Current Challenges and Opportunities for Improved Cannabidiol Solubility
Abstract
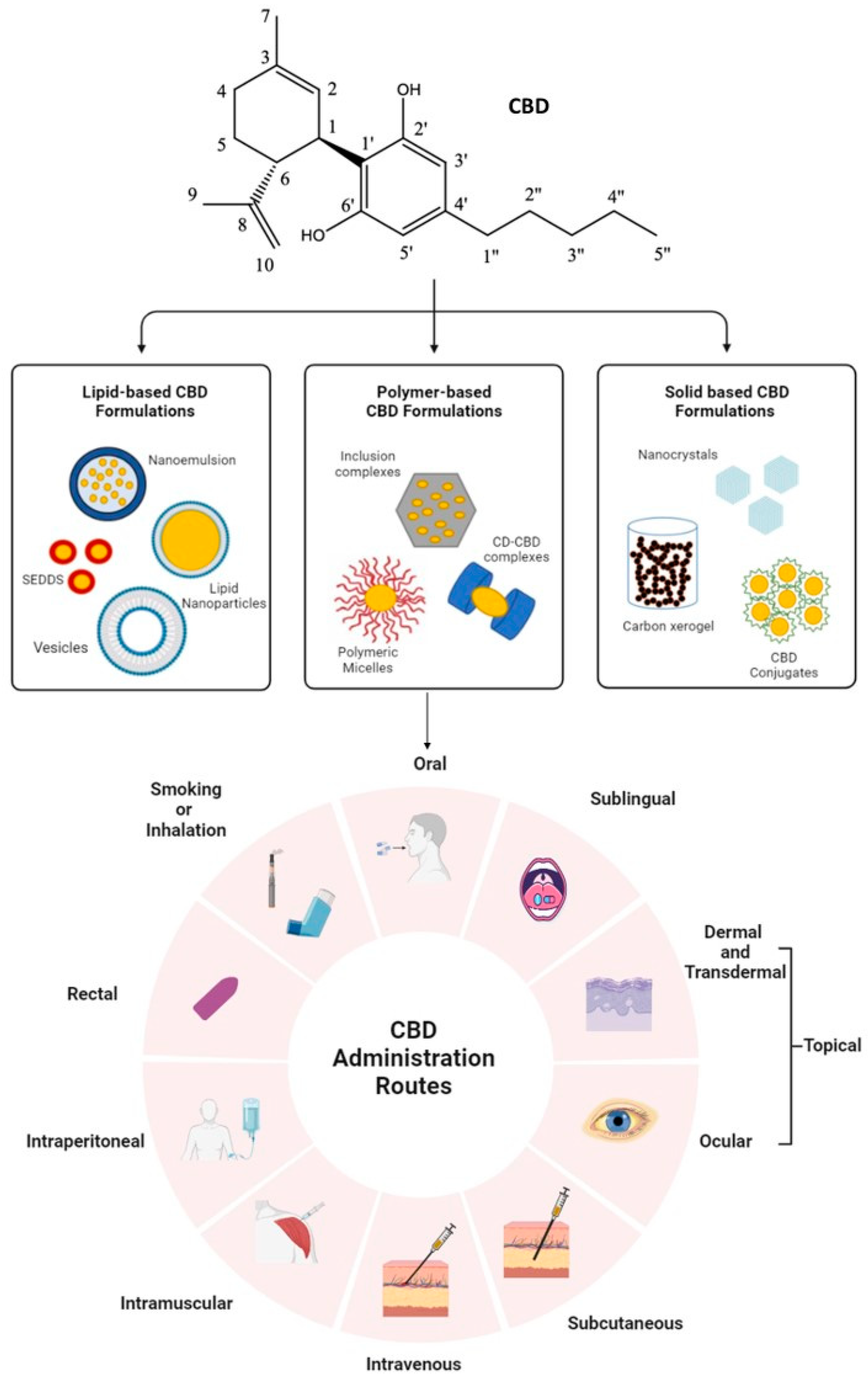
:1. Introduction
2. CBD Chemical Structure, Absorption, and Bioavailability
3. Factors Affecting the Bioavailability of CBD
4. Administration Routes of CBD
5. Approaches to Improve CBD Solubility and Bioavailability
6. Lipid-Based CBD Formulations
7. Polymer-Based CBD Inclusion Complexes
8. Solid-Based CBD Formulations
9. General Discussion
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Marinotti, O.; Sarill, M. Differentiating Full-Spectrum Hemp Extracts from CBD Isolates: Implications for Policy, Safety and Science. J. Diet. Suppl. 2020, 17, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.; Babalonis, S.; Walsh, S. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.; Abazia, D. Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharm. Ther. 2017, 42, 180. [Google Scholar]
- Balachandran, P.; Elsohly, M.; Hill, K. Cannabidiol interactions with medications, illicit substances, and alcohol: A comprehensive review. J. Gen. Intern. Med. 2021, 36, 2074–2084. [Google Scholar] [CrossRef]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.; Drago, F. Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Palrasu, M.; Wright, L.; Patel, M.; Leech, L.; Branch, S.; Harrelson, S.; Khan, S. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med. Cannabis Cannabinoids 2022, 5, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Laux, L.; Bebin, E.; Checketts, D.; Chez, M.; Flamini, R.; Marsh, E.; Miller, I.; Nichol, K.; Park, Y.; Segal, E. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res. 2019, 154, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.; Maguire, R.; Yates, A.; O’Sullivan, S. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Baban, B.; Khodadadi, H.; Salles, É.; Costigliola, V.; Morgan, J.; Hess, D.; Vaibhav, K.; Dhandapani, K.; Jack, C. Inflammaging and cannabinoids. Ageing Res. Rev. 2021, 72, 101487. [Google Scholar] [CrossRef]
- Takano, R.; Furumoto, K.; Shiraki, K.; Takata, N.; Hayashi, Y.; Aso, Y.; Yamashita, S. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs; prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharm. Res. 2008, 25, 2334–2344. [Google Scholar] [CrossRef]
- He, M.; Liu, A.; Shi, J.; Xu, Y.; Liu, Y. Multi-Omics Reveals the Effects of Cannabidiol on Gut Microbiota and Metabolic Phenotypes. Cannabis Cannabinoid Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Ibrahim, I.; Syamala, S.; Ayariga, J.; Xu, J.; Robertson, B.; Meenakshisundaram, S.; Ajayi, O. Modulatory Effect of Gut Microbiota on the Gut-Brain, Gut-Bone Axes, and the Impact of Cannabinoids. Metabolites 2022, 12, 1247. [Google Scholar] [CrossRef]
- Karoly, H.; Mueller, R.; Bidwell, L.; Hutchison, K. Cannabinoids and the Microbiota-Gut-Brain Axis: Emerging Effects of Cannabidiol and Potential Applications to Alcohol Use Disorders. Alcohol Clin. Exp. Res. 2020, 44, 340–353. [Google Scholar] [CrossRef]
