Abstract
The circadian rhythm, which is necessary for reproduction, is controlled by clock genes. In the mouse uterus, the oscillation of the circadian clock gene has been observed. The transcription of the core clock gene period (Per) and cryptochrome (Cry) is activated by the heterodimer of the transcription factor circadian locomotor output cycles kaput (Clock) and brain and muscle Arnt-like protein-1 (Bmal1). By binding to E-box sequences in the promoters of Per1/2 and Cry1/2 genes, the CLOCK-BMAL1 heterodimer promotes the transcription of these genes. Per1/2 and Cry1/2 form a complex with the Clock/Bmal1 heterodimer and inactivate its transcriptional activities. Endometrial BMAL1 expression levels are lower in human recurrent-miscarriage sufferers. Additionally, it was shown that the presence of BMAL1-depleted decidual cells prevents trophoblast invasion, highlighting the importance of the endometrial clock throughout pregnancy. It is widely known that hormone synthesis is disturbed and sterility develops in Bmal1-deficient mice. Recently, we discovered that animals with uterus-specific Bmal1 loss also had poor placental development, and these mice also had intrauterine fetal death. Furthermore, it was shown that time-restricted feeding controlled the uterine clock’s circadian rhythm. The uterine clock system may be a possibility for pregnancy complications, according to these results. We summarize the most recent research on the close connection between the circadian clock and reproduction in this review.
1. Introduction
In all mammalian species, including humans, circadian timing is essential for successful female reproduction. For instance, women who have irregular sleep or work schedules have lower fertility and higher chances of miscarriage [1]. Similar to humans, rats exhibit substantial anomalies in ovulation, fertility, and sexual drive as a result of changes to circadian rhythm [2,3]. Infertility/reproductive disorders are one of the main physiological features of animals with clock mutations. Arnt-like protein-1 (Bmal1) knockout in arginine vasopressin (AVP) neurons, kisspeptin neurons, GnRH neurons, or the whole body disrupts the timing and pattern of LH secretion, suggesting that the circadian clock system may integrate the HPG axis [4,5]. Circadian rhythms have been reported for the HPG axis in mice, rats, and humans [3,6,7,8]. In this context, clock genes are also expressed within the reproductive organs [9,10], and the rhythmic expression of Bmal1, circadian locomotor output cycles kaput (Clock), period (Per), and cryptochrome (Cry) within the uterus during pregnancy has been reported [11]. Moreover, mutations that alter the clock function can cause infertility in female mice [3,7,8]. Previous studies demonstrated that clock genes play important roles in regulating fertility [12,13,14], and those endocrine factors affect these clock genes [15,16,17]. Compared with the large amount of rodent data available, there have, however, been few studies on the relationship between fertility and clock disturbances in humans. Circadian clock dysfunction causes abnormalities in sleep, appetite, and emotional control [18,19,20,21,22]. Similarly, disturbances in circadian rhythms due to jet lag and night shift work are related to an increased frequency in menstrual cycle abnormalities, altered serum gonadotropin levels, and decreased fertility [23,24]. Meta-analyses have revealed associations between night shift work and an increased frequency of miscarriages [25,26]. Similar findings were noted in a study examining the miscarriage rate of pregnant flight attendants who worked during overnight hours [27]. Moreover, long-time workers whose schedules include night shifts during pregnancy have an increased risk of preterm delivery and low birth weight [28,29]. Given this, pregnant workers are no longer required to work at night when medically indicated in Europe and Japan.
The CLOCK-BMAL1 heterodimer induces transcription of Per1/2 and Cry1/2 genes by binding to E-box (CACGTG/T) regions in their promoters. Together with the Clock/Bmal1 heterodimer, Per1/2 and Cry1/2 form a complex that inhibits the transcriptional activity of CLOCK-BMAL1 [30,31]. Significant alterations in the circadian behavioral rhythms have been seen in Bmal1-knockout mice, Clock mutant mice, and Per- and Cry-deficient animals [32,33,34,35]. The suprachiasmatic nucleus (SCN) integrates information from the external light–dark cycle of the sun to entrain the cellular clocks of organs with the external environment [36,37,38]. The SCN is divided into two major parts: the core and the shell SCN. The core SCN contains the cell bodies of vasoactive intestinal polypeptide (VIP) neurons and the shell SCN contains the cell bodies of arginine vasopressin (AVP) neurons [39]. VIP neurons input onto gonadotropin-releasing hormone (GnRH) neurons in the preoptic area (POA), and AVP neurons input onto kisspeptin neurons in the anterior ventral periventricular (AVPV) nucleus [40]. It has been noted that the circadian rhythms of AVPV Kiss1 expression in Kiss1 neurons peaked coincident with LH, suggesting the interactions between the SCN and the reproductive neurons in the female hypothalamic–pituitary–gonad (HPG) axis [41]. The primary subject of this review is how circadian clock disorders cause these abnormalities in reproduction.
2. Effects of the Circadian Clock on the Hypothalamic–Pituitary–Gonadal (HPG) Axis and Reproduction
Female reproduction is under circadian control and temporal information is relayed through the HPG axis [13,42,43,44]. The 24 h rotation of the Earth produces regular patterns of environmental modifications, consisting of adjustments in light–dark, changes in temperature, risks of predation, and food availability [45]. The impact of molecular clocks on the HPG axis in relation to female reproduction is well known (Figure 1). The SCN regulates the circadian rhythm of Kiss1 expression in the AVPV [46]. Importantly, Bmal1 and other clock genes have also been identified in kisspeptin neurons [47].
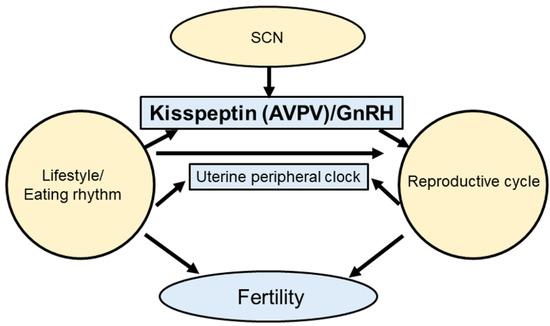
Figure 1.
Synchrony of the circadian clock and reproduction. The central clock controls kisspeptin secretion. Feeding rhythms also control hormone secretion. Moreover, circadian clocks and reproductive cycles affect fertility.
