 million reads per sample.
4.4. Transcriptome Data Analysis
The quality control and preprocessing of raw sequence data acquired from high-throughput sequencing pipelines were performed using FastQC and fastp [48] algorithms, which formulated the data for downstream analysis. After filtering out low-quality reads, raw reads were mapped to the human reference transcriptome (Ensembl; Homo sapiens version 86) using Kallisto version v0.48.0. [24]. The subsequent analyses were performed using the statistical computing environment, R version 4.2.1, R studio version 2022.07.2, and Bioconductor, version 3.14 [49]. Transcript quantification data were summarized to genes using TxImport packages [50] and normalized using the TMM method in EdgeR [51]. The normalized, filtered count data were variance stabilized using the voom (variance modeling at the observational level) function in EdgeR. Subsequently, the most exact process in EdgeR was used to perform differential gene expression analysis based on a negative binomial generalized linear model (GLM) applied to the count data (FDR ≤ 0.01, logFC ≥ 1).
4.5. Gene Set Enrichment and Gene Interaction Network Analysis
The commonly regulated differentially expressed genes on treatment with LC, PS, and STR were subjected to functional annotation and gene interaction network analysis using the FunRich algorithm [52]. FunRich performs comprehensive set functional enrichment, gaining biological insights from high-throughput experiments to identify overrepresented classes of genes.
4.6. Protein–Protein Interaction (PPI) Analysis
Protein–protein interactions are some of the crucial components that determine the cellular processes involved in normal as well as disease conditions. We used the STRING algorithm [53] to analyze protein interactions and assess the functional role of significant proteins from curated pathways. The STRING database provides known and predicted reliable protein–protein associations based on co-expression analysis, evolutionary signals, and ortholog-based evidence across organisms.
4.7. Quantitative Real-Time PCR Analysis
Quantitative real-time PCR was used to validate the selected differentially expressed (DE) genes retrieved from RNASeq analysis on treatment with cardiac glycosides. cDNA was synthesized from 2 µg total RNA using a verso cDNA synthesis kit (Thermo Fisher Scientific, Inc.) following the manufacturer’s instructions. The reaction mixture included 10 µL of 2X SYBR green qPCR master mix (Kapa Biosystems, Wilmington, MA, USA), nuclease-free water (Himedia), 100 ng RNA samples, and 100 ng RT primers. The primers were designed using Primer 3 and are listed in Supplementary Table S1. The PCR reactions were performed on the Roche light cycler® 480 system (Roche diagnostic corporation, Indianapolis, IN, USA) following specific experimental conditions, an initial denaturation step at 95 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 15 s, and extension at 72 °C for 15 s. This was followed by a final extension step at 95 °C for 1 min and a final annealing step at 60 °C for 1 min. The relative expression of each gene was normalized with an internal control β-actin and was calculated using the 2−ΔΔCT method. The experiments were further replicated in triplicate to ensure the accuracy and reliability of the results.
4.8. Immunoblotting Analysis
The effect of specific CGs at the translation level in a breast cancer model, MCF7 cells was assessed through immunoblotting or Western blot analysis. After a 24 h incubation period with the treatment, the cells were lysed in RIPA buffer containing a protease inhibitor cocktail (Merck, Darmstadt, Germany). The total protein concentrations were determined using the Bradford assay, and 20 µg of protein was loaded into each well of a 10% SDS-PAGE gel. After the electrophoresis, the gel was transferred to a PVDF membrane (Merck Millipore) using the Trans-Blot® Turbo TM blotting system (Bio-Rad, Hercules, CA, USA). The membrane was then blocked with a 3% Bovine Serum Albumin (BSA) solution in 1X Tris Buffered Saline-Tween (TBST) and subsequently incubated with the primary antibody overnight at 4 °C. Subsequently, the membrane was washed 3 to 4 times with 1X TBST and then incubated with the appropriate secondary antibody for an hour at room temperature. The primary antibodies used in this study were EGR1 Rabbit polyclonal antibody, MAPK1/ERK2 rabbit polyclonal antibody, Caspas-9 mouse polyclonal antibody, and p53 Rabbit polyclonal antibody (Origin India Pvt., Ltd., Mumbai, India). The chemiluminescent signal was detected and analyzed using a C-Digit chemiluminescent western blot scanner (LI-COR, Lincoln, NE, USA).
