Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition
Abstract
:1. Introduction
2. Results
2.1. Phenolic Acid and Total Dietary Fiber Content of Raw Grains
2.1.1. UPLC-MS/MS Characterization of PAs
2.1.2. Total PA and TDF Content of Raw Grains
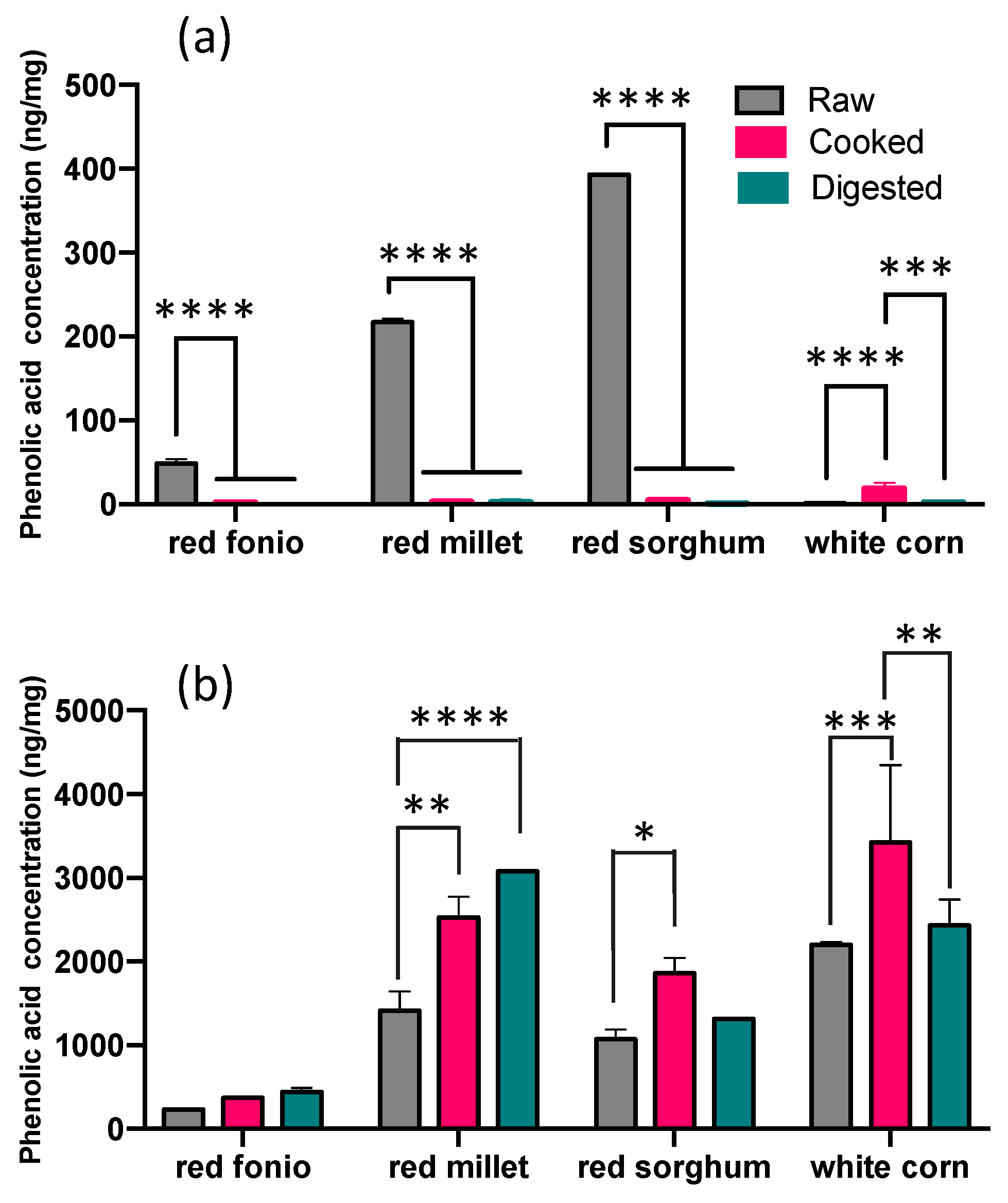
2.2. Impact of Cooking and Digestion on the Phenolic Acid Profile of Grains
Soluble and Bound PAs in Cooked and Digested Grains
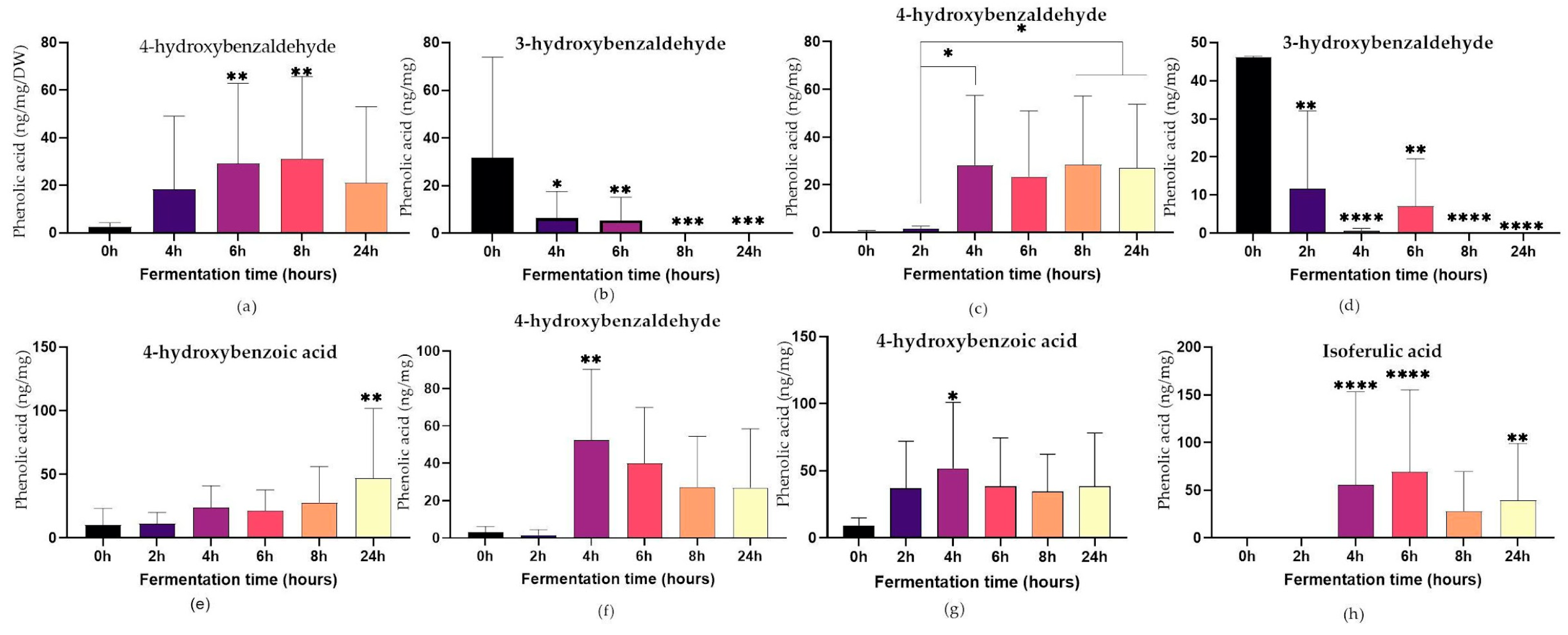
2.3. In Vitro Colonic Fermentation of Digested Grains
2.3.1. Phenolic Acid Content of Fermented Grains
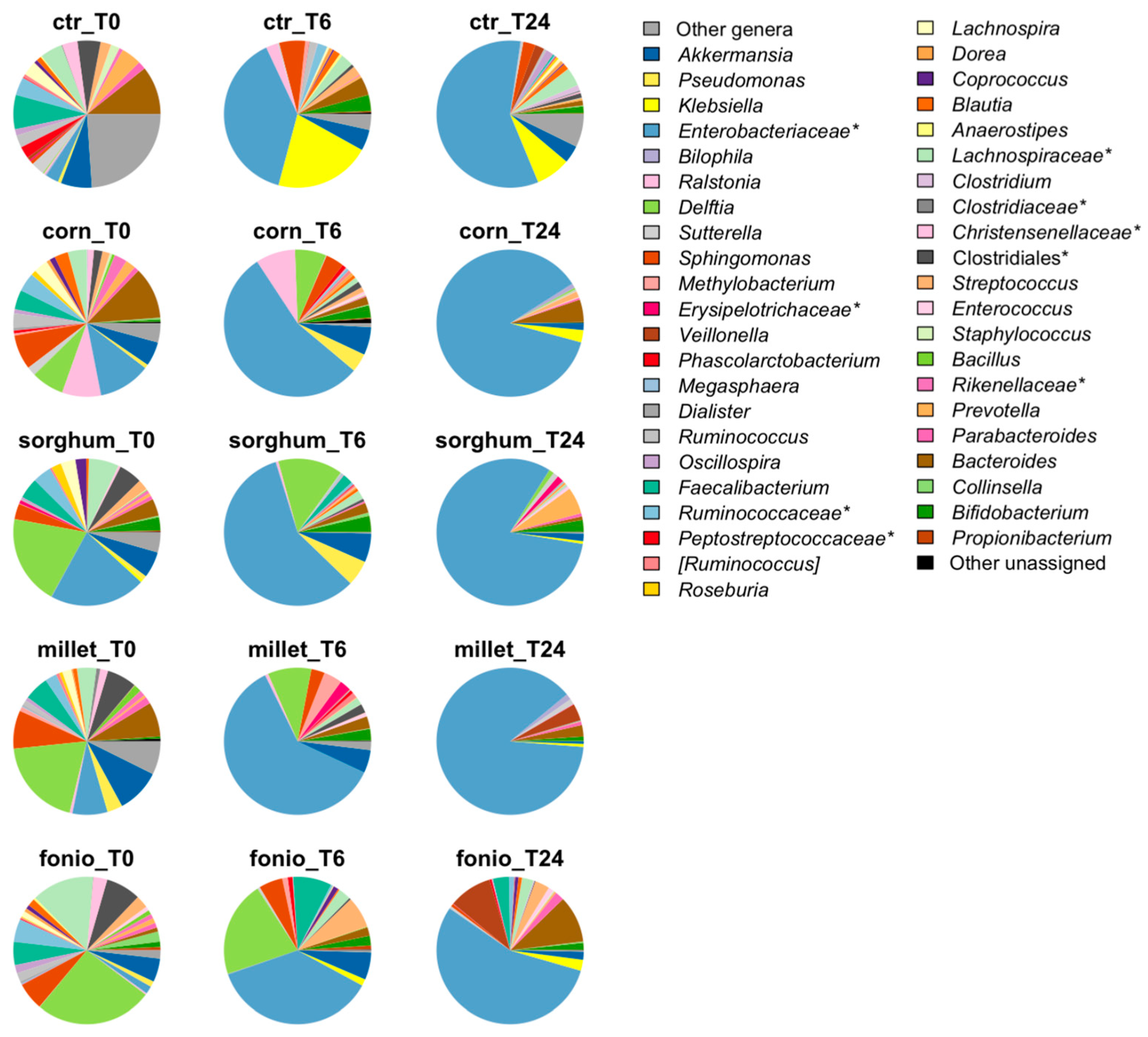
2.3.2. Impact of Digested Grains on Faecal-Derived Microbial Communities from Healthy Volunteers
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Wholegrains
4.3. Total Dietary Fibre (TDF) Determination
4.4. Wholegrain Processing (Cooking and Digestion)
4.5. Colonic Fermentation
4.5.1. Basal Media Composition
4.5.2. In Vitro Fermentation
4.6. PAs Extraction and Analysis
4.6.1. Solid Phase Extraction (SPE)
4.6.2. Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
4.7. Gut Microbiota Profiling
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Călinoiu, L.F.; Vodnar, D.C.; Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Mundia, C.W.; Secchi, S.; Akamani, K.; Wang, G. A regional comparison of factors affecting global sorghum production: The case of North America, Asia and Africa’s Sahel. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef]
- Cruz, J.-F.; Béavogui, F.; Dramé, D.; Diallo, T.A. Fonio, an African Cereal; CIRAD and IRAG Edition; CIRAD: Montpellier, France, 2016; 153p. [Google Scholar]
- National Research Council. Lost Crops of Africa; National Academies Press: Washington, DC, USA, 1996. [Google Scholar]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Fliedel, G.; Ouattara, M.; Grabulos, J.; Drame, D.; Cruz, J. Effect of Mechanical Bleaching on the Technological, Culinary and Nutritional Quality of Fonio, a West African Cereal; CIRAD: Montpellier, France, 2004. [Google Scholar]