2.1. Circadian Clock Regulation of the HPG Axis
Kisspeptin regulates the secretion of sex steroids such as estrogen through the HPG axis [48]. Kisspeptin signaling is necessary for the timing of reproductive activity, including the pulsatile and estrous cycle of GnRH [40,49]. It is known that GnRH secretion is regulated by circulating hormones, kisspeptin, and neurotransmitters [50,51,52,53,54]. Daily changes in GnRH cell responsiveness to kisspeptin have also been reported [55]. The sensitivity of the GnRH system to kisspeptin stimulation fluctuates significantly during the day, peaking in the afternoon [56]. Kisspeptin neurons are found in the AVPV and ARC nuclei of the hypothalamus, and the SCN controls the AVPV nucleus. AVPV kisspeptin neurons, whose activity is regulated by SCN signals in an E2-dependent manner, are responsible for controlling LH surge [41]. Although kisspeptin neurons in the ARC are more influenced by E2 and leptin than SCN signals [57,58], the effects of circadian dysregulation (e.g., skipping breakfast, shift work, and transmeridian travel) as a factor affecting infertility cannot be overlooked [59,60,61] (Figure 2). For the GPR54 receptor, kisspeptin serves as the endogenous agonist. It was determined that GPR54 expression can become rhythmic when E2 levels are raised, a behavior that appears to be controlled by intracellular ERβ receptors [62].
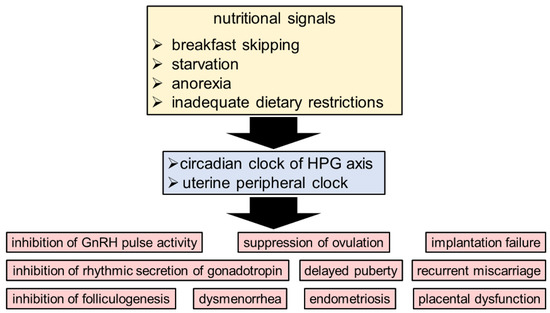
Figure 2.
The circadian clock system as a relationship between reproductive health and nutritional signals. The circadian rhythm in the HPG axis and uterus is negatively impacted by nutritional signals such as skipping breakfast, starvation, anorexia, and inadequate dietary restrictions.
2.2. Ovarian Circadian Clock
In the rat ovary, clock genes associated with the ovulation cycle have been identified. The day of proestrus sees a considerable increase in BMAL1 expression following the LH surge [15]. In follicular development, Per1 and Per2 mRNA are localized to steroidogenic cells in preantral, antral, and preovulatory follicles, corpora lutea, and interstitial glandular tissue by in situ hybridization histochemistry [63]. Furthermore, Per1 and Per2 mRNA and proteins oscillate in a circadian manner in follicles, granulosa cells, and theca cells. In contrast, LH promotes Per1 as well as Bmal1 expression in the ovary [15]. These clock genes display different amplitudes at different stages of the estrus cycle, suggesting endocrine control of the circadian clock [16]. Importantly, gonadotropins also control the ovarian clock, which is supported by experiments indicating that the administration of gonadotropins can synchronize isolated ovaries [64]. Using a Per1-luciferase reporter assay, circadian rhythms were noted in the ovaries, and clock gene phasing was observed in response to LH and FSH [64]. In addition, the ovaries of a mouse model of polycystic ovarian syndrome (PCOS) were shown to have an anomaly for the time of Per2 rhythm [65].
2.3. Endometrial Circadian Clock
An analysis of the relationship between the decidual circadian rhythm and recurrent miscarriage showed that BMAL1 expression in the human decidua during early pregnancy was decreased in patients that experienced recurrent miscarriage [66]. In particular, knockdown may impair the regulation of trophoblast invasion by decidual cells, disrupting proper placenta formation. Polymorphisms in the circadian clock genes are also associated with a higher risk of miscarriage, and gene variants were found in BMAL1 and NPAS2 [67]. Furthermore, progesterone is known to affect the peripheral endometrial clock rhythm in humans. When progesterone acts on the endometrium and decidualization occurs, the level of PER1 in the endometrium increases [68]. The microenvironment of the uterus responds to circadian rhythms and adapts to physiological functions. During pregnancy, the fetus is continuously exposed to hormonal and nutritional signals in the maternal endometrium [69,70]. These results suggest that the circadian rhythms play significant roles in reproduction.
2.4. Animal Studies on the Circadian Clock and Reproductive Function
Consistent with its expression pattern, global Bmal1 knockout mice were found to have significantly reduced ovulation compared with control mice [8]. Global Bmal1 knockout mice were also known to be infertile [5,8,71] (Table 1). Global Bmal1 knockout mice also showed delayed puberty and abnormal estrous cycles [5,72], and the deletion of Bmal1 was shown to reduce progesterone levels [5,73]. Later, the failure of embryo implantation in steroidogenic factor-1 (SF-1) expression-dependent Bmal1-deleted female mice (Bmal1SF1d/d) was shown to be rescued by P4 supplementation or normal ovarian transplantation, demonstrating that insufficient ovarian P4 production is one of the primary causes of infertility in Bmal1 knockout female mice [73]. Additional studies were carried out involving the conditional knockout of Bmal1 in ovarian granulosa cells or theca cells [7] (Table 1). Theca cells are the pacemakers that regulate ovulation timing and transient sensitivity to LH [14]. In these conditional knockout mice, transient susceptibility to LH was found in littermate controls and granulosa cell-specific Bmal1 knockout mice, but not in theca cell-specific Bmal1 knockouts [7,74]. This indicated that follicle development and ovulation are affected by circadian rhythm disfunction in theca cells (Table 1).
Recently, we generated mice with a conditional deletion (cKO) of uterine Bmal1 to examine the pathogenic functions of the uterine clock genes during pregnancy [75]. We found that cKO mice could achieve embryo implantation but could not maintain pregnancy. A histological analysis of their placentas showed that the maternal vascular spaces failed to form properly. In contrast to WT mice, cKO mice expressed scarce levels of the immunosuppressive NK marker CD161 in the spongiotrophoblast layer where maternal uNK cells are in close contact with the fetal trophoblast. These data suggest that Bmal1 plays a significant role in the reproductive organs (Table 1).

Table 1.
Distinct reproductive characteristics of Bmal1 mutant mice.
Table 1.
