Effect of Cannabidiol on Human Peripheral Blood Mononuclear Cells and CD4+ T Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of CBD on T Cell Functional Activation
2.1.1. Flow Cytometric Evaluation of CD4+ T Cells Intracellular Cytokine Content
2.1.2. TF mRNA Expression in Isolated Cultured PBMC
2.2. Effect of Treatment with CBD on Cytokine mRNA Expression and Protein Production in Cultured Human PBMC
2.3. Effect of Treatment with CBD on Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Substances
4.2. Peripheral Blood Mononuclear Cell (PBMC) Isolation and Functional Assays
4.2.1. PBMC Proliferation Assay
4.2.2. PBMC Cytokine Production
4.2.3. Transcriptional Factors’ mRNA Expression in PBMCs
4.3. Isolation and Proliferation Assay of T Regulatory and T Effector CD4+ T Lymphocytes
4.4. PBMC Stimulation and Intracellular Cytokine Staining of IFN-γ-, IL-4-, and IL-17A- Producing CD4+ T Cells
4.5. RNA Isolation and Real-Time Polymerase Chain Reaction
4.6. Cytokine Analysis by ELISA
4.7. Flow Cytometry
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, D.I. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur. J. Intern. Med. 2018, 49, 7. [Google Scholar] [CrossRef]
- Saviano, A.; Raucci, F.; Tallarico, M.; De Caro, C.; Di Martino, S.; Nesci, V.; Roberti, R.; Iannone, L.F.; Colia, A.L.; Dimonte, S.; et al. On behalf of SIF Working Group Pharmacognosy, Phytotherapy and nutraceuticals. Cannabidiol and the central nervous system: Translating into clinics. Pharmadvances 2021, 3, 369. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Kocot-Kępska, M.; Zajączkowska, R.; Mika, J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszyńska, A. Peripheral Mechanisms of Neuropathic Pain-the Role of Neuronal and Non-Neuronal Interactions and Their Implications for Topical Treatment of Neuropathic Pain. Pharmaceuticals 2021, 14, 77. [Google Scholar] [CrossRef]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400. [Google Scholar] [CrossRef]