- Elmes, M.; Kaczocha, M.; Berger, W.; Leung, K.; Ralph, B.; Wang, L.; Sweeney, J.; Miyauchi, J.; Tsirka, S.; Ojima, I.; et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef]
- Kaczocha, M.; Glaser, S.; Deutsch, D. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA 2009, 106, 6375–6380. [Google Scholar] [CrossRef]
- Duggan, P. The chemistry of cannabis and cannabinoids. Aust. J. Chem. 2021, 74, 369–387. [Google Scholar] [CrossRef]
- Hagan, K.; Varelas, P.; Zheng, H. Endocannabinoid System of the Blood–Brain Barrier: Current Understandings and Therapeutic Potentials. Cannabis Cannabinoid Res. 2022, 7, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Filer, C. Cannabinoid crystal polymorphism. J. Cannabis Res. 2022, 4, 23. [Google Scholar] [CrossRef]
- Mazzetti, C.; Ferri, E.; Pozzi, M.; Labra, M. Quantification of the content of cannabinol in commercially available e-liquids and studies on their thermal and photo-stability. Sci. Rep. 2020, 10, 3697. [Google Scholar] [CrossRef] [PubMed]
- Kosović, E.; Sýkora, D.; Kuchař, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtelova, G.; Nosková, K.; Turjap, M.; Sulcova, A.; Hanus, L.; Jurica, J. Cannabinoids and cytochrome P450 interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Chen, P.; Rogers, M. Opportunities and challenges in developing orally administered cannabis edibles. Curr. Opin. Food Sci. 2019, 28, 7–13. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Itin, C.; Barasch, D.; Domb, A.; Hoffman, A. Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef] [PubMed]
- Rabgay, K.; Waranuch, N.; Chaiyakunapruk, N.; Sawangjit, R.; Ingkaninan, K.; Dilokthornsakul, P. The effects of cannabis, cannabinoids, and their administration routes on pain control efficacy and safety: A systematic review and network meta-analysis. J. Am. Pharm. Assoc. 2020, 60, 225–234.e226. [Google Scholar] [CrossRef] [PubMed]
- Limpongsa, E.; Tabboon, P.; Pongjanyakul, T.; Jaipakdee, N. Preparation and Evaluation of Directly Compressible Orally Disintegrating Tablets of Cannabidiol Formulated Using Liquisolid Technique. Pharmaceutics 2022, 14, 2407. [Google Scholar] [CrossRef] [PubMed]
- Vlad, R.; Antonoaea, P.; Todoran, N.; Muntean, D.; Rédai, E.; Silași, O.; Tătaru, A.; Bîrsan, M.; Imre, S.; Ciurba, A. Pharmacotechnical and analytical preformulation studies for cannabidiol orodispersible tablets. Saudi Pharm. J. 2021, 29, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Vlad, R.; Antonoaea, P.; Todoran, N.; Rédai, E.; Bîrsan, M.; Muntean, D.; Imre, S.; Hancu, G.; Farczádi, L.; Ciurba, A. Development and Evaluation of Cannabidiol Orodispersible Tablets Using a 23-Factorial Design. Pharmaceutics 2022, 14, 1467. [Google Scholar] [CrossRef]
- Chan, J.; Duncan, R. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef]
- Bhat, T.A.; Kalathil, S.G.; Goniewicz, M.L.; Hutson, A.; Thanavala, Y. Not All Vaping Is the Same: Differential Pulmonary Effects of Vaping Cannabidiol Versus Nicotine. Thorax 2023, 2022, thoraxjnl. [Google Scholar]
