Abstract
Cutaneous melanoma (CM) is an increasingly significant public health concern. Due to alarming mortality rates and escalating incidence, it is crucial to understand its etiology and identify emerging biomarkers for improved diagnosis and treatment strategies. This review aims to provide a comprehensive overview of the multifactorial etiology of CM, underscore the importance of early detection, discuss the molecular mechanisms behind melanoma development and progression, and shed light on the role of the potential biomarkers in diagnosis and treatment. The pathogenesis of CM involves a complex interplay of genetic predispositions and environmental exposures, ultraviolet radiation exposure being the predominant environmental risk factor. The emergence of new biomarkers, such as novel immunohistochemical markers, gene mutation analysis, microRNA, and exosome protein expressions, holds promise for improved early detection, and prognostic and personalized therapeutic strategies.
1. Introduction
Cutaneous melanoma (CM) is a particularly aggressive form of cancer that originates from melanocytes, pigment-producing cells derived from the neural crest [1]. Despite representing a mere 4% of all skin cancers, CM accounts for up to 75% of skin cancer-related deaths [2]. However, with early detection and proper intervention, over 90% of the cases could be cured [3].
The pathogenesis of CM is multifactorial, involving both genetic and environmental factors [4]. Ultraviolet radiation (UVR), either from natural light or artificial sources, is the most important environmental risk factor for CM. Additionally, individuals with lighter complexion have the highest risk of developing CM due to lower levels of melanin which make these individuals more likely to develop sunburns. A higher number of nevi are also associated with an increased risk. A familial history of CM further increases this risk, possibly due to shared sun exposure behaviors or hereditary genetic mutations [5].
In this context, patient survival is strongly correlated with an early detection of the disease. Among various prognostic factors, the depth of invasion remains the most critical determinant of survival in numerous studies. Intraepidermal (in situ) melanomas can be cured by excision alone, and thin melanomas have minimal metastatic potential [6]. On the contrary, thick CMs still have very high mortality rates [7].
Despite its growing incidence, due to the advancements in diagnosis and management, the prognosis of CM has significantly increased. In this context, due to the ongoing challenges in melanoma prevention, diagnosis, and treatment, we performed a literature review discussing the latest progress in the diagnosis and management of CM, emphasizing the role of genetic testing, conventional immunohistochemistry, as well as several emerging biomarkers. These novel biomarkers such as microRNA and exosomes may significantly improve the prognosis of melanoma due to their potential to be used for non-invasive early diagnosis and monitoring, but also to become therapeutic targets.
2. Melanoma Pathogenesis
Ultraviolet radiation exposure is a major risk factor for CM due to the UVR capacity to damage DNA, causing somatic mutations [8]. The exposure can be classified as intermittent or chronic, the latter being mostly occupational. Both these exposure patterns are associated with an increased risk of CM, but it appears that the risk is higher for intermittent exposure [8,9]. This may be explained by the fact that intermittently exposed individuals have lower melanin levels and are more likely to develop sunburns [10]. Nevertheless, there are cutaneous melanomas, such as acral melanomas, which arise in skin that is not exposed to UVR. In this context, according to the 2023 WHO Classification of Tumors, cutaneous melanomas are classified as melanomas arising in sun-exposed skin and melanomas arising in sun-shielded sites (Table 1) [11].

Table 1.
WHO Classification of cutaneous melanomas.
Apart from UVR exposure, hereditary predisposition is another risk factor for cutaneous melanomas. However, familial cases encompass around 10% of all melanomas [12]. In this respect, several high-penetrance genes such as CDKN2A, CDK4, or BAP1 are the most mutated in hereditary melanomas [13]. Individuals with germline mutations in the CDKN2A, a tumor suppressor gene, have a very high lifetime risk of developing CM, this mutation being encountered in up to 40% of melanoma-prone families [12]. Nevertheless, these mutations are relatively rare and are responsible for just around 2% of all CM cases [8]. In addition to these high-penetrance genes, some medium-penetrance genes such as MITF and MC1R are also involved in hereditary CM [12]. Furthermore, MC1R can be considered a “melanoma modifier gene” as it also increases the penetrance of CDKN2A [14].
As hereditary melanomas are relatively rare, most cutaneous melanomas are characterized by a remarkably high burden of somatic genetic mutations [15,16]. Identifying these genetic mutations can serve both diagnostic and prognostic purposes. Genetic testing is particularly useful for the diagnosis of dedifferentiated CMs which lack typical morphological and immunohistochemical features. In such cases, the diagnosis can be established by identifying melanoma-specific mutations [17].
The most frequent mutations in CM affect genes involved in the aberrant activation of the RAS/RAF/MEK/ERK signaling pathway, also known as the mitogen-activated protein kinase (MAPK) pathway, and the phosphoinositol-3-kinase (PI3K)/AKT pathway [18]. These mutated genes include BRAF, NRAS, NF1, PTEN, KIT, TP53, CDKN2A, and TERT [19,20].
The MAPK pathway is involved in the transduction of extracellular signals to the nucleus, thus activating genes that regulate cell proliferation and differentiation [21,22]. This aberrant activation is responsible for several cellular dysfunctions, such as the deregulation of the cell cycle and inhibition of apoptosis [21,23,24]. MAPK is the most frequently dysregulated pathway in cutaneous melanoma [25]. Up to 90% of all melanoma cases exhibit an abnormal activation of the MAPK pathway. The second most frequently activated pathway in CM is the PI3K pathway which plays a crucial role in maintaining cellular homeostasis [26,27].
As the MAPK pathway is the most affected in CM, numerous mechanisms contribute to its abnormal signaling, including BRAF mutations [18,28]. Between 37% and 60% of cutaneous melanomas harbor a somatic mutation in this gene, with the highest frequency observed in CM associated with low CSD [29]. The majority of BRAF mutations in cutaneous melanoma are missense, resulting in amino acid substitutions at the valine 600 position. Approximately 80% are V600E mutations (glutamic acid substitution), while 5–12% are V600K mutations (lysine substitution). Less common mutations include V600D (valine to aspartic acid) or V600R (arginine substitution). Additionally, BRAF non-V600 mutations can occur in around 5% of cases [30]. The BRAF gene encodes a protein kinase with three distinct domains: two regulatory and one catalytic. The latter is involved in the phosphorylation of MEK and in maintaining the protein inactive through a hydrophobic interaction. [31]. In the BRAF V600E mutation, the hydrophobic valine residue is substituted by a polar, hydrophilic glutamic acid which induces a conformational change in the catalytic domain, resulting in a constitutively active kinase [32,33]. BRAF non-V600E mutations generally operate through a similar mechanism, enhancing BRAF kinase activity [33]. Acknowledging these mutations is clinically significant for treatment and prognosis. BRAF V600-mutated melanomas can be treated with BRAF/MEK inhibitors, with response rates higher in V600E-mutated cases compared to V600K-mutated cases. Furthermore, even though the evidence is still limited, BRAF non-V600-mutated melanomas may still benefit from BRAF/MEK inhibitors [30].
The second most prevalent cause of aberrant MAPK pathway signaling In cutaneous melanoma is attributed to activating mutations in the NRAS gene. These mutations occur in 15–30% of melanomas and are predominantly missense, most often affecting codon 61 [34,35]. These mutations perpetuate aberrant signaling through both the MAPK and PI3K pathways [18,36,37]. It is noteworthy that NRAS and BRAF mutations are generally considered to be mutually exclusive, although co-mutations have been observed in rare instances [37]. NRAS- and NRAS-BRAF-co-mutated melanomas have a less favorable prognosis than BRAF-mutated ones as there are no target therapies for NRAS mutations [17].
Neurofibromin 1 (NF1) is a tumor suppressor gene, mutated in 10–15% of CM, making it the third most common mutation in this pathology [38,39]. NF1 alterations are more frequent in melanomas associated with high CSD. These cases tend to possess a high mutational burden, including a co-occurrence of BRAF or NRAS mutations [19,40]. The NF1 protein serves as a regulator of the RAS family, attenuating downstream RAS signaling [41]. Consequently, loss-of-function mutations in NF1 result in the hyperactivation of NRAS, leading to increased signaling through both the MAPK and PI3K pathways [19,38,39,41]. Analyzing NF1 mutation status has some prognostic value even though there are no target therapies for NF1-mutated melanomas, but such cases respond favorably to immunotherapy [42]. Moreover, NF1 analysis can offer important diagnostic information as this mutation is particularly common in dedifferentiated lesions which can be difficult to diagnose otherwise [17].
The receptor tyrosine kinase KIT plays a crucial physiological role in the proliferation and survival of melanoma cells, through the PI3K and the MAPK signaling cascades. KIT mutations are found in 2–8% of melanoma cases and are more common in acral melanomas and melanomas associated with low CSD [43,44]. Recognizing these mutations is important as such cases can benefit from tyrosine kinase inhibitors [45].
Mutations in the TERT promoter confer a proliferative advantage to melanoma cells and are common in advanced disease, being associated with a less favorable prognosis. Nevertheless, this mutation could become a potential therapeutic target [46,47]. TP53-mutated melanomas are also associated with advanced disease [46]. Assessing the status of TP53 is important as these mutations have been associated with MAPK inhibitor resistance but they can also become potential therapeutic targets [48,49].