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory Potential of Cannabidiol in Multiple Sclerosis: A Systematic Review. J. Neuroimmune Pharmacol. 2021, 16, 251. [Google Scholar] [CrossRef]
- Gusho, C.A.; Court, T. Cannabidiol: A Brief Review of Its Therapeutic and Pharmacologic Efficacy in the Management of Joint Disease. Cureus 2020, 12, e7375. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Clauw, D.J.; Hauser, W. A cautious hope for cannabidiol (CBD) in rheumatology care. Arthritis Care Res. 2023, 75, 1371–1375. [Google Scholar] [CrossRef]
- Rodríguez Mesa, X.M.; Vergara, A.F.M.; Contreras Bolaños, L.A.; Guevara Moriones, N.; Mejía Piñeros, A.L.; Santander González, S.P. Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 2021, 6, 196. [Google Scholar] [CrossRef]
- Mabou Tagne, A.; Marino, F.; Legnaro, M.; Luini, A.; Pacchetti, B.; Cosentino, M. A Novel Standardized Cannabis sativa L. Extract and Its Constituent Cannabidiol Inhibit Human Polymorphonuclear Leukocyte Functions. Int. J. Mol. Sci. 2019, 20, 1833. [Google Scholar] [CrossRef]
- Cosentino, M.; Legnaro, M.; Luini, A.; Ferrari, M.; Sodergren, M.; Pacchetti, B.; Marino, F. Effect of cannabidiol on cyclooxygenase type 1 and 2 expression and function in human neutrophils. Cannabis Cannaabinoid Res. 2022. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445. [Google Scholar] [CrossRef]
- Loo, T.T.; Gao, Y.; Lazarevic, V. Transcriptional regulation of CD4+ TH cells that mediate tissue inflammation. J. Leukoc. Biol. 2018, 104, 1069–1085. [Google Scholar] [CrossRef]
- Liu, X.; Lee, Y.S.; Yu, C.R.; Egwuagu, C.E. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008, 180, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018, 201, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review on their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377. [Google Scholar] [CrossRef] [PubMed]