- Tijani, A.; Thakur, D.; Mishra, D.; Frempong, D.; Chukwunyere, U.; Puri, A. Delivering therapeutic cannabinoids via skin: Current state and future perspectives. J. Control Release 2021, 334, 427–451. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Rebibo, L.; Frušić-Zlotkin, M.; Ofri, R.; Nassar, T.; Benita, S. The dose-dependent effect of a stabilized cannabidiol nanoemulsion on ocular surface inflammation and intraocular pressure. Int. J. Pharm. 2022, 617, 121627. [Google Scholar] [CrossRef]
- Shilo-Benjamini, Y.; Cern, A.; Zilbersheid, D.; Hod, A.; Lavy, E.; Barasch, D.; Barenholz, Y. A case report of subcutaneously injected liposomal cannabidiol formulation used as a compassion therapy for pain management in a dog. Front. Vet. Sci. 2022, 550, 892306. [Google Scholar] [CrossRef]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F. Pharmacokinetic and behavioural profile of THC, CBD, and THC+ CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, S.; Li, Z.; Chen, K.; Fan, H.; Wang, Y.; Xie, Z.; Kou, L.; Zhang, S. Enhanced intramuscular bioavailability of cannabidiol using nanocrystals: Formulation, in vitro appraisal, and pharmacokinetics. AAPS Pharm. Sci. Tech. 2022, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: In Vitro and In Ovo assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Ramalho, Í.; Pereira, D.; Galvão, G.; Freire, D.; Amaral-Machado, L.; Alencar, É.; Egito, E. Current trends on cannabidiol delivery systems: Where are we and where are we going? Expert Opin. Drug Deliv. 2021, 18, 1577–1587. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Romero, I.; Male, D.; Slowing, K.; García-García, L.; Torres-Suárez, A. Cannabidiol Enhances the Passage of Lipid Nanocapsules across the Blood-Brain Barrier Both in Vitro and in Vivo. Mol. Pharm. 2019, 16, 1999–2010. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.; Torres-Suárez, A. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2019, 134, 126–137. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control Release 2003, 93, 377–387. [Google Scholar] [CrossRef]
- Di Bello, M.; Bloise, E.; Mazzetto, S.; Giuseppe, M. Formulation and chemical stability in aqueous media of cannabidiol embedded in cardanol-based nanovesicles. ACS Sustain. Chem. Eng. 2017, 5, 8870–8875. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Domb, A.; Hoffman, A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur. J. Pharm. Sci. 2017, 109, 21–30. [Google Scholar] [CrossRef]
- Rani, S.; Rana, R.; Saraogi, G.; Kumar, V.; Gupta, U. Self-Emulsifying Oral Lipid Drug Delivery Systems: Advances and Challenges. AAPS PharmSciTech 2019, 20, 129. [Google Scholar] [CrossRef]
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a New Formulation Based on Pro-Nano Dispersion Technology, Improves Oral Cannabinoids Bioavailability in Healthy Volunteers. J. Pharm. Sci. 2018, 107, 1423–1429. [Google Scholar] [CrossRef]