- Grains and Legumes Nutrition Council. Nutrient Database of Grains and Legumes Produced in Australia: Pilot Study Results; Grains and Legumes Nutrition Council: South Perth, Australia, 2014. [Google Scholar]
- Grains & Legumes Nutrition Council Ltd. Nutrient Composition of Grains & Pseudo Grains; Grains & Legumes Nutrition Council Ltd.: South Perth, Australia, 2012. [Google Scholar]
- Mohapatra, D.; Patel, A.S.; Kar, A.; Deshpande, S.S.; Tripathi, M.K. Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chem. 2019, 271, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O.; Bolaji, O.T.; Abegunde, T.A.; Idowu-Adebayo, F.; Tiamiyu, H.K.; Adebayo-Oyetoro, A.O. Nutritional composition and heavy metal profile of Nigerian rice varieties. Curr. Res. Nutr. Food Sci. 2019, 7, 576–583. [Google Scholar] [CrossRef]
- Ebuehi, O.A.T.; Oyewole, A.C. Effect of cooking and soaking on physical, nutrient composition and sensory evaluation of indigenous and foreign rice varieties in Nigeria. Afr. J. Biotechnol. 2007, 6, 1016–1020. [Google Scholar]
- Hiran, P.; Kerdchoechuen, O.; Laohakunjit, N. Combined effects of fermentation and germination on nutritional compositions, functional properties and volatiles of maize seeds. J. Cereal Sci. 2016, 71, 207–216. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Oluseyi, T.; Folarin, B.; Adegbola, R.; Olayinka, K. Proximate analysis, mineral element and total phenolic contents of some commonly consumed grains in Nigeria. Ilorin J. Sci. 2021, 8, 91–102. [Google Scholar]
- Popoola, O.O. Phenolic compounds composition and in vitro antioxidant activity of Nigerian Amaranthus viridis seed as affected by autoclaving and germination. Meas. Food 2022, 6, 100028. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Fact. 2020, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef]