The PTEN gene, a tumor suppressor gene, is commonly dysregulated in the vertical growth phase of melanoma and in metastatic lesions, occurring in 10–30% of cutaneous melanomas [18,50]. PTEN alterations tend to be mutually exclusive with NRAS mutations but often co-occur with mutations in BRAF [51,52]. This co-occurrence has been hypothesized to increase PI3K pathway activation [51,52], mimicking the effects of an NRAS-only activation [51,53]. Additionally, PTEN loss-of-function is involved in acquired resistance to BRAF inhibitors in BRAF-mutated melanomas [54]. As mentioned before, BRAF-mutated melanomas may respond to BRAF inhibitors. However, therapeutic success is often temporary, as patients usually experience disease progression at some point or may even exhibit primary resistance to this target therapy. In this respect, acquired genetic mutations affecting the MAPK and PI3K signaling pathways play a central role in resistance to both chemotherapy and targeted therapies [55,56,57]. In this context, targeted PTEN therapy could improve the outcomes of the patients [49]. Having taken everything into consideration, due to this extraordinary genetic heterogeneity of melanomas, a multi-faced diagnostic and therapeutic approach including the identification of molecular biomarkers and genetic aberrations is imperative for optimizing patient outcomes.
3. Diagnostic and Prognostic Immunohistochemical Markers in CM
Cutaneous melanomas can manifest a broad array of morphological characteristics, rendering them easily confusable with other neoplastic lesions on standard histopathological examination. Consequently, additional diagnostic tools, particularly immunohistochemical (IHC) staining methods, may be necessary, especially in instances where the histological sample is partial, or the differentiation status of the neoplasm is ambiguous [58].
Various melanocytic markers such as S100, HMB45, Melan A, tyrosinase, MITF, and SOX10 can aid in the detection and subtyping of melanoma [58,59,60]. The S100 marker stands out for its high sensitivity for melanomas of all subtypes, including desmoplastic melanoma [60,61]. However, it is important to note that while S100 demonstrates high sensitivity, its specificity is limited, given that it is also expressed in a range of other malignancies and normal cellular components, such as dendritic cells, certain macrophages, and Schwann cells in lymph nodes [62,63]. This lack of specificity can create diagnostic pitfalls by masking the presence of small metastatic melanoma foci amid other S100-expressing structures within lymph nodes [62,63]. Additionally, primary cutaneous, particularly dedifferentiated ones, and metastatic melanomas can, in rare cases, lack S100 expression [17]. In this context, Aisner D.L. et al. discovered that approximately 1% of metastatic melanoma specimens were devoid of S100 expression. The loss of S100 expression did not appear to correlate with any specific histological subtype or the anatomical site of metastasis [64].
HMB-45 and Melan-A/MART-1 are melanocyte-specific markers with considerable specificity. HMB-45 recognizes gp100, a component of the melanosomal complex, and is highly specific for melanoma [65,66,67]. HMB-45 is particularly useful in distinguishing between a benign and a malignant melanocytic tumor as nevi gradually lose HMB-45 due to their maturation process [67,68]. Nevertheless, its use is limited due to low sensitivity as it fails to stain a significant proportion of metastatic melanomas [69,70]. These numbers can be remarkably high even in primary cutaneous melanomas with divergent differentiation, when HMB-45 may be negative in up to 86% of the cases [17]. Melan-A expression is predominantly localized within the endoplasmic reticulum and melanosomes, thus having higher sensitivity compared to HMB-45. Melan-A is particularly useful in identifying isolated tumoral melanocytes in the dermis, which can reclassify a melanoma initially diagnosed as in situ to an invasive lesion [66,71,72]. Drabeni M. et al. reported an increased Breslow thickness in approximately 60% of cases when utilizing Melan-A compared to H&E staining alone [71]. Similarly, Megahed M. et al. found evidence of dermal invasion in 30 out of 104 cases that were initially classified as melanoma in situ based on H&E staining [72]. However, Melan-A analysis has its limitations. The formation of pseudomelanocytic nests—clusters of Melan-A positive cells at the dermo–epidermal junction—can confound the diagnosis of melanoma in situ in the presence of lichenoid inflammation [73]. The concomitant application of nuclear markers like MITF (microphthalmia-associated transcription factor) and SOX10 has been suggested as a solution [73]. SOX10 is significantly more specific than Melan-A (96% vs. 17%) in identifying epidermal melanocytes and consequently avoiding the overdiagnosis of melanoma in situ in sun-damaged skin [74]. Similarly, MITF is also superior to Melan-A for the correct diagnosis of solar lentigo [75]. Additionally, Melan-A also fails to stain most primary dedifferentiated cutaneous melanomas. SOX10 and MITF perform better in such cases but their sensitivity is still rather low at around 34% [17]. In this context, melanomas completely lacking expression of conventional melanocytic markers have been described in both primary and metastatic lesions [76]. In addition to their low sensitivity in dedifferentiated lesions, none of these markers is entirely specific for melanomas [17]. For example, clear-cell sarcomas express HMB-45, Melan-A, MITF, S100, and SOX10 [77,78]. PEComas, even though rarely located on the skin, express HMB-45 and MITF [79]. Malignant peripheral nerve sheet tumors express SOX10 and S100, and in rare cases can express Melan-A and tyrosinase [80]. As a consequence, several other immunohistochemical markers have been developed.
The NK1/C3 antibody is noteworthy for its ability to identify a specific cytoplasmic antigen prevalent in melanoma cells. The NK1/C3 antibody was synthesized at the Netherlands Cancer Institute, and although its target antigen was not initially known, it appears to be a glycoprotein located on the membranes of cytoplasmatic vesicles in melanoma cells [81,82]. However, its expression is not confined to melanoma alone. It is also detected in other melanocytic lesions including intradermal and compound nevi, congenital nevi, dysplastic nevi, blue nevi, and Spitz nevi [81]. Moreover, NK1/C3 is also highly sensitive for metastatic lesions [70]. Nevertheless, this antigen is also sporadically present in certain non-melanocytic neoplasms such as a subset of breast and prostate carcinomas, cellular neurothekeomas, granular cell tumors, and dendritic cells within lymph nodes [81,83,84]. Furthermore, the high cost associated with its use poses an additional impediment to its widespread use [85]. These constraints underscore the need for further research to either refine the specificity of NK1/C3 or to identify alternative, cost-effective markers with greater diagnostic precision. Future studies might aim to delineate the functional implications of the antigen identified by NK1/C3 in tumor pathogenesis, as this could provide additional insight into its potential roles as a therapeutic target or as part of a multi-marker diagnostic panel.
Immunohistochemical analysis for PRAME (preferentially expressed antigen in melanoma) has become increasingly used in the diagnosis of CM [86,87,88]. One study assessed the immunoexpression of PRAME in 400 melanocytic tumors, including primary melanomas, metastatic melanomas, and melanocytic nevi [86]. The study revealed diffuse nuclear immunoreactivity in over 80% of the metastatic (87%) and primary melanomas (83.2%). The expression was notably high in all subtypes except for desmoplastic melanomas (35%). Importantly, PRAME was expressed in both in situ and non-desmoplastic invasive components. Additionally, 86.4% of the melanocytic nevi investigated were entirely negative for this marker [86]. Furthermore, PRAME analysis is also useful for identifying melanocytic pseudonests in order to distinguish melanoma in situ from lichenoid dermatoses or keratoses [89]. PRAME expression also seems retained in primary dedifferentiated melanomas, but more studies are needed to confirm these findings [17]. Moreover, it is significantly less expressed in clear-cell sarcoma, PEComas, and other skin spindle-cell neoplasms that can be considered in the differential diagnosis of CM [90,91]. Therefore, PRAME is a constituent of a 23-gene array diagnostic assay used for cutaneous melanoma [92,93], and is one of two genes employed in a noninvasive molecular assay that aids clinicians in determining the necessity for biopsy in the case of melanocytic lesions [94]. PRAME is also highly useful for assessing metastatic lesions. For instance, a study comparing nodal nevi and melanoma metastases demonstrated that PRAME was expressed in 0% of the nevi and in 100% of the lymph node metastases [87]. Nevertheless, its expression is not confined to melanoma but is also present in various other malignancies, such as lung cancer, breast carcinoma and other gynecological malignancies, renal carcinoma, leukemia, synovial sarcoma, myxoid liposarcoma, and various other sarcomas [95,96,97,98]. Therefore, in the setting of metastatic disease, the diagnosis of melanoma should not solely rely on PRAME expression and should be confirmed by additional immunohistochemical or molecular analysis. However, PRAME analysis for metastatic melanoma may not possess just diagnostic, but also prognostic value as it may become a potential target for immunotherapy [99].
In addition to these markers, BRAF V600E immunohistochemical analysis is increasingly performed for primary cutaneous melanomas and metastatic lesions [100,101]. This method is particularly useful for diagnosing dedifferentiated melanomas that lack expression of conventional melanocytic markers and may aberrantly express other markers [102]. Another great advantage of immunohistochemical analysis is its considerably lower costs compared to molecular analysis while the results are similar, with a study reporting a sensitivity of 80.8% and specificity of 100% for immunohistochemistry [103]. In spite of these promising results, at present BRAF V600E immunohistochemical results should be confirmed by PCR analysis, as concordance between the two methods varies between 71.4 and 97% [101]. In the future, immunohistochemical methods for BRAF detection may significantly improve, providing increased diagnostic accuracy at lower costs.