Distinct reproductive characteristics of Bmal1 mutant mice.
| Mutant Mice | Phenotypes of Reproduction | References |
|---|---|---|
| Conventional Bmal1 KO | Delayed puberty; females have longer estrous cycles; infertile | [5,76] |
| Gonadotrope Bmal1 KO | Irregular estrous cycle; fertile | [7,77] |
| Granulosa cell Bmal1 KO (GCKO) | Normal ovarian morphology and a typical estrous cycle; fertile | [7] |
| Ovarian steroidogenic cells Bmal1 KO | Typical puberty; early pregnancy loss; infertile | [73] |
| Theca cell Bmal1 KO | Fewer offsprings and increased mating failure; regular estrous cycle; subfertile | [7] |
| Uterine Bmal1 KO | Reducing placental vascularization and causing fetal mortality within the uterus; subfertile | [75] |
Similarly, Per1 and Per2 knockout mice experienced reduced reproductive rates because of estrous cycle irregularities [78,79]. Moreover, in Per1-Per2 double knockout mice, the follicular reserve was depleted, resulting in infertility [80]. Mice with a dominant negative mutation in Clock (Clock Δ19/Δ19 mice) were generated to investigate the molecular mechanisms governing circadian clocks [81]. These mice are capable of producing the BMAL1-CLOCK dimer, but possess a defective form of the CLOCK protein that is unable to regulate Per and Cry expression, resulting in the loss of the feedback loop for circadian clock genes [81]. Clock Δ19/Δ19 mice are also overweight and develop symptoms of metabolic syndrome under high-fat diet (HFD) conditions [82]. This obesity-induced phenotype is associated with feeding during rest time. Untimely feeding is associated with obesity and excess body weight in mice and humans [83,84]. Fasting is also involved in circadian rhythm accommodation or dysregulation. Time-restricted feeding (TRF) in which food access is restricted to the dark phase has been reported to protect mice from obesity, fatty liver, hyperinsulinemia, and inflammation when they are fed an HFD [85,86,87]. Rodents fed an HFD ad libitum showed changes in circadian rhythms compared with rodents fed an HFD with TRF [85,88]. This suggests that feeding affects the circadian clock. In addition to the loss of a circadian rhythm, these mice were also reported to have increased risks of stillbirth and neonatal death compared with controls [89].
The pars tuberalis is situated between the anterior lobe of the pituitary gland and the median eminence. It has been demonstrated that melatonin acts as a photoperiodic signal, synchronizing an endogenous oscillator in the pars tuberalis to the photoperiod [90]. Thyroid-stimulating hormone beta (TSH) cells are found in the pars tuberalis, which also trigger the secretion of TSH. TSH promotes triiodothyronine synthesis, which helps gonadotropin-releasing hormone-I release, luteinizing hormone and follicle, stimulating hormone release [91]. Recent research has shown that pars tuberalis controls seasonal reproduction with its TSH secretion [92,93].
In diurnal primates, labor is often initiated at night, consistent with the increased sensitivity to oxytocin that causes pregnancy-related uterine contractions [94,95]. This suggests that circadian rhythms alter uterine sensitivity to oxytocin [96]. Furthermore, studies in rodents have shown that the uterus has a functional peripheral circadian clock [17,97,98]. It has also been suggested that embryo implantation and delivery are controlled by a peripheral circadian clock in the uterus [99,100]. Maternal myometrium and the bladder-specific deletion of Bmal1 cause the mistiming of labor onset [101]. While control mice gave birth early in the morning [29], maternal myometrium- and bladder-specific Bmal1 knockout mice had 28% more daytime births than control mice, demonstrating that the peripheral circadian clock is involved in the timing of labor [29]. These data suggest the importance of circadian clocks in reproduction.
3. The Circadian Clock System as a Link between Nutritional Signals and Reproduction
Reproduction is critical for species survival. Nevertheless, under certain environmental conditions, reproductive activity is suppressed. Many organisms, together with humans, adaptively reduce reproductive activity during periods of starvation and/or anorexia [102,103]. Inadequate dietary restrictions are known to adversely affect the rhythmic secretion of luteinizing hormone (LH) [4], ovarian development [5], and decreased human gonadotropin levels [104,105,106]. Food restriction inhibits both GnRH pulse activity and gonadotropin secretion, resulting in insufficient gonadotropin for folliculogenesis [107,108]. This ultimately results in delayed puberty and the suppression of ovulation when the food supply is insufficient [109].
Feeding rhythms are important for animals because food-entrainable oscillators are located within peripheral tissues, and these peripheral oscillators are independent of the SCN [110,111,112]. We found that time-restricted feeding regulates the circadian rhythm of the uterine clock that is synchronized throughout the uterine body [113]. Furthermore, we postulated that breakfast skipping impairs reproductive function by disrupting the circadian clock [114,115]. In modern society, breakfast skipping is a common habit. Previously, we discovered that skipping breakfast is related to dysmenorrhea [116], and later studies have also revealed a similar correlation between skipping breakfast and dysmenorrhea [117,118,119,120]. Experiments in mice were conducted in which feeding was limited to two meals per day at specified intervals (16 and 8 h). These studies found that the circadian clock was reset by a longer interval (16 h fast) than a shorter interval (8 h fast) between meals [88,121]. In general, breakfast corresponds to the start of one’s daily activities, and skipping breakfast interferes with circadian clocks [116,122,123,124]. This suggests that breakfast has the greatest impact on the chronobiology of the daily diet in humans, and skipping breakfast has been proposed to affect the reproductive system [120,125,126].
4. The Circadian Clock and Puberty
Proper timing of sexual maturation is necessary for reproduction [127,128,129]. Circadian regulation of the reproductive organs is associated with the timing of GnRH release and gonadotropin secretion, and these processes affect sexual maturation [77,130]. Moreover, human and animal puberty relies on complex endocrine regulation [131]. In European sea bass, a prolonged photoperiod delays or prevents puberty and the release of the hormones associated with reproduction [132,133]. One variable in female puberty is the age at menarche, and the timing of menarche is impacted by light. In women who are blind with loss of light perception, menarche occurs earlier than in women with normal light perception [134]. In addition, women are more likely to experience precocious puberty than men [135,136]. From a disease perspective, the associations between the timing of puberty and the risk of developing endometrial or breast cancer in women and prostate cancer in men have been described [137]. Thus, focusing on circadian rhythms may provide clues to preventing and/or treating these diseases.
Other factors affecting sexual maturation are endocrine-disrupting chemicals (EDCs). EDCs are substances that can mimic hormones in the body and are found in common household products. EDCs bind to hormone receptors and cause activation or suppression of natural hormones or alter the breakdown of natural hormones, thereby causing changes in normal hormonal signaling. Puberty is a complex developmental stage in which physical changes promote sexual maturation, and this process is sensitive to hormonal disruptions. EDCs have been reported to be involved in pubertal-onset variability [138] and can enter the body through drinking, eating, breathing, or direct contact [139]. Exposure to EDCs with estrogenic and/or anti-androgenic effects can disrupt the reproductive tract and sexual maturation [140]. Over the last 200 years, the timing of pubertal onset has changed. The age of menarche has been reduced from 17 in the early 19th century to 13 in the 1950s [141]. The liver of adult male Wistar rats treated with 4-hydroxy-2,3,3’,4’,5-pentachlorobiphenyl showed altered expression of the clock genes including BMAL1 [142]. Moreover, various studies have demonstrated changes in circadian clock-gene expression and the endocrine system after exposure to EDCs, and the importance of this is now clear [143,144,145].