- Makame, J.; De Kock, H.; Emmambux, N.M. Nutrient density of common African indigenous/local complementary porridge samples. LWT 2020, 133, 109978. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdelaal, E.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Hager, A.S.; Wolter, A.; Jacob, F.; Zannini, E.; Arendt, E.K. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal Sci. 2012, 56, 239–247. [Google Scholar] [CrossRef]
- Das, A.K.; Singh, V. Antioxidative free and bound phenolic constituents in pericarp, germ and endosperm of Indian dent (Zea mays var. indentata) and flint (Zea mays var. indurata) maize. J. Funct. Foods 2015, 13, 363–374. [Google Scholar]
- Ryu, D.; Koh, E. Influence of Cooking Methods on Free and Bound Phenolic Acids in Korean Black Rice. J. Food Process. Preserv. 2017, 41, e12873. [Google Scholar] [CrossRef]
- Hithamani, G.; Srinivasan, K. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef]
- N’Dri, D.; Mazzeo, T.; Zaupa, M.; Ferracane, R.; Fogliano, V.; Pellegrini, N. Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal African cereals. J. Sci. Food Agric. 2013, 93, 29–36. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanin, phenolics and antioxidant activity changes in purple waxy corn as affected by traditional cooking. Food Chem. 2014, 164, 510–517. [Google Scholar] [CrossRef]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’Asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, N.J.; González-Aguilar, G.A.; Rouzaud-Sández, O.; Robles-Sánchez, M. Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, 6–15. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gaytán-Martínez, M.; Preciado-Ortiz, R.; Mendoza, S.; Loarca-Piña, G. Bioaccessibility and antioxidant activity of free phenolic compounds and oligosaccharides from corn (Zea mays L.) and common bean (Phaseolus vulgaris L.) chips during in vitro gastrointestinal digestion and simulated colonic fermentation. Food Res. Int. 2017, 100, 304–311. [Google Scholar] [CrossRef]