4.9. Immunocytochemical Analysis of Protein Co-Localization
For the Assay, the MCF-7 cells were plated on a coverslip in 6-well plates with a density of 2 × 104 cells per well. The cells were incubated at 37 °C with 5% CO2 and further treated with lethal doses of lanatoside C, peruvoside, and strophanthin for 24 h. After the incubation period, the media containing the compounds was aspirated and the cells were washed with 1 × PBS (Phosphate Buffer Saline) about 3 times. The cells were then fixed with 4% paraformaldehyde, followed by permeabilization with 0.1% of Triton x-100 for 5 min, and further blocked with 3% BSA for an hour at room temperature. Afterward, the cells were incubated with the following primary antibodies, EGR1 Rabbit polyclonal antibody, MAPK1/ERK2 rabbit polyclonal antibody, and p53 Rabbit polyclonal antibody (Origin, India) overnight at 4 °C. The cells were further washed with 1× PBS and incubated in secondary antibody anti-mouse AlexaFluor-555/anti-rabbit AlexaFluor-488 for an hour at room temperature. To determine protein colocalization within cells, 0.5 µg mL−1 of DAPI was used to incubate cells in the dark for 10 min. The coverslips were subsequently mounted on glass slides coated with ProLong Gold Antifade Mountant and examined using a fluorescent microscope at a Magnification of 100× while also capturing images (Zeiss, Axio Observer, Oberkochen, Germany).
4.10. Molecular Docking
Molecular docking studies were performed to evaluate and validate the interaction of CG compounds with scrutinized gene products. To this end, the ligand and candidate target protein structures were extracted from PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on 25 June 2023) and RSCB protein data bank (https://www.rcsb.org/, accessed on 25 June 2023), respectively. The molecular docking was followed in PyRx version 0.9 [54] using the Auto-dock vina program. Subsequently, the docking results were analyzed and visualized using PyMol 2.5 and Discovery Studio Visualizer (v21.1.0.20298). The best docking confirmations were selected from 10 predicted poses, comparing the ranked binding affinity score and RMSD values.
4.11. Statistical Analysis
Differential gene expression (DEG) analysis and its accountability were tested in EdgeR. The systematic evaluation and interpretation of significant DEGs were performed in the R statistical environment considering p < 0.05. The means of fold change between multiple replicates of RT-qPCR results were compared using GraphPad Prism, version 9, using the analysis of variance (ANOVA) method. Western blot data were compared as mean ± SEM from two independent experiments (n = 2). Statistical significance of difference in drug-treated versus control cells was determined using the Student t-test.
5. Conclusions
In this study, we have substantially expanded our understanding of the effect of CGs in breast cancer by utilizing RNA sequencing. It is also worth noting that the study marks the first instance in which the action of CGs in cancer cell lines is studied through transcriptome-wide analysis of differentially expressed genes. Through the study, we obtained confirmatory results that supported previous findings, which showed the significance of the repression of cell proliferation through the MAPK/PI3K/AKT pathway and its influence on cell cycle and apoptosis. In addition, we found differential expression of downstream genes and transcription factors associated with the MAPK-PI3K pathway. The analysis provided evidence for the regulation of EGR1 through MAPK and the P53-dependent signaling cascade, which consecutively mediated cell toxicity (Figure 9). Notably, our findings demonstrate the significant regulation of MAPK, p53-dependent apoptosis, and downstream effectors on treatment with CGs. The importance of these observations is in pointing out the effectiveness of selected CGs in the treatment of breast cancer. In summary, we emphasize the anticancer potency of cardiac glycosides, specifically lanatoside C, peruvoside, and strophanthidin, in controlling cell proliferation, thereby indicating their effectiveness in treating breast cancer.
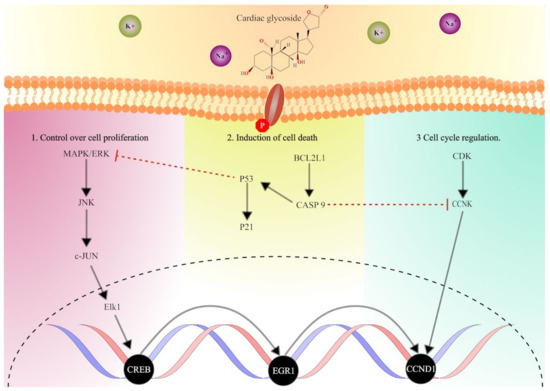
Figure 9.