- Angelina, A.; Pérez-Diego, M.; Maldonado, A.; Rückert, B.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A.; Palomares, O. The cannabinoid WIN55212-2 suppresses effector T-cell responses and promotes regulatory T cells in human tonsils. Allergy 2022, 77, 1029. [Google Scholar] [CrossRef]
- Pérez-Diego, M.; Angelina, A.; Martín-Cruz, L.; de la Rocha-Muñoz, A.; Maldonado, A.; Sevilla-Ortega, C.; Palomares, O. Cannabinoid WIN55,212-2 reprograms monocytes and macrophages to inhibit LPS-induced inflammation. Front. Immunol. 2023, 14, 1147520. [Google Scholar] [CrossRef]
- Sommer, A.; Winner, B.; Prots, I. The Trojan horse–neuroinflammatory impact of T cells in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, Y.; Bitar, L.; Zipp, F.; Vogelaar, C.F. Interleukin-4 as a therapeutic target. Pharmacol. Ther. 2023, 242, 108348. [Google Scholar] [CrossRef]
- Oestreich, K.J.; Weinmann, A.S. Transcriptional mechanisms that regulate T helper 1 cell differentiation. Curr. Opin. Immunol. 2012, 24, 191. [Google Scholar] [CrossRef]
- Chen, Z.; Laurence, A.; O’Shea, J.J. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin. Immunol. 2007, 19, 400. [Google Scholar] [CrossRef]
- Ho, I.C.; Tai, T.S.; Pai, S.Y. GATA3 and the T-cell lineage: Essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 2009, 9, 125. [Google Scholar] [CrossRef]
- Maier, E.; Duschl, A.; Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 2012, 42, 2827. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703. [Google Scholar] [CrossRef] [PubMed]
- Won, H.Y.; Hwang, E.S. Transcriptional modulation of regulatory T cell development by novel regulators NR4As. Arch. Pharm. Res. 2016, 39, 1530. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Comi, C.; Magistrelli, L.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Minafra, B.; Riboldazzi, G.; et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J. Neuroinflamm. 2018, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.; Terzaghi, M.; Storelli, E.; Magistrelli, L.; Comi, C.; Legnaro, M.; Mauri, M.; Marino, F.; Versino, M.; Cosentino, M. CD4+ T cell Transcription Factors in Idiopathic REM Sleep Behavior Disorder and Parkinson’s Disease. Mov. Disord. 2021, 36, 225. [Google Scholar] [CrossRef]