- Ruíz-García, Y.; Beres, C.; Chávez, D.W.H.; Pereira, D.C.d.S.; Santiago, M.C.P.A.; Godoy, R.d.O.; Gomes, F.d.S.; Antoniassi, R.; Tonon, R.V.; Cabral, L.M.C. In vitro digestion and colonic fermentation of an Alicante Bouschet (Vitis vinifera L.) skin extract. LWT 2022, 157, 113083. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Z.; Wu, G.; Cao, X.; Zhang, R.; Dong, L.; Huang, F.; Zhang, M.; Su, D. In vitro simulated digestion and colonic fermentation of lychee pulp phenolics and their impact on metabolic pathways based on fecal metabolomics of mice. Food Funct. 2021, 12, 203–214. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Zamora-Gasga, V.M.; Alvarez-Parrilla, E.; Ruíz-Valdiviezo, V.M.; Venema, K.; Sáyago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of tomato (Solanum lycopersicum L.) and husk tomato (Physalis ixocarpa Brot.): Phenolic compounds released and bioconverted by gut microbiota. Food Chem. 2021, 360, 130051. [Google Scholar] [CrossRef]
- Serra, A.; MacIà, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Hernández-Maldonado, L.M.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; Cárdenas-Castro, A.P.; Tovar, J.; Sáyago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of high dietary fiber and antioxidant-rich mango (Mangifera indica L.) “ataulfo”-based fruit bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chesson, A.; Provan, G.J.; Russell, W.R.; Scobbie, L.; Richardson, A.J.; Stewart, C. Hydroxycinnamic acids in the digestive tract of livestock and humans. J. Sci. Food Agric. 1999, 79, 373–378. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (poly)phenolic compounds in food fermentations by lactic acid bacteria: Novel insights into metabolic pathways and functional metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 710–737. [Google Scholar] [CrossRef]
- Bustamante, L.; Pastene, E.; Duran-Sandoval, D.; Vergara, C.; Von Baer, D.; Mardones, C. Pharmacokinetics of low molecular weight phenolic compounds in gerbil plasma after the consumption of calafate berry (Berberis microphylla) extract. Food Chem. 2018, 268, 347–354. [Google Scholar] [CrossRef]