5. Conclusions
In conclusion, elucidating the factors that modify circadian clocks in reproductive organs will provide clues to treating reproductive dysfunction. Moreover, it may suggest strategies for optimizing existing therapeutic interventions. We expect that the appropriate re-establishment of the networks governing circadian rhythms and the reproductive cycle in early life will help prevent future obstetric and gynecological diseases. The influence of circadian rhythms governing protein translation on the regenerative capacity of tissues must be considered in future studies of regeneration.
Author Contributions
Conceptualization, M.O., H.A., T.D., T.F. and H.F.; writing—original draft preparation, M.O.; writing—review and editing, T.F., M.M., Y.M. (Yasunari Mizumoto), T.I., K.K., T.H., S.N., N.T., N.S.-K., Y.M. (Yoshiko Maida), N.K., H.N. and H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Japan Agency for Medical Research and Development (no. 20gk0210016h0003), JSPS KAKENHI Grant Numbers JP19K09776 and JP22K09556, and the Japan Science and Technology Agency Health and Labor Sciences Research Grant (no. 18gk0110024h0002).
Acknowledgments
We thank Ai Sato (Kanazawa University) for her help. We also thank our department members for their helpful support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADHOGD | adolescent dietary habit-induced obstetric and gynecologic disease |
| Bmal1 | brain and muscle Arnt-like protein-1 |
| Clock | circadian locomotor output cycles kaput |
| Cry1 | cryptochrome circadian regulator 1 |
| Cry2 | cryptochrome circadian regulator 2 |
| Dbp | albumin D-binding protein |
| FSH | follicle-stimulating hormone |
| GnRH | gonadotropin-releasing hormone |
| HDP | hypertensive disorders of pregnancy |
| HPG | hypothalamic–pituitary–gonadal |
| LH | luteinizing hormone |
| PCOS | polycystic ovary syndrome |
| Per1 | period circadian regulator 1 |
| Per2 | period circadian regulator 2 |
| Per3 | period circadian regulator 3 |
| NR1D1 | nuclear receptor subfamily 1, group D, member 1 |
References
- Mahoney, M.M. Shift work, jet lag and female reproduction. Int. J. Endocrinol. 2010, 2010, 813764. [Google Scholar] [CrossRef] [PubMed]
- Summa, K.C.; Vitaterna, M.H.; Turek, F.W. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLoS ONE 2012, 7, e37668. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.H.; Olson, S.L.; Turek, F.W.; Levine, J.E.; Horton, T.H.; Takahashi, J.S. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 2004, 14, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Bittman, E.L. Circadian Function in Multiple Cell Types Is Necessary for Proper Timing of the Preovulatory LH Surge. J. Biol. Rhythm. 2019, 34, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Boden, M.J.; Varcoe, T.J.; Voultsios, A.; Kennaway, D.J. Reproductive biology of female Bmal1 null mice. Reproduction 2010, 139, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Sellix, M.T. Clocks underneath: The role of peripheral clocks in the timing of female reproductive physiology. Front. Endocrinol. 2013, 4, 91. [Google Scholar] [CrossRef]
- Mereness, A.L.; Murphy, Z.C.; Forrestel, A.C.; Butler, S.; Ko, C.; Richards, J.S.; Sellix, M.T. Conditional Deletion of Bmal1 in Ovarian Theca Cells Disrupts Ovulation in Female Mice. Endocrinology 2016, 157, 913–927. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, Y.; Xu, Y.; Zhou, C. Loss of Bmal1 decreases oocyte fertilization, early embryo development and implantation potential in female mice. Zygote 2016, 24, 760–767. [Google Scholar] [CrossRef]
- Perez, S.; Murias, L.; Fernandez-Plaza, C.; Diaz, I.; Gonzalez, C.; Otero, J.; Diaz, E. Evidence for clock genes circadian rhythms in human full-term placenta. Syst. Biol. Reprod. Med. 2015, 61, 360–366. [Google Scholar] [CrossRef]
- Muter, J.; Lucas, E.S.; Chan, Y.W.; Brighton, P.J.; Moore, J.D.; Lacey, L.; Quenby, S.; Lam, E.W.; Brosens, J.J. The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells. FASEB J. 2015, 29, 1603–1614. [Google Scholar] [CrossRef]
- Ratajczak, C.K.; Herzog, E.D.; Muglia, L.J. Clock gene expression in gravid uterus and extra-embryonic tissues during late gestation in the mouse. Reprod. Fertil. Dev. 2010, 22, 743–750. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Boden, M.J.; Varcoe, T.J. Circadian rhythms and fertility. Mol. Cell. Endocrinol. 2012, 349, 56–61. [Google Scholar] [CrossRef]
- Sen, A.; Hoffmann, H.M. Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol. Cell. Endocrinol. 2020, 501, 110655. [Google Scholar] [CrossRef]
- Pan, X.; Taylor, M.J.; Cohen, E.; Hanna, N.; Mota, S. Circadian Clock, Time-Restricted Feeding and Reproduction. Int. J. Mol. Sci. 2020, 21, 831. [Google Scholar] [CrossRef]
- Karman, B.N.; Tischkau, S.A. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol. Reprod. 2006, 75, 624–632. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Sellix, M.T.; Kudo, T.; Nakao, N.; Yoshimura, T.; Ebihara, S.; Colwell, C.S.; Block, G.D. Influence of the estrous cycle on clock gene expression in reproductive tissues: Effects of fluctuating ovarian steroid hormone levels. Steroids 2010, 75, 203–212. [Google Scholar] [CrossRef]
- Yaw, A.M.; Duong, T.V.; Nguyen, D.; Hoffmann, H.M. Circadian rhythms in the mouse reproductive axis during the estrous cycle and pregnancy. J. Neurosci. Res. 2021, 99, 294–308. [Google Scholar] [CrossRef]
- Mieda, M.; Okamoto, H.; Sakurai, T. Manipulating the Cellular Circadian Period of Arginine Vasopressin Neurons Alters the Behavioral Circadian Period. Curr. Biol. 2016, 26, 2535–2542. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.X.; Wang, G.; Dong, S.; Jiang, Y.; Spruyt, K.; Ling, J.; Zhu, Q.; Lee, T.M.; Jiang, F. Association of Sleep and Circadian Activity Rhythm with Emotional Face Processing among 12-month-old Infants. Sci. Rep. 2018, 8, 3200. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kumagai, H.; Skach, A.; Sato, M.; Yanagisawa, M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell 2013, 155, 1323–1336. [Google Scholar] [CrossRef]