- Atsmon, J.; Heffetz, D.; Deutsch, L.; Deutsch, F.; Sacks, H. Single-Dose Pharmacokinetics of Oral Cannabidiol Following Administration of PTL101: A New Formulation Based on Gelatin Matrix Pellets Technology. Clin. Pharmacol. Drug Dev. 2018, 7, 751–758. [Google Scholar] [CrossRef]
- Mitelpunkt, A.; Kramer, U.; Hausman Kedem, M.; Zilbershot Fink, E.; Orbach, R.; Chernuha, V.; Fattal-Valevski, A.; Deutsch, L.; Heffetz, D.; Sacks, H. The safety, tolerability, and effectiveness of PTL-101, an oral cannabidiol formulation, in pediatric intractable epilepsy: A phase II, open-label, single-center study. Epilepsy Behav. 2019, 987, 233–237. [Google Scholar] [CrossRef]
- Knaub, K.; Sartorius, T.; Dharsono, T.; Wacker, R.; Wilhelm, M.; Schön, C. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules 2019, 24, 2967. [Google Scholar] [CrossRef]
- Francke, N.; Schneider, F.; Baumann, K.; Bunjes, H. Formulation of Cannabidiol in Colloidal Lipid Carriers. Molecules 2021, 26, 1469. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef]
- Hernán Pérez de la Ossa, D.; Ligresti, A.; Gil-Alegre, M.; Aberturas, M.; Molpeceres, J.; Di Marzo, V.; Torres Suárez, A. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: Development, characterization and in vitro evaluation of their antitumoral efficacy. J. Control Release 2012, 161, 927–932. [Google Scholar] [CrossRef]
- Sosnik, A.; Shabo, R.; Halamish, H. Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery. Pharmaceutics 2021, 13, 2142. [Google Scholar] [CrossRef]
- Rao, Y.; Li, R.; Liu, S.; Meng, L.; Wu, Q.; Yuan, Q.; Liang, H.; Qin, M. Enhanced bioavailability and biosafety of cannabidiol nanomicelles for effective anti-inflammatory therapy. Particuology 2022, 69, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Sun, Y.; Freeman, K.; Mchenry, M.; Wang, C.; Guo, M. Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach. Foods 2022, 11, 376. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 35, 272–284. [Google Scholar] [CrossRef]
- Miranda, J.; Martins, T.; Veiga, F.; Ferraz, H. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Bethanis, K.; Koniari, E.; Christoforides, E.; Nikola, O.; Andreou, A.; Mantzou, A.; Chrousos, G.; Kanaka-Gantenbein, C.; Lambrou, G. Biophysical Studies and In Vitro Effects of Tumor Cell Lines of Cannabidiol and Its Cyclodextrin Inclusion Complexes. Pharmaceutics 2022, 14, 706. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhang, D.; Guo, M.; Lui, J.; Chen, X.; Guo, R.; Xu, Y.; Zhang, Q.; Liu, Y.; Guo, H.; et al. Structural analysis and cytotoxicity of host-guest inclusion complexes of cannabidiol with three native cyclodextrins. J. Drug Deliv. Sci. Technol. 2019, 51, 337–344. [Google Scholar] [CrossRef]