- Kristek, A.; Wiese, M.; Heuer, P.; Kosik, O.; Schär, M.Y.; Soycan, G.; Alsharif, S.; Kuhnle, G.G.C.; Walton, G.; Spencer, J.P.E. Oat bran, but not its isolated bioactive β-glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Br. J. Nutr. 2019, 121, 549–559. [Google Scholar] [CrossRef]
- Hunter, W.J.; Manter, D.K.; Van Der Lelie, D. Biotransformation of ferulic acid to 4-vinylguaiacol by Enterobacter soli and E. aerogenes. Curr. Microbiol. 2012, 65, 752–757. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Yang, H.-J. The release and catabolism of ferulic acid in plant cell wall by rumen microbes: A review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 219. [Google Scholar] [CrossRef]
- Keman, D.; Soyer, F. Antibiotic-resistant Staphylococcus aureus does not develop resistance to vanillic acid and 2-hydroxycinnamic acid after continuous exposure in vitro. ACS Omega 2019, 4, 15393–15400. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Van Rymenant, E.; Van Camp, J.; Pauwels, B.; Boydens, C.; Vanden Daele, L.; Beerens, K.; Brouckaert, P.; Smagghe, G.; Kerimi, A.; Williamson, G. Ferulic acid-4-O-sulfate rather than ferulic acid relaxes arteries and lowers blood pressure in mice. J. Nutr. Biochem. 2017, 44, 44–51. [Google Scholar] [CrossRef]
- Baeza, G.; Bachmair, E.-M.; Wood, S.; Mateos, R.; Bravo, L.; De Roos, B. The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food Funct. 2017, 8, 1333–1342. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06780. [Google Scholar] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis. Annex G AOAC Official Method 991.43. Total, Soluble and Insoluble Dietary Fibre in Foods; AOAC: Rockville, MD, USA, 1995; pp. 7–9. [Google Scholar]
- Li, L.; Peter, R.S.; Jane, L.W. Phenolic Acids in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]





| Raw Grain | Total PAs (ng/mg) FW | % Soluble PAs | % Bound PAs | TDF (g/100 g) |
|---|---|---|---|---|
| Red fonio | 281.4 ± 10.8 a | 17.2 ± 1.9 | 82.8 ± 1.9 | 6.7 |
| White fonio | 132.2 ± 12.1 a | 5.1 ± 0.6 | 94.9 ± 9.7 | 3 |
| Red millet | 1631.2 ± 227.5 b | 13.3 ± 0.2 | 86.7 ± 14.1 | 11.4 |