The main characteristics of the immunohistochemical markers discussed above are presented in Table 2.

Table 2.
Advantages and disadvantages of the main melanocytic markers.
Another pitfall in the immunohistochemical analysis of CM is that rare cases may display atypical IHC staining patterns. As mentioned before, these may include the aberrant expression of markers typically unrelated to melanocytes or the absence of conventional melanocytic markers, further complicating the diagnosis [104]. The heterogenous immunophenotypic profile in melanoma underscores the necessity for a multi-marker approach to the diagnosis of this malignancy. It also raises important questions about the biological mechanisms underlying the loss of conventional melanocytic markers during disease progression. These aspects could have significant implications for both prognosis and therapeutic choices. Future research might focus on understanding the molecular mechanism of marker loss, helping the development of more effective diagnostic tools and targeted therapies.
Finally, apart from their diagnostic value, immunohistochemical markers have also been analyzed as prognostic tools. Even though traditional markers may lose expression in dedifferentiated and metastatic lesions, it is not clear how this correlates with prognosis. For instance, primary dedifferentiated cutaneous melanomas and conventional CMs with similar prognostic factors have a similar overall prognosis [105]. In recent years, as PRAME expression has been associated with prognosis in patients with uveal melanomas [106], it has been studied as a potential prognostic marker in CM but it does not influence patient survival [107,108]. On the contrary, a higher expression of the proliferation marker, Ki67 has been associated with decreased survival in some studies [107,109]. A 2021 meta-analysis found a significant association between higher Ki67 expression and lower overall survival rates. However, no correlation was found between Ki67 expression and progression-free survival or recurrence-free survival [110] Having taken all these reports into consideration, Ki67 may be proved to be a useful prognostic factor in CM, but further studies are needed to validate these findings and establish a cut-off value associated with decreased survival.
4. Emerging Biomarkers in CM
Due to the extraordinarily heterogenous histopathological, immunohistochemical, and molecular landscape of CM, this disease continues to pose important challenges in terms of diagnosis and treatment. Therefore, several new biomarkers have become increasingly studied in recent years in the hope of improving the understanding of CM pathogenesis and management. Unlike immunohistochemical and genetic testing, these emerging biomarkers are expected to improve the early detection and subsequent monitoring of CM in rapid, cost-effective, and non-invasive ways as they can easily be analyzed from blood samples. Furthermore, these new biomarkers may also serve as potential therapeutic targets.
4.1. MicroRNA
MicroRNA (miRNAs) represent non-coding RNAs involved in degrading mRNAs [96]. They have been increasingly recognized as critical modulators of oncogenic processes, including various stages of cancer progression such as melanoma [111,112]. In melanomas, miRNA dysregulation is involved in promoting cell proliferation, resistance to apoptosis and invasion, angiogenesis, and metastasis [113]. Additionally, miRNAs have also been associated with resistance to BRAF and MAPK inhibitors [114,115]. In this context, due to their detectability in both intra- and extracellular compartments and their stable levels even in unfavorable conditions, miRNAs have attracted considerable attention as emerging biomarkers in oncology [116].
At present, miRNA levels can be assessed from various sources, such as resected primary or metastatic tumors, as well as arterial or venous plasma and serum. Importantly, the data derived from these different sources have shown no significant divergence. Depending on the type of cancer under investigation, abnormal miRNA expression profiles have been found to correlate with various disease stages, overall prognosis, tumor recurrence, and potential responsiveness to therapeutic interventions [111,117]. In patients with melanoma, different miRNAs can be either up- or down-regulated, and have been correlated with progression-free survival and overall survival [118,119,120]. Interestingly, serum levels of various microRNAs can discriminate between melanoma stages with increased accuracy compared to S100B or LDH [121]. For instance, miR-137, miR-148, and miR-182 downregulate MITF expression and promote tumor invasion [122]. miR-221 plasma levels are increased in melanoma patients, and are correlated with stage, recurrence, and disease progression [123]. Rigg E. et al. demonstrated that miR-146a-5p is overexpressed in melanoma brain metastases and its knockdown results in a reduction of metastatic lesions [124]. On the contrary, other types of microRNA such as mirR-211, miR-542 3p, or miR-152-3p are downregulated in invasive melanomas [125]. In vitro studies demonstrated that increasing miR-152-3p expression inhibits the proliferation and invasiveness of melanoma cells [126]. miR-542 3p is involved in epithelial-to-mesenchymal transition (ETM), and its experimental upregulation inhibited ETM and metastatic spread [127]. miR-143 also bears anti-tumoral effects as it has been linked to promoting apoptosis and inhibiting the proliferation of melanoma cells [128]. Similar anti-tumoral effects have been reported for miR-224-5p which can additionally overturn acquired resistance to BRAF inhibitors [129]. Several other microRNA seem to play a role in resistance to target or conventional chemotherapy. A downregulation of miR-7, miR-579 3p, and miR-126 3p was found in melanomas resistant to BRAF/MAPK inhibitors [130,131,132], while miR-31 downregulation is associated with chemoresistance [133]. Importantly, the experimental upregulation of miR-7 and miR-126 3p restored responses to BRAF inhibitors in melanoma cell lines [130,131].
Therefore, miRNA analysis could become a useful tool for monitoring melanoma progression after surgical excision and therapy, as well as a potential therapeutic target.
4.2. Exosomes
Exosomes are extracellular vesicles secreted by cells, encompassing a unique molecular signature that reflects the cell type from which they originate. Given their traceable cellular origins and facile isolation, exosomes can be regarded as potential biomarkers for diagnosis and prognosis in various cancers, including melanoma [134]. They are readily available through minimally invasive methods, as they can be isolated from a variety of biological fluids such as blood, plasma, urine, and cerebrospinal fluid [135]. Exosomes extracted from melanoma cell lines have been shown to contain distinct mRNA, miRNA, and protein profiles [134,135]. Exosome analysis can offer important diagnostic and prognostic information, as various exosomal components are significantly altered in cutaneous melanomas [134,136,137]. In this respect, Surman M. et al. found increased exosome concentrations in melanoma cases but those levels were not correlated with disease stage [134]. On the contrary, Boussadia Z. et al. reported a higher exosome concentration in metastatic melanoma compared to non-metastatic cases [138]. The complex relationship between exosomal components and melanoma progression is not entirely understood, but various mechanisms have been proposed. For instance, exosomes can carry and modulate the activity of matrix metalloproteinases (MMPs), as well as alter cell adhesion and activate fibroblasts to become cancer-associated fibroblasts, thus stimulating melanoma invasiveness [134,139,140,141]. Exosomal components have also been shown to enhance metastatic potential in CM by promoting epithelial-to-mesenchymal transition (ETM) [142], angiogenesis [134,143], and lymphangiogenesis [144]. As melanoma is particularly prone to brain metastases, exosomes may also at least partially explain this characteristic by damaging endothelial cells and the blood–brain barrier, and activating glial cells [145].
Some exosomal components have also been linked to resistance to therapy in CM, and targeting these molecules may improve therapeutic response [146]. In this context, exosomes can influence the melanoma microenvironment by altering the function of lymphocytes and stimulating tumor-associated macrophages (TAMs) to become M2-polarized and secrete pro-tumorigenic cytokines [147,148,149]. These effects can affect the response to immunotherapy, and targeting TAMs could improve the outcome of melanoma patients [150]. Furthermore, exosomes can be used as a means for administering therapy [151]. In this respect, exosomes containing BRAF siRNA were shown to have increased anti-tumoral activity compared to siBRAF in melanoma cell lines [152]. Similarly, cord-blood-derived exosomes produced significant genotoxicity and a decrease in survival time for melanoma cells and lymphocytes from melanoma patients, apparently by delivering anti-oncogenic miR-7. These results are particularly important as the exosome caused no significant damage to normal lymphocytes [153]. While these findings underscore the promising role of exosomes as diagnostic, prognostic, and treatment tools in melanoma, additional research is required to comprehensively delineate their utility. Future studies may aim to validate these biomarkers in larger patient cohorts, completely elucidate the roles of exosomal components in melanoma progression, and assess the feasibility of incorporating exosomal markers into existing diagnostic, prognostic, and therapeutic frameworks.
The complex effects of exosomal components in CM pathogenesis are presented in Figure 1.
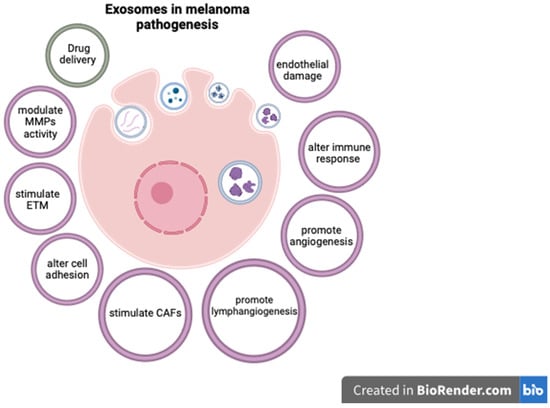
Figure 1.