- Perin, P.; Mabou Tagne, A.; Enrico, P.; Marino, F.; Cosentino, M.; Pizzala, R.; Boselli, C. Cannabinoids, Inner Ear, Hearing, and Tinnitus: A Neuroimmunological Perspective. Front. Neurol. 2020, 11, 505995. [Google Scholar] [CrossRef]
- Smith, R.C.; Sershen, H.; Janowsky, D.S.; Lajtha, A.; Grieco, M.; Gangoiti, J.A.; Gertsman, I.; Johnson, W.S.; Marcotte, T.D.; Davis, J.M. Changes in Expression of DNA-Methyltransferase and Cannabinoid Receptor mRNAs in Blood Lymphocytes After Acute Cannabis Smoking. Front. Psychiatry 2022, 13, 887700. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, S.A.; Thompson, A. UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomized placebo-controlled trial. Lancet 2003, 362, 1517. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Omar Viswanath, O. An update of current cannabis-based pharmaceuticals in pain medicine. Pain Ther. 2019, 8, 41. [Google Scholar] [CrossRef]
- Hameed, M.; Prasad, S.; Jain, E.; Dogrul, B.N.; Al-Oleimat, A.; Pokhrel, B.; Chowdhury, S.; Co, E.L.; Mitra, S.; Quinonez, J.; et al. Medical Cannabis for Chronic Nonmalignant Pain Management. Curr. Pain Headache Rep. 2023, 10, 57–63. [Google Scholar] [CrossRef]
- Watzl, B.; Scuderi, P.; Watson, R.R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int. J. Immunopharmacol. 1991, 13, 1091. [Google Scholar] [CrossRef]
- Cosentino, M.; Fietta, A.M.; Ferrari, M.; Rasini, E.; Bombelli, R.; Carcano, E.; Saporiti, F.; Meloni, F.; Marino, F.; Lecchini, S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 2007, 109, 632. [Google Scholar] [CrossRef] [PubMed]

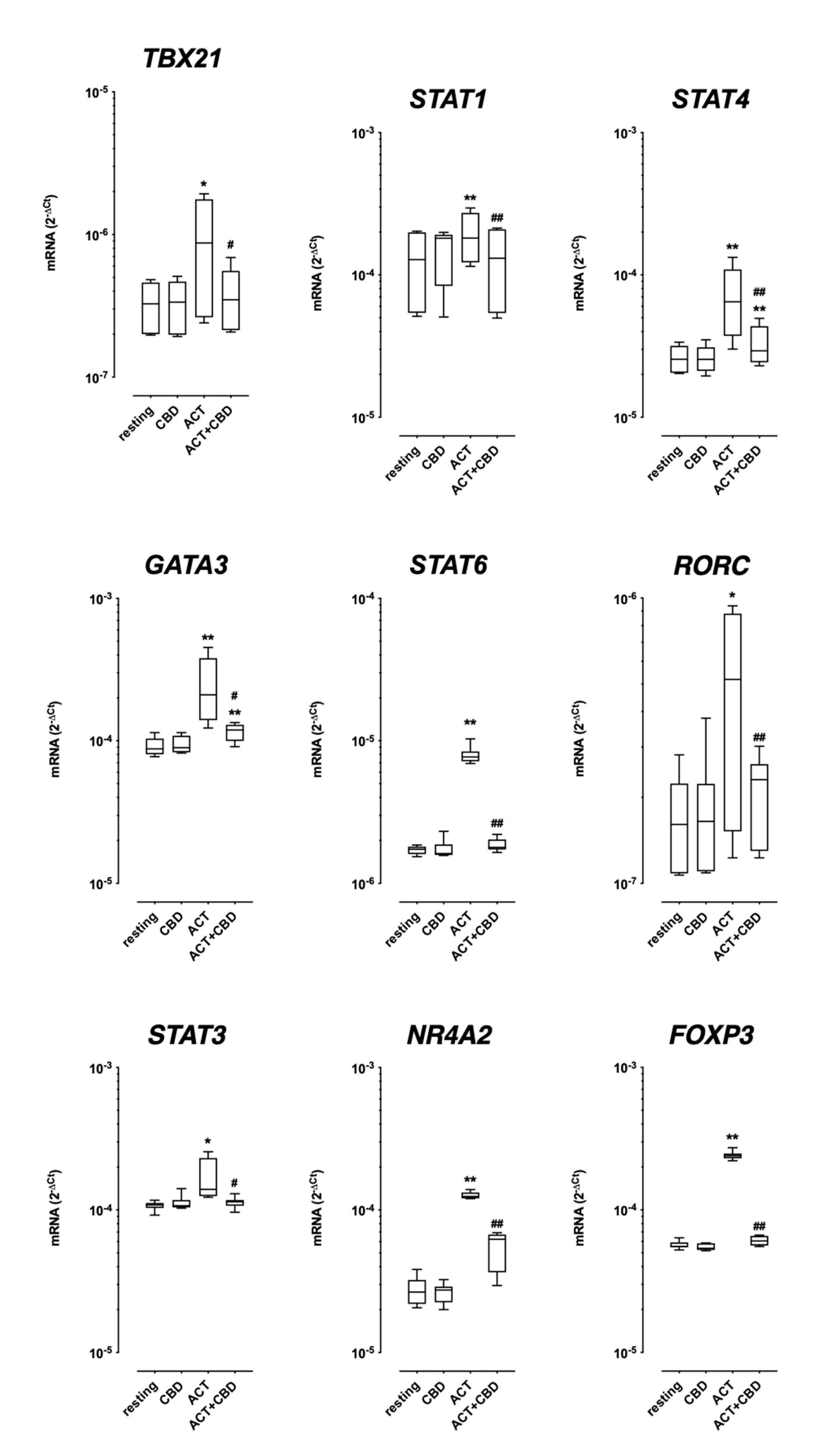
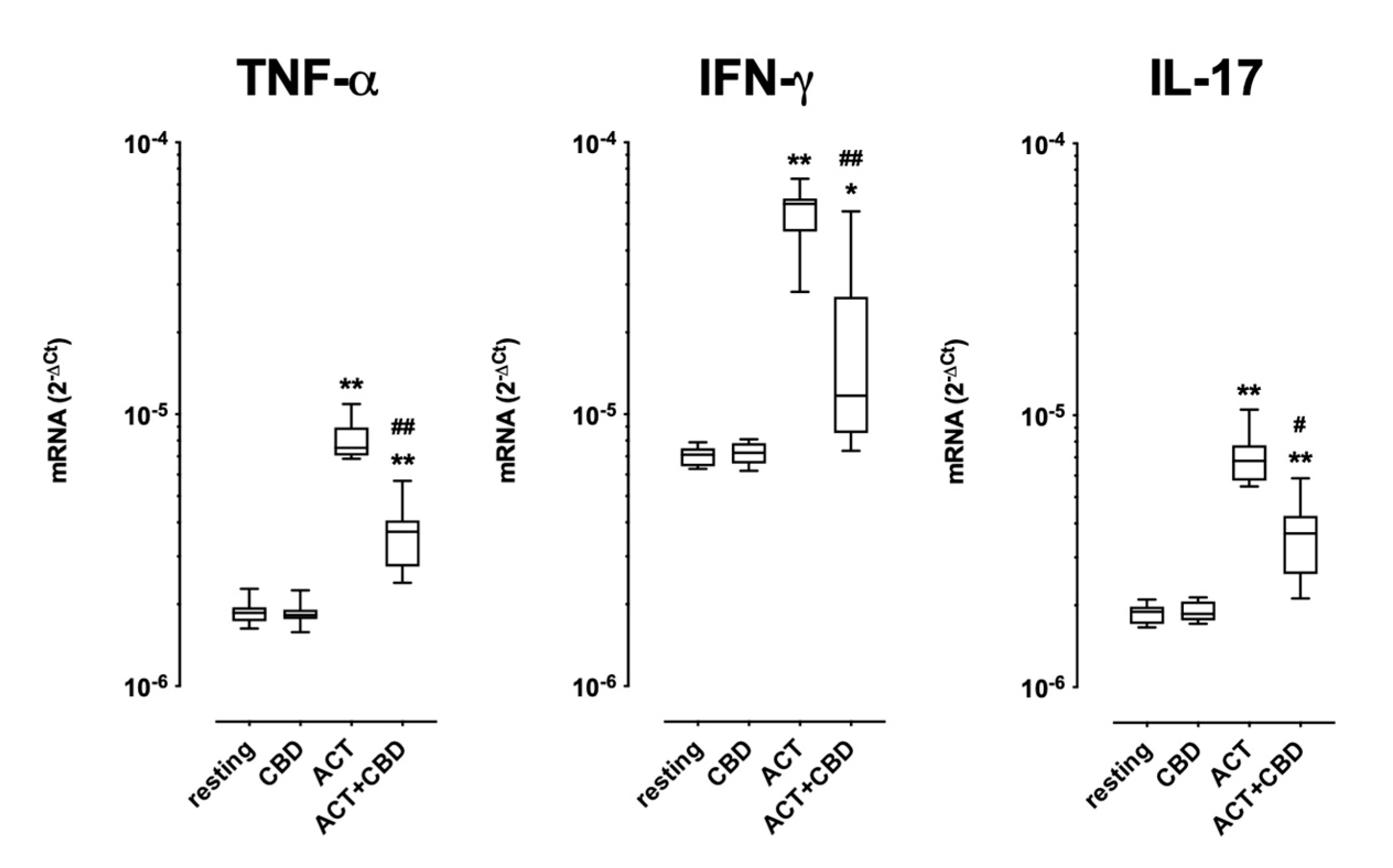
| Resting | Resting + CBD | Activated | P vs. Resting | Activated + CBD | P vs. Activated | |
|---|---|---|---|---|---|---|