| White millet | 1466.1 ± 187.1 b | 13.6 ± 1.0 | 86.4 ± 12.0 | 9.7 |
| Red sorghum | 1464.2 ± 184.2 b | 26.8 ± 0.0 | 73.2 ± 8.0 | 26.7 |
| White sorghum | 693.3 ± 38.0 c | 0.1 ± 0.0 | 99.9 ± 5.5 | 21.7 |
| White corn | 2202.5 ± 34.9 d | 0.1 ± 0.0 | 99.9 ± 1.6 | 14.3 |
| Ofada rice | 276.64 ± 25.31 a | 0.7 ± 0.3 | 99.3 ± 9.2 | 2.7 |
| Red Fonio | Red Millet | Red Sorghum | White Corn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | Raw | Cooked | Digested | Raw | Cooked | Digested | Raw | Cooked | Digested | Raw | Cooked | Digested |
| VA | 6.82 ± 1.33 | 1.02 ± 0.05 | 0.83 ± 0.12 | 37.60 ± 1.92 | 7.94 ± 1.23 | 8.20 ± 0.33 | 33.00 ± 3.52 | 4.60 ± 0.27 | 3.49 ± 0.45 | 5.87 ± 0.73 | 1.85 ± 0.66 | 1.28 ± 0.56 |
| IVA | ND | 0.00 ± 0.00 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 4-HBALD | 2.25 ± 0.14 | 3.47 ± 0.53 | 1.69 ± 0.10 | 7.63 ± 0.84 | 8.10 ± 1.13 | 9.19 ± 0.42 | 15.54 ± 0.31 | 7.52 ± 2.67 | 9.36 ± 0.70 | 4.37 ± 0.08 | 3.20 ± 0.18 | 3.01 ± 0.72 |
| 3-HBALD | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 4-HBA | 4.76 ± 0.41 | 2.57 ± 0.02 | 3.07 ± 0.03 | 43.58 ± 4.44 | 25.13 ± 0.63 | 21.22 ± 1.20 | 39.99 ± 0.02 | 41.57 ± 5.24 | 26.34 ± 2.10 | 2.03 ± 0.07 | 3.80 ± 0.24 | 1.92 ± 0.33 |
| 3-HBALD | 0.05 ± 0.07 | ND | 0.03 ± 0.04 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| SA | 0.46 ± 0.01 | 0.55 ± 0.04 | 0.24 ± 0.04 | 0.29 ± 0.05 | 0.30 ± 0.09 | 0.27 ± 0.05 | 0.43 ± 0.01 | 0.30 ± 0.06 | 0.13 ± 0.00 | 0.26 ± 0.06 | 1.14 ± 0.04 | 0.40 ± 0.00 |
| 4-HPA | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.26 ± 0.37 | ND | ND |
| 3-HPA | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2-HPA | 0.03 ± 0.04 | ND | ND | 0.41 ± 0.02 | ND | ND | ND | ND | ND | ND | ND | ND |
| VN | 0.50 ± 0.18 | 3.78 ± 1.15 | 2.32 ± 0.73 | 2.91 ± 0.20 | 13.92 ± 4.17 | 18.56 ± 1.49 | 1.08 ± 0.26 | 2.87 ± 1.82 | 2.94 ± 0.12 | 5.53 ± 0.21 | 9.57 ± 0.85 | 10.93 ± 3.58 |
| 3,4- and 3,5-DHBAs | 0.37 ± 0.04 | 0.44 ± 0.04 | 0.46 ± 0.02 | 2.04 ± 0.03 | 1.11 ± 0.08 | 1.33 ± 0.14 | 2.46 ± 0.02 | 1.85 ± 0.01 | 1.65 ± 0.23 | 0.06 ± 0.02 | 0.18 ± 0.01 | 0.10 ± 0.04 |