The role of exosomal components in CM (created with Biorender, https://www.biorender.com, accessed on 29 October 2023).
4.3. Melanoma-Inhibiting Activity
Melanoma-inhibiting activity (MIA) is a soluble protein overexpressed in melanoma cells and actively secreted into the extracellular environment, where it binds to various extracellular and cell surface proteins [154]. This protein was first identified in supernatants of melanoma cell lines and, in vitro, it was considered to possess growth-inhibiting activities [155]. Despite its paradoxical nomenclature, in vivo, elevated levels of MIA have been substantiated to promote invasive capabilities, extravasation, and metastatic spread [156]. Recent studies on murine melanocytes demonstrated that MIA is involved in cellular senescence, and its knockdown enhances cell proliferation [157].
In a study encompassing 176 cutaneous melanoma patients, a progressive escalation in serum MIA levels was observed in correlation with advanced stages of the disease. Only 18.5% of patients in stage I displayed elevated MIA levels, as opposed to 59% in stage IV [156]. Alegre E. et al. found significantly increased MIA levels in patients with metastatic CM compared to disease-free patients or healthy individuals [158]. Similar results were reported by various other authors [159,160,161]. Furthermore, increased MIA levels are significantly associated with decreased survival [158,162]. Moreover, MIA levels have also been correlated with melanoma recurrence [163]. Compared to LDH, measuring MIA concentrations is a more accurate method for identifying patients with advanced disease and for predicting metastatic spread in CM [160]. In this context, as MIA concentrations are correlated with disease severity, this protein may become a prognostic biomarker in cutaneous melanomas. Furthermore, MIA could also become a therapeutic target itself, as Schmidt J. et al. demonstrated that inhibiting MIA dimerization resulted in the reduction of melanoma metastases in murine models [154].
It is, however, crucial to recognize that serum MIA levels are not exclusively elevated in melanoma. Elevations have also been documented in lung cancer [164], while immunohistochemical analysis demonstrated positive MIA expression in lung, esophageal, and cervical cancers [159].
5. Conclusions
Cutaneous malignant melanoma is a prevalent and highly aggressive form of skin cancer that requires improved prevention, diagnosis, and treatment methods. Recognizing risk factors such as UVR exposure, genetics, and family history is crucial for prevention.
The role of traditional biomarkers remains essential in the diagnosis and monitoring of melanoma. However, the field is rapidly evolving with the identification of emerging biomarkers. MicroRNA, exosomes, and MIA offer insights into melanoma pathogenesis and progression, potentially serving as both diagnostic and therapeutic targets. These emerging biomarkers could be the key to more personalized and effective treatments, ultimately improving survival rates and quality of life for patients.
Author Contributions
Conceptualization, L.M.G. and M.C.; methodology, M.C.; software, D.-A.Ț.; validation, M.C.; formal analysis, L.M.G.; investigation, L.M.G. and D.-A.Ț.; resources, M.C.; data curation, D.-A.Ț.; writing—original draft preparation, L.M.G.; writing—review and editing, D.-A.Ț.; visualization, L.M.G.; supervision, D.-A.Ț.; project administration, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; Mccubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Orzan, O.A.; Șandru, A.; Jecan, C.R. Controversies in the diagnosis and treatment of early cutaneous melanoma. J. Med. Life 2015, 8, 132–141. [Google Scholar] [PubMed]
- Caini, S.; Gandini, S.; Sera, F.; Raimondi, S.; Fargnoli, M.C.; Boniol, M.; Armstrong, B.K. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur. J. Cancer. 2009, 45, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The Pathogenesis and Clinical Management of Cutaneous Melanoma: An Evidence-Based Review. J. Med. Imaging Radiat. Sci. 2019, 50, 460–469.e1. [Google Scholar] [CrossRef] [PubMed]
- Rigel, D.S.; Carucci, J.A. Malignant melanoma: Prevention, early detection, and treatment in the 21st century. CA Cancer J. Clin. 2000, 50, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Țăpoi, D.A.; Derewicz, D.; Gheorghișan-Gălățeanu, A.-A.; Dumitru, A.V.; Ciongariu, A.M.; Costache, M. The Impact of Clinical and Histopathological Factors on Disease Progression and Survival in Thick Cutaneous Melanomas. Biomedicines 2023, 11, 2616. [Google Scholar] [CrossRef] [PubMed]
- Cust, A.E.; Mishra, K.; Berwick, M. Melanoma—Role of the environment and genetics. Photochem. Photobiol. Sci. 2018, 17, 1853–1860. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer. 2005, 41, 45–60. [Google Scholar] [CrossRef]
- de Gruijl, F.R. UV adaptation: Pigmentation and protection against overexposure. Exp. Dermatol. 2017, 26, 557–562. [Google Scholar] [CrossRef]
- Elder, D.E.; Barnhill, R.Y. (Eds.) Chapter III: Melanocytic neoplasms. In WHO Classification of Tumours Editorial Board. Skin Tumours, 5th ed.; Forthcoming; International Agency for Research on Cancer: Lyon, France, 2023; Volume 12, Available online: https://tumourclassification.iarc.who.int/chapters/64 (accessed on 27 August 2023).
- Ribeiro Moura Brasil Arnaut, J.; Dos Santos Guimarães, I.; Evangelista Dos Santos, A.C.; de Moraes Lino da Silva, F.; Machado, J.R.; de Melo, A.C. Molecular landscape of Hereditary Melanoma. Crit. Rev. Oncol. Hematol. 2021, 164, 103425. [Google Scholar] [CrossRef] [PubMed]
- Tímár, J.; Ladányi, A. Molecular Pathology of Skin Melanoma: Epidemiology, Differential Diagnostics, Prognosis and Therapy Prediction. Int. J. Mol. Sci. 2022, 23, 5384. [Google Scholar] [CrossRef] [PubMed]
- Cakir, A.; Elcin, G.; Kilickap, S.; Gököz, Ö.; Taskiran, Z.E.; Celik, İ. Phenotypic and Genetic Features that Differ Between Hereditary and Sporadic Melanoma: Results of a Preliminary Study from a Single Center from Turkey. Dermatol. Pract. Concept. 2023, 13, e2023146. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Țăpoi, D.A.; Gheorghișan-Gălățeanu, A.-A.; Dumitru, A.V.; Ciongariu, A.M.; Furtunescu, A.R.; Marin, A.; Costache, M. Primary Undifferentiated/Dedifferentiated Cutaneous Melanomas—A Review on Histological, Immunohistochemical, and Molecular Features with Emphasis on Prognosis and Treatment. Int. J. Mol. Sci. 2023, 24, 9985. [Google Scholar] [CrossRef]
- Chappell, W.H.; Steelman, L.S.; Long, J.M.; Kempf, R.C.; Abrams, S.L.; Franklin, R.A.; Bäsecke, J.; Stivala, F.; Donia, M.; Fagone, P.; et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: Rationale and importance to inhibiting these pathways in human health. Oncotarget 2011, 2, 135–164. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef]
- Krauthammer, M.; Kong, Y.; Ha, B.H.; Evans, P.; Bacchiocchi, A.; McCusker, J.P.; Cheng, E.; Davis, M.J.; Goh, G.; Choi, M.; et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012, 44, 1006–1014. [Google Scholar] [CrossRef]
- Wellbrock, C.; Karasarides, M.; Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell. Biol. 2004, 5, 875–885. [Google Scholar] [CrossRef]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Zavala-Pompa, A.; Sequeira, J.H.; Shoji, M.; Sexton, D.G.; Cotsonis, G.; Cerimele, F.; Govindarajan, B.; Macaron, N.; Arbiser, J.L. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 2002, 8, 3728–3733. [Google Scholar] [PubMed]