- Page, A.J.; Christie, S.; Symonds, E.; Li, H. Circadian regulation of appetite and time restricted feeding. Physiol. Behav. 2020, 220, 112873. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.C.; Driver, H.S. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007, 8, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.C.; Whelan, E.A.; Lividoti Hibert, E.N.; Spiegelman, D.; Schernhammer, E.S.; Rich-Edwards, J.W. Rotating shift work and menstrual cycle characteristics. Epidemiology 2011, 22, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Stocker, L.J.; Macklon, N.S.; Cheong, Y.C.; Bewley, S.J. Influence of shift work on early reproductive outcomes: A systematic review and meta-analysis. Obstet. Gynecol. 2014, 124, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.; Jorgensen, K.T.; Bonzini, M.; Palmer, K.T. Miscarriage and occupational activity: A systematic review and meta-analysis regarding shift work, working hours, lifting, standing, and physical workload. Scand. J. Work Environ. Health 2013, 39, 325–334. [Google Scholar] [CrossRef]
- Grajewski, B.; Whelan, E.A.; Lawson, C.C.; Hein, M.J.; Waters, M.A.; Anderson, J.L.; MacDonald, L.A.; Mertens, C.J.; Tseng, C.Y.; Cassinelli, R.T., II; et al. Miscarriage among flight attendants. Epidemiology 2015, 26, 192–203. [Google Scholar] [CrossRef]
- Suzumori, N.; Ebara, T.; Matsuki, T.; Yamada, Y.; Kato, S.; Omori, T.; Saitoh, S.; Kamijima, M.; Sugiura-Ogasawara, M.; Japan Environment & Children’s Study Group. Effects of long working hours and shift work during pregnancy on obstetric and perinatal outcomes: A large prospective cohort study-Japan Environment and Children’s Study. Birth 2020, 47, 67–79. [Google Scholar] [CrossRef]
- Patil, D.; Enquobahrie, D.A.; Peckham, T.; Seixas, N.; Hajat, A. Retrospective cohort study of the association between maternal employment precarity and infant low birth weight in women in the USA. BMJ Open 2020, 10, e029584. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Abbas, S.; Ahmed, I.; Kudo, T.; Iqbal, M.; Lee, Y.J.; Fujiwara, T.; Ohkuma, M. A heavy metal tolerant novel bacterium, Bacillus malikii sp. nov., isolated from tannery effluent wastewater. Antonie Leeuwenhoek 2015, 108, 1319–1330. [Google Scholar] [CrossRef]
- Miller, B.H.; Olson, S.L.; Levine, J.E.; Turek, F.W.; Horton, T.H.; Takahashi, J.S. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol. Reprod. 2006, 75, 778–784. [Google Scholar] [CrossRef]
- Pendergast, J.S.; Oda, G.A.; Niswender, K.D.; Yamazaki, S. Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s). Proc. Natl. Acad. Sci. USA 2012, 109, 14218–14223. [Google Scholar] [CrossRef]
- De Bundel, D.; Gangarossa, G.; Biever, A.; Bonnefont, X.; Valjent, E. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front. Behav. Neurosci. 2013, 7, 152. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Whitmore, D.; Foulkes, N.S.; Sassone-Corsi, P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature 2000, 404, 87–91. [Google Scholar] [CrossRef]
- Bass, J. Circadian topology of metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef]
- Moore, R.Y.; Speh, J.C.; Leak, R.K. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002, 309, 89–98. [Google Scholar] [CrossRef]
- Putteeraj, M.; Soga, T.; Ubuka, T.; Parhar, I.S. A “Timed” Kiss Is Essential for Reproduction: Lessons from Mammalian Studies. Front. Endocrinol. 2016, 7, 121. [Google Scholar] [CrossRef]
- Robertson, J.L.; Clifton, D.K.; de la Iglesia, H.O.; Steiner, R.A.; Kauffman, A.S. Circadian regulation of Kiss1 neurons: Implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 2009, 150, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.A.; Moenter, S.M. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr. Rev. 2010, 31, 544–577. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.M.; Schmidt, C.X.; Brockmann, R.M.; Oster, H. Circadian regulation of endocrine systems. Auton. Neurosci. 2019, 216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Sellix, M.T. The Circadian Timing System and Environmental Circadian Disruption: From Follicles to Fertility. Endocrinology 2016, 157, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Van der Vinne, V.; Tachinardi, P.; Riede, S.J.; Akkerman, J.; Scheepe, J.; Daan, S.; Hut, R.A. Maximising survival by shifting the daily timing of activity. Ecol. Lett. 2019, 22, 2097–2102. [Google Scholar] [CrossRef]
- Smarr, B.L.; Morris, E.; de la Iglesia, H.O. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology 2012, 153, 2839–2850. [Google Scholar] [CrossRef]
- Chassard, D.; Bur, I.; Poirel, V.J.; Mendoza, J.; Simonneaux, V. Evidence for a Putative Circadian Kiss-Clock in the Hypothalamic AVPV in Female Mice. Endocrinology 2015, 156, 2999–3011. [Google Scholar] [CrossRef]
- Comninos, A.N.; Wall, M.B.; Demetriou, L.; Shah, A.J.; Clarke, S.A.; Narayanaswamy, S.; Nesbitt, A.; Izzi-Engbeaya, C.; Prague, J.K.; Abbara, A.; et al. Kisspeptin modulates sexual and emotional brain processing in humans. J. Clin. Investig. 2017, 127, 709–719. [Google Scholar] [CrossRef]
- Dror, T.; Franks, J.; Kauffman, A.S. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol. Reprod. 2013, 88, 146. [Google Scholar] [CrossRef]
- Saedi, S.; Khoradmehr, A.; Mohammad Reza, J.S.; Tamadon, A. The role of neuropeptides and neurotransmitters on kisspeptin/kiss1r-signaling in female reproduction. J. Chem. Neuroanat. 2018, 92, 71–82. [Google Scholar] [CrossRef]
- Kunimura, Y.; Iwata, K.; Ishigami, A.; Ozawa, H. Age-related alterations in hypothalamic kisspeptin, neurokinin B, and dynorphin neurons and in pulsatile LH release in female and male rats. Neurobiol. Aging. 2017, 50, 30–38. [Google Scholar] [CrossRef]
- Garcia, J.P.; Guerriero, K.A.; Keen, K.L.; Kenealy, B.P.; Seminara, S.B.; Terasawa, E. Kisspeptin and Neurokinin B Signaling Network Underlies the Pubertal Increase in GnRH Release in Female Rhesus Monkeys. Endocrinology 2017, 158, 3269–3280. [Google Scholar] [CrossRef]
- Qiu, J.; Nestor, C.C.; Zhang, C.; Padilla, S.L.; Palmiter, R.D.; Kelly, M.J.; Ronnekleiv, O.K. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 2016, 5, e16246. [Google Scholar] [CrossRef]