Hypothetical overview of the mechanistic impact of cardiac glycosides on multiple core oncogenic signaling pathways in breast cancer. Speculative examinations suggest that CGs influenced the MAPK pathway, p53 pathway, and CDK pathway, controlling cell growth, apoptosis, cell fate, cell cycle regulation, and cell division, respectively. The schematic representation includes (1) the upstream activators of MAPK/ERK signaling (2) regulation of p53-dependent cell death, and (3) modulators of cell cycle progression and cell division, alongside the downstream effectors of signaling pathways controlling cancer progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242115922/s1.
Author Contributions
Conceptualization, R.K.; Data curation, R.K. and H.P.; Formal analysis, R.K. and H.P.; Funding acquisition, R.K. and P.G.; Investigation, R.K.; Methodology, R.K. and H.P.; Project administration, R.K.; Resources, R.K. and P.G.; Supervision, R.K.; Validation, R.K., H.P. and P.G.; Visualization, R.K. and H.P.; Writing–original draft, R.K. and H.P.; Writing review & editing, R.K., H.P. and P.G. All authors have read and agreed to the published version of the manuscript.
Funding
Science and Engineering Research Board (SERB), Govt. of India, funds through the EMR (2016/003715/BBM) scheme supported this work and partially supported by 5R21MH128562-02 (PI: Roberson-Nay), 5R21AA029492-02 (PI: Roberson-Nay), CHRB-2360623 (PI: Das), NSF-2316003 (PI: Cano), VCU Quest (PI: Das), and VCU Breakthroughs (PI: Ghosh) funds awarded to P.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the Science and Engineering Research Board (SERB), Govt. of India, for financial assistance. HP thanks ICMR-SRF, Govt. of India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. Available online: https://www.frontiersin.org/articles/10.3389/fmed.2017.00227 (accessed on 27 September 2023). [CrossRef] [PubMed]
- Libson, S.; Lippman, M. A review of clinical aspects of breast cancer. Int. Rev. Psychiatry 2014, 26, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges, and recommendations. Nat. Rev. Drug. Discov. 2019, 18, E144–E146. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Herranz, N.; Sun, B.; Wagner, V.; Gallage, S.; Guiho, R.; Wolter, K.; Pombo, J.; Irvine, E.E.; Innes, A.J.; et al. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 2019, 1, 1074–1088. [Google Scholar] [CrossRef]
- Kumavath, R.; Paul, S.; Pavithran, H.; Paul, M.K.; Ghosh, P.; Barh, D.; Azevedo, V. Emergence of Cardiac Glycosides as Potential Drugs: Current and Future Scope for Cancer Therapeutics. Biomolecules 2021, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, tox-icokinetics, clinical signs, diagnosis, and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Plant-derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother. 2016, 84, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Dicato, M.; Diederich, M. Assembling the puzzle of anti-cancer mechanisms triggered by cardiac glycosides. Mitochondrion 2013, 13, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.F.Z.; Cerella, C.; Simões, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef]
- Geng, X.; Wang, F.; Tian, D.; Huang, L.; Streator, E.; Zhu, J.; Kurihara, H.; He, R.; Yao, X.; Zhang, Y.; et al. Cardiac glycosides inhibit cancer through Na/K-ATPase-dependent cell death induction. Biochem. Pharmacol. 2020, 182, 114226. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Ghosh, P.; Barh, D.; Lanatoside, C. Induces G2/M Cell Cycle Arrest and Suppresses Cancer Cell Growth by Attenuating MAPK, Wnt, JAK-STAT, and PI3K/AKT/mTOR Signaling Pathways. Biomolecules 2019, 9, 792. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Tan, T.Z.; Ampasala, D.R.; Kumar, A.P. Peruvoside targets apoptosis and au-tophagy through MAPK Wnt/β-catenin and PI3K/AKT/mTOR signaling pathways in human cancers. Life. Sci. 2020, 241, 117147. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.; Ghosh, P.; Kumavath, R. Strophanthidin Attenuates MAPK, PI3K/AKT/mTOR, and Wnt/β-Catenin Signaling Pathways in Human Cancers. Front. Oncol. 2020, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Campbell, C.; Venkitaraman, A.R.; Esposito, A. Pulsatile MAPK Signaling Modulates p53 Activity to Control Cell Fate Decisions at the G2 Checkpoint for DNA Damage. Cell. Rep. 2020, 30, 2083–2093.e5. [Google Scholar] [CrossRef]