- Wang, Y.F.; Jiang, C.C.; Kiejda, K.A.; Gillespie, S.; Zhang, X.D.; Hersey, P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin. Cancer Res. 2007, 13, 4934–4942. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Long, G.V.; Kefford, R.F.; Rizos, H. Targeting oncogenic BRAF and aberrant MAPK activation in the treatment of cutaneous melanoma. Crit. Rev. Oncol. Hematol. 2015, 96, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A. The role of the PI3K-AKT pathway in melanoma. Cancer J. 2012, 18, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Davis, E.J.; Johnson, D.B.; Sosman, J.A.; Chandra, S. Melanoma: What do all the mutations mean? Cancer 2018, 124, 3490–3499. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Richtig, G.; Hoeller, C.; Kashofer, K.; Aigelsreiter, A.; Heinemann, A.; Kwong, L.N.; Pichler, M.; Richtig, E. Beyond the BRAFV600E hotspot: Biology and clinical implications of rare BRAF gene mutations in melanoma patients. Br. J. Dermatol. 2017, 177, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.H.; Aiba, S.; Bröcker, E.B.; LeBoit, P.E.; et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Jakob, J.A.; Bassett, R.L., Jr.; Ng, C.S.; Curry, J.L.; Joseph, R.W.; Alvarado, G.C.; Rohlfs, M.L.; Richard, J.; Gershenwald, J.E.; Kim, K.B.; et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012, 118, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Giehl, K. Oncogenic Ras in tumour progression and metastasis. Biol. Chem. 2005, 386, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, I.V.; Gibney, G.T.; Smalley, K.S. NRAS mutant melanoma: Biological behavior and future strategies for therapeutic management. Oncogene 2013, 32, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Maertens, O.; Johnson, B.; Hollstein, P.; Frederick, D.T.; Cooper, Z.A.; Messiaen, L.; Bronson, R.T.; McMahon, M.; Granter, S.; Flaherty, K.; et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 2013, 3, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.R.; Theurillat, J.P.; Van Allen, E.; Wagle, N.; Hsiao, J.; Cowley, G.S.; Schadendorf, D.; Root, D.E.; Garraway, L.A. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov. 2013, 3, 350–362. [Google Scholar] [CrossRef]
- Gibney, G.T.; Smalley, K.S. An unholy alliance: Cooperation between BRAF and NF1 in melanoma development and BRAF inhibitor resistance. Cancer Discov. 2013, 3, 260–263. [Google Scholar] [CrossRef]
- Nissan, M.H.; Pratilas, C.A.; Jones, A.M.; Ramirez, R.; Won, H.; Liu, C.; Tiwari, S.; Kong, L.; Hanrahan, A.J.; Yao, Z.; et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014, 74, 2340–2350. [Google Scholar] [CrossRef]
- Thielmann, C.M.; Chorti, E.; Matull, J.; Murali, R.; Zaremba, A.; Lodde, G.; Jansen, P.; Richter, L.; Kretz, J.; Möller, I.; et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur. J. Cancer 2021, 159, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F., 3rd; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef] [PubMed]
- Handolias, D.; Salemi, R.; Murray, W.; Tan, A.; Liu, W.; Viros, A.; Dobrovic, A.; Kelly, J.; McArthur, G.A. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment. Cell Melanoma Res. 2010, 23, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Loras, A.; Gil-Barrachina, M.; Marqués-Torrejón, M.Á.; Perez-Pastor, G.; Martinez-Cadenas, C. UV-Induced Somatic Mutations Driving Clonal Evolution in Healthy Skin, Nevus, and Cutaneous Melanoma. Life 2022, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, W.; Mwamba, R.N.; Grullon, K.; Armstrong, M.; Zhao, P.; Hendren-Santiago, B.; Qin, K.H.; Li, A.J.; Hu, D.A.; Youssef, A.; et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022, 9, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Precazzini, F.; Hanžić, N.; Radić, M.; Gavioli, N.; Vlašić, I.; Ozretić, P.; Pinto, L.; Škreblin, L.; Barban, G.; et al. Altered Expression of Shorter p53 Family Isoforms Can Impact Melanoma Aggressiveness. Cancers 2021, 13, 5231. [Google Scholar] [CrossRef]
- Loureiro, J.B.; Raimundo, L.; Calheiros, J.; Carvalho, C.; Barcherini, V.; Lima, N.R.; Gomes, C.; Almeida, M.I.; Alves, M.G.; Costa, J.L.; et al. Targeting p53 for Melanoma Treatment: Counteracting Tumour Proliferation, Dissemination and Therapeutic Resistance. Cancers 2021, 13, 1648. [Google Scholar] [CrossRef]
- Wu, H.; Goel, V.; Haluska, F.G. PTEN signaling pathways in melanoma. Oncogene 2003, 22, 3113–3122. [Google Scholar] [CrossRef]
- Tsao, H.; Goel, V.; Wu, H.; Yang, G.; Haluska, F.G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Investig. Dermatol. 2004, 122, 337–341. [Google Scholar] [CrossRef]
- Stahl, J.M.; Cheung, M.; Sharma, A.; Trivedi, N.R.; Shanmugam, S.; Robertson, G.P. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003, 63, 2881–2890. [Google Scholar] [PubMed]
- Nogueira, C.; Kim, K.H.; Sung, H.; Paraiso, K.H.; Dannenberg, J.H.; Bosenberg, M.; Chin, L.; Kim, M. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene 2010, 29, 6222–6232. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hugo, W.; Kong, X.; Hong, A.; Koya, R.C.; Moriceau, G.; Chodon, T.; Guo, R.; Johnson, D.B.; Dahlman, K.B.; et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014, 4, 80–93. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Kempf, C.R.; Chappell, W.H.; Abrams, S.L.; Stivala, F.; Malaponte, G.; Nicoletti, F.; Libra, M.; Bäsecke, J.; et al. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J. Cell. Physiol. 2011, 226, 2762–2781. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, R.C.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging 2011, 3, 192–1222. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Franklin, R.A.; Montalto, G.; Cervello, M.; Libra, M.; Candido, S.; Malaponte, G.; et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: How mutations can result in therapy resistance and how to overcome resistance. Oncotarget 2012, 3, 1068–1111. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.L.; Glass, L.F.; Cruse, C.W.; Berman, C.; Ku, N.K.; Reintgen, D.S. Pathologic examination of the sentinel lymph node in malignant melanoma. Am. J. Surg. Pathol. 1999, 23, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.T.; Martini, C.; Brooks, T.; Prabhakaran, S.; Hopkins, A.M.; Ung, B.S.-Y.; Tang, J.; Caruso, M.C.; Brooks, R.D.; Johnson, I.R.D.; et al. Insights into Melanoma Clinical Practice: A Perspective for Future Research. Cancers 2023, 15, 4631. [Google Scholar] [CrossRef]
- Saleem, A.; Narala, S.; Raghavan, S.S. Immunohistochemistry in melanocytic lesions: Updates with a practical review for pathologists. Semin. Diagn. Pathol. 2022, 39, 239–247. [Google Scholar] [CrossRef]
- Marques, P.C.; Diniz, L.M.; Spelta, K.; Nogueira, P.S.E. Desmoplastic melanoma: A rare variant with challenging diagnosis. An. Bras. Dermatol. 2019, 94, 82–85. [Google Scholar] [CrossRef]
- Cochran, A.J.; Wen, D.R.; Morton, D.L. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am. J. Surg. Pathol. 1988, 12, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Sánchez, A. Recalcitrant primary cutaneous Rosai-Dorfman disease. Efficacy of sirolimus and intralesional methylprednisolone. Skin. Health Dis. 2023, 3, e273. [Google Scholar] [CrossRef] [PubMed]
- Aisner, D.L.; Maker, A.; Rosenberg, S.A.; Berman, D.M. Loss of S100 antigenicity in metastatic melanoma. Hum. Pathol. 2005, 36, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, Y.; Li, F.; Wang, J.; Mu, Y.; Mei, X.; Li, X.; Zhu, W.; Jin, X.; Yu, K. Expression of microphthalmia transcription factor, S100 protein, and HMB-45 in malignant melanoma and pigmented nevi. Biomed. Rep. 2016, 5, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.M.; Monea, M.; Olah, P.; Moraru, R.; Cotoi, O.S. The Importance of Immunohistochemistry in the Evaluation of Tumor Depth of Primary Cutaneous Melanoma. Diagnostics 2023, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Dass, S.E.; Huizenga, T.; Farshchian, M.; Mehregan, D.R. Comparison of SOX-10, HMB-45, and Melan-A in Benign Melanocytic Lesions. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.A. HMB45 protein expression and the immunohistochemical maturation in common blue nevi: A reappraisal. An. Bras. Dermatol. 2022, 97, 387–390. [Google Scholar] [CrossRef]
- Zand, S.; Buzney, E.; Duncan, L.M.; Dadras, S.S. Heterogeneity of Metastatic Melanoma: Correlation of MITF With Its Transcriptional Targets MLSN1, PEDF, HMB-45, and MART-1. Am. J. Clin. Pathol. 2016, 146, 353–360. [Google Scholar] [CrossRef][Green Version]
- Zubovits, J.; Buzney, E.; Yu, L.; Duncan, L.M. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum. Pathol. 2004, 35, 217–223. [Google Scholar] [CrossRef]