- Kalil, B.; Ribeiro, A.B.; Leite, C.M.; Uchoa, E.T.; Carolino, R.O.; Cardoso, T.S.; Elias, L.L.; Rodrigues, J.A.; Plant, T.M.; Poletini, M.O.; et al. The Increase in Signaling by Kisspeptin Neurons in the Preoptic Area and Associated Changes in Clock Gene Expression That Trigger the LH Surge in Female Rats Are Dependent on the Facilitatory Action of a Noradrenaline Input. Endocrinology 2016, 157, 323–335. [Google Scholar] [CrossRef]
- Adams, C.; Stroberg, W.; DeFazio, R.A.; Schnell, S.; Moenter, S.M. Gonadotropin-Releasing Hormone (GnRH) Neuron Excitability Is Regulated by Estradiol Feedback and Kisspeptin. J. Neurosci. 2018, 38, 1249–1263. [Google Scholar] [CrossRef]
- Williams, W.P., III; Jarjisian, S.G.; Mikkelsen, J.D.; Kriegsfeld, L.J. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology 2011, 152, 595–606. [Google Scholar] [CrossRef]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef]
- Navarro, V.M.; Castellano, J.M.; McConkey, S.M.; Pineda, R.; Ruiz-Pino, F.; Pinilla, L.; Clifton, D.K.; Tena-Sempere, M.; Steiner, R.A. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E202–E210. [Google Scholar] [CrossRef]
- Laposky, A.D.; Bradley, M.A.; Williams, D.L.; Bass, J.; Turek, F.W. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R2059–R2066. [Google Scholar] [CrossRef]
- Sutton, G.M.; Centanni, A.V.; Butler, A.A. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology 2010, 151, 1570–1580. [Google Scholar] [CrossRef]
- Ando, H.; Kumazaki, M.; Motosugi, Y.; Ushijima, K.; Maekawa, T.; Ishikawa, E.; Fujimura, A. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 2011, 152, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Tonsfeldt, K.J.; Goodall, C.P.; Latham, K.L.; Chappell, P.E. Oestrogen induces rhythmic expression of the Kisspeptin-1 receptor GPR54 in hypothalamic gonadotrophin-releasing hormone-secreting GT1-7 cells. J. Neuroendocrinol. 2011, 23, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J.; Georg, B.; Hannibal, J.; Hindersson, P.; Gras, S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 2006, 147, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Sellix, M.; Pezuk, P.; Menaker, M. Timing of the ovarian circadian clock is regulated by gonadotropins. Endocrinology 2009, 150, 4338–4347. [Google Scholar] [CrossRef] [PubMed]
- Mereness, A.L.; Murphy, Z.C.; Sellix, M.T. Developmental programming by androgen affects the circadian timing system in female mice. Biol. Reprod. 2015, 92, 88. [Google Scholar] [CrossRef]
- Lv, S.; Wang, N.; Ma, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Impaired decidualization caused by downregulation of circadian clock gene BMAL1 contributes to human recurrent miscarriage dagger. Biol. Reprod. 2019, 101, 138–147. [Google Scholar] [CrossRef]
- Kovanen, L.; Saarikoski, S.T.; Aromaa, A.; Lonnqvist, J.; Partonen, T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS ONE 2010, 5, e10007. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, N.; Bao, H.; Jiang, Y.; Yang, N.; Wu, K.; Wu, J.; Wang, H.; Kong, S.; Zhang, Y. Circadian gene PER1 senses progesterone signal during human endometrial decidualization. J. Endocrinol. 2019, 243, 229–242. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian clocks during embryonic and fetal development. Birth Defects Res. C Embryo Today 2007, 81, 204–214. [Google Scholar] [CrossRef]
- Akiyama, S.; Ohta, H.; Watanabe, S.; Moriya, T.; Hariu, A.; Nakahata, N.; Chisaka, H.; Matsuda, T.; Kimura, Y.; Tsuchiya, S.; et al. The uterus sustains stable biological clock during pregnancy. Tohoku J. Exp. Med. 2010, 221, 287–298. [Google Scholar] [CrossRef]
- Papacleovoulou, G.; Nikolova, V.; Oduwole, O.; Chambers, J.; Vazquez-Lopez, M.; Jansen, E.; Nicolaides, K.; Parker, M.; Williamson, C. Gestational disruptions in metabolic rhythmicity of the liver, muscle, and placenta affect fetal size. FASEB J. 2017, 31, 1698–1708. [Google Scholar] [CrossRef]
- Alvarez, J.D.; Hansen, A.; Ord, T.; Bebas, P.; Chappell, P.E.; Giebultowicz, J.M.; Williams, C.; Moss, S.; Sehgal, A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythm. 2008, 23, 26–36. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, B.P.; Shen, A.L.; Wallisser, J.A.; Krentz, K.J.; Moran, S.M.; Sullivan, R.; Glover, E.; Parlow, A.F.; Drinkwater, N.R.; et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl. Acad. Sci. USA 2014, 111, 14295–14300. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Xu, W.; Ying, J.; Qu, Y.; Jiang, X.; Zhang, A.; Yue, Y.; Zhou, R.; Ruan, T.; et al. Critical Roles of the Circadian Transcription Factor BMAL1 in Reproductive Endocrinology and Fertility. Front. Endocrinol. 2022, 13, 818272. [Google Scholar] [CrossRef]
- Ono, M.; Toyoda, N.; Kagami, K.; Hosono, T.; Matsumoto, T.; Horike, S.I.; Yamazaki, R.; Nakamura, M.; Mizumoto, Y.; Fujiwara, T.; et al. Uterine Deletion of Bmal1 Impairs Placental Vascularization and Induces Intrauterine Fetal Death in Mice. Int. J. Mol. Sci. 2022, 23, 7637. [Google Scholar] [CrossRef]
- Ratajczak, C.K.; Boehle, K.L.; Muglia, L.J. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology 2009, 150, 1879–1885. [Google Scholar] [CrossRef]
- Chu, A.; Zhu, L.; Blum, I.D.; Mai, O.; Leliavski, A.; Fahrenkrug, J.; Oster, H.; Boehm, U.; Storch, K.F. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology 2013, 154, 2924–2935. [Google Scholar] [CrossRef]
- Pilorz, V.; Steinlechner, S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction 2008, 135, 559–568. [Google Scholar] [CrossRef]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z.S.; Eichele, G.; Bradley, A.; et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105, 683–694. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, C.; Li, Y.; Jiang, H.; Yang, P.; Tang, J.; Xu, Y.; Wang, H.; He, Y. Loss-of-function mutations with circadian rhythm regulator Per1/Per2 lead to premature ovarian insufficiency dagger. Biol. Reprod. 2019, 100, 1066–1072. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; King, D.P.; Chang, A.M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.L.; Shostak, A.; Leliavski, A.; Tsang, A.H.; Johren, O.; Muller-Fielitz, H.; Landgraf, D.; Naujokat, N.; van der Horst, G.T.; Oster, H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1053–E1063. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Dolatshad, H.; Campbell, E.A.; O’Hara, L.; Maywood, E.S.; Hastings, M.H.; Johnson, M.H. Developmental and reproductive performance in circadian mutant mice. Hum. Reprod. 2006, 21, 68–79. [Google Scholar] [CrossRef]