- Mannila, J.; Järvinen, T.; Järvinen, K.; Jarho, P. Precipitation complexation method produces cannabidiol/beta-cyclodextrin inclusion complex suitable for sublingual administration of cannabidiol. J. Pharm. Sci. 2007, 96, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mannila, J.; Järvinen, T.; Järvinen, K.; Tarvainen, M.; Jarho, P. Effects of RM-beta-CD on sublingual bioavailability of Delta9-tetrahydrocannabinol in rabbits. Eur. J. Pharm. Sci. 2005, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Lv, P.; Zhang, Y.; Liao, R.; Liu, J.; Guo, R.; Chen, X.; Liao, X.; Gao, C.; Zhang, K.; et al. Self-Assembly System Based on Cyclodextrin for Targeted Delivery of Cannabidiol. Front. Chem. 2021, 9, 754832. [Google Scholar] [CrossRef] [PubMed]
- Viernstein, H.; Toegel, S.; Schueller, R. Fast Disintegrating Compositions Comprising Nabilone and Randomly Methylated β-Cyclodextrin. U.S. Patent 13/989,540, 31 May 2012. [Google Scholar]
- Kingsley, K.; Lee, S.; Greenbaum, E. Cannabinoid Formulations with Improved Solubility. U.S. Patent 2019003017, 31 January 2019. [Google Scholar]
- Dickman, D.; Levin, D. Crystalline Form of Cannabidiol. U.S. Patent 20170349518, 7 December 2017. [Google Scholar]
- MacLeman, P.; Mavlianov, M. Solid Self-Emulsifying Pharmaceutical Compositions Comprising Cannabinoids. WO2020024009, 6 February 2020. [Google Scholar]
- MacLeman, P.; Mavlianov, M. Free-Flowing Powder Compositions for Oral Solid Dosages Containing Cannabinoids. WO2020024011, 6 February 2020. [Google Scholar]
- Anastassov, G.; Changoer, L. Chewing Gum Composition Comprising Cannabinoids and Opioid Agonists and/or Antagonists. U.S. Patent 20180110730, 26 April 2018. [Google Scholar]
- Changoer, L.; Anastassov, G. Chewing Gum Composition Comprising Cannabinoids and Nicotine. U.S. Patent 10842786, 24 November 2017. [Google Scholar]
- Robson, P.; Guy, G.; Pertwee, R.; Jamontt, J. Use of Tetrahydrocannabinol and/or Cannabidiol for the Treatment of Inflammatory Bowel Disease. U.S. Patent 20100286098, 11 November 2007. [Google Scholar]
- De Vries, J.A.; Fernandez Cid, M.V.; Lopez, A.M.H. Granulate Containing Cannabinoid, Method for Its Manufacture and Oral Dosage Unit Comprising Such Granulate. U.S. Patent 20150132400, 14 May 2015. [Google Scholar]
- De Vries, J.; Fernandez Cid, M.; Heredia Lopez, A.; Eiroa Martinez, C. Compressed Tablet Containing Cannabidiol, Method for Its Manufacture and Use of Such Tablet in Oral Treatment of Psychosis or Anxiety Disorders. WO2015065179, 7 May 2015. [Google Scholar]
- Abdul Khalil, H.; Yahya, E.; Tajarudin, H.; Balakrishnan, V.; Nasution, H. Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications. Gels 2022, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Cortés, F.; Zapata, K.; Rojano, B.; Carrasco-Marín, F.; Gallego, J.; Hernández, M.; Franco, C. Dual-Purpose Materials Based on Carbon Xerogel Microspheres (CXMs) for Delayed Release of Cannabidiol (CBD) and Subsequent Aflatoxin Removal. Molecules 2019, 24, 3398. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.; Callahan, M. Formulations of Cannabinoids for the Treatment of Acne. CA3053503, 23 August 2018. [Google Scholar]