- Drabeni, M.; Lopez-Vilaró, L.; Barranco, C.; Trevisan, G.; Gallardo, F.; Pujol, R.M. Differences in tumor thickness between hematoxylin and eosin and Melan-A immunohistochemically stained primary cutaneous melanomas. Am. J. Dermatopathol. 2013, 35, 56–63. [Google Scholar] [CrossRef]
- Megahed, M.; Schön, M.; Selimovic, D.; Schön, M.P. Reliability of diagnosis of melanoma in situ. Lancet 2002, 359, 1921–1922. [Google Scholar] [CrossRef] [PubMed]
- Panse, G.; McNiff, J.M. Lichenoid dermatoses with pseudomelanocytic nests vs inflamed melanoma in situ: A comparative study. J. Cutan. Pathol. 2021, 48, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Argraves, M.; Kristjansson, A.; Ferenczi, K.; Dadras, S.S. A quantitative comparison between SOX10 and MART-1 immunostaining to detect melanocytic hyperplasia in chronically sun-damaged skin. J. Cutan. Pathol. 2018, 45, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Taube, J.M.; McCalmont, T.H.; Glusac, E.J. Quantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situ. J. Cutan. Pathol. 2011, 38, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Stoehr, R.; Hornung, A.; Popp, J.; Erdmann, M.; Heinzerling, L.; Hartmann, A. Dedifferentiated and Undifferentiated Melanomas: Report of 35 New Cases With Literature Review and Proposal of Diagnostic Criteria. Am. J. Surg. Pathol. 2021, 45, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Luzar, B.; Billings, S.D.; de la Fouchardiere, A.; Pissaloux, D.; Alberti, L.; Calonje, E. Compound Clear Cell Sarcoma of the Skin-A Potential Diagnostic Pitfall: Report of a Series of 4 New Cases and a Review of the Literature. Am. J. Surg. Pathol. 2020, 44, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Westover, C.; Bacchi, C.; Gru, A.A. Clear Cell Sarcoma with Cutaneous Presentation in a 4-Year-Old Boy. Am. J. Dermatopathol. 2020, 42, e131–e133. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Lospalluti, L.; Pacello, L.; Lettini, T.; Arezzo, F.; Loizzi, V.; Lupo, C.; Casatta, N.; Cormio, G.; et al. Primitive Cutaneous (P)erivascular (E)pithelioid (C)ell Tumour (PEComa): A New Case Report of a Rare Cutaneous Tumor. Genes 2022, 13, 1153. [Google Scholar] [CrossRef]
- Gaspard, M.; Lamant, L.; Tournier, E.; Valentin, T.; Rochaix, P.; Terrier, P.; Ranchere-Vince, D.; Coindre, J.M.; Filleron, T.; Le Guellec, S. Evaluation of eight melanocytic and neural crest-associated markers in a well-characterised series of 124 malignant peripheral nerve sheath tumours (MPNST): Useful to distinguish MPNST from melanoma? Histopathology 2018, 73, 969–982. [Google Scholar] [CrossRef]
- Mackie, R.M.; Campbell, I.; Turbitt, M.L. Use of NK1 C3 monoclonal antibody in the assessment of benign and malignant melanocytic lesions. J. Clin. Pathol. 1984, 37, 367–372. [Google Scholar] [CrossRef]
- Sulaimon, S.; Kitchell, B.; Ehrhart, E. Immunohistochemical detection of melanoma-specific antigens in spontaneous canine melanoma. J. Comp. Pathol. 2002, 127, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Orchard, G.; Wilson Jones, E. Immunocytochemistry in the diagnosis of malignant melanoma. Br. J. Biomed. Sci. 1994, 51, 44–56. [Google Scholar] [PubMed]
- Ramachandra, S.; Gillett, C.E.; Millis, R.R. A comparative immunohistochemical study of mammary and extramammary Paget’s disease and superficial spreading melanoma, with particular emphasis on melanocytic markers. Virchows Arch. 1996, 429, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Flotte, T.J.; Tanabe, K.K.; Gadd, M.A.; Cosimi, A.B.; Sober, A.J.; Mihm, M.C., Jr.; Duncan, L.M. Detection of microscopic melanoma metastases in sentinel lymph nodes. Cancer 1999, 86, 617–627. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME Expression in Melanocytic Tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Pulitzer, M.; Moy, A.P.; Hollmann, T.J.; Jungbluth, A.A.; Busam, K.J. Immunohistochemistry for PRAME in the Distinction of Nodal Nevi From Metastatic Melanoma. Am. J. Surg. Pathol. 2020, 44, 503–508. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Busam, K.J. PRAME Immunohistochemistry as an Ancillary Test for the Assessment of Melanocytic Lesions. Surg. Pathol. Clin. 2021, 14, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.F.; Panse, G.; McNiff, J.M. PRAME immunohistochemistry can distinguish melanocytic pseudonests of lichenoid reactions from melanoma in situ. J. Cutan. Pathol. 2023, 50, 450–454. [Google Scholar] [CrossRef]
- Hrycaj, S.M.; Szczepanski, J.M.; Zhao, L.; Siddiqui, J.; Thomas, D.G.; Lucas, D.R.; Patel, R.M.; Harms, P.W.; Bresler, S.C.; Chan, M.P. PRAME expression in spindle cell melanoma, malignant peripheral nerve sheath tumour, and other cutaneous sarcomatoid neoplasms: A comparative analysis. Histopathology 2022, 81, 818–825. [Google Scholar] [CrossRef]
- Kline, N.; Menge, T.D.; Hrycaj, S.M.; Andea, A.A.; Patel, R.M.; Harms, P.W.; Chan, M.P.; Bresler, S.C. PRAME Expression in Challenging Dermal Melanocytic Neoplasms and Soft Tissue Tumors with Melanocytic Differentiation. Am. J. Dermatopathol. 2022, 44, 404–410. [Google Scholar] [CrossRef]
- Clarke, L.E.; Flake, D.D., 2nd; Busam, K.; Cockerell, C.; Helm, K.; McNiff, J.; Reed, J.; Tschen, J.; Kim, J.; Barnhill, R.; et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer 2017, 123, 617–628. [Google Scholar] [CrossRef]
- Ko, J.S.; Matharoo-Ball, B.; Billings, S.D.; Thomson, B.J.; Tang, J.Y.; Sarin, K.Y.; Cai, E.; Kim, J.; Rock, C.; Kimbrell, H.Z.; et al. Diagnostic Distinction of Malignant Melanoma and Benign Nevi by a Gene Expression Signature and Correlation to Clinical Outcomes. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.K.; Jansen, B.; Ho, J.; Busam, K.J.; Gross, K.; Hansen, D.D.; Alsobrook, J.P., 2nd; Yao, Z.; Peck, G.L.; Gerami, P. Utility of a Noninvasive 2-Gene Molecular Assay for Cutaneous Melanoma and Effect on the Decision to Biopsy. JAMA Dermatol. 2017, 153, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.A.; Toland, A.E.; Scharschmidt, T.J.; Mayerson, J.L.; Guttridge, D.C.; Iwenofu, O.H. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod. Pathol. 2014, 27, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Iura, K.; Kohashi, K.; Hotokebuchi, Y.; Ishii, T.; Maekawa, A.; Yamada, Y.; Yamamoto, H.; Iwamoto, Y.; Oda, Y. Cancer-testis antigens PRAME and NY-ESO-1 correlate with tumour grade and poor prognosis in myxoid liposarcoma. J. Pathol. Clin. Res. 2015, 1, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Iura, K.; Maekawa, A.; Kohashi, K.; Ishii, T.; Bekki, H.; Otsuka, H.; Yamada, Y.; Yamamoto, H.; Harimaya, K.; Iwamoto, Y.; et al. Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum. Pathol. 2017, 61, 130–139. [Google Scholar] [CrossRef]
- Cammareri, C.; Beltzung, F.; Michal, M.; Vanhersecke, L.; Coindre, J.M.; Velasco, V.; Le Loarer, F.; Vergier, B.; Perret, R. PRAME immunohistochemistry in soft tissue tumors and mimics: A study of 350 cases highlighting its imperfect specificity but potentially useful diagnostic applications. Virchows Arch. 2023, 483, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zou, R.; Wang, J.; Wang, Z.W.; Zhu, X. The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer. Cell Prolif. 2020, 53, e12770. [Google Scholar] [CrossRef]
- Yakout, N.M.; Abdallah, D.M.; Abdelmonsif, D.A.; Kholosy, H.M.; Talaat, I.M.; Elsakka, O. BRAFV600E mutational status assessment in cutaneous melanocytic neoplasms in a group of the Egyptian population. Cancer Cell. Int. 2023, 23, 17. [Google Scholar] [CrossRef]
- Maksimaityte, V.; Reivytyte, R.; Milaknyte, G.; Mickys, U.; Razanskiene, G.; Stundys, D.; Kazenaite, E.; Valantinas, J.; Stundiene, I. Metastatic multifocal melanoma of multiple organ systems: A case report. World J. Clin. Cases 2022, 10, 10136–10145. [Google Scholar] [CrossRef]