- Baron, V.; Adamson, E.D.; Calogero, A.; Ragona, G.; Mercola, D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFβ1, PTEN, p53 and fibronectin. Cancer Gene Ther. 2006, 13, 115–124. [Google Scholar] [CrossRef]
- Trivedi, J.; Mohan, M.; Byrareddy, S.N. Drug repurposing approaches to combating viral infections. J. Clin. Med. 2020, 9, 3777. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Padey, B.; Terrier, O.; Rosa-Calatrava, M. Drug Repurposing Approaches for the Treatment of Influenza Viral Infection: Reviving Old Drugs to Fight Against a Long-Lived Enemy. Front. Immunol. 2019, 10, 531. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00531 (accessed on 27 September 2023). [CrossRef]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef]
- Rodrigues, R.; Duarte, D.; Vale, N. Drug Repurposing in Cancer Therapy: Influence of Patient’s Genetic Background in Breast Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 4280. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Barh, D.; Azevedo, V.; Ghosh, P. Anticancer and Antiviral Properties of Cardiac Glycosides: A Review to Explore the Mechanism of Actions. Molecules 2020, 25, 3596. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Cardiac Glycosides in Cancer Research and Cancer Therapy. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Newman, R.A.; Yang, P.; Pawlus, A.D.; Block, K.I. Cardiac Glycosides as Novel Cancer Therapeutic Agents. Mol. Interv. 2008, 8, 36. [Google Scholar] [CrossRef]
- Khatri, H.R.; Bhattarai, B.; Kaplan, W.; Li, Z.; Curtis Long, M.J.; Aye, Y.; Nagorny, P. Modular Total Synthesis and Cell-Based Anticancer Activity Evaluation of Ouabagenin and Other Cardiotonic Steroids with Varying Degrees of Oxygenation. J. Am. Chem. Soc. 2019, 141, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Triana-Martínez, F.; Picallos-Rabina, P.; Da Silva-Álvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, E.; Ped-rosa, P.; Ferreirós, A.; Barradas, M.; et al. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat. Commun. 2019, 10, 4731. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xiang, H.; Zong, X.; Yan, X.; Yu, Y.; Liu, G.; Zou, D.; Yang, H. CDK2-AP1 inhibits growth of breast cancer cells by regulating cell cycle and increasing docetaxel sensitivity in vivo and in vitro. Cancer. Cell. Int. 2014, 14, 130. [Google Scholar] [CrossRef]
- Duclot, F.; Kabbaj, M. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front. Behav. Neurosci. 2017, 11, 35. Available online: https://www.frontiersin.org/articles/10.3389/fnbeh.2017.00035 (accessed on 27 September 2023). [CrossRef] [PubMed]
- Koyani, C.N.; Kitz, K.; Rossmann, C.; Bernhart, E.; Huber, E.; Trummer, C.; Windischhofer, W.; Sattler, W.; Malle, E. Activation of the MAPK/Akt/Nrf2-Egr1/HO-1-GCLc axis protects MG-63 osteosarcoma cells against 15d-PGJ2-mediated cell death. Biochem. Pharmacol. 2016, 104, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.; Dieriks, V.B.; Rustenhoven, J.; Smyth, L.C.D.; Scotter, E.; Aalderink, M.; Feng, S.; Johnson, R.; Schweder, P.; Mee, E.; et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun. Biol. 2021, 4, 260. [Google Scholar] [CrossRef]