| 2,5-DHBA | ND | ND | 0.03 ± 0.05 | 0.03 ± 0.03 | 0.01 ± 0.02 | ND | ND | 0.05 ± 0.05 | 0.05 ± 0.02 | 0.02 ± 0.03 | 0.01 ± 0.02 | ND |
| 2,4-DHBA | 0.42 ± 0.08 | 2.07 ± 0.00 | 0.12 ± 0.16 | 0.30 ± 0.07 | 0.12 ± 0.04 | ND | 0.03 ± 0.04 | 0.01 ± 0.01 | ND | 0.76 ± 0.36 | 2.60 ± 0.00 | 0.98 ± 0.06 |
| pCA | 11.48 ± 3.10 | 19.79 ± 5.98 | 11.76 ± 1.66 | 298.47 ± 50.15 | 581.74 ± 21.79 | 554.45 ± 5.21 | 120.78 ± 15.74 | 117.67 ± 9.20 | 106.07 ± 47.90 | 209.83 ± 8.63 | 341.28 ± 19.74 | 284.05 ± 29.83 |
| GA | 0.02 ± 0.02 | 0.36 ± 0.02 | 0.45 ± 0.31 | 0.01 ± 0.01 | ND | ND | 0.01 ± 0.02 | 0.09 ± 0.12 | ND | ND | ND | ND |
| HA | ND | ND | 1.04 ± 0.19 | ND | ND | ND | 0.08 ± 0.11 | ND | ND | ND | ND | ND |
| CA | 7.94 ± 0.37 | 17.31 ± 0.12 | 23.38 ± 2.96 | 96.89 ± 5.16 | 127.87 ± 9.14 | 133.89 ± 7.81 | 252.30 ± 55.92 | 177.14 ± 24.65 | 156.17 ± 3.34 | 8.15 ± 0.38 | 22.96 ± 2.84 | 11.75 ± 4.87 |
| 3,4-DHCA | ND | ND | ND | ND | ND | ND | 0.03 ± 0.04 | 0.02 ± 0.03 | ND | 0.05 ± 0.07 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| HVA | 0.79 ± 1.12 | 15.79 ± 0.32 | 19.47 ± 2.79 | 100.20 ± 10.55 | 134.05 ± 3.52 | 123.71 ± 24.40 | 264.16 ± 63.75 | 173.96 | 146.22 ± 8.09 | 7.85 ± 0.10 | 29.23 ± 11.49 | 9.30 ± 13.15 |
| SYRALD | 0.20 ± 0.23 | ND | ND | 0.92 ± 0.14 | 2.30 ± 1.58 | 3.40 ± 0.02 | 0.06 ± 0.04 | ND | ND | 0.85 ± 0.08 | 0.06 ± 0.08 | 0.76 ± 1.07 |
| FA | 170.90 ± 8.50 | 232.81 ± 7.03 | 300.29 ± 56.19 | 880.57 ± 166.10 | 1500.71 ± 230.04 | 2038.59 ± 30.01 | 640.55 ± 35.17 | 1306.02 ± 120.10 | 828.01 ± 53.40 | 1797.34 ± 27.50 | 2884.67 ± 922.74 | 2023.40 ± 225.61 |
| IFA | 45.85 ± 0.11 | 55.81 ± 4.39 | 54.26 ± 14.21 | 74.69 ±.88 | 7.50 ± 1.02 | 49.71 ± 1.38 | 72.63 ± 10.06 | 8.33 ± 3.98 | 24.49 ± 2.81 | 124.92 ± 9.22 | 10.41 ± 1.75 | 29.50 ± 8.58 |
| HFA | ND | ND | 0.58 ± 0.82 | 0.04 ± 0.07 | ND | ND | ND | ND | ND | 0.01 ± 0.02 | ND | ND |
| SYRA | 17.52 ± 2.47 | 13.40 ± 2.92 | 19.80 ± 2.95 | 34.05 ± 0.95 | 74.21 ± 12.02 | 56.66 ± 8.31 | 8.52 ± 1.69 | 15.19 ± 1.86 | 8.98 ± 2.79 | 5.37 ± 1.40 | 42.49 ± 13.99 | 19.74 ± 7.14 |