- Rothrock, A.T.; Hameed, N.; Cho, W.C.; Nagarajan, P.; Ivan, D.; Torres-Cabala, C.A.; Prieto, V.G.; Curry, J.L.; Aung, P.P. BRAF V600E immunohistochemistry as a useful tool in the diagnosis of melanomas with ambiguous morphologies and immunophenotypes. J. Cutan. Pathol. 2023, 50, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Orchard, G.E.; Wojcik, K.; Rickaby, W.; Martin, B.; Semkova, K.; Shams, F.; Stefanato, C.M. Immunohistochemical detection of V600E BRAF mutation is a useful primary screening tool for malignant melanoma. Br. J. Biomed. Sci. 2019, 76, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Saliba, E.; Bhawan, J. Aberrant Expression of Immunohistochemical Markers in Malignant Melanoma: A Review. Dermatopathology 2021, 8, 359–370. [Google Scholar] [CrossRef]
- Ferreira, I.; Arends, M.J.; van der Weyden, L.; Adams, D.J.; Brenn, T. Primary de-differentiated, trans-differentiated and undifferentiated melanomas: Overview of the clinicopathological, immunohistochemical and molecular spectrum. Histopathology 2022, 80, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Han, L.M.; Lee, K.W.; Uludag, G.; Seider, M.I.; Afshar, A.R.; Bloomer, M.M.; Pekmezci, M. Prognostic Value of BAP1 and Preferentially Expressed Antigen in Melanoma (PRAME) Immunohistochemistry in Uveal Melanomas. Mod. Pathol. 2023, 36, 100081. [Google Scholar] [CrossRef]
- Parra, O.; Ma, W.; Li, Z.; Coffing, B.N.; Linos, K.; LeBlanc, R.E.; Momtahen, S.; Sriharan, A.; Cloutier, J.M.; Wells, W.A.; et al. PRAME expression in cutaneous melanoma does not correlate with disease-specific survival. J. Cutan. Pathol. 2023, 50, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, M.; Hahn, M.; Metzler, G.; Bauer, J.; Yazdi, A.S.; Keim, U.; Garbe, C.; Wagner, N.B.; Forchhammer, S. Diffuse PRAME Expression Is Highly Specific for Thin Melanomas in the Distinction from Severely Dysplastic Nevi but Does Not Distinguish Metastasizing from Non-Metastasizing Thin Melanomas. Cancers 2021, 13, 3864. [Google Scholar] [CrossRef]
- Du, Y.; Li, C.; Mao, L.; Wei, X.; Bai, X.; Chi, Z.; Cui, C.; Sheng, X.; Lian, B.; Tang, B.; et al. A nomogram incorporating Ki67 to predict survival of acral melanoma. J. Cancer Res. Clin. Oncol. 2023, 20, 13077–13085. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Z.; Shen, L.; Shen, L. Prognostic and Clinicopathological Value of Ki-67 in Melanoma: A Meta-Analysis. Front. Oncol. 2021, 11, 737760. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. MicroRNA Signature in Melanoma: Biomarkers and Therapeutic Targets. Front. Oncol. 2021, 11, 608987. [Google Scholar] [CrossRef]
- Poniewierska-Baran, A.; Słuczanowska-Głąbowska, S.; Małkowska, P.; Sierawska, O.; Zadroga, Ł.; Pawlik, A.; Niedźwiedzka-Rystwej, P. Role of miRNA in Melanoma Development and Progression. Int. J. Mol. Sci. 2023, 24, 201. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.E.; Bemis, L.; Norris, D.A.; Shellman, Y.G. miR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma. Physiol. Genom. 2013, 45, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, M.; Benito-Jardón, L.; Alonso, L.; Koetz-Ploch, L.; Hernando, E.; Teixidó, J. miR-204-5p and miR-211-5p Contribute to BRAF Inhibitor Resistance in Melanoma. Cancer Res. 2018, 78, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Motti, M.L.; Minopoli, M.; Di Carluccio, G.; Ascierto, P.A.; Carriero, M.V. MicroRNAs as Key Players in Melanoma Cell Resistance to MAPK and Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 4544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, H.; Gao, Y.; Zhang, W. Secretory miRNAs as novel cancer biomarkers. Biochim. Biophys. Acta 2012, 1826, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Wang, C.; Deng, T.; Liang, H.; Wang, Y.; Huang, D.; Fan, Q.; Wang, X.; Ning, T.; et al. Serum miRNA expression profile as a prognostic biomarker of stage II/III colorectal adenocarcinoma. Sci. Rep. 2015, 5, 12921. [Google Scholar] [CrossRef] [PubMed]
- Hanniford, D.; Zhong, J.; Koetz, L.; Gaziel-Sovran, A.; Lackaye, D.J.; Shang, S.; Pavlick, A.; Shapiro, R.; Berman, R.; Darvishian, F.; et al. A miRNA-Based Signature Detected in Primary Melanoma Tissue Predicts Development of Brain Metastasis. Clin. Cancer Res. 2015, 21, 4903–4912. [Google Scholar] [CrossRef]
- Stark, M.S.; Klein, K.; Weide, B.; Haydu, L.E.; Pflugfelder, A.; Tang, Y.H.; Palmer, J.M.; Whiteman, D.C.; Scolyer, R.A.; Mann, G.J.; et al. The Prognostic and Predictive Value of Melanoma-related MicroRNAs Using Tissue and Serum: A MicroRNA Expression Analysis. EBioMedicine 2015, 2, 671–680. [Google Scholar] [CrossRef]
- Antonova, E.; Hambikova, A.; Shcherbakov, D.; Sukhov, V.; Vysochanskaya, S.; Fadeeva, I.; Gorshenin, D.; Sidorova, E.; Kashutina, M.; Zhdanova, A.; et al. Determination of Common microRNA Biomarker Candidates in Stage IV Melanoma Patients and a Human Melanoma Cell Line: A Potential Anti-Melanoma Agent Screening Model. Int. J. Mol. Sci. 2023, 24, 9160. [Google Scholar] [CrossRef]
- Vitiello, M.; D’Aurizio, R.; Poliseno, L. Biological role of miR-204 and miR-211 in melanoma. Oncoscience 2018, 5, 248–251. [Google Scholar] [CrossRef][Green Version]
- Varrone, F.; Caputo, E. The miRNAs Role in Melanoma and in Its Resistance to Therapy. Int. J. Mol. Sci. 2020, 21, 878. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.-Y.; Li, P.; He, Q.-Y.; Luo, C.-Q. Circulating miR-221 Expression Level and Prognosis of Cutaneous Malignant Melanoma. Experiment 2014, 20, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Rigg, E.; Wang, J.; Xue, Z.; Lunavat, T.R.; Liu, G.; Hoang, T.; Parajuli, H.; Han, M.; Bjerkvig, R.; Nazarov, P.V.; et al. Inhibition of extracellular vesicle-derived miR-146a-5p decreases progression of melanoma brain metastasis via Notch pathway dysregulation in astrocytes. J. Extracell. Vesicles 2023, 12, e12363. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.E.; Khaled, M.; Netanely, D.; Schubert, S.; Golan, T.; Buxbaum, A.; Janas, M.M.; Postolsky, B.; Goldberg, M.S.; Shamir, R.; et al. Transcription factor/microRNA axis blocks melanoma invasion program by miR-211 targeting NUAK1. J. Investig. Dermatol. 2014, 134, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Li, R.; Liu, L.; Ni, X.; Shi, Y.; Xia, Y.; Wang, J.; Lu, F.; Xu, B. Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR-152-3p. Oncotarget 2017, 8, 85401–85414. [Google Scholar] [CrossRef] [PubMed]
- Rang, Z.; Yang, G.; Wang, Y.W.; Cui, F. miR-542-3p suppresses invasion and metastasis by targeting the proto-oncogene serine/threonine protein kinase, PIM1, in melanoma. Biochem. Biophys. Res. Commun. 2016, 474, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Nabipoorashrafi, S.A.; Shomali, N.; Sadat-Hatamnezhad, L.; Mahami-Oskouei, M.; Mahmoudi, J.; Shotorbani, B.S.; Akbari, M.; Xu, H. miR-143 acts as an inhibitor of migration and proliferation as well as an inducer of apoptosis in melanoma cancer cells in vitro. IUBMB Life 2020, 72, 2034–2044. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, H.; Lu, F.; Peng, H.; Luan, W. miR-224-5p acts as a tumour suppressor and reverses the resistance to BRAF Inhibitor In melanoma through directly targeting PAK4 to block the MAPK pathway. Pathol. Res. Pract. 2023, 249, 154772. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Sun, Y.; Zhang, Y.; Dong, L.; Shen, C.; Yang, L.; Yang, M.; Li, Y.; Shen, G.; et al. miR-7 reverses the resistance to BRAFi in melanoma by targeting EGFR/IGF-1R/CRAF and inhibiting the MAPK and PI3K/AKT signaling pathways. Oncotarget 2016, 7, 53558–53570. [Google Scholar] [CrossRef]
- Caporali, S.; Amaro, A.; Levati, L.; Alvino, E.; Lacal, P.M.; Mastroeni, S.; Ruffini, F.; Bonmassar, L.; Antonini Cappellini, G.C.; Felli, N.; et al. miR-126-3p down-regulation contributes to dabrafenib acquired resistance in melanoma by up-regulating ADAM9 and VEGF-A. J. Exp. Clin. Cancer Res. 2019, 38, 272. [Google Scholar] [CrossRef]