- Titus, H.E.; Xu, H.; Robinson, A.P.; Patel, P.A.; Chen, Y.; Fantini, D.; Eaton, V.; Karl, M.; Garrison, E.D.; Rose, I.V.L.; et al. Re-purposing the cardiac glycoside digoxin to stimulate myelin regeneration in chemically-induced and im-mune-mediated mouse models of multiple sclerosis. Glia 2022, 70, 1950–1970. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, J.; Chen, J.; Kuo, K.T.; Tang, J.; Gao, H.; Chen, L.; Chen, Z.; Meng, Z. New therapeutic aspects of steroidal cardiac glycosides: The anticancer properties of Huachansu and its main active constituent Bufalin. Cancer. Cell. Int. 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Che, Y.; Wang, G.; Xia, Q. CDK2AP1 Influences Immune Infiltrates and Serves as a Prognostic Indicator for Hepatocellular Carcinoma. Front. Genet. 2022, 13, 937310. Available online: https://www.frontiersin.org/articles/10.3389/fgene.2022.937310 (accessed on 27 September 2023). [CrossRef] [PubMed]
- Wang, B.; Guo, H.; Yu, H.; Chen, Y.; Xu, H.; Zhao, G. The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 2021, 11, 642547. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2021.642547 (accessed on 27 September 2023). [CrossRef]
- Li, N.; Xu, H.; Ou, Y.; Feng, Z.; Zhang, Q.; Zhu, Q.; Cai, Z. LPS-induced CXCR7 expression promotes gastric Cancer proliferation and migration via the TLR4/MD-2 pathway. Diagn Pathol. 2019, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fry, E.A. Tumor suppression by the EGR1, DMP1, ARF, p53, and PTEN network. Cancer. Investig. 2018, 36, 520–536. [Google Scholar] [CrossRef]
- Shan, J.; Dudenhausen, E.; Kilberg, M.S. Induction of early growth response gene 1 (EGR1) by endoplasmic reticulum stress is mediated by the extracellular regulated kinase (ERK) arm of the MAPK pathways. Biochim. Biophys. Acta. Mol. Cell. Res. 2019, 1866, 371–381. [Google Scholar] [CrossRef]
- Ten Hoeve, A.L.; Hakimi, M.-A.; Barragan, A. Sustained Egr-1 Response via p38 MAP Kinase Signaling Modulates Early Immune Responses of Dendritic Cells Parasitized by Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2019, 9, 349. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Tan, X.; Liu, W.; He, X.; Pan, D.; Li, E.; Xu, L.; Long, L. Friend or Foe: Regulation, Downstream Effectors of RRAD in Cancer. Biomolecules 2023, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-H.; Su, Y.-C.; Lin, S.-F.; Lin, P.-R.; Wu, C.-L.; Tung, C.-L.; Li, C.-F.; Shieh, G.-S.; Shiau, A.-L. Oct4 upregulates osteopontin via Egr1 and is associated with poor outcome in human lung cancer. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Ameri, A.H.; Wang, S.; Jansson, K.H.; Casey, O.M.; Yang, Q.; Beshiri, M.L.; Fang, L.; Lake, R.G.; Agarwal, S.; et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene 2019, 38, 6241–6255. [Google Scholar] [CrossRef]
- Saha, S.K.; Islam, S.M.R.; Saha, T.; Nishat, A.; Biswas, P.K.; Gil, M.; Nkenyereye, L.; El-Sappagh, S.; Islam, M.S.; Cho, S.-G. Prognostic role of EGR1 in breast cancer: A systematic review. BMB Rep. 2021, 54, 497–504. [Google Scholar] [CrossRef]
- Yu, J.; Baron, V.; Mercola, D.; Mustelin, T.; Adamson, E.D. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell. Death. Differ. 2007, 14, 436–446. [Google Scholar] [CrossRef]
- Yoon, T.M.; Kim, S.-A.; Lee, D.H.; Lee, J.K.; Park, Y.-L.; Lee, K.-H.; Chung, I.-J.; Joo, Y.-E.; Lim, S.C. EGR1 regulates radiation-induced apoptosis in head and neck squamous cell carcinoma. Oncol. Rep. 2015, 33, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yu, M.; Zhu, Y.; Liu, D.; Wu, Q.; Hu, Y. EGR-1/ASPP1 inter-regulatory loop promotes apoptosis by inhibiting cyto-protective autophagy. Cell. Death. Dis. 2017, 8, e2869. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates im-prove gene-level inferences. F1000Research 2016, 4, 1521. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with in-creased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic. Acids. Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology: Methods and Protocols; Hempel, J.E., Williams, C.H., Hong, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 243–250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).