- Wagner, G.C.; Johnston, J.D.; Tournier, B.B.; Ebling, F.J.; Hazlerigg, D.G. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus). Eur. J. Neurosci. 2007, 25, 485–490. [Google Scholar] [CrossRef]
- Kosonsiriluk, S.; Mauro, L.J.; Chaiworakul, V.; Chaiseha, Y.; El Halawani, M.E. Photoreceptive oscillators within neurons of the premammillary nucleus (PMM) and seasonal reproduction in temperate zone birds. Gen. Comp. Endocrinol. 2013, 190, 149–155. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yasuo, S.; Watanabe, M.; Iigo, M.; Yamamura, T.; Hirunagi, K.; Ebihara, S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 2003, 426, 178–181. [Google Scholar] [CrossRef]
- Nakao, N.; Ono, H.; Yamamura, T.; Anraku, T.; Takagi, T.; Higashi, K.; Yasuo, S.; Katou, Y.; Kageyama, S.; Uno, Y.; et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 2008, 452, 317–322. [Google Scholar] [CrossRef]
- Olcese, J.; Lozier, S.; Paradise, C. Melatonin and the circadian timing of human parturition. Reprod. Sci. 2013, 20, 168–174. [Google Scholar] [CrossRef]
- Olcese, J. Circadian clocks and pregnancy. Front. Endocrinol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Sharkey, J.T.; Puttaramu, R.; Word, R.A.; Olcese, J. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 2009, 94, 421–427. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Sellix, M.T.; Menaker, M.; Block, G.D. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1025–E1031. [Google Scholar] [CrossRef]
- Loh, D.H.; Kuljis, D.A.; Azuma, L.; Wu, Y.; Truong, D.; Wang, H.B.; Colwell, C.S. Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J. Biol. Rhythm. 2014, 29, 355–369. [Google Scholar] [CrossRef]
- Gamble, K.L.; Resuehr, D.; Johnson, C.H. Shift work and circadian dysregulation of reproduction. Front. Endocrinol. 2013, 4, 92. [Google Scholar] [CrossRef]
- Miller, B.H.; Takahashi, J.S. Central circadian control of female reproductive function. Front. Endocrinol. 2013, 4, 195. [Google Scholar] [CrossRef]
- Ratajczak, C.K.; Asada, M.; Allen, G.C.; McMahon, D.G.; Muglia, L.M.; Smith, D.; Bhattacharyya, S.; Muglia, L.J. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod. Fertil. Dev. 2012, 24, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, A.; Sabbadin, C.; Minardi, S.; Favaro, A.; Dona, G.; Bordin, L.; Ambrosini, G.; Armanini, D. Persistent amenorrhea and decreased DHEAS to cortisol ratio after recovery from anorexia nervosa. Gynecol. Endocrinol. 2017, 33, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Luisi, S.; Ciani, V.; Podfigurna-Stopa, A.; Lazzeri, L.; De Pascalis, F.; Meczekalski, B.; Petraglia, F. Serum anti-Mullerian hormone, inhibin B, and total inhibin levels in women with hypothalamic amenorrhea and anorexia nervosa. Gynecol. Endocrinol. 2012, 28, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Booth, P.J.; Cosgrove, J.R.; Foxcroft, G.R. Endocrine and metabolic responses to realimentation in feed-restricted prepubertal gilts: Associations among gonadotropins, metabolic hormones, glucose and uteroovarian development. J. Anim. Sci. 1996, 74, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, H.; Pasquier, A.; Mounier, A.M.; Prunier, A. Influence of feed restriction during lactation on gonadotropic hormones and ovarian development in primiparous sows. J. Anim. Sci. 1998, 76, 856–863. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Iranmanesh, A.; Evans, W.S.; Lizarralde, G.; Thorner, M.O.; Vance, M.L. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J. Clin. Endocrinol. Metab. 1993, 76, 587–593. [Google Scholar]
- Brito, L.F.; Barth, A.D.; Rawlings, N.C.; Wilde, R.E.; Crews, D.H.J.; Boisclair, Y.R.; Ehrhardt, R.A.; Kastelic, J.P. Effect of feed restriction during calfhood on serum concentrations of metabolic hormones, gonadotropins, testosterone, and on sexual development in bulls. Reproduction 2007, 134, 171–181. [Google Scholar] [CrossRef]
- Brito, L.F.; Barth, A.D.; Rawlings, N.C.; Wilde, R.E.; Crews, D.H.J.; Mir, P.S.; Kastelic, J.P. Effect of nutrition during calfhood and peripubertal period on serum metabolic hormones, gonadotropins and testosterone concentrations, and on sexual development in bulls. Domest. Anim. Endocrinol. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Tropp, J.; Markus, E.J. Effects of mild food deprivation on the estrous cycle of rats. Physiol. Behav. 2001, 73, 553–559. [Google Scholar] [CrossRef]
- Stephan, F.K. Phase shifts of circadian rhythms in activity entrained to food access. Physiol. Behav. 1984, 32, 663–671. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994, 18, 171–195. [Google Scholar] [CrossRef]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef]
- Hosono, T.; Ono, M.; Daikoku, T.; Mieda, M.; Nomura, S.; Kagami, K.; Iizuka, T.; Nakata, R.; Fujiwara, T.; Fujiwara, H.; et al. Time-Restricted Feeding Regulates Circadian Rhythm of Murine Uterine Clock. Curr. Dev. Nutr. 2021, 5, nzab064. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nakata, R. Skipping breakfast is associated with reproductive dysfunction in post-adolescent female college students. Appetite 2010, 55, 714–717. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ono, M.; Mieda, M.; Yoshikawa, H.; Nakata, R.; Daikoku, T.; Sekizuka-Kagami, N.; Maida, Y.; Ando, H.; Fujiwara, H. Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis-Possible Involvement of Clock System. Nutrients 2020, 12, 1294. [Google Scholar] [CrossRef]
- Fujiwara, T. Skipping breakfast is associated with dysmenorrhea in young women in Japan. Int. J. Food Sci. Nutr. 2003, 54, 505–509. [Google Scholar] [CrossRef]
- Angelin, P.; Dileep, D.; Manju, T.; Veena, M.; Pradeep, D.; Amreen, K.; Soumitra, S. Effect of Skipping Breakfast on Young Girls’ Menstruation. Ind. J. Youth Adol. Health 2017, 4, 17–20. [Google Scholar]