- Diaz-Martinez, M.; Benito-Jardon, L.; Teixido, J. New insights in melanoma resistance to BRAF inhibitors: A role for microRNAs. Oncotarget 2018, 9, 35374–35375. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, Y.; Liu, Y.; Zhang, X.; Li, F.; Li, L.; Wang, J. The miR-31-SOX10 axis regulates tumor growth and chemotherapy resistance of melanoma via PI3K/AKT pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Jankowska, U.; Wilczak, M.; Przybyło, M. Similarities and Differences in the Protein Composition of Cutaneous Melanoma Cells and Their Exosomes Identified by Mass Spectrometry. Cancers 2023, 15, 1097. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Immunosuppressive functions of melanoma cell-derived exosomes in plasma of melanoma patients. Front. Cell Dev. Biol. 2023, 10, 1080925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, P.; Shi, J.; Kou, D.; Bai, X. Exosome-delivered circRPS5 inhibits the progression of melanoma via regulating the miR-151a/NPTX1 axis. PLoS ONE 2023, 18, e0287347. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, M.; Liao, L.; Gao, S.; Wang, Y. Plasma exosome-derived connexin43 as a promising biomarker for melanoma patients. BMC Cancer 2023, 23, 242. [Google Scholar] [CrossRef] [PubMed]
- Boussadia, Z.; Lamberti, J.; Mattei, F.; Pizzi, E.; Puglisi, R.; Zanetti, C.; Pasquini, L.; Fratini, F.; Fantozzi, L.; Felicetti, F.; et al. Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. J. Exp. Clin. Cancer Res. 2018, 37, 245. [Google Scholar] [CrossRef]
- Surman, M.; Kędracka-Krok, S.; Hoja-Łukowicz, D.; Jankowska, U.; Drożdż, A.; Stępień, E.Ł.; Przybyło, M. Mass Spectrometry-Based Proteomic Characterization of Cutaneous Melanoma Ectosomes Reveals the Presence of Cancer-Related Molecules. Int. J. Mol. Sci. 2020, 21, 2934. [Google Scholar] [CrossRef]
- Lattmann, E.; Levesque, M.P. The Role of Extracellular Vesicles in Melanoma Progression. Cancers 2022, 14, 3086. [Google Scholar] [CrossRef]
- Strnadová, K.; Pfeiferová, L.; Přikryl, P.; Dvořánková, B.; Vlčák, E.; Frýdlová, J.; Vokurka, M.; Novotný, J.; Šáchová, J.; Hradilová, M.; et al. Exosomes produced by melanoma cells significantly influence the biological properties of normal and cancer-associated fibroblasts. Histochem. Cell Biol. 2022, 157, 153–172. [Google Scholar] [CrossRef]
- Xiao, D.; Barry, S.; Kmetz, D.; Egger, M.; Pan, J.; Rai, S.N.; Qu, J.; McMasters, K.M.; Hao, H. Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. 2016, 376, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, A.; Laurenzana, A.; Menicacci, B.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Guasti, D.; Paoli, P.; Serratì, S.; Mocali, A.; et al. uPAR-expressing melanoma exosomes promote angiogenesis by VE-Cadherin, EGFR and uPAR overexpression and rise of ERK1,2 signaling in endothelial cells. Cell Mol. Life Sci. 2021, 78, 3057–3072. [Google Scholar] [CrossRef] [PubMed]
- García-Silva, S.; Benito-Martín, A.; Nogués, L.; Hernández-Barranco, A.; Mazariegos, M.S.; Santos, V.; Hergueta-Redondo, M.; Ximénez-Embún, P.; Kataru, R.P.; Lopez, A.A.; et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat. Cancer 2021, 2, 1387–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, Y.; Chen, W.; Zhang, M.; Qin, J. Malignant melanoma-derived exosomes induce endothelial damage and glial activation on a human BBB chip model. Biosensors 2022, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, Z.; Li, Y.; Li, Y.; Zhang, Y.; Gui, R.; Cui, Y.; Zhang, Q.; Qian, L.; Xiong, Y.; et al. Exosome-Derived microRNA: Implications in Melanoma Progression, Diagnosis and Treatment. Cancers 2023, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tang, F.; Li, J.; Yu, H.; Wu, M.; Wu, Y.; Zeng, H.; Hou, K.; Zhang, Q. Tumor-derived exosomes: The emerging orchestrators in melanoma. Biomed. Pharmacother. 2022, 149, 112832. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, D.; Lützkendorf, J.; Moritz, R.K.C.; Wersig, T.; Mäder, K.; Müller, L.P.; Sunderkötter, C. Melanoma-Derived Exosomal miR-125b-5p Educates Tumor Associated Macrophages (TAMs) by Targeting Lysosomal Acid Lipase A (LIPA). Cancers 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, L.; Jiang, Y.; Qian, Q. TIM-3 shuttled by MV3 cells-secreted exosomes inhibits CD4+ T cell immune function and induces macrophage M2 polarization to promote the growth and metastasis of melanoma cells. Transl. Oncol. 2022, 18, 101334. [Google Scholar] [CrossRef]
- Zhou, Q.; Fang, T.; Wei, S.; Chai, S.; Yang, H.; Tao, M.; Cao, Y. Macrophages in melanoma: A double-edged sword and targeted therapy strategies (Review). Exp. Ther. Med. 2022, 24, 640. [Google Scholar] [CrossRef]
- Gu, Y.; Du, Y.; Jiang, L.; Tang, X.; Li, A.; Zhao, Y.; Lang, Y.; Liu, X.; Liu, J. αvβ3 integrin-specific exosomes engineered with cyclopeptide for targeted delivery of triptolide against malignant melanoma. J. Nanobiotechnol. 2022, 20, 384. [Google Scholar] [CrossRef]
- Gao, H.; Lao, Y.; Zhang, J.; Ding, B. Dendritic Cell-Derived Exosomes Driven Drug Co-Delivery Biomimetic Nanosystem for Effective Combination of Malignant Melanoma Immunotherapy and Gene Therapy. Drug Des. Devel. Ther. 2023, 17, 2087–2106. [Google Scholar] [CrossRef]
- Naeem, P.; Baumgartner, A.; Ghaderi, N.; Sefat, F.; Alhawamdeh, M.; Heidari, S.; Shahzad, F.; Swaminathan, K.; Akhbari, P.; Isreb, M.; et al. Anticarcinogenic impact of extracellular vesicles (exosomes) from cord blood stem cells in malignant melanoma: A potential biological treatment. J. Cell. Mol. Med. 2023, 27, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Riechers, A.; Stoll, R.; Amann, T.; Fink, F.; Spruss, T.; Gronwald, W.; König, B.; Hellerbrand, C.; Bosserhoff, A.K. Targeting melanoma metastasis and immunosuppression with a new mode of melanoma inhibitory activity (MIA) protein inhibition. PLoS ONE 2012, 7, 37941. [Google Scholar] [CrossRef] [PubMed]
- Bogdahn, U.; Apfel, R.; Hahn, M.; Gerlach, M.; Behl, C.; Hoppe, J.; Martin, R. Autocrine tumor cell growth-inhibiting activities from human malignant melanoma. Cancer Res. 1989, 49, 5358–5363. [Google Scholar] [PubMed]
- Bolovan, L.M.; Ceausu, M.; Stanciu, A.E.; Panait, M.E.; Busca, A.; Hotnog, C.M.; Bleotu, C.; Gales, L.N.; Georgescu, M.T.; Prunoiu, V.M.; et al. Correlation Studies between S100 Protein Level and Soluble MIA or Tissue MelanA and gp100 (HMB45) Expression in Cutaneous Melanoma. J. Pers. Med. 2023, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, L.; Lamm, S.; Henz, I.; Kappelmann-Fenzl, M.; Haferkamp, S.; Meierjohann, S.; Hellerbrand, C.; Kuphal, S.; Bosserhoff, A.K. Role of melanoma inhibitory activity in melanocyte senescence. Pigment. Cell Melanoma Res. 2019, 32, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Alegre, E.; Zubiri, L.; Perez-Gracia, J.L.; González-Cao, M.; Soria, L.; Martín-Algarra, S.; González, A. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin. Chim. Acta 2016, 454, 28–32. [Google Scholar] [CrossRef]
- Sasahira, T.; Kirita, T.; Nishiguchi, Y.; Kurihara, M.; Nakashima, C.; Bosserhoff, A.K.; Kuniyasu, H. A comprehensive expression analysis of the MIA gene family in malignancies: MIA gene family members are novel, useful markers of esophageal, lung, and cervical squamous cell carcinoma. Oncotarget 2016, 7, 31137–31152. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Jiang, L.; Xu, J.; Ren, A.; Lin, Y.; Yao, G. The value of melanoma inhibitory activity and LDH with melanoma patients in a Chinese population. Medicine 2021, 100, e24840. [Google Scholar] [CrossRef]
- Odashiro, M.; Hans Filho, G.; Pereira, P.R.; Castro, A.R.; Stief, A.C.; Pontes, E.R.; Odashiro, A.N. Melanoma inhibitory activity in Brazilian patients with cutaneous melanoma. An. Bras. Dermatol. 2015, 90, 327–332. [Google Scholar] [CrossRef]
- Fan, S.; Liu, X.; Wu, Y.; Li, K.; Zhao, X.; Lin, W.; Liu, J. Prognostic Value of Lactate Dehydrogenase, Melanoma Inhibitory Protein, and S-100B Protein in Patients with Malignant Melanoma. Evid. Based Complement Altern. Med. 2022, 2022, 9086540. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Gupta, R.K.; Ye, X.; Hsueh, E.C.; Morton, D.L. Melanoma-inhibiting activity assay predicts survival in patients receiving a therapeutic cancer vaccine after complete resection of American Joint Committee on Cancer Stage III Melanoma. Ann. Surg. Oncol. 2004, 11, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.H.; Li, D.; Xie, Z.H.; Shen, Q.B. The clinical significance of MIA gene in tumorigenesis of lung cancer. Neoplasma 2020, 67, 660–667. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).