- Cooper, E.; Callahan, M. Formulations of Cannabinoids for the Treatment of Psoriasis. WO2018148787, 23 August 2018. [Google Scholar]
- Cooper, E.; Callahan, M. Formulations of Cannabinoids for the Treatment of Dermatitis and Inflammatory Skin Diseases. WO2018148785, 23 August 2018. [Google Scholar]
- Heussler, H.; Cohen, J.; Silove, N.; Tich, N.; Bonn-Miller, M.; Du, W.; O’Neill, C.; Sebree, T. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J. Neurodev. Disord. 2019, 11, 16. [Google Scholar] [CrossRef]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlović, D.; Dujić, Ž.; Ainslie, P. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef]
- Harris, J.; Bentley, M.; Moreadith, R.; Viegas, T.; Fang, Z.; Yoon, K.; Weimer, R.; Dizman, B.; Nordstierna, L. Tuning drug release from polyoxazoline-drug conjugates. Eur. Polym. J. 2019, 120, 109241. [Google Scholar] [CrossRef]
- Hershberger, P.; Arlen, P. Cannabinoid Conjugate Molecules. WO2020263893, 30 December 2020. [Google Scholar]
- Salzman, A.; Flower, K.; Garner, C.; Jagtap, P.; Musa, S. Cbd Prodrugs, Compositions, and Methods of Administering cbd and cbd Prodrugs. WO2018096504, 31 May 2018. [Google Scholar]
- De Prá, M.; Vardanega, R.; Loss, C. Lipid-based formulations to increase cannabidiol bioavailability: In vitro digestion tests, pre-clinical assessment and clinical trial. Int. J. Pharm. 2021, 609, 121159. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Carneiro, S.; Costa Duarte, F.; Heimfarth, L.; Siqueira Quintans, J.; Quintans-Júnior, L.; Veiga Júnior, V.; Neves de Lima, Á. Cyclodextrin-Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- di Cagno, M. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2017, 22, 1. [Google Scholar] [CrossRef]
- Hirlekar, R.; Sonawane, S.; Kadam, V. Studies on the effect of water-soluble polymers on drug-cyclodextrin complex solubility. AAPS PharmSciTech 2009, 10, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Brzeziński, M.; Socka, M.; Baśko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, K.R.; Alghalayini, A.; Valenzuela, S.M. Current Challenges and Opportunities for Improved Cannabidiol Solubility. Int. J. Mol. Sci. 2023, 24, 14514. https://doi.org/10.3390/ijms241914514
Hossain KR, Alghalayini A, Valenzuela SM. Current Challenges and Opportunities for Improved Cannabidiol Solubility. International Journal of Molecular Sciences. 2023; 24(19):14514. https://doi.org/10.3390/ijms241914514
Chicago/Turabian StyleHossain, Khondker Rufaka, Amani Alghalayini, and Stella M. Valenzuela. 2023. "Current Challenges and Opportunities for Improved Cannabidiol Solubility" International Journal of Molecular Sciences 24, no. 19: 14514. https://doi.org/10.3390/ijms241914514
APA StyleHossain, K. R., Alghalayini, A., & Valenzuela, S. M. (2023). Current Challenges and Opportunities for Improved Cannabidiol Solubility. International Journal of Molecular Sciences, 24(19), 14514. https://doi.org/10.3390/ijms241914514