- Abu Helwa, H.A.; Mitaeb, A.A.; Al-Hamshri, S.; Sweileh, W.M. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. BMC Women’s Health 2018, 18, 18. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, L.; Chen, L.; Kaminga, A.C.; Xu, H. Prevalence and Risk Factors Associated with Primary Dysmenorrhea among Chinese Female University Students: A Cross-sectional Study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 15–22. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ono, M.; Iizuka, T.; Sekizuka-Kagami, N.; Maida, Y.; Adachi, Y.; Fujiwara, H.; Yoshikawa, H. Breakfast Skipping in Female College Students Is a Potential and Preventable Predictor of Gynecologic Disorders at Health Service Centers. Diagnostics 2020, 10, 476. [Google Scholar] [CrossRef]
- Shibata, S.; Tahara, Y.; Hirao, A. The adjustment and manipulation of biological rhythms by light, nutrition and abused drugs. Adv. Drug Deliv. Rev. 2010, 62, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Bajalan, Z.; Alimoradi, Z.; Moafi, F. Nutrition as a Potential Factor of Primary Dysmenorrhea: A Systematic Review of Observational Studies. Gynecol. Obstet. Investig. 2019, 84, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Merino, J.; Lane, J.M.; Song, Y.; Smith, C.E.; Tanaka, T.; McKeown, N.M.; Tucker, C.; Sun, D.; Bartz, T.M.; et al. Genome-wide association study of breakfast skipping links clock regulation with food timing. Am. J. Clin. Nutr. 2019, 110, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Nakata, R.; Ono, M.; Mieda, M.; Ando, H.; Daikoku, T.; Fujiwara, H. Time Restriction of Food Intake During the Circadian Cycle Is a Possible Regulator of Reproductive Function in Postadolescent Female Rats. Curr. Dev. Nutr. 2019, 3, nzy093. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nakata, R. Current problems of food intake in young women in Japan: Their influence on female reproductive function. Reprod. Med. Biol. 2004, 3, 107–114. [Google Scholar] [CrossRef]
- Simonneaux, V.; Bahougne, T.; Angelopoulou, E. Daily rhythms count for female fertility. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 505–519. [Google Scholar] [CrossRef]
- Sellix, M.T.; Murphy, Z.C.; Menaker, M. Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology 2013, 154, 1636–1647. [Google Scholar] [CrossRef]
- Huhtaniemi, I. Mutations along the pituitary-gonadal axis affecting sexual maturation: Novel information from transgenic and knockout mice. Mol. Cell. Endocrinol. 2006, 254–255, 84–90. [Google Scholar] [CrossRef]
- Resuehr, H.E.; Resuehr, D.; Olcese, J. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol. Cell. Endocrinol. 2009, 311, 120–125. [Google Scholar] [CrossRef]
- Perry, J.R.; Day, F.; Elks, C.E.; Sulem, P.; Thompson, D.J.; Ferreira, T.; He, C.; Chasman, D.I.; Esko, T.; Thorleifsson, G.; et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014, 514, 92–97. [Google Scholar] [CrossRef]
- Bayarri, M.J.; Rodriguez, L.; Zanuy, S.; Madrid, J.A.; Sanchez-Vazquez, F.J.; Kagawa, H.; Okuzawa, K.; Carrillo, M. Effect of photoperiod manipulation on the daily rhythms of melatonin and reproductive hormones in caged European sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 2004, 136, 72–81. [Google Scholar] [CrossRef]
- Bayarri, M.J.; Zanuy, S.; Yilmaz, O.; Carrillo, M. Effects of continuous light on the reproductive system of European sea bass gauged by alterations of circadian variations during their first reproductive cycle. Chronobiol. Int. 2009, 26, 184–199. [Google Scholar] [CrossRef]
- Flynn-Evans, E.E.; Stevens, R.G.; Tabandeh, H.; Schernhammer, E.S.; Lockley, S.W. Effect of light perception on menarche in blind women. Ophthalmic Epidemiol. 2009, 16, 243–248. [Google Scholar] [CrossRef]
- De Vries, L.; Kauschansky, A.; Shohat, M.; Phillip, M. Familial central precocious puberty suggests autosomal dominant inheritance. J. Clin. Endocrinol. Metab. 2004, 89, 1794–1800. [Google Scholar] [CrossRef]
- Wehkalampi, K.; Widen, E.; Laine, T.; Palotie, A.; Dunkel, L. Patterns of inheritance of constitutional delay of growth and puberty in families of adolescent girls and boys referred to specialist pediatric care. J. Clin. Endocrinol. Metab. 2008, 93, 723–728. [Google Scholar] [CrossRef]
- Day, F.R.; Thompson, D.J.; Helgason, H.; Chasman, D.I.; Finucane, H.; Sulem, P.; Ruth, K.S.; Whalen, S.; Sarkar, A.K.; Albrecht, E.; et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017, 49, 834–841. [Google Scholar] [CrossRef]
- Livadas, S.; Chrousos, G.P. Molecular and Environmental Mechanisms Regulating Puberty Initiation: An Integrated Approach. Front. Endocrinol. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Beszterda, M.; Franski, R. Endocrine disruptor compounds in environment: As a danger for children health. Pediatr. Endocrinol. Diabetes Metab. 2018, 24, 88–95. [Google Scholar] [CrossRef]
- Fudvoye, J.; Lopez-Rodriguez, D.; Franssen, D.; Parent, A.S. Endocrine disrupters and possible contribution to pubertal changes. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101300. [Google Scholar] [CrossRef]
- Sorensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Mogensen, S.S.; Juul, A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm. Res. Paediatr. 2012, 77, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Iida, M.; Agusa, T.; Takaguchi, K.; Fujii, S.; Nomiyama, K.; Iwata, H. Effects of 4-Hydroxy-2,3,3’,4’,5-Pentachlorobiphenyl (4-OH-CB107) on Liver Transcriptome in Rats: Implication in the Disruption of Circadian Rhythm and Fatty Acid Metabolism. Toxicol. Sci. 2018, 165, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, S.; Mirbahai, L.; Castiglioni, S.; Fent, K. Transcriptional and physiological responses induced by binary mixtures of drospirenone and progesterone in zebrafish (Danio rerio). Environ. Sci. Technol. 2014, 48, 3523–3531. [Google Scholar] [CrossRef]
- Zhao, Y.; Castiglioni, S.; Fent, K. Synthetic progestins medroxyprogesterone acetate and dydrogesterone and their binary mixtures adversely affect reproduction and lead to histological and transcriptional alterations in zebrafish (Danio rerio). Environ. Sci. Technol. 2015, 49, 4636–4645. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xiong, C.; Liu, J.; Hu, B.; Zheng, L. Chronic bisphenol A exposure alters behaviors of zebrafish (Danio rerio). Environ. Pollut. 2015, 206, 275–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).