| SNA | 11.05 ± 0.85 | 6.06 ± 0.34 | 4.79 ± 0.03 | 50.61 ± 7.23 | 45.83 ± 5.66 | 58.54 ± 5.99 | 12.53 ± 0.98 | 6.19 ± 1.75 | 3.63 ± 0.75 | 28.96 ± 7.44 | 91.58 ± 3.00 | 36.20 ± 15.77 |
| TOTAL | 281.40 ± 10.78 | 375.23 ± 8.25 | 444.62 ± 45.25 | 1631.24 ± 227.46 | 2530.83 ± 248.11 | 3077.71 ± 2.20 | 1464.17 ± 184.19 | 1863.37 ± 185.37 | 1317.51 ± 2.21 | 2202.51 ± 34.93 | 3445.04 ± 913.04 | 2433.33 ± 311.17 |
| Cereal Grain | Initial Weight (g) | Volume of Water Used (mL) | Cooking Time (min) | Cooling Time (min) | Final Weight (g) |
|---|---|---|---|---|---|
| Red fonio | 101.13 | 600 | 15 | 15 | 551.86 |
| Red millet | 100.29 | 550 | 36 | 15 | 178.92 |
| Red sorghum | 100.61 | 500 | 43 | 10 | 176.89 |
| White corn | 100.33 | 750 | 79 | 10 | 152.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obayiuwana, O.A.; Behrends, V.; Calle-Patino, Y.; Barone, M.; Turroni, S.; Brigidi, P.; Costabile, A.; Corona, G. Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition. Int. J. Mol. Sci. 2023, 24, 14111. https://doi.org/10.3390/ijms241814111
Obayiuwana OA, Behrends V, Calle-Patino Y, Barone M, Turroni S, Brigidi P, Costabile A, Corona G. Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition. International Journal of Molecular Sciences. 2023; 24(18):14111. https://doi.org/10.3390/ijms241814111
Chicago/Turabian StyleObayiuwana, Oghenerukevwe Anne, Volker Behrends, Yolanda Calle-Patino, Monica Barone, Silvia Turroni, Patrizia Brigidi, Adele Costabile, and Giulia Corona. 2023. "Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition" International Journal of Molecular Sciences 24, no. 18: 14111. https://doi.org/10.3390/ijms241814111
APA StyleObayiuwana, O. A., Behrends, V., Calle-Patino, Y., Barone, M., Turroni, S., Brigidi, P., Costabile, A., & Corona, G. (2023). Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition. International Journal of Molecular Sciences, 24(18), 14111. https://doi.org/10.3390/ijms241814111