| TNF-α | 24.3 ± 16.6 | 39.9 ± 23.9 | 2010.4 ± 1176.7 | 0.0005 | 2437.1 ± 1555.7 | ns |
| IFN-γ | 5.9 ± 2.4 | 5.5 ± 2.4 | 1251.3 ± 1631.8 | 0.039 | 1088.2 ± 1521.9 | ns |
| IL-17 | Under LOD | Under LOD | 36.8 ± 24.4 | 0.04 * | 33.6 ± 24.6 | ns |
| R | CBD | P vs. R | ACT | P vs. Resting | ACT + CBD | P vs. ACT Alone |
|---|---|---|---|---|---|---|
| 0.56 ± 0.30 | 0.78 ± 0.48 | ns | 50.90 ± 22.80 | <0.01 | 60.40 ± 21.20 | <0.05 |
| R | CBD | P vs. R | ACT Teff | P vs. R | ACT Teff/Treg | P vs. ACT Alone | ACT Teff/Treg + CBD | P vs. ACT Teff/Treg |
|---|---|---|---|---|---|---|---|---|
| 0.60 ± 0.20 | 0.58 ± 0.50 | ns | 71.40 ± 6.01 | <0.01 | 5.23 ± 2.38 | <0.001 | 6.67 ± 2.89 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furgiuele, A.; Marino, F.; Rasini, E.; Legnaro, M.; Luini, A.; Albizzati, M.G.; di Flora, A.; Pacchetti, B.; Cosentino, M. Effect of Cannabidiol on Human Peripheral Blood Mononuclear Cells and CD4+ T Cells. Int. J. Mol. Sci. 2023, 24, 14880. https://doi.org/10.3390/ijms241914880
Furgiuele A, Marino F, Rasini E, Legnaro M, Luini A, Albizzati MG, di Flora A, Pacchetti B, Cosentino M. Effect of Cannabidiol on Human Peripheral Blood Mononuclear Cells and CD4+ T Cells. International Journal of Molecular Sciences. 2023; 24(19):14880. https://doi.org/10.3390/ijms241914880
Chicago/Turabian StyleFurgiuele, Alessia, Franca Marino, Emanuela Rasini, Massimiliano Legnaro, Alessandra Luini, Maria Giulia Albizzati, Alessia di Flora, Barbara Pacchetti, and Marco Cosentino. 2023. "Effect of Cannabidiol on Human Peripheral Blood Mononuclear Cells and CD4+ T Cells" International Journal of Molecular Sciences 24, no. 19: 14880. https://doi.org/10.3390/ijms241914880
APA StyleFurgiuele, A., Marino, F., Rasini, E., Legnaro, M., Luini, A., Albizzati, M. G., di Flora, A., Pacchetti, B., & Cosentino, M. (2023). Effect of Cannabidiol on Human Peripheral Blood Mononuclear Cells and CD4+ T Cells. International Journal of Molecular Sciences, 24(19), 14880. https://doi.org/10.3390/ijms241914880







